3. Oleocanthal in the Mediterranean Diet: Bioavailability, First-Pass Metabolism, and Clinical Drug Interaction Considerations
MD is a well-established dietary pattern characterized by a high intake of vegetables, legumes, fresh fruits, non-refined cereals, nuts, and olive oil—particularly EVOO. It also includes the moderate consumption of fish and dairy, a low intake of red meats, and moderate ethanol consumption, primarily from red wine during meals. In Mediterranean countries, EVOO is a staple, consumed daily alongside main dishes such as vegetables and legumes, enhancing both flavor and nutrient bioavailability via the lipid-mediated absorption of fat-soluble polyphenols [
35]. Epidemiological studies and clinical trials have consistently linked adherence to the MD with a reduced risk of various malignancies and lower cancer mortality rates, with the most robust evidence observed in breast cancer (summarized in
Table 1). Given that EVOO is a fundamental component of the MD, its bioactive constituents—particularly OC—are of significant interest in elucidating the molecular mechanisms underlying the MD’s protective effects against cancer. EVOO serves as a natural carrier for OC, with epidemiological studies correlating EVOO-rich diets with reduced cancer incidence [
36,
37], details of which are shown in
Table 1. Understanding this dietary framework helps contextualize OC’s bioavailability, absorption, and real-world impact, as dietary fat enhances polyphenol uptake by facilitating micelle formation and lymphatic transport [
35]. Furthermore, linking the MD’s protective effects to molecular mechanisms of OC can bridge epidemiological findings with biochemical pathways, reinforcing its potential as a dietary-based cancer prevention strategy.
While the MD is widely recognized for its health benefits, including potential anti-cancer effects, it is important to note that OC cannot be singled out as the sole bioactive component responsible for these effects. The diet consists of various nutraceuticals, and the specific contribution of OC remains difficult to quantify due to its high variability in EVOO, ranging from <100 mg/kg to >1000 mg/kg depending on agronomic and processing factors [
30,
31,
56]. The OC content in EVOO varies significantly (
Table 2) due to several factors, including the following:
- (a)
Olive Cultivar: Certain cultivars, such as Coratina, exhibit substantially higher OC concentrations (78.2 µg/mL), whereas others, such as Taggiasca, contain significantly lower levels (8.3 µg/mL).
- (b)
Geographical Location: Italian EVOOs have been reported to contain up to 191.8 µg/mL OC, whereas EVOOs from the United States typically exhibit lower levels (~22.6 µg/mL).
- (c)
Agricultural Techniques: Increased irrigation reduces OC content, suggesting that water stress conditions enhance OC biosynthesis via activation of phenylpropanoid pathways.
- (d)
Olive Maturity and Harvest Time: OC concentration is dependent on the degree of ripeness at harvest, with early harvests yielding EVOO with higher phenolic content due to increased secoiridoid biosynthesis in unripe drupes [
57].
- (e)
Processing Methods: EVOO extraction techniques influence OC retention, with two-phase centrifugation preserving higher OC levels compared to three-phase methods by minimizing water contact and hydrolysis.
- (f)
Storage Conditions and Thermal Stability: The chemical stability of OC is affected by exposure to oxygen, light, and temperature fluctuations, though OC remains relatively stable when initially present in high concentrations (>200 mg/kg), which inhibit oxidative degradation [
57].
These factors therefore are responsible for the inconsistent dosing of OC. Despite extensive preclinical evaluations, clinical pharmacokinetic data on OC remain conspicuously limited. To date, no large-scale human trials have systematically quantified OC plasma concentrations, metabolite profiles, or bioavailability kinetics following dietary intake or purified OC supplementation [
58]. These data gaps hinder the translational potential of OC and underscore the urgent need for well-designed, dose escalation clinical studies to define its pharmacokinetic and pharmacodynamic parameters in human subjects.
Additionally, OC undergoes significant hydrolysis in the gastrointestinal tract, with only 16–33% remaining intact after 4.5 h, as shown in rat models using LC-ESI-LTQ-Orbitrap-MS quantification [
58]. While EVOO shows promise as a dietary prevention strategy, its therapeutic application is limited by the lack of precise dosing due to the factors mentioned above. These variations make it challenging to determine a standardized OC intake within the MD framework. Further research is needed to identify the optimal oil source, processing methods, and consumption guidelines to ensure consistent bioactive effects.
Even if a standardized OC content could be achieved in dietary interventions, its bioavailability and metabolism present additional challenges that must be addressed before definitive conclusions can be drawn regarding its therapeutic effects. OC’s systemic bioavailability is restricted by limited absorption in the gastrointestinal tract, compounded by its poor aqueous solubility (logP ~2.8), which limits dissolution and uptake. Once absorbed, OC undergoes extensive first-pass metabolism, reducing its systemic circulation levels. Its metabolism occurs through both Phase I (CYP450-mediated hydroxylation/hydrogenation) and Phase II (UGT-mediated glucuronidation) enzymatic processes [
58]. Phase I metabolism primarily involves hydrogenation and hydroxylation reactions that modify its chemical structure, whereas Phase II metabolism includes conjugation reactions, particularly glucuronidation, which enhances its water solubility for excretion but may also influence its bioactivity. Additionally, OC interacts with circulating compounds such as amino acids, forming conjugates like OC–glycine, which may alter its biological effects [
59], though further research is needed to elucidate these interactions.
OC demonstrates relatively limited intestinal absorption, as evidenced by its low effective permeability coefficient (2.23 ± 3.16 × 10
−5 cm/s) and apparent permeability coefficient (4.12 ± 2.33 × 10
−6 cm/s) [
60]. The extent of absorption, reflected by the area under the mesenteric blood–time curve, is also lower compared to highly permeable reference compounds like levofloxacin. This suggests moderate-to-low oral bioavailability, although higher levels are expected to reach human plasma compared to rat plasma, indicating potential species differences in absorption and metabolism [
61].
The amphiphilic characteristics of OC result in partitioning between oily and aqueous phases during digestion, with a tendency toward higher concentration in the aqueous phase (68.7%) due to its polar functional groups [
58]. Following oral administration, OC concentration in the stomach decreases significantly over time (36% reduction at 2 h and 74% reduction at 4.5 h), while intestinal concentrations show similar but less pronounced reductions (16% at 2 h and 33% at 4.5 h). These findings suggest extensive pre-systemic metabolism [
58].
At the molecular level, OC undergoes a series of Phase I metabolic transformations. These include hydrogenation reactions mediated by NADPH-dependent aldo-keto reductases (AKRs) located in the small intestine epithelium, resulting in reduced metabolites (OC + H
2) [
62]. In parallel, hydroxylation reactions are likely catalyzed by cytochrome P450 enzymes, leading to hydroxylated derivatives (OC + OH) [
58]. Additionally, OC undergoes hydration reactions, forming hydrated metabolites (OC + H
2O), which have been identified in plasma and various tissues [
63]. Certain metabolites may undergo combined modifications, such as hydroxylation and hydration (OC + OH + H
2O), further supporting the complexity of OC metabolism [
58].
The distribution of these metabolites is tissue specific. The hydrogenated metabolite (OC + H
2) exhibits maximum concentrations (C
max) in plasma, liver, and heart at 2 h post-administration, while its levels peak in the stomach and small intestine at 1 h and in the kidney at 4.5 h [
58]. In contrast, the metabolite OC + OH + H
2O has been identified in various tissues except the stomach and small intestine, suggesting hepatic formation after initial gastrointestinal metabolism [
58].
Following Phase I metabolism, OC metabolites undergo Phase II conjugation reactions, predominantly glucuronidation. Phase II metabolism results in the formation of glucuronide conjugates of hydrogenated and hydrated metabolites, including OC + H
2 + glucuronic acid and OC + H
2O + glucuronic acid, the latter representing one of the two main circulating metabolites [
58,
60,
64]. These reactions are catalyzed by UDP-glucuronosyltransferases (UGTs), which transfer glucuronic acid from UDP-glucuronic acid to the hydroxyl groups of OC metabolites, enhancing water solubility and facilitating elimination [
65]. While hepatic glucuronidation constitutes the primary clearance route, significant UGT activity in the kidney and intestine also contributes to extrahepatic metabolism [
58,
66,
67].
Interestingly, no sulfate metabolites of OC have been identified in human studies [
68]. This absence may be attributed to potential inhibition of sulfotransferases by OC or the preferential and higher-capacity nature of glucuronidation over sulfation, particularly at higher doses.
These findings collectively illustrate the complexity of OC’s first-pass metabolism, characterized by limited intestinal absorption, extensive pre-systemic metabolic processing, and significant conjugation reactions that contribute to its low systemic bioavailability.
A critical consideration in this context is the potential impact of drug–drug interactions on OC metabolism, particularly in oncology settings where polypharmacy is common.
Table 3 indicates anti-cancer drugs and their potential interactions with OC metabolism. Both CYP450 and UGT-mediated interactions may influence OC pharmacokinetics and therapeutic outcomes. Cytochrome P450 enzymes, involved in Phase I metabolism, can be inhibited or induced by co-administered drugs, thereby affecting the hydroxylation and hydrogenation of OC. The induction of CYP enzymes could lead to the enhanced metabolism and reduced systemic availability of OC, while inhibition may increase plasma concentrations and potentially its bioactivity.
Similarly, glucuronidation, the predominant Phase II metabolic pathway for OC, is susceptible to modulation by several anti-cancer agents. The inhibition of UGT enzymes may reduce OC clearance, leading to elevated plasma levels and increased pharmacological effects. Conversely, the induction of UGT expression may accelerate metabolic clearance, potentially compromising OC’s therapeutic efficacy [
68].
Several drugs commonly prescribed in cancer therapy or supportive care settings have been identified as potential modulators of OC metabolism. Tyrosine kinase inhibitors such as imatinib, hormone receptor modulators like tamoxifen, and antiepileptics including valproic acid, carbamazepine, and phenytoin are known to interact with CYP450 and UGT enzymes, thereby altering the metabolic fate of OC [
69,
70,
71]. Corticosteroids such as dexamethasone, frequently co-administered with chemotherapy, are potent inducers of CYP3A4 and UGT enzymes and may accelerate OC metabolism [
72,
73,
74].
Furthermore, OC has demonstrated pharmacodynamic interactions with several anti-cancer drugs, enhancing or protecting against their cytotoxic effects. Studies have shown that OC exhibits synergistic activity with paclitaxel, tamoxifen, lapatinib, doxorubicin, and dacarbazine in various cancer cell lines [
75,
76,
77,
78]. Additionally, Oleuropein, another phenolic compound found in olive oil, has been reported to reduce cyclophosphamide-induced toxicity in animal models, suggesting a potential protective role for OC as well [
79].
