Omics Sciences in Regular Physical Activity
Abstract
1. Introduction
2. Bibliographic Search Method
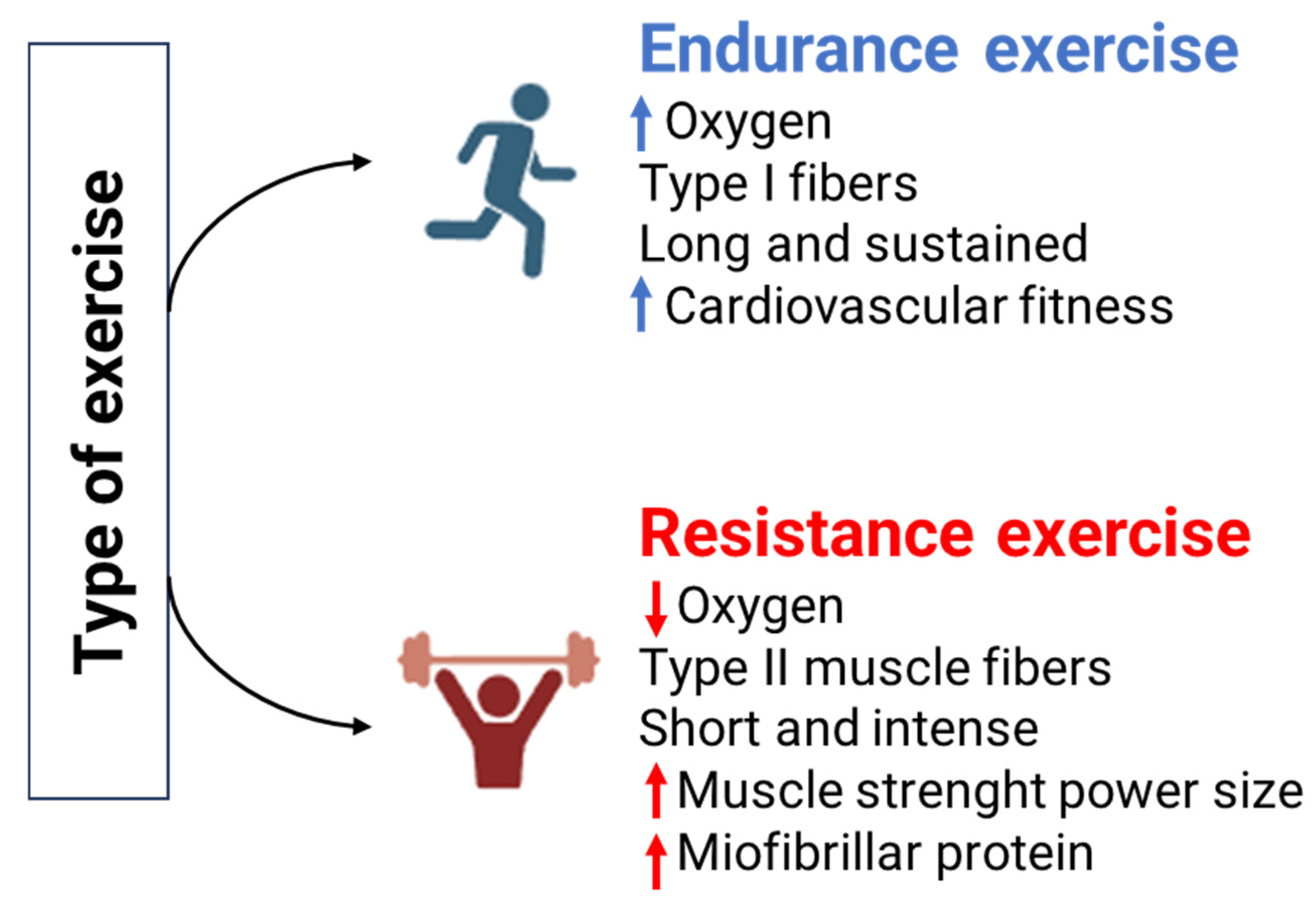
3. Role of Intensity, Volume, and Type of Activity on Regular Physical Exercise
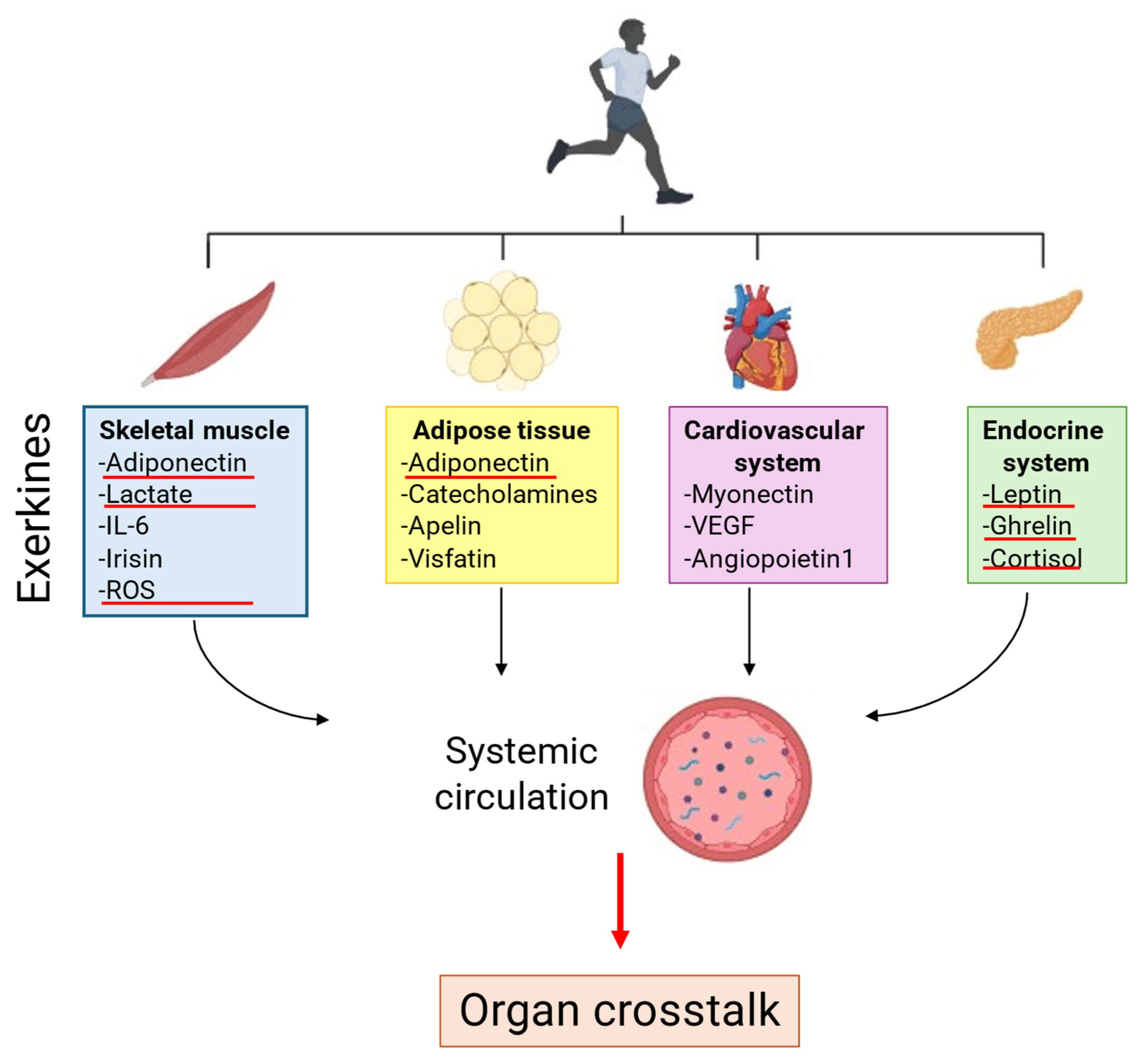
4. Role of Exerkines on Regular Physical Exercise
5. Omics Sciences
5.1. Genomics
5.2. Epigenomics
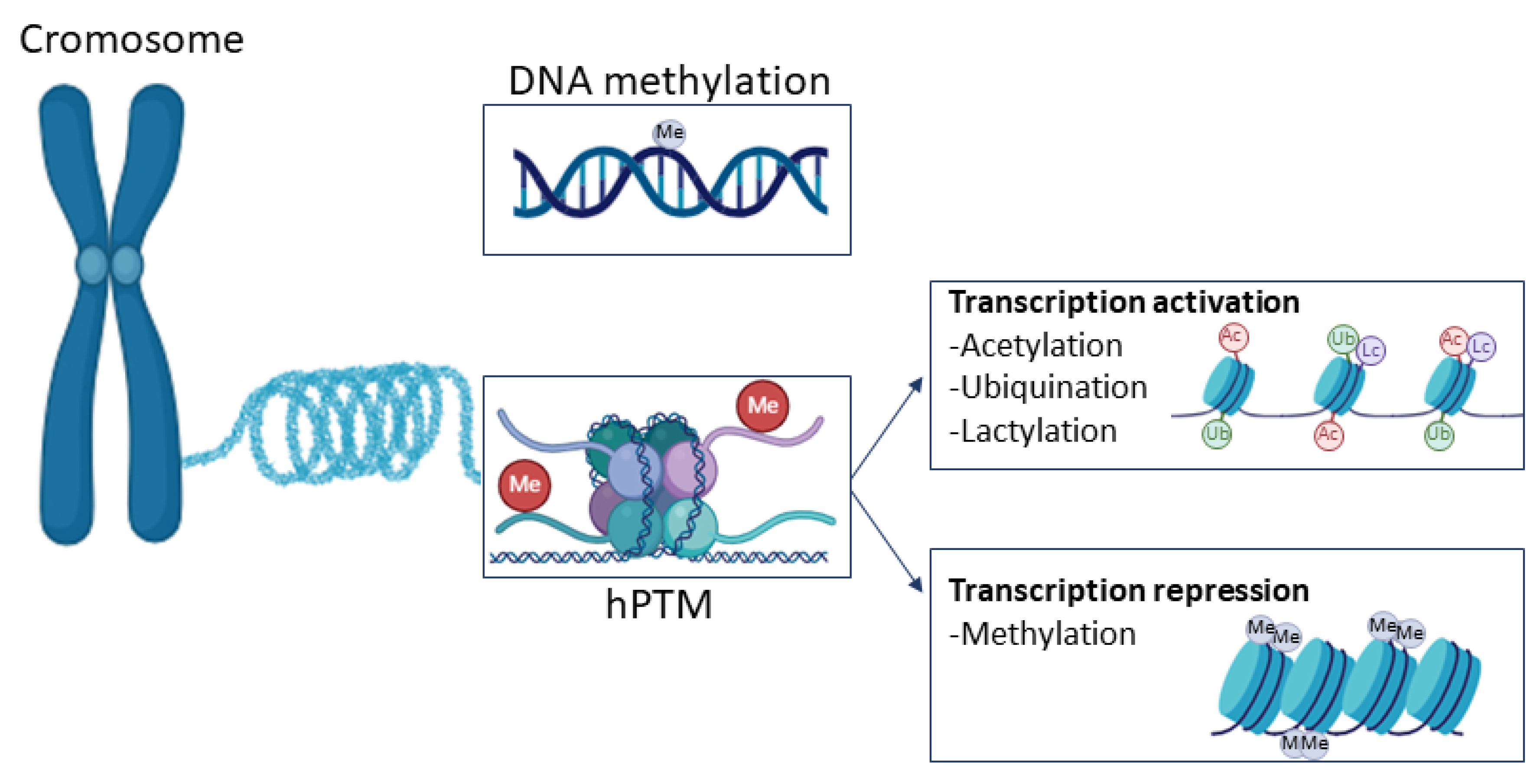
5.2.1. DNA Methylation
5.2.2. Histone Post-Translational Modification
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Dimauro et al. [34] | 2020 | Both | Exercise influences DNA methylation and histone modifications. | Histones, DNA methylation, and redox-related molecules during exercise. |
| Jacques et al. [35] | 2019 | Both | DNA methylation and histone changes in human skeletal muscle following exercise. | DNA hypomethylation marks genes such as PGC-1a, TFAM, MEF2A, and PDK4, along with histone modifications such as H3K36 and H3K9/14 acetylation. |
| Voisin et al. [36] | 2015 | Both | Exercise training affects DNA methylation. | p15, ASC, PGC-1α, PDK4, TFAM, PPAR-δ, citrate synthase, MEF2A, MYOD1. |
| Landen et al. [37] | 2023 | Both | Physiological and molecular sex differences in muscle response to exercise training. | DNA methylation of hormone-related transcription factors as the androgen receptor and the estrogen receptor. |
| Fabre et al. [38] | 2018 | Both | DNA methylation in human adipose tissue after exercise. | DNA methylation in ADRA2A, FOSL1, METRNL, RARA, CBLB, GPR132, and RELT. |
| Geiger et al. [39] | 2024 | Both | DNA methylation of exercise-responsive genes differs between trained and untrained individuals. | Exercise-responsive genes MYH7 and MYL3, high methylation in transcription factors such as FOXO3, CREB5, and PGC-1α. |
| Turner et al. [40] | 2020 | Both | DNA methylation of HOX genes. | Hypermethylated state in genes KIF15, DYRK2, FHL2, MRPS33, ABCA17P, and HOX genes. |
| Li et al. [44] | 2020 | Both | Glis1 induces epigenome-metabolome signaling cascade influenced by exercise. | Glis1 and modulation of glycolytic gene expression. |
5.3. Transcriptomics
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| De Sanctis et al. [51] | 2021 | Aerobic | Non-coding RNAs differentiating in skeletal muscles in elderly trained in endurance vs. resistance exercise. | Non-coding RNAs: miR-20a-5p and miR-106-5p shared 108 mRNA involved in the regulation of TGF-beta, p53, FoxO, Hippo, WNT, MAPK, and HIF-1. |
| Hecksteden et al. [52] | 2016 | Aerobic | Non-coding RNAs in sports training. | Non-coding RNAs hsa-miR 513b-5p, hsa-miR-140-5p, hsa-miR-650, and hsa-miR 3620-3p regulate vascular endothelial growth factor A pathway. |
| Domańska-Senderowska et al. [53] | 2019 | Both | MicroRNA profiles and their adaptive response to exercise training. | Non-coding RNAs microRNA-1, -21, -23a, -124, -125b, -133a/b, -144, -145, -206, -486, and -696 regulate the IGF1/PI3K/AKT/mTOR signaling pathway. |
| Dimauro et al. [34] | 2020 | Both | Exercise in redox homeostasis and epigenetic regulation in skeletal muscle. | Non-coding RNAs: miR-1, miR-16, miR-21, miR-26a, miR-29a, miR-126, miR-133a, miR-133b, and miR-206, miR-210, miR-221, 328, miR-378, miR-451, miR-494, miR-21, miR-221, miR-20a, miR-146a, miR-133, and miR-222. |
5.4. Metabolomics
5.5. Proteomics
6. Bioinformatic Tools
7. Conclusions
8. Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magherini, F.; Fiaschi, T.; Marzocchini, R.; Mannelli, M.; Gamberi, T.; Modesti, P.A.; Modesti, A. Oxidative Stress in Exercise Training: The Involvement of Inflammation and Peripheral Signals. Free Radic. Res. 2019, 53, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Tipton, C.M. The History of “Exercise Is Medicine” in Ancient Civilizations. Adv. Physiol. Educ. 2014, 38, 109–117. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Bergeron, M.F.; Lee, E.C.; Mershon, J.E.; Armstrong, E.M. Overtraining Syndrome as a Complex Systems Phenomenon. Front. Netw. Physiol. 2021, 1, 794392. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Spiteri, T.; Piggott, B.; Bonhotal, J.; Haff, G.G.; Joyce, C. Monitoring and Managing Fatigue in Basketball. Sports 2018, 6, 19. [Google Scholar] [CrossRef]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Gomes, C.; Almeida, J.A.; Franco, O.L.; Petriz, B. Omics and the Molecular Exercise Physiology. Adv. Clin. Chem. 2020, 96, 55–84. [Google Scholar]
