1. Introduction
Epidural analgesia using local anesthetics, with or without the addition of opioids, is primarily applied for pain relief during vaginal delivery. Ropivacaine has several advantages over bupivacaine, such as an improved safety profile and less cardiovascular and nervous system toxicity [
1]. The occurrence of intrapartum fever is observed quite frequently and was originally attributed to clinical infections or an extensive inflammatory state at the timepoint of the induction of labor. In 1989, Fusi et al. provided the first evidence of a connection between epidural analgesia and an increase in body temperature [
2], a phenomenon that was termed epidural-related maternal fever (ERMF) [
3]. The incidence of ERMF varies depending on the type of study and the definition of fever (>37.5 °C, >38 °C, >39 °C) from the single-digit range to almost 50% [
3]. As causal explanations were not immediately at hand, ERMF did not receive much further attention until adverse outcomes for mother and child became evident. Several studies have investigated different types of anesthetic delivery, the effect of adjunct opioids, and anesthetic dosing [
3]. Meanwhile, it became clear that epidural analgesia is an independent risk factor for intrapartum fever [
4]. However, the pathophysiology of this is still insufficiently explored, and several theories have been proposed. As the occurrence of fever is not observed in non-pregnant patients who receive epidural analgesia for surgery, a causal relationship must be linked to the physiology of pregnancy, such as the mechanisms of thermoregulation or an extensive inflammatory state. The latter is a principal feature of labor, but the additional effects of the epidural anesthetic can ultimately lead to a rise in body temperature. Evidence for the latter has been found in several studies showing that ropivacaine induces the release of pyrogenic pro-inflammatory cytokines and prostaglandins from endothelial cells [
5] and that bupivacaine reduces leucocyte caspase-1 and plasma levels of the antipyrogenic factor interleukin-1-receptor antagonist (IL-1ra) [
6].
In order to further dive into the elucidation of the molecular mechanisms leading to ERMF, we investigated the role of DUSP9 and PHLPP1 in the present study. We followed this path because, in a previous study from our research group [
5], we have demonstrated that the mitogen-activated protein kinase (MAPK) signaling and the AKT-kinase pathway were affected by ropivacaine. The DUSP family of phosphatases and PHLPPs, together with other phosphatases, control the activation of these pathways, and DUSP9 is known to have an important functional role in the placenta.
MAPKs are serine/threonine-specific protein kinases that regulate gene expression, proliferation, cell survival/apoptosis, and differentiation, and comprise three families: Extracellular-signal-Regulated Kinase (ERK1/2 and ERK5-BMK pathways), c-Jun N-terminal kinase (JNK), and p38 mitogen-activated protein kinase. These kinases become activated by a cascade of upstream kinase activities, ultimately leading to the phosphorylation of a threonine-x-tyrosine motif. Their activity is swiftly switched off by dephosphorylation of this motif by a number of phosphatases, including serine/threonine, tyrosine, and dual-specificity MAPK phosphatases (MAPK-DUSPs; MKP) [
7,
8]. The MAPK-DUSP family comprises 10 members, which contain a kinase-interaction motif, in contrast to another group of atypical DUSPs. Class I inducible nuclear MAPK-DUSPs include DUSPs 1, 2, 4, and 5. Class II (DUSPs 6, 7, and 9) are mainly cytoplasmic and have a substrate preference for ERK. Class III (DUSPs 8, 10, and 16) shuttle between the nucleus and the cytoplasm, and dephosphorylate preferably p38 and JNK. Initially, it was believed that members of the MAPK-DUSPs are functionally redundant. However, it became evident that their expression varies in different tissues, that specific subtypes play distinct roles in diseases, and that individual DUSP knock-out mice display characteristic phenotypes. In vitro binding assays show that the preferred MAPK substrate depends on structural details in the binding region [
9]. The expression of DUSP genes and the concomitant outcome are not only dependent on extracellular stimuli (ligands) and receptors, but also depend on the cell type.
DUSP9 is expressed from its gene on the X chromosome, and the protein is primarily expressed in the placenta, in addition to the kidney and adipose tissue. DUSP9 seems to be important for placental development and function, as knock-out mice display a phenotype of placental insufficiency [
10].
Similar to MAPKs, AKT-kinase—with its three isoforms, AKT1, AKT2, and AKT3—has a central role in cellular metabolism, proliferation, and survival. AKT is sequentially activated by upstream kinases on amino acids Thr-450, Thr-308, and Ser-473 and reciprocally inactivated by PH-domain and Leucine-rich repeat phosphatases (PHLPP1α, PHLPP1/SCOP, and PHLPP2) on Ser-473, protein serine/threonine phosphatase-2A (PP-2A), and others on Thr-308, and by PP-1 on Thr-450 [
11]. PHLPP variants play an important role as tumor suppressors in various types of cancers. Both MAPKs and AKT are very important for placental development and function, with significant crosstalk between these signaling pathways [
12].
