Comprehensive Anatomical Staging Predicts Clinical Progression in Mild Cognitive Impairment: A Data-Driven Approach
Abstract
1. Introduction
2. Results
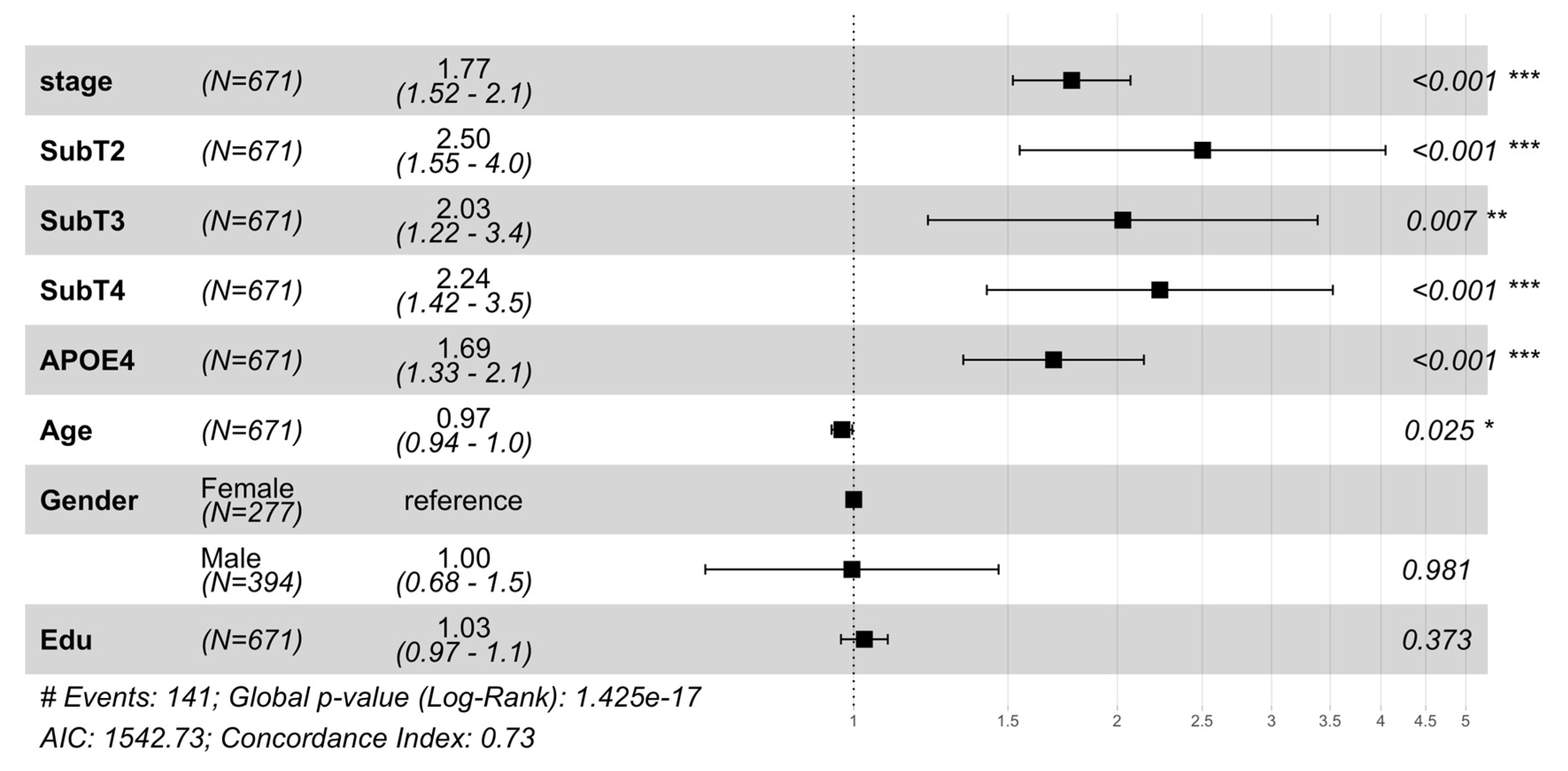
2.1. MCI to AD Conversion Risk: Stage, Subtype, and Neuroanatomy
2.2. Progression of AD Biomarkers and Brain Atrophy
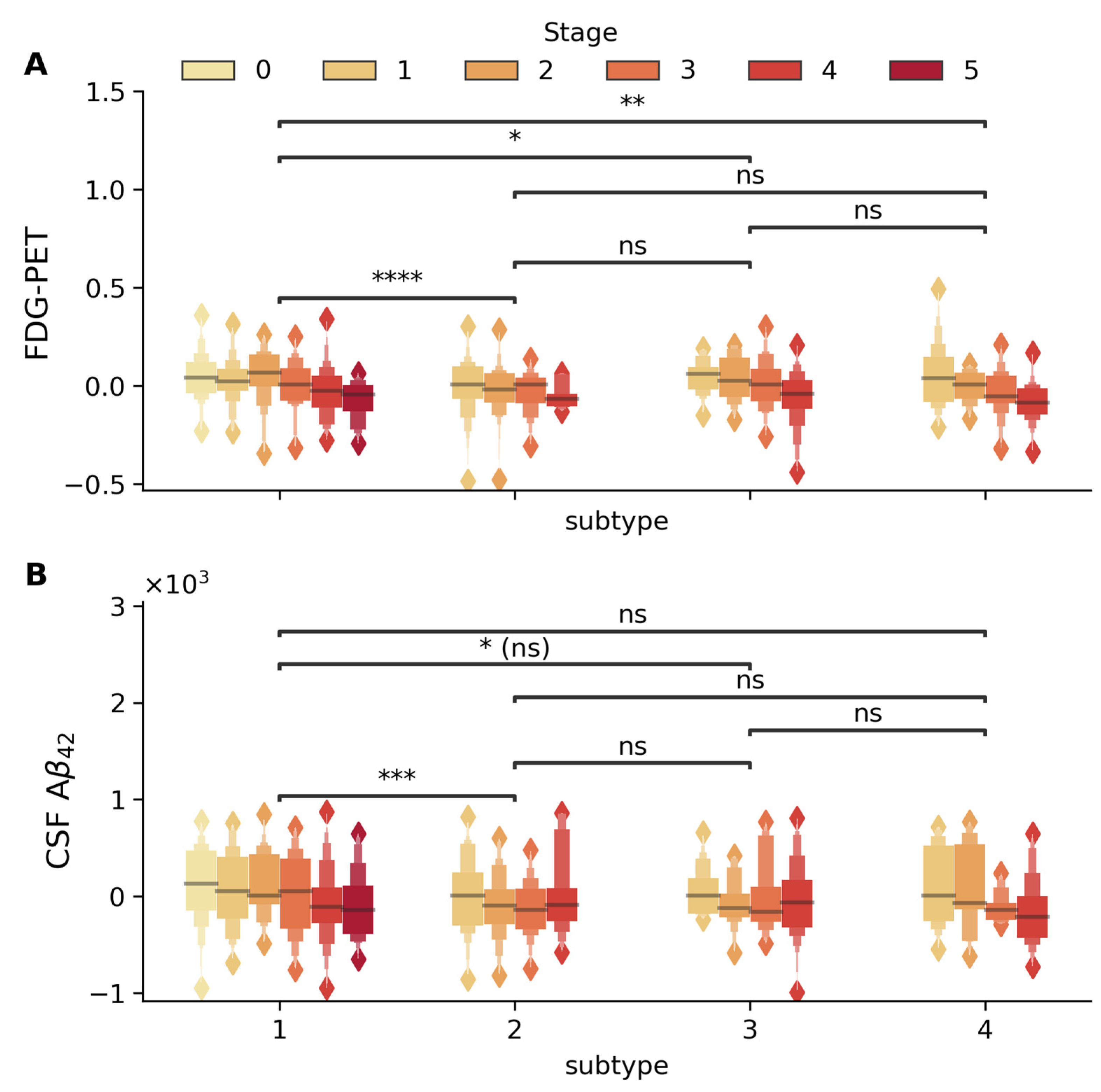
2.2.1. Glucose Metabolism and Amyloid Pathology Subtype-Specific Patterns
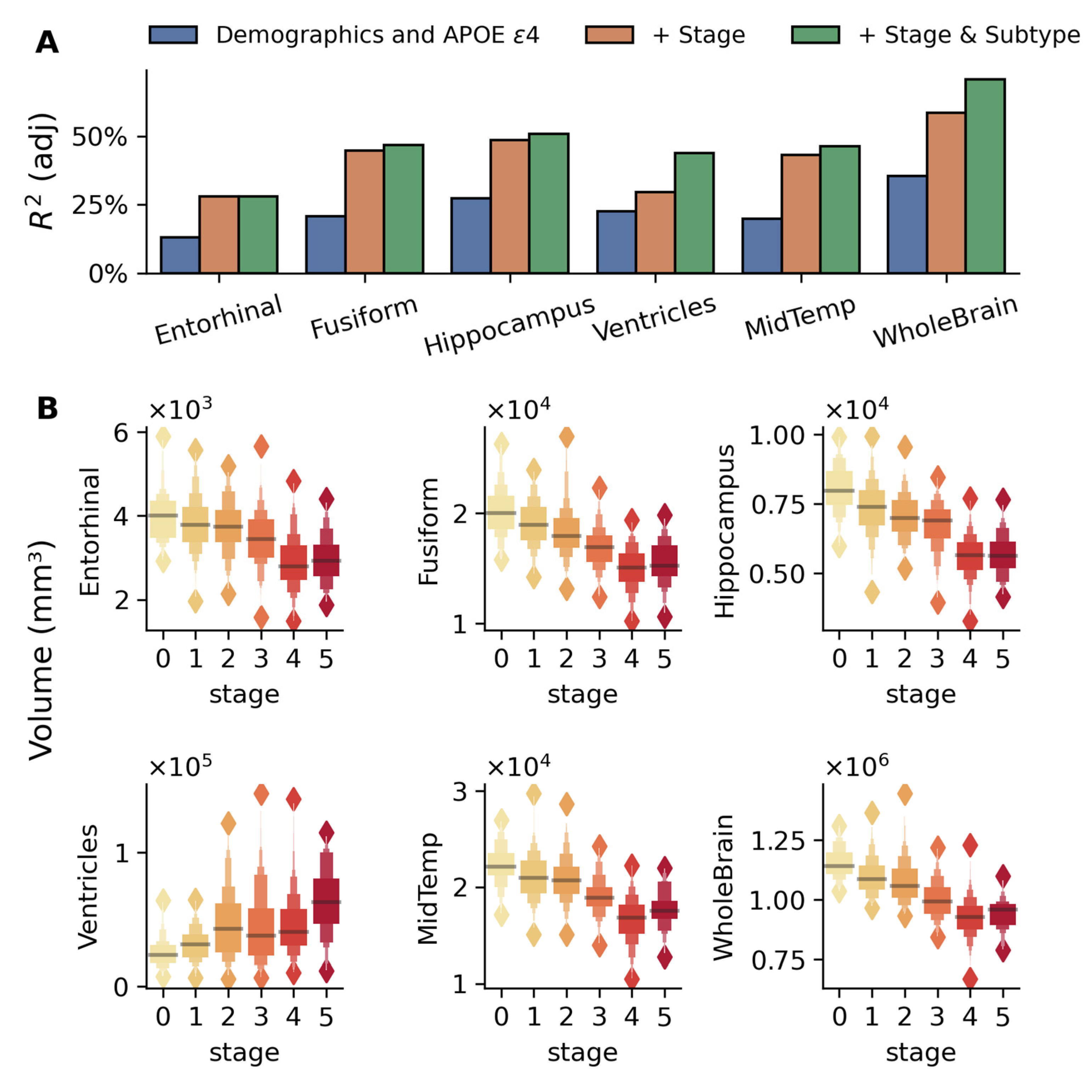
2.2.2. Regional Brain Volume Changes
2.3. Stage-Dependent Changes in Cognitive Performance
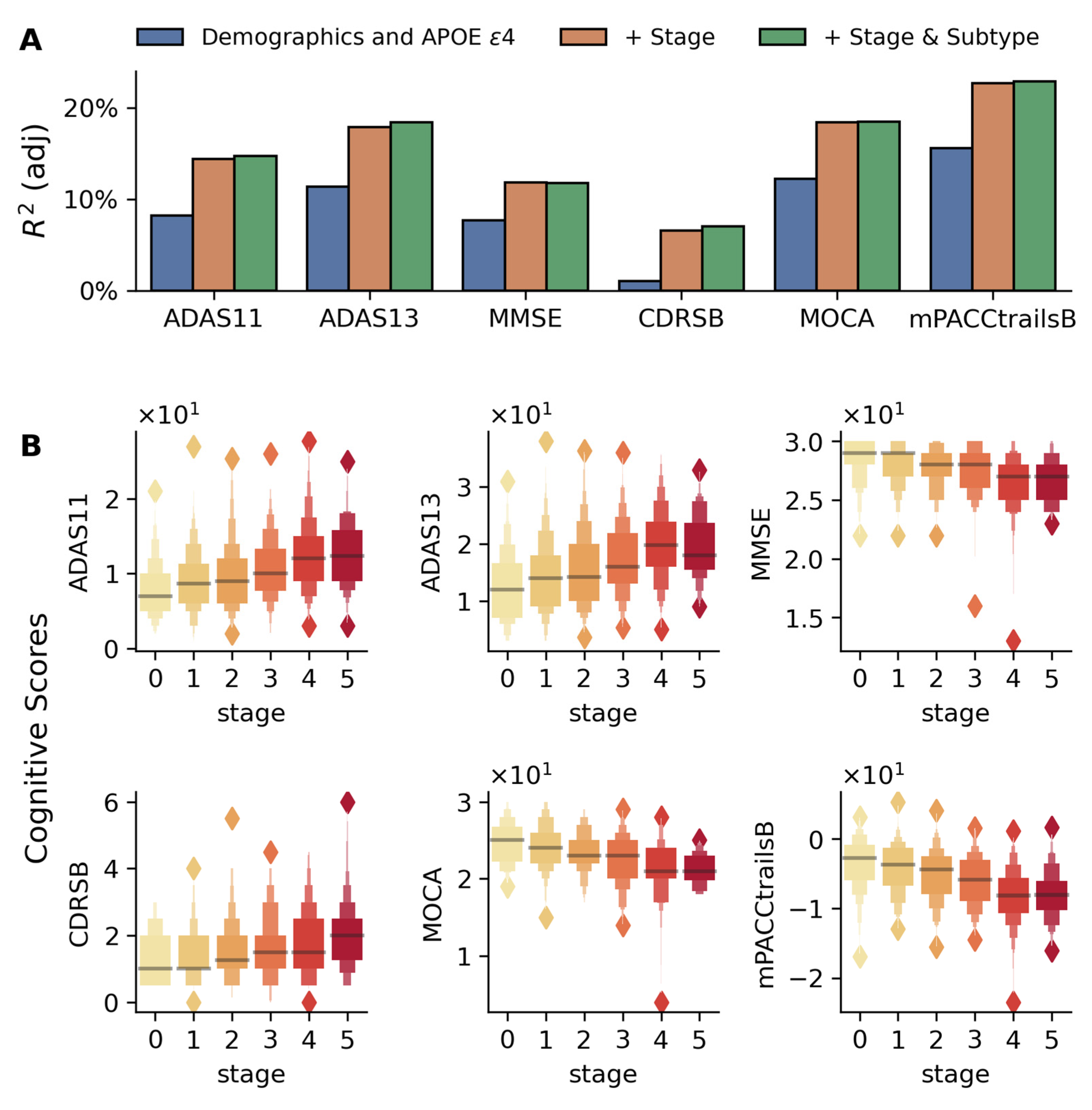
2.3.1. Global Cognitive Measures
2.3.2. Learning and Memory Tests
2.4. Visuospatial Processing, Motor Planning, and Executive Function
2.4.1. Trail Making Test (Processing Speed, Executive Control)
2.4.2. Boston Naming Test (Visual Recognition, Confrontational Naming)
2.4.3. Geometric Construction (Visuoconstructional Skills)
2.4.4. Clock Drawing (Visuoconstructional Skills and Executive Function)
