MiR 329/449 Suppresses Cell Proliferation, Migration and Synergistically Sensitizes GBM to TMZ by Inhibiting Src/FAK, NF-kB, and Cyclin D1 Activity
Abstract
1. Introduction
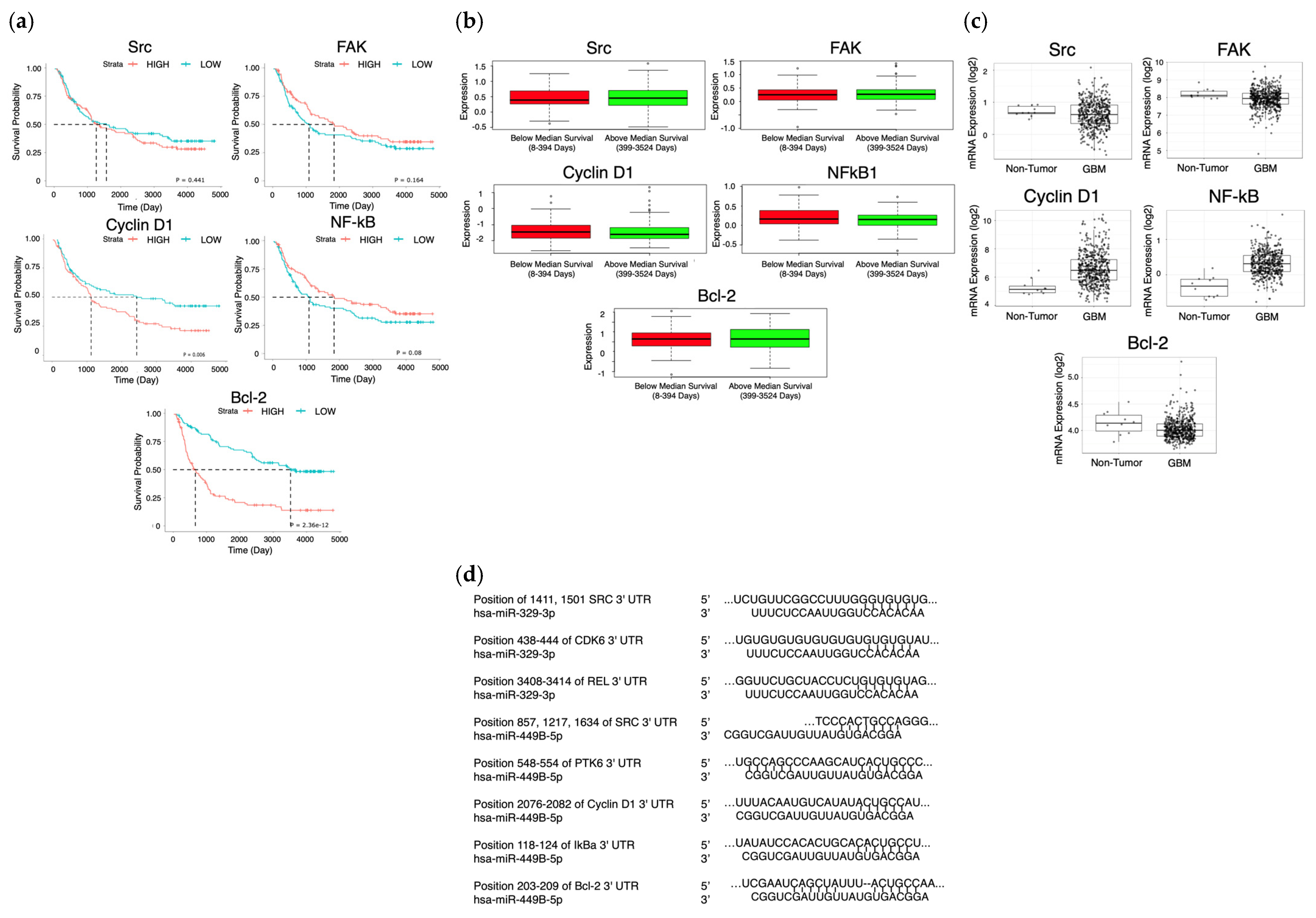
2. Results
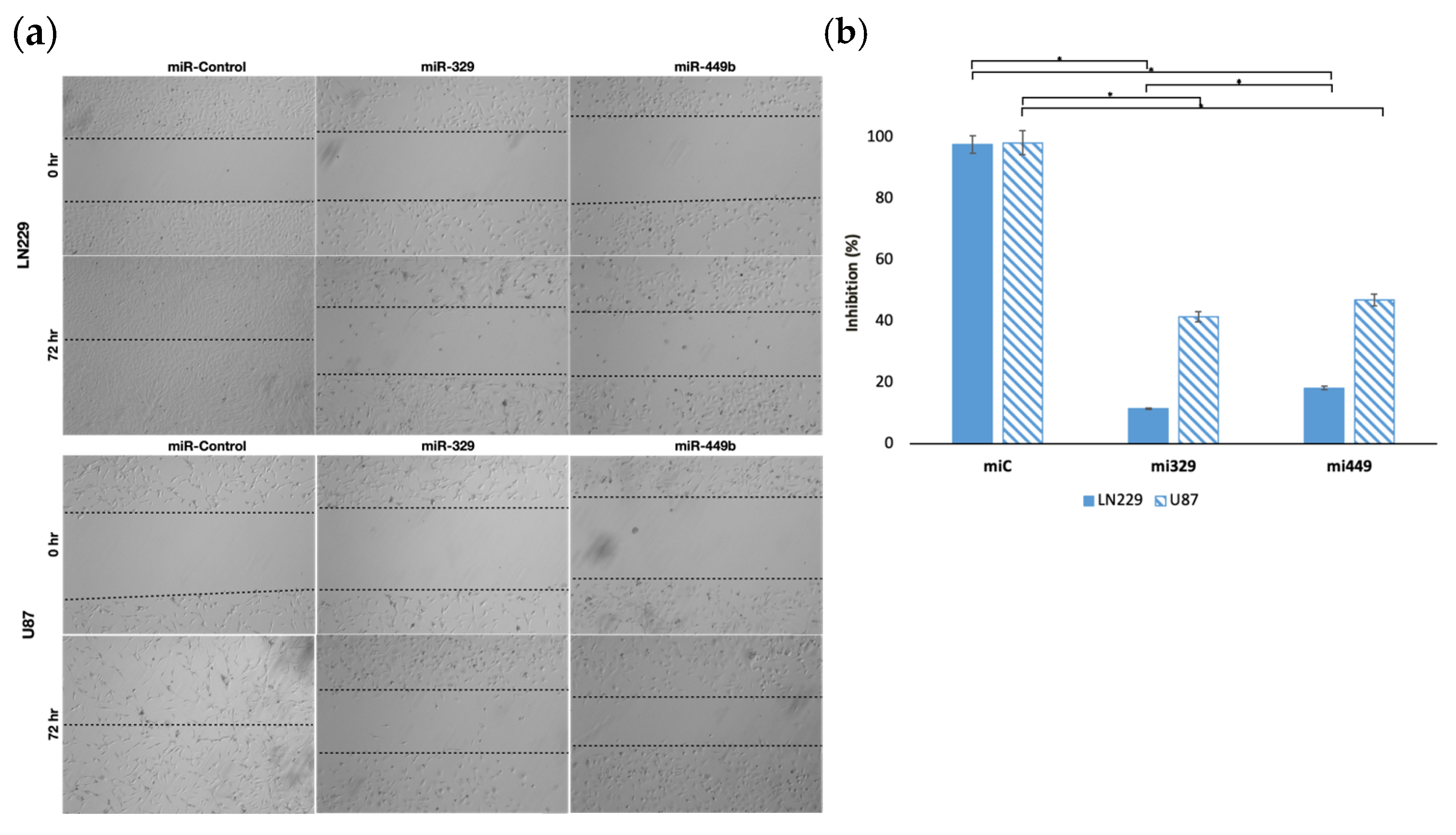
2.1. MiR-329 and miR-449b Expression Impairs GBM Cell Migration
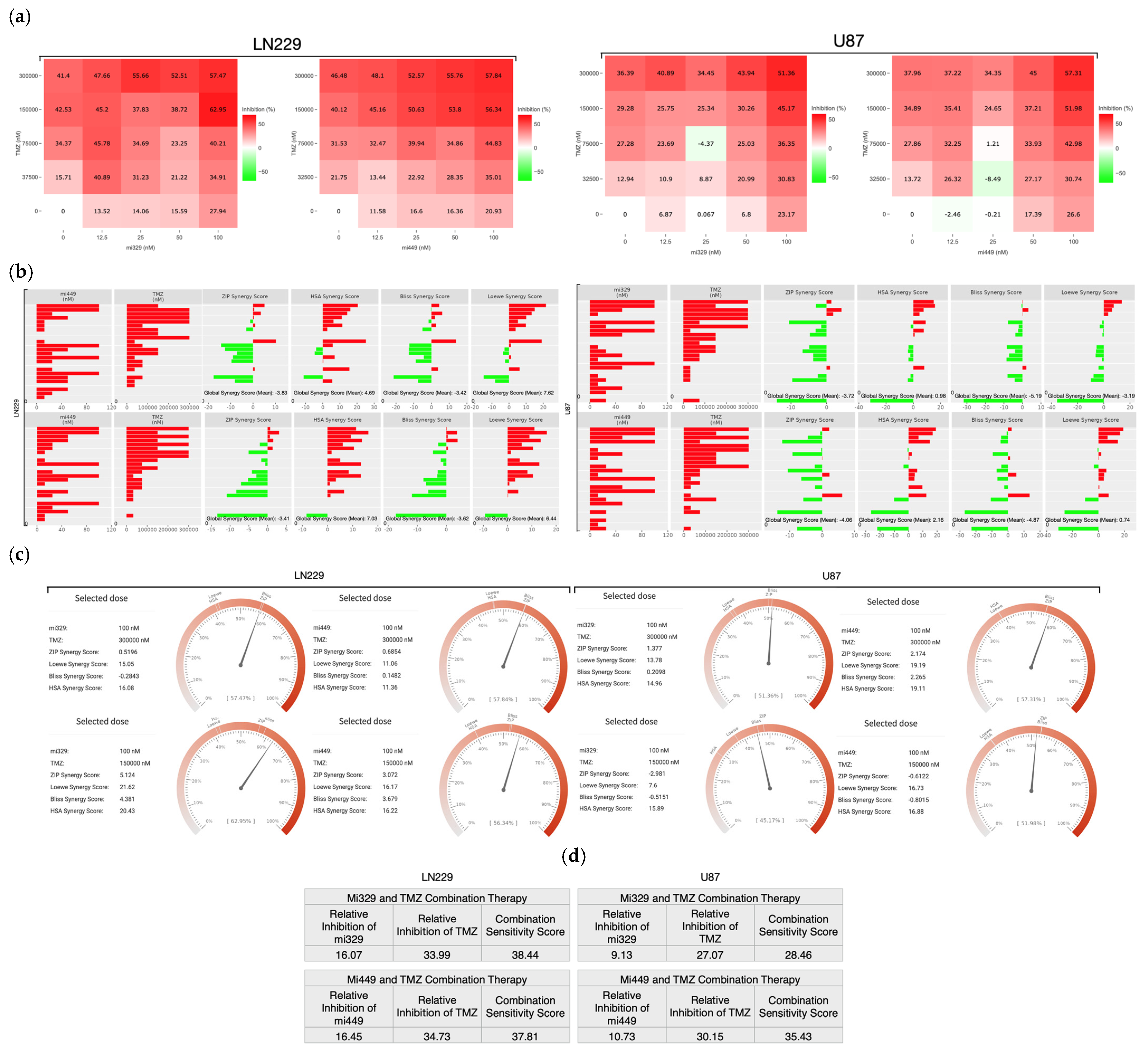
2.2. Synergistic Concentrations of TMZ and miR Treatments Optimized for 3D Applications in GBM
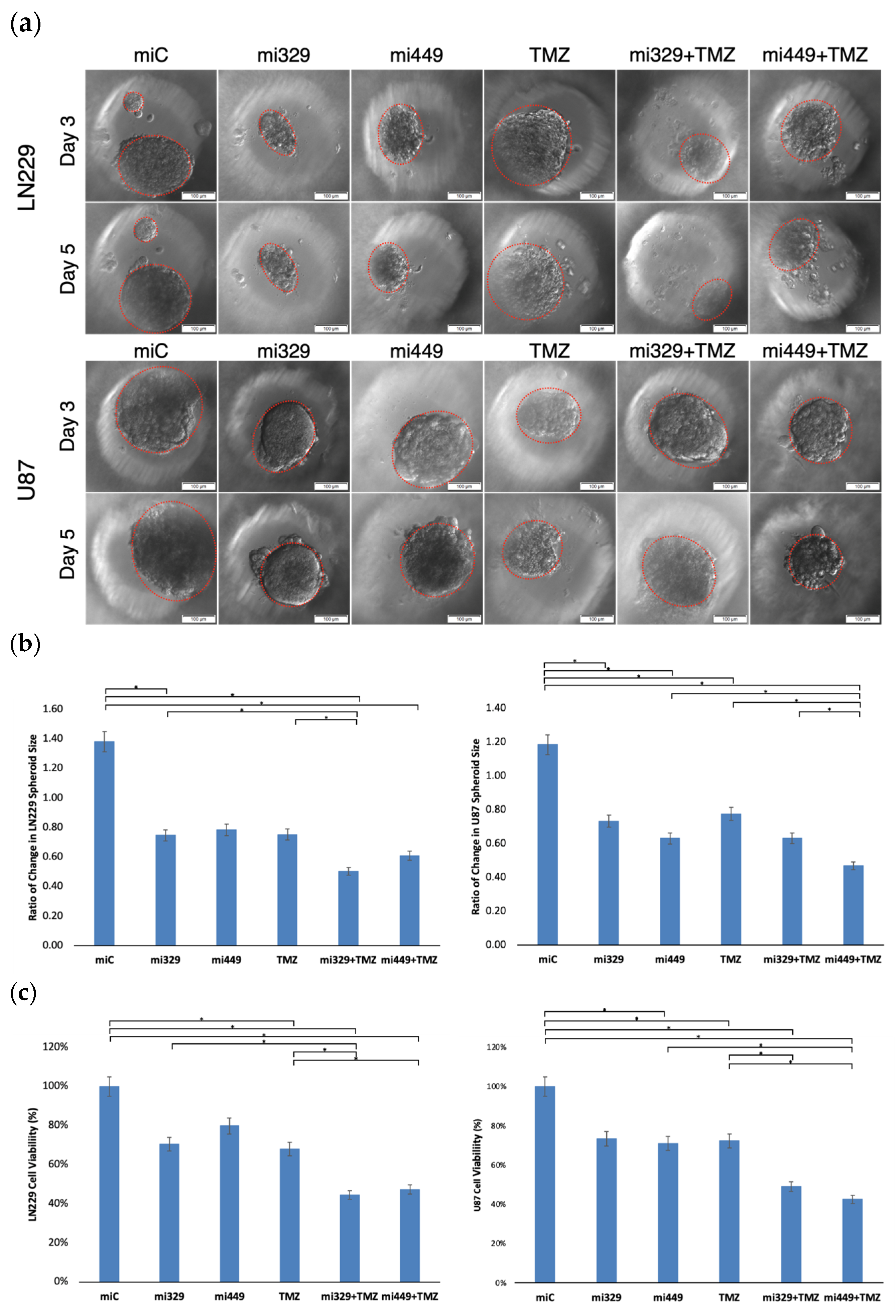
2.3. Synergistic Application of TMZ and miR Treatments to PEGDA Microwell GBM Spheroids’ Reduced Viability of GBM Cells
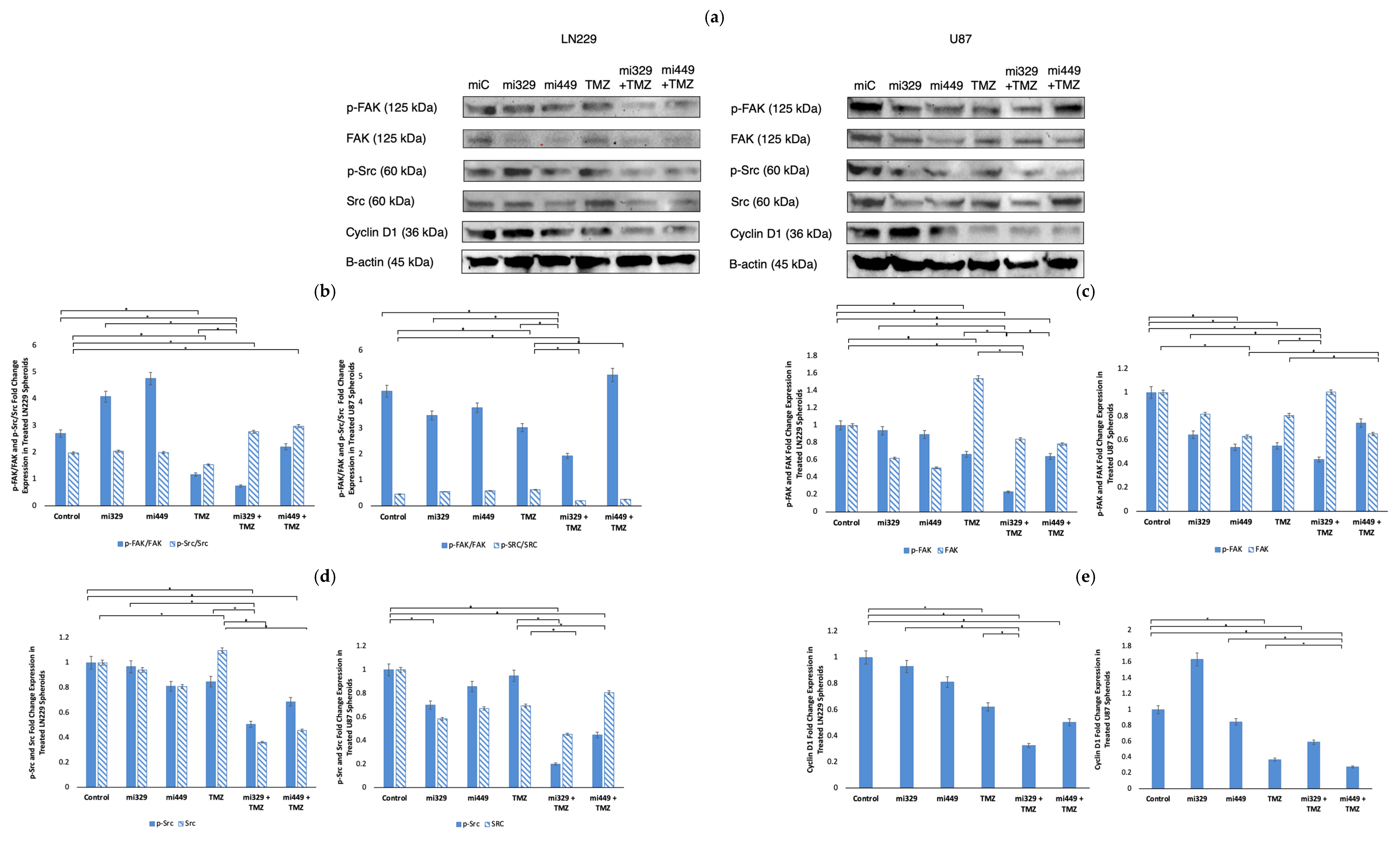
2.4. Src/Fak Pathway Downregulated in GBM Spheroids by Synergistic Concentrations of TMZ and miR Treatments
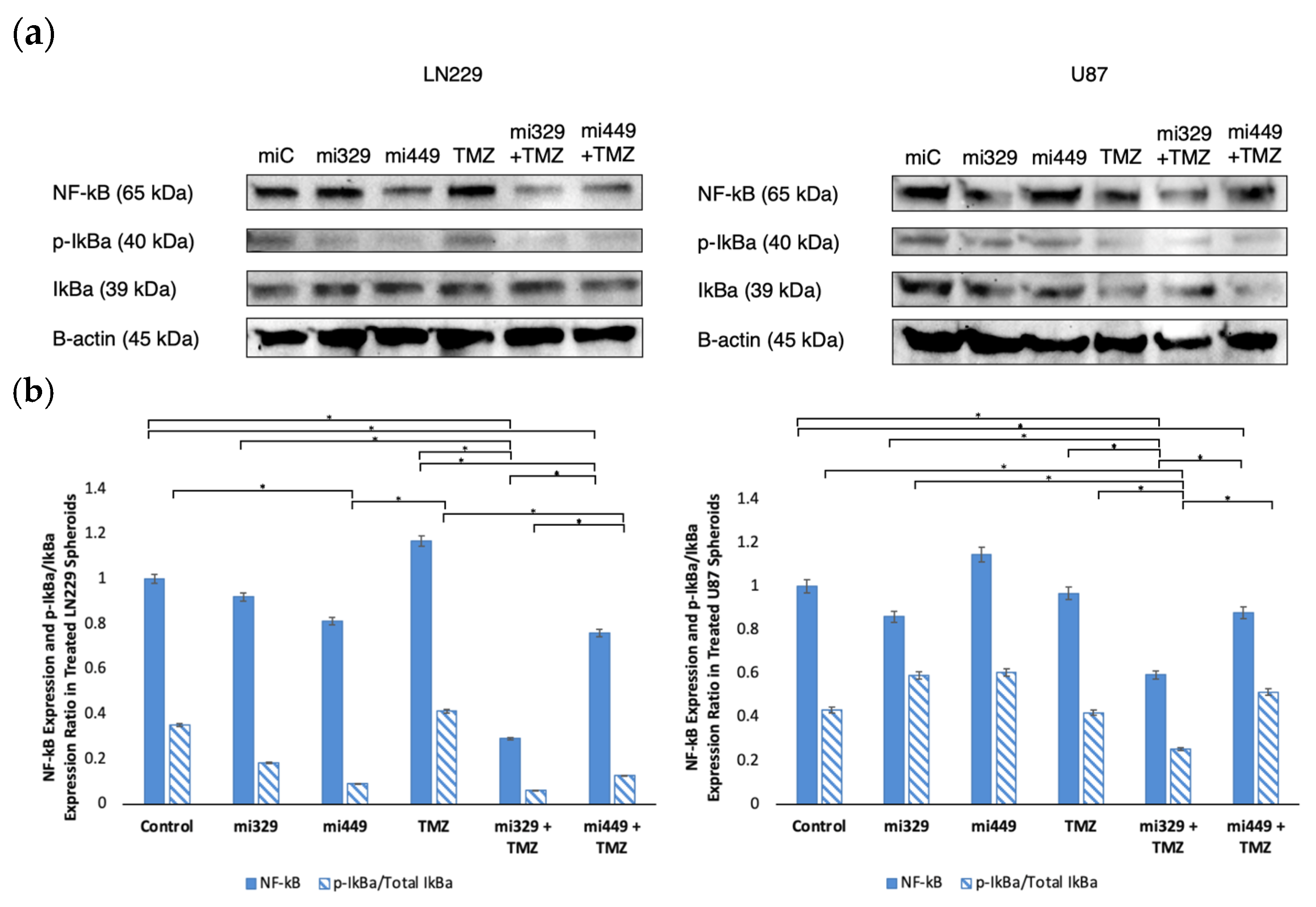
2.5. NF-kB Activity Downregulated in GBM Spheroids by Synergistic Concentrations of TMZ and miR Treatments
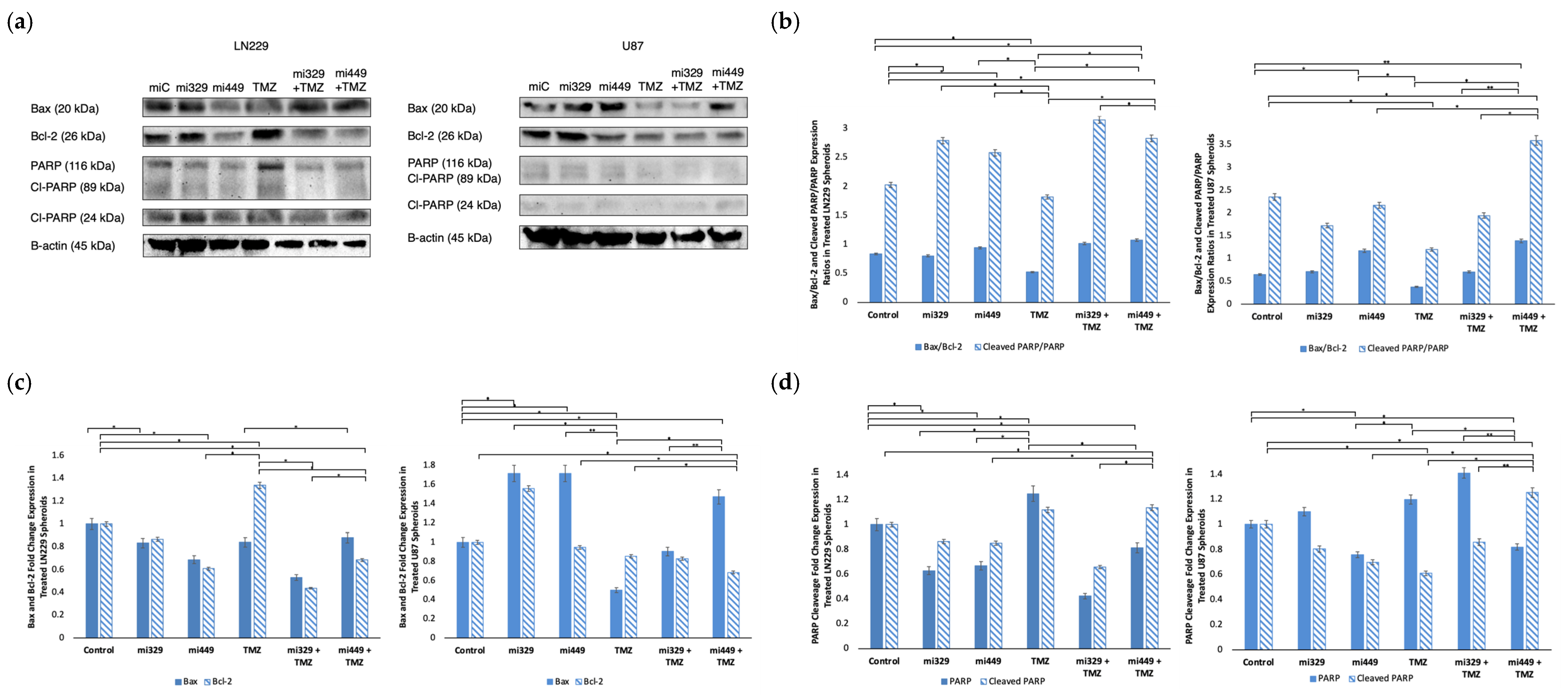
2.6. Apoptosis Signaling Promoted in GBM Spheroids by Synergistic Concentrations of TMZ and miR Treatments
3. Discussion
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Cell Lines and Cell Culture
4.3. MiR Transfection
4.4. Optimization, Synergy Assay, and TMZ Administration
4.5. Microwell Fabrication and Cell Seeding
4.6. Cell Viability and Spheroid Size Quantification
4.7. Wound Healing Assay
4.8. Western Blot
4.9. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMZ | Temozolomide |
| GBM | Glioblastoma |
| CGGA | Chinese Glioma Genome Atlas |
| miR | Micro RNA |
| mRNA | Messenger RNA |
| PEGDA | Poly(ethylene glycol) dimethyl acrylate |
| ZIP | Zero interaction potency |
