The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes
Abstract
1. Introduction
2. Results
2.1. Characteristics of Studied Subgroups
2.2. Laboratory Examination
2.3. Biomarkers Examination
2.4. Pulsatility and Blood Pressure Indices
2.5. Correlations Between Variables
2.5.1. Correlations Between Lipids and Biomarkers
2.5.2. Correlations Between Age and Biomarkers
2.5.3. Correlations Between Pulsatility Indices, Age, and Lipids
2.5.4. Correlations Between Pulsatility Indices and Biomarkers
3. Discussion
Limitations
4. Materials and Methods
4.1. Study Design and Population
4.2. Laboratory Analyses
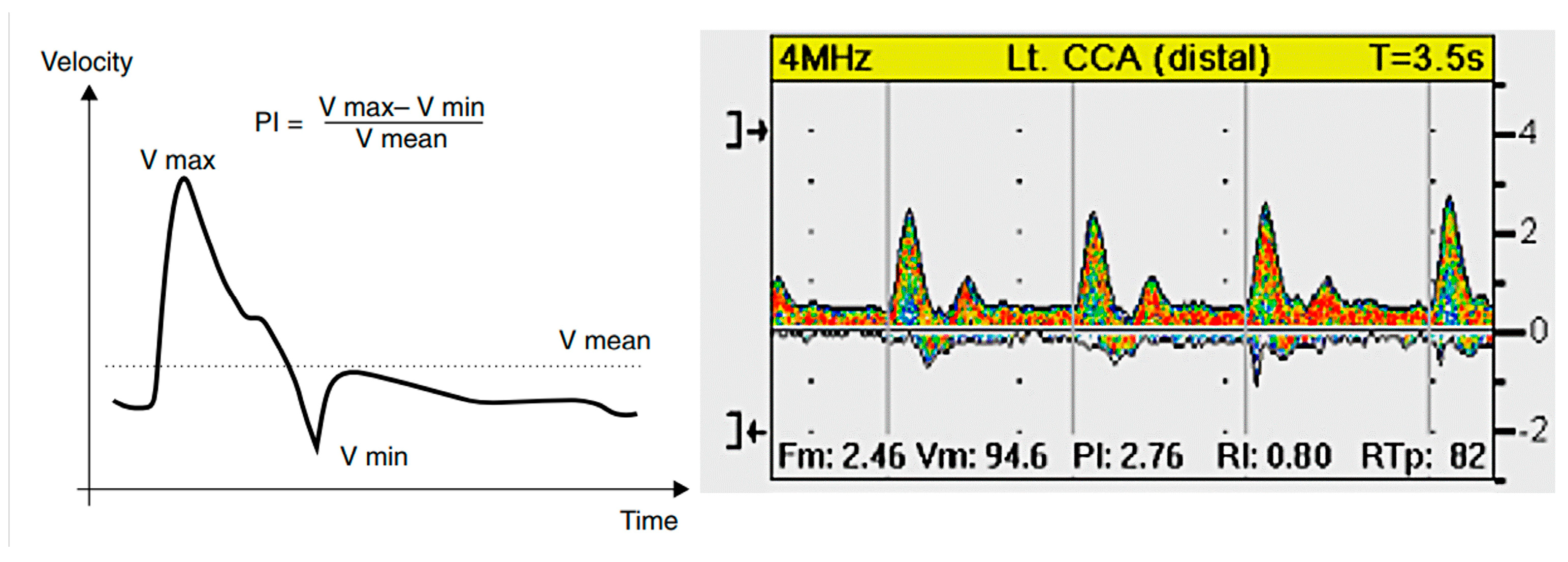
4.3. Pulsatility and Blood Pressure Indices
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Damm, J.A.; Dalgas-Madsen, A.; Bech, A.M.K.; Pilgaard, K.A.; Pociot, F.; Hansen, T.W.; Johannesen, J. Associations Between Arterial Stiffness and Metabolic Target in Children and Adolescents with Type 1 Diabetes Treated in a Modern Setting. Pediatr. Diabetes 2024, 2024, 5528717. [Google Scholar] [CrossRef] [PubMed]
- Naskret, D.; Kulecki, M.; Kaminski, M.; Kasprzak, D.; Lachowski, P.; Flotynska, J.; Anderko, I.; Lasowski, B.; Zozulinska-Ziolkiewicz, D.; Uruska, A. How to Improve Cardiovascular Risk Assessment in Type 1 Diabetes? The Role of Vascular Age and Arterial Stiffness. Pol. Arch. Intern. Med. 2025, 135, 16956. [Google Scholar] [CrossRef]
- Carvalho-Ribeiro, I.A.; Cunha, L.C.F.; Ribeiro, L.R.; Lima, M.N.; Ferreira-Silva, B.A.; Rajão, J.S.; Bittencourt, J.C.; Pinheiro, J.F.A.; Rodrigues-Machado, M.d.G. Adolescents and Young Adults with Type 1 Diabetes Present Changes in Arterial Compliance and Resistance and Increased Arterial Stiffness. Pediatr. Diabetes 2024, 2024, 9919121. [Google Scholar] [CrossRef]
- Brady, R.P.; Urbina, E.M.; Gao, Z.; Dabelea, D.; Lustigova, E.; Marcovina, S.; Mottl, A.K.; Pihoker, C.; Reynolds, K.; Sancrainte, L.; et al. Arterial Stiffness Is Related to Diabetes–Associated Microvascular Complications: The SEARCH for Diabetes in Youth Study. Diabetes Care 2025, 48, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Teixeira, A.; Cardoso, J.; Borges, T.; Caldas Afonso, A.; Correia-Costa, L. Association between Proinflammatory Cytokines and Arterial Stiffness in Type 1 Diabetic Adolescents. J. Pediatr. Endocrinol. Metab. 2024, 37, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Marcon, D.; Tagetti, A.; Piona, C.; Giontella, A.; Bortolotti, S.; Bonafini, S.; Carletti, L.; Morandi, A.; Trombetta, M.; Maffeis, C.; et al. Markers of Subclinical Vascular Damage in Young Adults with Type 1 Diabetes Mellitus: The Role of Central Blood Pressure. J. Hypertens. 2022, 40, 2469–2475. [Google Scholar] [CrossRef]