2.5. Verbal Fluency and Semantic Memory
2.6. Premorbid Verbal Ability
2.7. Daily Function, Self-Awareness, and Well-Being
2.7.1. Activities of Daily Living and Self-Awareness
2.7.2. Mood and Life Satisfaction
3. Discussion
3.1. Prognostic Utility of a Data-Driven Disease Staging Framework
3.2. Novel Clinical Contributions and Model Scalability
3.3. Validation Across Independent Modalities and Cohorts
- Cognitive Performance: Stage-dependent decline was observed in global cognitive scores (Figure 4). Specific impairments in learning, memory (Figure 5), executive function, and semantic memory (Figure 6 and Figure S7) were consistent with hallmark patterns of AD. Notably, category fluency decline occurred primarily via reduced production rather than increased errors, with sex-specific effects evident in the vegetable category only.
3.4. Subtype-Specific Prognosis and Implications for Clinical Trials
- Subtype 1 (Subcortical-First Pattern): This subtype shows early involvement of subcortical structures (caudate, pallidum) and ventricular systems before affecting classical AD regions like the hippocampus and entorhinal cortex (which change in the final stage). This pattern suggests a vascular or mixed pathology variant, where subcortical changes may reflect cerebrovascular disease or different tau/amyloid deposition patterns. The late involvement of medial temporal structures aligns with better-preserved memory function observed in this subtype.
- Subtype 2 (Executive–Cortical Pattern): Early frontal and posterior cingulate involvement followed by classic medial temporal progression mirrors the “outside–in” cortical pattern described in atypical AD variants. The late-stage hippocampal/entorhinal changes suggest this represents an executive-predominant phenotype where tau pathology may follow different cortical networks before reaching classical memory circuits. This aligns with reports of AD patients presenting with executive dysfunction rather than memory impairment.
- Subtype 3 (Disconnection Pattern): The early corpus callosum and bilateral thalamic involvement reflects white matter tract vulnerability and connectivity hub disruption. This pattern suggests tau spreads via trans-synaptic mechanisms along major white matter pathways, consistent with the recent understanding of tau propagation through neural networks. The relatively late hippocampal involvement indicates preserved memory networks until advanced stages.
- Subtype 4 (Frontal–Executive Pattern): Extensive early frontal involvement with very late medial temporal changes represents the most atypical progression pattern. This may reflect primary age-related tauopathy (PART) or suspected non-Alzheimer pathophysiology (SNAP), where tau pathology predominantly affects frontal networks. The pattern resembles behavioral variant frontotemporal dementia in early stages, highlighting diagnostic challenges in atypical AD presentations.
