Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review
Abstract
1. Introduction
2. Method
2.1. Eligibility Criteria
2.2. Search Strategy
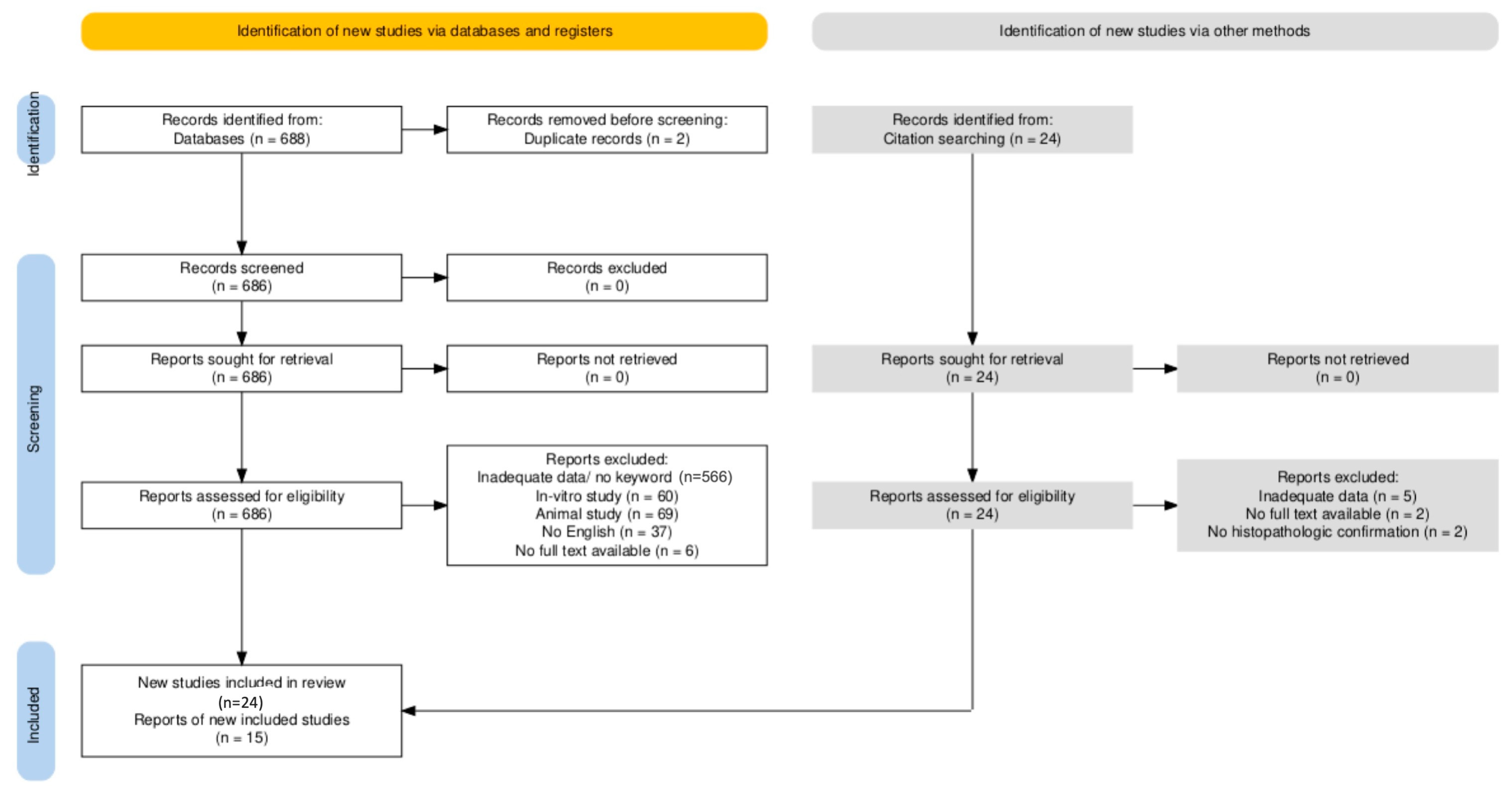
2.3. Selection Process
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Synthesis Method
3. Results
| Author, Year | Study Design | Patients | Median Age | Cancer Type | Parameters |
|---|---|---|---|---|---|
| Xu et al. 2022 [9] | Retrospective | 164/200 | 55/59 | DHGTC/PDTC | SR, ETE, LNI, DM |
| Panchangam et al. 2022 [14] | Retrospective | 29 | 54 | PDTC | SR, MTS, ETE, LNI, DM |
| Aslan et al. 2014 [23] | Retrospective | 29 | 64.5 | ATC | SR, MTS, ETE, DM |
| Brignardello et al. 2014 [24] | Retrospective | 55 | 73.15 | ATC | SR, ETE, DM |
| Duan et al. 2019 [25] | Retrospective | 41/25 | 51/64 | PDTC/ATC | MTS, ETE, LNI, DM |
| Evans et al. 2024 [26] | Retrospective Case–control | 41 | 67.4 | ATC | SR, DM |
| Fouchardiere et al. 2018 [27] | Retrospective | 104 | 62 | PDTC | SR, ETE, LNI, DM |
| Glaser et al. 2016 [28] | Retrospective | 3552 | ATC | SR, LNI, DM | |
| Gu et al. 2024 [29] | Retrospective | 15/42 | 52/64.5 | PDTC/ATC | MTS, ETE, LNI, DM |
| Ibrahimpasic et al. 2014 [30] | Retrospective Case–control | 91 | 59 | PDTC | SR, ETE, LNI, DM |
| Jeong et al. 2023 [31] | Retrospective | 14 | 47 | DHGTC | MTS, ETE, LNI, DM |
| Jin et al. 2022 [32] | Retrospective | 970 | PDTC | LNI, DM | |
| Kersting et al. 2021 [33] | Retrospective | 51 | 58.5 | PDTC | SR, ETE, DM |
| Kunte et al. 2022 [34] | Retrospective | 19 | 60 | PDTC | SR, LNI, DM |
| Landa et al. 2016 [35] | Retrospective | 84/33 | 55/66 | PDTC/ATC | DM |
| Patil et al. 2025 [36] | Retrospective | 106 | 54 | DHGTC/PDTC | SR, LNI, DM |
| Paunovic et al. 2016 [37] | Retrospective | 150 | ATC | SR | |
| Saito et al. 2024 [38] | Retrospective | 102 | 73 | ATC | LNI |
| Sherman et al. 2011 [39] | Retrospective Case–control | 75 | 68 | ATC | SR, ETE, DM |
| Swaak-Kragten et al. 2011 [40] | Retrospective Case–control | 37 | 63 | ATC | SR |
| Thompson et al. 2023 [41] | Retrospective | 17/24 | 64/58 | DHGTC/PDTC | MTS, ETE, LNI, DM |
| Tondi Resta et al. 2024 [42] | Retrospective | 32 | 52.6 | DHGTC | MTS, ETE, LNI, DM |
| Wendler et al. 2016 [43] | Retrospective | 100 | 70.5 | ATC | MTS, LNI, DM |
| Wong et al. 2019 [44] | Retrospective | 47 | 57 | PDTC | MTS, ETE, LNI, DM |
| Wu et al. 2023 [45] | Retrospective | 97 | 70 | ATC | LNI, DM |
| Xu et al. 2023 [46] | Retrospective | 210 | 60 | PDTC | MTS, ETE, LNI |
| Yu et al. 2017 [47] | Retrospective | 18 | 62 | PDTC | SR, MTS, ETE, LNI, DM |
| Ravi et al. 2019 [48] | Retrospective | 14 | 71.4 | ATC | MTS, LNI, DM |
| Author, Year | Type of Cancer | Patients | Yielded Parameters | Molecular Technique | |||||
|---|---|---|---|---|---|---|---|---|---|
| BRAF | RAS | TERT | TP53 | PTEN | PIK3CA | ||||
| Bonhomme et al. 2017 [49] | ATC | 94 | x | x | x | x | x | x | NGS, FISH |
| Duan et al. 2019 [25] | PDTC | 41 | x | x | x | x | x | x | NGS |
| ATC | 25 | x | x | x | x | x | x | ||
| Fouchardière et al. 2018 [27] | PDTC | 104 | x | x | x | PCR | |||
| Gu et al. 2024 [29] | PDTC | 9 | x | x | x | Sanger Seq. | |||
| ATC | 24 | x | x | x | |||||
| Landa et al. 2016 [35] | PDTC | 84 | x | x | x | x | x | x | NGS (Target Seq—MSK-IMPACT) |
| ATC | 33 | x | x | x | x | x | |||
| Latteyer et al. 2016 [50] | ATC | 30 | x | x | x | NGS | |||