- Warburton, D.E.R.; Bredin, S.S.D. Health benefits of physical activity: A systematic review of current systematic reviews. Curr. Opin. Cardiol. 2017, 32, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights From Exercise Science. Front. Physiol. 2020, 11, 604274. [Google Scholar] [CrossRef]
- Nayor, M.; Shah, R.V.; Miller, P.E.; Blodgett, J.B.; Tanguay, M.; Pico, A.R.; Murthy, V.L.; Malhotra, R.; Houstis, N.E.; Deik, A.; et al. Metabolic Architecture of Acute Exercise Response in Middle-Aged Adults in the Community. Circulation 2020, 142, 1905–1924. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Betts, J.; Sale, C.; Pope, M.; Budge, H.; Symonds, M.E. Exercise-Induced ‘Browning’ of Adipose Tissues. Metabolism 2018, 81, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Morville, T.; Sahl, R.E.; Moritz, T.; Helge, J.W.; Clemmensen, C. Plasma Metabolome Profiling of Resistance Exercise and Endurance Exercise in Humans. Cell Rep. 2020, 33, 108554. [Google Scholar] [CrossRef]
- Kim, D.S.; Wheeler, M.T.; Ashley, E.A. The Genetics of Human Performance. Nat. Rev. Genet. 2022, 23, 40–54. [Google Scholar] [CrossRef]
- Roberts, M.D.; Ruple, B.A.; Godwin, J.S.; McIntosh, M.C.; Chen, S.-Y.; Kontos, N.J.; Agyin-Birikorang, A.; Michel, M.; Plotkin, D.L.; Mattingly, M.L.; et al. A Novel Deep Proteomic Approach in Human Skeletal Muscle Unveils Distinct Molecular Signatures Affected by Aging and Resistance Training. Aging 2024, 16, 6631–6651. [Google Scholar] [CrossRef] [PubMed]
- Sabaratnam, R.; Wojtaszewski, J.F.P.; Højlund, K. Factors Mediating Exercise-induced Organ Crosstalk. Acta Physiol. 2022, 234, e13766. [Google Scholar] [CrossRef]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K.; et al. Adipose-Derived Circulating MiRNAs Regulate Gene Expression in Other Tissues. Nature 2017, 542, 450–455. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Pourteymour, S.; Eckardt, K.; Holen, T.; Langleite, T.; Lee, S.; Jensen, J.; Birkeland, K.I.; Drevon, C.A.; Hjorth, M. Global MRNA Sequencing of Human Skeletal Muscle: Search for Novel Exercise-Regulated Myokines. Mol. Metab. 2017, 6, 352–365. [Google Scholar] [CrossRef]
- Catoire, M.; Mensink, M.; Kalkhoven, E.; Schrauwen, P.; Kersten, S. Identification of Human Exercise-Induced Myokines Using Secretome Analysis. Physiol. Genom. 2014, 46, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Pucino, V.; Bombardieri, M.; Pitzalis, C.; Mauro, C. Lactate at the Crossroads of Metabolism, Inflammation, and Autoimmunity. Eur. J. Immunol. 2017, 47, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sá Filho, A.S.; Barsanulfo, S.R.; Faria, S.S.; Inacio, P.A.; Ayatizadeh, F.; Machado, S. Exerkines: A Crosstalk between Lactate Production, Exercise and Mental Health. CNS Neurol. Disord. Drug Targets 2023, 23, 1057–1060. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a Myokine and Exerkine: Drivers and Signals of Physiology and Metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate Shuttles in Nature. Biochem. Soc. Trans. 2002, 30, 258–264. [Google Scholar] [CrossRef]
- Pal, M.; Febbraio, M.A.; Whitham, M. From Cytokine to Myokine: The Emerging Role of Interleukin-6 in Metabolic Regulation. Immunol. Cell Biol. 2014, 92, 331–339. [Google Scholar] [CrossRef]
- Tanisawa, K.; Wang, G.; Seto, J.; Verdouka, I.; Twycross-Lewis, R.; Karanikolou, A.; Tanaka, M.; Borjesson, M.; Di Luigi, L.; Dohi, M.; et al. Sport and Exercise Genomics: The FIMS 2019 Consensus Statement Update. Br. J. Sports Med. 2020, 54, 969–975. [Google Scholar] [CrossRef]
- Pitsiladis, Y.P.; Tanaka, M.; Eynon, N.; Bouchard, C.; North, K.N.; Williams, A.G.; Collins, M.; Moran, C.N.; Britton, S.L.; Fuku, N.; et al. Athlome Project Consortium: A Concerted Effort to Discover Genomic and Other “Omic” Markers of Athletic Performance. Physiol. Genom. 2016, 48, 183–190. [Google Scholar] [CrossRef]
- Semenova, E.A.; Hall, E.C.R.; Ahmetov, I.I. Genes and Athletic Performance: The 2023 Update. Genes 2023, 14, 1235. [Google Scholar] [CrossRef]