3.5. Translational Value and Feasibility
3.6. Limitations and Future Directions
4. Materials and Methods
4.1. Study Data
4.2. Disease Progression Modeling
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAS | Alzheimer’s Disease Assessment Scale |
| AIC | Akaike Information Criterion |
| ANART | American National Adult Reading Test |
| CDR-SB | Clinical Dementia Rating Sum of Boxes |
| C-Index | Concordance Index |
| Cox PH | Cox Proportional Hazards Model |
| CSF | Cerebrospinal Fluid |
| Ecog-Pt | Everyday Cognition (Patient Score) |
| Ecog-SP | Everyday Cognition (Study Partner Score) |
| FAQ | Functional Activities Questionnaire |
| FDG | Fluorodeoxyglucose |
| MCI | Mild Cognitive Impairment |
| MoCA | Montreal Cognitive Assessment |
| MMSE | Mini-Mental State Examination |
| PACC | Preclinical Alzheimer’s Cognitive Composite |
| PET | Positron Emission Tomography |
| RAVLT | Rey’s Auditory Verbal Learning Test |
| s-SuStaIn | Scaling Subtype and Stage Inference |
| SuStaIn | Subtype and Stage Inference |
Appendix A
Appendix A.1
| Biomarker | Differences Across Stages | Difference Across Subtypes |
| CSF Aβ42 | Yes | Yes (Subtype 1 vs. Subtype 2) |
| FDG-PET | Yes | Yes (Subtype 1 vs. Subtype 2) |
| Brain Volumetrics | ||
| Entorhinal Cortex | Yes | No |
| Fusiform | Yes | Yes (Subtype 1 vs. Subtype 2) |
| Hippocampus | Yes | Yes (Subtype 1 vs. Subtype 4) |
| Middle Temporal | Yes | Yes (Subtype 1 vs. Subtype 2) |
| Ventricles | Yes | Yes (Subtype 1 vs. Subtype 2) (Subtype 1 vs. Subtype 3) (Subtype 1 vs. Subtype 4) |
| Whole Brain | Yes | Yes (Subtype 1 vs. Subtype 2) |
| Neurocognitive Assessments | ||
| ADAS11 | Yes | Yes (Subtype 1 vs. Subtype 4) |
| ADAS13 | Yes | Yes (Subtype 1 vs. Subtype 2) |
| MMSE | Yes | No |
| MoCA | Yes | No |
| mPACCTrailB | Yes | No |
| CDRSB | Yes | Yes (Subtype 1 vs. Subtype 2) |
Appendix A.2
| Characteristics | Subtype1 | Subtype2 | Subtype3 | Subtype4 | p-Value (χ2) |
| Psychiatric | 280 (35.4%) | 105 (30.9%) | 87 (32.7%) | 87 (33.1%) | 0.5 |
| Neurological (non-AD) | 227 (28.7%) | 107 (31.5%) | 72 (27.0%) | 79 (30.0%) | 0.64 |
| Head, Eyes, Ears, Nose and Throat | 471 (59.6%) | 223 (65.8%) | 173 (65.0%) | 173 (65.8%) | 0.1 |
| Cardiovascular | 520 (35.4%) | 243 (30.9%) | 184 (32.7%) | 172 (33.1%) | 0.2 |
| Respiratory | 167 (21.1%) | 74 (21.8%) | 56 (21.1%) | 59 (22.4%) | 0.9 |
| Hepatic | 20 (2.53%) | 17 (5.0%) | 13 (4.9%) | 12 (4.6%) | 0.1 |
| Dermatologic-Connective Tissue | 225 (28.5%) | 128 (37.8%) | 81 (30.5%) | 86 (32.69%) | 0.02 * |
| Musculoskeletal | 534 (67.6%) | 215 (63.4%) | 182 (68.4%) | 174 (66.1%) | 0.51 |
| Endocrine-Metabolic | 349 (44.2%) | 122 (36.0%) | 113 (42.5%) | 121 (46.0%) | 0.043 * |
| Gastrointestinal | 361 (45.7%) | 145 (42.8%) | 123 (46.2%) | 113 (43.0%) | 0.7 |
| Hematopoietic-Lymphatic | 66 (8.4%) | 28 (8.3%) | 25 (9.4%) | 29 (11.0%) | 0.057 |
| Renal-Genitourinary | 320 (40.5%) | 162 (47.8%) | 105 (39.5%) | 126 (47.9%) | 0.027 * |
| Allergies or Drug Sensitivities | 332 (42.0%) | 136 (40.1%) | 112 (42.1%) | 115 (43.7%) | 0.85 |
| Alcohol Abuse | 22 (2.8%) | 16 (4.7%) | 21 (7.9%) | 13 (4.9%) | 0.004 * |
| Drug Abuse | 8 (1.0%) | 3 (0.8%) | 1 (0.3%) | 1 (0.4%) | 0.63 |
| Smoking | 280 (35.4%) | 143 (42.2%) | 132 (49.6%) | 96 (36.5%) | 0.0003 ** |
| Malignancy | 154 (19.5%) | 86 (25.4%) | 72 (27.1%) | 73 (27.75%) | 0.0064 * |
| Skin and Appendages | 118 (14.9%) | 78 (23.0%) | 38 (14.3%) | 48 (18.3%) | 0.005 * |
| History of Hypertension | 369 (46.7%) | 180 (53.1%) | 139 (52.3%) | 113 (43.0%) | 0.035 * |
| Hachinski Score | |||||
| 0 | 391 | 147 | 117 | 139 | |
| 1 | 351 | 166 | 133 | 108 | |
| 2 | 33 | 12 | 4 | 9 | |
| 3 | 13 | 12 | 12 | 6 | |
| 4 | 2 | 2 | 0 | 1 | 0.048 |
References