ERMF is a phenomenon only occurring in parturition, which is why we focused on cell types of the placenta, the decidua, and the umbilical cord in this study.
The placenta is a highly complex organ that develops in parallel to the fetus and forms the basis for an intact pregnancy. It represents the connection between mother and child, supplies the fetus with oxygen and nutrients, ensures the removal of metabolic end-products, and guarantees protection of the semi-allogeneic fetus against the maternal immune system [
13,
14,
15]. The epithelial placental cells are called trophoblasts, which can develop differently depending on their localization. The functional units of the placenta are the placental villi, tree-like structures with a villous core consisting of stromal, immune, and endothelial cells surrounded by the bi-layered trophoblast epithelium. Floating placental villi protrude into the intervillous space, where they are in direct contact with the maternal blood. Underlying mononuclear cytotrophoblasts (CTBs) of these villi fuse and form a continuous multinucleated cell layer on the outside, the syncytiotrophoblasts (STs). The STs, bathed in maternal blood, are responsible for molecule transfer from the mother to the child, and vice versa, and the secretion of pregnancy-maintaining hormones, such as human chorionic gonadotrophin [
16]. On the other hand, so-called anchoring villi attach to the maternal decidua basalis (DB), the part of the endometrium during pregnancy where implantation takes place. CTBs proliferate here, form cell columns, and gradually detach from the villous tips, migrating into the maternal uterus. This differentiation process gives rise to extravillous trophoblasts (EVTs) and is accompanied by the expression of EVT-specific marker genes, such as HLA-G, and the loss of the proliferative capacity [
17,
18]. EVTs either invade the decidual stroma as deep as the first third of the myometrium to become multinucleated trophoblast giant cells in that region, or infiltrate and remodel maternal vessels, such as spiral arteries, thereby replacing the endothelial cells to adapt utero–placental blood flow [
19,
20,
21].
In this study, we show that ropivacaine specifically regulates the expression of dual-specificity phosphatase DUSP9 and PHLPP1 in different subtypes of trophoblasts of the human placenta, thereby contributing to an increased inflammatory state, including the release of pyrogens.
3. Discussion
In this study, we followed up previous investigations with the aim of better understaning the phenomenon of epidural-related maternal fever. The latter represents a challenge in obstetric anesthesiology, since it is suggested to potentially have detrimental consequences for the health of mother and child [
23,
24].
When epidural analgesia is chosen for pain relief in child birth, the anesthesiologist inserts a catheter into the epidural space for the delivery of local anesthetics such as ropivacaine or bupivacaine, with or without opioids, as needed. In a significant number of patients, the anesthetic induces a rise in body temperature after 4–6 h, which is not caused by the injection technique or some kind of infection, but by effects of the drugs themselves [
3]. Interestingly, this observation does not occur in other types of surgeries in non-pregnant patients. In those cases, rather hypothermia frequently develops. Therefore, it is readily apparent that the febrile response in ERMF is most likely due to pregnancy and labor.
Evidence from clinical studies indicates that inflammatory events must be an important part of ERMF, as women with initially higher serum levels of pro-inflammatory cytokine and lower anti-pyrogenic cytokine IL-1ra are more likely to develop ERMF [
25]. Also, anti-inflammatory glucocorticoids reduce the incidence of ERMF [
26]. Previous in vitro studies have indicated several aspects of sterile inflammation, such as the induction of endogenous pyrogen (IL-6, PGE2) release from vascular cells, the upregulation of cell adhesion molecules for the docking of immune cells, and the release of damage-associated molecular patterns (DAMPs). In addition, oxidative stress, which is at least partly caused by a downregulation of cellular antioxidants, such as superoxide dismutases, thioredoxins, and other ROS-dissipating molecules, could play a role [
5]. In a previous study, we also observed that ropivacaine shuts off ERK1/2 and AKT activity for an extended period of time. Both signaling pathways play an important role in the function of the placenta and maintain significant crosstalk [
12,
27]. The activity of ERK and AKT is determined by their phosphorylation status. These enzymes are activated by upstream kinases and are switched off by different phosphatases. In this study, we focused on the phosphatases DUSPs and PHLPP modulating ERK and AKT activity. The DUSP family is heterogenous, with some substrate preferences, but also has the capability to compensate for other isoforms [
28]. PHLPP occurs in three isoforms: PHLPP1α, PHLPP1β/SCOP, and PHLPP2.