- Tougaard, N.H.; Theilade, S.; Winther, S.A.; Tofte, N.; Ahluwalia, T.S.; Hansen, T.W.; Rossing, P.; Frimodt-Møller, M. Carotid-Femoral Pulse Wave Velocity as a Risk Marker for Development of Complications in Type 1 Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e017165. [Google Scholar] [CrossRef]
- Dahl-Jørgensen, K.; Larsen, J.R.; Hanssen, K.F. Atherosclerosis in Childhood and Adolescent Type 1 Diabetes: Early Disease, Early Treatment? Diabetologia 2005, 48, 1445–1453. [Google Scholar] [CrossRef]
- Bharathy, P.S.; Delhikumar, C.G.; Rajappa, M.; Sahoo, J.; Anantharaj, A. Early Markers of Atherosclerosis in Children and Adolescents with Type 1 Diabetes Mellitus. Indian. J. Pediatr. 2023, 90, 227–232. [Google Scholar] [CrossRef]
- Shah, A.S.; Wadwa, R.P.; Dabelea, D.; Hamman, R.F.; D’Agostino, R.; Marcovina, S.; Daniels, S.R.; Dolan, L.M.; Fino, N.F.; Urbina, E.M. Arterial Stiffness in Adolescents and Young Adults with and without Type 1 Diabetes: The SEARCH CVD Study. Pediatr. Diabetes 2015, 16, 367–374. [Google Scholar] [CrossRef]
- Dost, A.; Molz, E.; Krebs, A.; Bechtold, S.; Kapellen, T.; Rohrer, T.; Raile, K.; Fritsch, M.; Schwab, K.O.; Holl, R. Pulse Pressure in Children and Adolescents with Type 1 Diabetes Mellitus in Germany and Austria. Pediatr. Diabetes 2014, 15, 236–243. [Google Scholar] [CrossRef]
- Luirink, I.K.; Wiegman, A.; Kusters, D.M.; Hof, M.H.; Groothoff, J.W.; de Groot, E.; Kastelein, J.J.P.; Hutten, B.A. 20–Year Follow–up of Statins in Children with Familial Hypercholesterolemia. N. Engl. J. Med. 2019, 381, 1547–1556. [Google Scholar] [CrossRef]
- Urbina, E.M.; Wadwa, R.P.; Davis, C.; Snively, B.M.; Dolan, L.M.; Daniels, S.R.; Hamman, R.F.; Dabelea, D. Prevalence of Increased Arterial Stiffness in Children with Type 1 Diabetes Mellitus Differs by Measurement Site and Sex: The SEARCH for Diabetes in Youth Study. J. Pediatr. 2010, 156, 731–737.e1. [Google Scholar] [CrossRef]
- Miller, R.G.; Orchard, T.J.; Costacou, T. Risk Factors Differ by First Manifestation of Cardiovascular Disease in Type 1 Diabetes. Diabetes Res. Clin. Pract. 2020, 163, 108141. [Google Scholar] [CrossRef] [PubMed]
- Efficacy of Cholesterol–Lowering Therapy in 18,686 People with Diabetes in 14 Randomised Trials of Statins: A Meta–Analysis. Lancet 2008, 371, 117–125. [CrossRef] [PubMed]