| Pozdeyev et al. 2018 [51] | ATC | 196 | x | x | x | x | NGS (MSK-IMPACT) | ||
| Ravi et al. 2019 [48] | ATC | 8 | x | x | x | x | x | x | WES RNA-Seq |
| Saito et al. 2024 [38] | ATC | 102 | x | x | x | x | NGS (database) | ||
| Scholfield et al. 2025 [52] | DHGTC | 252 | x | x | x | x | NGS | ||
| Stenman et al. 2021 [53] | ATC | 8 | x | x | x | x | x | WGS RNA-Seq | |
| Takano et al. 2007 [54] | ATC | 20 | x | Sanger seq | |||||
| Tiedje et al. 2017 [55] | ATC | 118 | x | x | x | x | x | NGS | |
| Toda et al. 2024 [56] | PDTC | 104 | x | x | NGS (database) | ||||
| ATC | 130 | x | x | x | x | ||||
| Torous et al. 2024 [57] | DHGTC | 40 | x | NGS | |||||
| Wong et al. 2021 [44] | DHGTC | 12 | x | x | x | x | NGS | ||
| ATC | 33 | x | x | x | x | ||||
| Xu et al. 2022 [9] | DHGTC | 164 | x | x | x | x | x | x | NGS (MSK-IMPACT) |
| PDTC | 87 | x | x | x | x | x | x | ||
| Xu et al. 2023 [46] | PDTC | 87 | x | x | x | x | |||
| Yamazaki et al. 2024 [58] | PDTC | 51 | x | x | x | x | x | x | NGS (databse) |
| ATC | 110 | x | x | x | x | x | x | ||
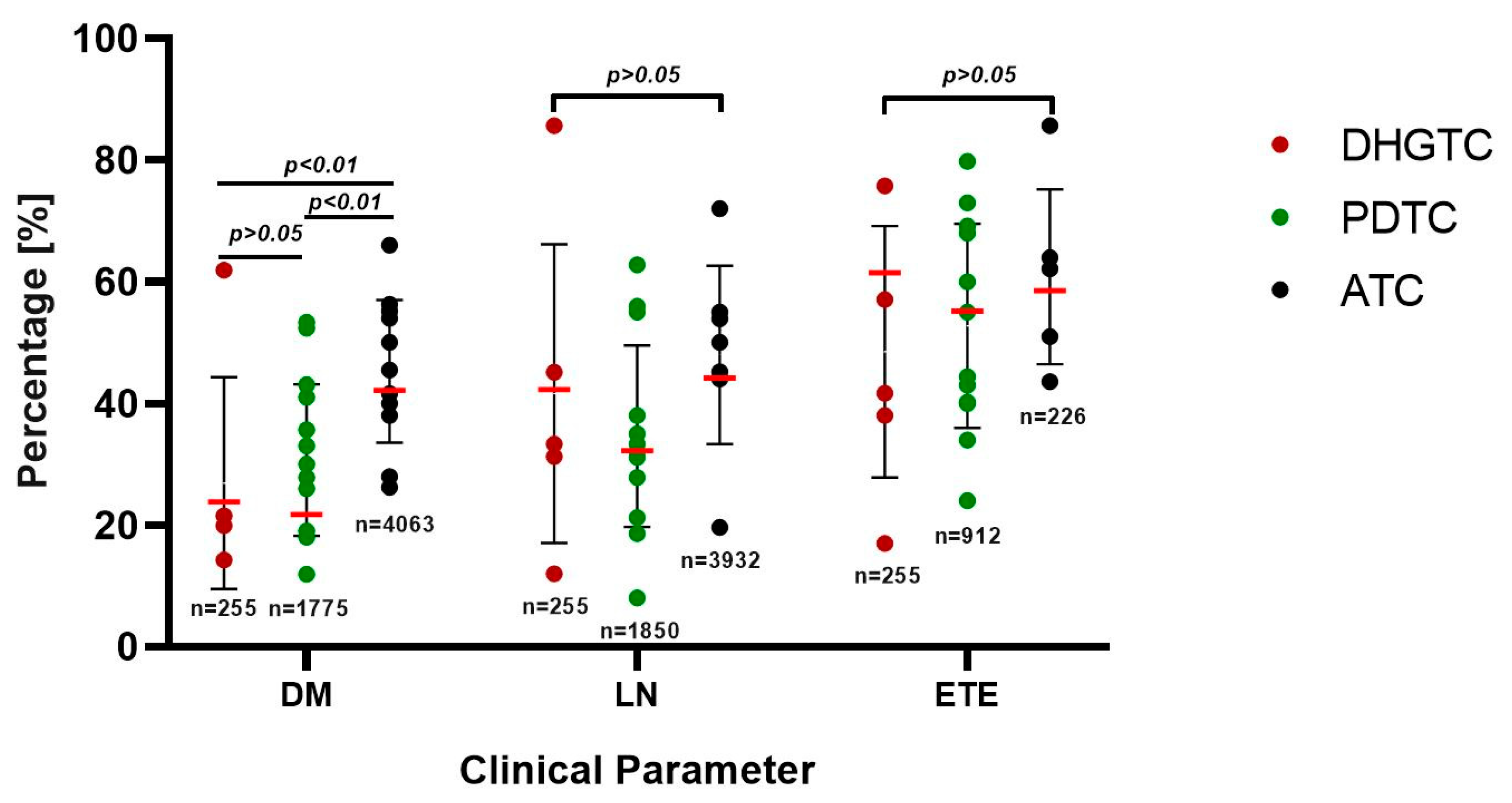
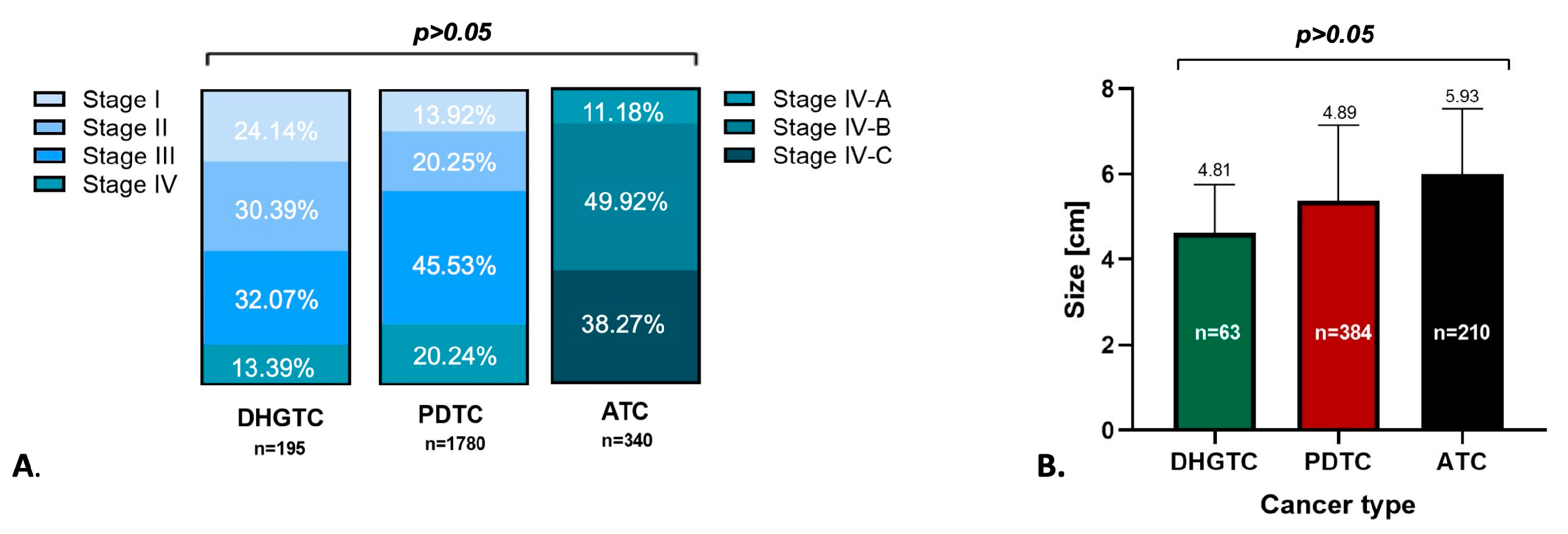
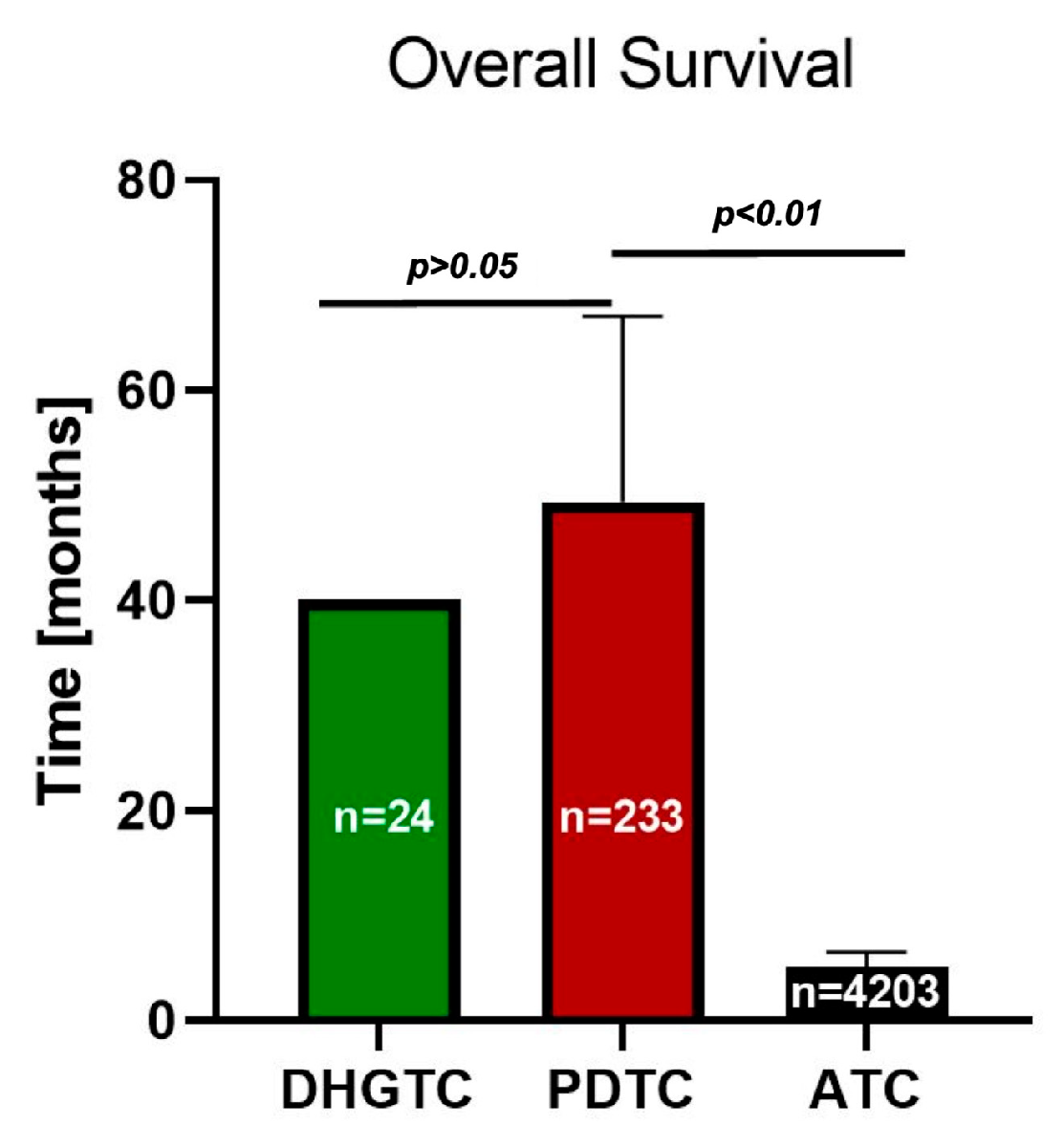
3.1. Clinical Outcome and Prognostic Considerations
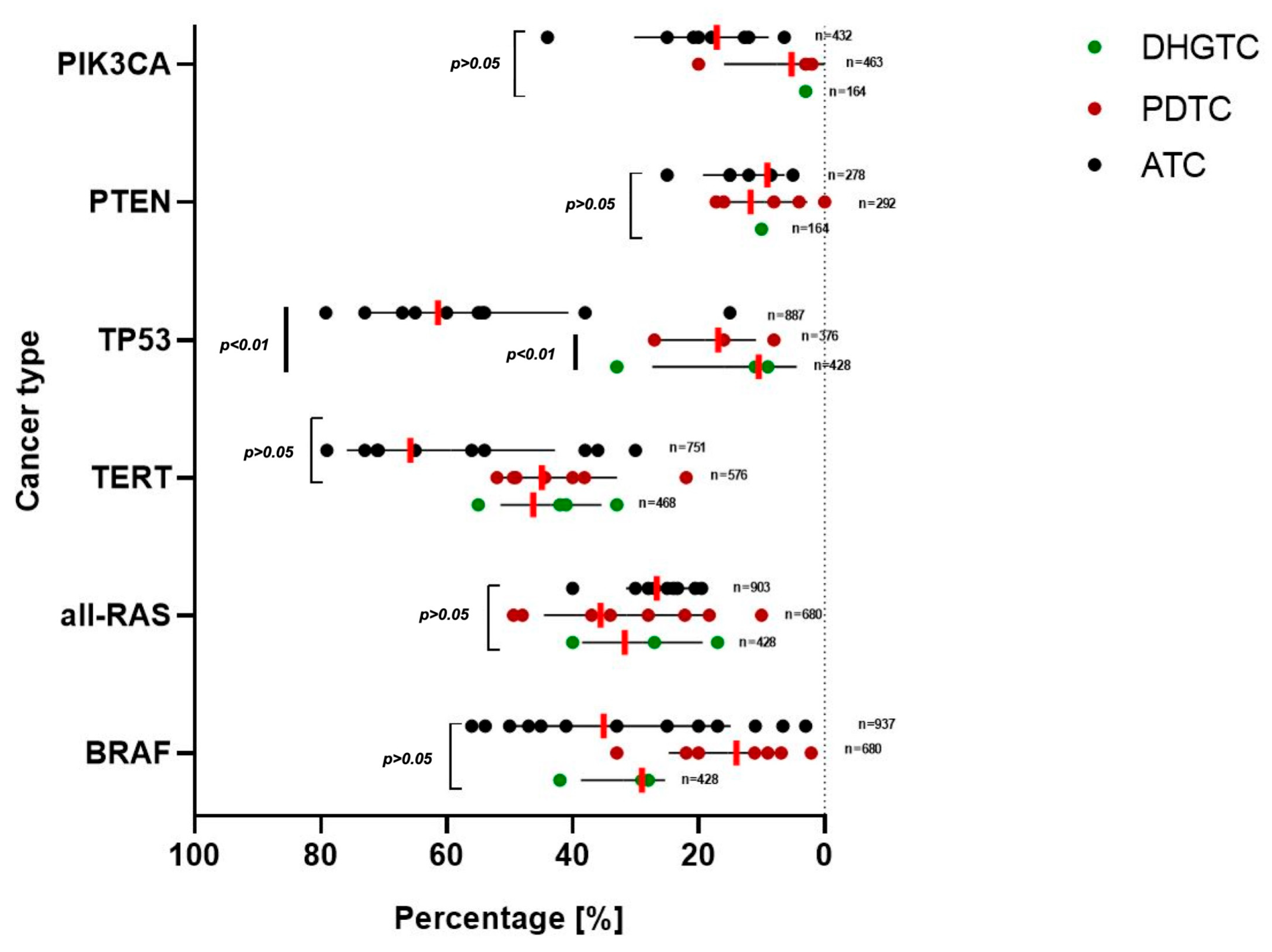
3.2. Molecular Landscape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AJCC | American Joint Committee for Cancer |
| ATC | Anaplastic thyroid carcinoma |
| DHGTC | Differentiated high-grade thyroid carcinoma |
| DTC | Differentiated thyroid carcinoma |
| FISH | Fluorescent in situ hybridization |
| NGS | New generation sequencing |
| OS | Overall Survival |
| PDTC | Poorly differentiated thyroid carcinoma |
| RAI | Radioactive iodine |
| TNM | Tumor node metastasis |
| WHO | World Health Organisation |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024. [Google Scholar]
- Li, M.; Meheus, F.; Polazzi, S.; Delafosse, P.; Borson-Chazot, F.; Seigneurin, A.; Simon, R.; Combes, J.-D.; Maso, L.D.; Colonna, M.; et al. The Economic Cost of Thyroid Cancer in France and the Corresponding Share Associated with Treatment of Overdiagnosed Cases. Value Health 2023, 26, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Chiba, T. Molecular Pathology of Thyroid Tumors: Essential Points to Comprehend Regarding the Latest WHO Classification. Biomedicines 2024, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Juhlin, C.C.; Mete, O.; Baloch, Z.W. The 2022 WHO classification of thyroid tumors: Novel concepts in nomenclature and grading. Endocr.-Relat. Cancer 2023, 30, e220293. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours [Internet], 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 10. [Google Scholar]
- Boudina, M.; Zisimopoulou, E.; Xirou, P.; Chrisoulidou, A. Aggressive Types of Malignant Thyroid Neoplasms. J. Clin. Med. 2024, 13, 6119. [Google Scholar] [CrossRef]
- Dal Maso, L.; Tavilla, A.; Pacini, F.; Serraino, D.; van Dijk, B.A.C.; Chirlaque, M.D.; Capocaccia, R.; Larrañaga, N.; Colonna, M.; Agius, D.; et al. Survival of 86,690 patients with thyroid cancer: A population-based study in 29 European countries from EUROCARE-5. Eur. J. Cancer 2017, 77, 140–152. [Google Scholar] [CrossRef]
- Poma, A.M.; Macerola, E.; Ghossein, R.A.; Tallini, G.; Basolo, F. Prevalence of Differentiated High-Grade Thyroid Carcinoma Among Well-Differentiated Tumors: A Systematic Review and Meta-Analysis. Thyroid 2024, 34, 314–323. [Google Scholar] [CrossRef]
