Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective
Abstract
1. Introduction
2. AD Risk Factors, Genetics and Etiology
2.1. Risk Factors
2.2. Genetics
2.3. Etiology
3. Neurotransmitter- and Ion-Based AD Hypotheses and Therapeutics
3.1. Cholinergic Hypothesis and Therapeutics
3.1.1. Cholinergic Hypothesis
3.1.2. Cholinergic-Based Therapeutics
3.2. Glutamate Excitotoxicity Hypothesis and Therapeutics
3.2.1. Glutamate-Based Hypothesis
3.2.2. Glutamate-Based Therapeutics
3.3. Serotoninergic Hypothesis and Therapeutics
3.3.1. Serotoninergic Hypothesis
3.3.2. Serotoninergic-Based Therapeutics
3.4. Calcium Signaling Hypothesis and Therapeutics
3.4.1. Calcium Signaling Hypothesis
3.4.2. Calcium-Signaling Modulators
4. Peptide-Based AD Hypotheses and Therapeutics
4.1. Amyloid Deposits Hypotheses and Therapeutics
4.1.1. Amyloid Hypothesis (Amyloid Cascade)
4.1.2. Aβ-Targeted Therapeutics
4.2. Tau Hypothesis and Therapeutics
4.2.1. Tau Hypothesis
4.2.2. Tau-Targeted Therapies
5. AD Aspecific Hypotheses and Therapeutics
5.1. Neuroinflammation-Based Hypotheses and Therapeutics
5.1.1. Neuroinflammation
5.1.2. Therapeutics for Neuroinflammation
5.2. Mitochondrial Cascade Hypothesis and Therapeutics
5.2.1. Mitochondrial Cascade Hypothesis
5.2.2. Targeting Oxidative Stress and Mitochondrial Dysfunction
5.3. Vascular Hypothesis and Therapeutics
5.3.1. Vascular Hypothesis
5.3.2. Therapeutics for Vascular-Based Diseases
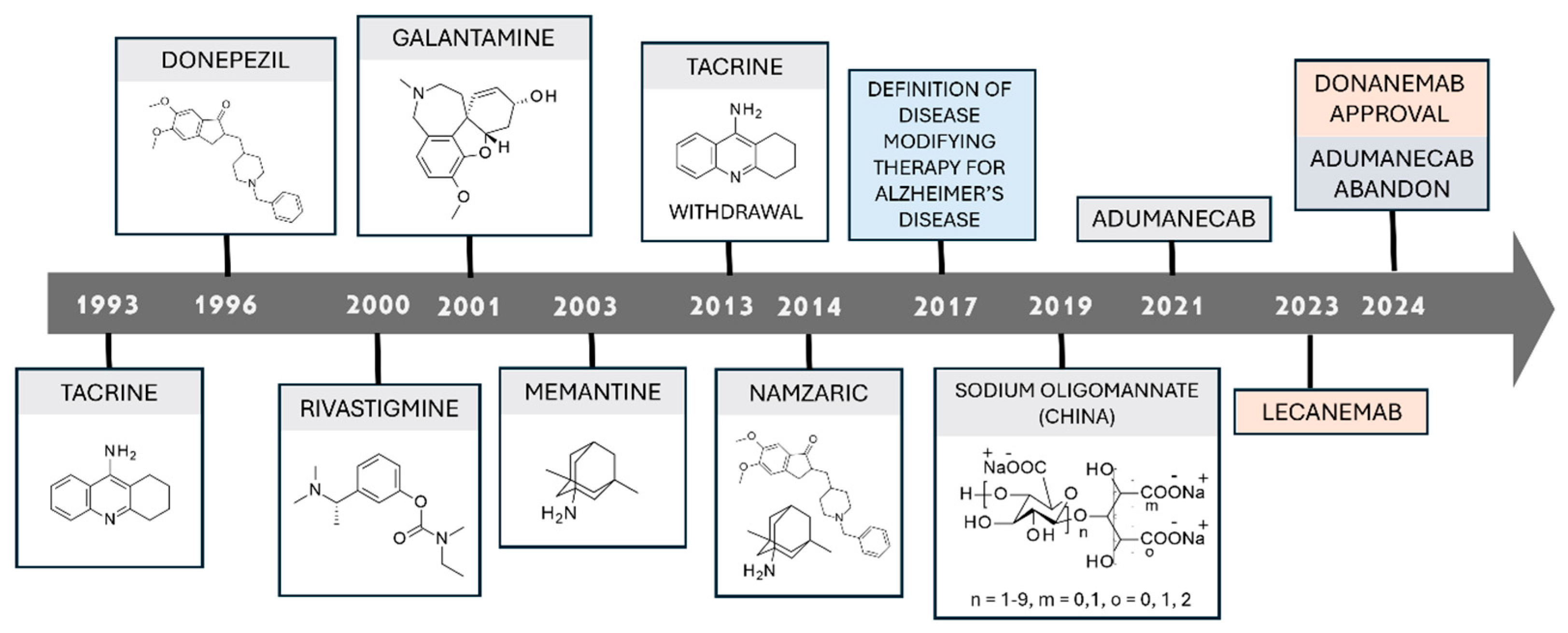
6. FDA-Approved Drugs for Alzheimer’s Disease
6.1. AChEIs-Approved Drugs
6.2. NMDAR Antagonists as Approved Drugs
6.3. Monoclonal Antibodies
7. Therapeutic Approaches: Lights and Shadows
8. Medicinal Chemist Opportunities and Challenges
8.1. Mutated Proteins Interventions
8.2. Neurotrasmitter- and Ion-Based Interventions
8.3. Calcium Signaling Modulator Interventions
8.4. Peptide-Based Level Interventions
9. Innovative Strategies and Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarano, N. Druggable Targets to Contrast Alzheimer’s Disease: Molecular Modelling Approaches for the Drug Design Process; University of Genoa: Genoa, Italy, 2025. [Google Scholar]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Tahami Monfared, A.A.; Byrnes, M.J.; White, L.A.; Zhang, Q. Alzheimer’s Disease: Epidemiology and Clinical Progression. Neurol. Ther. 2022, 11, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Luo, Z.; Xiao, L.; Huang, D.; Lv, J.; Wang, J.; Liu, X.; Ouyang, S.; Xu, Q.; Zhu, H.; Zou, K. Global, Regional, and National Burden and Trends of Alzheimer’s Disease and Related Dementias from 1990 to 2021: Results from the Global Burden of Disease Study 2021. SSRN 2024. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Available online: https://wonder.cdc.gov/ (accessed on 9 October 2024).
- Mendez, M.F. Early-Onset Alzheimer Disease. Neurol. Clin. 2017, 35, 263–281. [Google Scholar] [CrossRef]
- Aisen, P.S.; Cummings, J.; Jack, C.R.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the Path to 2025: Understanding the Alzheimer’s Disease Continuum. Alzheimer’s Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s Disease: Definition, Natural History, and Diagnostic Criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Rafii, M.S.; Aisen, P.S. Detection and Treatment of Alzheimer’s Disease in Its Preclinical Stage. Nat. Aging 2023, 3, 520–531. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer Disease. 2024 Feb 12. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Kelly, J.F.; Aggarwal, N.T.; Shah, R.C.; Wilson, R.S. Neuropathology of Older Persons without Cognitive Impairment from Two Community-Based Studies. Neurology 2006, 66, 1837–1844. [Google Scholar] [CrossRef]
- Jack, C.R.; Andrews, J.S.; Beach, T.G.; Buracchio, T.; Dunn, B.; Graf, A.; Hansson, O.; Ho, C.; Jagust, W.; McDade, E.; et al. Revised Criteria for Diagnosis and Staging of Alzheimer’s Disease: Alzheimer’s Association Workgroup. Alzheimer’s Dement. 2024, 20, 5143–5169. [Google Scholar] [CrossRef] [PubMed]
- Juganavar, A.; Joshi, A.; Shegekar, T. Navigating Early Alzheimer’s Diagnosis: A Comprehensive Review of Diagnostic Innovations. Cureus 2023, 15, e44937. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H.M. Rate of Conversion from Prodromal Alzheimer’s Disease to Alzheimer’s Dementia: A Systematic Review of the Literature. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 320–332. [Google Scholar] [CrossRef]
- Canevelli, M.; Grande, G.; Lacorte, E.; Quarchioni, E.; Cesari, M.; Mariani, C.; Bruno, G.; Vanacore, N. Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, C.; Massano, J.; Freitas, A. Hospital Admissions 2000–2014: A Retrospective Analysis of 288 096 Events in Patients with Dementia. Arch. Gerontol. Geriatr. 2018, 77, 150–157. [Google Scholar] [CrossRef]
- Maciejewska, K.; Czarnecka, K.; Szymański, P. A Review of the Mechanisms Underlying Selected Comorbidities in Alzheimer’s Disease. Pharmacol. Rep. 2021, 73, 1565–1581. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. The Impact of Disease Comorbidities in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 631770. [Google Scholar] [CrossRef]
- Jellinger, K.A. Recent Update on the Heterogeneity of the Alzheimer’s Disease Spectrum. J. Neural. Transm. 2022, 129, 1–24. [Google Scholar] [CrossRef]
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The Epidemiology of Alzheimer’s Disease Modifiable Risk Factors and Prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia Prevention, Intervention, and Care: 2024 Report of the Lancet Standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef]
- Jeong, J.H.; Na, H.R.; Choi, S.H.; Kim, J.; Na, D.L.; Seo, S.W.; Chin, J.; Park, S.A.; Kim, E.-J.; Han, H.J.; et al. Group- and Home-Based Cognitive Intervention for Patients with Mild Cognitive Impairment: A Randomized Controlled Trial. Psychother. Psychosom. 2016, 85, 198–207. [Google Scholar] [CrossRef]
- Brasure, M.; Desai, P.; Davila, H.; Nelson, V.A.; Calvert, C.; Jutkowitz, E.; Butler, M.; Fink, H.A.; Ratner, E.; Hemmy, L.S.; et al. Physical Activity Interventions in Preventing Cognitive Decline and Alzheimer-Type Dementia. Ann. Intern. Med. 2018, 168, 30. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Mroczko, J.; Rutkowski, P.; Mroczko, B. Molecular Aspects of a Diet as a New Pathway in the Prevention and Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 10751. [Google Scholar] [CrossRef] [PubMed]
- Straubmeier, M.; Behrndt, E.-M.; Seidl, H.; Özbe, D.; Luttenberger, K.; Gräßel, E. Non-Pharmacological Treatment in People With Cognitive Impairment. Dtsch. Arztebl. Int. 2017, 114, 815. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.; Youngberg, W. Clinical Lifestyle Medicine Strategies for Preventing and Reversing Memory Loss in Alzheimer’s. Am. J. Lifestyle Med. 2018, 12, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E. Reversal of Cognitive Decline: A Novel Therapeutic Program. Aging 2014, 6, 707–717. [Google Scholar] [CrossRef]
- Roach, J.C.; Rapozo, M.K.; Hara, J.; Glusman, G.; Lovejoy, J.; Shankle, W.R.; Hood, L. A Remotely Coached Multimodal Lifestyle Intervention for Alzheimer’s Disease Ameliorates Functional and Cognitive Outcomes. J. Alzheimer’s Dis. 2023, 96, 591–607. [Google Scholar] [CrossRef]
- Andrade-Guerrero, J.; Santiago-Balmaseda, A.; Jeronimo-Aguilar, P.; Vargas-Rodríguez, I.; Cadena-Suárez, A.R.; Sánchez-Garibay, C.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Cardenas-Aguayo, M.-C.; Diaz-Cintra, S.; et al. Alzheimer’s Disease: An Updated Overview of Its Genetics. Int. J. Mol. Sci. 2023, 24, 3754. [Google Scholar] [CrossRef]
- Andrews, S.J.; Renton, A.E.; Fulton-Howard, B.; Podlesny-Drabiniok, A.; Marcora, E.; Goate, A.M. The Complex Genetic Architecture of Alzheimer’s Disease: Novel Insights and Future Directions. EBioMedicine 2023, 90, 104511. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Petit, D.; Fernández, S.G.; Zoltowska, K.M.; Enzlein, T.; Ryan, N.S.; O’Connor, A.; Szaruga, M.; Hill, E.; Vandenberghe, R.; Fox, N.C.; et al. Aβ Profiles Generated by Alzheimer’s Disease Causing PSEN1 Variants Determine the Pathogenicity of the Mutation and Predict Age at Disease Onset. Mol. Psychiatry 2022, 27, 2821–2832. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.V.F.; de Loures, C.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; Carvalho, M. das G. Alzheimer’s Disease: Risk Factors and Potentially Protective Measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef] [PubMed]
- Muratore, C.R.; Rice, H.C.; Srikanth, P.; Callahan, D.G.; Shin, T.; Benjamin, L.N.P.; Walsh, D.M.; Selkoe, D.J.; Young-Pearse, T.L. The Familial Alzheimer’s Disease APPV717I Mutation Alters APP Processing and Tau Expression in IPSC-Derived Neurons. Hum. Mol. Genet. 2014, 23, 3523–3536. [Google Scholar] [CrossRef]
- Levin, J.; Hasan, A.; Alejandre, I.A.; Lorenzi, I.; Mall, V.; Rohrer, T.R. Diseases Affecting Middle-Aged and Elderly Individuals With Trisomy 21. Dtsch. Arztebl. Int. 2023, 120, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Deane, R.; Sagare, A.; Hamm, K.; Parisi, M.; Lane, S.; Finn, M.B.; Holtzman, D.M.; Zlokovic, B.V. ApoE Isoform–Specific Disruption of Amyloid β Peptide Clearance from Mouse Brain. J. Clin. Investig. 2008, 118, 4002–4013. [Google Scholar] [CrossRef]
- Jiang, Q.; Lee, C.Y.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L.; et al. ApoE Promotes the Proteolytic Degradation of Aβ. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef]
- Qin, Q.; Teng, Z.; Liu, C.; Li, Q.; Yin, Y.; Tang, Y. TREM2, Microglia, and Alzheimer’s Disease. Mech. Ageing Dev. 2021, 195, 111438. [Google Scholar] [CrossRef]
- De Roeck, A.; Van Broeckhoven, C.; Sleegers, K. The Role of ABCA7 in Alzheimer’s Disease: Evidence from Genomics, Transcriptomics and Methylomics. Acta Neuropathol. 2019, 138, 201–220. [Google Scholar] [CrossRef]
- Lambert, E.; Saha, O.; Soares Landeira, B.; Melo de Farias, A.R.; Hermant, X.; Carrier, A.; Pelletier, A.; Gadaut, J.; Davoine, L.; Dupont, C.; et al. The Alzheimer Susceptibility Gene BIN1 Induces Isoform-Dependent Neurotoxicity through Early Endosome Defects. Acta Neuropathol. Commun. 2022, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.-L.; Tan, M.-S.; Tan, L.; Yu, J.-T. The Role of TDP-43 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Patassini, S.; Begley, P.; Church, S.; Waldvogel, H.J.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Cerebral Deficiency of Vitamin B5 (d-Pantothenic Acid; Pantothenate) as a Potentially-Reversible Cause of Neurodegeneration and Dementia in Sporadic Alzheimer’s Disease. Biochem. Biophys. Res. Commun. 2020, 527, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, H.B. Targeting Fyn Kinase in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 369–376. [Google Scholar] [CrossRef]
- Liu, S.-L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.-T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef]