- Mysliwiec, M.; Bandura, M.; Anna Wołoszyn-Durkiewicz, A.; Hennig, M.; Walczak, M.; Peregut Pogorzelski, J.; Sykut-Cegielska, J.; Miszczak-Knecht, M.; Chlebus, K.; Wasąg, B.; et al. 2024 Polish Recommendations for the Management of Familial Hypercholesterolemia in Children and Adolescents. Arch. Med. Sci. 2024, 20, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Maahs, D.M.; Ogden, L.G.; Dabelea, D.; Snell-Bergeon, J.K.; Daniels, S.R.; Hamman, R.F.; Rewers, M. Association of Glycaemia with Lipids in Adults with Type 1 Diabetes: Modification by Dyslipidaemia Medication. Diabetologia 2010, 53, 2518–2525. [Google Scholar] [CrossRef]
- Rawshani, A.; Sattar, N.; Franzén, S.; Rawshani, A.; Hattersley, A.T.; Svensson, A.M.; Eliasson, B.; Gudbjörnsdottir, S. Excess Mortality and Cardiovascular Disease in Type 1 Diabetes in Relation to Age at Onset: A Nationwide Study of 27,195 Young Adults with Diabetes. Lancet 2018, 392, 477. [Google Scholar] [CrossRef]
- Skrivarhaug, T.; Bangstad, H.-J.; Stene, L.C.; Sandvik, L.; Hanssen, K.F.; Joner, G. Long–Term Mortality in a Nationwide Cohort of Childhood–Onset Type 1 Diabetic Patients in Norway. Diabetologia 2006, 49, 298–305. [Google Scholar] [CrossRef]
- Davies, M.J.; Gordon, J.L.; Gearing, A.J.H.; Pigott, R.; Woolf, N.; Katz, D.; Kyriakopoulos, A. The Expression of the Adhesion Molecules ICAM-1, VCAM–1, PECAM, and E–selectin in Human Atherosclerosis. J. Pathol. 1993, 171, 223–229. [Google Scholar] [CrossRef]
- O’Brien, K.D.; McDonald, T.O.; Chait, A.; Allen, M.D.; Alpers, C.E. Neovascular Expression of E–Selectin, Intercellular Adhesion Molecule–1, and Vascular Cell Adhesion Molecule–1 in Human Atherosclerosis and Their Relation to Intimal Leukocyte Content. Circulation 1996, 93, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Cook-Mills, J.M.; Marchese, M.E.; Abdala-Valencia, H. Vascular Cell Adhesion Molecule–1 Expression and Signaling During Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid. Redox Signal. 2011, 15, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Jha, H.C.; Divya, A.; Prasad, J.; Mittal, A. Plasma Circulatory Markers in Male and Female Patients with Coronary Artery Disease. Heart Lung 2010, 39, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.T.W.; (Smith) Tomic, M.M.; Chen, P.C.Y.; Miguelino, E.; Li, C.-S.; Devaraj, S. Correlation of Microvascular Abnormalities and Endothelial Dysfunction in Type–1 Diabetes Mellitus (T1DM): A Real–Time Intravital Microscopy Study. Clin. Hemorheol. Microcirc. 2009, 42, 285–295. [Google Scholar] [CrossRef]