The molecular basis of these interactions may involve OC’s inhibition of cyclooxygenase enzymes (COX-1 and COX-2), modulation of the c-MET receptor tyrosine kinase pathway, and induction of oxidative stress through reactive oxygen species (ROS) generation. These mechanisms may potentiate the efficacy of co-administered anti-cancer agents, mitigate chemotherapy-associated toxicity, or overcome drug resistance [
80,
81,
82].
Recently, we have demonstrated that OC significantly downregulates the expression of PAR-2 at both the mRNA and protein levels in CRC cell lines [
83]. This novel finding underscores the possibility that OC may not only exert direct anti-inflammatory and anti-cancer effects but may also indirectly influence drug disposition through the modulation of PAR-2-mediated pathways.
Emerging evidence indicates that PAR-2 signaling is intricately involved in regulating the expression and function of several ATP-binding cassette (ABC) transporters and solute carrier (SLC) transporters, which govern drug efflux and influx mechanisms. For instance, PAR-2 activation shows the potential to upregulate the expression of P-glycoprotein (ABCB1) [
84,
85], Breast Cancer Resistance Protein (BCRP/ABCG2) [
86,
87], and Multidrug Resistance-associated Proteins (MRPs, particularly MRP2/ABCC2 and MRP4/ABCC4) [
88,
89,
90,
91]. These transporters play pivotal roles in determining the pharmacokinetics of various chemotherapeutic agents by mediating their cellular efflux, thereby contributing to drug resistance and therapeutic failure in oncology settings.
Given that OC attenuates PAR-2 expression, it is plausible to hypothesize that it may concurrently modulate the activity or expression of these efflux transporters, potentially enhancing the intracellular retention and efficacy of anti-cancer agents. Notably, drugs such as doxorubicin, paclitaxel, vincristine, etoposide, and methotrexate are substrates of these transporters and are frequently compromised by transporter-mediated efflux mechanisms [
92,
93,
94].
This observation opens a promising avenue for future investigation. Specifically, it would be pertinent to examine whether the OC-mediated downregulation of PAR-2 results in decreased expression or functional activity of ABC transporters, thereby augmenting the intracellular accumulation and cytotoxicity of co-administered anti-cancer drugs. Such studies could include detailed pharmacokinetic profiling, transporter expression analysis, and functional efflux assays in relevant cancer models. Furthermore, exploring the impact of OC on the SLC family transporters—which facilitates drug uptake—would provide a comprehensive understanding of its role in modulating drug disposition and therapeutic outcomes.
Beyond OC’s intrinsic bioavailability challenges and issues associated with drug–drug interactions, its interaction with other dietary components in the MD may further modulate its metabolism and function. In fact, interplay between OC and MD components extends beyond simple conjugation reactions, involving dynamic competition between endogenous and dietary nucleophiles for OC’s electrophilic α,β-unsaturated aldehydes. Recent mechanistic studies demonstrate OC’s pH-dependent reactivity profile, with accelerated adduct formation occurring in the acidic gastric environment (pH 3.5) compared to intestinal conditions (pH 7), potentially directing metabolite formation pathways through compartment-specific reactions [
59]. This pH-mediated selectivity explains the preferential formation of oleocysteine derivatives in the stomach versus the intestinal generation of oleoglutathione conjugates, creating distinct metabolite pools with differential absorption kinetics and tissue distribution patterns. Competitive binding assays reveal that sulfur-containing amino acids (cysteine, methionine) exhibit 3–5× higher reaction rates with OC compared to neutral or aromatic residues [
95], suggesting MD components like garlic (rich in cysteine derivatives) may profoundly influence OC’s metabolic fate.
The MD’s characteristic combination of wine polyphenols and OC creates a complex reaction milieu where proline-rich wine peptides compete with plasma amino acids for OC conjugation. Kinetic studies using model digestive systems show wine-derived tripeptides (e.g., γ-glutamyl-cysteinyl-glycine) form stable OC adducts 40% faster than free amino acids [
59], potentially redirecting OC from hepatic glucuronidation pathways toward novel metabolite production. Notably, oleoserine derivatives demonstrate enhanced blood–brain barrier permeability compared to native OC in ex vivo models [
58], suggesting dietary modulation could optimize OC’s neuroprotective effects while altering systemic exposure levels.
Emerging evidence indicates MD-derived lipids may competitively bind OC’s aldehyde groups through Schiff base formation, creating transient complexes that delay gastric absorption but enhance intestinal lymphatic uptake. In simulated digestion models, co-administration with omega-3 fatty acids (abundant in MD fish sources) increases OC’s micellar incorporation by 60% while reducing free aldehyde availability for amino acid conjugation [
30]. This lipid-mediated shielding effect preserves OC’s redox-sensitive phenolic groups during gastric transit, potentially explaining observed synergies between olive oil and fish consumption in Mediterranean populations [
95].
Advanced delivery systems specifically address OC’s multi-compartmental metabolic challenges through molecular encapsulation strategies. Novel phytosome formulations incorporating phosphatidylcholine and cholesterol derivatives demonstrate a 90% protection of OC’s reactive aldehydes during simulated digestion, while achieving 3× higher hepatic accumulation compared to free OC in preclinical models [
30]. Phase inversion temperature-engineered nanoemulsions show particular promise for intestinal-targeted release, leveraging bile salt interactions to preferentially deliver OC conjugates to enterocyte absorption pathways over passive diffusion mechanisms [
58].
Section Summary:
This section situates OC within the MD, highlighting its contribution to the MD’s cancer-protective effects and addressing the wide variability in OC content due to olive cultivar, geography, harvest timing, and processing methods. Although EVOO serves as a natural delivery matrix, OC exhibits limited systemic bioavailability owing to poor aqueous solubility, extensive pre-systemic metabolism, and complex Phase I/II biotransformation, including glucuronidation and conjugation with dietary nucleophiles. The section also delineates how MD components—such as sulfur-rich amino acids, wine-derived peptides, and omega-3 lipids—influence OC’s metabolic fate, absorption kinetics, and pharmacodynamic potential. Notably, these interactions may alter the formation of bioactive conjugates, impact transporter-mediated drug disposition, and contribute to interindividual variability in therapeutic outcomes. Finally, emerging delivery technologies such as phytosomes and nanoemulsions are introduced as potential solutions to optimize OC’s stability, absorption, and tissue targeting.
7. Oleocanthal in Hereditary Cancer Predisposition
OC presents itself as a compelling candidate in the realm of dietary chemoprevention, particularly for individuals bearing hereditary predispositions to malignancy. Its potential extends beyond mere epidemiological associations, offering a mechanistic basis for attenuating carcinogenesis in genetically susceptible cohorts.
Among these, carriers of BRCA1/2 mutations, who face an elevated risk of breast and ovarian malignancies due to impaired DNA repair mechanisms [
140,
141], may particularly benefit from OC’s anti-inflammatory and oxidative stress-modulating properties. Given that heightened oxidative burden is a critical driver of genomic instability in BRCA1/2-deficient cells [
142,
143], OC’s ability to quell ROS and modulate apoptotic pathways suggests a plausible adjunctive role alongside existing prophylactic strategies, such as surgical risk-reduction, lifestyle interventions, and targeted therapies, including Poly (ADP-ribose) polymerase (PARP) inhibitors. Given the inherent challenges of resistance to PARP inhibitors, there is growing interest in identifying dietary and pharmacological agents that may enhance their efficacy or mitigate adverse effects [
144]. OC, with its anti-inflammatory and oxidative stress-modulating properties, emerges as a plausible adjunct in this context. As oxidative stress plays a dual role in both sensitizing cancer cells to DNA damage and promoting tumorigenesis, OC’s ability to ROS levels could influence PARP inhibitor responses.
Similarly, Lynch syndrome patients, characterized by mismatch repair (MMR) deficiencies that predispose them to colorectal, endometrial, and various extracolonic neoplasms, stand to gain from OC’s documented capacity to suppress pro-tumorigenic inflammatory pathways, including PAR-2-associated pathways [
80,
133,
145]. Chronic inflammation plays a pivotal role in accelerating neoplastic transformation in MMR-deficient tissues, with pathways such as NF-κB and STAT3 driving sustained cellular proliferation and immune evasion [
146]. OC’s ability to modulate these key molecular cascades, particularly when incorporated into a high-fiber, polyphenol-rich diet, presents an intriguing avenue for delaying tumor progression or reducing cumulative cancer risk in these individuals.
Beyond these well-characterized hereditary syndromes, OC may exert protective effects in other high-risk groups, including individuals with familial adenomatous polyposis (FAP) and those suffering from chronic inflammatory conditions such as inflammatory bowel disease (IBD), chronic gastritis, and pancreatitis—conditions that significantly elevate lifetime malignancy risk. Given its NSAID-like properties, OC’s role in mitigating chronic inflammation, restoring gut homeostasis, and potentially influencing hormone receptor activity renders it a promising, albeit underexplored, intervention in hormone-driven malignancies, including estrogen-sensitive breast and endometrial cancers. Nevertheless, while preclinical evidence underscores OC’s potential as a dietary chemopreventive agent [
147], its translational application necessitates rigorous clinical investigation to determine optimal dosing, bioavailability enhancement strategies, and possible interactions with existing therapeutic regimens. A refined understanding of OC’s pharmacokinetics, particularly within genetically predisposed cohorts, will be paramount in establishing its efficacy as a viable adjunct in precision oncology and preventative medicine.
Section Summary:
This section proposes OC as a promising dietary chemopreventive agent for individuals with hereditary cancer predispositions. Due to its anti-inflammatory and oxidative stress-modulating properties, OC may benefit BRCA1/2 mutation carriers by mitigating oxidative burden and supporting DNA repair. Similarly, OC’s suppression of pro-tumorigenic inflammatory pathways (e.g., PAR-2, NF-κB, STAT3) suggests its utility for Lynch syndrome patients and other high-risk groups with chronic inflammation. While preclinical evidence is encouraging, clinical investigation is essential to determine optimal dosing and integrate OC into precision oncology and preventative strategies for these cohorts.