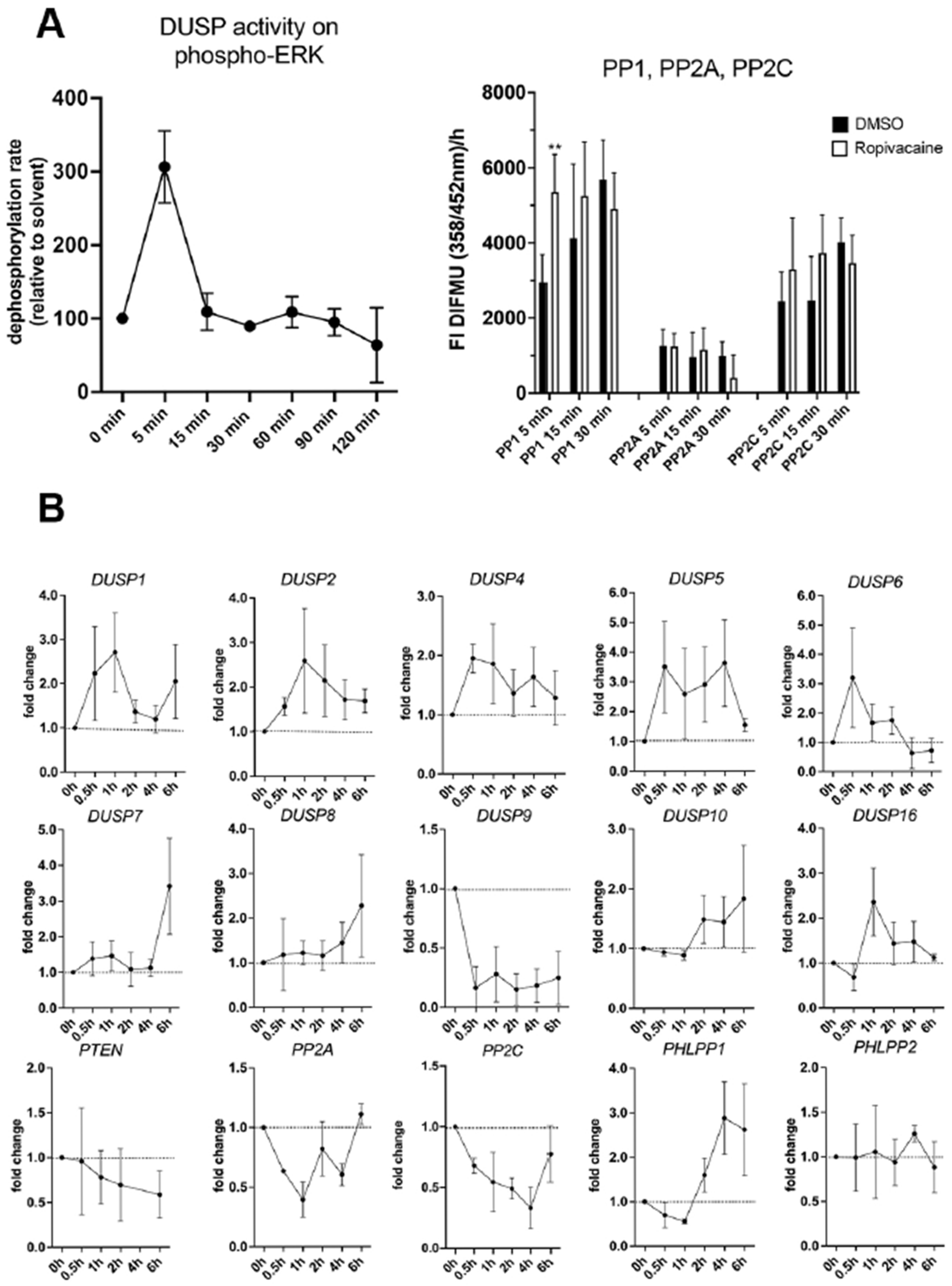
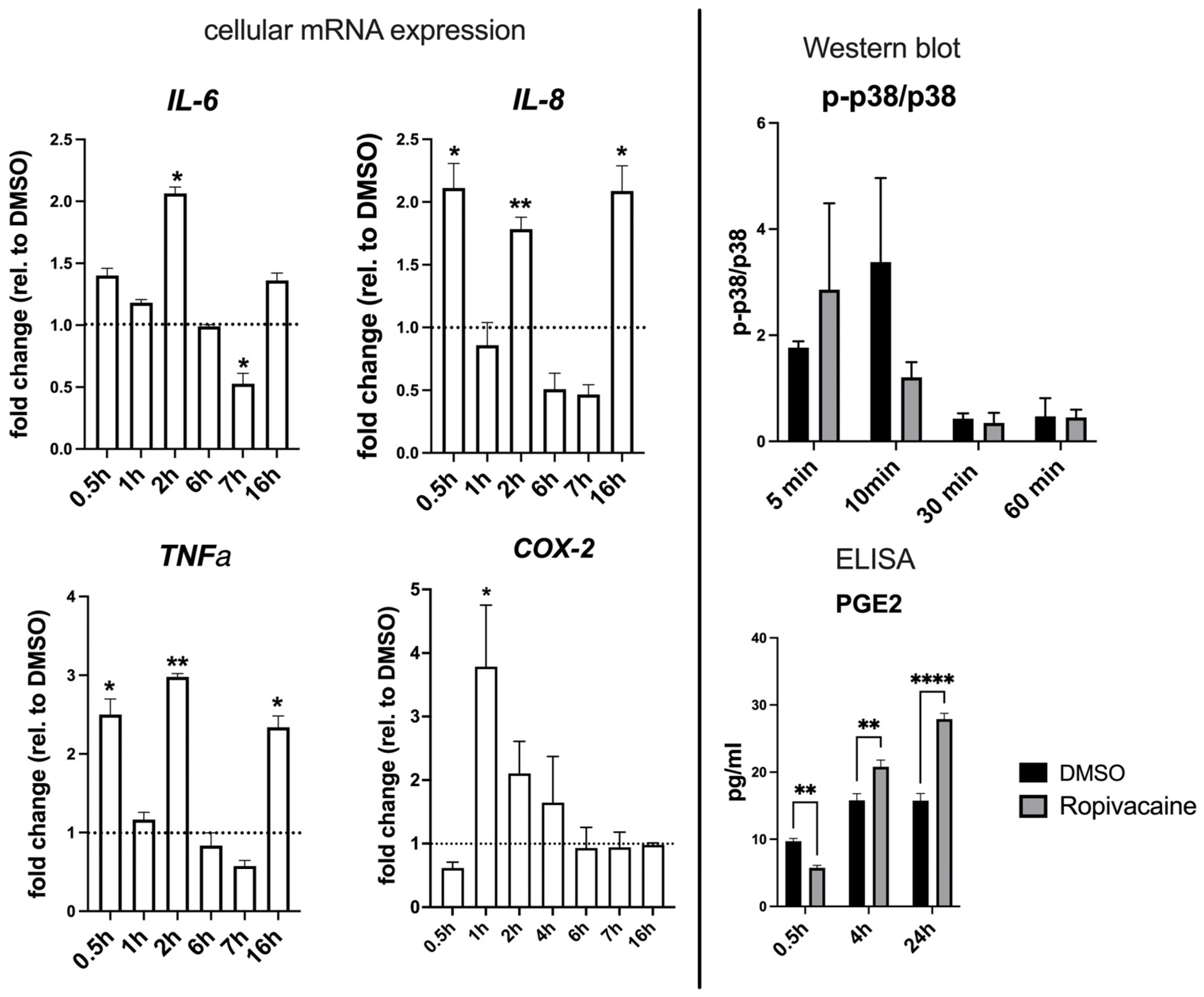
We performed initial experiments with HUVECs as a model for vascular endothelial cells of fetal origin. We found that ropivacaine induces a rapid transient increase in DUSP enzymatic activities, as well as PP1 activity, which dephosphorylates AKT in the first place. A specific PHLPP activity assay was not available. Over the next couple of hours, we saw an increase in the mRNA expression of the inducible DUSPs 1, 2, 4, and 5; a later increase in the expression of DUSP6 and 16; and an exceptional decrease to about only 10% of the basal levels in the expression of DUSP9 mRNA from 0.5 h onwards, until at least 6 h. In contrast to PHLPP2, the PHLPP1 isoform was upregulated 3-fold from 2 h onwards.
For several reasons, the drastic downregulation of DUSP9 by ropivacaine caught our specific attention, as DUSP9 has an important role in placental function during gestation, but is dispensable for embryonic development [
10]. DUSP9 expression is high in the first-trimester placenta, but it later declines until parturition [
29]. DUSP9 levels are significantly lower in the placentae of women with pre-eclampsia [
29]. A protective effect against gestational diabetes is also debated, as DUSP9 affects insulin resistance and generally has a complex role in insulin signaling, glucose uptake, and related metabolic processes [
30]. DUSP9 is also expressed in several types of blood and immune cells [
31]. The immune cell type plasmacytoid dendritic cells express high levels of DUSP9, and its levels seem to correlate with a high production of type I interferon by these cells [
32].