- Jones, S.C.; Banks, R.E.; Haidar, A.; Gearing, A.J.; Hemingway, I.K.; Ibbotson, S.H.; Dixon, M.F.; Axon, A.T. Adhesion Molecules in Inflammatory Bowel Disease. Gut 1995, 36, 724–730. [Google Scholar] [CrossRef]
- Øymar, K.; Bjerknes, R. Differential Patterns of Circulating Adhesion Molecules in Children with Bronchial Asthma and Acute Bronchiolitis. Pediatr. Allergy Immunol. 1998, 9, 73–79. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, Y.; Lu, Z.; Luo, C.; Shi, Y.; Zeng, Q.; Cao, Y.; Liu, L.; Wang, X.; Ji, Q. Decreased Plasma IL-35 Levels Are Related to the Left Ventricular Ejection Fraction in Coronary Artery Diseases. PLoS ONE 2012, 7, e52490. [Google Scholar] [CrossRef]
- Sha, X.; Meng, S.; Li, X.; Xi, H.; Maddaloni, M.; Pascual, D.W.; Shan, H.; Jiang, X.; Wang, H.; Yang, X. Interleukin–35 Inhibits Endothelial Cell Activation by Suppressing MAPK–AP–1 Pathway. J. Biol. Chem. 2015, 290, 19307–19318. [Google Scholar] [CrossRef] [PubMed]
- Park, A.C.; Huang, G.; Jankowska-Gan, E.; Massoudi, D.; Kernien, J.F.; Vignali, D.A.; Sullivan, J.A.; Wilkes, D.S.; Burlingham, W.J.; Greenspan, D.S. Mucosal Administration of Collagen V Ameliorates the Atherosclerotic Plaque Burden by Inducing Interleukin 35–Dependent Tolerance. J. Biol. Chem. 2016, 291, 3359–3370. [Google Scholar] [CrossRef]
- Yan, A.; Zhang, Y.; Wang, X.; Cui, Y.; Tan, W. Interleukin 35 Regulates Interleukin 17 Expression and T Helper 17 in Patients with Proliferative Diabetic Retinopathy. Bioengineered 2022, 13, 13293–13299. [Google Scholar] [CrossRef]
- Giannopoulou, E.Z.; Doundoulakis, I.; Antza, C.; Christoforidis, A.; Haidich, A.B.; Kotsis, V.; Stabouli, S. Subclinical Arterial Damage in Children and Adolescents with Type 1 Diabetes: A Systematic Review and Meta–Analysis. Pediatr. Diabetes 2019, 20, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Neubauer-Geryk, J.; Wielicka, M.; Kozera, G.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. Common Carotid Pulsatility Is Deteriorated by Autoimmune Thyroiditis in Children with Type 1 Diabetes Mellitus—A Pilot Study. Physiol. Rep. 2020, 8, e14518. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, M.; Urbano, F.; Filippello, A.; Di Mauro, S.; Scamporrino, A.; Miano, N.; Coppolino, G.; L’Episcopo, G.; Leggio, S.; Scicali, R.; et al. Increased Platelet Reactivity and Proinflammatory Profile Are Associated with Intima–Media Thickness and Arterial Stiffness in Prediabetes. J. Clin. Med. 2022, 11, 2870. [Google Scholar] [CrossRef]