- Ahmetov, I.I.; Egorova, E.S.; Gabdrakhmanova, L.J.; Fedotovskaya, O.N. Genes and Athletic Performance: An Update. Med. Sport. Sci. 2016, 61, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Durmic, T.S.; Zdravkovic, M.D.; Djelic, M.N.; Gavrilovic, T.D.; Djordjevic Saranovic, S.A.; Plavsic, J.N.; Mirkovic, S.V.; Batinic, D.V.; Antic, M.N.; Mihailovic, Z.R.; et al. Polymorphisms in ACE and ACTN3 Genes and Blood Pressure Response to Acute Exercise in Elite Male Athletes from Serbia. Tohoku J. Exp. Med. 2017, 243, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, H.; Chen, K.; Wu, B.; Liu, H. Association of Polymorphisms Rs1800012 in COL1A1 with Sports-Related Tendon and Ligament Injuries: A Meta-Analysis. Oncotarget 2017, 8, 27627–27634. [Google Scholar] [CrossRef]
- Dimauro, I.; Paronetto, M.P.; Caporossi, D. Exercise, Redox Homeostasis and the Epigenetic Landscape. Redox Biol. 2020, 35, 101477. [Google Scholar] [CrossRef]
- Jacques, M.; Hiam, D.; Craig, J.; Barrès, R.; Eynon, N.; Voisin, S. Epigenetic Changes in Healthy Human Skeletal Muscle Following Exercise– a Systematic Review. Epigenetics 2019, 14, 633–648. [Google Scholar] [CrossRef]
- Voisin, S.; Eynon, N.; Yan, X.; Bishop, D.J. Exercise Training and DNA Methylation in Humans. Acta Physiol. 2015, 213, 39–59. [Google Scholar] [CrossRef]
- Landen, S.; Hiam, D.; Voisin, S.; Jacques, M.; Lamon, S.; Eynon, N. Physiological and Molecular Sex Differences in Human Skeletal Muscle in Response to Exercise Training. J. Physiol. 2023, 601, 419–434. [Google Scholar] [CrossRef]
- Fabre, O.; Ingerslev, L.R.; Garde, C.; Donkin, I.; Simar, D.; Barrès, R. Exercise Training Alters the Genomic Response to Acute Exercise in Human Adipose Tissue. Epigenomics 2018, 10, 1033–1050. [Google Scholar] [CrossRef] [PubMed]
- Geiger, C.; Needhamsen, M.; Emanuelsson, E.B.; Norrbom, J.; Steindorf, K.; Sundberg, C.J.; Reitzner, S.M.; Lindholm, M.E. DNA Methylation of Exercise-Responsive Genes Differs between Trained and Untrained Men. BMC Biol. 2024, 22, 147. [Google Scholar] [CrossRef]
- Turner, D.C.; Gorski, P.P.; Maasar, M.F.; Seaborne, R.A.; Baumert, P.; Brown, A.D.; Kitchen, M.O.; Erskine, R.M.; Dos-Remedios, I.; Voisin, S.; et al. DNA Methylation across the Genome in Aged Human Skeletal Muscle Tissue and Muscle-Derived Cells: The Role of HOX Genes and Physical Activity. Sci. Rep. 2020, 10, 15360. [Google Scholar] [CrossRef]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic Control of Methylation and Acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Fairlie, E.; Garnham, A.P.; Hargreaves, M. Exercise-induced Histone Modifications in Human Skeletal Muscle. J. Physiol. 2009, 587, 5951–5958. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, K.; Wang, T.; Wu, Y.; Xing, G.; Chen, M.; Hao, Z.; Zhang, C.; Zhang, J.; Ma, B.; et al. Glis1 Facilitates Induction of Pluripotency via an Epigenome–Metabolome–Epigenome Signalling Cascade. Nat. Metab. 2020, 2, 882–892. [Google Scholar] [CrossRef]
- Meng, X.; Baine, J.M.; Yan, T.; Wang, S. Comprehensive Analysis of Lysine Lactylation in Rice (Oryza sativa) Grains. J. Agric. Food Chem. 2021, 69, 8287–8297. [Google Scholar] [CrossRef]
- Lamas, A.; Regal, P.; Vázquez, B.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Transcriptomics: A Powerful Tool to Evaluate the Behavior of Foodborne Pathogens in the Food Production Chain. Food Res. Int. 2019, 125, 108543. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.C.; de Gonzalo-Calvo, D.; Toro, R.; Fernandes, T.; Theisen, D.; Wang, D.-Z.; Devaux, Y. Non-Coding RNAs and Exercise: Pathophysiological Role and Clinical Application in the Cardiovascular System. Clin. Sci. 2018, 132, 925–942. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Yao, R.-W.; Wang, Y.; Chen, L.-L. Cellular Functions of Long Noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef]
- De Sanctis, P.; Filardo, G.; Abruzzo, P.M.; Astolfi, A.; Bolotta, A.; Indio, V.; Di Martino, A.; Hofer, C.; Kern, H.; Löfler, S.; et al. Non-Coding RNAs in the Transcriptional Network That Differentiates Skeletal Muscles of Sedentary from Long-Term Endurance- and Resistance-Trained Elderly. Int. J. Mol. Sci. 2021, 22, 1539. [Google Scholar] [CrossRef] [PubMed]