| HSA | Highest single agent |
| TMSPMA | 3-(Trimethoxy-silyl) propyl methacrylate |
| PBS | Phosphate buffered solution |
| BSA | Bovine serum albumin |
| TBS-T | Tris-buffered saline with polysorbate (tween) 20 |
References
- Esemen, Y.; Awan, M.; Parwez, R.; Baig, A.; Rahman, S.; Masala, I.; Franchini, S.; Giakoumettis, D. Molecular Pathogenesis of Glioblastoma in Adults and Future Perspectives: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2607. [Google Scholar] [CrossRef] [PubMed]
- Chekenya, M.; Pilkington, G.J. NG2 precursor cells in neoplasia: Functional, histogenesis and therapeutic implications for malignant brain tumours. J. Neurocytol. 2002, 31, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Eisenbarth, D.; Wang, Y.A. Glioblastoma heterogeneity at single cell resolution. Oncogene 2023, 42, 2155–2165. [Google Scholar] [CrossRef]
- Urbańska, K.; Sokołowska, J.; Szmidt, M.; Sysa, P. Glioblastoma multiforme—An overview. Contemp. Oncol. 2014, 18, 307–312. [Google Scholar] [CrossRef]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in Glioblastoma Therapy: An Update on Current Approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Wang, X.; Jia, L.; Jin, X.; Liu, Q.; Cao, W.; Gao, X.; Yang, M.; Sun, B. NF-κB inhibitor reverses temozolomide resistance in human glioma TR/U251 cells. Oncol. Lett. 2015, 9, 2586–2590. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.-Y.; Cho, B.J.; Choi, E.J.; Kim, J.-H.; Chie, E.K.; Wu, H.-G.; Kim, I.H.; Paek, S.H.; Kim, J.-S.; Kim, I.A. The Effect of Chemoradiotherapy with SRC Tyrosine Kinase Inhibitor, PP2 and Temozolomide on Malignant Glioma Cells In Vitro and In Vivo. Cancer Res. Treat. 