- Panova-Noeva, M.; Arnold, N.; Hermanns, M.I.; Prochaska, J.H.; Schulz, A.; Spronk, H.M.; Binder, H.; Pfeiffer, N.; Beutel, M.; Blankenberg, S.; et al. Mean Platelet Volume and Arterial Stiffness–Clinical Relationship and Common Genetic Variability. Sci. Rep. 2017, 7, 40229. [Google Scholar] [CrossRef]
- Smigoc Schweiger, D.; Battelino, T.; Groselj, U. Sex–Related Differences in Cardiovascular Disease Risk Profile in Children and Adolescents with Type 1 Diabetes. Int. J. Mol. Sci. 2021, 22, 10192. [Google Scholar] [CrossRef]
- Martínez, D.; Castro, A.; Merino, P.M.; López, P.; Lardone, M.C.; Iñiguez, G.; Cassorla, F.; Codner, E. Oestrogen Activity of the Serum in Adolescents with Type 1 Diabetes. Diabet. Med. 2016, 33, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Marshall, Z.A.; Mackintosh, K.A.; Lewis, M.J.; Ellins, E.A.; McNarry, M.A. Association of Physical Activity Metrics with Indicators of Cardiovascular Function and Control in Children with and without Type 1 Diabetes. Pediatr. Diabetes 2021, 22, 320–328. [Google Scholar] [CrossRef]
- Marshall, Z.A.; Mackintosh, K.A.; Gregory, J.W.; McNarry, M.A. Using Compositional Analysis to Explore the Relationship between Physical Activity and Cardiovascular Health in Children and Adolescents with and without Type 1 Diabetes. Pediatr. Diabetes 2022, 23, 115–125. [Google Scholar] [CrossRef]
- Castro-Correia, C.; Moura, C.; Mota, C.; Santos-Silva, R.; Areias, J.C.; Calhau, C.; Fontoura, M. Arterial Stiffness in Children and Adolescents with and without Continuous Insulin Infusion. J. Pediatr. Endocrinol. Metab. 2019, 32, 837–841. [Google Scholar] [CrossRef]
- Libman, I.; Haynes, A.; Lyons, S.; Pradeep, P.; Rwagasor, E.; Tung, J.Y.; Jefferies, C.A.; Oram, R.A.; Dabelea, D.; Craig, M.E. Clinical Practice Consensus Guidelines 2022: Definition, Epidemiology, and Classification of Diabetes in Children and Adolescents. Pediatr. Diabetes 2022, 23, 1160–1174. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Wielicka, M.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Impact of Metabolic Memory on Immune Profile in Young Patients with Uncomplicated Type 1 Diabetes. Int. J. Mol. Sci. 2024, 25, 3190. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; McCoy, R.G.; Aleppo, G.; Balapattabi, K.; Beverly, E.A.; Early, B.; Bruemmer, D.; Echouffo-Tcheugui, J.B.; Ekhlaspour, L.; Garg, R.; et al. 6. Glycemic Goals and Hypoglycemia: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48, S128–S145. [Google Scholar] [CrossRef]