- Habes, M.; Grothe, M.J.; Tunc, B.; McMillan, C.; Wolk, D.A.; Davatzikos, C. Disentangling Heterogeneity in Alzheimer’s Disease and Related Dementias Using Data-Driven Methods. Biol. Psychiatry 2020, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, N.M.E.; Tijms, B.M.; Koene, T.; Barkhof, F.; Teunissen, C.E.; Wolfsgruber, S.; Wagner, M.; Kornhuber, J.; Peters, O.; Cohn-Sheehy, B.I.; et al. Cognitive subtypes of probable Alzheimer’s disease robustly identified in four cohorts. Alzheimer’s Dement. 2017, 13, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Jackson, R.; Paul, G.; Shi, J.; Sabbagh, M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert. Opin. Investig. Drugs 2017, 26, 735–739. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Wiste, H.J.; Vemuri, P.; Weigand, S.D.; Senjem, M.L.; Zeng, G.; Bernstein, M.A.; Gunter, J.L.; Pankratz, V.S.; Aisen, P.S.; et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain 2010, 133, 3336–3348. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Mathys, H.; Boix, C.A.; Akay, L.A.; Xia, Z.; Davila-Velderrain, J.; Ng, A.P.; Jiang, X.; Abdelhady, G.; Galani, K.; Mantero, J.; et al. Single-cell multiregion dissection of Alzheimer’s disease. Nature 2024, 632, 858–868. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef]
- Xie, J.; Tandon, R.; Mitchell, C.S. Network Diffusion-Constrained Variational Generative Models for Investigating the Molecular Dynamics of Brain Connectomes Under Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 1062. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Wolk, D.A.; Alzheimer’s Disease Neuroimaging Initiative. Dysexecutive versus amnesic phenotypes of very mild Alzheimer’s disease are associated with distinct clinical, genetic and cortical thinning characteristics. J. Neurol. Neurosurg. Psychiatry 2011, 82, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fonteijn, H.M.; Clarkson, M.J.; Modat, M.; Barnes, J.; Lehmann, M.; Ourselin, S.; Fox, N.C.; Alexander, D.C. An event-based disease progression model and its application to familial Alzheimer’s disease. Inf. Process. Med. Imaging 2011, 22, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Kirkpatrick, A.; Mitchell, C.S. sEBM: Scaling Event Based Models to Predict Disease Progression via Implicit Biomarker Selection and Clustering. Inf. Process. Med. Imaging 2023, 13939, 208–221. [Google Scholar] [CrossRef]
- Young, A.L.; Marinescu, R.V.; Oxtoby, N.P.; Bocchetta, M.; Yong, K.; Firth, N.C.; Cash, D.M.; Thomas, D.L.; Dick, K.M.; Cardoso, J.; et al. Uncovering the heterogeneity and temporal complexity of neurodegenerative diseases with Subtype and Stage Inference. Nat. Commun. 2018, 9, 4273. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Lah, J.J.; Mitchell, C.S. s-SuStaIn: Scaling subtype and stage inference via simultaneous clustering of subjects and biomarkers. In Proceedings of the Fifth Conference on Health, Inference, and Learning, New York, NY, USA, 27–28 June 2024; pp. 461–476. [Google Scholar]
- Oxtoby, N.P.; Alexander, D.C.; Euro, P.C. Imaging plus X: Multimodal models of neurodegenerative disease. Curr. Opin. Neurol. 2017, 30, 371–379. [Google Scholar] [CrossRef]
- Tondo, G.; Carli, G.; Santangelo, R.; Mattoli, M.V.; Presotto, L.; Filippi, M.; Magnani, G.; Iannaccone, S.; Cerami, C.; Perani, D.; et al. Biomarker-based stability in limbic-predominant amnestic mild cognitive impairment. Eur. J. Neurol. 2021, 28, 1123–1133. [Google Scholar] [CrossRef]
- Young, A.L.; Oxtoby, N.P.; Garbarino, S.; Fox, N.C.; Barkhof, F.; Schott, J.M.; Alexander, D.C. Data-driven modelling of neurodegenerative disease progression: Thinking outside the black box. Nat. Rev. Neurosci. 2024, 25, 111–130. [Google Scholar] [CrossRef]
- Caminiti, S.P.; De Francesco, S.; Tondo, G.; Galli, A.; Redolfi, A.; Perani, D.; Alzheimer’s Disease Neuroimaging Initiative; Interceptor Project. FDG-PET markers of heterogeneity and different risk of progression in amnestic MCI. Alzheimers Dement. 2024, 20, 159–172. [Google Scholar] [CrossRef]
- Choi, S.E.; Mukherjee, S.; Gibbons, L.E.; Sanders, R.E.; Jones, R.N.; Tommet, D.; Mez, J.; Trittschuh, E.H.; Saykin, A.; Lamar, M.; et al. Development and validation of language and visuospatial composite scores in ADNI. Alzheimer’s Dement. 2020, 6, e12072. [Google Scholar] [CrossRef]
