A Systematic Review on Microbial Profiling Techniques in Goat Milk: Implications for Probiotics and Shelf-Life
Abstract
1. Introduction
2. Results and Discussion
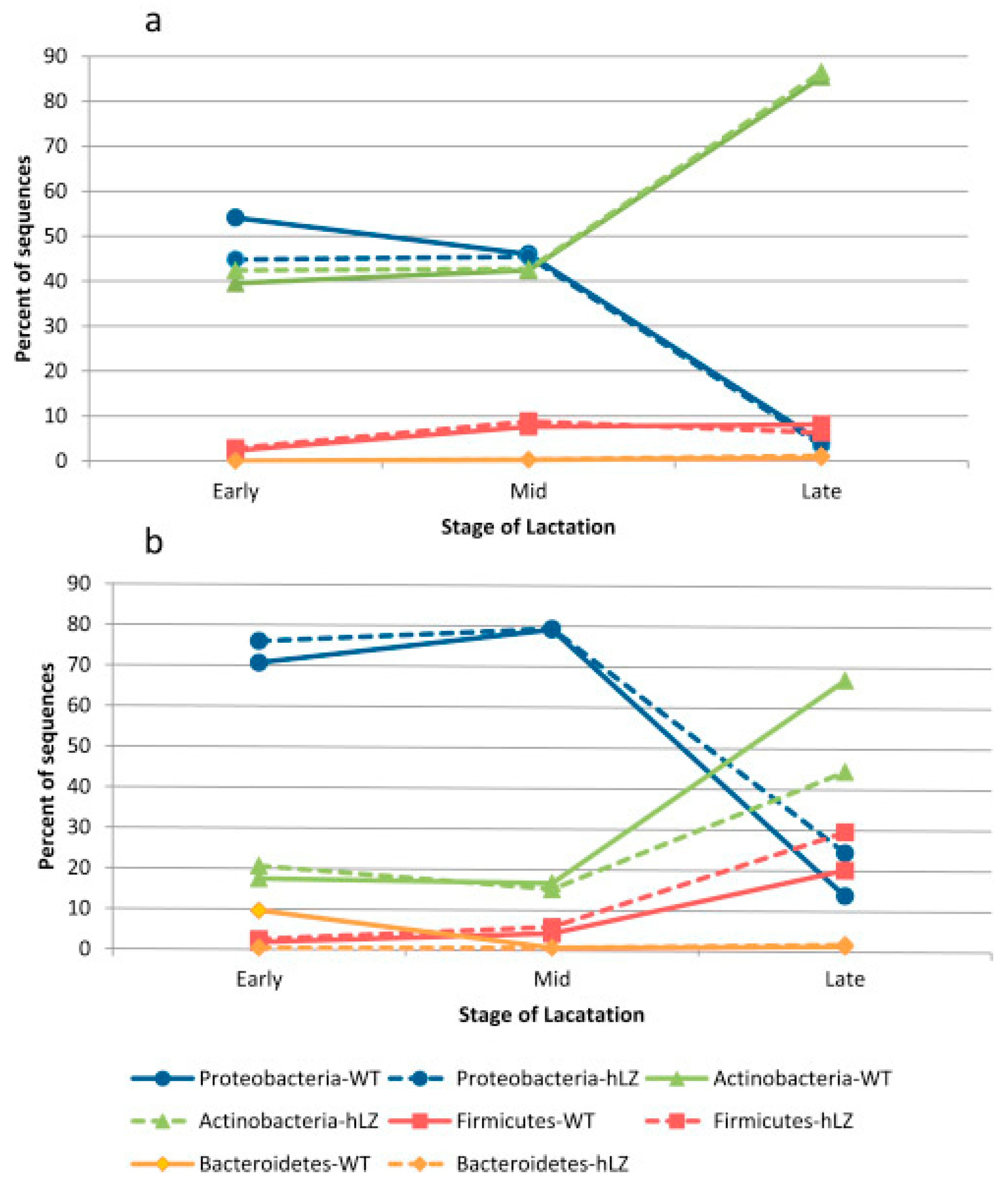
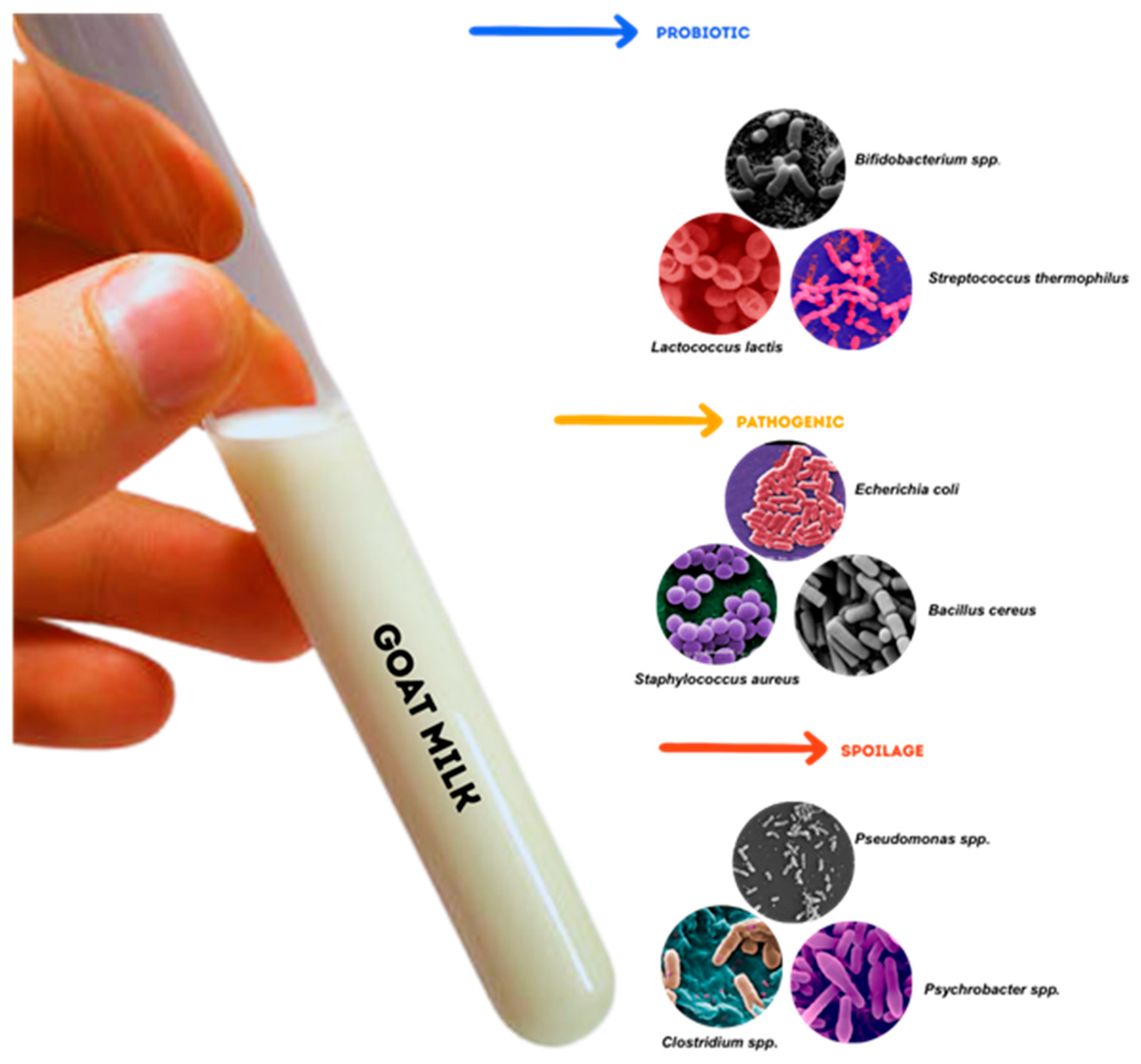
2.1. Microbial Communities in Goat Milk
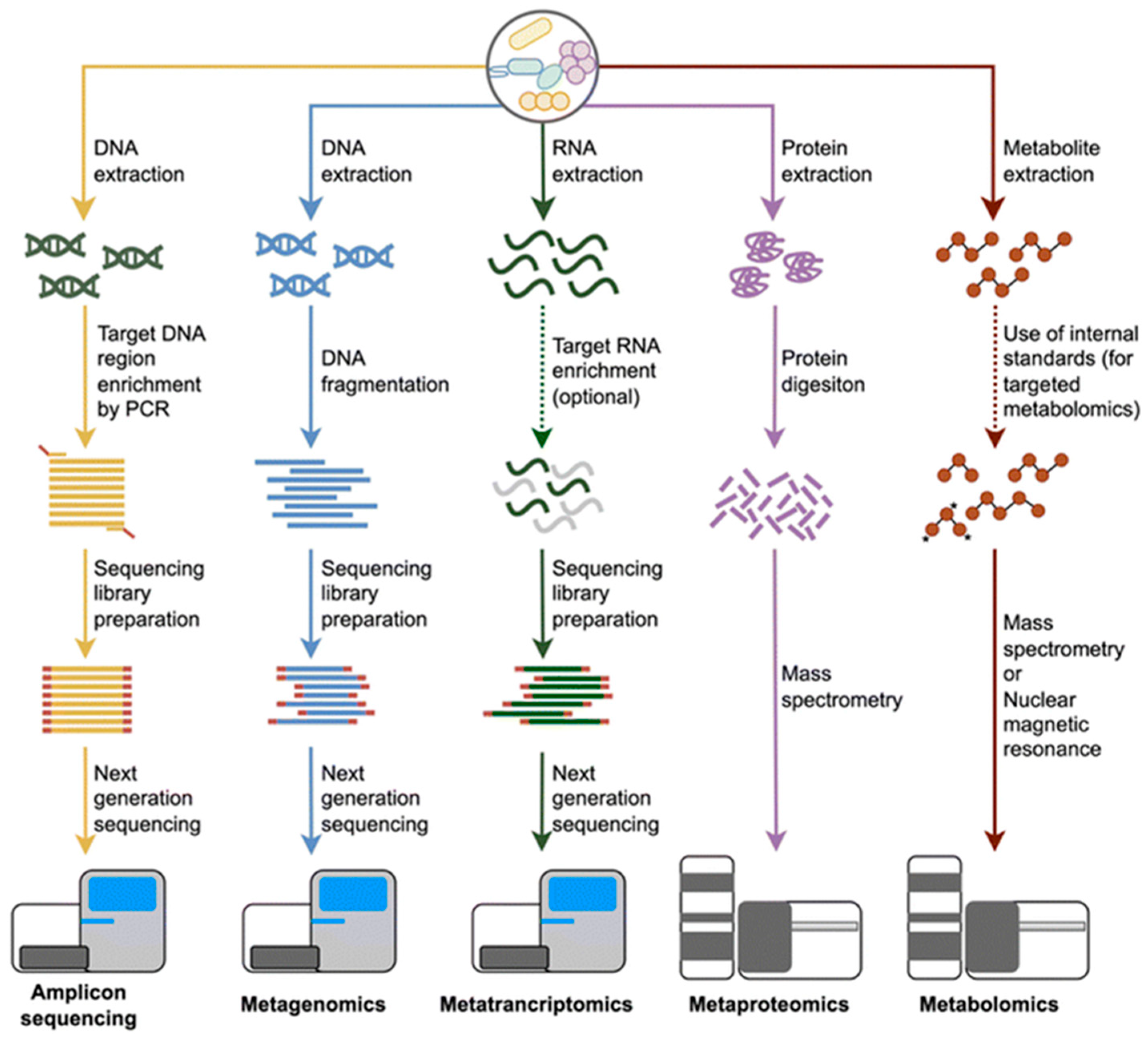
2.2. Microbial Profiling Techniques
2.2.1. 16S rRNA Sequencing
| Technique | Cost Level | Level of Resolution | Microbial Detection Sensitivity | Sample Needs | Application |
|---|---|---|---|---|---|
| 16S rRNA Sequencing | Low | Taxonomic identification at genus level; limited species resolution | Moderate | Moderate DNA yield | Used to study shifts in microbial diversity across lactation stages [4]; identification of Lactobacillus plantarum with probiotic traits [8] |
| Shotgun Metagenomics | High | High-resolution taxonomic profiling down to strain level; functional gene detection | High | High-quality, high-yield DNA | Detected antimicrobial resistance genes in Staphylococcus spp. in Brazilian goat milk [32] |
| Metatranscriptomics | Very high | Gene expression profiling of active microbial functions in real time | High | RNA integrity is crucial | Identified spoilage gene activation under cold storage [16] |
| Metabolomics | Medium-high | Functional protein identification: links taxa to metabolic pathways | Moderate-high | Metabolites-preserved samples | Tracked microbial protein markers in goat cheese spoilage and fermentation [33,34] |
| Metaproteomics | High | Detection of microbial metabolites reflects community functionality | Variable | Fresh, high-protein-yield samples | Assessed bioactive compounds (e.g., GABA, SCFAs) in goat milk kefir after fermentation and heating [35] |
2.2.2. Shotgun Metagenomics
2.2.3. Metatranscriptomics
2.2.4. Metaproteomics
2.2.5. Metabolomics
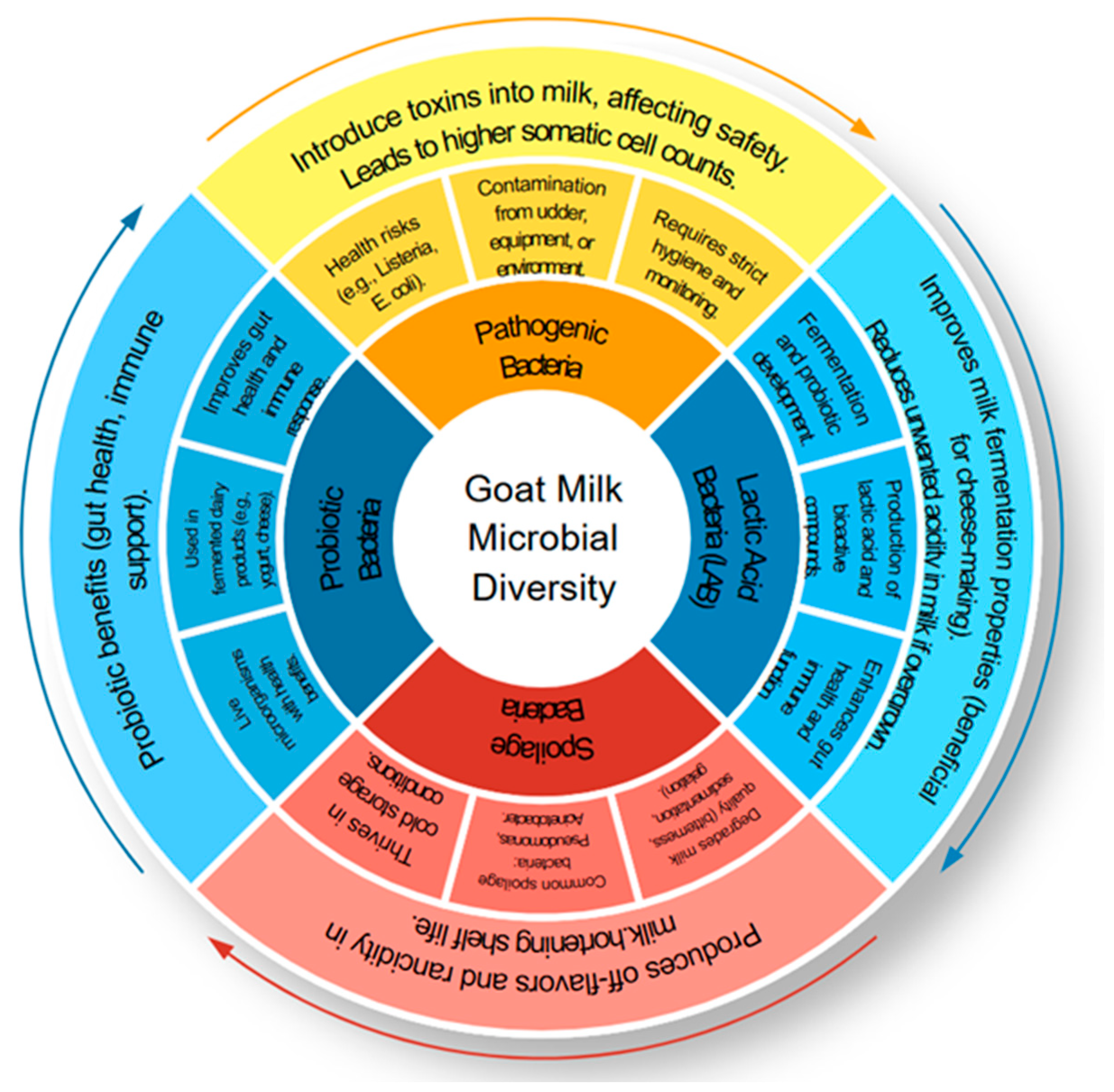
2.3. Spoilage and Shelf-Life of Goat Milk
2.4. Goat Milk Microbiota Probiotic Potential
3. Methods
4. Conclusions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Veettil, V.N.; Chitra, A.V. Probiotic Lactic Acid Bacteria from Goat’s Milk Potential Producer of Bacteriocin: Evidence from Liquid Chromatography-Mass Spectrometry. J. Pure Appl. Microbiol. 2022, 16, 305–317. [Google Scholar] [CrossRef]
- Kamilari, E.; Anagnostopoulos, D.A.; Papademas, P.; Efthymiou, M.; Tretiak, S.; Tsaltas, D. Snapshot of Cyprus Raw Goat Milk Bacterial Diversity via 16S rDNA High-Throughput Sequencing; Impact of Cold Storage Conditions. Fermentation 2020, 6, 100. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Lei, F.; Wang, B.; Jiang, S.; Peng, Q.; Zhang, J.; Shao, Y. Bacterial Diversity in Goat Milk from the Guanzhong Area of China. J. Dairy. Sci. 2017, 100, 7812–7824. [Google Scholar] [CrossRef] [PubMed]
- McInnis, E.A.; Kalanetra, K.M.; Mills, D.A.; Maga, E.A. Analysis of Raw Goat Milk Microbiota: Impact of Stage of Lactation and Lysozyme on Microbial Diversity. Food Microbiol. 2015, 46, 121–131. [Google Scholar] [CrossRef]
- Lauková, A.; Micenková, L.; Grešáková, Ľ.; Maďarová, M.; Simonová, M.P.; Focková, V.; Ščerbová, J. Microbiome Associated with Slovak Raw Goat Milk, Trace Minerals, and Vitamin E Content. Int. J. Food Sci. 2022, 2022, 1–8. [Google Scholar] [CrossRef]