2016, 48, 687–697. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Caner, A.; Asik, E.; Ozpolat, B. SRC Signaling in Cancer and Tumor Microenvironment. Adv. Exp. Med. Biol. 2021, 1270, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hamurcu, Z.; Kahraman, N.; Ashour, A.; Ozpolat, B. FOXM1 transcriptionally regulates expression of integrin β1 in triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 163, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Tluli, O.; Al-Maadhadi, M.; Al-Khulaifi, A.A.; Akomolafe, A.F.; Al-Kuwari, S.Y.; Al-Khayarin, R.; Maccalli, C.; Pedersen, S. Exploring the Role of microRNAs in Glioma Progression, Prognosis, and Therapeutic Strategies. Cancers 2023, 15, 4213. [Google Scholar] [CrossRef] [PubMed]
- Shea, A.; Harish, V.; Afzal, Z.; Chijioke, J.; Kedir, H.; Dusmatova, S.; Roy, A.; Ramalinga, M.; Harris, B.; Blancato, J.; et al. MicroRNAs in glioblastoma multiforme pathogenesis and therapeutics. Cancer Med. 2016, 5, 1917–1946. [Google Scholar] [CrossRef]
- Ying, S.-Y.; Chang, D.C.; Lin, S.-L. The MicroRNA (miRNA): Overview of the RNA Genes that Modulate Gene Function. Mol. Biotechnol. 2008, 38, 257–268. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front. Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Szczepanek, J.; Skorupa, M.; Tretyn, A. MicroRNA as a Potential Therapeutic Molecule in Cancer. Cells 2022, 11, 1008. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Fan, Y.; Avci, N.G.; Nguyen, D.T.; Dragomir, A.; Akay, Y.; Xu, F.; Akay, M. Engineering a High-Throughput 3-D In Vitro Glioblastoma Model. IEEE J. Transl. Eng. Health Med. 2015, 3, 4300108. [Google Scholar] [CrossRef]