- Xu, B.; David, J.; Dogan, S.; Landa, I.; Katabi, N.; Saliba, M.; Khimraj, A.; Sherman, E.J.; Tuttle, R.M.; Tallini, G.; et al. Primary High-Grade Non-Anaplastic Thyroid Carcinoma: A Retrospective Study of 364 Cases. Histopathology 2022, 80, 322–337. [Google Scholar] [CrossRef]
- Dong, W.; Okamoto, T.; Ji, X.; Xiang, J.; Zhang, D.; Zhang, P.; Zhang, H. Conditional Survival Rate Estimates for Anaplastic Thyroid Cancer Beyond the First Year: An Analysis of SEER Data (2004–2019). Thyroid 2023, 33, 523–526. [Google Scholar] [CrossRef]
- Pavlidis, E.T.; Pavlidis, T.E. Role of prophylactic central neck lymph node dissection for papillary thyroid carcinoma in the era of de-escalation. World J. Clin. Oncol. 2023, 14, 247–258. [Google Scholar] [CrossRef]
- Pavlidis, E.T.; Galanis, I.N.; Pavlidis, T.E. Update on current diagnosis and management of anaplastic thyroid carcinoma. World J. Clin. Oncol. 2023, 14, 570–583. [Google Scholar] [CrossRef]
- Ibrahimpasic, T.; Ghossein, R.; Shah, J.P.; Ganly, I. Poorly Differentiated Carcinoma of the Thyroid Gland: Current Status and Future Prospects. Thyroid 2019, 29, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Panchangam, R.B.; Puthenveetil, P.; Mayilvaganan, S. Prognostic Impact of Focal Poorly Differentiated Areas in Follicular Differentiated Thyroid Cancer: Is It a Distinct Entity from Poorly Differentiated Thyroid Cancer? Indian J. Surg. Oncol. 2022, 13, 157–163. [Google Scholar] [CrossRef]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Moon, S.R.; Seok, J.Y.; Lee, J.H.; Nam, S.; Chung, Y.S. Characterization of the genomic alterations in poorly differentiated thyroid cancer. Sci. Rep. 2023, 13, 19154. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef] [PubMed]
- Cleere, E.F.; Prunty, S.; O’Neill, J.P. Anaplastic thyroid cancer:Improved understanding of what remains a deadly disease. Surgeon 2024, 22, e48–e53. [Google Scholar] [CrossRef]
- Hlozek, J.; Pekova, B.; Rotnagl, J.; Holy, R.; Astl, J. Genetic Changes in Thyroid Cancers and the Importance of Their Preoperative Detection in Relation to the General Treatment and Determination of the Extent of Surgical Intervention—A Review. Biomedicines 2022, 10, 1515. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Murtha, T.D.; Dong, W.; Paulsson, J.O.; Choi, J.; Barbieri, A.L.; Brown, T.C.; Kunstman, J.W.; Larsson, C.; Prasad, M.L.; et al. Comprehensive Genetic Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent of Histology. J. Clin. Endocrinol. Metab. 2018, 103, 2640–2650. [Google Scholar] [CrossRef]
- Fumet, J.D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Taissoun Aslan, Z.A.; Granados-García, M.; Luna-Ortiz, K.; Guerrero-Huerta, F.J.; Gómez-Pedraza, A.; Ñamendys-Silva, S.A.; Meneses-García, A.; Ordoñez-Mosquera, J.M. Anaplastic thyroid cancer: Multimodal treatment results. Ecancermedicalscience 2014, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Brignardello, E.; Palestini, N.; Felicetti, F.; Castiglione, A.; Piovesan, A.; Gallo, M.; Freddi, M.; Ricardi, U.; Gasparri, G.; Ciccone, G.; et al. Early Surgery and Survival of Patients with Anaplastic Thyroid Carcinoma: Analysis of a Case Series Referred to a Single Institution Between 1999 and 2012. Thyroid 2014, 24, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, Y.; Hu, P.; Gao, J.; Ying, J.; Xu, W.; Zhao, D.; Wang, Z.; Ye, J.; Lizaso, A.; et al. Mutational profiling of poorly differentiated and anaplastic thyroid carcinoma by the use of targeted next-generation sequencing. Histopathology 2019, 75, 890–899. [Google Scholar] [CrossRef]