- Cao, X.; Fang, Y.; Bandan, P.; Suo, L.; Jiacuo, G.; Wu, Y.; Cuoji, A.; Zhuoga, D.; Chen, Y.; Ji, D.; et al. Age-Specific Composition of Milk Microbiota in Tibetan Sheep and Goats. Appl. Microbiol. Biotechnol. 2024, 108, 411. [Google Scholar] [CrossRef]
- Jyoti; Singh, N.A. Unveiling the Culturable Bacterial Diversity, Pathogens and Impact of Seasonality on Microflora of Raw Goat Milk: A Phylogenetic Outlook. Int. Dairy. J. 2024, 153, 105904. [Google Scholar] [CrossRef]
- Makete, G.; Aiyegoro, O.A.; Thantsha, M.S. Isolation, Identification and Screening of Potential Probiotic Bacteria in Milk from South African Saanen Goats. Probiotics Antimicrob. Proteins 2017, 9, 246–254. [Google Scholar] [CrossRef]
- Taheri, F.; Ownagh, A.; Mardani, K. Phylogenetic and Molecular Analysis Based on Genes 16S-RRNA, OMPA and POMP to Identify Chlamydia Abortus Infection Occurrence at the Milk Samples of Goats and Sheep in West Azerbaijan of Iran. Iran J. Microbiol. 2021, 13, 480. [Google Scholar] [CrossRef]
- Sotohy, S.A.; Elnaker, Y.F.; Omar, A.M.; Alm Eldin, N.K.; Diab, M.S. Prevalence, Antibiogram and Molecular Characterization of Listeria Monocytogenes from Ruminants and Humans in New Valley and Beheira Governorates, Egypt. BMC Vet. Res. 2024, 20, 297. [Google Scholar] [CrossRef]
- Maleke, M.S.; Adefisoye, M.A.; Doorsamy, W.; Adebo, O.A. Processing, Nutritional Composition and Microbiology of Amasi: A Southern African Fermented Milk Product. Sci. Afr. 2021, 12, e00795. [Google Scholar] [CrossRef]
- Nam, N.N.; Do, H.D.K.; Loan Trinh, K.T.; Lee, N.Y. Metagenomics: An Effective Approach for Exploring Microbial Diversity and Functions. Foods 2023, 12, 2140. [Google Scholar] [CrossRef]
- Abd Rahman, M.R.; Hassan, Z.; Hassan, M.S.; Hashim, R.; Arifin, N.; Abdul Manap, M.N. Preliminary Metabolomic Analysis of Goat Milk from Different Breeds Using Mass Spectrometry. Int. J. Adv. Sci. Eng. Inf. Technol. 2015, 5, 181–191. [Google Scholar] [CrossRef]
- Salama, A.A.K.; Contreras-Jodar, A.; Love, S.; Mehaba, N.; Such, X.; Caja, G. Milk Yield, Milk Composition, and Milk Metabolomics of Dairy Goats Intramammary-Challenged with Lipopolysaccharide under Heat Stress Conditions. Sci. Rep. 2020, 10, 5055. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi-Omics Revealed the Long-Term Effect of Ruminal Keystone Bacteria and the Microbial Metabolome on Lactation Performance in Adult Dairy Goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Fanning, S.; Proos, S.; Jordan, K.; Srikumar, S. A Review on the Applications of Next Generation Sequencing Technologies as Applied to Food-Related Microbiome Studies. Front. Microbiol. 2017, 8, 1829. [Google Scholar]
- Zhang, L.; Chen, F.X.; Zeng, Z.; Xu, M.; Sun, F.; Yang, L.; Bi, X.; Lin, Y.; Gao, Y.J.; Hao, H.X.; et al. Advances in Metagenomics and Its Application in Environmental Microorganisms. Front. Microbiol. 2021, 12, 766364. [Google Scholar] [CrossRef]
- Niyazbekova, Z.; Yao, X.T.; Liu, M.J.; Bold, N.; Tong, J.Z.; Chang, J.J.; Wen, Y.; Li, L.; Wang, Y.; Chen, D.K.; et al. Compositional and Functional Comparisons of the Microbiota in the Colostrum and Mature Milk of Dairy Goats. Animals 2020, 10, 1955. [Google Scholar] [CrossRef]