- Mendieta, M.; Avci, N.G.; Pandurangi, R.; Akay, Y.M.; Akay, M. Targeted Sensitization of Glioblastoma Multiforme Using AAAPT Technology. IEEE Open J. Eng. Med. Biol. 2023, 4, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Pustchi, S.E.; Avci, N.G.; Akay, Y.M.; Akay, M. Astrocytes Decreased the Sensitivity of Glioblastoma Cells to Temozolomide and Bay 11-7082. Int. J. Mol. Sci. 2020, 21, 7154. [Google Scholar] [CrossRef] [PubMed]
- Avci, N.G.; Fan, Y.; Dragomir, A.; Akay, Y.M.; Akay, M. Investigating the Influence of HUVECs in the Formation of Glioblastoma Spheroids in High-Throughput Three-Dimensional Microwells. IEEE Trans. NanoBiosci. 2015, 14, 790–796. [Google Scholar] [CrossRef]
- Mendieta, M.; Hatami, M.; Singh, M.; Fard, S.S.; Dehshiri, M.; Schill, A.; Nevozhay, D.; Aglyamov, S.; Ozpolat, B.; Sokolov, K.V.; et al. Non-Invasive Measurement of Elasticity in Glioblastoma Multiforme Validates Decreased TMZ Sensitivity in Astrocyte Co-Culture. IEEE Open J. Eng. Med. Biol. 2025, 26, 1–10. [Google Scholar] [CrossRef]
- Xia, H.; Avci, N.G.; Akay, Y.; Esquenazi, Y.; Schmitt, L.H.; Tandon, N.; Zhu, J.-J.; Akay, M. Temozolomide in Combination With NF-κB Inhibitor Significantly Disrupts the Glioblastoma Multiforme Spheroid Formation. IEEE Open J. Eng. Med. Biol. 2020, 1, 9–16. [Google Scholar] [CrossRef]
- SynergyFinder Plus: Toward Better Interpretation and Annotation of Drug Combination Screening Datasets|Genomics, Proteomics & Bioinformatics|Oxford Academic. Available online: https://academic.oup.com/gpb/article/20/3/587/7198175?login=false (accessed on 1 April 2025).