- Nasb, M.; Tao, W.; Chen, N. Alzheimer’s Disease Puzzle: Delving into Pathogenesis Hypotheses. Aging Dis. 2024, 15, 43–73. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L.; Beer, B.; Lippa, A.S. The Cholinergic Hypothesis of Geriatric Memory Dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Chen, Z.-R.; Huang, J.-B.; Yang, S.-L.; Hong, F.-F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Shinotoh, H.; Namba, H.; Fukushi, K.; Nagatsuka, S.-I.; Tanaka, N.; Aotsuka, A.; Ota, T.; Tanada, S.; Irie, T. Progressive Loss of Cortical Acetylcholinesterase Activity in Association with Cognitive Decline in Alzheimer’s Disease: A Positron Emission Tomography Study. Ann. Neurol. 2000, 48, 194–200. [Google Scholar] [CrossRef]
- Campanari, M.-L.; García-Ayllón, M.-S.; Belbin, O.; Galcerán, J.; Lleó, A.; Sáez-Valero, J. Acetylcholinesterase Modulates Presenilin-1 Levels and γ-Secretase Activity. J. Alzheimer’s Dis. 2014, 41, 911–924. [Google Scholar] [CrossRef]
- Cacabelos, R.; Torrellas, C.; Carrera, I.; Cacabelos, P.; Corzo, L.; Fernández-Novoa, L.; Tellado, I.; Carril, J.C.; Aliev, G. Novel Therapeutic Strategies for Dementia. CNS Neurol. Disord. Drug Targets 2016, 15, 141–241. [Google Scholar] [CrossRef]
- Amenta, F.; Carotenuto, A.; Fasanaro, A.M.; Rea, R.; Traini, E. The ASCOMALVA (Association between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in Alzheimer’s Disease) Trial: Interim Results after Two Years of Treatment. J. Alzheimer’s Dis. 2014, 42, S281–S288. [Google Scholar] [CrossRef]
- Carotenuto, A.; Fasanaro, A.M.; Manzo, V.; Amenta, F.; Traini, E. Association Between the Cholinesterase Inhibitor Donepezil and the Cholinergic Precursor Choline Alphoscerate in the Treatment of Depression in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2022, 6, 235–243. [Google Scholar] [CrossRef]
- Sterling, J.; Herzig, Y.; Goren, T.; Finkelstein, N.; Lerner, D.; Goldenberg, W.; Miskolczi, I.; Molnar, S.; Rantal, F.; Tamas, T.; et al. Novel Dual Inhibitors of AChE and MAO Derived from Hydroxy Aminoindan and Phenethylamine as Potential Treatment for Alzheimer’s Disease. J. Med. Chem. 2002, 45, 5260–5279. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Geffen, Y.; Rabinowitz, J.; Thomas, R.G.; Schmidt, R.; Ropele, S.; Weinstock, M. Low-Dose Ladostigil for Mild Cognitive Impairment. Neurology 2019, 93, e1474–e1484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-Y.; Chen, T.-K.; Zhou, J.-T.; He, S.-Y.; Yang, H.-Y.; Chen, Y.; Qu, W.; Feng, F.; Sun, H.-P. Dual GSK-3β/AChE Inhibitors as a New Strategy for Multitargeting Anti-Alzheimer’s Disease Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhou, J.; Wang, Y.; Chen, L.; Duan, Y.; Huang, J.; Liu, C.; Chen, Y.; Liu, W.; Sun, H.; et al. Rational Design and Biological Evaluation of a New Class of Thiazolopyridyl Tetrahydroacridines as Cholinesterase and GSK-3 Dual Inhibitors for Alzheimer’s Disease. Eur. J. Med. Chem. 2020, 207, 112751. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Zheng, L.; Feng, K.-W.; Wang, H.-T.; Xu, J.-P.; Zhou, Z.-Z. Discovery of Novel 2,3-Dihydro-1H-Inden-1-Ones as Dual PDE4/AChE Inhibitors with More Potency against Neuroinflammation for the Treatment of Alzheimer’s Disease. Eur. J. Med. Chem 2022, 238, 114503. [Google Scholar] [CrossRef]
- Silva, G.M.; Barcelos, M.P.; Poiani, J.G.C.; da Hage-Melim, L.I.S.; da Silva, C.H.T.P. Allosteric Modulators of Potential Targets Related to Alzheimer’s Disease: A Review. ChemMedChem 2019, 14, 1467–1483. [Google Scholar] [CrossRef]
- Chatzidaki, A.; Millar, N.S. Allosteric Modulation of Nicotinic Acetylcholine Receptors. Biochem. Pharmacol. 2015, 97, 408–417. [Google Scholar] [CrossRef]
- Maelicke, A.; Albuquerque, E.X. Allosteric Modulation of Nicotinic Acetylcholine Receptors as a Treatment Strategy for Alzheimer’s Disease. Eur. J. Pharmacol. 2000, 393, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Melancon, B.J.; Tarr, J.C.; Panarese, J.D.; Wood, M.R.; Lindsley, C.W. Allosteric Modulation of the M1 Muscarinic Acetylcholine Receptor: Improving Cognition and a Potential Treatment for Schizophrenia and Alzheimer’s Disease. Drug Discov. Today 2013, 18, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Callahan, P.M.; Hutchings, E.J.; Kille, N.J.; Chapman, J.M.; Terry, A.V. Positive Allosteric Modulator of Alpha 7 Nicotinic-Acetylcholine Receptors, PNU-120596 Augments the Effects of Donepezil on Learning and Memory in Aged Rodents and Non-Human Primates. Neuropharmacology 2013, 67, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Potasiewicz, A.; Krawczyk, M.; Gzielo, K.; Popik, P.; Nikiforuk, A. Positive Allosteric Modulators of Alpha 7 Nicotinic Acetylcholine Receptors Enhance Procognitive Effects of Conventional Anti-Alzheimer Drugs in Scopolamine-Treated Rats. Behavioural. Brain Res. 2020, 385, 112547. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kos, T.; Potasiewicz, A.; Popik, P. Positive Allosteric Modulation of Alpha 7 Nicotinic Acetylcholine Receptors Enhances Recognition Memory and Cognitive Flexibility in Rats. Eur. Neuropsychopharmacol. 2015, 25, 1300–1313. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Faron-Gorecka, A.; Popik, P.; Nikiforuk, A. Repeated Treatment with Alpha 7 Nicotinic Acetylcholine Receptor Ligands Enhances Cognitive Processes and Stimulates Erk1/2 and Arc Genes in Rats. Behav. Brain Res. 2021, 409, 113338. [Google Scholar] [CrossRef]
- Wang, X.; Bell, I.M.; Uslaner, J.M. Activators of A7 NAChR as Potential Therapeutics for Cognitive Impairment. In Behavioral Pharmacology of the Cholinergic System; Springer: Cham, Switzerland, 2020; pp. 209–245. [Google Scholar]
- Nguyen, H.T.M.; van der Westhuizen, E.T.; Langmead, C.J.; Tobin, A.B.; Sexton, P.M.; Christopoulos, A.; Valant, C. Opportunities and Challenges for the Development of M1 Muscarinic Receptor Positive Allosteric Modulators in the Treatment for Neurocognitive Deficits. Br. J. Pharmacol. 2024, 181, 2114–2142. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining Cholinergic Therapy for Alzheimer’s Disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Maragos, W.F.; Greenamyre, J.T.; Penney, J.B.; Young, A.B. Glutamate Dysfunction in Alzheimer’s Disease: An Hypothesis. Trends Neurosci. 1987, 10, 65–68. [Google Scholar] [CrossRef]
- Behl, C. The Glutamatergic Hypothesis of Alzheimer’s Disease. In Alzheimer’s Disease Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 93–107. [Google Scholar]
- Soares, C.; Da Ros, L.U.; Machado, L.S.; Rocha, A.; Lazzarotto, G.; Carello-Collar, G.; De Bastiani, M.A.; Ferrari-Souza, J.P.; Lussier, F.Z.; Souza, D.O.; et al. The Glutamatergic System in Alzheimer’s Disease: A Systematic Review with Meta-Analysis. Mol. Psychiatry 2024, 29, 2261–2273. [Google Scholar] [CrossRef]
- Jacob, C.P.; Koutsilieri, E.; Bartl, J.; Neuen-Jacob, E.; Arzberger, T.; Zander, N.; Ravid, R.; Roggendorf, W.; Riederer, P.; Grünblatt, E. Alterations in Expression of Glutamatergic Transporters and Receptors in Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2007, 11, 97–116. [Google Scholar] [CrossRef]
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Calcium-Dependent Excitotoxicity. J. Mol. Med. 2000, 78, 3–13. [Google Scholar] [CrossRef]
- Conway, M.E. Alzheimer’s Disease: Targeting the Glutamatergic System. Biogerontology 2020, 21, 257–274. [Google Scholar] [CrossRef] [PubMed]
- McShane, R.; Westby, M.J.; Roberts, E.; Minakaran, N.; Schneider, L.; Farrimond, L.E.; Maayan, N.; Ware, J.; Debarros, J. Memantine for Dementia. Cochrane Database Syst. Rev. 2019, CD003154. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Paradigm Shift in Neuroprotection by NMDA Receptor Blockade: Memantine and Beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Talantova, M.; Sanz-Blasco, S.; Zhang, X.; Xia, P.; Akhtar, M.W.; Okamoto, S.; Dziewczapolski, G.; Nakamura, T.; Cao, G.; Pratt, A.E.; et al. Aβ Induces Astrocytic Glutamate Release, Extrasynaptic NMDA Receptor Activation, and Synaptic Loss. Proc. Natl. Acad. Sci. USA 2013, 110, E2518–E2527. [Google Scholar] [CrossRef]
- Peterson, A.R.; Binder, D.K. Post-Translational Regulation of GLT-1 in Neurological Diseases and Its Potential as an Effective Therapeutic Target. Front. Mol. Neurosci. 2019, 12, 164. [Google Scholar] [CrossRef]
- Hefendehl, J.K.; LeDue, J.; Ko, R.W.Y.; Mahler, J.; Murphy, T.H.; MacVicar, B.A. Mapping Synaptic Glutamate Transporter Dysfunction in Vivo to Regions Surrounding Aβ Plaques by IGluSnFR Two-Photon Imaging. Nat. Commun. 2016, 7, 13441. [Google Scholar] [CrossRef]
- Yimer, E.M.; Hishe, H.Z.; Tuem, K.B. Repurposing of the β-Lactam Antibiotic, Ceftriaxone for Neurological Disorders: A Review. Front. Neurosci. 2019, 13, 236. [Google Scholar] [CrossRef]
- Matos-Ocasio, F.; Hernández-López, A.; Thompson, K.J. Ceftriaxone, a GLT-1 Transporter Activator, Disrupts Hippocampal Learning in Rats. Pharmacol. Biochem. Behav. 2014, 122, 118–121. [Google Scholar] [CrossRef]
- Fumagalli, E.; Funicello, M.; Rauen, T.; Gobbi, M.; Mennini, T. Riluzole Enhances the Activity of Glutamate Transporters GLAST, GLT1 and EAAC1. Eur. J. Pharmacol. 2008, 578, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Phase 2/3 Study of Troriluzole Shows No Statistically Significant Effects on Alzheimer Disease. Available online: https://Practicalneurology.Com/News/Phase-23-Study-of-Troriluzole-Shows-No-Statistically-Significant-Effects-on-Alzheimer-Disease#:~:Text=A%20phase%202%2F3%20clinical%20trial%20%28NCT03605667%29%20of%20troriluzole,For%20symptomatic%20treatment%20of%20mild-to-Moderate%20Alzheimer%20disease%20%28AD%29 (accessed on 6 December 2024).
- Zschocke, J.; Bayatti, N.; Clement, A.M.; Witan, H.; Figiel, M.; Engele, J.; Behl, C. Differential Promotion of Glutamate Transporter Expression and Function by Glucocorticoids in Astrocytes from Various Brain Regions. J. Biol. Chem. 2005, 280, 34924–34932. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Brito, V.; Küppers, E.; Beyer, C. Regulation of Glutamate Transporter GLAST and GLT-1 Expression in Astrocytes by Estrogen. Mol. Brain Res. 2005, 138, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Spampinato, S.F.; Sortino, M.A. Estrogen and Alzheimer’s Disease: Still an Attractive Topic despite Disappointment from Early Clinical Results. Eur. J. Pharmacol. 2017, 817, 51–58. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Pinky, P.D.; Bloemer, J.; Ghanei, N.; Suppiramaniam, V.; Amin, R. Signaling Mechanisms of Selective PPARγ Modulators in Alzheimer’s Disease. PPAR Res. 2018, 2018, 2010675. [Google Scholar] [CrossRef]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Craft, S.; Landreth, G.; Linnamägi, Ü.; Sawchak, S. Rosiglitazone Monotherapy in Mild-to-Moderate Alzheimer’s Disease: Results from a Randomized, Double-Blind, Placebo-Controlled Phase III Study. Dement. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef]
- ALZFORUM-THERAPEUTICS. Available online: https://Www.Alzforum.Org/Therapeutics/Rosiglitazone (accessed on 6 December 2024).