- Ryu, S.; Park, W.S.; Yun, B.; Shin, M.; Go, G.-W.; Kim, J.N.; Oh, S.; Kim, Y. Diversity and Characteristics of Raw Milk Microbiota from Korean Dairy Farms Using Metagenomic and Culturomic Analysis. Food Control 2021, 127, 108160. [Google Scholar] [CrossRef]
- Polveiro, R.C.; Vidigal, P.M.P.; de Oliveira Mendes, T.A.; Yamatogi, R.S.; da Silva, L.S.; Fujikura, J.M.; Da Costa, M.M.; Moreira, M.A.S. Distinguishing the Milk Microbiota of Healthy Goats and Goats Diagnosed with Subclinical Mastitis, Clinical Mastitis, and Gangrenous Mastitis. Front. Microbiol. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Barzideh, Z.; Siddiqi, M.; LaPointe, G. Taxonomy, Sequence Variance and Functional Profiling of the Microbial Community of Long-Ripened Cheddar Cheese Using Shotgun Metagenomics. Microorganisms 2023, 11, 2052. [Google Scholar] [CrossRef] [PubMed]
- Spanamberg, A.; Ramos, J.P.; Leoncini, O.; Hartz Alves, S.; Valente, P. High Frequency of Potentially Pathogenic Yeast Species in Goat’s Raw Milk and Creamed Cheese in Southern Brazil. Acta Sci. Vet. 2009, 37, 133–141. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne Pathogens in Raw Milk and Cheese of Sheep and Goat Origin: A Meta-Analysis Approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Nuhriawangsa, A.D.M.; Kartikasari, L.R.; Hadi, R.F.; Wati, A.K.; Hertanto, B.S. Microbiological Profile of Fresh Goat Milk: Impact of Goat Farmer Practices in “Taruna Mukti” Goat Farmer Group, Sragen, Central-Java. IOP Conf. Ser. Mater. Sci. Eng. 2019, 633, 012010. [Google Scholar] [CrossRef]
- Islam, M.Z.; Uddin, M.E.; Rahman, M.T.; Islam, M.A.; Harun-ur-Rashid, M. Isolation and Characterization of Dominant Lactic Acid Bacteria from Raw Goat Milk: Assessment of Probiotic Potential and Technological Properties. Small Rumin. Res. 2021, 205, 106532. [Google Scholar] [CrossRef]
- Yang, Z.; Ni, L.; Tian, W.; Chi, H. Screening and Identification of Goat-Milk-Derived Lactic Acid Bacteria with Bacteriocin-like Activity and Probiotic Potentials. Microorganisms 2023, 11, 849. [Google Scholar] [CrossRef]
- Reuben, R.C.; Torres, C. Integrating the Milk Microbiome Signatures in Mastitis: Milk-Omics and Functional Implications. World J. Microbiol. Biotechnol. 2025, 41, 41. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, M.; Muth, T. Integrated Multi-Omics Analyses of Microbial Communities: A Review of the Current State and Future Directions. Mol. Omics 2023, 19, 607–623. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.; Lei, Z. Meta-Omics Insights in the Microbial Community Profiling and Functional Characterization of Fermented Foods. Trends Food Sci. Technol. 2017, 65, 23–31. [Google Scholar] [CrossRef]
- Kasi Rao, M.; Sunkad, G. Metaomics Approaches to Unravel the Functioning of Multispecies Microbial Communities. In Microbiome Drivers of Ecosystem Function; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–416. ISBN 9780443191213. [Google Scholar]
- Zhang, S.; Chen, J.; Gao, F.; Su, W.; Li, T.; Wang, Y. Foodomics as a Tool for Evaluating Food Authenticity and Safety from Field to Table: A Review. Foods 2025, 14, 15. [Google Scholar] [CrossRef]