- Evans, L.K.; Chen, H.; Labib, M.T.; Cronkite, D.A.; Yu, A.C.; Ashendouek, M.; Elashoff, D.; Chai-Ho, W.; Wong, D.J.; John, M.S. Improved Survival of Advanced-Stage Anaplastic Thyroid Cancer with Systemic Therapy. Laryngoscope 2025, 135, 478–484. [Google Scholar] [CrossRef]
- de la Fouchardière, C.; Decaussin-Petrucci, M.; Berthiller, J.; Descotes, F.; Lopez, J.; Lifante, J.-C.; Peix, J.-L.; Giraudet, A.-L.; Delahaye, A.; Masson, S.; et al. Predictive factors of outcome in poorly differentiated thyroid carcinomas. Eur. J. Cancer 2018, 92, 40–47. [Google Scholar] [CrossRef]
- Glaser, S.M.; Mandish, S.F.; Gill, B.S.; Balasubramani, G.K.; Clump, D.A.; Beriwal, S. Anaplastic thyroid cancer: Prognostic factors, patterns of care, and overall survival. Head Neck 2016, 38, E2083–E2090. [Google Scholar] [CrossRef]
- Gu, H.; Wang, J.; Ran, W.; Li, G.; Hu, S.; Zhao, H.; Wang, X.; Wang, J. Anaplastic and poorly differentiated thyroid carcinomas: Genetic evidence of high-grade transformation from differentiated thyroid carcinoma. J. Pathol. Clin. Res. 2024, 10, e356. [Google Scholar] [CrossRef] [PubMed]
- Ibrahimpasic, T.; Ghossein, R.; Carlson, D.L.; Nixon, I.; Palmer, F.L.; Shaha, A.R.; Patel, S.G.; Tuttle, R.M.; Shah, J.P.; Ganly, I. Outcomes in Patients with Poorly Differentiated Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2014, 99, 1245–1252. [Google Scholar] [CrossRef]
- Jeong, S.I.; Kim, W.; Yu, H.W.; Choi, J.Y.; Ahn, C.H.; Moon, J.H.; Choi, S.I.; Cha, W.; Jeong, W.-J.; Park, S.Y.; et al. Incidence and Clinicopathological Features of Differentiated High-Grade Thyroid Carcinomas: An Institutional Experience. Endocr. Pathol. 2023, 34, 287–297. [Google Scholar] [CrossRef]
- Jin, S.; Liu, H.; Yang, J.; Zhou, J.; Peng, D.; Liu, X.; Zhang, H.; Zeng, Z.; Ye, Y.-N. Development and validation of a nomogram model for cancer-specific survival of patients with poorly differentiated thyroid carcinoma: A SEER database analysis. Front. Endocrinol. 2022, 13, 882279. [Google Scholar] [CrossRef]
- Kersting, D.; Seifert, R.; Kessler, L.; Herrmann, K.; Theurer, S.; Brandenburg, T.; Dralle, H.; Weber, F.; Umutlu, L.; Führer-Sakel, D.; et al. Predictive Factors for RAI-Refractory Disease and Short Overall Survival in PDTC. Cancers 2021, 13, 1728. [Google Scholar] [CrossRef] [PubMed]
- Kunte, S.; Sharett, J.; Wei, W.; Nasr, C.; Prendes, B.; Lamarre, E.; Ku, J.; Lorenz, R.R.; Scharpf, J.; Burkey, B.B.; et al. Poorly Differentiated Thyroid Carcinoma: Single Institution Series of Outcomes. Anticancer Res. 2022, 42, 2531–2539. [Google Scholar] [CrossRef]
- Landa, I.; Ibrahimpasic, T.; Boucai, L.; Sinha, R.; Knauf, J.A.; Shah, R.H.; Dogan, S.; Ricarte-Filho, J.C.; Krishnamoorthy, G.P.; Xu, B.; et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016, 126, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Johari, M.; Rabade, K.; Rane, S.; Mittal, N.; Bal, M. 535 Clinicopathological Spectrum of High-Grade Follicular Cell-Derived Non-Anaplastic Thyroid Carcinoma: A Series of 106 Cases. Lab. Investig. 2025, 105, 102765. [Google Scholar] [CrossRef]