- Coleman, E.S.; Dennis, J.C.; Braden, T.D.; Judd, R.L.; Posner, P. Insulin Treatment Prevents Diabetes-Induced Alterations in Astrocyte Glutamate Uptake and GFAP Content in Rats at 4 and 8 Weeks of Diabetes Duration. Brain Res. 2010, 1306, 131–141. [Google Scholar] [CrossRef]
- Hölscher, C. First Clinical Data of the Neuroprotective Effects of Nasal Insulin Application in Patients with Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, S33–S37. [Google Scholar] [CrossRef]
- Craft, S.; Raman, R.; Chow, T.W.; Rafii, M.S.; Sun, C.-K.; Rissman, R.A.; Donohue, M.C.; Brewer, J.B.; Jenkins, C.; Harless, K.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia. JAMA Neurol. 2020, 77, 1099. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hirano, A. Nucleus Raphe Dorsalis in Alzheimer’s Disease: Neurofibrillary Tangles and Loss of Large Neurons. Ann. Neurol. 1985, 17, 573–577. [Google Scholar] [CrossRef]
- Ebinger, G.; Bruyland, M.; Martin, J.J.; Herregodts, P.; Cras, P.; Michotte, Y.; Gommé, L. Distribution of Biogenic Amines and Their Catabolites in Brains from Patients with Alzheimer’s Disease. J. Neurol. Sci. 1987, 77, 267–283. [Google Scholar] [CrossRef]
- Vakalopoulos, C. Alzheimer’s Disease: The Alternative Serotonergic Hypothesis of Cognitive Decline. J. Alzheimer’s Dis. 2017, 60, 859–866. [Google Scholar] [CrossRef]
- Lai, M.K.; Tsang, S.W.; Alder, J.T.; Keene, J.; Hope, T.; Esiri, M.M.; Francis, P.T.; Chen, C.P. Loss of Serotonin 5-HT2A Receptors in the Postmortem Temporal Cortex Correlates with Rate of Cognitive Decline in Alzheimer’s Disease. Psychopharmacology 2005, 179, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Busceti, C.L.; Di Pietro, P.; Riozzi, B.; Traficante, A.; Biagioni, F.; Nisticò, R.; Fornai, F.; Battaglia, G.; Nicoletti, F.; Bruno, V. 5-HT2C Serotonin Receptor Blockade Prevents Tau Protein Hyperphosphorylation and Corrects the Defect in Hippocampal Synaptic Plasticity Caused by a Combination of Environmental Stressors in Mice. Pharmacol. Res. 2015, 99, 258–268. [Google Scholar] [CrossRef]
- Arjona, A.A.; Pooler, A.M.; Lee, R.K.; Wurtman, R.J. Effect of a 5-HT2C Serotonin Agonist, Dexnorfenfluramine, on Amyloid Precursor Protein Metabolism in Guinea Pigs. Brain Res. 2002, 951, 135–140. [Google Scholar] [CrossRef]
- Ban, J.Y.; Seong, Y.H. Blockade of 5-HT3 Receptor with MDL72222 and Y25130 Reduces β-Amyloid Protein (25–35)-Induced Neurotoxicity in Cultured Rat Cortical Neurons. Eur. J. Pharmacol. 2005, 520, 12–21. [Google Scholar] [CrossRef]
- Cochet, M.; Donneger, R.; Cassier, E.; Gaven, F.; Lichtenthaler, S.F.; Marin, P.; Bockaert, J.; Dumuis, A.; Claeysen, S. 5-HT4 Receptors Constitutively Promote the Non-Amyloidogenic Pathway of APP Cleavage and Interact with ADAM10. ACS Chem. Neurosci. 2013, 4, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Nirogi, R.; Mohammed, A.R.; Shinde, A.K.; Gagginapally, S.R.; Kancharla, D.M.; Ravella, S.R.; Bogaraju, N.; Middekadi, V.R.; Subramanian, R.; Palacharla, R.C.; et al. Discovery and Preclinical Characterization of Usmarapride (SUVN-D4010): A Potent, Selective 5-HT4 Receptor Partial Agonist for the Treatment of Cognitive Deficits Associated with Alzheimer’s Disease. J. Med. Chem. 2021, 64, 10641–10665. [Google Scholar] [CrossRef] [PubMed]
- Maillet, M.; Robert, S.; Lezoualc’h, F. New Insights into Serotonin 5-HT4 Receptors: A Novel Therapeutic Target for Alzheimer’s Disease? Curr. Alzheimer Res. 2004, 1, 79–85. [Google Scholar] [CrossRef]
- Benhamú, B.; Martín-Fontecha, M.; Vázquez-Villa, H.; Pardo, L.; López-Rodríguez, M.L. Serotonin 5-HT6 Receptor Antagonists for the Treatment of Cognitive Deficiency in Alzheimer’s Disease. J. Med. Chem. 2014, 57, 7160–7181. [Google Scholar] [CrossRef] [PubMed]
- Hashemi-Firouzi, N.; Shahidi, S.; Soleimani-Asl, S.; Komaki, A. 5-Hydroxytryptamine Receptor 6 Antagonist, SB258585 Exerts Neuroprotection in a Rat Model of Streptozotocin-Induced Alzheimer’s Disease. Metab. Brain Dis. 2018, 33, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Lennon, J.C.; Malkaram, S.A.; Zeng, Y.; Fisher, D.W.; Dong, H. Serotonergic System, Cognition, and BPSD in Alzheimer’s Disease. Neurosci. Lett. 2019, 704, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yan, J.; Zhou, P.; Li, J.; Gao, H.; Xia, Y.; Wang, Q. Neurotransmitter Receptors and Cognitive Dysfunction in Alzheimer’s Disease and Parkinson’s Disease. Prog. Neurobiol. 2012, 97, 1–13. [Google Scholar] [CrossRef]
- Jans, L.A.W.; Riedel, W.J.; Markus, C.R.; Blokland, A. Serotonergic Vulnerability and Depression: Assumptions, Experimental Evidence and Implications. Mol. Psychiatry 2007, 12, 522–543. [Google Scholar] [CrossRef]
- Whitney, M.S.; Shemery, A.M.; Yaw, A.M.; Donovan, L.J.; Glass, J.D.; Deneris, E.S. Adult Brain Serotonin Deficiency Causes Hyperactivity, Circadian Disruption, and Elimination of Siestas. J. Neurosci. 2016, 36, 9828–9842. [Google Scholar] [CrossRef]
- Maillet, A.; Krack, P.; Lhommée, E.; Météreau, E.; Klinger, H.; Favre, E.; Le Bars, D.; Schmitt, E.; Bichon, A.; Pelissier, P.; et al. The Prominent Role of Serotonergic Degeneration in Apathy, Anxiety and Depression in de Novo Parkinson’s Disease. Brain 2016, 139, 2486–2502. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Gil-Bea, F.J.; Diez-Ariza, M.; Chen, C.P.L.-H.; Francis, P.T.; Lasheras, B.; Ramirez, M.J. Cholinergic–Serotonergic Imbalance Contributes to Cognitive and Behavioral Symptoms in Alzheimer’s Disease. Neuropsychologia 2005, 43, 442–449. [Google Scholar] [CrossRef]
- Schechter, L.E.; Smith, D.L.; Rosenzweig-Lipson, S.; Sukoff, S.J.; Dawson, L.A.; Marquis, K.; Jones, D.; Piesla, M.; Andree, T.; Nawoschik, S.; et al. Lecozotan (SRA-333): A Selective Serotonin 1A Receptor Antagonist That Enhances the Stimulated Release of Glutamate and Acetylcholine in the Hippocampus and Possesses Cognitive-Enhancing Properties. J. Pharmacol. Exp. Ther. 2005, 314, 1274–1289. [Google Scholar] [CrossRef]
- Patat, A.; Parks, V.; Raje, S.; Plotka, A.; Chassard, D.; Coz, F. Le Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of Ascending Single and Multiple Doses of Lecozotan in Healthy Young and Elderly Subjects. Br. J. Clin. Pharmacol. 2009, 67, 299–308. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, C.; Lv, J.; Zhu, X.; Jiang, X.; Lu, W.; Lu, Y.; Tang, Z.; Wang, J.; Shen, X. Antiallergic Drug Desloratadine as a Selective Antagonist of 5HT2A Receptor Ameliorates Pathology of Alzheimer’s Disease Model Mice by Improving Microglial Dysfunction. Aging Cell 2021, 20, e13286. [Google Scholar] [CrossRef]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Komaki, A.; Asl, S.S. The Effect of NAD-299 and TCB-2 on Learning and Memory, Hippocampal BDNF Levels and Amyloid Plaques in Streptozotocin-Induced Memory Deficits in Male Rats. Psychopharmacology 2018, 235, 2809–2822. [Google Scholar] [CrossRef]
- Baranger, K.; Giannoni, P.; Girard, S.D.; Girot, S.; Gaven, F.; Stephan, D.; Migliorati, M.; Khrestchatisky, M.; Bockaert, J.; Marchetti-Gauthier, E.; et al. Chronic Treatments with a 5-HT 4 Receptor Agonist Decrease Amyloid Pathology in the Entorhinal Cortex and Learning and Memory Deficits in the 5xFAD Mouse Model of Alzheimer’s Disease. Neuropharmacology 2017, 126, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Gaven, F.; de Bundel, D.; Baranger, K.; Marchetti-Gauthier, E.; Roman, F.S.; Valjent, E.; Marin, P.; Bockaert, J.; Rivera, S.; et al. Early Administration of RS 67333, a Specific 5-HT4 Receptor Agonist, Prevents Amyloidogenesis and Behavioral Deficits in the 5XFAD Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2013, 5, 96. [Google Scholar] [CrossRef] [PubMed]
- Freret, T.; Lelong-Boulouard, V.; Lecouflet, P.; Hamidouche, K.; Dauphin, F.; Boulouard, M. Co-Modulation of an Allosteric Modulator of Nicotinic Receptor-Cholinesterase Inhibitor (Galantamine) and a 5-HT4 Receptor Agonist (RS-67333): Effect on Scopolamine-Induced Memory Deficit in the Mouse. Psychopharmacology 2017, 234, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Shacham, S.; Milgram, B.; Araujo, J.; Ragazzino, M.; Mohler, E.; Marantz, Y.; Mercedes, L.; Noiman, S. O3–05–03: PRX–03140: A Novel 5–HT4 Partial Agonist with a Dual Cholinergic/Disease–Modifying Mechanism for the Treatment of Alzheimer Disease. Alzheimer’s Dement. 2006, 2, S62. [Google Scholar] [CrossRef]
- Megerian, J.T. Results of a Phase 2A Study of a Novel 5HT4 Agonist for the Treatment of Alzheimer’s Disease. In Proceedings of the American Society for Experimental Neuro Therapeutics Annual Meeting, Arlington, TX, USA, 6 March 2009. [Google Scholar]
- Nirogi, R.; Bhyrapuneni, G.; Muddana, N.R.; Goyal, V.K.; Pandey, S.K.; Mohammed, A.R.; Ravula, J.; Jetta, S.; Palacharla, V.R.C. First-in-Human Studies to Evaluate the Safety, Tolerability, and Pharmacokinetics of a Novel 5-HT4 Partial Agonist, SUVN-D4010, in Healthy Adult and Elderly Subjects. Clin. Drug Investig. 2021, 41, 469–482. [Google Scholar] [CrossRef]
- Quiedeville, A.; Boulouard, M.; Hamidouche, K.; Da Silva Costa-Aze, V.; Nee, G.; Rochais, C.; Dallemagne, P.; Fabis, F.; Freret, T.; Bouet, V. Chronic Activation of 5-HT4 Receptors or Blockade of 5-HT6 Receptors Improve Memory Performances. Behav. Brain Res. 2015, 293, 10–17. [Google Scholar] [CrossRef]
- Wicke, K.; Haupt, A.; Bespalov, A. Investigational Drugs Targeting 5-HT6 Receptors for the Treatment of Alzheimer’s Disease. Expert Opin. Investig. Drugs 2015, 24, 1515–1528. [Google Scholar] [CrossRef]
- Nirogi, R.; Jayarajan, P.; Shinde, A.; Mohammed, A.R.; Grandhi, V.R.; Benade, V.; Goyal, V.K.; Abraham, R.; Jasti, V.; Cummings, J. Progress in Investigational Agents Targeting Serotonin-6 Receptors for the Treatment of Brain Disorders. Biomolecules 2023, 13, 309. [Google Scholar] [CrossRef]
- Minabe, Y.; Shirayama, Y.; Hashimoto, K.; Routledge, C.; Hagan, J.J.; Ashby, C.R. Effect of the Acute and Chronic Administration of the Selective 5-HT6 Receptor Antagonist SB-271046 on the Activity of Midbrain Dopamine Neurons in Rats: An in Vivo Electrophysiological Study. Synapse 2004, 52, 20–28. [Google Scholar] [CrossRef]
- Comery, T.A.; Aschmies, S.; Haydar, S.; Hughes, Z.; Huselton, C.; Kowal, D.; Kramer, A.; McFarlane, G.; Monaghan, M.; Smith, D.; et al. P3-329: SAM-531, N,N-dimethyl-3-{[3-(1-naphthylsulfonyl)-1H-indazol-5-yl]Oxy} Propan-1-amine, a Novel Serotonin-6 Receptor Antagonist with Preclinical Pro-cognitive Efficacy. Alzheimer’s Dement. 2010, 6, 309. [Google Scholar] [CrossRef]
- Brisard, C.; Safirstein, B.; Booth, K.; Hua, L.; Brault, Y.; Raje, S.; Leventer, S. P1-457: Safety, Tolerability, and Preliminary Efficacy of SAM-531, a 5HT-6 Antagonist, in Subjects with Mild-to-moderate Alzheimer’s Disease: Results from a Phase 2a Study. Alzheimer’s Dement. 2010, 6, S311. [Google Scholar] [CrossRef]
- Arnt, J.; Bang-Andersen, B.; Grayson, B.; Bymaster, F.P.; Cohen, M.P.; DeLapp, N.W.; Giethlen, B.; Kreilgaard, M.; McKinzie, D.L.; Neill, J.C.; et al. Lu AE58054, a 5-HT6 Antagonist, Reverses Cognitive Impairment Induced by Subchronic Phencyclidine in a Novel Object Recognition Test in Rats. Int. J. Neuropsychopharmacol. 2010, 13, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Amat-Foraster, M.; Leiser, S.C.; Herrik, K.F.; Richard, N.; Agerskov, C.; Bundgaard, C.; Bastlund, J.F.; de Jong, I.E.M. The 5-HT6 Receptor Antagonist Idalopirdine Potentiates the Effects of Donepezil on Gamma Oscillations in the Frontal Cortex of Anesthetized and Awake Rats without Affecting Sleep-Wake Architecture. Neuropharmacology 2017, 113, 45–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atri, A.; Frölich, L.; Ballard, C.; Tariot, P.N.; Molinuevo, J.L.; Boneva, N.; Windfeld, K.; Raket, L.L.; Cummings, J.L. Effect of Idalopirdine as Adjunct to Cholinesterase Inhibitors on Change in Cognition in Patients With Alzheimer Disease. JAMA 2018, 319, 130. [Google Scholar] [CrossRef]
- Lang, F.M.; Mo, Y.; Sabbagh, M.; Solomon, P.; Boada, M.; Jones, R.W.; Frisoni, G.B.; Grimmer, T.; Dubois, B.; Harnett, M.; et al. Intepirdine as Adjunctive Therapy to Donepezil for Mild-to-moderate Alzheimer’s Disease: A Randomized, Placebo-controlled, Phase 3 Clinical Trial (MINDSET). Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12136. [Google Scholar] [CrossRef]
- Nirogi, R.; Ieni, J.; Goyal, V.K.; Ravula, J.; Jetta, S.; Shinde, A.; Jayarajan, P.; Benade, V.; Palacharla, V.R.C.; Dogiparti, D.K.; et al. Effect of Masupirdine (SUVN-502) on Cognition in Patients with Moderate Alzheimer’s Disease: A Randomized, Double-blind, Phase 2, Proof-of-concept Study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12307. [Google Scholar] [CrossRef]
- Shahidi, S.; Asl, S.S.; Komaki, A.; Hashemi-Firouzi, N. The Effect of Chronic Stimulation of Serotonin Receptor Type 7 on Recognition, Passive Avoidance Memory, Hippocampal Long-Term Potentiation, and Neuronal Apoptosis in the Amyloid β Protein Treated Rat. Psychopharmacology 2018, 235, 1513–1525. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Komaki, A.; Soleimani Asl, S.; Shahidi, S. The Effects of the 5-HT7 Receptor on Hippocampal Long-Term Potentiation and Apoptosis in a Rat Model of Alzheimer’s Disease. Brain Res. Bull. 2017, 135, 85–91. [Google Scholar] [CrossRef]