- Usyk, M.; Peters, B.A.; Karthikeyan, S.; McDonald, D.; Sollecito, C.C.; Vazquez-Baeza, Y.; Shaffer, J.P.; Gellman, M.D.; Talavera, G.A.; Daviglus, M.L.; et al. Comprehensive Evaluation of Shotgun Metagenomics, Amplicon Sequencing, and Harmonization of These Platforms for Epidemiological Studies. Cell Reports Methods 2023, 3, 100391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.S.; Bu, S.H.; Liu, J.X.; Niu, C.; Wang, L.; Yuan, H.; Zhang, L.; Song, Y. Label-Free-Based Proteomics Analysis Reveals Differential Proteins of Sheep, Goat and Cow Milk. J. Dairy. Sci. 2024, 107, 8908–8918. [Google Scholar] [CrossRef]
- Gouveia, D.; Pible, O.; Culotta, K.; Jouffret, V.; Geffard, O.; Chaumot, A.; Degli-Esposti, D.; Armengaud, J. Combining Proteogenomics and Metaproteomics for Deep Taxonomic and Functional Characterization of Microbiomes from a Non-Sequenced Host. NPJ Biofilms Microbiomes 2020, 6, 23. [Google Scholar] [CrossRef]
- Sharma, H.; Ramanathan, R. Gas Chromatography-Mass Spectrometry Based Metabolomic Approach to Investigate the Changes in Goat Milk Yoghurt during Storage. Food Res. Int. 2021, 140, 110072. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Silva Júnior, A.E.; Vasconcelos, P.C.; Saraiva, M.M.S.; Filho, L.S.; Silva, N.M.V.; Givisiez, P.E.N.; Oliveira, C.J.B. Antimicrobial Susceptibility Profiles of Staphylococcus Spp. Contaminating Raw Goat Milk. Vet. World 2021, 14, 1074–1079. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An Ultra-Fast Single-Node Solution for Large and Complex Metagenomics Assembly via Succinct de Bruijn Graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Franzosa, E.; Tickle, T.L.; Scholz, M.; Weingart, G.; Pasolli, E.; Tett, A.; Huttenhower, C.; Segata, N. MetaPhlAn2 for Enhanced Metagenomic Taxonomic Profiling. Nat. Methods 2015, 12, 902–903. [Google Scholar] [CrossRef]
- Keegan, K.P.; Glass, E.M.; Meyer, F. MG-RAST, a Metagenomics Service for Analysis of Microbial Community Structure and Function. In: Methods in Molecular Biology. Microb. Environ. Genom. MEG 2016, 1399, 207–233. [Google Scholar]
- Zhang, Y.; Thompson, K.N.; Branck, T.; Yan, Y.; Nguyen, L.H.; Franzosa, E.A.; Huttenhower, C. Metatranscriptomics for the Human Microbiome and Microbial Community Functional Profiling. Annu. Rev. Biomed. Data Sci. 2025, 27, 279–311. [Google Scholar] [CrossRef]
- Tveit, A.T.; Urich, T.; Svenning, M.M. Metatranscriptomic Analysis of Arctic Peat Soil Microbiota. Appl. Environ. Microbiol. 2014, 80, 5761–5772. [Google Scholar] [CrossRef]
- Stewart, F.J. Preparation of Microbial Community CDNA for Metatranscriptomic Analysis in Marine Plankton. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 531, pp. 187–218. ISBN 9780124078635. [Google Scholar]
- Xuan, R.; Wang, J.; Zhao, X.; Li, Q.; Wang, Y.; Du, S.; Duan, Q.; Guo, Y.; Ji, Z.; Chao, T. Transcriptome Analysis of Goat Mammary Gland Tissue Reveals the Adaptive Strategies and Molecular Mechanisms of Lactation and Involution. Int. J. Mol. Sci. 2022, 23, 14424. [Google Scholar] [CrossRef] [PubMed]
