Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment
Abstract
1. Introduction
2. Epidemiology
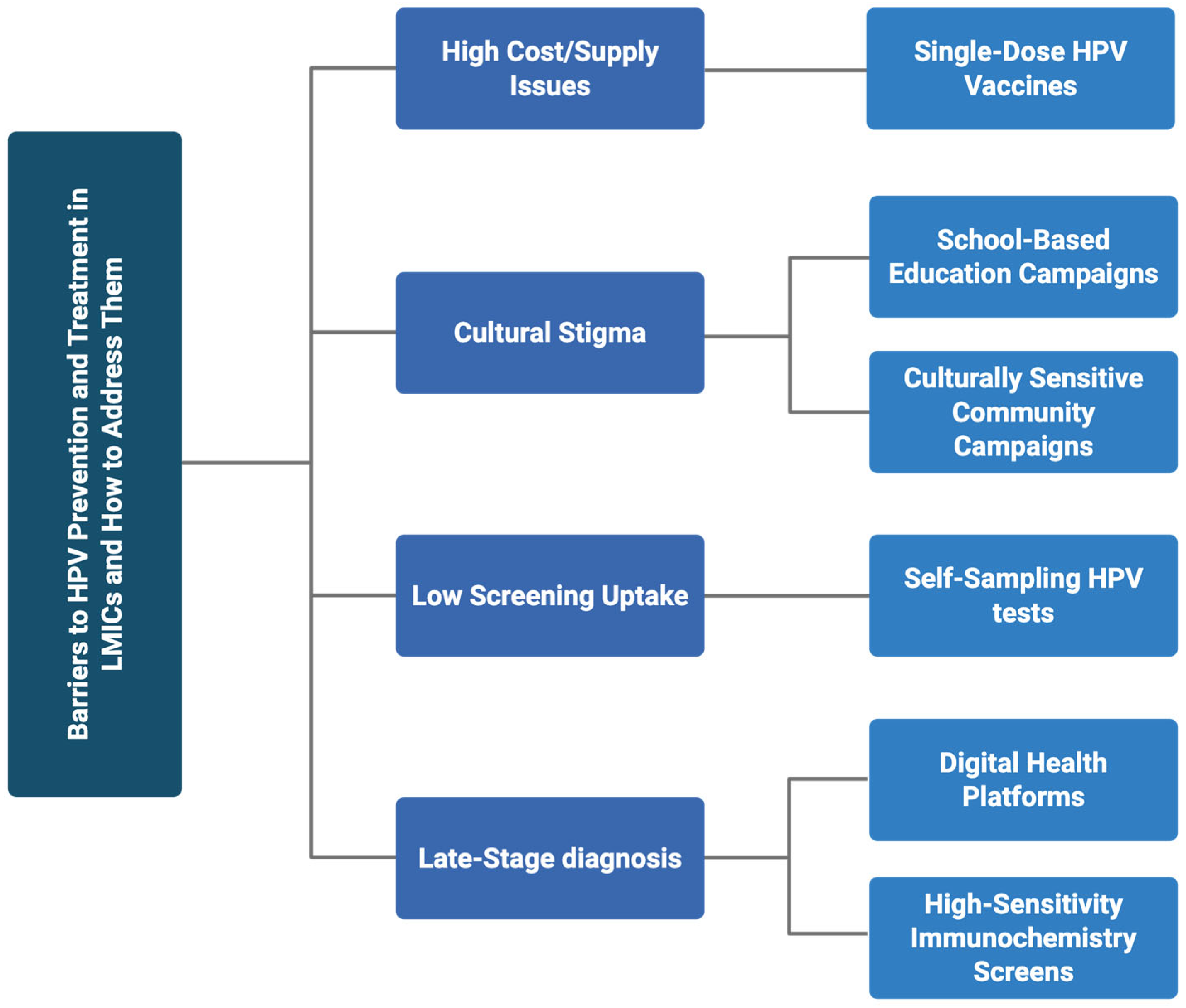
3. Multilevel Barriers to HPV Prevention and Treatment in LMICs
4. Types of HPV-Induced Cancers
5. Mechanism of HPV-Induced Cancers
6. Treatment
7. Methods of Prevention
8. Recent Advances
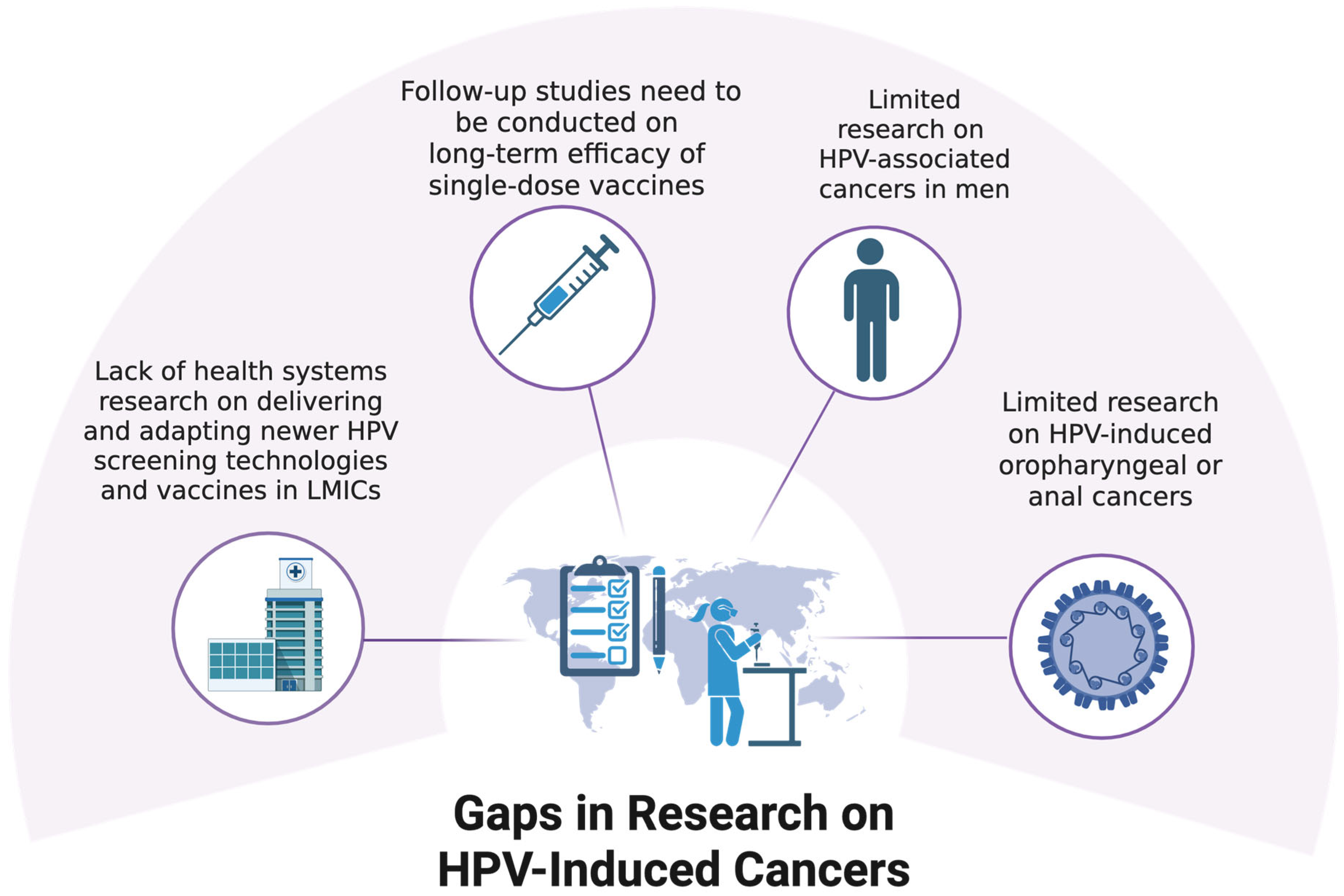
9. Gaps in Research on HPV-Induced Cancers
Author Contributions
Funding
Conflicts of Interest
References
- Dabán-López, P.; Fernández-Martínez, N.F.; Petrova, D.; Rodríguez-Barranco, M.; Jiménez-Moleón, J.J.; Gutierrez, J.; Sánchez, M.J. Epidemiology of human papillomavirus-associated anogenital cancers in Granada: A three-decade population-based study. Front. Public Health 2023, 11, 1205170. [Google Scholar] [CrossRef] [PubMed]
- Longworth, M.S.; Laimins, L.A. Pathogenesis of human papillomaviruses in differentiating epithelia. Microbiol. Mol. Biol. Rev. 2004, 68, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Mpunga, T.; Chantal Umulisa, M.; Tenet, V.; Rugwizangoga, B.; Milner, D.A., Jr.; Munyanshongore, C.; Heideman, D.A.M.; Bleeker, M.C.G.; Tommasino, M.; Franceschi, S.; et al. Human papillomavirus genotypes in cervical and other HPV-related anogenital cancer in Rwanda, according to HIV status. Int. J. Cancer 2020, 146, 1514–1522. [Google Scholar] [CrossRef]
- Falcaro, M.; Soldan, K.; Ndlela, B.; Sasieni, P. Effect of the HPV vaccination programme on incidence of cervical cancer and grade 3 cervical intraepithelial neoplasia by socioeconomic deprivation in England: Population based observational study. BMJ 2024, 385, e077341. [Google Scholar] [CrossRef] [PubMed]
- Goldstone, S.E. Human papillomavirus (HPV) vaccines in adults: Learnings from long-term follow-up of quadrivalent HPV vaccine clinical trials. Hum. Vaccines Immunother. 2023, 19, 2184760. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Tota, J.E.; Chevarie-Davis, M.; Richardson, L.A.; Devries, M.; Franco, E.L. Epidemiology and burden of HPV infection and related diseases: Implications for prevention strategies. Prev. Med. 2011, 53 (Suppl. 1), S12–S21. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Rowley, J.; Alemany, L.; Arbyn, M.; Giuliano, A.R.; Markowitz, L.E.; Broutet, N.; Taylor, M. Global and regional estimates of genital human papillomavirus prevalence among men: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e1345–e1362. [Google Scholar] [CrossRef]
- Bruzzesi, E.; Gandini, F.; Diotallevi, S.; Lolatto, R.; Cernuschi, M.; Candela, C.; Raccagni, A.R.; Passini, F.; Tamburini, A.M.; Burioni, R.; et al. High Prevalence of High-Risk HPV Among People with and Without HIV: Insights into Risk Factors for Tailored Screening Approaches. Microorganisms 2024, 12, 2571. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lázaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef] [PubMed]