- Yadav, B.; Wennerberg, K.; Aittokallio, T.; Tang, J. Searching for Drug Synergy in Complex Dose–Response Landscapes Using an Interaction Potency Model. Comput. Struct. Biotechnol. J. 2015, 13, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- The Toxicity of Poisons Applied Jointly1—BLISS—1939—Annals of Applied Biology—Wiley Online Library. Available online: https://onlinelibrary-wiley-com.ezproxy.lib.uh.edu/doi/abs/10.1111/j.1744-7348.1939.tb06990.x (accessed on 1 April 2025).
- Kashif, M.; Andersson, C.; Mansoori, S.; Larsson, R.; Nygren, P.; Gustafsson, M.G. Bliss and Loewe interaction analyses of clinically relevant drug combinations in human colon cancer cell lines reveal complex patterns of synergy and antagonism. Oncotarget 2017, 8, 103952–103967. [Google Scholar] [CrossRef]
- Tang, J.; Wennerberg, K.; Aittokallio, T. What is synergy? The Saariselkä agreement revisited. Front. Pharmacol. 2015, 6, 181. [Google Scholar] [CrossRef]
- Malyutina, A.; Majumder, M.M.; Wang, W.; Pessia, A.; Heckman, C.A.; Tang, J. Drug combination sensitivity scoring facilitates the discovery of synergistic and efficacious drug combinations in cancer. PLoS Comput. Biol. 2019, 15, e1006752. [Google Scholar] [CrossRef]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Tan, L.; He, B.; Liu, Z.; Xu, R. MiRNA-329 targeting E2F1 inhibits cell proliferation in glioma cells. J. Transl. Med. 2013, 11, 172. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, X.; He, X.; Ma, W.; Wang, J.; Zhou, Q.; Li, M.; Yu, S. MicroRNA-449b-5p suppresses the growth and invasion of breast cancer cells via inhibiting CREPT-mediated Wnt/β-catenin signaling. Chem. Biol. Interact. 2019, 302, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef]