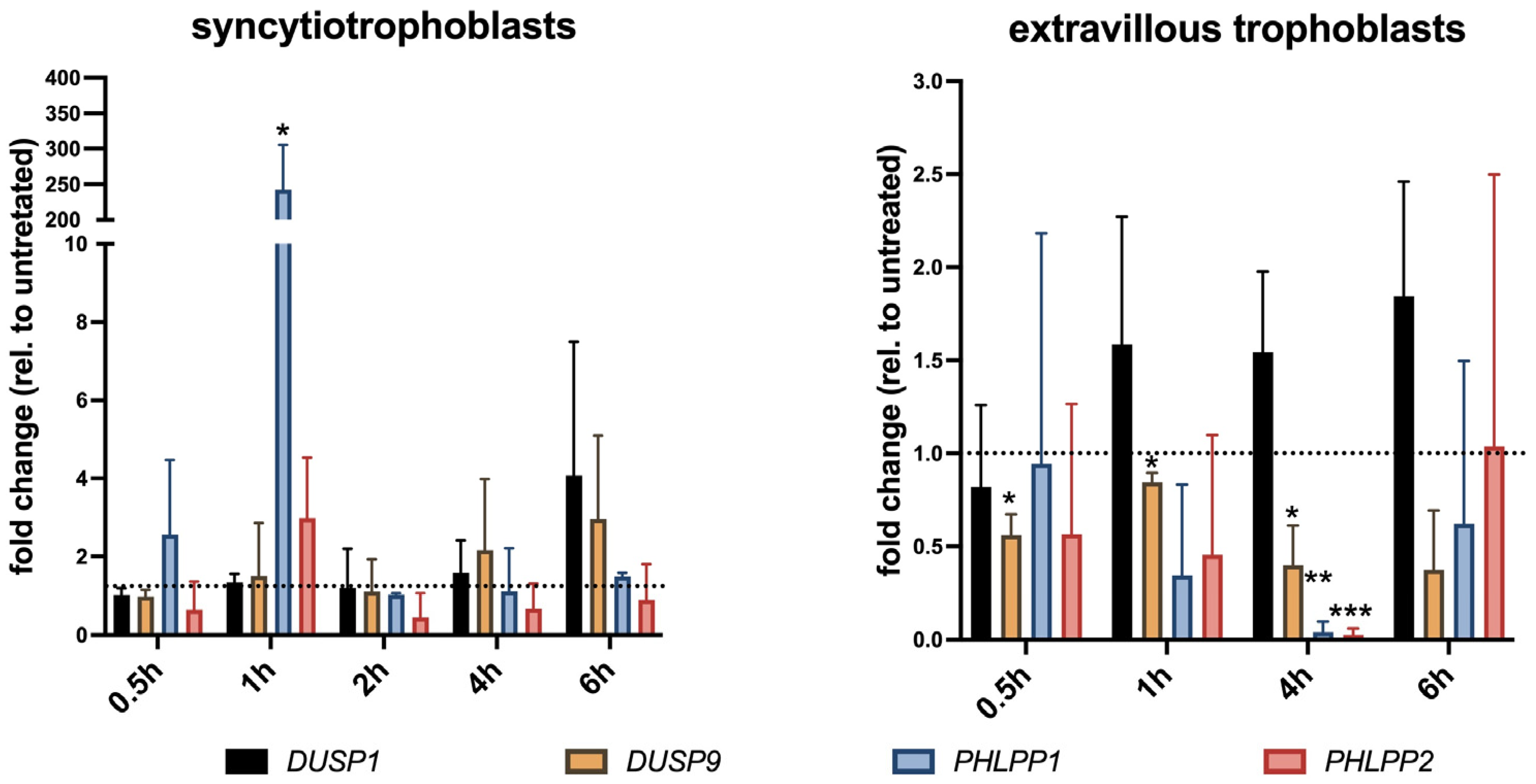
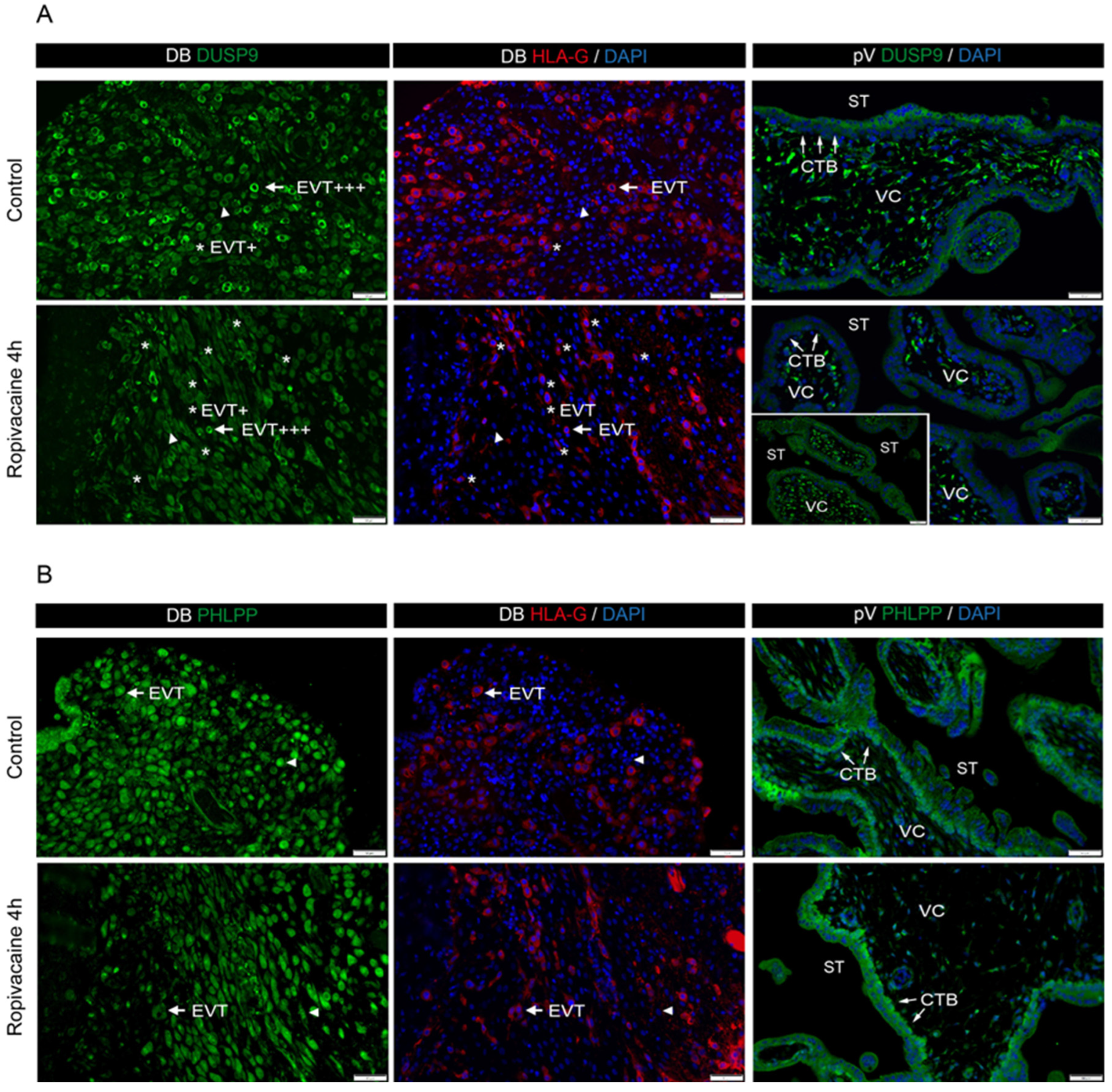
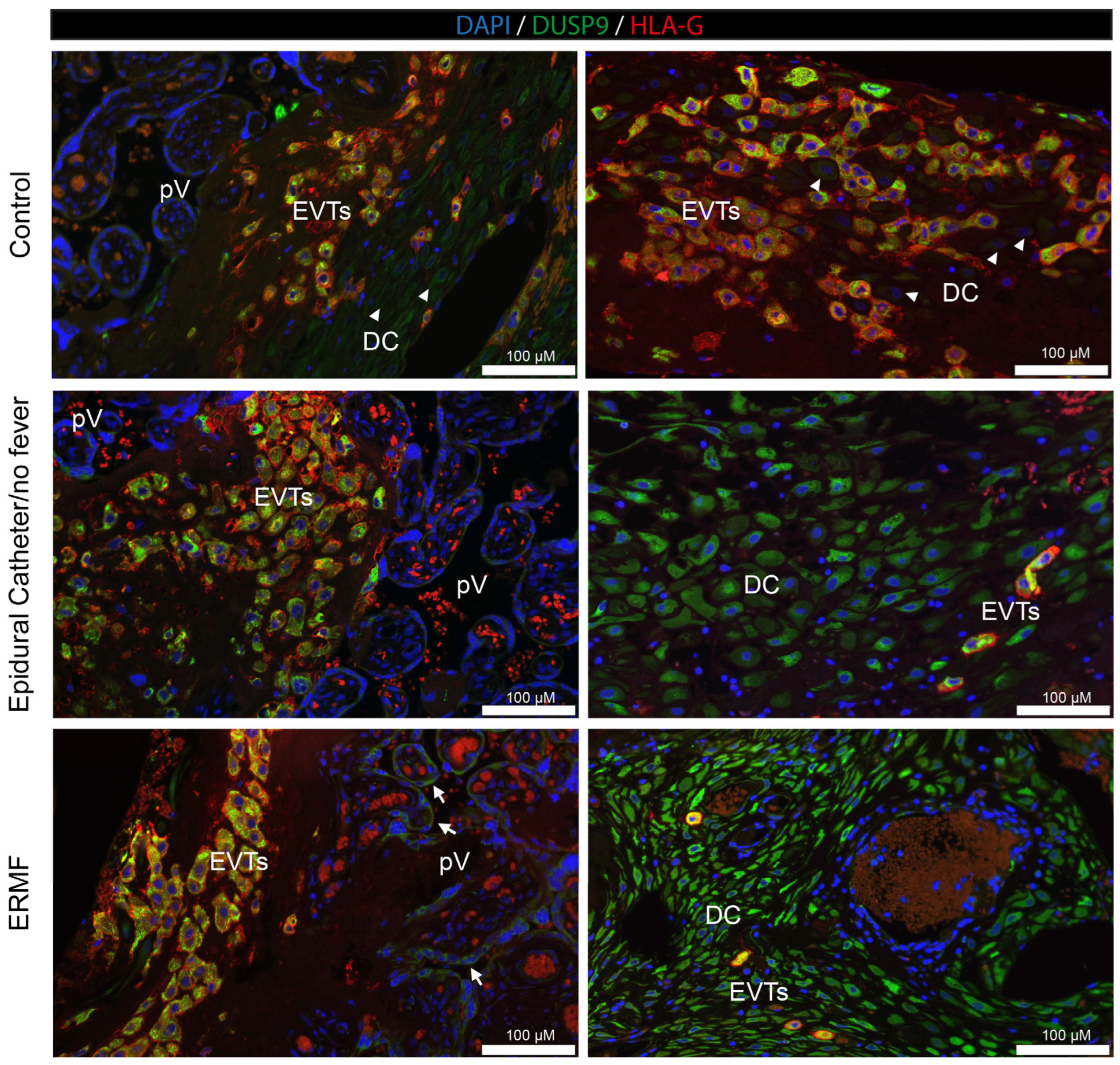
In addition to vascular cells, we investigated DUSP9 and PHLPP in placental trophoblasts in the culture and in placental tissue derived from the first trimester of pregnancy. Finally, we analyzed DUSP9 levels in term placentae collected after parturition from women with and without epidural analgesia with ropivacaine. STs and extravillous trophoblasts responded differently to ropivacaine treatment, both in in vitro cultures and in patients suffering from ERMF. Ropivacaine showed a trend to increase DUSP9 mRNA in cultured STs and led to elevated protein levels in the STs of ERMF patients. However, first-trimester placental explant tissues treated with ropivacaine displayed both positive and negative areas of STs, which may be due to additional influences, such as stimuli from neighboring cells.
Similar to HUVECs, primary extravillous trophoblasts responded with a significant downregulation of
DUSP9 mRNA to almost undetectable levels at 4 h of exposure to ropivacaine. This downregulation might be at least partly regulated by miRNAs targeting DUSP9 mRNA, which is a mechanism already found in other contexts. We identified some miRNA candidates that might target DUSP9 in concert; however, due to the small available sample