- Getachew, S.; Getachew, E.; Gizaw, M.; Ayele, W.; Addissie, A.; Kantelhardt, E.J. Cervical cancer screening knowledge and barriers among women in Addis Ababa, Ethiopia. PLoS ONE 2019, 14, e0216522. [Google Scholar] [CrossRef] [PubMed]
- Scarinci, I.C.; Beech, B.M.; Kovach, K.W.; Bailey, T.L. An examination of sociocultural factors associated with cervical cancer screening among low-income Latina immigrants of reproductive age. J. Immigr. Health 2003, 5, 119–128. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Farajimakin, O. Barriers to Cervical Cancer Screening: A Systematic Review. Cureus 2024, 16, e65555. [Google Scholar] [CrossRef]
- Slavkovsky, R.; Callen, E.; Pecenka, C.; Mvundura, M. Costs of human papillomavirus vaccine delivery in low- and middle-income countries: A systematic review. Vaccine 2024, 42, 1200–1210. [Google Scholar] [CrossRef]
- Ochalek, J.; Abbas, K.; Claxton, K.; Jit, M.; Lomas, J. Assessing the value of human papillomavirus vaccination in Gavi-eligible low-income and middle-income countries. BMJ Glob. Health 2020, 5, e003006. [Google Scholar] [CrossRef]
- Srinath, A.; van Merode, F.; Rao, S.V.; Pavlova, M. Barriers to cervical cancer and breast cancer screening uptake in low- and middle-income countries: A systematic review. Health Policy Plan 2023, 38, 509–527. [Google Scholar] [CrossRef]
- Mabelele, M.M.; Materu, J.; Ng’ida, F.D.; Mahande, M.J. Knowledge towards cervical cancer prevention and screening practices among women who attended reproductive and child health clinic at Magu district hospital, Lake Zone Tanzania: A cross-sectional study. BMC Cancer 2018, 18, 565. [Google Scholar] [CrossRef]
- Castrillo-Diez, J.L.; Rivera-Santiago, C.; Ávila-Flores, S.M.; Barrera-Barrera, S.A.; Barrera-Saldaña, H.A. Findings and Challenges in Replacing Traditional Uterine Cervical Cancer Diagnosis with Molecular Tools in Private Gynecological Practice in Mexico. Viruses 2024, 16, 887. [Google Scholar] [CrossRef] [PubMed]
- Ogbolu, M.O.; Eniade, O.D.; Majiya, H.; Kozlovszky, M. Factors Associated with HPV Genital Warts: A Self-Reported Cross-Sectional Study among Students and Staff of a Northern University in Nigeria. Viruses 2024, 16, 902. [Google Scholar] [CrossRef] [PubMed]
- Alkhamis, F.H.; Alabbas, Z.A.S.; Al Mulhim, J.E.; Alabdulmohsin, F.F.; Alshaqaqiq, M.H.; Alali, E.A. Prevalence and Predictive Factors of Cervical Cancer Screening in Saudi Arabia: A Nationwide Study. Cureus 2023, 15, e49331. [Google Scholar] [CrossRef] [PubMed]