- Dige, M.S.; Gurao, A.; Singh, L.P.; Chitkara, M.; Singh, M.K.; Dass, G.; Verma, A.K.; Pundir, R.K.; Kataria, R.S. Transcriptomic Analysis Reveals Molecular Insights into Lactation Dynamics in Jakhrana Goat Mammary Gland. BMC Genomics 2024, 25, 874. [Google Scholar] [CrossRef]
- Jia, W.; Liu, Y.; Shi, L. Integrated Metabolomics and Lipidomics Profiling Reveals Beneficial Changes in Sensory Quality of Brown Fermented Goat Milk. Food Chem. 2021, 364, 130378. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, M. Metaproteomics: Much More than Measuring Gene Expression in Microbial Communities. mSystems 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Tanca, A.; Palomba, A.; Pisanu, S.; Deligios, M.; Fraumene, C.; Manghina, V.; Pagnozzi, D.; Addis, M.F.; Uzzau, S. A Straightforward and Efficient Analytical Pipeline for Metaproteome Characterization. Microbiome 2014, 2, 49. [Google Scholar] [CrossRef]
- Soggiu, A.; Piras, C.; Mortera, S.L.; Alloggio, I.; Urbani, A.; Bonizzi, L.; Roncada, P. Unravelling the Effect of Clostridia Spores and Lysozyme on Microbiota Dynamics in Grana Padano Cheese: A Metaproteomics Approach. J. Proteomics 2016, 147, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Roncada, P.; Rodrigues, P.M.; Bonizzi, L.; Soggiu, A. Proteomics in Food: Quality, Safety, Microbes, and Allergens. Proteomics 2016, 16, 799–815. [Google Scholar] [CrossRef]
- Olumee-Shabon, Z.; Boehmer, J.L. Proteomic Analysis of Goat Milk. In Goat Science; InTech: Houston, TX, USA, 2018. [Google Scholar]
- Tilocca, B.; Soggiu, A.; Iavarone, F.; Greco, V.; Putignani, L.; Ristori, M.V.; Macari, G.; Spina, A.A.; Morittu, V.M.; Ceniti, C.; et al. The Functional Characteristics of Goat Cheese Microbiota from a One-Health Perspective. Int. J. Mol. Sci. 2022, 23, 14131. [Google Scholar] [CrossRef]
- Suh, J.H. Critical Review: Metabolomics in Dairy Science – Evaluation of Milk and Milk Product Quality. Food Res. Int. 2022, 154, 110984. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Nagana Gowda, G.A.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. Nmr Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.M.; Xu, L.Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Scano, P.; Murgia, A.; Pirisi, F.M.; Caboni, P. A Gas Chromatography-Mass Spectrometry-Based Metabolomic Approach for the Characterization of Goat Milk Compared with Cow Milk. J. Dairy. Sci. 2014, 97, 6057–6066. [Google Scholar] [CrossRef]
- Kandasamy, S.; Park, W.S.; Bae, I.S.; Yoo, J.; Yun, J.; Hoa, V.B.; Ham, J.S. HRMAS-NMR-Based Metabolomics Approach to Discover Key Differences in Cow and Goat Milk Yoghurt Metabolomes. Foods 2024, 13, 3483. [Google Scholar] [CrossRef]
- Goldansaz, S.A.; Guo, A.C.; Sajed, T.; Steele, M.A.; Plastow, G.S.; Wishart, D.S. Livestock Metabolomics and the Livestock Metabolome: A Systematic Review. PLoS ONE 2017, 12, e0177675. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Saipriya, K.; Singh, A.K.; Singh, R.; Meena, G.S.; Khetra, Y.; Sharma, H. A Metabolomics Approach to Establish the Relationship between the Techno-Functional Properties and Metabolome of Indian Goat Yoghurt. Foods 2024, 13, 913. [Google Scholar] [CrossRef]