- Hecksteden, A.; Leidinger, P.; Backes, C.; Rheinheimer, S.; Pfeiffer, M.; Ferrauti, A.; Kellmann, M.; Sedaghat, F.; Meder, B.; Meese, E.; et al. MiRNAs and Sports: Tracking Training Status and Potentially Confounding Diagnoses. J. Transl. Med. 2016, 14, 219. [Google Scholar] [CrossRef]
- Domańska-Senderowska, D.; Laguette, M.-J.; Jegier, A.; Cięszczyk, P.; September, A.; Brzeziańska-Lasota, E. MicroRNA Profile and Adaptive Response to Exercise Training: A Review. Int. J. Sports Med. 2019, 40, 227–235. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of Bioactive Metabolites Using Activity Metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Pintus, R.; Dessì, A.; Noto, A.; Sardo, S.; Finco, G.; Corsello, G.; Fanos, V. Sportomics: Metabolomics Applied to Sports. The New Revolution? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11011–11019. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Nieman, D.C.; Pence, B.D. Exercise Immunology: Future Directions. J. Sport. Health Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary Modulation of Oxylipins in Cardiovascular Disease and Aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef]
- de Bus, I.; Witkamp, R.; Zuilhof, H.; Albada, B.; Balvers, M. The Role of N-3 PUFA-Derived Fatty Acid Derivatives and Their Oxygenated Metabolites in the Modulation of Inflammation. Prostaglandins Other Lipid Mediat. 2019, 144, 106351. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular Inflammatory and Resolving Lipid Profile Responses to an Acute Bout of Resistance Exercise in Men. Physiol. Rep. 2019, 7, e14108. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle Damage and Inflammation during Recovery from Exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Egan, B.; Hawley, J.A.; Zierath, J.R. SnapShot: Exercise Metabolism. Cell Metab. 2016, 24, 342–342.e1. [Google Scholar] [CrossRef]
- Santone, C.; Dinallo, V.; Paci, M.; D’Ottavio, S.; Barbato, G.; Bernardini, S. Saliva Metabolomics by NMR for the Evaluation of Sport Performance. J. Pharm. Biomed. Anal. 2014, 88, 441–446. [Google Scholar] [CrossRef]
- Luti, S.; Militello, R.; Pinto, G.; Illiano, A.; Amoresano, A.; Chiappetta, G.; Marzocchini, R.; Modesti, P.A.; Pratesi, S.; Pazzagli, L.; et al. Chronic Training Induces Metabolic and Proteomic Response in Male and Female Basketball Players: Salivary Modifications during In-Season Training Programs. Healthcare 2023, 11, 241. [Google Scholar] [CrossRef]
- Ntovas, P.; Loumprinis, N.; Maniatakos, P.; Margaritidi, L.; Rahiotis, C. The Effects of Physical Exercise on Saliva Composition: A Comprehensive Review. Dent. J. 2022, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.A.S.; Mussavira, S.; Bindhu, O.S. Clinical and Diagnostic Utility of Saliva as a Non-Invasive Diagnostic Fluid: A Systematic Review. Biochem. Med. 2015, 25, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Pitti, E.; Petrella, G.; Di Marino, S.; Summa, V.; Perrone, M.; D’Ottavio, S.; Bernardini, A.; Cicero, D.O. Salivary Metabolome and Soccer Match: Challenges for Understanding Exercise Induced Changes. Metabolites 2019, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, T.; Lacome, M.; Fanos, V.; Martera, G.; Cione, E.; Cannataro, R. Metabolomics in Team-Sport Athletes: Current Knowledge, Challenges, and Future Perspectives. Proteomes 2022, 10, 27. [Google Scholar] [CrossRef]
- Gamberi, T.; Magherini, F.; Fiaschi, T.; Valocchia, E.; Raugei, G.; Modesti, P.A.; Messori, L.; Modesti, A. Systems Biology of the Proteomic Analysis of Cytotoxic Gold Compounds in A2780 Ovarian Cancer Cell Line: A Network Analysis. Jacobs J. Bioinform. Proteom. 2016, 1, 004. [Google Scholar]
- Birhanu, A.G. Mass Spectrometry-Based Proteomics as an Emerging Tool in Clinical Laboratories. Clin. Proteom. 2023, 20, 32. [Google Scholar] [CrossRef]
- Franco-Martínez, L.; González-Hernández, J.M.; Horvatić, A.; Guillemin, N.; Cerón, J.J.; Martínez-Subiela, S.; Sentandreu, M.Á.; Brkljačić, M.; Mrljak, V.; Tvarijonaviciute, A.; et al. Differences on Salivary Proteome at Rest and in Response to an Acute Exercise in Men and Women: A Pilot Study. J. Proteom. 2020, 214, 103629. [Google Scholar] [CrossRef] [PubMed]