- Quintero-Villegas, A.; Valdés-Ferrer, S.I. Role of 5-HT7 Receptors in the Immune System in Health and Disease. Mol. Med. 2020, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Calcium Hypothesis of Alzheimer’s Disease. Pflug. Arch. 2010, 459, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef]
- Ge, M.; Zhang, J.; Chen, S.; Huang, Y.; Chen, W.; He, L.; Zhang, Y. Role of Calcium Homeostasis in Alzheimer’s Disease. Neuropsychiatr. Dis. Treat. 2022, 18, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Popugaeva, E.; Pchitskaya, E.; Bezprozvanny, I. Dysregulation of Neuronal Calcium Homeostasis in Alzheimer’s Disease—A Therapeutic Opportunity? Biochem. Biophys. Res. Commun. 2017, 483, 998–1004. [Google Scholar] [CrossRef]
- O’Day, D.H. Calmodulin Binding Proteins and Alzheimer’s Disease: Biomarkers, Regulatory Enzymes and Receptors That Are Regulated by Calmodulin. Int. J. Mol. Sci. 2020, 21, 7344. [Google Scholar] [CrossRef]
- Demuro, A.; Mina, E.; Kayed, R.; Milton, S.C.; Parker, I.; Glabe, C.G. Calcium Dysregulation and Membrane Disruption as a Ubiquitous Neurotoxic Mechanism of Soluble Amyloid Oligomers. J. Biol. Chem. 2005, 280, 17294–17300. [Google Scholar] [CrossRef]
- Texidó, L.; Martín-Satué, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid β Peptide Oligomers Directly Activate NMDA Receptors. Cell Calcium 2011, 49, 184–190. [Google Scholar] [CrossRef]
- Ishii, M.; Hiller, A.J.; Pham, L.; McGuire, M.J.; Iadecola, C.; Wang, G. Amyloid-Beta Modulates Low-Threshold Activated Voltage-Gated L-Type Calcium Channels of Arcuate Neuropeptide Y Neurons Leading to Calcium Dysregulation and Hypothalamic Dysfunction. J. Neurosci. 2019, 39, 8816–8825. [Google Scholar] [CrossRef]
- Um, J.W.; Kaufman, A.C.; Kostylev, M.; Heiss, J.K.; Stagi, M.; Takahashi, H.; Kerrisk, M.E.; Vortmeyer, A.; Wisniewski, T.; Koleske, A.J.; et al. Metabotropic Glutamate Receptor 5 Is a Coreceptor for Alzheimer Aβ Oligomer Bound to Cellular Prion Protein. Neuron 2013, 79, 887–902. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Bacskai, B.J. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 2021, 44, 136–151. [Google Scholar] [CrossRef]
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired Mitochondrial Dynamics and Abnormal Interaction of Amyloid Beta with Mitochondrial Protein Drp1 in Neurons from Patients with Alzheimer’s Disease: Implications for Neuronal Damage. Hum. Mol. Genet. 2011, 20, 2495–2509. [Google Scholar] [CrossRef]
- Pérez, M.J.; Ponce, D.P.; Aranguiz, A.; Behrens, M.I.; Quintanilla, R.A. Mitochondrial Permeability Transition Pore Contributes to Mitochondrial Dysfunction in Fibroblasts of Patients with Sporadic Alzheimer’s Disease. Redox. Biol. 2018, 19, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Rodriguez, M.; Kharitonova, E.K.; Bacskai, B.J. Therapeutic Strategies to Target Calcium Dysregulation in Alzheimer’s Disease. Cells 2020, 9, 2513. [Google Scholar] [CrossRef] [PubMed]
- Schampel, A.; Kuerten, S. Danger: High Voltage—The Role of Voltage-Gated Calcium Channels in Central Nervous System Pathology. Cells 2017, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, B.; Segurado, R.; Kennelly, S.; Olde Rikkert, M.G.M.; Howard, R.; Pasquier, F.; Börjesson-Hanson, A.; Tsolaki, M.; Lucca, U.; Molloy, D.W.; et al. Nilvadipine in Mild to Moderate Alzheimer Disease: A Randomised Controlled Trial. PLoS. Med. 2018, 15, e1002660. [Google Scholar] [CrossRef]
- Kodis, E.J.; Choi, S.; Swanson, E.; Ferreira, G.; Bloom, G.S. N-methyl-D-aspartate Receptor–Mediated Calcium Influx Connects Amyloid-β Oligomers to Ectopic Neuronal Cell Cycle Reentry in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 1302–1312. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Mizoguchi, Y.; Ohgidani, M.; Imamura, Y.; Murakawa-Hirachi, T.; Nabeta, H.; Tateishi, H.; Kato, T.A.; Monji, A. Donepezil Suppresses Intracellular Ca2+ Mobilization through the PI3K Pathway in Rodent Microglia. J. Neuroinflamm. 2017, 14, 258. [Google Scholar] [CrossRef]
- Gupta, P.; Tiwari, S.; Singh, A.; Pal, A.; Mishra, A.; Singh, S. Rivastigmine Attenuates the Alzheimer’s Disease Related Protein Degradation and Apoptotic Neuronal Death Signalling. Biochem. J. 2021, 478, 1435–1451. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.A.; Heimala, K.; Wonnacott, S. The Allosteric Potentiation of Nicotinic Acetylcholine Receptors by Galantamine Is Transduced into Cellular Responses in Neurons: Ca 2+ Signals and Neurotransmitter Release. Mol. Pharmacol. 2003, 64, 1217–1226. [Google Scholar] [CrossRef]
- Kastanenka, K.V.; Bussiere, T.; Shakerdge, N.; Qian, F.; Weinreb, P.H.; Rhodes, K.; Bacskai, B.J. Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. J. Neurosci. 2016, 36, 12549–12558. [Google Scholar] [CrossRef]
- Takada, S.H.; Ikebara, J.M.; de Sousa, E.; Cardoso, D.S.; Resende, R.R.; Ulrich, H.; Rückl, M.; Rüdiger, S.; Kihara, A.H. Determining the Roles of Inositol Trisphosphate Receptors in Neurodegeneration: Interdisciplinary Perspectives on a Complex Topic. Mol. Neurobiol. 2017, 54, 6870–6884. [Google Scholar] [CrossRef]
- Chiantia, G.; Hidisoglu, E.; Marcantoni, A. The Role of Ryanodine Receptors in Regulating Neuronal Activity and Its Connection to the Development of Alzheimer’s Disease. Cells 2023, 12, 1236. [Google Scholar] [CrossRef]
- Lacampagne, A.; Liu, X.; Reiken, S.; Bussiere, R.; Meli, A.C.; Lauritzen, I.; Teich, A.F.; Zalk, R.; Saint, N.; Arancio, O.; et al. Post-Translational Remodeling of Ryanodine Receptor Induces Calcium Leak Leading to Alzheimer’s Disease-like Pathologies and Cognitive Deficits. Acta Neuropathol. 2017, 134, 749–767. [Google Scholar] [CrossRef]
- Oulès, B.; Del Prete, D.; Greco, B.; Zhang, X.; Lauritzen, I.; Sevalle, J.; Moreno, S.; Paterlini-Bréchot, P.; Trebak, M.; Checler, F.; et al. Ryanodine Receptor Blockade Reduces Amyloid-β Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. J. Neurosci. 2012, 32, 11820–11834. [Google Scholar] [CrossRef]
- Peng, J.; Liang, G.; Inan, S.; Wu, Z.; Joseph, D.J.; Meng, Q.; Peng, Y.; Eckenhoff, M.F.; Wei, H. Dantrolene Ameliorates Cognitive Decline and Neuropathology in Alzheimer Triple Transgenic Mice. Neurosci. Lett. 2012, 516, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, B.; Liu, C.; Liang, G.; Liu, W.; Pickup, S.; Meng, Q.; Tian, Y.; Li, S.; Eckenhoff, M.F.; et al. Long-Term Dantrolene Treatment Reduced Intraneuronal Amyloid in Aged Alzheimer Triple Transgenic Mice. Alzheimer. Dis. Assoc. Disord. 2015, 29, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Blasco, S.; Calvo-Rodríguez, M.; Caballero, E.; Garcia-Durillo, M.; Nunez, L.; Villalobos, C. Is It All Said for NSAIDs in Alzheimer’s Disease? Role of Mitochondrial Calcium Uptake. Curr. Alzheimer Res. 2018, 15, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Vinogradova, D.; Neganova, M.E.; Serkova, T.P.; Sokolov, V.V.; Bachurin, S.O.; Shevtsova, E.F.; Abramov, A.Y. Pharmacological Sequestration of Mitochondrial Calcium Uptake Protects Neurons Against Glutamate Excitotoxicity. Mol. Neurobiol. 2019, 56, 2244–2255. [Google Scholar] [CrossRef]
- Shevtsova, E.F.; Angelova, P.R.; Stelmashchuk, O.A.; Esteras, N.; Vasil’eva, N.A.; Maltsev, A.V.; Shevtsov, P.N.; Shaposhnikov, A.V.; Fisenko, V.P.; Bachurin, S.O.; et al. Pharmacological Sequestration of Mitochondrial Calcium Uptake Protects against Dementia and β-Amyloid Neurotoxicity. Sci. Rep. 2022, 12, 12766. [Google Scholar] [CrossRef]
- Villard, V.; Espallergues, J.; Keller, E.; Vamvakides, A.; Maurice, T. Anti-Amnesic and Neuroprotective Potentials of the Mixed Muscarinic Receptor/Sigma 1 (Σ1) Ligand ANAVEX2-73, a Novel Aminotetrahydrofuran Derivative. J. Psychopharmacol. 2011, 25, 1101–1117. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- ANAVEX®2-73 (Blarcamesine) AVATAR Phase 3 Trial Met Primary and Secondary Efficacy Endpoints. Available online: https://www.anavex.com/post/anavex-2-73-blarcamesine-avatar-phase-3-trial-met-primary-and-secondary-efficacy-endpoints#:~:text=Primary%20and%20all%20secondary%20efficacy%20and%20safety%20endpoints,%3D%200.010%29%20and%20CGI-I%20%28p%20%3D%200.037%29%20re (accessed on 6 December 2024).
- Macfarlane, S.; Cecchi, M.; Moore, D.; Maruff, P.; Zografidis, T.; Missling, C. P4-356: Safety and Efficacy 31 Week Data of Anavex 2-73 in a Phase 2A Study in Mild-Moderate Alzheimer’S Disease Patients. Alzheimer’s Dement. 2016, 12, P1174. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Van Giau, V.; Bagyinszky, E.; Yang, Y.S.; Youn, Y.C.; An, S.S.A.; Kim, S.Y. Genetic Analyses of Early-Onset Alzheimer’s Disease Using next Generation Sequencing. Sci. Rep. 2019, 9, 8368. [Google Scholar] [CrossRef]
- Shoghi-Jadid, K.; Small, G.W.; Agdeppa, E.D.; Kepe, V.; Ercoli, L.M.; Siddarth, P.; Read, S.; Satyamurthy, N.; Petric, A.; Huang, S.-C.; et al. Localization of Neurofibrillary Tangles and Beta-Amyloid Plaques in the Brains of Living Patients With Alzheimer Disease. Am. J. Geriatr. Psychiatry 2002, 10, 24–35. [Google Scholar] [CrossRef]
- Trejo-Lopez, J.A.; Yachnis, A.T.; Prokop, S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics 2022, 19, 173–185. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s Disease: Pathophysiology and Therapeutic Strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, J.Y.; Lee, T.; Nam, D.; Jeon, C.-J.; Kim, J.B. SPON1 Can Reduce Amyloid Beta and Reverse Cognitive Impairment and Memory Dysfunction in Alzheimer’s Disease Mouse Model. Cells 2020, 9, 1275. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, F.; Li, W.; Abumaria, N. TRPM7 Kinase Activity Induces Amyloid-β Degradation to Reverse Synaptic and Cognitive Deficits in Mouse Models of Alzheimer’s Disease. Sci. Signal 2023, 16, eade6325. [Google Scholar] [CrossRef]
- Li, C.; Ebrahimi, A.; Schluesener, H. Drug Pipeline in Neurodegeneration Based on Transgenic Mice Models of Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 116–140. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Pike, K.E.; Chételat, G.; Ellis, K.A.; Mulligan, R.S.; Bourgeat, P.; Ackermann, U.; Jones, G.; Szoeke, C.; Salvado, O.; et al. Longitudinal Assessment of Aβ and Cognition in Aging and Alzheimer Disease. Ann. Neurol. 2011, 69, 181–192. [Google Scholar] [CrossRef]
- Ferreira, S.T.; Lourenco, M.V.; Oliveira, M.M.; De Felice, F.G. Soluble Amyloid-β Oligomers as Synaptotoxins Leading to Cognitive Impairment in Alzheimer’s Disease. Front. Cell Neurosci. 2015, 9, 191. [Google Scholar] [CrossRef]
- De Strooper, B.; Vassar, R.; Golde, T. The Secretases: Enzymes with Therapeutic Potential in Alzheimer Disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Vellas, B.; Sol, O.; Snyder, P.J.; Ousset, P.-J.; Haddad, R.; Maurin, M.; Lemarie, J.-C.; Desire, L.; Pando, M.P. EHT0202 in Alzheimers Disease: A 3-Month, Randomized, Placebo- Controlled, Double-Blind Study. Curr. Alzheimer Res. 2011, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Endres, K.; Fahrenholz, F.; Lotz, J.; Hiemke, C.; Teipel, S.; Lieb, K.; Tüscher, O.; Fellgiebel, A. Increased CSF APPs-α Levels in Patients with Alzheimer Disease Treated with Acitretin. Neurology 2014, 83, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Bansoad, A.V.; Singh, R.; Khatik, G.L. BACE1: A Key Regulator in Alzheimer’s Disease Progression and Current Development of Its Inhibitors. Curr. Neuropharmacol. 2022, 20, 1174–1193. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Yang, F.; Sivils, A.; Cegielski, V.; Chu, X.-P. Amylin and Secretases in the Pathology and Treatment of Alzheimer’s Disease. Biomolecules 2022, 12, 996. [Google Scholar] [CrossRef]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A Phase 3 Trial of Semagacestat for Treatment of Alzheimer’s Disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Frisardi, V.; Imbimbo, B.P.; Capurso, C.; Logroscino, G.; Sancarlo, D.; Seripa, D.; Vendemiale, G.; Pilotto, A.; Solfrizzi, V. REVIEW: Γ-Secretase Inhibitors for the Treatment of Alzheimer’s Disease: The Current State. CNS Neurosci. Ther. 2010, 16, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Shelton, C.C.; Zhu, L.; Chau, D.; Yang, L.; Wang, R.; Djaballah, H.; Zheng, H.; Li, Y.-M. Modulation of γ-Secretase Specificity Using Small Molecule Allosteric Inhibitors. Proc. Natl. Acad. Sci. USA 2009, 106, 20228–20233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, X.; Li, L.; Zheng, J. Inhibition of Amyloid-β Aggregation in Alzheimer’s Disease. Curr. Pharm. Des. 2014, 20, 1223–1243. [Google Scholar] [CrossRef]
- Maity, D. Inhibition of Amyloid Protein Aggregation Using Selected Peptidomimetics. ChemMedChem 2023, 18, e202200499. [Google Scholar] [CrossRef]