- Gari, A.; Ghazzawi, M.A.; Ghazzawi, S.A.; Alharthi, S.M.; Yanksar, E.A.; Almontashri, R.M.; Batarfi, R.; Kinkar, L.I.; Baradwan, S. Knowledge about cervical cancer risk factors and human papilloma virus vaccine among Saudi women of childbearing age: A community-based cross-sectional study from Saudi Arabia. Vaccine X 2023, 15, 100361. [Google Scholar] [CrossRef]
- Al-Darwish, A.A.; Al-Naim, A.F.; Al-Mulhim, K.S.; Al-Otaibi, N.K.; Morsi, M.S.; Aleem, A.M. Knowledge about cervical cancer early warning signs and symptoms, risk factors and vaccination among students at a medical school in Al-Ahsa, Kingdom of Saudi Arabia. Asian Pac. J. Cancer Prev. 2014, 15, 2529–2532. [Google Scholar] [CrossRef]
- Aldawood, E.; Dabbagh, D.; Alharbi, S.; Alzamil, L.; Faqih, L.; Alshurafa, H.H.; Dabbagh, R. HPV Vaccine Knowledge and Hesitancy Among Health Colleges’ Students at a Saudi University. J. Multidiscip. Healthc. 2023, 16, 3465–3476. [Google Scholar] [CrossRef]
- Guimaraes, M.J.; Macieira, R.; Azevedo, F.; Lisboa, C. Association between HPV infection and penile cancer and penile intraepithelial neoplasia: A retrospective observational study. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 186–190. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Vaccarella, S.; Lortet-Tieulent, J.; Plummer, M.; Franceschi, S.; Bray, F. Worldwide trends in cervical cancer incidence: Impact of screening against changes in disease risk factors. Eur. J. Cancer 2013, 49, 3262–3273. [Google Scholar] [CrossRef]
- Kang, Y.J.; Smith, M.; Canfell, K. Anal cancer in high-income countries: Increasing burden of disease. PLoS ONE 2018, 13, e0205105. [Google Scholar] [CrossRef]
- Moscicki, A.B.; Ma, Y.; Farhat, S.; Jay, J.; Hanson, E.; Benningfield, S.; Jonte, J.; Godwin-Medina, C.; Wilson, R.; Shiboski, S. Natural history of anal human papillomavirus infection in heterosexual women and risks associated with persistence. Clin. Infect. Dis. 2014, 58, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Akagi, K.; Li, J.; Broutian, T.R.; Padilla-Nash, H.; Xiao, W.; Jiang, B.; Rocco, J.W.; Teknos, T.N.; Kumar, B.; Wangsa, D.; et al. Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res. 2014, 24, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Rossi, N.M.; Dai, J.; Xie, Y.; Wangsa, D.; Heselmeyer-Haddad, K.; Lou, H.; Boland, J.F.; Yeager, M.; Orozco, R.; Freites, E.A.; et al. Extrachromosomal Amplification of Human Papillomavirus Episomes Is a Mechanism of Cervical Carcinogenesis. Cancer Res. 2023, 83, 1768–1781. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, G.; Leventakou, D.; Saltiel, D.R.; Zervoudi, E.; Logotheti, E.; Pettas, S.; Karagianni, K.; Daiou, A.; Hatzistergos, K.E.; Dafou, D.; et al. HPV16 E6 Oncogene Contributes to Cancer Immune Evasion by Regulating PD-L1 Expression through a miR-143/HIF-1a Pathway. Viruses 2024, 16, 113. [Google Scholar] [CrossRef]