8. Oleocanthal as a Modulator of Metabolic Programming and Tumor Bioenergetics
Metabolic reprogramming constitutes a fundamental adaptation in malignancy, wherein tumor cells remodel their bioenergetic and biosynthetic networks to sustain proliferation, evade apoptosis, and adapt to fluctuating microenvironmental conditions. The Warburg effect, characterized by a preferential reliance on aerobic glycolysis despite the availability of oxygen, has long been considered a cornerstone of cancer metabolism [
148,
149]. However, a more nuanced understanding of tumor bioenergetics has revealed that mitochondrial metabolism, lipid biosynthesis, and redox homeostasis all contribute to the metabolic plasticity that underlies cancer cell survival. In this context, the potential role of OC in metabolic regulation remains an emerging but compelling area of study. Our own investigations, demonstrating OC-mediated restoration of mitochondrial membrane potential (MMP) in LPS-stimulated chondrocytes [
150], provide a mechanistic foundation for exploring its impact on cancer cell metabolism, particularly in the context of oxidative stress resilience, mitochondrial function, and metabolic flux through anabolic pathways.
The influence of OC on glycolysis and the Warburg effect remains largely speculative, though evidence from olive polyphenol-enriched extracts, such as oleuropein-containing olive leaf extract (OLEO), suggests a regulatory effect on glucose uptake and glycolytic flux. The downregulation of glucose transporters (GLUT1, GLUT3) and key glycolytic enzymes such as pyruvate kinase M2 (PKM2) has been observed in melanoma and CRC models treated with olive-derived compounds, suggesting a metabolic shift away from glycolysis as the primary source of ATP [
151]. Given the structural similarities between OC and other olive polyphenols, it is plausible that OC disrupts glucose metabolism at a transcriptional or post-translational level, potentially via the suppression of HIF-1α and MYC, two master regulators of glycolytic reprogramming [
152]. If OC is indeed capable of impairing glucose uptake and PKM2-mediated conversion of phosphoenolpyruvate to pyruvate, this would have profound consequences for cancer cell proliferation and lactate-driven immunosuppression. Furthermore, the metabolic shift induced by OC may force reliance on mitochondrial respiration, creating a novel therapeutic vulnerability in tumors that retain functional oxidative phosphorylation (OXPHOS).
Beyond glycolysis, OC may also intersect with the PPP, a critical metabolic axis that fuels anabolic growth and redox homeostasis in cancer cells. The PPP serves as an alternative glucose metabolism pathway, diverting glucose-6-phosphate from glycolysis towards ribose-5-phosphate production for nucleotide biosynthesis and NADPH generation for antioxidant defense. Tumors exhibiting high oxidative stress, such as those driven by MYC amplification or KRAS mutations, often display PPP upregulation as a compensatory survival mechanism, allowing for the sustained nucleotide synthesis and glutathione (GSH)-mediated detoxification of ROS [
153]. If OC exerts metabolic control over glycolysis, it is reasonable to hypothesize that it simultaneously disrupts the PPP by restricting glucose flux into this pathway, thereby depriving tumor cells of essential precursors for DNA replication and antioxidant defense. Such an effect would render tumor cells more susceptible to oxidative damage, metabolic stress, and apoptotic induction.
In line with this, studies on SH-SY5Y cell lines have demonstrated that OC counteracts oxidative stress in non-cancerous contexts by increasing cell viability, reducing ROS production, and elevating intracellular levels of reduced glutathione (GSH). Proteomic analysis revealed that OC significantly modulates 19 proteins in the presence of H
2O
2, upregulating proteins related to the proteasome, the chaperone heat shock protein 90, and the antioxidant enzyme peroxiredoxin 1 [
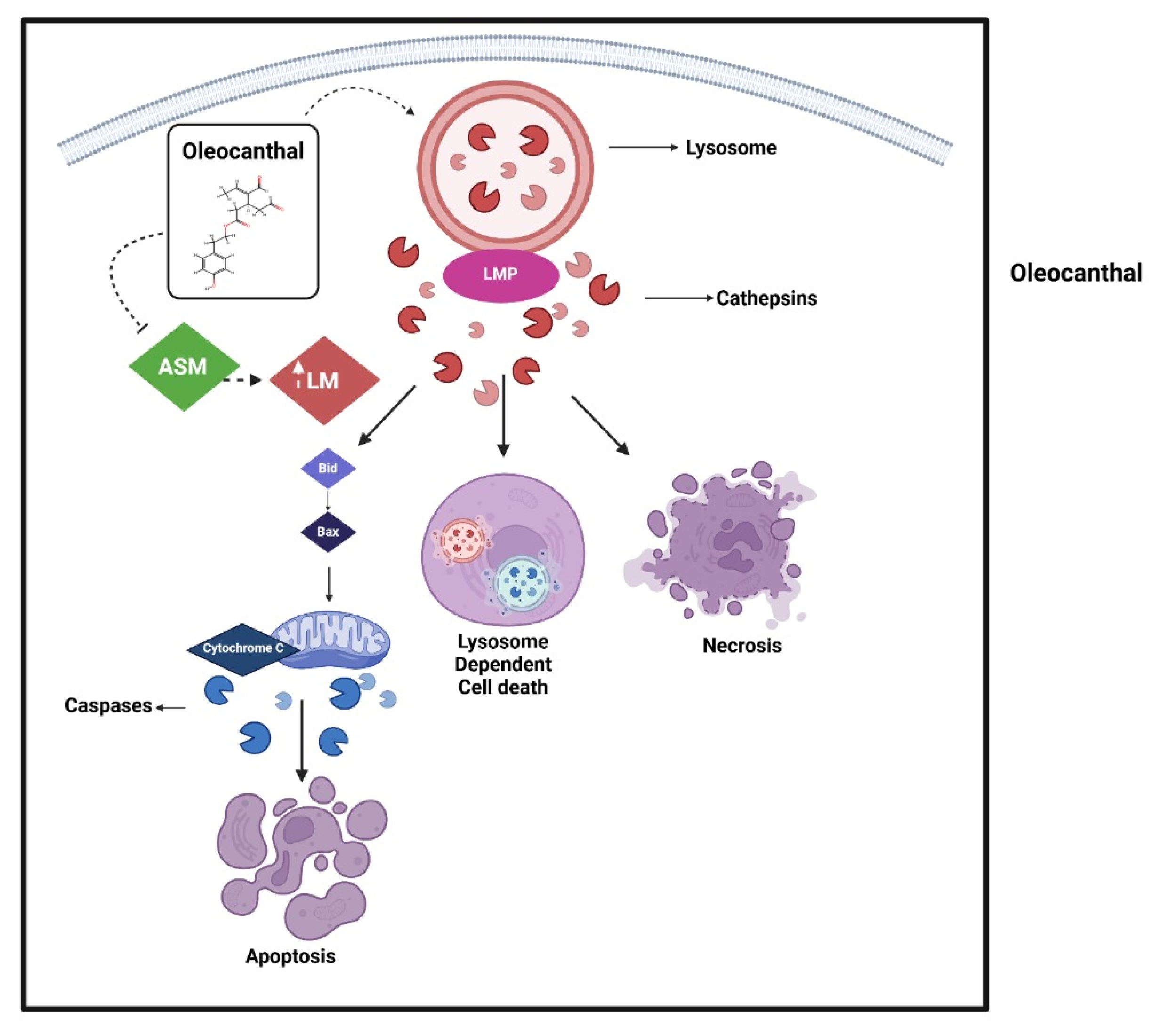
154]. However, in cancer cells, as discussed previously OC induces lysosomal membrane permeabilization and causes potential ROS generation, selectively increasing oxidative stress in malignant cells while sparing non-cancerous cells. This selective action suggests that OC may exacerbate oxidative stress in tumor cells by disrupting their redox balance, potentially through interference with PPP-derived NADPH production.
Given that OC has been shown to mediate mitochondrial membrane stabilization in inflammatory settings [
150], it is conceivable that OC could further modulate metabolic redox control in cancer cells by heightening oxidative stress and impairing their ability to cope with metabolic and oxidative challenges. This mechanism aligns with the hypothesis that OC disrupts the PPP, depriving tumor cells of NADPH and GSH-mediated antioxidant defenses, thereby rendering them more vulnerable to oxidative damage and apoptosis.
The impact of OC on the tricarboxylic acid (TCA) cycle and OXPHOS remains less well-defined, yet its ability to regulate mitochondrial integrity suggests that it may influence TCA cycle flux and mitochondrial ATP production. Although most rapidly proliferating cancer cells suppress OXPHOS in favor of glycolysis, therapy-resistant populations, circulating tumor cells, and cancer stem-like cells frequently exhibit enhanced mitochondrial metabolism, allowing for survival under metabolic stress [
155]. The perturbation of mitochondrial membrane potential by OC may influence electron transport chain (ETC) efficiency, leading to bioenergetic stress and impaired tumor survival. Whether OC enhances mitochondrial oxidative metabolism as a pro-differentiation strategy, similar to retinoic acid in myeloid malignancies, remains an intriguing possibility that warrants further exploration [
156]. Notably, OC induces ROS in cancer cells, leading to mitochondrial membrane depolarization and the disruption of mitochondrial integrity, which facilitates cytochrome c release into the cytosol. Released cytochrome c binds to apoptotic protease-activating factor 1 (APAF1), forming the apoptosome, a complex that activates caspase-9 and subsequently executioner caspases like caspase-3/7, driving apoptosis [
157]. OC treatment increases caspase-3/7 activity, cleaving downstream targets such as PARP, a DNA repair enzyme, to execute programmed cell death, as observed in hepatocellular carcinoma (HCC) and CRC cells. Additionally, OC enhances γ-H2AX expression, a marker of DNA double-strand breaks, amplifying apoptotic signals alongside cytochrome c-mediated pathways [
80]. While no direct link exists between OC and succinate dehydrogenase (SDH), both influence cancer cell survival via ROS, metabolism, and epigenetic regulation. Interestingly, OC and SDH deficiency exert opposing epigenetic effects: OC reactivates silenced genes through HDAC/SMYD2 inhibition [
133], while SDH loss drives hypermethylation via TET suppression [
158]. This interplay highlights the potential for combined therapies targeting both histone modifications and DNA methylation in cancers with metabolic–epigenetic vulnerabilities.
The role of OC in lipid metabolism, an often-overlooked aspect of tumor bioenergetics, presents another promising avenue for investigation. Tumor cells exhibit heightened reliance on fatty acid oxidation (FAO) and lipid biosynthesis, allowing them to sustain membrane synthesis, oncogenic signaling, and metabolic flexibility under hypoxia or nutrient deprivation [
159]. Olive oil-derived compounds, particularly oleic acid, have demonstrated lipid-modulating properties, though the precise effects of OC on cancer lipid metabolism remain undefined. Given the essential role of sterol regulatory element-binding protein 1 (SREBP-1) and fatty acid synthase (FASN) in tumor lipogenesis [
160], it is plausible that OC may interfere with lipid biosynthetic pathways, thereby depriving tumor cells of essential precursors for proliferation and survival. Equally compelling is the hypothesis that OC may inhibit carnitine palmitoyltransferase 1 (CPT1A), the rate-limiting enzyme in mitochondrial fatty acid oxidation, thereby restricting an alternative fuel source that many therapy-resistant tumors exploit. The possibility that OC impairs lipid homeostasis to induce metabolic stress should be investigated in cancer models that exhibit heightened lipid droplet formation and FAO dependence, such as triple-negative breast cancer, prostate cancer, and pancreatic ductal adenocarcinoma.