- Caboni, P.; Murgia, A.; Porcu, A.; Manis, C.; Ibba, I.; Contu, M.; Scano, P. A Metabolomics Comparison between Sheep’s and Goat’s Milk. Food Res. Int. 2019, 119, 869–875. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, R.; Terriente-Palacios, C.; García-Olmo, J.; Osorio, S.; Rodríguez-Ortega, M.J. Combined Metabolomic and NIRS Analyses Reveal Biochemical and Metabolite Changes in Goat Milk Kefir under Different Heat Treatments and Fermentation Times. Biomolecules 2024, 14, 816. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.; Liu, B.; Gong, P.; Shi, C.; Zhu, L.; Zhao, J.; Yao, W.; Liu, Q.; Luo, J. Widely Targeted Metabolomic Analysis Revealed the Diversity in Milk from Goats, Sheep, Cows, and Buffaloes and Its Association with Flavor Profiles. Foods 2024, 13, 1365. [Google Scholar] [CrossRef]
- Scatamburlo, T.M.; Yamazi, A.K.; Cavicchioli, V.Q.; Pieri, F.A.; Nero, L.A. Spoilage Potential of Pseudomonas Species Isolated from Goat Milk. J. Dairy. Sci. 2015, 98, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Majumder, R.; Rout, P.; Hossain, S. Unveiling the Significance of Psychrotrophic Bacteria in Milk and Milk Product Spoilage—A Review. Microbe 2024, 2, 100034. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial Safety of Milk Production and Fermented Dairy Products in Africa. Microorganisms 2020, 8, 752. [Google Scholar] [CrossRef] [PubMed]
- Dagdu, R.; Jayawant, D.; Mandal, S.P.; Jadhav, A. Spoilage and Preservation of Milk and Milk. Products: A Review. Jetir 2019, 6, 173–179. Available online: https://www.researchgate.net/publication/346606004 (accessed on 28 April 2025).
- Tan, S.F.; Chin, N.L.; Tee, T.P.; Chooi, S.K. Physico-Chemical Changes, Microbiological Properties, and Storage Shelf Life of Cow and Goat Milk from Industrial High-Pressure Processing. Processes 2020, 8, 697. [Google Scholar] [CrossRef]
- Ma, H.; Li, J.; Xi, X.; Xu, H.; Wuri, L.; Bian, Y.; Yu, Z.; Ren, M.; Duo, L.; Sun, Y.; et al. Evaluation of Bacterial Contamination in Goat Milk Powder Using PacBio Single Molecule Real-Time Sequencing and Droplet Digital PCR. J. Food Prot. 2018, 81, 1791–1799. [Google Scholar] [CrossRef]
- Yalew, K.; Pang, X.; Huang, S.; Zhang, S.; Yang, X.; Xie, N.; Wang, Y.; Lv, J.; Li, X. Recent Development in Detection and Control of Psychrotrophic Bacteria in Dairy Production: Ensuring Milk Quality. Foods 2024, 13, 2908. [Google Scholar] [CrossRef]
- de Paiva Anciens Ramos, G.L.; dos Santos Nascimento, J. Characterization of Acinetobacter Spp. from Raw Goat Milk. Ciencia Rural. 2019, 49, e20190404. [Google Scholar] [CrossRef]
- Júnior, J.C.R.; Junior, P.I.T.; Oliveira, A.L.M.; Rios, E.A.; Tamanini, R.; Beloti, V. Proteolytic and Lipolytic Potential of Pseudomonas Spp. From Goat and Bovine Raw Milk. Pesqui. Vet. Bras. 2018, 38, 1577–1583. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The Complex Microbiota of Raw Milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A Review on the General Cheese Processing Technology, Flavor Biochemical Pathways and the Influence of Yeasts in Cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Pawtowski, A.; Auhustsinava-Galerne, L.; Frotté, N.; Baroncelli, R.; Deniel, F.; Coton, E.; Mounier, J. Diversity of Spoilage Fungi Associated with Various French Dairy Products. Int. J. Food Microbiol. 2017, 241, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Sperber, W.H. Microbiological Spoilage of Acidified Specialty Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 285–299. [Google Scholar]