- Hwang, S.G.; Lee, D.; Kim, J.; Seo, T.; Choe, J. Human papillomavirus type 16 E7 binds to E2F1 and activates E2F1-driven transcription in a retinoblastoma protein-independent manner. J. Biol. Chem. 2002, 277, 2923–2930. [Google Scholar] [CrossRef]
- Hong, S.; Laimins, L.A. Manipulation of the innate immune response by human papillomaviruses. Virus Res. 2017, 231, 34–40. [Google Scholar] [CrossRef]
- Ntuli, L.; Mtshali, A.; Mzobe, G.; Liebenberg, L.J.; Ngcapu, S. Role of Immunity and Vaginal Microbiome in Clearance and Persistence of Human Papillomavirus Infection. Front. Cell. Infect. Microbiol. 2022, 12, 927131. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Li, C. The role of TLRs in cervical cancer with HPV infection: A review. Signal Transduct. Target. Ther. 2017, 2, 17055. [Google Scholar] [CrossRef]
- Goodwin, E.C.; Yang, E.; Lee, C.J.; Lee, H.W.; DiMaio, D.; Hwang, E.S. Rapid induction of senescence in human cervical carcinoma cells. Proc. Natl. Acad. Sci. USA 2000, 97, 10978–10983. [Google Scholar] [CrossRef]
- Purohit, S.; Zhi, W.; Ferris, D.G.; Alverez, M.; Tran, L.K.H.; Tran, P.M.H.; Dun, B.; Hopkins, D.; Santos, B.D.; Ghamande, S.; et al. Senescence-Associated Secretory Phenotype Determines Survival and Therapeutic Response in Cervical Cancer. Cancers 2020, 12, 2899. [Google Scholar] [CrossRef]
- Ellerbrock, T.V.; Chiasson, M.A.; Bush, T.J.; Sun, X.W.; Sawo, D.; Brudney, K.; Wright, T.C., Jr. Incidence of cervical squamous intraepithelial lesions in HIV-infected women. JAMA 2000, 283, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Suzuki, T.; Nakamura, M.; Yamamoto, M.; Iizuka, T.; Ono, M.; Kagami, K.; Kasama, H.; Kanda, T.; Sakai, Y.; et al. Androgen promotes squamous differentiation of atypical cells in cervical intraepithelial neoplasia via an ELF3-dependent pathway. Cancer Med. 2023, 12, 10816–10828. [Google Scholar] [CrossRef] [PubMed]
- Dunne, E.F.; Unger, E.R.; Sternberg, M.; McQuillan, G.; Swan, D.C.; Patel, S.S.; Markowitz, L.E. Prevalence of HPV infection among females in the United States. JAMA 2007, 297, 813–819. [Google Scholar] [CrossRef]
- Partridge, J.M.; Hughes, J.P.; Feng, Q.; Winer, R.L.; Weaver, B.A.; Xi, L.F.; Stern, M.E.; Lee, S.K.; O’Reilly, S.F.; Hawes, S.E.; et al. Genital human papillomavirus infection in men: Incidence and risk factors in a cohort of university students. J. Infect. Dis. 2007, 196, 1128–1136. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, M.; Nie, H.; Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccines Immunother. 2019, 15, 1111–1122. [Google Scholar] [CrossRef]