- Neubauer-Geryk, J.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. Soluble P–Selectin as an Indicator of Cutaneous Microangiopathy in Uncomplicated Young Patients with Type 1 Diabetes. Life 2024, 14, 1587. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| A Group (LDL < 100 mg/dL) n = 24 | B Group (LDL ≥ 100 mg/dL) n = 35 | ||
| Males, n (%) | 12 (50) | 15 (42.9) | 0.56 |
| BMI [kg/m2] | 20.4 (16.8–29.6) | 20.3 (14.5–26.8) | 0.9 |
| Age [years] | 16.1 (11.3–18)/16 ± 1.8 | 14.9 (8.4–18.0)/14.8 ± 2.5 | 0.1 |
| Onset of diabetes [age] | 9.6 (1.8–13.6)/8.5 ± 4.1 | 7.1 (1.2–12.7)/6.8 ± 3.3 | 0.1 |
| Diabetes duration [years] | 6.9 (1.2–15.9)/7.5 ± 4.6 | 8.7 (1.7–14.4)/8.1 ± 3.4 | 0.5 |
| Insulin dose units/24 h | 45 (21–70) | 45 (20–100) | 0.9 |
| Insulin dose units/kg | 0.8 (0.4–1.1) | 0.8 (0.5–1.4) | 0.13 |
| Treatment with pump [%] | 50 (0–100) | 78 (0–98) | 0.45 |
| HbA1c current [%] | 7.8 (6.2–11.3) | 8.1 (5.9–13.4) | 0.3 |
| Episodes of mild hypoglycemia [N/last month] | 10 (2–15) | 10 (0–30) | 0.83 |
| Episodes of severe hypoglycemia [N/last year] | 0 (0–1) | 0 (0–1) | 0.74 |
| Celiac disease, n [%] | 2 (8.3) | 4 (11.4) | 0.99 |
| Autoimmune thyroiditis, n [%] | 5 (21) | 7 (20) | 0.80 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| A Group (LDL < 100 mg/dL) n = 24 | B Group (LDL ≥ 100 mg/dL) n = 35 | ||
| CRP [mg/L] | 0.3 (0.1–1.3) | 0.4 (0.1–4.9) | 0.5 |
| Serum creatinine [mg/dL] | 0.77 (0.5–0.95) | 0.6 (0.5–0.9) | 0.03 |
| Creatinine clearance [mL/min] | 98 (77–117) | 107 (78–147) | 0.08 |
| Albuminuria [mg/dL] | 7.1 (2.5–88) | 6.7 (2.5–27) | 0.5 |
| Total cholesterol [mg/dL] | 148 (125–190) | 193 (159–288) | <0.001 |
| Cholesterol LDL [mg/dL] | 89 (61–99) | 118 (102–188) | <0.001 |
| Cholesterol HDL [mg/dL] | 54 (33–83) | 60 (39–90) | 0.18 |
| Triglycerides [mg/dL] | 69 (34–154) | 78 (38–294) | 0.24 |
| TSH [mlU/L] | 1.9 (0.7–4.2) | 1.9 (1–5.1) | 0.7 |
| fT4 [pmol/L] | 12.9 (10.8–15.0) | 12.5 (9–15) | 0.3 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| A Group (LDL < 100 mg/dL) n = 24 | B Group (LDL ≥ 100 mg/dL) n = 35 | ||
| Anti–inflammatory cytokines | |||
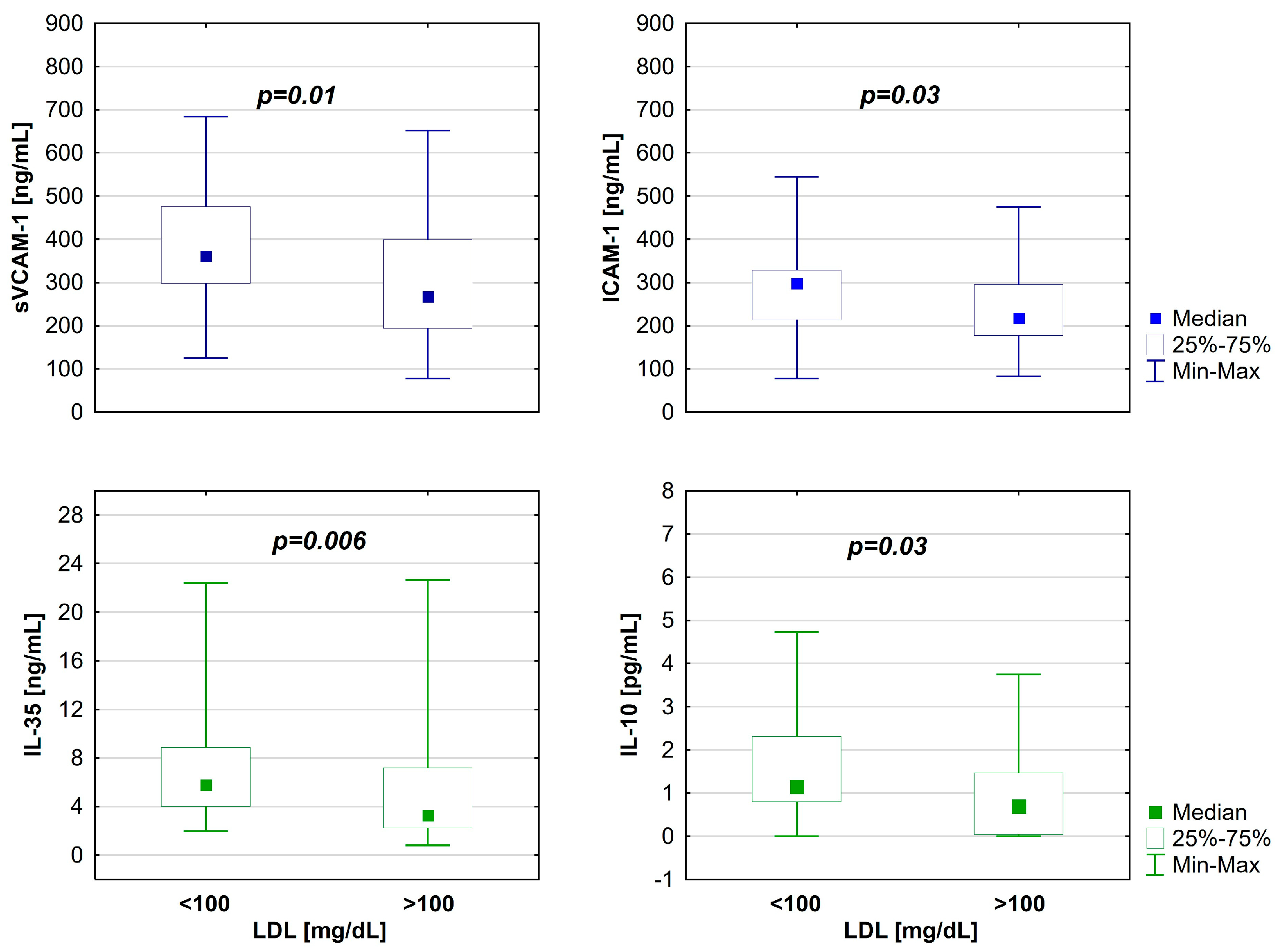
| IL-35 [ng/mL] | 5.8 (2–22.4) | 3.3 (0.8–22.7) | 0.006 |
| IL-4 [pg/mL] | 5.7 (0–29) | 6.4 (0–22.9) | 0.59 |
| IL-10 [pg/mL] | 1.2 (0–4.7) | 0.7 (0–3.8) | 0.03 |
| Pro–inflammatory cytokines | |||
| TNF–α [pg/mL] | 1.7 (0–6.8) | 2.5 (0–5.9) | 0.45 |
| IL-12 [pg/mL] | 3.0 (0–16.3) | 2.5 (0–15.7) | 0.85 |