To fully elucidate the metabolic effects of OC, future investigations must leverage multi-omic platforms capable of integrating metabolic flux analysis, transcriptomics, and proteomics, such as the study performed by Guitsi et al., although this was in context of cancer [
154]. A combination of mass spectrometry-based metabolomics and isotope-labeled tracing experiments would allow for a precise quantification of OC’s impact on glucose metabolism, PPP activity, TCA cycle flux, and lipid anabolism. Functional validation using CRISPR-based knockout models targeting PKM2, G6PD (the rate-limiting enzyme of the PPP), CPT1A, and IDH1 could define whether OC’s metabolic effects are enzyme-specific or mediated through broader signaling pathways. Given the intricate interplay between metabolism and therapy resistance, it will also be crucial to explore synergistic interactions between OC and metabolic inhibitors, such as 2-deoxyglucose (a glycolytic inhibitor), metformin (an OXPHOS disruptor), and FASN inhibitors. Furthermore, in vivo studies employing tumor xenografts and patient-derived organoid models will be essential in determining whether OC exerts a clinically relevant impact on tumor metabolism and therapeutic response.
The emergent landscape of metabolic oncology underscores the need for novel agents capable of targeting tumor bioenergetic plasticity. OC, with its apparent ability to modulate mitochondrial integrity, restrict glucose metabolism, and potentially interfere with lipid and PPP flux, represents an intriguing, albeit underexplored, metabolic disruptor in cancer. Our findings on OC-mediated mitochondrial membrane potential restoration in inflammatory settings lend support to the hypothesis that OC stabilizes mitochondrial function while simultaneously inducing bioenergetic stress in tumor cells. As the field progresses, rigorous mechanistic studies will be required to determine whether OC can be effectively leveraged as a metabolic modulator in precision oncology, with a particular emphasis on its role in targeting the metabolic vulnerabilities of therapy-resistant malignancies.
Section Summary:
This section explores OC’s role in disrupting cancer metabolic reprogramming. OC is hypothesized to impair glycolysis and the pentose PPP, depriving cancer cells of essential resources and enhancing oxidative stress. While its direct impact on TCA cycle/OXPHOS needs further study, OC induces mitochondrial depolarization, contributing to apoptosis. OC may also interfere with lipid metabolism, stressing anabolic pathways. Our findings on OC-mediated mitochondrial membrane potential restoration suggest its potential as a metabolic disruptor. Future multi-omic and in vivo studies are crucial to validate OC’s efficacy in targeting cancer metabolic vulnerabilities.
9. Gut Microbiome, Cancer, and Oleocanthal
OC’s disruption of tumor metabolism intersects critically with its gut microbiome modulation—a dual mechanism rooted in its polyphenolic properties. We now examine how OC’s bidirectional microbial interactions influence both gastrointestinal and systemic malignancies.
The gut microbiome plays a pivotal role in maintaining gastrointestinal and systemic health, with dysbiosis—an imbalance in microbial composition—being implicated in local and distant cancer development. Due to its ability to influence immune responses, cancer growth, the effectiveness of immune checkpoint inhibitors, immunotherapy, and the avoidance of its negative effects, gut microbiota play a significant role in the management of cancer [
161,
162]. The term “immuno-oncology-microbiome” (IOM) refers to the immune-mediated interactions between immune cells, tumors, and microorganisms in the TME [
163]. According to the IOM, there are primarily two types of interactions between the host microbiome and tumor: (1) interactions involving gut microbes, which influence the host immune system and affect both local and distant tumor growth and survival; and (2) interactions involving intertumoral microbes, which either reside in the TME or tumor/immune cells to influence tumor progression and anti-tumor immunity [
164]. On the other hand, of all illnesses, dysbiosis of the gut microbiota is directly linked to the development of tumors, both in regional gastrointestinal malignancies and other, distant tumors [
163]. The multifaceted function of the gut microbiome in cancer prevention, carcinogenesis, and anti-cancer therapy is highlighted by metabolomics and metagenomics research [
164].
Polyphenols, have emerged as critical modulators of gut health and microbiota composition, exerting prebiotic, antimicrobial, and anti-inflammatory effects that may have profound implications for cancer prevention and treatment [
165]. Numerous polyphenols have demonstrated gut-modulating properties, with specific mechanistic insights emerging from both in vitro and in vivo models [
166,
167].
Polyphenols and gut bacteria have a reciprocal interaction [
165]. Although phenolic compounds can positively impact the composition and function of gut microbial populations, gut microbiota is engaged in the bioconversion of polyphenols, modifying their stability, metabolism, and bioactivity [
168]. This exemplifies the mutually beneficial interaction between gut bacteria and polyphenols in cancer prevention. Gut microbiota is the key to the biotransformation of dietary polyphenols into bioactive molecules that initiate and modulate molecular and physiological processes involved in the prevention of cancer. On the other side, polyphenols serve as an army for gut homeostasis against pathogens and enhance gut health. In this way, polyphenols may target cancerous cells through the modulation of microbe-derived molecular mechanisms (summarized in
Figure 5).
Additionally, polyphenols can have an impact on the intestinal ecology and maintain the balance of the gut’s microbes by acting as both a prebiotic and an antimicrobial agent that prevents pathogenic bacteria like E. coli, Clostridium perfringens, Clostridium histolyticum, and H. pylori from growing and colonizing the body [
168]. Furthermore, polyphenols affect the metabolites produced by the microbiota such as SCFA, luminal oxygen levels, mucus production, the intestinal immune system and inflammation, and intestinal integrity. Similarly, bacteria mediate the metabolism of tea polyphenols (TPs), for example, by removing the gallic acid moiety and ring fission to release phenolic acid catabolites, whereas TPs may contribute to the modification of bacterial profiles that enhance gut barrier function, for example, by increasing intestinal permeability to reduce inflammation—reshaping microbial composition—and associated metabolites that exert systemic protection on host metabolism [
169].
The gut microbiota can also affect the bioaccessibility and bioavailability of polyphenols through the carbon-to-carbon separation of aromatic and heterocyclic rings in flavonoids, as well as through the dehydroxylation, decarboxylation, and/or hydrogenation of alkene moieties [
170]. There is mounting evidence that the gut microbiome plays a critical role in the metabolism and absorption of polyphenols and is thought to be the primary driver of much of the interindividual variation in their bioavailability [
171]. The occurrence of metabolic phenotypes in the population can be linked to the various production patterns of polyphenol metabolites obtained by the gut microbiota. Because the gut microbiota substantially metabolizes polyphenols, interindividual variances in bacteria-converting species may play a role in interperson variability.
There is mounting evidence that conventional chemotherapy can considerably benefit from the addition of some natural dietary polyphenols [
172,
173,
174]. One polyphenol whose capacity to prevent BC has been studied is carnosol. In vitro and in vivo investigations have revealed that this chemical dramatically reduces invasion and metastasis. Carnosol reduces matrix metalloproteinase (MMP)-9 activity and the STAT3 signaling pathway by degrading the STAT3 protein in a proteasome-dependent manner to inhibit BC and the STAT3 signaling pathway, respectively [
175].
When discussing OC, it is important to keep in mind that protected by the monounsaturated fatty acid triglycerides matrix of EVOO, simple EVOO tyrosol and hydroxytyrosol-based phenolics can be rapidly absorbed in the small intestine, unlike the more complex phenolics, which can reach, at least in part, the large intestine, where they can interact with host gut microbiota (GM) [
176]. Consequently, it is important to understand if OC would modulate GM taxonomical distribution and metabotypes independent of systemic OC metabolic fate following absorption. Here, we can also note that absorption of lipid-entrained OC via the chylomicron route is of likely importance, opening the possibility for enterohepatic cycling, which to our knowledge remains to be addressed. In the context of the above, its important to note that in a single-dose safety study of OC, Swiss albino mice tolerated oral doses of up to 500 mg/kg, whereas the intraperitoneal LD50 of OC was estimated to be in the range of 164–524 mg/kg [
177]. This disparity clearly shows the great prospective importance of roles played by GM in the safety of OC and EVOO phenolics with oral administration/consumption.
The study [
178] identified over 50 bacterial species significantly suppressed by OC across all samples, while 28 species exhibited marked increases. Notably, members of the Bacteroidia class, particularly
Bacteroides fragilis, have been implicated in colorectal carcinogenesis. Enterotoxigenic
Bacteroides fragilis (ETBF) produces a toxin that compromises epithelial barrier integrity, induces DNA damage, and fosters a pro-inflammatory microenvironment conducive to tumorigenesis. Its role in CRC is well-documented, given its ability to modulate immune responses and drive chronic inflammation [
179,
180]. Intriguingly,
Bacteroidia class was among the most suppressed bacterial species in OC-treated mice, suggesting a potential anti-cancer benefit associated with OC consumption.
Another beneficial gut bacterium modulated by OC in previous studies is
Monoglobus pectinilyticus, a member of the
Ruminococcaceae family [
181].
M. pectinilyticus plays a crucial role in human colonic metabolism as a specialized pectin-degrading bacterium. Its unique glycobiome enables efficient pectin degradation, generating polysaccharide degradation products (PDPs) that serve as substrates for other gut microbes [
182]. This metabolic activity is critical for maintaining gut homeostasis and may influence cancer risk by modulating microbial composition and function. While
M. pectinilyticus itself has not been directly linked to CRC, its role in fostering a balanced gut microbiome and producing anti-inflammatory metabolites could contribute to protective effects against colorectal carcinogenesis.
The gut microbiome plays a pivotal role in CRC pathogenesis, with microbial dysbiosis leading to chronic inflammation, the production of carcinogenic metabolites, and the disruption of the mucosal barrier [
183]. By selectively promoting beneficial bacteria while suppressing pro-carcinogenic species, OC may exert a favorable impact on gut microbiota composition, thereby reducing CRC risk.