- Balfoussia, E.; Skenderi, K.; Tsironi, M.; Anagnostopoulos, A.K.; Parthimos, N.; Vougas, K.; Papassotiriou, I.; Tsangaris, G.T.; Chrousos, G.P. A Proteomic Study of Plasma Protein Changes under Extreme Physical Stress. J. Proteom. 2014, 98, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Philp, A.; Rowland, T.; Perez-Schindler, J.; Schenk, S. Understanding the Acetylome: Translating Targeted Proteomics into Meaningful Physiology. Am. J. Physiol. Cell Physiol. 2014, 307, C763–C773. [Google Scholar] [CrossRef]
- Gehlert, S.; Klinz, F.; Willkomm, L.; Schiffer, T.; Suhr, F.; Bloch, W. Intense Resistance Exercise Promotes the Acute and Transient Nuclear Translocation of Small Ubiquitin-Related Modifier (SUMO)-1 in Human Myofibres. Int. J. Mol. Sci. 2016, 17, 646. [Google Scholar] [CrossRef]
- Gorini, G.; Gamberi, T.; Fiaschi, T.; Mannelli, M.; Modesti, A.; Magherini, F. Irreversible Plasma and Muscle Protein Oxidation and Physical Exercise. Free Radic. Res. 2019, 53, 126–138. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Mégarbane, B.; Rezaee, R. Molecular Mechanisms Mediating Adaptation to Exercise. Adv. Exp. Med. Biol. 2020, 1228, 45–61. [Google Scholar] [CrossRef]
- Wilson, G.M.; Blanco, R.; Coon, J.J.; Hornberger, T.A. Identifying Novel Signaling Pathways: An Exercise Scientists Guide to Phosphoproteomics. Exerc. Sport. Sci. Rev. 2018, 46, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of Metabolomic Data: Tools, Current Strategies and Future Challenges for Omics Data Integration. Brief. Bioinform. 2016, bbw031. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, A.; Chana, I. Computational Techniques and Tools for Omics Data Analysis: State-of-the-Art, Challenges, and Future Directions. Arch. Comput. Methods Eng. 2021, 28, 4595–4631. [Google Scholar] [CrossRef]
- Kumar, D.; Bansal, G.; Narang, A.; Basak, T.; Abbas, T.; Dash, D. Integrating Transcriptome and Proteome Profiling: Strategies and Applications. Proteomics 2016, 16, 2533–2544. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Pan, D.; Wang, X.; Xu, Y.; Yan, J.; Wang, L.; Yang, X.; Yang, M.; Liu, G.-P. Applications of Multi-Omics Analysis in Human Diseases. MedComm 2023, 4, e315. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, M.; da Veiga Leprevost, F.; Squizzato, S.; Park, Y.M.; Haug, K.; Carroll, A.J.; Spalding, D.; Paschall, J.; Wang, M.; et al. Discovering and Linking Public Omics Data Sets Using the Omics Discovery Index. Nat. Biotechnol. 2017, 35, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Park, S.; Kim, S.; Chae, H. Machine Learning-Based Analysis of Multi-Omics Data on the Cloud for Investigating Gene Regulations. Brief. Bioinform. 2021, 22, 66–76. [Google Scholar] [CrossRef]
- Amar, D.; Gay, N.R.; Jean-Beltran, P.M.; Bae, D.; Dasari, S.; Dennis, C.; Evans, C.R.; Gaul, D.A.; Ilkayeva, O.; Ivanova, A.A.; et al. Temporal Dynamics of the Multi-Omic Response to Endurance Exercise Training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef]
- Bersanelli, M.; Mosca, E.; Remondini, D.; Giampieri, E.; Sala, C.; Castellani, G.; Milanesi, L. Methods for the Integration of Multi-Omics Data: Mathematical Aspects. BMC Bioinform. 2016, 17, S15. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, M. A User-Friendly Visualization Tool for Multi-Omics Data. Proteomics 2020, 20, 2000136. [Google Scholar] [CrossRef]
- Ahmed, Z.; Wan, S.; Zhang, F.; Zhong, W. Artificial Intelligence for Omics Data Analysis. BMC Methods 2024, 1, 4. [Google Scholar] [CrossRef]
- Misra, B.B.; Langefeld, C.; Olivier, M.; Cox, L.A. Integrated Omics: Tools, Advances and Future Approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling Time Is Crucial for Measurement of Aerobic Exercise-Induced Oxidative Stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef]
- Sanford, J.A.; Nogiec, C.D.; Lindholm, M.E.; Adkins, J.N.; Amar, D.; Dasari, S.; Drugan, J.K.; Fernández, F.M.; Radom-Aizik, S.; Schenk, S.; et al. Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise. Cell 2020, 181, 1464–1474. [Google Scholar] [CrossRef]
- Barker, K.; Eickmeyer, S. Therapeutic Exercise. Med. Clin. N. Am. 2020, 104, 189–198. [Google Scholar] [CrossRef] [PubMed]