- Konar, M.; Ghosh, D.; Samanta, S.; Govindaraju, T. Combating Amyloid-Induced Cellular Toxicity and Stiffness by Designer Peptidomimetics. RSC Chem. Biol. 2022, 3, 220–226. [Google Scholar] [CrossRef]
- Samanta, S.; Rajasekhar, K.; Ramesh, M.; Murugan, N.A.; Alam, S.; Shah, D.; Clement, J.P.; Govindaraju, T. Naphthalene Monoimide Derivative Ameliorates Amyloid Burden and Cognitive Decline in a Transgenic Mouse Model of Alzheimer’s Disease. Adv. Ther. 2021, 4, 2000225. [Google Scholar] [CrossRef]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bullock, R.; Love, S.; Neal, J.W.; et al. Long-Term Effects of Aβ42 Immunisation in Alzheimer’s Disease: Follow-up of a Randomised, Placebo-Controlled Phase I Trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau Pathology Is an Initiating Factor in Sporadic Alzheimer’s Disease. Alzheimer’s Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Bejanin, A.; Schonhaut, D.R.; La Joie, R.; Kramer, J.H.; Baker, S.L.; Sosa, N.; Ayakta, N.; Cantwell, A.; Janabi, M.; Lauriola, M.; et al. Tau Pathology and Neurodegeneration Contribute to Cognitive Impairment in Alzheimer’s Disease. Brain 2017, 140, 3286–3300. [Google Scholar] [CrossRef]
- Ge, X.; Qiao, Y.; Choi, J.; Raman, R.; Ringman, J.M.; Shi, Y. Enhanced Association of Tau Pathology and Cognitive Impairment in Mild Cognitive Impairment Subjects with Behavior Symptoms. J. Alzheimer’s Dis. 2022, 87, 557–568. [Google Scholar] [CrossRef]
- Mielke, M.M.; Hagen, C.E.; Wennberg, A.M.V.; Airey, D.C.; Savica, R.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R.; Petersen, R.C.; et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017, 74, 1073. [Google Scholar] [CrossRef]
- Guillozet, A.L.; Weintraub, S.; Mash, D.C.; Mesulam, M.M. Neurofibrillary Tangles, Amyloid, and Memory in Aging and Mild Cognitive Impairment. Arch. Neurol. 2003, 60, 729. [Google Scholar] [CrossRef]
- Wolfe, M.S. Tau Mutations in Neurodegenerative Diseases. J. Biol. Chem. 2009, 284, 6021–6025. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-β and Tau. JAMA Neurol. 2014, 71, 505. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological Stageing of Alzheimer-Related Changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Schönheit, B.; Zarski, R.; Ohm, T.G. Spatial and Temporal Relationships between Plaques and Tangles in Alzheimer-Pathology. Neurobiol. Aging 2004, 25, 697–711. [Google Scholar] [CrossRef]
- Braak, H.; Zetterberg, H.; Del Tredici, K.; Blennow, K. Intraneuronal Tau Aggregation Precedes Diffuse Plaque Deposition, but Amyloid-β Changes Occur before Increases of Tau in Cerebrospinal Fluid. Acta Neuropathol. 2013, 126, 631–641. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-β-Independent Regulators of Tau Pathology in Alzheimer Disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Maphis, N.; Xu, G.; Kokiko-Cochran, O.N.; Jiang, S.; Cardona, A.; Ransohoff, R.M.; Lamb, B.T.; Bhaskar, K. Reactive Microglia Drive Tau Pathology and Contribute to the Spreading of Pathological Tau in the Brain. Brain 2015, 138, 1738–1755. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, D.G.; Dickson, D.W.; Josephs, K.A.; Litvan, I. Progressive Supranuclear Palsy and Corticobasal Degeneration. In Frontotemporal Dementias: Emerging Milestones of the 21st Century; Springer: Cham, Switzerland, 2021; pp. 151–176. [Google Scholar]
- Lovestone, S.; Boada, M.; Dubois, B.; Hüll, M.; Rinne, J.O.; Huppertz, H.-J.; Calero, M.; Andrés, M.V.; Gómez-Carrillo, B.; León, T.; et al. A Phase II Trial of Tideglusib in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 75–88. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Nygaard, H.B.; Chen, K.; Donohue, M.C.; Raman, R.; Rissman, R.A.; Brewer, J.B.; Koeppe, R.A.; Chow, T.W.; Rafii, M.S.; et al. Effect of AZD0530 on Cerebral Metabolic Decline in Alzheimer Disease. JAMA Neurol. 2019, 76, 1219. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Huang, H.; Li, Y.; Jiang, L.; Wang, Y.; Jin, H.; Jiao, N.; Zhang, L.; Zhang, L.; Liu, Z. Multistage Screening Reveals 3-Substituted Indolin-2-One Derivatives as Novel and Isoform-Selective c-Jun N-Terminal Kinase 3 (JNK3) Inhibitors: Implications to Drug Discovery for Potential Treatment of Neurodegenerative Diseases. J. Med. Chem. 2019, 62, 6645–6664. [Google Scholar] [CrossRef]
- Wang, X.-L.; Xiong, Y.; Yang, Y.; Tuo, Q.; Wang, X.; Chen, R.; Tian, Q.; Zhang, Z.; Yan, X.; Yang, Z.; et al. A Novel Tacrine-Dihydropyridine Hybrid (-)SCR1693 Induces Tau Dephosphorylation and Inhibits Aβ Generation in Cells. Eur. J. Pharmacol. 2015, 754, 134–139. [Google Scholar] [CrossRef]
- Zhang, X.; Hernandez, I.; Rei, D.; Mair, W.; Laha, J.K.; Cornwell, M.E.; Cuny, G.D.; Tsai, L.-H.; Steen, J.A.J.; Kosik, K.S. Diaminothiazoles Modify Tau Phosphorylation and Improve the Tauopathy in Mouse Models. J. Biol. Chem. 2013, 288, 22042–22056. [Google Scholar] [CrossRef]
- Soumbasis, A.; Eldeeb, M.A.; Ragheb, M.A.; Zorca, C.E. Dephosphorylation Targeting Chimaera (DEPTAC): Targeting Tau Proteins in Tauopathies. Curr. Protein Pept. Sci. 2022, 23, 129–132. [Google Scholar] [CrossRef]
- Ballatore, C.; Brunden, K.R.; Huryn, D.M.; Trojanowski, J.Q.; Lee, V.M.-Y.; Smith, A.B. Microtubule Stabilizing Agents as Potential Treatment for Alzheimer’s Disease and Related Neurodegenerative Tauopathies. J. Med. Chem. 2012, 55, 8979–8996. [Google Scholar] [CrossRef]
- Ancidoni, A.; Bacigalupo, I.; Remoli, G.; Lacorte, E.; Piscopo, P.; Sarti, G.; Corbo, M.; Vanacore, N.; Canevelli, M. Anticancer Drugs Repurposed for Alzheimer’s Disease: A Systematic Review. Alzheimer’s Res. Ther. 2021, 13, 96. [Google Scholar] [CrossRef]
- Brunden, K.R.; Zhang, B.; Carroll, J.; Yao, Y.; Potuzak, J.S.; Hogan, A.-M.L.; Iba, M.; James, M.J.; Xie, S.X.; Ballatore, C.; et al. Epothilone D Improves Microtubule Density, Axonal Integrity, and Cognition in a Transgenic Mouse Model of Tauopathy. J. Neurosci. 2010, 30, 13861–13866. [Google Scholar] [CrossRef]
- Guo, B.; Huang, Y.; Gao, Q.; Zhou, Q. Stabilization of Microtubules Improves Cognitive Functions and Axonal Transport of Mitochondria in Alzheimer’s Disease Model Mice. Neurobiol. Aging 2020, 96, 223–232. [Google Scholar] [CrossRef]
- Robles-Gómez, Á.A.; Ordaz, B.; Lorea-Hernández, J.-J.; Peña-Ortega, F. Deleterious and Protective Effects of Epothilone-D Alone and in the Context of Amyloid β- and Tau-Induced Alterations. Front. Mol. Neurosci. 2023, 16, 1198299. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, J.; Ramirez, L.-M.; Gümüşdil, E.; Pokhrel, P.; Man, V.H.; Ye, H.; Han, Y.; Liu, Y.; Li, P.; et al. Methylene Blue Accelerates Liquid-to-Gel Transition of Tau Condensates Impacting Tau Function and Pathology. Nat. Commun. 2023, 14, 5444. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J.; et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimer’s Dis. 2017, 61, 435–457. [Google Scholar] [CrossRef]
- Gauthier, S.; Feldman, H.H.; Schneider, L.S.; Wilcock, G.K.; Frisoni, G.B.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Wischik, D.J.; et al. Efficacy and Safety of Tau-Aggregation Inhibitor Therapy in Patients with Mild or Moderate Alzheimer’s Disease: A Randomised, Controlled, Double-Blind, Parallel-Arm, Phase 3 Trial. Lancet 2016, 388, 2873–2884. [Google Scholar] [CrossRef]
- Kontsekova, E.; Zilka, N.; Kovacech, B.; Novak, P.; Novak, M. First-in-Man Tau Vaccine Targeting Structural Determinants Essential for Pathological Tau–Tau Interaction Reduces Tau Oligomerisation and Neurofibrillary Degeneration in an Alzheimer’s Disease Model. Alzheimer’s Res. Ther. 2014, 6, 44. [Google Scholar] [CrossRef]
- Rajamohamedsait, H.; Rasool, S.; Rajamohamedsait, W.; Lin, Y.; Sigurdsson, E.M. Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice. Sci. Rep. 2017, 7, 17034. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N.; et al. ADAMANT: A Placebo-Controlled Randomized Phase 2 Study of AADvac1, an Active Immunotherapy against Pathological Tau in Alzheimer’s Disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Davtyan, H.; Chen, W.W.; Zagorski, K.; Davis, J.; Petrushina, I.; Kazarian, K.; Cribbs, D.H.; Agadjanyan, M.G.; Blurton-Jones, M.; Ghochikyan, A. MultiTEP Platform-Based DNA Epitope Vaccine Targeting N-Terminus of Tau Induces Strong Immune Responses and Reduces Tau Pathology in THY-Tau22 Mice. Vaccine 2017, 35, 2015–2024. [Google Scholar] [CrossRef]
- West, T.; Hu, Y.; Verghese, P.B.; Bateman, R.J.; Braunstein, J.B.; Fogelman, I.; Budur, K.; Florian, H.; Mendonca, N.; Holtzman, D.M. Preclinical and Clinical Development of ABBV-8E12, a Humanized Anti-Tau Antibody, for Treatment of Alzheimer’s Disease and Other Tauopathies. J. Prev. Alzheimers Dis. 2017, 4, 236–241. [Google Scholar] [CrossRef]
- Sopko, R.; Golonzhka, O.; Arndt, J.; Quan, C.; Czerkowicz, J.; Cameron, A.; Smith, B.; Murugesan, Y.; Gibbons, G.; Kim, S.-J.; et al. Characterization of Tau Binding by Gosuranemab. Neurobiol. Dis. 2020, 146, 105120. [Google Scholar] [CrossRef]
- Budur, K.; West, T.; Braunstein, J.B.; Fogelman, I.; Bordelon, Y.M.; Litvan, I.; Roberson, E.D.; Hu, H.; Verghese, P.B.; Bateman, R.J.; et al. Results of a phase 1, single ascending dose, placebo-controlled study of abbv-8e12 in patients with progressive supranuclear palsy and phase 2 study design in early Alzheimer’s disease. Alzheimer’s Dement. 2017, P599. [Google Scholar] [CrossRef]
- Shulman, M.; Kong, J.; O’Gorman, J.; Ratti, E.; Rajagovindan, R.; Viollet, L.; Huang, E.; Sharma, S.; Racine, A.M.; Czerkowicz, J.; et al. TANGO: A Placebo-Controlled Randomized Phase 2 Study of Efficacy and Safety of the Anti-Tau Monoclonal Antibody Gosuranemab in Early Alzheimer’s Disease. Nat. Aging 2023, 3, 1591–1601. [Google Scholar] [CrossRef]
- ALZFORUM-THERAPEUTICS (E2814). Available online: https://Www.Alzforum.Org/Therapeutics/E2814 (accessed on 5 December 2024).
- Chu, T.-T.; Gao, N.; Li, Q.-Q.; Chen, P.-G.; Yang, X.-F.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Specific Knockdown of Endogenous Tau Protein by Peptide-Directed Ubiquitin-Proteasome Degradation. Cell Chem. Biol. 2016, 23, 453–461. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zheng, J.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A Novel Small-Molecule PROTAC Selectively Promotes Tau Clearance to Improve Cognitive Functions in Alzheimer-like Models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef]
- Chen, Z.; Balachandran, Y.L.; Chong, W.P.; Chan, K.W.Y. Roles of Cytokines in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 5803. [Google Scholar] [CrossRef]
- Jorda, A.; Campos-Campos, J.; Iradi, A.; Aldasoro, M.; Aldasoro, C.; Vila, J.M.; Valles, S.L. The Role of Chemokines in Alzheimer’s Disease. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1383–1390. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Merighi, S.; Nigro, M.; Travagli, A.; Gessi, S. Microglia and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 12990. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, Neuroinflammation, and Beta-Amyloid Protein in Alzheimer’s Disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Liao, Y.; Xing, Q.; Li, Q.; Zhang, J.; Pan, R.; Yuan, Z. Astrocytes in Depression and Alzheimer’s Disease. Front. Med. 2021, 15, 829–841. [Google Scholar] [CrossRef]
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways. Cells 2021, 10, 540. [Google Scholar] [CrossRef]
- Grammas, P. Inflammatory Factors Are Elevated in Brain Microvessels in Alzheimer’s Disease. Neurobiol. Aging 2001, 22, 837–842. [Google Scholar] [CrossRef]
- Piette, F.; Belmin, J.; Vincent, H.; Schmidt, N.; Pariel, S.; Verny, M.; Marquis, C.; Mely, J.; Hugonot-Diener, L.; Kinet, J.-P.; et al. Masitinib as an Adjunct Therapy for Mild-to-Moderate Alzheimer’s Disease: A Randomised, Placebo-Controlled Phase 2 Trial. Alzheimer’s Res. Ther. 2011, 3, 16. [Google Scholar] [CrossRef]
- Dubois, B.; López-Arrieta, J.; Lipschitz, S.; Doskas, T.; Spiru, L.; Moroz, S.; Venger, O.; Vermersch, P.; Moussy, A.; Mansfield, C.D.; et al. Masitinib for Mild-to-Moderate Alzheimer’s Disease: Results from a Randomized, Placebo-Controlled, Phase 3, Clinical Trial. Alzheimer’s Res. Ther. 2023, 15, 39. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Cecon, E.; Dam, J.; Pei, Z.; Jockers, R.; Burns, L.H. Simufilam Reverses Aberrant Receptor Interactions of Filamin A in Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 13927. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Pei, Z.; Lee, K.-C.; Lopez-Brignoni, E.; Nikolov, B.; Crowley, C.A.; Marsman, M.R.; Barbier, R.; Friedmann, N.; Burns, L.H. PTI-125 Reduces Biomarkers of Alzheimer’s Disease in Patients. J. Prev. Alzheimers Dis. 2020, 7, 256–264. [Google Scholar] [CrossRef]
- Cassava Sciences Announces Positive Top-Line Clinical Results in Phase 2 Study Evaluating Simufilam in Alzheimer’s Disease. Available online: https://Www.Cassavasciences.Com/News-Releases/News-Release-Details/Cassava-Sciences-Announces-Positive-Top-Line-Clinical-Results (accessed on 6 December 2024).