- Crane, P.K.; Carle, A.; Gibbons, L.E.; Insel, P.; Mackin, R.S.; Gross, A.; Jones, R.N.; Mukherjee, S.; Curtis, S.M.; Harvey, D.; et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012, 6, 502–516. [Google Scholar] [CrossRef]
- Chatzikostopoulos, A.; Moraitou, D.; Tsolaki, M.; Masoura, E.; Papantoniou, G.; Sofologi, M.; Papaliagkas, V.; Kougioumtzis, G.; Papatzikis, E. Episodic Memory in Amnestic Mild Cognitive Impairment (aMCI) and Alzheimer’s Disease Dementia (ADD): Using the “Doors and People” Tool to Differentiate between Early aMCI-Late aMCI-Mild ADD Diagnostic Groups. Diagnostics 2022, 12, 1768. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, D.; Gainotti, G.; Di Giuda, D.; Vita, M.G.; Cocciolillo, F.; Lacidogna, G.; Guglielmi, V.; Masullo, C.; Giordano, A.; Marra, C. Predicting progression of amnesic MCI: The integration of episodic memory impairment with perfusion SPECT. Psychiatry Res. Neuroimaging 2018, 271, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Warren, S.L.; Moustafa, A.A.; Alashwal, H.; Alzheimer’s Disease Neuroimaging Initiative. Harnessing forgetfulness: Can episodic-memory tests predict early Alzheimer’s disease? Exp. Brain Res. 2021, 239, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; Moss, M.B.; Tanzi, R.; Jones, K. Preclinical prediction of AD using neuropsychological tests. J. Int. Neuropsychol. Soc. 2001, 7, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ewers, M.; Walsh, C.; Trojanowski, J.Q.; Shaw, L.M.; Petersen, R.C.; Jack, C.R., Jr.; Feldman, H.H.; Bokde, A.L.; Alexander, G.E.; Scheltens, P.; et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol. Aging 2012, 33, 1203–1214. [Google Scholar] [CrossRef]
- Knesevich, J.W.; LaBarge, E.; Edwards, D. Predictive value of the Boston Naming Test in mild senile dementia of the Alzheimer type. Psychiatry Res. 1986, 19, 155–161. [Google Scholar] [CrossRef]
- Pravata, E.; Tavernier, J.; Parker, R.; Vavro, H.; Mintzer, J.E.; Spampinato, M.V. The neural correlates of anomia in the conversion from mild cognitive impairment to Alzheimer’s disease. Neuroradiology 2016, 58, 59–67. [Google Scholar] [CrossRef]
- Buchhave, P.; Stomrud, E.; Warkentin, S.; Blennow, K.; Minthon, L.; Hansson, O. Cube copying test in combination with rCBF or CSF A beta 42 predicts development of Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2008, 25, 544–552. [Google Scholar] [CrossRef]
- Palmqvist, S.; Hansson, O.; Minthon, L.; Londos, E. The usefulness of cube copying for evaluating treatment of Alzheimer’s disease. Am. J. Alzheimer’s Dis. Other Dement. 2008, 23, 439–446. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, E.S.; Jeong, S.H.; Sohn, E.H.; Lee, T.Y.; Lee, A.Y. Longitudinal changes in clock drawing test (CDT) performance according to dementia subtypes and severity. Arch. Gerontol. Geriatr. 2011, 53, e179–e182. [Google Scholar] [CrossRef]
- Umegaki, H.; Suzuki, Y.; Yamada, Y.; Komiya, H.; Watanabe, K.; Nagae, M.; Kuzuya, M. Association of the Qualitative Clock Drawing Test with Progression to Dementia in Non-Demented Older Adults. J. Clin. Med. 2020, 9, 2850. [Google Scholar] [CrossRef]
- Sakurai, H.; Hanyu, H.; Murakami, M.; Kume, K.; Takata, Y.; Onuma, T.; Akai, T.; Iwamoto, T. The category “animals” is more appropriate than the category “vegetables” to measure semantic category fluency. Geriatr. Gerontol. Int. 2011, 11, 374–375. [Google Scholar] [CrossRef] [PubMed]
- McGurn, B.; Starr, J.M.; Topfer, J.A.; Pattie, A.; Whiteman, M.C.; Lemmon, H.A.; Whalley, L.J.; Deary, I.J. Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology 2004, 62, 1184–1186. [Google Scholar] [CrossRef]
- Devanand, D.P.; Liu, X.; Brown, P.J. Impact of Functional Deficits in Instrumental Activities of Daily Living in Mild Cognitive Impairment: A Clinical Algorithm to Predict Progression to Dementia. Alzheimer Dis. Assoc. Disord. 2017, 31, 55–61. [Google Scholar] [CrossRef]