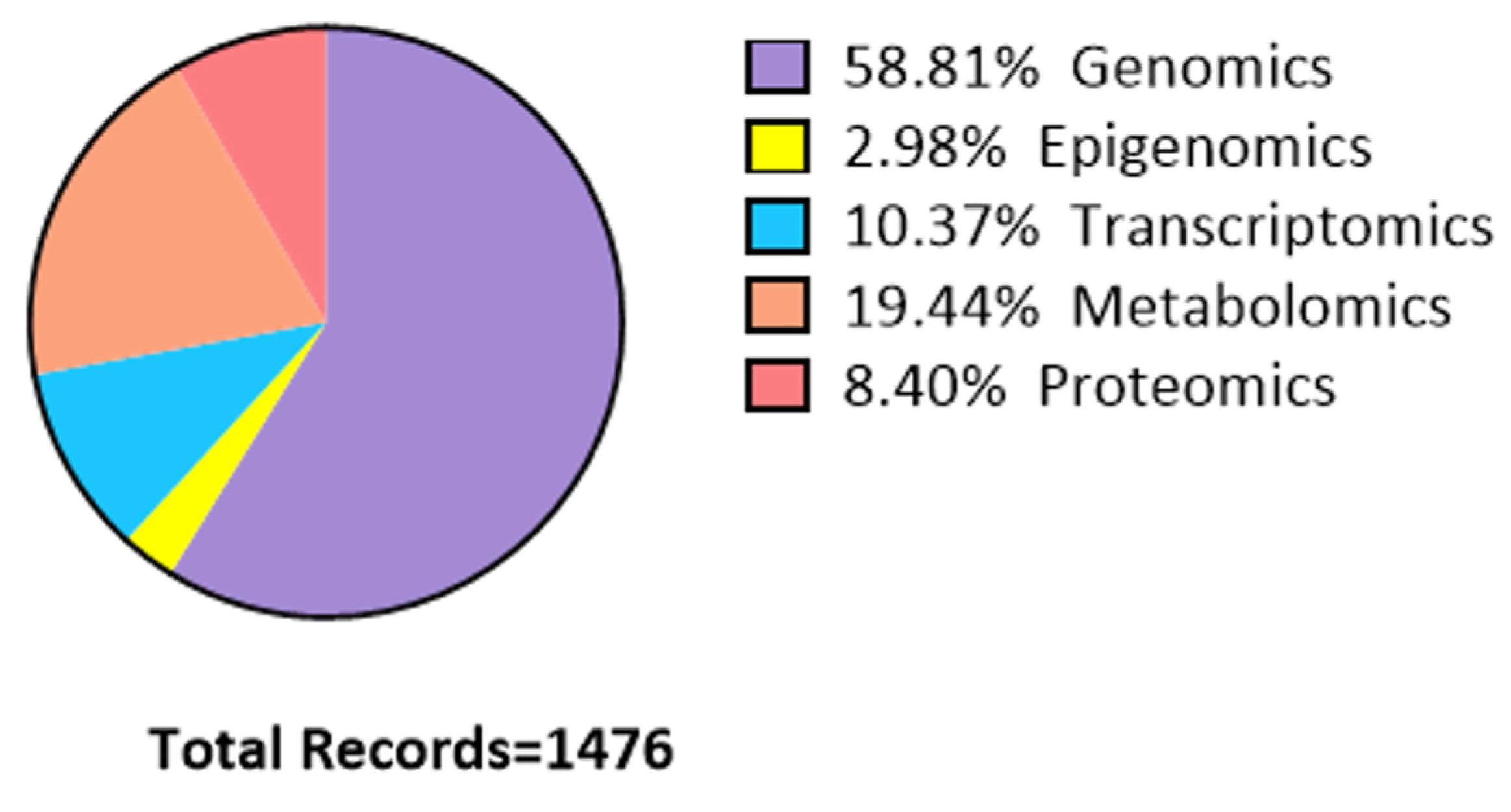
| Keywords | Total | 2014–2024 | Free Full Text | English | Humans | 19–44 Years Old | Records After Duplicate Remove |
|---|---|---|---|---|---|---|---|
| Omics AND exercise | 392 | 349 | 282 | 281 | 145 | 30 | 40 |
| Omics AND PA | 509 | 456 | 362 | 360 | 187 | 40 | |
| Genomics AND exercise | 7135 | 4901 | 3527 | 3520 | 2163 | 634 | 868 |
| Genomics AND PA | 12,178 | 7510 | 5441 | 5427 | 3126 | 845 | |
| Epigenomics AND exercise | 281 | 251 | 178 | 177 | 133 | 34 | 44 |
| Epigenomics AND PA | 426 | 380 | 282 | 277 | 199 | 43 | |
| Transcriptomics AND exercise | 1760 | 1340 | 1057 | 1057 | 481 | 129 | 153 |
| Transcriptomics AND PA | 2391 | 1826 | 1427 | 1426 | 643 | 150 | |
| Metabolomics AND exercise | 1768 | 1605 | 1188 | 1183 | 653 | 221 | 287 |
| Metabolomics AND PA | 2328 | 2133 | 1592 | 1585 | 854 | 286 | |
| Proteomics AND exercise | 1328 | 1069 | 781 | 778 | 359 | 102 | 124 |
| Proteomics AND PA | 1981 | 1574 | 1160 | 1153 | 536 | 122 |
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Warburg et al. [9] | 2017 | Both | Health benefits, cardiovascular improvements, and longevity. | Not specific to molecules, focused on general health benefits and physiological effects of exercise. |
| Nederveen et al. [10] | 2020 | Both | Role of extracellular vesicles and exosomes in exercise science in cell communication and adaptation. | Exosomes, extracellular vesicles, and various proteins involved in cellular communication post-exercise. |
| Nayor et al. [11] | 2020 | Both | Metabolic response to acute exercise in middle-aged adults. | Metabolites: amino acids, lipids, glucose, lactate, and several hormones involved in metabolic response. |
| Aldiss et al. [12] | 2018 | Aerobic | Influence of exercise on adipose tissue. | Brown adipose tissue markers, UCP1 (uncoupling protein 1), and other adipokines involved in thermogenesis. |
| Morville et al. [13] | 2020 | Both | Metabolic shifts based on exercise type. | Metabolites: amino acids (e.g., BCAA), fatty acids, glucose, and other metabolites linked to exercise metabolism. |
| Kim et al. [14] | 2022 | Both | Genetic factors influencing human performance to endurance and resistance exercise. | Genes: PPARs (peroxisome proliferator-activated receptors), FNDC5 (fibronectin type III domain-containing protein 5), and ACE (angiotensin-converting enzyme). |
| Roberts et al. [15] | 2024 | Anaerobic | Molecular signatures in human skeletal muscle in response to resistance training. | Proteins: MYH (myosin heavy chains), actin, PGC-1α (peroxisome proliferator-activated receptor gamma coactivator), IGF-1 (insulin-like growth factor 1). |
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Nederveen et al. [10] | 2020 | Both | Role of extracellular vesicles in tissue communication and adaptation. | Exosomes, extracellular vesicles, miRNAs, and proteins involved in cell signaling. |
| Sabaratnam et al. [16] | 2022 | Both | Exercise-induced organ crosstalk involving muscle. | Myokines (e.g., IL-6, irisin), adipokines, and cytokines involved in muscle–organ interactions. |
| Thomou et al. [17] | 2017 | Both | Circulating miRNAs from adipose tissue regulate gene expression in distant tissues. | miRNAs from adipose tissue, including miR-30a-5p, miR-27a-3p, and miR-155. |
| Whitham et al. [18] | 2018 | Both | Extracellular vesicles as mediators of tissue crosstalk during exercise. | Extracellular vesicles, miRNAs, proteins like IL-6, and adipokines. |
| Severinsen et al. [19] | 2020 | Both | The emerging roles of myokines in muscle–organ crosstalk, inflammation, metabolism, and muscle growth. | Myokines (e.g., IL-6, irisin, FGF21), and adipokines influencing metabolism. |
| Pourteymour et al. [20] | 2017 | Aerobic | Identification of novel exercise-regulated myokines of human skeletal muscle. | Myokines: IL-6, irisin, FGF21. |
| Catoire et al. [21] | 2014 | Both | Identification of exercise-induced myokines and their regulatory role in metabolism. | Myokines: IL-6, irisin, FGF21, and other factors regulating fat metabolism and muscle growth. |
| Chow et al. [22] | 2022 | Both | Role of exerkines on released signaling molecules during exercise. | IL-6, FGF21, irisin, BDNF influencing metabolism, stress responses, and disease. |