- Paunovic, I.R.; Sipetic, S.B.; Zoric, G.V.; Diklic, A.D.; Savic, D.V.; Marinkovic, J.; Zivaljevic, V.R. Survival and Prognostic Factors of Anaplastic Thyroid Carcinoma. Acta Chir. Belg. 2015, 115, 62–67. [Google Scholar] [CrossRef]
- Saito, Y.; Kage, H.; Kobayashi, K.; Kamogashira, T.; Fukuoka, O.; Yamamura, K.; Yamashita, S.; Tanabe, M.; Oda, K.; Kondo, K. Comprehensive genomic profiling from C-CAT database unveiled over 80% presence of oncogenic drivers in anaplastic thyroid carcinoma including BRAF, RAS family, NF1, and FGFR1. Clin. Endocrinol. 2024, 101, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Sherman, E.J.; Lim, S.H.; Ho, A.L.; Ghossein, R.A.; Fury, M.G.; Shaha, A.R.; Rivera, M.; Lin, O.; Wolden, S.; Lee, N.Y.; et al. Concurrent doxorubicin and radiotherapy for anaplastic thyroid cancer: A critical re-evaluation including uniform pathologic review. Radiother. Oncol. 2011, 101, 425–430. [Google Scholar] [CrossRef]
- Swaak-Kragten, A.T.; de Wilt, J.H.W.; Schmitz, P.I.M.; Bontenbal, M.; Levendag, P.C. Multimodality treatment for anaplastic thyroid carcinoma—Treatment outcome in 75 patients. Radiother. Oncol. 2009, 92, 100–104. [Google Scholar] [CrossRef]
- Thompson, L.D.R. High Grade Differentiated Follicular Cell-Derived Thyroid Carcinoma Versus Poorly Differentiated Thyroid Carcinoma: A Clinicopathologic Analysis of 41 Cases. Endocr. Pathol. 2023, 34, 234–246. [Google Scholar] [CrossRef]
- Resta, I.T.; Gubbiotti, M.A.; Montone, K.T.; Livolsi, V.A.; Baloch, Z.W. Differentiated high grade thyroid carcinomas: Diagnostic consideration and clinical features. Hum. Pathol. 2024, 144, 53–60. [Google Scholar] [CrossRef]
- Wendler, J.; Kroiss, M.; Gast, K.; Kreissl, M.C.; Allelein, S.; Lichtenauer, U.; Blaser, R.; Spitzweg, C.; Fassnacht, M.; Schott, M.; et al. Clinical presentation, treatment and outcome of anaplastic thyroid carcinoma: Results of a multicenter study in Germany. Eur. J. Endocrinol. 2016, 175, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Lorch, J.H.; Alexander, E.K.; Marqusee, E.; Cho, N.L.; Nehs, M.A.; Doherty, G.M.; Barletta, J.A. Prognostic Significance of Extent of Invasion in Poorly Differentiated Thyroid Carcinoma. Thyroid 2019, 29, 1255–1261. [Google Scholar] [CrossRef]
- Wu, S.S.; Lamarre, E.D.; Yalamanchali, A.; Brauer, P.R.; Hong, H.; Reddy, C.A.; Yilmaz, E.; Woody, N.; Ku, J.A.; Prendes, B.; et al. Association of Treatment Strategies and Tumor Characteristics with Overall Survival Among Patients with Anaplastic Thyroid Cancer: A Single-Institution 21-Year Experience. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 300–309. [Google Scholar] [CrossRef]
- Xu, B.; Lubin, D.J.; Dogan, S.; Ghossein, R.A.; Viswanathan, K. Significance of oncocytic features in poorly differentiated thyroid carcinoma—A bi-institutional experience. Virchows Arch. 2023, 482, 479–491. [Google Scholar] [CrossRef]
- Yu, M.G.; Rivera, J.; Jimeno, C. Poorly Differentiated Thyroid Carcinoma: 10-Year Experience in a Southeast Asian Population. Endocrinol. Metab. 2017, 32, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Yang, M.; Gretarsson, S.; Jansson, C.; Mylona, N.; Sydow, S.R.; Woodward, E.L.; Ekblad, L.; Wennerberg, J.; Paulsson, K. Identification of Targetable Lesions in Anaplastic Thyroid Cancer by Genome Profiling. Cancers 2019, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, B.; Godbert, Y.; Perot, G.; Al Ghuzlan, A.; Bardet, S.; Belleannée, G.; Crinière, L.; Do Cao, C.; Fouilloux, G.; Guyetant, S.; et al. Molecular Pathology of Anaplastic Thyroid Carcinomas: A Retrospective Study of 144 Cases. Thyroid 2017, 27, 682–692. [Google Scholar] [CrossRef]