- Song, Z.; Zou, K.; Zou, L. Immune checkpoint blockade for locally advanced or recurrent/metastatic cervical cancer: An update on clinical data. Front. Oncol. 2022, 12, 1045481. [Google Scholar] [CrossRef]
- Tsuda, N.; Watari, H.; Ushijima, K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin. J. Cancer Res. 2016, 28, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Ferreira da Silva, I.; Ferreira da Silva, I.; Koifman, R.J. Cervical Cancer Treatment Delays and Associated Factors in a Cohort of Women From a Developing Country. J. Glob. Oncol. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Arbyn, M.; Raifu, A.O.; Weiderpass, E.; Bray, F.; Anttila, A. Trends of cervical cancer mortality in the member states of the European Union. Eur. J. Cancer 2009, 45, 2640–2648. [Google Scholar] [CrossRef]
- Ekaterina, K.; Irakli, K.; Elene, K.; Ana, M.; Mariam, A. A Comparative Study of Conventional Pap Smear and Liquid-Based Cytology. Health Sci. Rep. 2025, 8, e70768. [Google Scholar] [CrossRef] [PubMed]
- Poli, U.R.; Bidinger, P.D.; Gowrishankar, S. Visual Inspection with Acetic Acid (VIA) Screening Program: 7 Years Experience in Early Detection of Cervical Cancer and Pre-Cancers in Rural South India. Indian J. Community Med. 2015, 40, 203–207. [Google Scholar] [CrossRef]
- Cubie, H.A.; Canham, M.; Moore, C.; Pedraza, J.; Graham, C.; Cuschieri, K. Evaluation of commercial HPV assays in the context of post-treatment follow-up: Scottish Test of Cure Study (STOCS-H). J. Clin. Pathol. 2014, 67, 458–463. [Google Scholar] [CrossRef]
- Di Gennaro, G.; Licata, F.; Trovato, A.; Bianco, A. Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front. Public Health 2022, 10, 1003461. [Google Scholar] [CrossRef]
- de Sanjose, S.; Quint, W.G.; Alemany, L.; Geraets, D.T.; Klaustermeier, J.E.; Lloveras, B.; Tous, S.; Felix, A.; Bravo, L.E.; Shin, H.R.; et al. Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.M.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Harper, D.M.; Leodolter, S.; Tang, G.W.; Ferris, D.G.; Steben, M.; Bryan, J.; et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 2007, 356, 1928–1943. [Google Scholar] [CrossRef]
- Paz-Zulueta, M.; Álvarez-Paredes, L.; Rodríguez Díaz, J.C.; Parás-Bravo, P.; Andrada Becerra, M.E.; Rodríguez Ingelmo, J.M.; Ruiz García, M.M.; Portilla, J.; Santibañez, M. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer 2018, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Bénard, É.; Drolet, M.; Laprise, J.F.; Gingras, G.; Jit, M.; Boily, M.C.; Bloem, P.; Brisson, M. Potential population-level effectiveness of one-dose HPV vaccination in low-income and middle-income countries: A mathematical modelling analysis. Lancet Public Health 2023, 8, e788–e799. [Google Scholar] [CrossRef]