size and the putative heterogeneity of cultures, it did not reach absolute statistical significance. EVTs derive from proliferating cytotrophoblasts, undergo EMT, and start to invade the decidua like tumor cells, where they are either interstitial EVTs or invade the vascular system to remodel spiral arteries and adopt endothelial-like phenotypes. We also observed a decrease in DUSP9 immunostaining in the EVTs from the first-trimester DB explant tissue at 4 h of exposure to ropivacaine. Concordantly, Western blot analyses of the corresponding tissues revealed a downregulation of DUSP9 protein levels in the presence of ropivacaine for 4 h. In term placentae from ERMF patients, the EVTs with strong DUSP9 staining prevailed, and only a few EVTs seemed to be DUSP9-negative. However, it must be mentioned that, in the control group, populations of EVTs with different expression levels of DUSP9 were also identified. Moreover, only the fraction of DB tissue that is attached to the placenta is accessible for investigations. The basal portion of the DB remains in the mother after placental expulsion. Hence, analyses of EVTs at deeper invasion sites are not feasible [
33].
PHLPP1 expression was time-dependently induced by ropivacaine in HUVECs, cultured STs, and on the protein level in the syncytium of the first-trimester placental explants. In cultured EVTs, PHLPP1 mRNA was transiently reduced, which was not detected on the protein level in the decidua basalis of the first-trimester explants at the investigated timepoints, nor in the Western blots of the corresponding decidual tissues upon ropivacaine treatment.
Cultured EVTs further exhibited a strong upregulation of pro-inflammatory cytokines IL-6, IL-8, and TNFα, as well as COX-2, and delayed PGE2 production. Also, in this aspect, these cells respond similarly to HUVECs. The miRNome of the cultured EVTs exposed to ropivacaine displayed a plethora of affected stress-related and inflammatory pathways, as well as signaling pathways typical for EVT signaling.
The upregulation of miRNAs, which repress DUSP9 mRNA, such as miR-1246 and miR-132-3p, has been found previously to be involved in such pro-inflammatory events (see discussion below). DUSP9 expression is regulated on various levels, including transcriptionally, post-transcriptionally, and post-translationally [
34,
35]. The transcriptional regulation of DUSP9 is known to be mediated by HIF-1α, the EFS family, BMP4 via SMAD1/5, and SMAD4. Post-translational stabilization of DUSP9 has been shown to be mediated by the long non-coding RNA
LincU [
36]. As post-transcriptional regulators, several miRNAs have been described, such as miR-1246, miR-212, miR-133b, miR-4458, and miR-4510 [
37].