- Comert Onder, F.; Kahraman, N.; Bellur Atici, E.; Cagir, A.; Kandemir, H.; Tatar, G.; Taskin Tok, T.; Kara, G.; Karliga, B.; Durdagi, S.; et al. Target-Driven Design of a Coumarinyl Chalcone Scaffold Based Novel EF2 Kinase Inhibitor Suppresses Breast Cancer Growth In Vivo. ACS Pharmacol. Transl. Sci. 2021, 4, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, S.; Ning, T.; Xu, H.; Guan, B. SNHG7 Facilitates Glioblastoma Progression by Functioning as a Molecular Sponge for MicroRNA-449b-5p and Thereby Increasing MYCN Expression. Technol. Cancer Res. Treat. 2020, 19, 1533033820945802. [Google Scholar] [CrossRef]
- Guan, Y.; Cao, Z.; Du, J.; Liu, T.; Wang, T. Circular RNA circPITX1 knockdown inhibits glycolysis to enhance radiosensitivity of glioma cells by miR-329-3p/NEK2 axis. Cancer Cell Int. 2020, 20, 80. [Google Scholar] [CrossRef]
- Yu, L.; Gui, S.; Liu, Y.; Qiu, X.; Qiu, B.; Zhang, X.; Pan, J.; Fan, J.; Qi, S.; Zhang, G. Long intergenic non-protein coding RNA 00475 silencing acts as a tumor suppressor in glioma under hypoxic condition by impairing microRNA-449b-5p-dependent AGAP2 up-regulation. Ther. Adv. Med. Oncol. 2020, 12, 1758835920940936. [Google Scholar] [CrossRef]
- Avci, N.G.; Ebrahimzadeh-Pustchi, S.; Akay, Y.M.; Esquenazi, Y.; Tandon, N.; Zhu, J.-J.; Akay, M. NF-κB inhibitor with Temozolomide results in significant apoptosis in glioblastoma via the NF-κB(p65) and actin cytoskeleton regulatory pathways. Sci. Rep. 2020, 10, 13352. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Carroll, L.J.