| Pucino et al. [23] | 2017 | Aerobic | Lactate as a key molecule released during exercise. | Lactate. |
| Sá Filho et al. [24] | 2023 | Both | Lactate as mediator on mental health. | Lactate, neurotransmitters, and miRNAs involved in brain-muscle communication. |
| Brooks et al. [25] | 2022 | Both | Lactate as a myokine influencing metabolism during exercise. | Lactate, IL-6, FGF21, and other myokines. |
| Pal et al. [27] | 2014 | Both | Role of IL-6 in metabolic regulation after exercise. | IL-6, signaling molecules related to muscle metabolism. |
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Tanisawa et al. [28] | 2020 | Both | Genetic factors influencing physical performance and adaptation to exercise. | ACE, PPARA, ACTN3, VEGF. |
| Pitsiladis et al. [29] | 2016 | Both | Markers that predict athletic performance. | ACTN3, ACE, PPARA, VEGF, FTO. |
| Semenova et al. [30] | 2023 | Both | Genes involved in athletic performance, endurance, strength, and recovery. | Genetic variants in ACTN3, ACE, BDNF, PPARA, and myostatin (MSTN). |
| Ahmetov et al. [31] | 2016 | Both | Polymorphisms in genes related to endurance and strength. | ACE, ACTN3, VEGF, FTO, myostatin, PPARA. |
| Durmic et al. [32] | 2017 | Anaerobic | Polymorphisms in ACE and ACTN3 genes in response to acute exercise. | ACE (Angiotensin-converting enzyme), ACTN3 (Alpha-actinin-3). |
| Wang et al. [33] | 2017 | Both | COL1A1 gene polymorphisms and their association with tendon and ligament injuries in athletes. | COL1A1 gene polymorphisms. |
| Sample | Advantages | Disadvantages |
|---|---|---|
| Blood | Allows the study of all endogenous metabolites secreted by different tissues and could be used for all methods of analysis. | Very invasive, difficult to evaluate the origin of metabolites identified, can be easily degraded due to the presence of enzymes in the sample. |
| Saliva | Easy to pick up and handle. Reflects the state of the body. | Could be affected by the condition of mouth, such as the presence of bacteria, and may show lower concentrations of endogenous metabolites compared to blood. |
| Urine | Easy to pick up and handle. Contains stable metabolites and both endogenous and exogenous compounds. | Could be affected by diet and external factors such as bacteria. The presence of urea and salts can be a problem for MS. |
| Tissue | Allows the study local metabolites detectable at high concentrations | Very invasive, limitation in the amount of sample that can be taken. |
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Bongiovanni et al. [55] | 2019 | Both | “Sportomics” linked to performance analysis. | Summary of the most important studies of metabolites related to exercise performance and biomarkers for sports performance. |
| Nieman et al. [57] | 2020 | Both | Exercise immunology. | Lipid and Krebs cycle metabolites, n-6/n-3PUFA oxylipins. |
| Vella et al. [60] | 2019 | Aerobic | Lipid profile responses to resistance exercise. | Lipid profiles: cyclooxygenase (COX)-derived thromboxanes and prostaglandins (PGE2), lipoxygenase (LOX), monohydroxy-eicosatetraenoic acids (5-HETE, 12-HETE, 15-HETE), monohydroxy-docosahexaenoic acids (4 HDoHE, 7-HDoHE, 14-HDoHE). CYP pathway-derived epoxy- and dihydroxy-eicosa trienoic acids (5,6-EpETrE, 11,12-DiHETrE and 14,15-DiHETrE). |
| Egan et al. [62] | 2016 | Aerobic | Exercise metabolism and its effects on muscle energy systems and performance. | Metabolites related to aerobic metabolism, lipid oxidation, glucose metabolism, and mitochondrial function. |
| Santone et al. [63] | 2014 | Both | Saliva metabolomics by NMR in sport performance. | Salivary metabolites, amino acids, lipids, and lactate, glucose, glycerol, and citrate. |
| Luti et al. [64] | 2023 | Both | Chronic training effects on metabolic and proteomic responses to exercise. | Salivary metabolites, amino acids (lysine, valine, glycine, tyrosine) citric acid, taurine. |
| Ntovas et al. [65] | 2022 | Both | Effects of physical exercise on saliva composition. | Salivary metabolites, biomarkers of exercise intensity (alpha amylase, salivary cortisol IgA, IgM, IgG), melatonin, lactate, testosterone, and oral health indicators. |