We analyzed the miRNAome of cultured EVTs, with and without treatment with ropivacaine, for 1 h and 4 h in order to gain some insights into the regulation of DUSP9 and other molecular events occurring in this cell type. The altered miRNAome was analyzed using Pathway Enrichment analysis, which revealed typical pathways known to be affected by ropivacaine treatment (oxidative stress, HIF-1α, AKT signaling, and inflammasome), in the context of DUSP9 regulation (glucose transport, insulin signaling, GLUT4 translocation, interferon signaling, and SMAD transcriptional activity) and EVT signaling (TGFβ-SMAD, NOTCH, Wnt, etc.). In this analysis, we could only include a small sample size, as this material is extremely scarce and ethically restricted. Therefore, we could only work with miRNAs that reached an uncorrected
p-value of less than 0.05. A closer look at miRNAs with
DUSP9, as a predicted target gene, revealed a trend of ropivacaine-mediated upregulation for the following miRNAs: miR-1246 (
p = 0.09), miR-132-3p (
p = 0.18), miR-3622a-5p (
p = 0.67), and miR-212-3p (
p = 0.76)—the last two with already poor statistical quality. DUSP9 regulation by miR-132-3p has recently been described in the context of inflammation. Zhong et al. described that this mechanism putatively mediates the enhancement of inflammation in the amnion involving p38 signaling and increases the expression of COX-2 and PGE2, potentially causing preterm birth [
22].
The analysis of term placentae from a clinical study revealed another interesting finding. In this study, probands were stratified into three groups: a control group without an epidural catheter; group 2 with an epidural catheter, but no fever; and group 3, including patients who developed an increased body temperature upon epidural analgesia with ropivacaine. It must be considered that the collection of these placentae occurred many hours after the insertion of the epidural catheter. During this time, the regulation of protein expression can be different compared to that of our observation periods of only up to 6 h. However, the ERMF group showed characteristic details that were different from those of the other groups, since DUSP9 was strongly upregulated in the STs of the placental villi. Moreover, the decidual stroma cells were negative for DUSP9 in the control group but became strongly positive upon the application of ropivacaine via the epidural catheter. The reason for this observation remains unsolved and needs further investigation. Whether these characteristics in the ERMF group are causal to a rise in body temperature or a consequence can currently not be distinguished. Further mechanistic studies of these aspects could include the treatment of naïve term placental tissue with ropivacaine and the consecutive analysis of DUSP9 expression—maybe even on the single cell level. Also, DUSP9 expression could be knocked out or overexpressed in primary tissue to analyze the functional consequences. Animal experiments have already shown that DUSP9 knock-out in the germline is detrimental to labyrinth development and, therefore, placental function [
10]. However, the human placenta is anatomically and physiologically different from that of rodents, and conclusions cannot be translated easily.
Apart from its local role in regulating AKT in the placenta, we would like to add another hypothetical aspect of PHLPP1 in this context. PHLPP1β’s alias is SCOP (suprachiasmatic nucleus (SCN) circadian oscillatory protein). The SCN in the ventral hypothalamus is the master regulator of the circadian clock, where PHLPP1β and p-ERK are cyclically upregulated and downregulated, respectively, triggered by light as the stimulus [
38]. The circadian clock also regulates the core body temperature. The thermoregulatory center is located in the ventral medial preoptic area of the hypothalamus. Adjacent is found the organum vasculosum of the lamina terminalis (OVLT), which lacks a blood–brain barrier [
39]. Therefore, circulatory substances, such as endogenous pyrogens (IL-1, IL-6, TNFα, IFN, and PGE2) and drugs can directly act on the OVLT and raise the body’s core temperature. It is feasible that ropivacaine can reach the OVLT via the blood and could induce changes in PHLPP1 expression in the hypothalamus, thereby resetting the circadian clock, which theoretically could leverage control over the body temperature, resulting in either hyper- or hypothermia. Interestingly, in non-pregnant patients, epidural anesthesia occasionally induces hypothermia. This perspective demands independent investigations, but can serve as a working hypothesis for future studies. The birth process per se goes hand in hand with local inflammatory processes at the feto–maternal interface. Several studies, including our own, have shown that epidural anesthetics additionally enhance the inflammatory state by the release of pro-inflammatory cytokines and prostaglandins (for instance, from the endothelial cells and trophoblasts of the placenta), possibly with additional assistance from the immune system. The already diverse local inflammatory events might sum up with the effects of the anesthetic to pass a threshold in certain individuals, resulting in an elevated body temperature.