- Avila, A.; Cordero, J.; Ibilah, O.; Frietze, G.; Moya, E.M. Hispanic Survivors and Caregivers of Human Papillomavirus-Associated Cancers: Lived Experiences in a U.S.-Mexico Border Community. Health Educ. Behav. 2023, 50, 595–603. [Google Scholar] [CrossRef]
- Fisher, C.L.; Mullis, M.D.; McFarlane, A.; Hansen, M.D.; Vilaro, M.J.; Bylund, C.L.; Wiggins, L.; Corbitt, H.; Staras, S.A.S. Promoting Rural-Residing Parents’ Receptivity to HPV Vaccination: Targeting Messages and Mobile Clinic Implementation. Vaccines 2024, 12, 712. [Google Scholar] [CrossRef]
- Francis, D.B.; Cates, J.R.; Wagner, K.P.G.; Zola, T.; Fitter, J.E.; Coyne-Beasley, T. Communication technologies to improve HPV vaccination initiation and completion: A systematic review. Patient Educ. Couns. 2017, 100, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Brisson, M.; Kim, J.J.; Canfell, K.; Drolet, M.; Gingras, G.; Burger, E.A.; Martin, D.; Simms, K.T.; Bénard, É.; Boily, M.C.; et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: A comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020, 395, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Petersen, Z.; Jaca, A.; Ginindza, T.G.; Maseko, G.; Takatshana, S.; Ndlovu, P.; Zondi, N.; Zungu, N.; Varghese, C.; Hunting, G.; et al. Barriers to uptake of cervical cancer screening services in low-and-middle-income countries: A systematic review. BMC Womens Health 2022, 22, 486. [Google Scholar] [CrossRef] [PubMed]
- Rossman, A.H.; Reid, H.W.; Pieters, M.M.; Mizelle, C.; von Isenburg, M.; Ramanujam, N.; Huchko, M.J.; Vasudevan, L. Digital Health Strategies for Cervical Cancer Control in Low- and Middle-Income Countries: Systematic Review of Current Implementations and Gaps in Research. J. Med. Internet Res. 2021, 23, e23350. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleezada, Z.N.; Patel, I.; Yusuf, N. Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment. Int. J. Mol. Sci. 2025, 26, 5581. https://doi.org/10.3390/ijms26125581
Aleezada ZN, Patel I, Yusuf N. Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment. International Journal of Molecular Sciences. 2025; 26(12):5581. https://doi.org/10.3390/ijms26125581
Chicago/Turabian StyleAleezada, Zahab N., Ishika Patel, and Nabiha Yusuf. 2025. "Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment" International Journal of Molecular Sciences 26, no. 12: 5581. https://doi.org/10.3390/ijms26125581
APA StyleAleezada, Z. N., Patel, I., & Yusuf, N. (2025). Understanding HPV-Induced Cancers and Investigating the Barriers Faced by Low- and Middle-Income Countries in Prevention and Treatment. International Journal of Molecular Sciences, 26(12), 5581. https://doi.org/10.3390/ijms26125581