In contrast,
Prevotella species demonstrate context-dependent roles in cancer, functioning as either pro-tumorigenic pathobionts or protective commensals depending on microbial community dynamics and host immune interactions. In OC-treated groups, three
Prevotella strains were notably suppressed. The role of
Prevotella in CRC remains complex—while some species, such as
Prevotella intermedia, have been enriched in colorectal adenocarcinoma tissues and are associated with enhanced CRC cell migration and invasion, others may exert protective effects.
P. intermedia has been implicated in the malignant transformation of colorectal adenomas, particularly in synergy with
Fusobacterium nucleatum, highlighting its potential contribution to CRC progression and metastasis. Conversely, higher
Prevotella abundance in the gut microbiota has also been correlated with reduced CRC progression and lower mortality rates, underscoring its multifaceted role in colorectal oncogenesis [
184,
185].
As mentioned previously OC’s modulation of gut microbiota extends beyond local gastrointestinal effects; in breast cancer,
Prevotella copri has been identified as significantly enriched in the gut microbiota of breast cancer patients. Mechanistically,
P. copri promotes breast cancer growth by metabolizing tryptophan, leading to a depletion of indole-3-pyruvic acid (IPyA), a naturally occurring anti-cancer agent. The reduction in IPyA subsequently inactivates the AMPK signaling pathway, facilitating tumor growth [
186].
Furthermore, OC’s potential influence on distal tumors through gut–brain and gut–liver axes should be noted. Microbial metabolites modulated by OC—particularly SCFAs and tryptophan derivatives such as kynurenine—directly shape the brain tumor microenvironment by regulating anti-tumor immunity and inflammatory signaling. Additionally, OC’s correction of gut dysbiosis may restore immune balance, counteracting the pro-tumorigenic shift in regulatory and effector immune populations that drives brain cancer progression [
187]. Quesada et al. (2023) demonstrated in an ex vivo mouse model that OC intake significantly alters gut microbiota composition, enriching beneficial bacteria linked to SCFA production, such as butyrate producers (
Faecalibacterium spp.) [
178]. This aligns with Farràs et al. (2020), who highlighted that olive oil phenolics like OC elevate SCFAs (e.g., butyrate), which exert anti-inflammatory and anti-tumorigenic effects systemically [
176]. The central mechanisms through which butyrate modulates the immune system is by promoting the differentiation and function of regulatory T cells (Tregs), a subset of immune cells known for their role in maintaining immune tolerance [
188]. Butyrate influences the epigenetic regulation of Tregs by inhibiting histone deacetylases (HDACs), which enhances the expression of genes essential for Treg development and function [
189]. This mechanism helps maintain immune balance while suppressing pro-inflammatory responses that promote tumor-associated inflammation and cancer progression. On the other hand, direct evidence linking OC to kynurenine modulation remains lacking; its suppression of
Prevotella copri (a tryptophan-metabolizing pathobiont), however, suggests a plausible indirect role in limiting kynurenine-driven neurotoxicity and tumor immune escape, as observed in glioblastoma (GBM)-associated dysbiosis.
Investigations into the gut microbial ecosystem of GBM patients, the most aggressive brain malignancy, have revealed significant dysbiosis. Studies show GBM patients often exhibit higher microbial diversity and bacterial overgrowth, marked by an increase in Proteobacteria (e.g.,
Escherichia coli). Elevated levels of
Enterobacteriaceae,
Bacteroidaceae (including
Bacteroides vulgatus), and
Lachnospiraceae are observed, alongside notably lower levels of beneficial genera such as
Faecalibacterium and certain
Prevotella species [
187]. Given OC’s documented ability to enrich beneficial bacteria like
Faecalibacterium (a key butyrate producer) [
178] and suppress problematic groups such as
Bacteroides and certain
Prevotella strains, these findings collectively suggest that OC could potentially modulate the profound gut dysbiosis seen in GBM patients. By restoring microbial balance, OC may help counteract the pro-tumorigenic environment influenced by these specific bacterial alterations, opening new avenues for understanding and potentially treating GBM through gut microbiome modulation.
Similarly, OC’s ability to modulate gut dysbiosis presents a promising therapeutic avenue for hepatocellular carcinoma (HCC), a malignancy significantly influenced by the gut–liver axis [
190]. HCC pathogenesis is driven by intestinal dysbiosis and compromised gut barrier function, facilitating bacterial translocation and chronic hepatic inflammation. HCC-associated dysbiosis is characterized by reduced microbial α-diversity, depleted commensal genera like
Lactobacillus and
Bifidobacterium, and an overrepresentation of pathogenic taxa such as
Enterobacteriaceae,
Enterococcus,
Bacteroides, and
Ruminococcaceae [
191]. OC, through its demonstrated capacity to suppress problematic bacteria (e.g.,
Bacteroides) and foster beneficial ones (e.g.,
Monoglobus pectinilyticus and
Faecalibacterium), may help restore this crucial balance. Furthermore, OC’s influence on microbial metabolites like SCFAs and bile acids (BAs) is critical. Dysbiosis-driven alterations in BA profiles, particularly the downregulation of FXR, contribute to hepatotoxicity and activate pro-inflammatory signaling in HCC [
192,
193]. Additionally, exogenous SCFA administration in a hepatitis B virus (HBV)-induced murine model of HCC has shown to significantly reduce the number of dysplastic nodules and tumor burden in HBx-transgenic mice [
194]. Corroborating these findings, stool microbiome analyses in HCC patients have revealed a marked depletion of SCFA-producing bacterial genera, such as
Lachnospira,
Ruminococcus, and
Butyricicoccus, suggesting a disrupted SCFA-mediated regulatory axis in human HCC pathophysiology [
195]. OC’s role in rebalancing the microbiome could thus help normalize BA metabolism and enhance protective SCFA levels, thereby mitigating chronic inflammation and disrupting carcinogenic pathways in the liver, offering a novel approach to HCC prevention and treatment.
Collectively, these findings highlight the intricate interplay between OC and gut microbiota in the context of cancer. By selectively suppressing pro-tumorigenic bacteria, such as B. fragilis and specific Prevotella strains, while fostering beneficial microbial populations like Faecalibacterium spp. and M. pectinilyticus, OC may exert a protective role in cancer prevention. These results underscore the therapeutic potential of OC as a modulator of gut microbiota composition, paving the way for further investigation into its application in cancer treatment and prevention.
Given the growing body of evidence, OC’s interaction with the gut microbiota presents a promising avenue for cancer prevention and treatment. Its ability to modulate bacterial populations, suppress pro-carcinogenic species, and promote gut homeostasis highlights its potential as a natural therapeutic agent. Future research should aim to elucidate the precise molecular mechanisms underlying these effects and explore its clinical applications in oncology.
Section Summary:
This section elucidates OC dual mechanism in cancer modulation: direct effects and critical interactions with the gut microbiome. Dysbiosis is pivotal in cancer development, influencing immunity and therapy. Polyphenols, including OC, reciprocally modulate gut health, acting as prebiotics and antimicrobials, influencing the microbial metabolism of SCFAs, and impacting OC’s systemic fate. Studies show that OC suppresses pro-carcinogenic bacteria like Bacteroides fragilis and specific Prevotella strains while promoting beneficial ones like Monoglobus pectinilyticus. This selective modulation helps reduce cancer risk, notably in colorectal cancer, and extends to modulating breast cancer through Prevotella copri and glioblastoma and HCC via the gut–brain and gut–liver axis and SCFA production. Collectively, OC’s therapeutic potential as a gut microbiota modulator for cancer prevention and treatment is underscored.
11. Future Directions, Therapeutic Potential, and Critical Research Priorities
11.1. Translating PAR-2 Modulation by Oleocanthal into Chondrosarcoma Therapeutics
Our preliminary findings demonstrating the ability of OC to downregulate PAR-2 expression in both transcriptomic and proteomic assays warrant exploration of its therapeutic applicability beyond inflammatory cartilage pathologies. Specifically, there is a compelling mechanistic rationale to extend this PAR-2 modulatory effect to chondrosarcoma (CHS), a malignant cartilage tumor characterized by profound ECM remodeling, immune evasion, and stemness-associated dedifferentiation. Emerging evidence identifies PAR-2 as a critical mediator of inflammation-driven cartilage degeneration, and recent work has confirmed OC’s capacity to suppress PAR-2 expression alongside downstream inflammatory cascades in osteoarthritic chondrocytes [
150]. Given the chondrocytic origin shared by osteoarthritis (OA) and CHS, and the central role of PAR-2 in tumorigenic inflammation and matrix degradation, this mechanistic axis represents a promising therapeutic target in CHS.
A priority research direction involves elucidating the role of PAR-2/NF-κB crosstalk in regulating CHS stemness and progression. CHS is frequently driven by IDH1/2 mutations that induce global DNA hypermethylation and promote SOX9-mediated dedifferentiation programs [
134]. It is hypothesized that PAR-2 activation in CHS may stabilize β-catenin through calcium-dependent disheveled (DVL) phosphorylation, amplifying Wnt/β-catenin signaling to sustain cancer stem cell populations. Future studies should investigate whether OC disrupts this PAR-2/RAC1/β-catenin axis in IDH-mutant CHS models, leveraging its dual capacity to downregulate PAR-2 [
150] and preserve repressive H3K27me3 epigenetic marks [
132] to counteract CpG island hypermethylation at tumor suppressor loci such as CDKN2A.
Additionally, OC’s previously demonstrated ability to stabilize collagen type II alpha 1 chain (COL2A1) expression in OA chondrocytes [
150] may hold relevance in CHS, where ECM remodeling and collagen mutations create an immunosuppressive tumor niche enriched in M2 macrophages [
134]. Notably, COL2A1 truncation mutations (e.g., p.Gly1170Val) in CHS impair matrix integrity and immune infiltration. OC-mediated restoration of COL2A1 expression may counteract this process, thereby enhancing CD8
+ T cell infiltration. Parallel investigations should focus on evaluating OC’s impact on PAR-2-driven secretion of chemokines such as CCL2/MCP-1, which contribute to tumor-associated macrophage (TAM) recruitment and immune evasion. Single-cell RNA sequencing of OC-treated CHS xenografts would provide high-resolution insight into the modulation of tumor–immune interactions.
From a metabolic perspective, IDH1/2 mutations in CHS promote the accumulation of the oncometabolite 2-hydroxyglutarate (2-HG), impairing mitochondrial electron transport chain (ETC) activity and contributing to cellular dedifferentiation [
30]. Our preliminary data demonstrating OC’s capacity to preserve mitochondrial membrane potential (ΔΨm) in LPS-stimulated chondrocytes [
150] suggest a possible metabolic synergy between OC and IDH-targeted therapies (e.g., AG-120). Future studies employing CHS organoid models should assess whether OC-mediated restoration of ETC function and suppression of PAR-2/IL-1β-driven glycolytic reprogramming enhances the efficacy of IDH inhibitors and sensitizes tumors to DNA repair inhibitors, such as PARP inhibitors, through the attenuation of 2-HG-induced DNA repair defects (e.g., FANCD2 mono-ubiquitination).