- Zhang, C.; Griciuc, A.; Hudry, E.; Wan, Y.; Quinti, L.; Ward, J.; Forte, A.M.; Shen, X.; Ran, C.; Elmaleh, D.R.; et al. Cromolyn Reduces Levels of the Alzheimer’s Disease-Associated Amyloid β-Protein by Promoting Microglial Phagocytosis. Sci. Rep. 2018, 8, 1144. [Google Scholar] [CrossRef]
- Kulkarni, B.; Kumar, D.; Cruz-Martins, N.; Sellamuthu, S. Role of TREM2 in Alzheimer’s Disease: A Long Road Ahead. Mol. Neurobiol. 2021, 58, 5239–5252. [Google Scholar] [CrossRef]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-Human TREM2 Induces Microglia Proliferation and Reduces Pathology in an Alzheimer’s Disease Model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef]
- van Lengerich, B.; Zhan, L.; Xia, D.; Chan, D.; Joy, D.; Park, J.I.; Tatarakis, D.; Calvert, M.; Hummel, S.; Lianoglou, S.; et al. A TREM2-Activating Antibody with a Blood–Brain Barrier Transport Vehicle Enhances Microglial Metabolism in Alzheimer’s Disease Models. Nat. Neurosci. 2023, 26, 416–429. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 Receptor System Reverses Amyloid-Induced Memory Deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Magham, S.V.; Thaggikuppe krishnamurthy, P.; Shaji, N.; Mani, L.; Balasubramanian, S. Cannabinoid Receptor 2 Selective Agonists and Alzheimer’s Disease: An Insight into the Therapeutic Potentials. J. Neurosci. Res. 2021, 99, 2888–2905. [Google Scholar] [CrossRef]
- Kiraly, M.; Foss, J.F.; Giordano, T. Neuroinflammation, Its Role in Alzheimer’s Disease and Therapeutic Strategies. J. Prev. Alzheimer’s Dis. 2023, 10, 686–698. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Swerdlow, R.H. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: A Current Overview. J. Alzheimer’s Dis. 2023, 92, 751–768. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria Dysfunction in the Pathogenesis of Alzheimer’s Disease: Recent Advances. Mol. Neurodegener. 2020, 15, 30. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, F. The Association of Tau With Mitochondrial Dysfunction in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 163. [Google Scholar] [CrossRef]
- Huang, Z.; Yan, Q.; Wang, Y.; Zou, Q.; Li, J.; Liu, Z.; Cai, Z. Role of Mitochondrial Dysfunction in the Pathology of Amyloid-β. J. Alzheimer’s Dis. 2020, 78, 505–514. [Google Scholar] [CrossRef]
- Leuner, K.; Müller, W.E.; Reichert, A.S. From Mitochondrial Dysfunction to Amyloid Beta Formation: Novel Insights into the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2012, 46, 186–193. [Google Scholar] [CrossRef]
- Burtscher, J.; Romani, M.; Bernardo, G.; Popa, T.; Ziviani, E.; Hummel, F.C.; Sorrentino, V.; Millet, G.P. Boosting Mitochondrial Health to Counteract Neurodegeneration. Prog. Neurobiol. 2022, 215, 102289. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Geetha, T.; Broderick, T.L.; Babu, J.R. The Role of Dietary Antioxidants and Their Potential Mechanisms in Alzheimer’s Disease Treatment. Metabolites 2023, 13, 438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.; Ma, A.; You, J.; Miao, J.; Li, J. Natural Antioxidants: An Effective Strategy for the Treatment of Alzheimer’s Disease at the Early Stage. J. Agric. Food Chem. 2024, 72, 11854–11870. [Google Scholar] [CrossRef]
- Collins, A.E.; Saleh, T.M.; Kalisch, B.E. Naturally Occurring Antioxidant Therapy in Alzheimer’s Disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Kontush, A.; Schekatolina, S. Vitamin E in Neurodegenerative Disorders: Alzheimer’s Disease. Ann. N. Y. Acad Sci. 2004, 1031, 249–262. [Google Scholar] [CrossRef]
- Hertzog da Silva Leme, A.G.; Cardoso, B.R. Selenium and Alzheimer’s Disease. In Genetics, Neurology, Behavior, and Diet in Dementia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 739–748. [Google Scholar]
- Holland, T.M.; Agarwal, P.; Wang, Y.; Leurgans, S.E.; Bennett, D.A.; Booth, S.L.; Morris, M.C. Dietary Flavonols and Risk of Alzheimer Dementia. Neurology 2020, 94, e1749–e1756. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Dong, Q.-X.; Zhu, J.; Huang, Y.; Hou, S.; Yu, X.; Liu, R. Rutin Prevents Tau Pathology and Neuroinflammation in a Mouse Model of Alzheimer’s Disease. J. Neuroinflamm. 2021, 18, 131. [Google Scholar] [CrossRef]
- Punmiya, A.; Prabhu, A. Structural Fingerprinting of Pleiotropic Flavonoids for Multifaceted Alzheimer’s Disease. Neurochem. Int. 2023, 163, 105486. [Google Scholar] [CrossRef]
- Buglio, D.S.; Marton, L.T.; Laurindo, L.F.; Guiguer, E.L.; Araújo, A.C.; Buchaim, R.L.; de Goulart, R.A.; Rubira, C.J.; Barbalho, S.M. The Role of Resveratrol in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review. J. Med. Food 2022, 25, 797–806. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Fillit, H.M.; Dacks, P.A.; McKeehan, N. Evaluation of the neuroprotective potential of n-acetylcysteine for prevention and treatment of cognitive aging and dementia. J. Prev. Alzheimers Dis. 2017, 4, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, M. Brain-targeted N-Acetyl Cysteine-loaded Nanoparticle for AD Treatment. Alzheimer’s Dement. 2022, 18, e069329. [Google Scholar] [CrossRef]
- McManus, M.J.; Murphy, M.P.; Franklin, J.L. The Mitochondria-Targeted Antioxidant MitoQ Prevents Loss of Spatial Memory Retention and Early Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2011, 31, 15703–15715. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Kandimalla, R. Mitochondria-Targeted Small Molecule SS31: A Potential Candidate for the Treatment of Alzheimer’s Disease. Hum. Mol. Genet. 2017, 26, 1483–1496. [Google Scholar] [CrossRef]
- Srivastava, A.; Johnson, M.; Renna, H.A.; Sheehan, K.M.; Ahmed, S.; Palaia, T.; Pinkhasov, A.; Gomolin, I.H.; De Leon, J.; Reiss, A.B. Therapeutic Potential of P110 Peptide: New Insights into Treatment of Alzheimer’s Disease. Life 2023, 13, 2156. [Google Scholar] [CrossRef]
- Joshi, A.U.; Saw, N.L.; Shamloo, M.; Mochly-Rosen, D. Drp1/Fis1 Interaction Mediates Mitochondrial Dysfunction, Bioenergetic Failure and Cognitive Decline in Alzheimer’s Disease. Oncotarget 2018, 9, 6128–6143. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy Inhibits Amyloid-β and Tau Pathology and Reverses Cognitive Deficits in Models of Alzheimer’s Disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, S.; Maezawa, I.; Trushin, S.; Minhas, P.; Pinto, M.; Jin, L.-W.; Prasain, K.; Nguyen, T.D.T.; Yamazaki, Y.; et al. Modulation of Mitochondrial Complex I Activity Averts Cognitive Decline in Multiple Animal Models of Familial Alzheimer’s Disease. EBioMedicine 2015, 2, 294–305. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, J.C.; Mussivan, T. Can Disturbed Brain Microcirculation Cause Alzheimer’s Disease? Neurol. Res. 1993, 15, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; de Wit, N.M.; van der Flier, W.M.; de Vries, H.E. The Blood Brain Barrier in Alzheimer’s Disease. Vascul. Pharmacol. 2017, 89, 12–18. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Markus, H.S. Vascular Risk Factors and Alzheimer’s Disease. BMC Med. 2014, 12, 218. [Google Scholar] [CrossRef]
- Perry, G.; Smith, M.A.; McCann, C.E.; Siedlak, S.L.; Jones, P.K.; Friedland, R.P. Cerebrovascular Muscle Atrophy Is a Feature of Alzheimer’s Disease. Brain Res. 1998, 791, 63–66. [Google Scholar] [CrossRef]
- Cheng, Z.; Dai, L.; Wu, Y.; Cao, Y.; Chai, X.; Wang, P.; Liu, C.; Ni, M.; Gao, F.; Wang, Q.; et al. Correlation of Blood–Brain Barrier Leakage with Cerebral Small Vessel Disease Including Cerebral Microbleeds in Alzheimer’s Disease. Front. Neurol. 2023, 14, 1077860. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral Blood Flow Decrease as an Early Pathological Mechanism in Alzheimer’s Disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Aliev, G.; Palacios, H.H.; Walrafen, B.; Lipsitt, A.E.; Obrenovich, M.E.; Morales, L. Brain Mitochondria as a Primary Target in the Development of Treatment Strategies for Alzheimer Disease. Int. J. Biochem. Cell Biol. 2009, 41, 1989–2004. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Xuan, K.; Zhao, T.; Qu, G.; Liu, H.; Chen, X.; Sun, Y. The Efficacy of Statins in the Treatment of Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trial. Neurol. Sci. 2020, 41, 1391–1404. [Google Scholar] [CrossRef]
- Petek, B.; Häbel, H.; Xu, H.; Villa-Lopez, M.; Kalar, I.; Hoang, M.T.; Maioli, S.; Pereira, J.B.; Mostafaei, S.; Winblad, B.; et al. Statins and Cognitive Decline in Patients with Alzheimer’s and Mixed Dementia: A Longitudinal Registry-Based Cohort Study. Alzheimer’s Res. Ther. 2023, 15, 220. [Google Scholar] [CrossRef]
- Schultz, B.G.; Patten, D.K.; Berlau, D.J. The Role of Statins in Both Cognitive Impairment and Protection against Dementia: A Tale of Two Mechanisms. Transl. Neurodegener. 2018, 7, 5. [Google Scholar] [CrossRef]
- Suraweera, C.; de Silva, V.; Hanwella, R. Simvastatin-Induced Cognitive Dysfunction: Two Case Reports. J. Med. Case Rep. 2016, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Alsubaie, N.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Alharbi, B.; De Waard, M.; Sabatier, J.-M.; Saad, H.M.; Batiha, G.E.-S. Statins Use in Alzheimer Disease: Bane or Boon from Frantic Search and Narrative Review. Brain Sci. 2022, 12, 1290. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Troiano, V.; La Porta, R. Statins in Alzheimer’s Disease (AD). Eur. J. Clin. Pharmacol. 2022, 78, 1201–1202. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mayer, C.L.; Morelli, D.; Millard, S.P.; Raskind, W.H.; Petrie, E.C.; Cherrier, M.; Fagan, A.M.; Raskind, M.A.; Peskind, E.R. Effect of Simvastatin on CSF Alzheimer Disease Biomarkers in Cognitively Normal Adults. Neurology 2017, 89, 1251–1255. [Google Scholar] [CrossRef]
- Tzimopoulou, S.; Cunningham, V.J.; Nichols, T.E.; Searle, G.; Bird, N.P.; Mistry, P.; Dixon, I.J.; Hallett, W.A.; Whitcher, B.; Brown, A.P.; et al. A Multi-Center Randomized Proof-of-Concept Clinical Trial Applying [18F]FDG-PET for Evaluation of Metabolic Therapy with Rosiglitazone XR in Mild to Moderate Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 22, 1241–1256. [Google Scholar] [CrossRef]
- Nelson, M.L.; Pfeifer, J.A.; Hickey, J.P.; Collins, A.E.; Kalisch, B.E. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1042. [Google Scholar] [CrossRef]
- Moonga, I.; Niccolini, F.; Wilson, H.; Pagano, G.; Politis, M. Hypertension Is Associated with Worse Cognitive Function and Hippocampal Hypometabolism in Alzheimer’s Disease. Eur. J. Neurol. 2017, 24, 1173–1182. [Google Scholar] [CrossRef]
- Wharton, W.; Stein, J.H.; Korcarz, C.; Sachs, J.; Olson, S.R.; Zetterberg, H.; Dowling, M.; Ye, S.; Gleason, C.E.; Underbakke, G.; et al. The Effects of Ramipril in Individuals at Risk for Alzheimer’s Disease: Results of a Pilot Clinical Trial. J. Alzheimer’s Dis. 2012, 32, 147–156. [Google Scholar] [CrossRef]
- Ouk, M.; Wu, C.-Y.; Rabin, J.S.; Jackson, A.; Edwards, J.D.; Ramirez, J.; Masellis, M.; Swartz, R.H.; Herrmann, N.; Lanctôt, K.L.; et al. The Use of Angiotensin-Converting Enzyme Inhibitors vs. Angiotensin Receptor Blockers and Cognitive Decline in Alzheimer’s Disease: The Importance of Blood-Brain Barrier Penetration and APOE Ε4 Carrier Status. Alzheimer’s Res. Ther. 2021, 13, 43. [Google Scholar] [CrossRef]
- Ye, R.; Hu, Y.; Yao, A.; Yang, Y.; Shi, Y.; Jiang, Y.; Zhang, J. Impact of Renin-Angiotensin System-Targeting Antihypertensive Drugs on Treatment of Alzheimer’s Disease: A Meta-Analysis. Int. J. Clin. Pract. 2015, 69, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target Ther. 2024, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Crismon, M.L. Tacrine: First Drug Approved for Alzheimer’s Disease. Ann. Pharmacother. 1994, 28, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, Ó.M.; Ismaili, L.; Iriepa, I.; Diez-Iriepa, D.; Chabchoub, F.; Marco-Contelles, J.; Pérez, M. Tacrines as Therapeutic Agents for Alzheimer’s Disease. V. Recent Developments. Chem. Rec. 2021, 21, 162–174. [Google Scholar] [CrossRef]
- Benjamin, B.; Burns, A. Donepezil for Alzheimer’s Disease. Expert Rev. Neurother. 2007, 7, 1243–1249. [Google Scholar] [CrossRef]
- Jann, M.W. Rivastigmine, a New-Generation Cholinesterase Inhibitor for the Treatment of Alzheimer’s Disease. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2000, 20, 1–12. [Google Scholar] [CrossRef]
- Nguyen, K.; Hoffman, H.; Chakkamparambil, B.; Grossberg, G.T. Evaluation of Rivastigmine in Alzheimer’s Disease. Neurodegener. Dis. Manag. 2021, 11, 35–48. [Google Scholar] [CrossRef]
- Prvulovic, D.; Hampel, H.; Pantel, J. Galantamine for Alzheimer’s Disease. Expert Opin. Drug Metab. Toxicol. 2010, 6, 345–354. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Ren, J. Basic Information about Memantine and Its Treatment of Alzheimer’s Disease and Other Clinical Applications. Ibrain 2023, 9, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Fox, N. Defining disease modifying therapy for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2017, 4, 109. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; McShane, R.; Lindesay, J.; Ritchie, C.; Baldwin, A.; Barber, R.; Burns, A.; Dening, T.; Findlay, D.; Holmes, C.; et al. Donepezil and Memantine for Moderate-to-Severe Alzheimer’s Disease. N. Engl. J. Med. 2012, 366, 893–903. [Google Scholar] [CrossRef] [PubMed]
- Nourelden, A.Z.; Kamal, I.; Tawfik, A.G.; Hagrass, A.I.; Fathallah, A.H.; Zaazouee, M.S. Safety and Efficacy of Sodium Oligomannate in Patients with Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Egypt J. Neurol. Psychiatr. Neurosurg. 2023, 59, 82. [Google Scholar] [CrossRef]
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443. [Google Scholar] [CrossRef]
- Hoy, S.M. Lecanemab: First Approval. Drugs 2023, 83, 359–365. [Google Scholar] [CrossRef]
- Kang, C. Donanemab: First Approval. Drugs 2024, 84, 1313–1318. [Google Scholar] [CrossRef]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef]
- Novak, M.; Vajrychova, M.; Koutsilieri, S.; Sismanoglou, D.-C.; Kobrlova, T.; Prchal, L.; Svobodova, B.; Korabecny, J.; Zarybnicky, T.; Raisova-Stuchlikova, L.; et al. Tacrine First-Phase Biotransformation and Associated Hepatotoxicity: A Possible Way to Avoid Quinone Methide Formation. ACS Chem. Biol. 2023, 18, 1993–2002. [Google Scholar] [CrossRef]
- Bubley, A.; Erofeev, A.; Gorelkin, P.; Beloglazkina, E.; Majouga, A.; Krasnovskaya, O. Tacrine-Based Hybrids: Past, Present, and Future. Int. J. Mol. Sci. 2023, 24, 1717. [Google Scholar] [CrossRef]
- Bryson, H.M.; Benfield, P. Donepezil. Drugs Aging 1997, 10, 234–239. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, V.; Sharma, S. Donepezil. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- FDA Approved Drug Products: Aricept/Aricept ODT (Donepezil Hydrochloride) Tablets for Oral Use. Available online: https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2018/020690s042,021720s014,022568s011lbl.Pdf (accessed on 6 December 2024).
- Birks, J.S.; Harvey, R.J. Donepezil for Dementia Due to Alzheimer’s Disease. Cochrane Database Syst. Rev. 2018, 2018, CD001190. [Google Scholar] [CrossRef]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s Disease Current Therapies, Novel Drug Delivery Systems and Future Directions for Better Disease Management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef]
- Desai, A.K.; Grossberg, G.T. Rivastigmine for Alzheimer’s Disease. Expert Rev. Neurother. 2005, 5, 563–580. [Google Scholar] [CrossRef]
- Lilienfeld, S. Galantamine—A Novel Cholinergic Drug with a Unique Dual Mode of Action for the Treatment of Patients with Alzheimer’s Disease. CNS Drug Rev. 2002, 8, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, M.; García, A.G.; Marco-Contelles, J.; López, M.G. An Update on the Pharmacology of Galantamine. Expert Opin. Investig. Drugs 2007, 16, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- FDA Approval. Available online: https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2015/021615s021lbl.Pdf (accessed on 6 December 2024).
- Kalola, U.K.; Patel, P.; Nguyen, H. Galantamine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; M. Ribeiro, F. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Moreta, M.P.-G.; Burgos-Alonso, N.; Torrecilla, M.; Marco-Contelles, J.; Bruzos-Cidón, C. Efficacy of Acetylcholinesterase Inhibitors on Cognitive Function in Alzheimer’s Disease. Review of Reviews. Biomedicines 2021, 9, 1689. [Google Scholar] [CrossRef]
- Salehipour, A.; Bagheri, M.; Sabahi, M.; Dolatshahi, M.; Boche, D. Combination Therapy in Alzheimer’s Disease: Is It Time? J. Alzheimer’s Dis. 2022, 87, 1433–1449. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Liu, R.; Huang, Y.; Zhang, N.; Zhang, R. Memantine, Donepezil, or Combination Therapy—What Is the Best Therapy for Alzheimer’s Disease? A Network Meta-Analysis. Brain Behav. 2020, 10, e01831. [Google Scholar] [CrossRef]
- Chen, H.; Pellegrini, J.; Aggarwal, S.; Lei, S.; Warach, S.; Jensen, F.; Lipton, S. Open-Channel Block of N-Methyl-D-Aspartate (NMDA) Responses by Memantine: Therapeutic Advantage against NMDA Receptor-Mediated Neurotoxicity. J. Neurosci. 1992, 12, 4427–4436. [Google Scholar] [CrossRef]
- Kavirajan, H. Memantine: A Comprehensive Review of Safety and Efficacy. Expert Opin. Drug Saf. 2009, 8, 89–109. [Google Scholar] [CrossRef]
- Rossom, R.; Adityanjee; Dysken, M. Efficacy and Tolerability of Memantine in the Treatment of Dementia. Am. J. Geriatr. Pharmacother. 2004, 2, 303–312. [Google Scholar] [CrossRef]
- Escamilla, S.; Sáez-Valero, J.; Cuchillo-Ibáñez, I. NMDARs in Alzheimer’s Disease: Between Synaptic and Extrasynaptic Membranes. Int. J. Mol. Sci. 2024, 25, 10220. [Google Scholar] [CrossRef]
- McKeage, K. Memantine. CNS Drugs 2009, 23, 881–897. [Google Scholar] [CrossRef]
- Lo, D.; Grossberg, G.T. Use of Memantine for the Treatment of Dementia. Expert Rev. Neurother. 2011, 11, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S. Lack of Evidence for the Efficacy of Memantine in Mild Alzheimer Disease. Arch. Neurol. 2011, 68, 991. [Google Scholar] [CrossRef] [PubMed]
- Rammes, G.; Rupprecht, R.; Ferrari, U.; Zieglgänsberger, W.; Parsons, C.G. The N-Methyl-d-Aspartate Receptor Channel Blockers Memantine, MRZ 2/579 and Other Amino-Alkyl-Cyclohexanes Antagonise 5-HT3 Receptor Currents in Cultured HEK-293 and N1E-115 Cell Systems in a Non-Competitive Manner. Neurosci. Lett. 2001, 306, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Kuns, B.; Rosani, A.; Patel, P.; Varghese, D. Memantine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Neațu, M.; Covaliu, A.; Ioniță, I.; Jugurt, A.; Davidescu, E.I.; Popescu, B.O. Monoclonal Antibody Therapy in Alzheimer’s Disease. Pharmaceutics 2023, 16, 60. [Google Scholar] [CrossRef]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S.; et al. Structural and Kinetic Basis for the Selectivity of Aducanumab for Aggregated Forms of Amyloid-β. Sci. Rep. 2018, 8, 6412. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The Antibody Aducanumab Reduces Aβ Plaques in Alzheimer’s Disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Herring, W.L.; Gould, I.G.; Fillit, H.; Lindgren, P.; Forrestal, F.; Thompson, R.; Pemberton-Ross, P. Predicted Lifetime Health Outcomes for Aducanumab in Patients with Early Alzheimer’s Disease. Neurol. Ther. 2021, 10, 919–940. [Google Scholar] [CrossRef]
- Kuller, L.H.; Lopez, O.L. ENGAGE and EMERGE: Truth and Consequences? Alzheimer’s Dement. 2021, 17, 692–695. [Google Scholar] [CrossRef]
- Biogen Abandons Aducanumab, Pivots Focus to Lecanemab for Alzheimer Disease. Available online: https://Www.Ajmc.Com/View/Biogen-Abandons-Aducanumab-Pivots-Focus-to-Lecanemab-for-Alzheimer-Disease (accessed on 6 December 2024).