- Premasiri, D.L.D.A.S.; Rajawardana, D.U.; Muddannayake, D.C.; Hewajulige, I.G.N. Isolation, Characterization and Identification of Industrially Beneficial Probiotic Lactic Acid Bacteria from Goat Milk. J. Agric. Sci. 2021, 16, 369–382. [Google Scholar] [CrossRef]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of Autochthonous Lactobacilli from Goat Dairy Products with Probiotic Potential for Metabolic Diseases. Heliyon 2022, 8, e10462. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Baines, S.K.; Balthazar, C.F.; Cruz, A.G.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Wittwer, A.E.; Naumovski, N.; et al. Probiotics in Goat Milk Products: Delivery Capacity and Ability to Improve Sensory Attributes. Compr. Rev. Food Sci. Food Saf. 2019, 18, 867–882. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Niu, H.; Tian, X.; Tian, H.; Yao, W.; He, H.; Shi, H.; Li, C.; Luo, J. Goat Milk Improves Glucose Metabolism in Type 2 Diabetic Mice and Protects Pancreatic β-Cell Functions. Mol. Nutr. Food Res. 2024, 68, e2200842. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, L.; Jia, F.; Yan, Y.; Xiong, F.; Hidayat, K.; Li, Y. Effect of Probiotic Fermented Milk Supplementation on Glucose and Lipid Metabolism Parameters and Inflammatory Markers in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Biology 2024, 13, 641. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering Probiotic Microorganisms: In Vitro, in Vivo, Genetic and Omics Approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Lerma, A.; García-Burgos, M.; Alférez, M.J.M.; Crespo-Pérez, J.V.; Pérez-Carrasco, V.; Ortiz-Gonzalez, M.; Linde-Rodriguez, Á.; Sanchez-Martin, V.; Soriano, M.; Garcia-Salcedo, J.A.; et al. Fermented Goat’s Milk Contributes to the Recovery of Iron Deficiency Anemia via Modulation of the Gut Microbiome. J. Agric. Food Chem. 2023, 71, 15668–15679. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]

| Technique | Principle | Advantages | Limitations | Reference |
|---|---|---|---|---|
| Metagenomics | Sequencing of microbial DNA from environmental samples | Identifies unculturable species, provides comprehensive microbial diversity | Requires advanced bioinformatics | [12] |
| Metabolomics | Analysis of metabolites in milk | Identifies biomarkers for quality and spoilage with high sensitivity | Complex data interpretation requires specialised equipment | [13] |
| Metatranscriptomics | Sequencing of RNA to study gene expression in microbial communities | Provides insights into active microbial functions and metabolic pathways | High-cost, complex data analysis | [16] |
| Next-Generation Sequencing (NGS) | High-throughput sequencing of microbial genomes | Hugh’s resolution identifies strain-level diversity | Expensive, requires computational resources | [17] |
| Bacterial Group | Genera/Species | Function | Potential Impact | Reference |
|---|---|---|---|---|