- Latteyer, S.; Tiedje, V.; König, K.; Ting, S.; Heukamp, L.C.; Meder, L.; Schmid, K.W.; Führer, D.; Moeller, L.C. Targeted next-generation sequencing for TP53, RAS, BRAF, ALK and NF1 mutations in anaplastic thyroid cancer. Endocrine 2016, 54, 733–741. [Google Scholar] [CrossRef]
- Pozdeyev, N.; Gay, L.M.; Sokol, E.S.; Hartmaier, R.; Deaver, K.E.; Davis, S.; French, J.D.; Borre, P.V.; LaBarbera, D.V.; Tan, A.-C.; et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin. Cancer Res. 2018, 24, 3059–3068. [Google Scholar] [CrossRef]
- Scholfield, D.W.; Xu, B.; Levyn, H.; Eagan, A.; Shaha, A.R.; Shah, J.P.; Tuttle, R.M.; Fagin, J.A.; Wong, R.J.; Patel, S.G.; et al. High-Grade Follicular Cell-Derived Non-Anaplastic Thyroid Carcinoma: Correlating Extent of Invasion and Mutation Profile with Oncologic Outcome. Thyroid 2025, 35, 153–165. [Google Scholar] [CrossRef]
- Stenman, A.; Yang, M.; Paulsson, J.O.; Zedenius, J.; Paulsson, K.; Juhlin, C.C. Pan-genomic sequencing reveals actionable cdkn2a/2b deletions and kataegis in anaplastic thyroid carcinoma. Cancers 2021, 13, 6340. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Ito, Y.; Hirokawa, M.; Yoshida, H.; Miyauchi, A. BRAFV600E mutation in anaplastic thyroid carcinomas and their accompanying differentiated carcinomas. Br. J. Cancer 2007, 96, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Tiedje, V.; Ting, S.; Herold, T.; Synoracki, S.; Latteyer, S.; Moeller, L.C.; Zwanziger, D.; Stuschke, M.; Fuehrer, D.; Schmid, K.W. NGS based identification of mutational hotspots for targeted therapy in anaplastic thyroid carcinoma. Oncotarget 2017, 8, 42613–42620. [Google Scholar] [CrossRef]
- Toda, S.; Hiroshima, Y.; Iwasaki, H.; Masudo, K. Genomic Landscape and Clinical Features of Advanced Thyroid Carcinoma: A National Database Study in Japan. J. Clin. Endocrinol. Metab. 2024, 109, 2784–2792. [Google Scholar] [CrossRef]
- Torous, V.F.; Jitpasutham, T.; Baloch, Z.; Cantley, R.L.; Kerr, D.A.; Liu, X.; Maleki, Z.; Merkin, R.; Nosé, V.; Pantanowitz, L.; et al. Cytologic features of differentiated high-grade thyroid carcinoma: A multi-institutional study of 40 cases. Cancer Cytopathol. 2024, 132, 525–536. [Google Scholar] [CrossRef]
- Yamazaki, H.; Kunisaki, C.; Sugimori, M.; Rino, Y.; Saito, A. Genetic landscape of 482 thyroid carcinomas: Analysis with the national datacenter for cancer genomic medicine in Japan. Endocrine 2024, 85, 766–776. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2002, 18, e1230. [Google Scholar] [CrossRef]
- Harahap, A.S.; Roren, R.S.; Imtiyaz, S. A Comprehensive Review and Insights into the New Entity of Differentiated High-Grade Thyroid Carcinoma. Curr. Oncol. 2024, 31, 3311–3328. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, A.; Kopczyński, J.; Gąsior-Perczak, D.; Pałyga, I.; Kowalik, A.; Chrapek, M.; Hejnold, M.; Góźdź, S.; Kowalska, A. Poorly differentiated thyroid cancer in the context of the revised 2015 American Thyroid Association Guidelines and the Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System (eighth edition). Clin. Endocrinol. 2019, 91, 331–339. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Andrianus, S.; Sakhi, R.; Zhang, Q.; Montone, K.; Jalaly, J.B.; Baloch, Z. Does the presence of capsule influence prognosis in poorly differentiated thyroid carcinoma? Hum. Pathol. 2023, 136, 96–104. [Google Scholar] [CrossRef]