Furthermore, CHS progression is associated with STAT3 hyperactivation via IL-6/JAK2 signaling, which drives dedifferentiation through SOX4-mediated transcriptional programs [
134]. Given prior evidence of OC’s capacity to inhibit PAR-2-dependent STAT3 phosphorylation at Ser727 in OA models [
217], it is plausible that OC may disrupt this pro-tumorigenic STAT3–PAR-2 feedforward loop in CHS. Experimental validation employing CRISPR-Cas9-mediated PAR-2 knockout in established CHS cell lines (SW1353, JJ012) will be instrumental in delineating whether OC’s anti-tumorigenic effects extend beyond PAR-2-dependent mechanisms.
One of the principal challenges in CHS therapy is the dense collagenous stroma, which impedes effective drug penetration and contributes to chemoresistance. OC’s amphiphilic structure lends itself to nanotherapeutic encapsulation strategies. Specifically, lipid–polymer hybrid nanoparticles (LPHNs) functionalized with CHS-specific surface ligands (e.g., anti-CD44v6) could be engineered for OC delivery, facilitating tumor-specific uptake and stromal penetration. Preclinical validation should assess whether such OC-LPHNs improve doxorubicin accumulation in CHS xenografts while simultaneously attenuating PAR-2/RANKL-driven osteolytic activity [
150].
To operationalize these strategies, we propose a translational roadmap comprising three phases:
Phase 1: Validation of OC-mediated PAR-2 suppression and downstream transcriptomic alterations in IDH1-R132C mutant CHS patient-derived organoids, employing multiplexed Nanostring PanCancer Pathways panels.
Phase 2: Evaluation of the combinatorial efficacy of OC with demethylating agents such as decitabine (DAC) to exploit potential epigenetic synergy—specifically, DAC’s capacity to reverse IDH-mediated DNA hypermethylation coupled with OC’s stabilization of H3K27me3 at oncogenic loci [
132].
Phase 3: Development of non-invasive imaging modalities through the synthesis of PAR-2/COL2A1 dual-targeted positron emission tomography (PET) tracers (e.g., 68Ga-OC-DOTA conjugates) to monitor OC’s therapeutic efficacy and ECM remodeling capacity in vivo.
Collectively, these future investigations will clarify the therapeutic relevance of PAR-2 suppression by OC in chondrosarcoma and establish a framework for the clinical translation of OC-based multimodal therapies targeting tumor inflammation, stemness, metabolism, and the immunosuppressive microenvironment.
11.2. Translating Oleocanthal’s PAR-2/TNF-α/Calcium Signaling Modulation into Colorectal Cancer Therapeutics
Our preliminary investigations demonstrating OC’s ability to downregulate PAR-2 and TNF-α expression in CRC models [
83] provide a strong mechanistic rationale to further explore its therapeutic potential in modulating key oncogenic signaling cascades. Given the established role of PAR-2-mediated calcium flux and TNF-α-induced inflammation in sustaining CRC progression, EMT, and immune evasion, we propose a comprehensive translational strategy to leverage OC’s multimodal actions.
A principal research priority is to delineate OC’s capacity to disrupt the PAR-2–TNF-α–ERK1/2–TROP-2 axis, which orchestrates metastatic dissemination in CRC. Building upon our findings, systematic in vitro studies should evaluate OC’s dose-dependent inhibition (20–150 µg/mL) of TROP-2 expression and ERK1/2 phosphorylation in HT-29 and Caco-2 cell lines under TNF-α stimulation (5–20 ng/mL). Functional validation through TROP-2 knockdown using siRNA in combination with OC treatment should be performed, assessing resultant effects on cell invasion (Transwell assays) and matrix degradation (MMP-2 and MMP-9 activity by zymography) [
218]. Further mechanistic depth should be achieved by pharmacologically inhibiting ERK1/2 (e.g., SCH772984) to establish whether OC’s anti-invasive effects are ERK-dependent.
Additionally, OC’s modulation of calcium signaling in CRC cells [
83] merits detailed evaluation. Calcium flux serves as a critical mediator of Wnt/β-catenin and NFAT pathway activation, both of which are central to CRC progression. Future studies should employ live-cell calcium imaging using Fluo-4 AM to characterize OC-induced alterations in intracellular calcium dynamics during EMT in CRC spheroids. Parallel assays should assess OC’s effects on β-catenin nuclear translocation (via immunofluorescence) and LEF/TCF transcriptional activity, particularly under conditions of ER calcium depletion induced by thapsigargin. Translationally, combination studies evaluating OC with calcium channel blockers (e.g., verapamil) in APC-mutant patient-derived organoids (PDOs) should be prioritized to target store-operated calcium entry (SOCE)-driven proliferative signaling.
OC’s regulatory effects on the TME also warrant investigation. Given that TNF-α signaling amplifies IL-6 and IL-8 secretion in the CRC TME, fostering apt conditions for tumor invasion [
219], it is imperative to evaluate OC’s capacity to attenuate this cytokine axis. Co-culture models using HT-29 cells and THP-1-derived macrophages can be employed to assess the dose-dependent suppression of IL-6 and IL-8 (via multiplex Luminex assays) alongside flow cytometric profiling of M2 macrophage markers (CD206
+). Spatial transcriptomic analyses of OC-treated CRC xenografts should further resolve the compartmental localization of PAR-2, TNF-α, and cytokine expression within stromal and epithelial niches. Correlative studies linking OC-induced cytokine shifts with PD-L1 expression and CD8
+ T cell infiltration across microsatellite-stable (MSS) and microsatellite instability-high (MSI-H) CRC subtypes will offer insights into OC’s immunomodulatory potential.
A critical area for mechanistic exploration is the role of PAR-2–calcium crosstalk in sustaining CRC stemness. PAR-2 activation is known to maintain LGR5+ CRC stem cells via calcium-dependent YAP/TAZ signaling. It is therefore pertinent to assess whether OC disrupts this signaling axis by treating primary CRC stem cells (CD133+/CD44+) with OC in the presence or absence of the PAR-2 agonist SLIGRL-NH2, followed by an evaluation of stemness markers (OCT4, NANOG) and organoid-forming capacity. Real-time imaging of calcium oscillations in stem-like niches can be performed using genetically encoded FRET-based calcium biosensors (GCaMP6s) to visualize OC’s inhibitory effects. Furthermore, combinatorial studies with Wnt pathway inhibitors (e.g., LGK974) in RSPO fusion-positive PDOs may offer a synergistic therapeutic strategy.
Beyond these signaling effects, OC’s reported ability to preserve H3K27me3 marks [
83] opens avenues for epigenetic intervention. Chromatin immunoprecipitation sequencing (ChIP-seq) should be utilized to assess H3K27ac and H3K27me3 enrichment at PAR-2 (F2RL1) and TNF-α promoter loci following OC treatment. Complementary CRISPR-dCas9-KRAB-mediated silencing of PAR-2, combined with OC administration, can clarify additive effects on TNF-α suppression (quantified via ELISA). Additionally, DNA methylation profiling using the Illumina EPIC array will help identify epigenetic biomarkers predictive of OC responsiveness in CRC PDOs.
Given the physical and biochemical barriers of CRC desmoplasia that limit OC bioavailability, nanotherapeutic strategies are warranted. The development of fibroblast activation protein (FAP)-targeted liposomes encapsulating OC (L-OC) is proposed, leveraging FAP’s overexpression in CRC CAFs [
83]. L-OC penetration and biodistribution should be assessed in decellularized CRC extracellular matrix scaffolds using mass spectrometry imaging and orthotopic patient-derived xenograft (PDX) models. For theranostic applications, the radiolabeling of L-OC with
68Ga-DOTA will enable the PET/CT monitoring of PAR-2–rich metastatic lesions.
Finally, OC’s potential to overcome therapeutic resistance through synergy with TNF-α–ERK1/2-directed therapies should be investigated. Preclinical screening should evaluate the combinatorial efficacy of OC with ERK1/2 inhibitors (e.g., ulixertinib) in Consensus Molecular Subtype 4 (CMS4) CRC models characterized by high TNF-α expression. Single-cell RNA sequencing (scRNA-seq) of circulating tumor cells (CTCs) treated with OC should map shifts in the TNF-α–NF-κB–IL-8 axis, elucidating OC’s capacity to mitigate pro-metastatic signaling trajectories.
To operationalize these strategies, we again propose a translational roadmap comprising three phases:
Phase 1: Validate OC’s inhibition of the PAR-2/TNF-α/calcium signaling triad in APC/KRAS-mutant PDOs (n = 50), employing high-content imaging platforms (e.g., Operetta CLS).
Phase 2: Conduct preclinical combinatorial trials evaluating OC in conjunction with SOCE inhibitors (e.g., CM2489) in CRC liver metastasis PDX models.
Phase 3: Develop OC-loaded hydrogel-based delivery systems for locoregional application in murine models of CRC-associated peritoneal carcinomatosis, assessing therapeutic efficacy and stromal remodeling.
These future directions will comprehensively delineate the mechanistic underpinnings of OC’s multimodal action in CRC and lay the foundation for the rational development of OC-based adjunctive therapies targeting key inflammatory and metabolic vulnerabilities in colorectal carcinogenesis.
11.3. Advancing Oleocanthal’s Clinical Potential
To realize OC’s clinical potential, several research priorities will need to be addressed. Systematic pharmacokinetic–pharmacodynamic (PK-PD) modeling comparing various delivery routes (oral, IV, and nanoparticle-mediated) in murine models will be essential to quantify biodistribution and tumor accumulation. Dual-loaded systems combining OC with chemotherapeutics (e.g., paclitaxel) in nanocarriers should be explored to synergize lysosomal disruption (OC) and microtubule inhibition (paclitaxel).
Comprehensive toxicology profiles will assess OC’s effects on normal lysosomes, particularly in renal and hepatic tissues. Scaling up liposome/nanoparticle production under GMP standards will be critical for translational trials. Future studies will prioritize in vivo PK-PD correlations, scalable nanomanufacturing, and combinatorial approaches with immunotherapies.
The integration of advanced delivery systems with molecular targeting strategies will hold transformative potential for enhancing OC’s anti-cancer efficacy. By addressing these challenges, OC could transition from a dietary chemopreventive agent to a cornerstone of precision oncology.