- Yadollahikhales, G.; Rojas, J.C. Anti-Amyloid Immunotherapies for Alzheimer’s Disease: A 2023 Clinical Update. Neurotherapeutics 2023, 20, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A Randomized, Double-Blind, Phase 2b Proof-of-Concept Clinical Trial in Early Alzheimer’s Disease with Lecanemab, an Anti-Aβ Protofibril Antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lancet, T. Lecanemab for Alzheimer’s Disease: Tempering Hype and Hope. Lancet 2022, 400, 1899. [Google Scholar] [CrossRef]
- Qiao, Y.; Chi, Y.; Zhang, Q.; Ma, Y. Safety and Efficacy of Lecanemab for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Aging Neurosci. 2023, 15, 1169499. [Google Scholar] [CrossRef]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s Disease: Targeting β-Amyloid and Beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Alawode, D.O.T.; Heslegrave, A.J.; Fox, N.C.; Zetterberg, H. Donanemab Removes Alzheimer’s Plaques: What Is Special about Its Target? Lancet Healthy Longev. 2021, 2, e395–e396. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease. JAMA 2023, 330, 512. [Google Scholar] [CrossRef]
- FDA’s Decision to Approve New Treatment for Alzheimer’s Disease. Available online: https://Www.Fda.Gov/Drugs/Our-Perspective/Fdas-Decision-Approve-New-Treatment-Alzheimers-Disease (accessed on 6 December 2024).
- Ackley, S.F.; Zimmerman, S.C.; Brenowitz, W.D.; Tchetgen Tchetgen, E.J.; Gold, A.L.; Manly, J.J.; Mayeda, E.R.; Filshtein, T.J.; Power, M.C.; Elahi, F.M.; et al. Effect of Reductions in Amyloid Levels on Cognitive Change in Randomized Trials: Instrumental Variable Meta-Analysis. BMJ 2021, 372, n156. [Google Scholar] [CrossRef]
- Anandakrishnan, R.; Tobey, H.; Nguyen, S.; Sandoval, O.; Klein, B.G.; Costa, B.M. Cranial Manipulation Affects Cholinergic Pathway Gene Expression in Aged Rats. J. Osteopath. Med. 2022, 122, 95–103. [Google Scholar] [CrossRef]
- Rapaka, D.; Adiukwu, P.C.; Bitra, V.R. Experimentally Induced Animal Models for Cognitive Dysfunction and Alzheimer’s Disease. MethodsX 2022, 9, 101933. [Google Scholar] [CrossRef]
- Raina, P.; Santaguida, P.; Ismaila, A.; Patterson, C.; Cowan, D.; Levine, M.; Booker, L.; Oremus, M. Effectiveness of Cholinesterase Inhibitors and Memantine for Treating Dementia: Evidence Review for a Clinical Practice Guideline. Ann. Intern. Med. 2008, 148, 379. [Google Scholar] [CrossRef]
- Singh, Y.P.; Kumar, H. Recent Advances in Medicinal Chemistry of Memantine Against Alzheimer’s Disease. Chem. Biol. Drug Des. 2024, 104, e14638. [Google Scholar] [CrossRef]
- Kishi, T.; Matsunaga, S.; Oya, K.; Nomura, I.; Ikuta, T.; Iwata, N. Memantine for Alzheimer’s Disease: An Updated Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2017, 60, 401–425. [Google Scholar] [CrossRef]
- Chakroborty, S.; Stutzmann, G.E. Calcium Channelopathies and Alzheimer’s Disease: Insight into Therapeutic Success and Failures. Eur. J. Pharmacol. 2014, 739, 83–95. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-Amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Bazzari, F.H.; Bazzari, A.H. BACE1 Inhibitors for Alzheimer’s Disease: The Past, Present and Any Future? Molecules 2022, 27, 8823. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Altomare, D.; Thal, D.R.; Ribaldi, F.; van der Kant, R.; Ossenkoppele, R.; Blennow, K.; Cummings, J.; van Duijn, C.; Nilsson, P.M.; et al. The Probabilistic Model of Alzheimer Disease: The Amyloid Hypothesis Revised. Nat. Rev. Neurosci. 2022, 23, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Three Dimensions of the Amyloid Hypothesis: Time, Space and “Wingmen”. Nat. Neurosci. 2015, 18, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Granzotto, A.; Sensi, S.L. Once upon a Time, the Amyloid Cascade Hypothesis. Ageing Res. Rev. 2024, 93, 102161. [Google Scholar] [CrossRef]
- Salemme, S.; Ancidoni, A.; Locuratolo, N.; Piscopo, P.; Lacorte, E.; Canevelli, M.; Vanacore, N. Advances in Amyloid-Targeting Monoclonal Antibodies for Alzheimer’s Disease: Clinical and Public Health Issues. Expert Rev. Neurother. 2023, 23, 1113–1129. [Google Scholar] [CrossRef]
- Lacorte, E.; Ancidoni, A.; Zaccaria, V.; Remoli, G.; Tariciotti, L.; Bellomo, G.; Sciancalepore, F.; Corbo, M.; Lombardo, F.L.; Bacigalupo, I.; et al. Safety and Efficacy of Monoclonal Antibodies for Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Published and Unpublished Clinical Trials. J. Alzheimer’s Dis. 2022, 87, 101–129. [Google Scholar] [CrossRef]
- Slomski, A. Anti-Tau Antibody Semorinemab Fails to Slow Alzheimer Disease. JAMA 2022, 328, 415. [Google Scholar] [CrossRef]
- Mullard, A. Anti-Tau Antibody Stumbles in Phase II Alzheimer Trial. Nat. Rev. Drug Discov. 2024, 23, 883. [Google Scholar] [CrossRef]
- Dubey, S.; Singh, E. Antioxidants: An Approach for Restricting Oxidative Stress Induced Neurodegeneration in Alzheimer’s Disease. Inflammopharmacology 2023, 31, 717–730. [Google Scholar] [CrossRef]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Herring, W.J.; Ceesay, P.; Snyder, E.; Bliwise, D.; Budd, K.; Hutzelmann, J.; Stevens, J.; Lines, C.; Michelson, D. Polysomnographic Assessment of Suvorexant in Patients with Probable Alzheimer’s Disease Dementia and Insomnia: A Randomized Trial. Alzheimer’s Dement. 2020, 16, 541–551. [Google Scholar] [CrossRef]
- Medications for Memory, Cognition and Dementia-Related Behaviors. Available online: https://Www.Alz.Org/Alzheimers-Dementia/Treatments/Medications-for-Memory (accessed on 6 December 2024).
- Sultzer, D.L.; Davis, S.M.; Tariot, P.N.; Dagerman, K.S.; Lebowitz, B.D.; Lyketsos, C.G.; Rosenheck, R.A.; Hsiao, J.K.; Lieberman, J.A.; Schneider, L.S. Clinical Symptom Responses to Atypical Antipsychotic Medications in Alzheimer’s Disease: Phase 1 Outcomes From the CATIE-AD Effectiveness Trial. Am. J. Psychiatry 2008, 165, 844–854. [Google Scholar] [CrossRef]
- Arai, H.; Nakamura, Y.; Taguchi, M.; Kobayashi, H.; Yamauchi, K.; Schneider, L.S. Mortality Risk in Current and New Antipsychotic Alzheimer’s Disease Users: Large Scale Japanese Study. Alzheimer’s Dement. 2016, 12, 823–830. [Google Scholar] [CrossRef]
- Siwek, M.; Wojtasik-Bakalarz, K.; Krupa, A.J.; Chrobak, A.A. Brexpiprazole—Pharmacologic Properties and Use in Schizophrenia and Mood Disorders. Brain Sci. 2023, 13, 397. [Google Scholar] [CrossRef] [PubMed]
- FDA Approval. Available online: https://Www.Accessdata.Fda.Gov/Drugsatfda_docs/Label/2023/205422s009lbl.Pdf (accessed on 6 December 2024).
- Ballard, C. Brexpiprazole for the Treatment of Agitation and Aggression in Alzheimer Disease. JAMA Neurol. 2023, 80, 1272. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Clark, E.D.; Antonsdottir, I.M.; Porsteinsson, A.P. Brexpiprazole for Agitation Associated With Dementia Due to Alzheimer’s Disease. J. Am. Med. Dir. Assoc. 2024, 25, 105173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Zhang, J.; Peng, W.; Li, T.; Zhang, J. Efficacy of Selective Serotonin Reuptake Inhibitors-Related Antidepressants in Alzheimer’s Disease: A Meta-Analysis. Eur. J. Med. Res. 2024, 29, 438. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Huang, J.; Huang, S.; Bai, Y.; Li, Y.; Huang, W. Efficacy of Antidepressant Drugs in the Treatment of Depression in Alzheimer Disease Patients: A Systematic Review and Network Meta-Analysis. J. Psychopharmacol. 2021, 35, 901–909. [Google Scholar] [CrossRef]
- Costello, H.; Roiser, J.P.; Howard, R. Antidepressant Medications in Dementia: Evidence and Potential Mechanisms of Treatment-Resistance. Psychol. Med. 2023, 53, 654–667. [Google Scholar] [CrossRef]
- Dudas, R.; Malouf, R.; McCleery, J.; Dening, T. Antidepressants for Treating Depression in Dementia. Cochrane Database Syst. Rev. 2018, 2018, CD003944. [Google Scholar] [CrossRef]
- Padovani, A.; Antonini, A.; Barone, P.; Bellelli, G.; Fagiolini, A.; Ferini Strambi, L.; Sorbi, S.; Stocchi, F. Exploring Depression in Alzheimer’s Disease: An Italian Delphi Consensus on Phenomenology, Diagnosis, and Management. Neurol. Sci. 2023, 44, 4323–4332. [Google Scholar] [CrossRef]
- Gallagher, D.; Herrmann, N. Antiepileptic Drugs for the Treatment of Agitation and Aggression in Dementia: Do They Have a Place in Therapy? Drugs 2014, 74, 1747–1755. [Google Scholar] [CrossRef]
- Nagata, T.; Shinagawa, S.; Nakajima, S.; Noda, Y.; Mimura, M. Pharmacological Management of Behavioral Disturbances in Patients with Alzheimer’s Disease. Expert Opin. Pharmacother. 2020, 21, 1093–1102. [Google Scholar] [CrossRef]
- Mintzer, J.; Lanctôt, K.L.; Scherer, R.W.; Rosenberg, P.B.; Herrmann, N.; van Dyck, C.H.; Padala, P.R.; Brawman-Mintzer, O.; Porsteinsson, A.P.; Lerner, A.J.; et al. Effect of Methylphenidate on Apathy in Patients with Alzheimer Disease. JAMA Neurol. 2021, 78, 1324. [Google Scholar] [CrossRef]
- Moser, C.; Guschtschin-Schmidt, N.; Silber, M.; Flum, J.; Muhle-Goll, C. Substrate Selection Criteria in Regulated Intramembrane Proteolysis. ACS Chem. Neurosci. 2024, 15, 1321–1334. [Google Scholar] [CrossRef]
- Pester, O.; Barrett, P.J.; Hornburg, D.; Hornburg, P.; Pröbstle, R.; Widmaier, S.; Kutzner, C.; Dürrbaum, M.; Kapurniotu, A.; Sanders, C.R.; et al. The Backbone Dynamics of the Amyloid Precursor Protein Transmembrane Helix Provides a Rationale for the Sequential Cleavage Mechanism of γ-Secretase. J. Am. Chem. Soc. 2013, 135, 1317–1329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lemmin, T.; Dimitrov, M.; Fraering, P.C.; Dal Peraro, M. Perturbations of the Straight Transmembrane α-Helical Structure of the Amyloid Precursor Protein Affect Its Processing by γ-Secretase. J. Biol. Chem. 2014, 289, 6763–6774. [Google Scholar] [CrossRef] [PubMed]
- Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Lopera, F.; Jimenez-Del-Rio, M. Cholinergic-like Neurons Carrying PSEN1 E280A Mutation from Familial Alzheimer’s Disease Reveal Intraneuronal SAPPβ Fragments Accumulation, Hyperphosphorylation of TAU, Oxidative Stress, Apoptosis and Ca2+ Dysregulation: Therapeutic Implications. PLoS ONE 2020, 15, e0221669. [Google Scholar] [CrossRef] [PubMed]