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Three-Dimensional (3D) in vitro cell culture protocols to enhance glioblastoma research. PLoS ONE 2023, 18, e0276248. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Li, X.; Yang, S.; Yue, S.; Zhang, J.; Yang, X.; Zhu, T.; Jiang, R.; Yang, W. Downregulation of Src enhances the cytotoxic effect of temozolomide through AKT in glioma. Oncol. Rep. 2013, 29, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Stettner, M.R.; Wang, W.; Nabors, L.B.; Bharara, S.; Flynn, D.C.; Grammer, J.R.; Gillespie, G.Y.; Gladson, C.L. Lyn Kinase Activity Is the Predominant Cellular Src Kinase Activity in Glioblastoma Tumor Cells. Cancer Res. 2005, 65, 5535–5543. [Google Scholar] [CrossRef]
- Cirotti, C.; Contadini, C.; Barilà, D. SRC Kinase in Glioblastoma: News from an Old Acquaintance. Cancers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef]
- Torrisi, F.; Vicario, N.; Spitale, F.M.; Cammarata, F.P.; Minafra, L.; Salvatorelli, L.; Russo, G.; Cuttone, G.; Valable, S.; Gulino, R.; et al. The Role of Hypoxia and SRC Tyrosine Kinase in Glioblastoma Invasiveness and Radioresistance. Cancers 2020, 12, 2860. [Google Scholar] [CrossRef] [PubMed]
- Cahill, K.E.; Morshed, R.A.; Yamini, B. Nuclear factor-κB in glioblastoma: Insights into regulators and targeted therapy. Neuro-Oncol. 2016, 18, 329–339. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018, 25, 46–55. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, M.; Uno, A.; Tanimichi, A.; Nobeyama, A.; Mori, M.; Yamada, S.; Negi, S.; Bu, X.; Kato, J.; et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2020, 296, 100046. [Google Scholar] [CrossRef]