While the preclinical evidence for OC’s anti-cancer potential is robust and multifaceted, clinical validation remains conspicuously limited. To date, a small number of registered trials—such as NCT03528603 and NCT02902913—have assessed oleocanthal-rich EVOO primarily in the context of platelet reactivity and metabolic modulation, while others (e.g., NCT04215367) have explored its effects in chronic lymphocytic leukemia as part of broader dietary interventions. Importantly, none of these studies isolate OC as a therapeutic agent or rigorously evaluate its role in oncology-specific outcomes. This paucity of clinical trials underscores a critical translational gap and reinforces the need for dedicated, well-powered interventional studies that assess OC’s safety, pharmacokinetics, and therapeutic efficacy in cancer. Addressing this unmet need will be pivotal in transitioning OC from a nutraceutical of dietary interest to a viable adjunctive agent in precision oncology.
11.4. Strategy for Investigating Protein Expression in Cancer Cells Using Proteomics and Oleocanthal
Future research should employ a multi-tiered experimental approach, integrating advanced mass spectrometry-based proteomics, bioinformatics, and functional validation assays to elucidate OC’s molecular effects on tumor progression and signaling pathways. The first phase will involve selecting clinically relevant cancer cell lines, including colorectal (HCT116, SW620, LS174T), breast (MCF-7, MDA-MB-231), liver (HepG2, Huh7), lung (A549, H460), and prostate (PC-3, LNCaP) cancer models, alongside normal epithelial controls for differential analysis. These cells will be maintained under standard culture conditions (RPMI/DMEM + 10% FBS + 1% Pen/Strep) and exposed to increasing OC concentrations (0, 5, 10, 25, 50 µM) over 24–72 h to establish a dose–response curve for proteomic interrogation.
Upon OC treatment, cells will undergo protein extraction using RIPA or urea-based lysis buffers to maximize the solubilization of cytoplasmic, nuclear, and membrane-bound proteins. Protein quantification via BCA or Bradford assay will precede in-solution tryptic digestion, incorporating reduction (DTT), alkylation (IAA), and enzymatic digestion (trypsin or Lys-C) to generate peptides suitable for high-resolution LC-MS/MS analysis. Global proteomic profiling will be conducted using label-free quantification (LFQ) on an Orbitrap-based mass spectrometer or via TMT/iTRAQ-based multiplexed proteomics for comparative quantification across treatment conditions. Data acquisition in a data-dependent acquisition (DDA) or data-independent acquisition (DIA) mode will maximize peptide coverage and enable accurate protein quantitation.
In the second phase, targeted proteomics strategies such as Selected Reaction Monitoring (SRM) and Parallel Reaction Monitoring (PRM) should validate the differential expression of proteins associated with key oncogenic pathways, including NF-κB, PI3K/AKT, MAPK, apoptosis regulation (Bcl-2, Bax, Caspase-3, PARP-1), and EMT markers. Post-translational modification (PTM) profiling will assess changes in phosphoproteomics (using TiO2 or IMAC enrichment), acetylation, ubiquitination, and glycosylation following OC treatment. This step is crucial for delineating OC’s mechanistic impact at a post-translational regulatory level, particularly in relation to PAR-2 signaling in inflammation-associated tumorigenesis.
In the third phase, validation assays such as Western blotting (WB) for pathway-specific protein analysis, qRT-PCR for transcriptomic correlation, immunoprecipitation (IP) for protein–protein interaction studies, and immunofluorescence (IF) coupled with confocal microscopy to assess subcellular localization of key differentially expressed proteins (DEPs) should be conducted. This phase will also entail the dissemination of functional assays, including MTT/BrdU cell proliferation assays, Annexin V/PI apoptosis detection by flow cytometry, wound healing and transwell migration assays, colony formation efficiency studies, and ROS detection via DCFDA fluorescence, to establish the phenotypic consequences of OC-induced proteomic alterations. Parallelly, high-content imaging and live-cell tracking will monitor real-time cellular responses to OC treatment.
In the fourth phase, data processing and pathway enrichment analyses will be conducted using MaxQuant, Perseus, and Scaffold for proteomic data normalization and visualization, followed by Ingenuity Pathway Analysis (IPA), DAVID, and KEGG enrichment mapping to identify statistically significant perturbations in key oncogenic networks. STRING-based protein–protein interaction (PPI) mapping will infer molecular connectivity and predict novel OC-modulated targets.
Furthermore, to ensure translational relevance, patient-derived organoid models should be utilized for further validation, and CRISPR/Cas9-mediated knockout studies targeting OC-regulated proteins will ascertain their functional necessity in oncogenesis. Finally, in vivo validation in xenograft mouse models should be conducted to determine whether OC-mediated proteomic alterations translate into tumor growth inhibition and metastasis suppression.
This integrative strategy will bridge MS-based proteomics with bioinformatics, functional validation, and translational oncology, positioning OC as a promising modulator of oncogenic signaling pathways and a potential therapeutic candidate in cancer research.
11.5. AI-Driven Oleocanthal Target Identification in Cancer Therapeutics
Future research should harness artificial intelligence (AI) for OC target identification in oncology through a computationally intensive, multimodal framework integrating deep learning architectures, high-dimensional multi-omics data processing, and predictive pharmacogenomics. AI-driven methodologies will leverage transformer-based models (e.g., BioBERT, GPT-4Bio) for literature mining, convolutional neural networks (CNNs) for structural protein–ligand interaction analysis, and graph neural networks (GNNs) for dynamic protein–protein interaction (PPI) modeling, facilitating the precise identification of OC-regulated molecular targets.
High-throughput proteomic mass spectrometry data (LC-MS/MS) will be processed using Fourier transform-based spectral deconvolution algorithms coupled with machine learning-based feature engineering (e.g., random forest, XGBoost) to extract differentially expressed proteins (DEPs) following OC treatment. Tandem affinity purification (TAP) and cross-linking mass spectrometry (XL-MS) datasets will be subjected to unsupervised clustering models (e.g., hierarchical clustering, t-SNE, UMAP) to establish OC-driven interaction perturbations within the PPI landscape. Deep variational autoencoders (VAEs) will facilitate dimensionality reduction in transcriptomic and epigenomic datasets, enabling the extraction of latent biological signals correlating OC exposure with oncogenic pathway suppression.
Molecular docking and dynamic simulation pipelines will employ hybrid physics-informed neural networks (PINNs) and quantum mechanics/molecular mechanics (QM/MM) simulations to predict OC’s binding kinetics with potential target proteins. AlphaFold2-based protein structure predictions will be integrated with generative adversarial networks (GANs) for ligand-based virtual screening, refining OC-protein docking precision. AI-enhanced binding free energy calculations (MM/PBSA and MM/GBSA models) will inform target validation, ranking protein targets based on predicted OC-binding thermodynamics.
Federated learning models will analyze heterogeneous datasets from multi-omics repositories (e.g., TCGA, CPTAC, LINCS L1000, PRIDE), training meta-classifiers that predict OC efficacy across diverse cancer subtypes. Multi-omics Bayesian networks will uncover causative OC-driven molecular rewiring by modeling perturbation–response trajectories. Causal inference frameworks (e.g., DoWhy, CausalML) will differentiate direct OC-mediated target effects from secondary adaptive responses, ensuring precise mechanistic delineation.
Post hoc interpretability techniques such as SHapley Additive exPlanations (SHAP), Integrated Gradients, and attention-based saliency maps will validate AI-derived target prioritization, ensuring alignment with experimental pharmacodynamics. This integrative AI-driven pipeline will advance systems pharmacology by refining OC’s therapeutic target identification, enabling rational drug repurposing and the development of OC-based precision oncology therapeutics with enhanced efficacy and translational relevance.
11.6. Computational Roadmap for Target Identification and Binding Characterization of Oleocanthal
While previous studies have primarily focused on assessing OC’s effects on downstream molecular markers in various cancer cell lines, direct investigations into its interactions with specific receptors or target molecules remain unexplored. Although research such as that by Cusimano et al. has demonstrated OC-induced apoptosis through markers like PARP cleavage, caspase activation, and γH2AX expression in liver (HepG2, Huh7, Hep3B, PLC/PRF/5) and colon (HT29, SW480) cancer cell lines, the precise molecular interactions between OC and its potential targets have not been characterized.
Recognizing this gap, our lab recently explored the direct interaction between OC and PAR2 in CRC cell lines (Caco-2, HT-29). Observing OC-induced PAR2 downregulation through Western blotting and RT-PCR, we hypothesized a potential direct interaction, which was later validated in silico (readers are referred to the article by Patnaik et al. for specific details [
83]).
Building upon the preliminary evidence of OC’s interaction with PAR-2, future investigations should adopt an integrative, scalable, and translational computational–experimental strategy to delineate the direct molecular interactome of OC across multiple oncogenic targets. This approach will not only validate observed downstream signaling perturbations but will also facilitate rational design of OC-based therapeutic interventions.
The proposed strategy initiates with sequence-based structural modeling. High-confidence amino acid sequences of candidate OC targets will be retrieved from the UniProt database and subjected to structure prediction using AlphaFold v2.3 or later versions. Structural modeling will be performed using MMseqs2-based multiple sequence alignment against UniRef90, MGnify, and PDB70 datasets, yielding five structural models per target ranked by confidence metrics, including pLDDT scores, predicted aligned error (PAE) matrices, and per-residue pIDDT scores. Transmembrane regions and structured domains with pLDDT > 90 will be prioritized for downstream analysis, while flexible loop regions (pLDDT < 50) will be earmarked for further refinement using Rosetta Membrane protocols or cryo-EM-guided model fitting to enhance atomic-level resolution.
Once structural models are validated, molecular docking analyses will be performed using both blind and focused docking strategies. Platforms such as CB-Dock2, AutoDock Vina (v1.2.0), and GNINA (a deep learning–augmented docking engine) will be employed to predict potential OC binding pockets and binding poses. Ligand preparation will incorporate geometry optimization and energy minimization using the OMEGA toolkit, followed by conversion to PDBQT format for docking studies. Binding poses will be ranked based on binding free energy (ΔG), ligand efficiency indices, and cavity-based scoring functions (PC-score).