- Jung, T.S.; Kim, T.Y.; Kim, K.W.; Oh, Y.L.; Park, D.J.; Cho, B.Y.; Shong, Y.K.; Kim, W.B.; Park, Y.J.; Jung, J.H.; et al. Clinical Features and Prognostic Factors for Survival in Patients with Poorly Differentiated Thyroid Carcinoma and Comparison to the Patients with the Aggressive Variants of Papillary Thyroid Carcinoma. Endocr. J. 2007, 54, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Lam, A.K.Y. Anaplastic thyroid carcinoma: Updates on WHO classification, clinicopathological features and staging. Histol. Histopathol. 2021, 36, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Jannin, A.; Escande, A.; Al Ghuzlan, A.; Blanchard, P.; Hartl, D.; Chevalier, B.; Deschamps, F.; Lamartina, L.; Lacroix, L.; Dupuy, C.; et al. Anaplastic Thyroid Carcinoma: An Update. Cancers 2022, 14, 1061. [Google Scholar] [CrossRef]
- Tiedje, V.; Stuschke, M.; Weber, F.; Dralle, H.; Moss, L.; Führer, D. Anaplastic thyroid carcinoma: Review of treatment protocols. Endocr.-Relat. Cancer. 2018, 25, R153–R161. [Google Scholar] [CrossRef] [PubMed]
- Zivaljevic, V.; Tausanovic, K.; Paunovic, I.; Diklic, A.; Kalezic, N.; Zoric, G.; Sabljak, V.; Vekic, B.; Zivic, R.; Marinkovic, J.; et al. Age as a Prognostic Factor in Anaplastic Thyroid Cancer. Int. J. Endocrinol. 2014, 2014, 240513. [Google Scholar] [CrossRef]
- Ahn, J.; Song, E.; Oh, H.-S.; Song, D.E.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Jeon, M.J. Low Lymphocyte-to-Monocyte Ratios Are Associated with Poor Overall Survival in Anaplastic Thyroid Carcinoma Patients. Thyroid 2019, 29, 824–829. [Google Scholar] [CrossRef]
- Volante, M.; Lam, A.K.; Papotti, M.; Tallini, G. Molecular Pathology of Poorly Differentiated and Anaplastic Thyroid Cancer: What Do Pathologists Need to Know? Endocr. Pathol. 2021, 32, 63–76. [Google Scholar] [CrossRef]
- Tong, J.; Ruan, M.; Jin, Y.; Fu, H.; Cheng, L.; Luo, Q.; Liu, Z.; Lv, Z.; Chen, L. Poorly differentiated thyroid carcinoma: A clinician’s perspective. Eur. Thyroid J. 2022, 11, e220021. [Google Scholar] [CrossRef]
- Shaul, Y.D.; Seger, R. The MEK/ERK cascade: From signaling specificity to diverse functions. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2007, 1773, 1213–1226. [Google Scholar] [CrossRef]
- Sykorova, V.; Dvorakova, S.; Vcelak, J.; Vaclavikova, E.; Halkova, T.; Kodetova, D.; Lastuvka, P.; Betka, J.; Vlcek, P.; Reboun, M.; et al. Search for New Genetic Biomarkers in Poorly Differentiated and Anaplastic Thyroid Carcinomas Using Next Generation Sequencing. Anticancer Res. 2015, 35, 2029–2036. [Google Scholar]
- Singh, A.; Ham, J.; Po, J.W.; Niles, N.; Roberts, T.; Lee, C.S. The Genomic Landscape of Thyroid Cancer Tumourigenesis and Implications for Immunotherapy. Cells 2021, 10, 1082. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.Y.; Kim, S.-M.; Chang, H.-J.; Lee, Y.S.; Park, C.S.; Chang, H.-S. Long-term survival of patients with anaplastic thyroid cancer after multimodal treatment. Transl. Cancer Res. 2020, 9, 5430–5436. [Google Scholar] [CrossRef]