- Pustchi, S.E.; Waits, C.M.K.; Avci, N.G.; Mcgill, L.; Akay, Y.M.; Akay, M. The Influence of Human Astrocyte-Conditioned Media on Glioblastoma Multiforme Response to Temozolomide and Bay 11-7082. IEEE Access 2022, 10, 12882–12888. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta, M.; Bandegi, M.; Biltekin, E.; Akay, Y.M.; Ozpolat, B.; Akay, M. MiR 329/449 Suppresses Cell Proliferation, Migration and Synergistically Sensitizes GBM to TMZ by Inhibiting Src/FAK, NF-kB, and Cyclin D1 Activity. Int. J. Mol. Sci. 2025, 26, 5533. https://doi.org/10.3390/ijms26125533
Mendieta M, Bandegi M, Biltekin E, Akay YM, Ozpolat B, Akay M. MiR 329/449 Suppresses Cell Proliferation, Migration and Synergistically Sensitizes GBM to TMZ by Inhibiting Src/FAK, NF-kB, and Cyclin D1 Activity. International Journal of Molecular Sciences. 2025; 26(12):5533. https://doi.org/10.3390/ijms26125533
Chicago/Turabian StyleMendieta, Megan, Mehrdad Bandegi, Ezgi Biltekin, Yasemin M. Akay, Bulent Ozpolat, and Metin Akay. 2025. "MiR 329/449 Suppresses Cell Proliferation, Migration and Synergistically Sensitizes GBM to TMZ by Inhibiting Src/FAK, NF-kB, and Cyclin D1 Activity" International Journal of Molecular Sciences 26, no. 12: 5533. https://doi.org/10.3390/ijms26125533
APA StyleMendieta, M., Bandegi, M., Biltekin, E., Akay, Y. M., Ozpolat, B., & Akay, M. (2025). MiR 329/449 Suppresses Cell Proliferation, Migration and Synergistically Sensitizes GBM to TMZ by Inhibiting Src/FAK, NF-kB, and Cyclin D1 Activity. International Journal of Molecular Sciences, 26(12), 5533. https://doi.org/10.3390/ijms26125533