| Pitti et al. [67] | 2019 | Both | Salivary metabolome changes due to exercise-induced stress. | Exercise-induced metabolites: amino acids, acetate, creatine, dimethylamine, ethanol, ethanolamine, formate, fumarate, glycerol, lactate, ornithine. |
| Bongiovanni et al. [68] | 2022 | Both | Metabolomics in team-sport athletes. | Metabolites in urine and fecal samples: adenine, creatine, glutamine, carnitine, arachidonic acid, plasmalogen, cortisol, lysine, tyrosine, leucine, valine, and isoleucine, glutamate, beta-citryl-glutamate, 5-oxoproline, fatty acid-carnitines and acylated carnitines, trimethylamine-N-oxide (TMAO), dimethylglycine, O-acetyl carnitine, proline, betaine, acetoacetate, 3-hydroxy-isovaleric acid, acetone, N-methyl nicotinate, N-methyl nicotinamide, phenylacetylglutamine (PAG), 3-methylhistidine, trimethylamine (TMA), short-chain fatty acids, as well as methylamine, glycerate, allantoin, and succinate. |
| Author | Year | Aerobic or Anaerobic Exercise | Main Findings | Main Molecules Identified |
|---|---|---|---|---|
| Franco-Martínez et al. [71] | 2020 | Anaerobic | Salivary proteome in response to acute exercise in men and women. | Men: keratin, ceruloplasmin, IgLambda, catalase, suprabasin, annexin A1. |
| Balfoussia et al. [72] | 2014 | Anaerobic | Plasma proteome under extreme physical stress. | Plasma proteins (albumin, fibrinogen), stress response proteins, oxidative stress biomarkers. |
| Gehlert et al. [74] | 2016 | Aerobic | Small ubiquitin-related modifier (SUMO)-1 in human myofibers. | SUMO-1 myofiber proteins: Lamina-A, actina, perinuclear region of myonuclei. |
| Gorini et al. [75] | 2019 | Both | Protein oxidation during exercise. | Oxidized plasma and muscle proteins (carbonylated proteins). |
| Gholamnezhad et al. [76] | 2020 | Both | Signaling pathways and muscle recovery. | Exercise adaptation-related proteins, signaling molecules (Rapamycin, myostatin/Smad) recovery biomarkers. |
| Wilson et al. [77] | 2018 | Both | Phosphoproteomics. | Phosphorylated proteins, signaling molecules (kinases). |
| Tool | Main Functionality | Key Features | Best for |
|---|---|---|---|
| MetaboAnalyst | Web-based platform for comprehensive analysis of metabolomics data | - Data preprocessing - Statistical analysis - Pathway analysis - Visualization tools | Metabolomics data analysis and visualization |
| MergeOmics | Integration and analysis of multiomics data (e.g., genomics, proteomics, and metabolomics) | - Multiomics integration - Differential analysis - Pathway enrichment analysis | Multiomics data integration and analysis |
| Cytoscape | Open-source software for network analysis and visualization | - Network visualization - Integration of omics data into networks - Supports large networks | Network analysis, visualization, and biological data integration |
| InCroMAP | Network-based analysis for integrative omics data analysis (omics and clinical data) | - Multiomics integration - Clinical outcome prediction - Network analysis | Integrative omics and clinical data analysis |
| 3Omics | Tool for the integrative analysis of multiomics data (omics, clinical, and phenotypic) | - Multiomics data integration - Predictive modeling - Pathway analysis - Data visualization | Integrative analysis of multiomics data with clinical information |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Militello, R.; Luti, S.; Modesti, A. Omics Sciences in Regular Physical Activity. Int. J. Mol. Sci. 2025, 26, 5529. https://doi.org/10.3390/ijms26125529
Militello R, Luti S, Modesti A. Omics Sciences in Regular Physical Activity. International Journal of Molecular Sciences. 2025; 26(12):5529. https://doi.org/10.3390/ijms26125529
Chicago/Turabian StyleMilitello, Rosamaria, Simone Luti, and Alessandra Modesti. 2025. "Omics Sciences in Regular Physical Activity" International Journal of Molecular Sciences 26, no. 12: 5529. https://doi.org/10.3390/ijms26125529
APA StyleMilitello, R., Luti, S., & Modesti, A. (2025). Omics Sciences in Regular Physical Activity. International Journal of Molecular Sciences, 26(12), 5529. https://doi.org/10.3390/ijms26125529