| Beneficial bacteria | Lactococcus, Lactobacillus, Leuconostoc | Plays a crucial role in fermentation, producing lactic acid and enhancing probiotic potential. | Enhances fermentation, improves probiotic potential, and extends shelf-life | [5,6] |
| Bifidobacterium, Curtobacterium | Improves the content of short-chain fatty acids and medium-chain fatty acids in fermented milk. | Contributes to gut health, potential probiotic | [5,6] | |
| Spoilage bacteria | Pseudomonas, Bacillus, Staphylococcus | Produces extracellular enzymes that digest milk proteins and fats, leading to spoilage. | Causes spoilage, impacts milk safety and quality | [3,7] |
| Enterobacter, Pseudomonas | Commonly found in raw milk; its role in spoilage and fermentation is under investigation. | Can spoil milk, impacts quality | [3,19] | |
| Pathogenic bacteria | Escherichia. sp, Staphylococcus aureus | Associated with milk safety concerns, but also part of the natural microbiota. | Causes health risks, spoilage issues | [7,20] |
| Mycoplasma sp. | Pathogenic, spoilage | Associated with mastitis, reduces microbial diversity | [20] |
| Spoilage Bacteria | Effects on Milk Quality | Reference |
|---|---|---|
| Bacillus cereus | Potential food spoilage and foodborne illness risk, quantified at 6.3 × 104 copies/g in goat milk powder. | [69] |
| Cronobacter spp. | Food safety risk, quantified at 1.0 × 104 copies/g in goat milk powder. | [69] |
| Pseudomonas fluorescens | Proteolytic activity leading to spoilage; observed at refrigeration (7 °C) and ambient temperatures (25–35 °C). | [64] |
| Pyschrotrophic bacteria | Spoilage in pasteurised goat milk, exceeding permissible limits during storage. | [68] |
| Pseudomonas spp. | Causes flavour, texture, and nutritional degradation in milk and milk products. | [67] |
| E. coli | Contributes to spoilage, altering flavour and safety. | [67] |
| Staphylococcus aureus | Spoilage and potential foodborne illness risk. | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monareng, N.J.; Ncube, K.T.; van Rooi, C.; Modiba, M.C.; Mtileni, B. A Systematic Review on Microbial Profiling Techniques in Goat Milk: Implications for Probiotics and Shelf-Life. Int. J. Mol. Sci. 2025, 26, 5551. https://doi.org/10.3390/ijms26125551
Monareng NJ, Ncube KT, van Rooi C, Modiba MC, Mtileni B. A Systematic Review on Microbial Profiling Techniques in Goat Milk: Implications for Probiotics and Shelf-Life. International Journal of Molecular Sciences. 2025; 26(12):5551. https://doi.org/10.3390/ijms26125551
Chicago/Turabian StyleMonareng, Nare Jessica, Keabetswe T. Ncube, Charles van Rooi, Mamokoma C. Modiba, and Bohani Mtileni. 2025. "A Systematic Review on Microbial Profiling Techniques in Goat Milk: Implications for Probiotics and Shelf-Life" International Journal of Molecular Sciences 26, no. 12: 5551. https://doi.org/10.3390/ijms26125551
APA StyleMonareng, N. J., Ncube, K. T., van Rooi, C., Modiba, M. C., & Mtileni, B. (2025). A Systematic Review on Microbial Profiling Techniques in Goat Milk: Implications for Probiotics and Shelf-Life. International Journal of Molecular Sciences, 26(12), 5551. https://doi.org/10.3390/ijms26125551