- Violetis, O.; Konstantakou, P.; Spyroglou, A.; Xydakis, A.; Kekis, P.B.; Tseleni, S.; Kolomodi, D.; Konstadoulakis, M.; Mastorakos, G.; Theochari, M.; et al. The Long Journey towards Personalized Targeted Therapy in Poorly Differentiated Thyroid Carcinoma (PDTC): A Case Report and Systematic Review. J. Pers. Med. 2024, 14, 654. [Google Scholar] [CrossRef]
- Kong, N.; Xu, Q.; Zhang, Z.; Cui, A.; Tan, S.; Bai, N. Age Influences the Prognosis of Anaplastic Thyroid Cancer Patients. Front. Endocrinol. 2021, 12, 704596. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Ruffilli, I.; La Motta, C.; Paparo, S.R.; Patrizio, A.; Vita, R.; Benvenga, S.; Materazzi, G.; et al. Novel treatments for anaplastic thyroid carcinoma. Gland Surg. 2020, 9 (Suppl. 1), S28–S42. [Google Scholar] [CrossRef]
- De Leo, S.; Trevisan, M.; Fugazzola, L. Recent advances in the management of anaplastic thyroid cancer. Thyroid. Res. 2020, 13, 17. [Google Scholar] [CrossRef]
- da Silva, T.N.; Rodrigues, R.; Saramago, A.; Pires, C.; Rito, M.; Horta, M.; Martins, C.; Leite, V.; Cavaco, B.M. Target therapy for BRAF mutated anaplastic thyroid cancer: A clinical and molecular study. Eur. J. Endocrinol. 2023, 188, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shugard, E.; Khanafshar, E.; Quivey, J.M.; Garsa, A.A.; Yom, S.S. Association Between BRAF V600E Mutation and Decreased Survival in Patients Locoregionally Irradiated for Anaplastic Thyroid Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, E356. [Google Scholar] [CrossRef]
- Riccio, I.; Laforteza, A.; Landau, M.B.; Hussein, M.H.; Linhuber, J.; Staav, J.; Issa, P.P.; Toraih, E.A.; Kandil, E. Decoding RAS mutations in thyroid cancer: A meta-analysis unveils specific links to distant metastasis and increased mortality. Am. J. Otolaryngol. 2025, 46, 104570. [Google Scholar] [CrossRef]
- Laha, D.; Nilubol, N.; Boufraqech, M. New Therapies for Advanced Thyroid Cancer. Front. Endocrinol. 2020, 11, 82. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Chen, Z.; Yang, J.; Ma, Y.; Cai, H.; Shan, C.; Lv, Z.; Zhang, X. Wild-Type P53 Induces Sodium/Iodide Symporter Expression Allowing Radioiodide Therapy in Anaplastic Thyroid Cancer. Cell. Physiol. Biochem. 2017, 43, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Peuget, S.; Zhou, X.; Selivanova, G. Translating p53-based therapies for cancer into the clinic. Nat. Rev. Cancer 2024, 24, 192–215. [Google Scholar] [CrossRef] [PubMed]
- Chai, D.; Wang, J.; Fan, C.; Lim, J.-M.; Wang, X.; Neeli, P.; Yu, X.; Young, K.H.; Li, Y. Remodeling of anti-tumor immunity with antibodies targeting a p53 mutant. J. Hematol. Oncol. 2024, 17, 45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schipor, S.; Publik, M.A.; Manda, D.; Ceausu, M. Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5535. https://doi.org/10.3390/ijms26125535
Schipor S, Publik MA, Manda D, Ceausu M. Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(12):5535. https://doi.org/10.3390/ijms26125535
Chicago/Turabian StyleSchipor, Sorina, Mihai Alin Publik, Dana Manda, and Mihail Ceausu. 2025. "Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review" International Journal of Molecular Sciences 26, no. 12: 5535. https://doi.org/10.3390/ijms26125535
APA StyleSchipor, S., Publik, M. A., Manda, D., & Ceausu, M. (2025). Aggressive Thyroid Carcinomas Clinical and Molecular Features: A Systematic Review. International Journal of Molecular Sciences, 26(12), 5535. https://doi.org/10.3390/ijms26125535