For the highest-scoring binding modes, structural and energetic stability will be evaluated using all-atom molecular dynamics (Mol D) simulations in explicit solvent environments. Mol D simulations will be performed using GROMACS (v2023 or later) with CHARMM36m or AMBER ff19SB force fields, spanning simulation trajectories of 200–500 ns. Parameters such as root mean square deviation (RMSD), root mean square fluctuation (RMSF), and hydrogen bond occupancy will be analyzed to assess complex stability. Free energy calculations, including Molecular Mechanics/Poisson–Boltzmann Surface Area (MM/PBSA) or Free Energy Perturbation (FEP) methods, will be employed to quantify the thermodynamic favorability of OC–target interactions.
To ensure translational validity, in silico predictions will be experimentally verified using the site-directed mutagenesis of key interacting residues, followed by binding affinity measurements through surface plasmon resonance (SPR), microscale thermophoresis (MST), or biolayer interferometry (BLI). In parallel, downstream functional assays, such as reporter gene assays, Western blotting, and cell viability studies, will corroborate the biological relevance of identified interactions.
Critically, this computational pipeline is designed to be modular and expandable, enabling the systematic interrogation of OC’s binding interactions beyond PAR-2 to include other validated oncogenic targets such as receptor tyrosine kinases (e.g., c-Met, EGFR), epigenetic modulators (e.g., histone acetyltransferases), and multidrug resistance transporters (e.g., ABCB1, ABCG2). Complementary cheminformatics tools, such as SwissTargetPrediction and the Similarity Ensemble Approach (SEA), may further assist in target identification and prioritization. This integrative strategy will facilitate the construction of a comprehensive OC–target interaction atlas, laying the groundwork for the rational development of OC-derived multi-targeted anti-cancer therapeutics.
Section Summary:
This section outlines OC’s significant therapeutic potential and critical research priorities for its clinical translation, emphasizing combination therapies. Future efforts will focus on translating OC’s PAR-2 modulation for chondrosarcoma and its PAR-2/TNF-α/calcium signaling effects for colorectal cancer, both involving detailed mechanistic studies and nanotherapeutic delivery. Broader advancements require comprehensive PK-PD modeling, toxicology, and scalable nanomanufacturing for clinical trials. Crucially, AI-driven target identification and detailed computational roadmaps will define OC’s precise molecular interactome. Despite robust preclinical data, limited clinical validation underscores the urgent need for dedicated interventional studies to transition OC from a dietary compound to a cornerstone of precision oncology.
12. Material and Methods
12.1. Literature Search Strategy
A comprehensive and systematic literature search was performed to identify and collate relevant studies investigating the molecular mechanisms underlying the anti-cancer effects of OC. The search strategy was conducted in accordance with the PRISMA guidelines to ensure methodological transparency. PubMed (National Library of Medicine, Bethesda, MD, USA) was employed as the primary database due to its extensive coverage of biomedical and life science literature. The search period was restricted from January 2005 to January 2025, based on the landmark discovery of OC’s chemical structure and biological activity by Beauchamp et al. in 2005 [
32], which provided the foundational basis for subsequent mechanistic studies on OC.
A combination of Medical Subject Headings (MeSH) terms and free-text keywords were used in various Boolean combinations to maximize the retrieval of relevant publications. The search terms included but were not limited to the following: “Oleocanthal”, “Mediterranean Diet”, “Cancer”, “Tumor Suppression”, “Molecular Mechanisms”, “Apoptosis”, “Protease-Activated Receptor-2”, “Lysosomal Membrane Permeabilization”, “c-Met”, “STAT3”, “Epigenetic Modulation”, “Drug Resistance”, and “Metastasis”. Search filters were applied to restrict the retrieval to English-language, peer-reviewed, original research articles, clinical studies, and high-quality reviews containing primary experimental data. To ensure exhaustive coverage, additional hand-searching was conducted using the citation tracking of key articles and cross-referencing in Google Scholar and Scopus databases.
To contextualize the translational potential of OC as part of dietary interventions, a parallel search was performed on the ClinicalTrials.gov registry to identify completed or ongoing clinical trials evaluating the impact of the MD on cancer-related outcomes. Search terms used included “Mediterranean Diet”, “Cancer”, “Nutraceutical”, “Polyphenol”, and “Biomarker”. No restrictions were applied with regard to cancer type, geographical location, or study design. This search aimed to bridge preclinical mechanistic evidence with potential clinical applications of MD, particularly emphasizing its high OC content.
12.2. Eligibility Criteria and Study Selection
All identified records were subjected to an eligibility assessment based on pre-defined inclusion and exclusion criteria. Studies were included if they fulfilled the following conditions: (i) original experimental research (in vitro, in vivo, or ex vivo) evaluating the molecular mechanisms of OC in the context of cancer biology; (ii) studies elucidating the impact of OC on cellular signaling pathways, gene expression, apoptosis, metastasis, or drug resistance; and (iii) clinical trials and dietary intervention studies reporting cancer-related outcomes with clear association to the MD, where OC was recognized as a bioactive constituent.
Exclusion criteria encompassed the following: (i) review articles without primary data, commentaries, editorials, conference abstracts, and letters; (ii) studies focusing on olive oil phenolics other than OC without mechanistic evaluation of OC itself; and (iii) publications not available in English.
Two independent reviewers (SJ and YB; lead authors in this study) screened all titles and abstracts retrieved from the database search. Full-text articles were then assessed to ensure compliance with the inclusion criteria. Any discrepancies in study selection were resolved by discussion and consensus.
12.3. Data Extraction and Synthesis
A standardized data extraction form was developed to systematically collect relevant information from the eligible studies. Extracted variables included the following: (i) publication details (first author, year, journal); (ii) experimental design (cell lines used, animal models, human studies); (iii) sample size; (iv) cancer type and molecular targets investigated; (v) methodological platforms employed (e.g., Western blotting, RT-PCR, flow cytometry, transcriptomics, molecular docking, molecular dynamics simulations); (vi) key mechanistic findings; and (vii) limitations and quality indicators of the study. Data extraction was conducted independently by both reviewers (SJ and YB; lead authors in this study), with cross-validation for accuracy and consistency. Any disagreements were discussed until a consensus was achieved.
Given the methodological and experimental heterogeneity across the included studies, a formal quantitative meta-analysis was not feasible. Instead, a qualitative, narrative synthesis approach was employed to thematically organize and summarize the molecular pathways and biological processes modulated by OC. Studies were categorized according to mechanistic domains such as lysosomal membrane permeabilization, STAT3 inhibition, PAR-2 downregulation, c-Met inhibition, epigenetic modulation, and drug–drug interaction potential.
12.4. Chemical Structure Visualization
The 2D chemical structures of OC, oleacein, oleuropein, and hydroxytyrosol were retrieved from the PubChem database “
https://pubchem.ncbi.nlm.nih.gov (accessed on 24 May 2025)” using their respective Compound Identification Numbers (CIDs). Structural files were downloaded in SDF format to preserve stereochemical and functional group information. These files were then imported into MarvinSketch (ChemAxon, Budapest, Hungary; version 24.3.0), a cheminformatics drawing tool, where the structures were rendered, annotated, and exported as high-resolution images for inclusion in the manuscript. Care was taken to preserve bond geometries, stereochemistry, and functional group labeling to ensure accurate representation of the molecular scaffolds.
12.5. Ethical Considerations
This review exclusively utilized secondary data extracted from publicly accessible, peer-reviewed publications and clinical trial registries. Therefore, institutional ethical approval was not required. The study adhered to established ethical standards for systematic literature reviews and complied with PRISMA guidelines.
12.6. Limitations
This review is subject to several inherent limitations. First, the search was confined to English-language publications, which may introduce language bias and limit the inclusion of potentially relevant studies published in other languages. Second, the heterogeneity of experimental models, dosing strategies, and outcome measures among the included studies precluded the execution of a formal meta-analysis. Third, publication bias cannot be excluded, as studies reporting negative or inconclusive results may be underrepresented in the published literature. Lastly, although relevant clinical trials assessing the MD were identified, the lack of trials specifically evaluating OC as an isolated intervention limits the translational extrapolation of preclinical findings to clinical practice.
13. Conclusions
In summary, OC emerges as a multifunctional, mechanistically versatile nutraceutical with significant promise in precision oncology. Through its pleiotropic modulation of cancer hallmarks—including lysosomal membrane permeabilization, suppression of oncogenic signaling cascades (c-MET/STAT3, PAR-2, COX-2/mPGES-1), inhibition of EMT and angiogenesis, and epigenetic remodeling—OC exerts selective cytotoxicity towards malignant cells while sparing non-cancerous tissues. Its ability to recalibrate tumor-promoting immune phenotypes, restore calcium homeostasis, and reprogram the tumor microenvironment underscores its potential not only as a cytotoxic agent but also as an immune and metabolic modulator.
However, OC’s clinical translation has been hindered by significant pharmacokinetic limitations, including poor oral bioavailability, rapid metabolism, and inadequate tumor targeting. Recent advances in delivery strategies—ranging from liposomal encapsulation and polymer-based nanoparticles to exosome-mediated delivery, microneedle-assisted transdermal systems, and peptide–drug conjugates—have shown promise in circumventing these limitations, enhancing OC’s stability, tissue specificity, and mechanistic precision. Furthermore, emerging data on OC’s bidirectional modulation of the gut microbiota, exemplified by suppression of oncogenic Bacteroides fragilis and enrichment of anti-inflammatory Monoglobus spp., add an additional layer of complexity to its systemic anti-cancer effects, potentially bridging tumor metabolism and host microbiome interactions.
Critically, our comprehensive analysis consolidates the preclinical evidence supporting OC’s synergistic potential with established chemotherapeutic, targeted, and immunotherapeutic agents across diverse malignancies—including prostate, pancreatic, colorectal, breast, and hematological cancers. By destabilizing key resistance mechanisms (e.g., AR-V7 splice variants, STAT3 activation, NLRP3 inflammasome assembly, and Bcl-2/Mcl-1-mediated apoptosis resistance), OC potentiates the efficacy of first-line and salvage therapies while simultaneously mitigating off-target toxicities.
Future research efforts must now focus on systematic pharmacokinetic profiling, validation in robust preclinical models—including organoid and patient-derived xenograft systems—and the development of GMP-grade delivery formulations optimized for human trials. Moreover, the elucidation of OC’s pharmacogenomic interactions and its impact on transporter-mediated drug efflux warrants further investigation to fully leverage its chemosensitizing capabilities.
Taken together, the converging lines of molecular, pharmacological, and translational evidence presented in this review firmly establish OC as a next-generation, dietary-derived anti-cancer candidate. Its integration into precision oncology frameworks, through rational combination regimens and advanced delivery technologies, holds considerable potential to augment therapeutic outcomes, particularly in inflammation-driven, drug-resistant, and metabolically reprogrammed malignancies.