- Vik, A.; Kocinski, M.; Rye, I.; Lundervold, A.J.; Lundervold, A.S. Functional activity level reported by an informant is an early predictor of Alzheimer’s disease. BMC Geriatr. 2023, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Thabtah, F.; Spencer, R.; Ye, Y. The correlation of everyday cognition test scores and the progression of Alzheimer’s disease: A data analytics study. Health Inf. Sci. Syst. 2020, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Lo, R.Y.; Hubbard, A.E.; Shaw, L.M.; Trojanowski, J.Q.; Petersen, R.C.; Aisen, P.S.; Weiner, M.W.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging Initiative. Longitudinal change of biomarkers in cognitive decline. Arch. Neurol. 2011, 68, 1257–1266. [Google Scholar] [CrossRef]
- Seppala, T.T.; Koivisto, A.M.; Hartikainen, P.; Helisalmi, S.; Soininen, H.; Herukka, S.K. Longitudinal changes of CSF biomarkers in Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 25, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Levey, A.I.; Lah, J.J.; Seyfried, N.T.; Mitchell, C.S. Machine Learning Selection of Most Predictive Brain Proteins Suggests Role of Sugar Metabolism in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 92, 411–424. [Google Scholar] [CrossRef]
- Tandon, R.; Zhao, L.; Watson, C.M.; Sarkar, N.; Elmor, M.; Heilman, C.; Sanders, K.; Hales, C.M.; Yang, H.; Loring, D.W.; et al. Stratifying risk of Alzheimer’s disease in healthy middle-aged individuals with machine learning. Brain Commun. 2025, 7, fcaf121. [Google Scholar] [CrossRef]
- Shand, C.; Markiewicz, P.J.; Cash, D.M.; Alexander, D.C.; Donohue, M.C.; Barkhof, F.; Oxtoby, N.P. Heterogeneity in Preclinical Alzheimer’s Disease Trial Cohort Identified by Image-based Data-Driven Disease Progression Modelling. medRxiv 2023. [Google Scholar] [CrossRef]
- Marinescu, R.V.; Oxtoby, N.P.; Young, A.L.; Bron, E.E.; Toga, A.W.; Weiner, M.W.; Barkhof, F.; Fox, N.C.; Golland, P.; Klein, S.; et al. TADPOLE Challenge: Accurate Alzheimer’s disease prediction through crowdsourced forecasting of future data. In Predictive Intelligence in Medicine; Springer: Cham, Switzerland, 2019; Volume 11843, pp. 1–10. [Google Scholar] [CrossRef]
- Young, A.L.; Oxtoby, N.P.; Daga, P.; Cash, D.M.; Fox, N.C.; Ourselin, S.; Schott, J.M.; Alzheimer’s Disease Neuroimaging Initiative. A data-driven model of biomarker changes in sporadic Alzheimer’s disease. Brain 2014, 137, 2564–2577. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Nordberg, A.; Westman, E. Biological subtypes of Alzheimer disease: A systematic review and meta-analysis. Neurology 2020, 94, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Aksman, L.M.; Wijeratne, P.A.; Oxtoby, N.P.; Eshaghi, A.; Shand, C.; Altmann, A.; Alexander, D.C.; Young, A.L. pySuStaIn: A Python implementation of the Subtype and Stage Inference algorithm. SoftwareX 2021, 16, 100811. [Google Scholar] [CrossRef]
- Eshaghi, A.; Young, A.L.; Wijeratne, P.A.; Prados, F.; Arnold, D.L.; Narayanan, S.; Guttmann, C.R.G.; Barkhof, F.; Alexander, D.C.; Thompson, A.J.; et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat. Commun. 2021, 12, 2078. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandon, R.; Mei, Y.; Lah, J.J.; Mitchell, C.S. Comprehensive Anatomical Staging Predicts Clinical Progression in Mild Cognitive Impairment: A Data-Driven Approach. Int. J. Mol. Sci. 2025, 26, 5514. https://doi.org/10.3390/ijms26125514
Tandon R, Mei Y, Lah JJ, Mitchell CS. Comprehensive Anatomical Staging Predicts Clinical Progression in Mild Cognitive Impairment: A Data-Driven Approach. International Journal of Molecular Sciences. 2025; 26(12):5514. https://doi.org/10.3390/ijms26125514
Chicago/Turabian StyleTandon, Raghav, Yajun Mei, James J. Lah, and Cassie S. Mitchell. 2025. "Comprehensive Anatomical Staging Predicts Clinical Progression in Mild Cognitive Impairment: A Data-Driven Approach" International Journal of Molecular Sciences 26, no. 12: 5514. https://doi.org/10.3390/ijms26125514
APA StyleTandon, R., Mei, Y., Lah, J. J., & Mitchell, C. S. (2025). Comprehensive Anatomical Staging Predicts Clinical Progression in Mild Cognitive Impairment: A Data-Driven Approach. International Journal of Molecular Sciences, 26(12), 5514. https://doi.org/10.3390/ijms26125514