| IL-18 [pg/mL] | 65 (34.6–146) | 77.2 (44.8–127.8) | 0.26 |
| ratio TNF–α/IL-35 | 0.28 (0–3.44) | 0.76 (0–2.97) | 0.058 |
| Vascular and inflammatory biomarkers | |||
| Serum angiogenin [ng/mL] | 307.5 (112–985) | 294.2 (124–799.6) | 0.82 |
| VEGF [pg/mL] | 232.2 (43–465) | 212.8 (55.6–546.2) | 0.63 |
| sVCAM–1 [ng/mL] | 361.5 (124.8–683.7) | 267 (76.9–652) | 0.01 |
| ICAM-1 [ng/mL] | 297 (77.9–544.6) | 217.2 (82.7–475.5) | 0.03 |
| sP–Selectin [ng/mL] | 293.9 (98.4–731.6) | 275 (107–625) | 0.61 |
| AGEs [pg/mL] | 18,563 (6356–39,420) | 19,340 (5480–39,870) | 0.78 |
| sRAGE [pg/mL] | 1336 (1072.9–2482.9) | 1286.8 (788.3–2482.9) | 0.65 |
| Characteristics | Diabetic Patients | p | |
|---|---|---|---|
| A Group (LDL < 100 mg/dL) n = 24 | B Group (LDL ≥ 100 mg/dL) n = 35 | ||
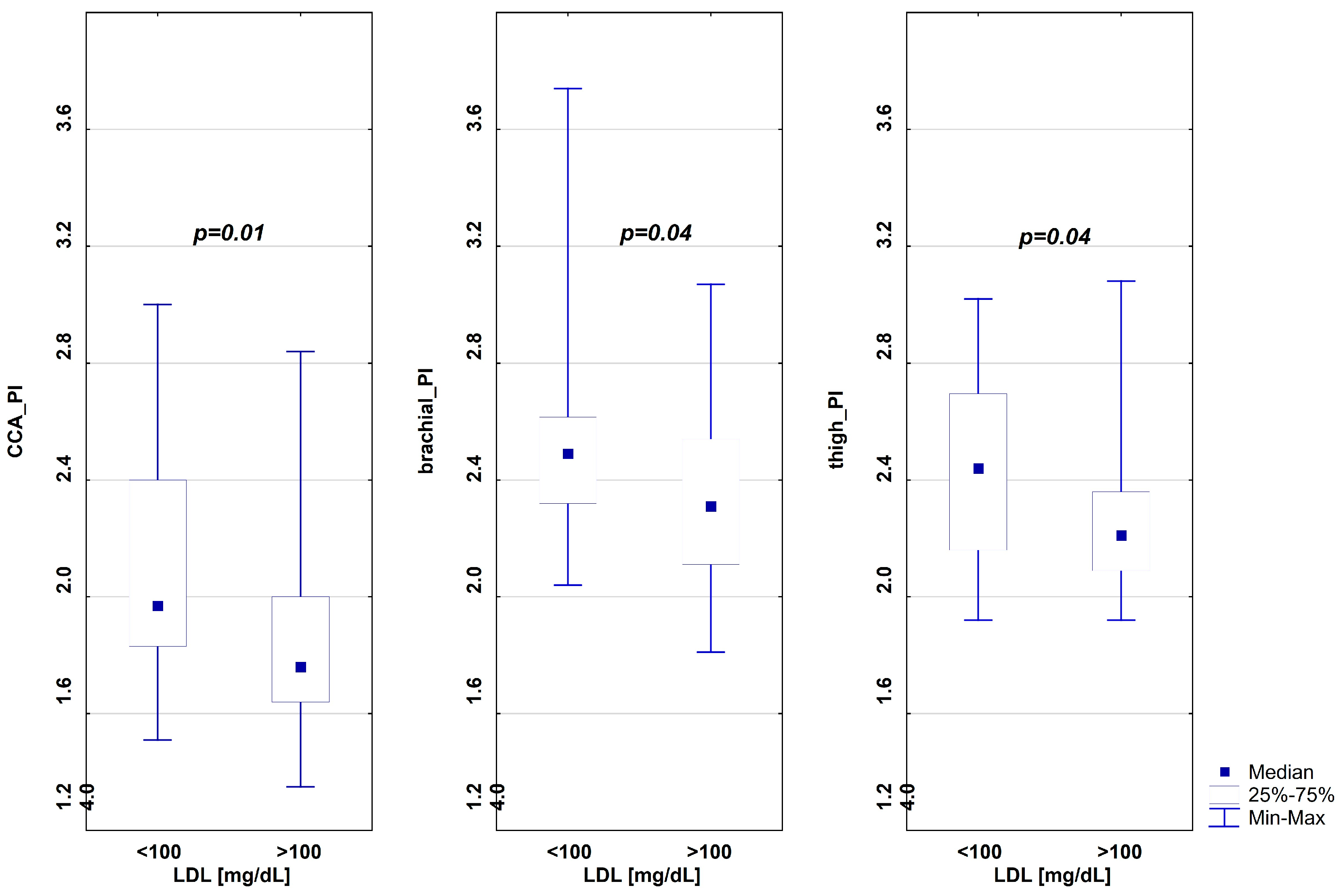
| CCA_PI | 2 (1.5–3) | 1.8 (1.4–2.8) | 0.01 |
| brachial_PI | 2.5 (2–3.7) | 2.3 (1.8–3.1) | 0.04 |
| thigh_PI | 2.4 (1.9–3) | 2.23 (1.9–3.1) | 0.03 |
| above_knee_PI | 2.3 (1.8–3.5) | 2.2 (1.9–2.8) | 0.10 |
| below_knee_PI | 2.6 (2–3.6) | 2.4 (2.1–3.6) | 0.06 |
| ankle_PI | 2.5 (2–3.8) | 2.4 (2 –3.3) | 0.11 |
| Blood pressure and heart rate | |||
| SBP (mmHg) | 107 (89–124) | 107 (84–126) | 1.0 |
| DBP (mmHg) | 59 (49–70) | 60 (49–76) | 0.8 |
| PP (mmHg) | 47 (32–70) | 47 (34–66) | 0.72 |
| HR (beats/minutes) | 78 (63–97) | 84 (57–111) | 0.1 |
| Parameters | Total Cholesterol | Cholesterol LDL | Cholesterol HDL | Triglycerides | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| IL-10 | −0.31 | 0.02 | −0.29 | 0.03 | – | ns | – | ns |
| IL-35 | −0.27 | 0.04 | −0.29 | 0.03 | – | ns | – | ns |
| sVCAM–1 | −0.29 | 0.03 | −0.31 | 0.02 | – | ns | – | ns |
| ICAM-1 | – | ns | −0.26 | 0.047 | – | ns | – | ns |
| AGEs | – | ns | – | ns | 0.29 | 0.03 | – | ns |
| Parameters | Age | T1D Onset | T1D Duration | Total Cholesterol | Cholesterol LDL | Cholesterol HDL | Triglycerides | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| CCA_PI | – | ns | – | ns | – | ns | −0.46 | <0.001 | −0.42 | 0.001 | −0.26 | 0.04 | – | ns |
| brachial_PI | – | ns | – | ns | – | ns | −0.37 | 0.004 | −0.36 | 0.005 | – | ns | – | ns |
| thigh_PI | – | ns | – | ns | – | ns | −0.31 | 0.02 | −0.31 | 0.02 | – | ns | – | ns |
| above_knee_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| below_knee_PI | 0.55 | <0.001 | – | ns | 0.27 | 0.04 | −0.31 | 0.02 | −0.31 | 0.02 | – | ns | – | ns |
| ankle_PI | 0.62 | <0.001 | – | ns | – | ns | – | ns | −0.26 | 0.046 | – | ns | – | ns |
| Parameters | IL-35 | IL-10 | IL-12 | sVCAM–1 | ICAM-1 | Angiogenin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Whole study group | ||||||||||||
| CCA_PI | – | ns | 0.27 | 0.04 | – | ns | – | ns | – | ns | – | ns |
| brachial_PI | – | ns | – | ns | – | ns | – | ns | 0.31 | 0.02 | – | ns |
| thigh_PI | – | ns | – | ns | 0.27 | 0.04 | – | ns | – | ns | – | ns |
| above_knee_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| below_knee_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| ankle_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| Subgroup A | ||||||||||||
| CCA_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| brachial_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| thigh_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| above_knee_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| below_knee_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| ankle_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| Subgroup B | ||||||||||||
| CCA_PI | – | ns | – | ns | 0.37 | 0.03 | – | ns | – | ns | – | ns |
| brachial_PI | – | ns | – | ns | – | ns | – | ns | 0.42 | 0.01 | −0.36 | 0.03 |
| thigh_PI | – | ns | – | ns | – | ns | – | ns | – | ns | – | ns |
| above_knee_PI | −0.41 | 0.01 | – | ns | – | ns | −0.34 | 0.049 | – | ns | – | ns |
| below_knee_PI | −0.48 | 0.003 | – | ns | – | ns | −0.36 | 0.03 | – | ns | – | ns |
| ankle_PI | −0.38 | 0.03 | – | ns | – | ns | −0.44 | 0.009 | – | ns | – | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neubauer-Geryk, J.; Myśliwiec, M.; Zorena, K.; Bieniaszewski, L. The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes. Int. J. Mol. Sci. 2025, 26, 5513. https://doi.org/10.3390/ijms26125513
Neubauer-Geryk J, Myśliwiec M, Zorena K, Bieniaszewski L. The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes. International Journal of Molecular Sciences. 2025; 26(12):5513. https://doi.org/10.3390/ijms26125513
Chicago/Turabian StyleNeubauer-Geryk, Jolanta, Małgorzata Myśliwiec, Katarzyna Zorena, and Leszek Bieniaszewski. 2025. "The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes" International Journal of Molecular Sciences 26, no. 12: 5513. https://doi.org/10.3390/ijms26125513
APA StyleNeubauer-Geryk, J., Myśliwiec, M., Zorena, K., & Bieniaszewski, L. (2025). The Relationship Between Cholesterol Level, Cytokine Profile, and Arterial Stiffness in Young Patients with Uncomplicated Type 1 Diabetes. International Journal of Molecular Sciences, 26(12), 5513. https://doi.org/10.3390/ijms26125513