- Giraldo-Berrio, D.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Sildenafil Reverses the Neuropathological Alzheimer’s Disease Phenotype in Cholinergic-Like Neurons Carrying the Presenilin 1 E280A Mutation. J. Alzheimer’s Dis. 2024, 99, 639–656. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, D.; Luo, R.; Wu, Y.; Zhou, H.; Kong, L.; Bi, R.; Yao, Y. A Systematic Integrated Analysis of Brain Expression Profiles Reveals YAP1 and Other Prioritized Hub Genes as Important Upstream Regulators in Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 215–229. [Google Scholar] [CrossRef]
- Elshazly, S.M.; Elhassanny, A.E.M.; Mahmoud, N.M. Cilostazol Protects against Acetic Acid-Induced Colitis in Rats: Possible Role for CAMP/SIRT1 Pathway. Eur. J. Pharmacol. 2020, 881, 173234. [Google Scholar] [CrossRef]
- Gad, E.S.; Aldossary, S.A.; El-Ansary, M.R.; Abd El-Galil, M.M.; Abd-El-Hamid, A.H.; El-Ansary, A.R.; Hassan, N.F. Cilostazol Counteracts Mitochondrial Dysfunction in Hepatic Encephalopathy Rat Model: Insights into the Role of CAMP/AMPK/SIRT1/ PINK-1/Parkin Hub and p-CREB /BDNF/ TrkB Neuroprotective Trajectory. Eur. J. Pharmacol. 2025, 987, 177194. [Google Scholar] [CrossRef]
- Li, Z.; Martens, Y.A.; Ren, Y.; Jin, Y.; Sekiya, H.; Doss, S.V.; Kouri, N.; Castanedes-Casey, M.; Christensen, T.A.; Miller Nevalainen, L.B.; et al. APOE Genotype Determines Cell-Type-Specific Pathological Landscape of Alzheimer’s Disease. Neuron 2025, 113, 1380–1397.e7. [Google Scholar] [CrossRef]
- Huang, S.; Xu, P.; Shen, D.-D.; Simon, I.A.; Mao, C.; Tan, Y.; Zhang, H.; Harpsøe, K.; Li, H.; Zhang, Y.; et al. GPCRs Steer Gi and Gs Selectivity via TM5-TM6 Switches as Revealed by Structures of Serotonin Receptors. Mol. Cell 2022, 82, 2681–2695.e6. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhao, Q.; Qi, J.; Wang, Y.; Han, W.; Chen, Z.; Cong, Y.; Wang, S. Structural Insights into Constitutive Activity of 5-HT6 Receptor. Proc. Natl. Acad. Sci. USA 2023, 120, e2209917120. [Google Scholar] [CrossRef] [PubMed]
- Djebari, S.; Jiménez-Herrera, R.; Iborra-Lázaro, G.; Jiménez-Díaz, L.; Navarro-López, J.D. Social and Contextual Memory Impairments Induced by Amyloid-β Oligomers Are Rescued by Sigma-1 Receptor Activation. Biomed. Pharmacother. 2025, 184, 117914. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Meunier, J.; Feng, B.; Ieni, J.; Monaghan, D.T. Interaction with Σ1 Protein, but Not N-Methyl-d-Aspartate Receptor, Is Involved in the Pharmacological Activity of Donepezil. J. Pharmacol. Exp. Ther. 2006, 317, 606–614. [Google Scholar] [CrossRef]
- Yang, G.; Zhou, R.; Guo, X.; Yan, C.; Lei, J.; Shi, Y. Structural Basis of γ-Secretase Inhibition and Modulation by Small Molecule Drugs. Cell 2021, 184, 521–533.e14. [Google Scholar] [CrossRef]
- Guo, X.; Li, H.; Lu, X.; Liu, H.; U, K.; Yan, C.; Lei, J.; Huang, J.; Zhou, R.; Shi, Y. Structural Basis of Human γ-Secretase Inhibition by Anticancer Clinical Compounds. Nat. Struct. Mol. Biol. 2025, 32, 719–728. [Google Scholar] [CrossRef]
- Mazanetz, M.P.; Cheng, R.K.Y.; Rowan, F.; Laughton, C.A.; Barker, J.J.; Fischer, P.M. Crystal Structure of Human Glycogen Synthase Kinase 3 Beta (GSK3b) in Complex with Inhibitor 142. Worldwide Protein Data Bank 2012. [Google Scholar] [CrossRef]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 155–175. [Google Scholar] [CrossRef]
- Vitek, M.P.; Araujo, J.A.; Fossel, M.; Greenberg, B.D.; Howell, G.R.; Rizzo, S.J.S.; Seyfried, N.T.; Tenner, A.J.; Territo, P.R.; Windisch, M.; et al. Translational Animal Models for Alzheimer’s Disease: An Alzheimer’s Association Business Consortium Think Tank. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2020, 6, e12114. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Estrada, L.; Sanchez-Mejias, E.; Sanchez-Varo, R.; Garcia-Leon, J.A.; Nuñez-Diaz, C.; Davila, J.C.; Vitorica, J.; LaFerla, F.M.; Moreno-Gonzalez, I.; Gutierrez, A.; et al. Animal and Cellular Models of Alzheimer’s Disease: Progress, Promise, and Future Approaches. Neuroscientist 2022, 28, 572–593. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhang, Y.; Wang, J.-Z. Updates in Alzheimer’s Disease: From Basic Research to Diagnosis and Therapies. Transl. Neurodegener. 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P. Viral Hypothesis and Antiviral Treatment in Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 55. [Google Scholar] [CrossRef]
- Whitson, H.E.; Colton, C.; El Khoury, J.; Gate, D.; Goate, A.; Heneka, M.T.; Kaddurah-Daouk, R.; Klein, R.S.; Shinohara, M.L.; Sisodia, S.; et al. Infection and Inflammation: New Perspectives on Alzheimer’s Disease. Brain Behav. Immun. Health 2022, 22, 100462. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Shen, Y.; Li, H.; Rausch, W.-D.; Huang, X. Iron Dyshomeostasis and Ferroptosis: A New Alzheimer’s Disease Hypothesis? Front. Aging Neurosci. 2022, 14, 830569. [Google Scholar] [CrossRef]
- Rudge, J.D. A New Hypothesis for Alzheimer’s Disease: The Lipid Invasion Model. J. Alzheimers Dis. Rep. 2022, 6, 129–161. [Google Scholar] [CrossRef]
- Keizer, H.G.; Brands, R.; Oosting, R.S.; Seinen, W. The Carnitine Palmitoyl-Transferase 2 Cascade Hypothesis for Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 97, 553–558. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Dawbarn, D. The Neurotrophins and Their Role in Alzheimers Disease. Curr. Neuropharmacol. 2011, 9, 559–573. [Google Scholar] [CrossRef] [PubMed]
- González Cordero, E.M.; Cuevas-Budhart, M.A.; Pérez Morán, D.; Trejo Villeda, M.A.; Gomez-Del-Pulgar Ga-Madrid, M. Relationship Between the Gut Microbiota and Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. 2022, 87, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current Pharmacotherapy and Multi-Target Approaches for Alzheimer’s Disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef] [PubMed]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.; Pooe, O.J. Potential Impact of the Multi-Target Drug Approach in the Treatment of Some Complex Diseases. Drug Des. Devel Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef]
- Monzani, F.; Pasqualetti, G.; Tognini, S.; Calsolaro, V.; Polini, A. Potential Drug-Drug Interactions in Alzheimer Patients with Behavioral Symptoms. Clin. Interv. Aging 2015, 10, 1457. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Designed Multiple Ligands. An Emerging Drug Discovery Paradigm. J. Med. Chem. 2005, 48, 6523–6543. [Google Scholar] [CrossRef]
- Ataei, B.; Hokmabadi, M.; Asadi, S.; Asadifard, E.; Aghaei Zarch, S.M.; Najafi, S.; Bagheri-Mohammadi, S. A Review of the Advances, Insights, and Prospects of Gene Therapy for Alzheimer’s Disease: A Novel Target for Therapeutic Medicine. Gene 2024, 912, 148368. [Google Scholar] [CrossRef]
- Iqubal, A.; Iqubal, M.K.; Khan, A.; Ali, J.; Baboota, S.; Haque, S.E. Gene Therapy, A Novel Therapeutic Tool for Neurological Disorders: Current Progress, Challenges and Future Prospective. Curr. Gene Ther. 2020, 20, 184–194. [Google Scholar] [CrossRef]
- Mandel, R.J. CERE-110, an Adeno-Associated Virus-Based Gene Delivery Vector Expressing Human Nerve Growth Factor for the Treatment of Alzheimer’s Disease. Curr. Opin Mol. Ther. 2010, 12, 240–247. [Google Scholar]
- Piedrahita, D.; Hernández, I.; López-Tobón, A.; Fedorov, D.; Obara, B.; Manjunath, B.S.; Boudreau, R.L.; Davidson, B.; LaFerla, F.; Gallego-Gómez, J.C.; et al. Silencing of CDK5 Reduces Neurofibrillary Tangles in Transgenic Alzheimer’s Mice. J. Neurosci. 2010, 30, 13966–13976. [Google Scholar] [CrossRef]
- Liu, N.; Liang, X.; Chen, Y.; Xie, L. Recent Trends in Treatment Strategies for Alzheimer’s Disease and the Challenges: A Topical Advancement. Ageing Res. Rev. 2024, 94, 102199. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, Y. Applications of Nanoparticles in Alzheimer’s Disease. J. Alzheimer’s Dis. 2023, 96, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Fatima, M.T.; Islam, Z.; Khan, R.H.; Uversky, V.N.; Salahuddin, P. Nanoparticle Formulations in the Diagnosis and Therapy of Alzheimer’s Disease. Int. J. Biol. Macromol. 2019, 130, 515–526. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Akhtar, M.d.J.; Al Balushi, K.; Safi, S.Z.; Azmi, S.N.H.; Khan, S.A. The Emerging Role of Proteolysis Targeting Chimeras (PROTACs) in the Treatment of Alzheimer’s Disease. Med. Chem. Res. 2023, 32, 601–616. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Swetha, R.; Kumar, D.; Gangaram, G.P.; Singh, R.; Gutti, G.; Jana, S.; Kumar, D.; Kumar, A.; Singh, S.K. Protein-Protein Interactions and Aggregation Inhibitors in Alzheimer’s Disease. Curr. Top Med. Chem. 2019, 19, 501–533. [Google Scholar] [CrossRef]
- Ma, C.; Ye, Y.; Shi, X.; Li, N.; Mu, Z.; Tan, T.; Yin, H.; Dai, J.; Liu, Y.; Chen, H. Photobiomodulation Mitigates Blood–Brain Barrier Disruption in APP/PS1 Mouse Model of Alzheimer’s Disease by Activating the AMPK Pathway. Alzheimer’s Res. Ther. 2025, 17, 141. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y.-J.; Guo, J. Biofluid Biomarkers of Alzheimer’s Disease: Progress, Problems, and Perspectives. Neurosci. Bull. 2022, 38, 677–691. [Google Scholar] [CrossRef]
- Jiang, A. Challenges in Early Diagnosis and Treatment of Alzheimer’s Disease. Highlights Sci. Eng. Technol. 2023, 74, 713–718. [Google Scholar] [CrossRef]
- Twiss, E.; McPherson, C.; Weaver, D.F. Global Diseases Deserve Global Solutions: Alzheimer’s Disease. Neurol. Int. 2025, 17, 92. [Google Scholar] [CrossRef]
- Gong, C.X.; Liu, F.; Iqbal, K. Multifactorial Hypothesis and Multi-Targets for Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, S107–S117. [Google Scholar]
- Niu, Y.; Lin, P. Advances of Computer-Aided Drug Design (CADD) in the Development of Anti-Azheimer’s-Disease Drugs. Drug Discov. Today 2023, 28, 103665. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, X. Alzheimer’s Disease Drug Development Based on Computer-Aided Drug Design. Eur. J. Med. Chem. 2016, 121, 851–863. [Google Scholar] [CrossRef]
- Mullard, A. FDA Approves Third Anti-Amyloid Antibody for Alzheimer Disease. Nat. Rev. Drug Discov. 2024, 23, 571. [Google Scholar] [CrossRef]
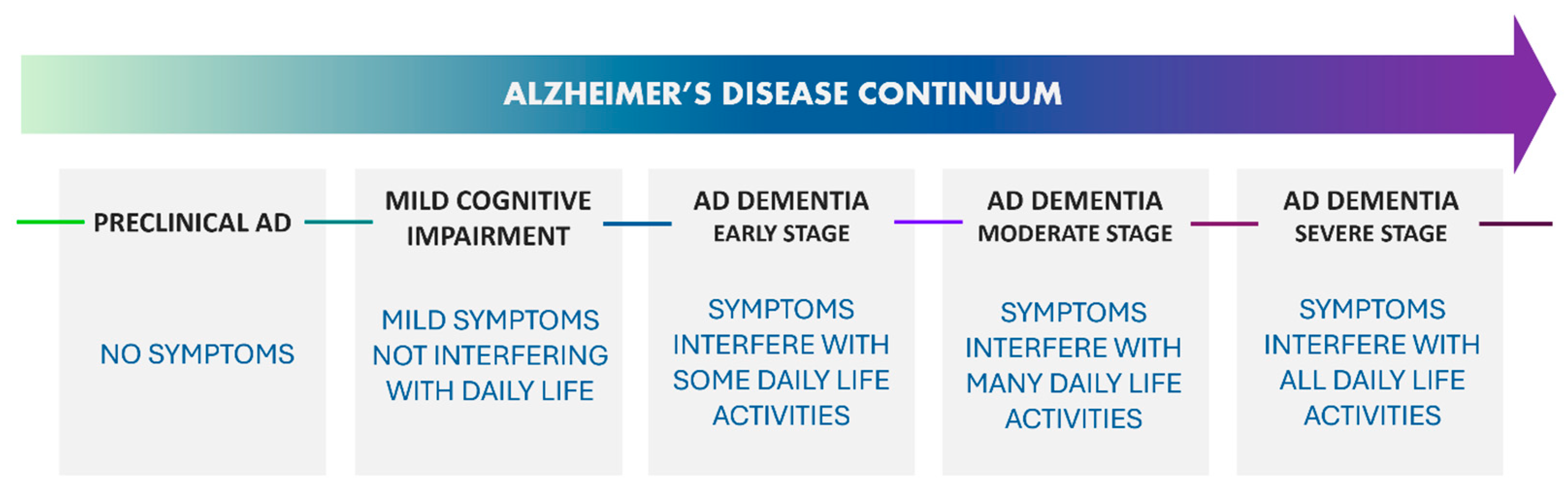
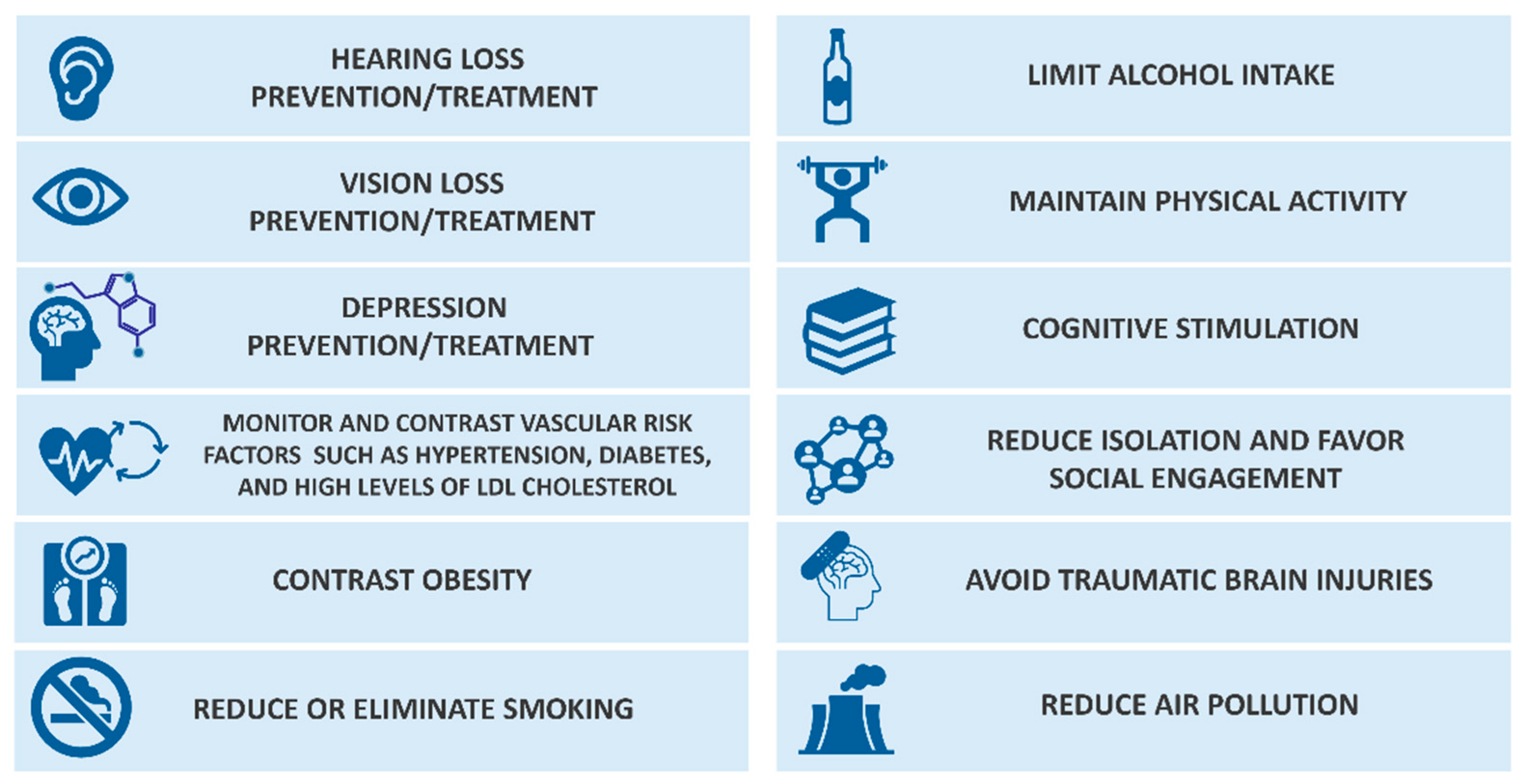
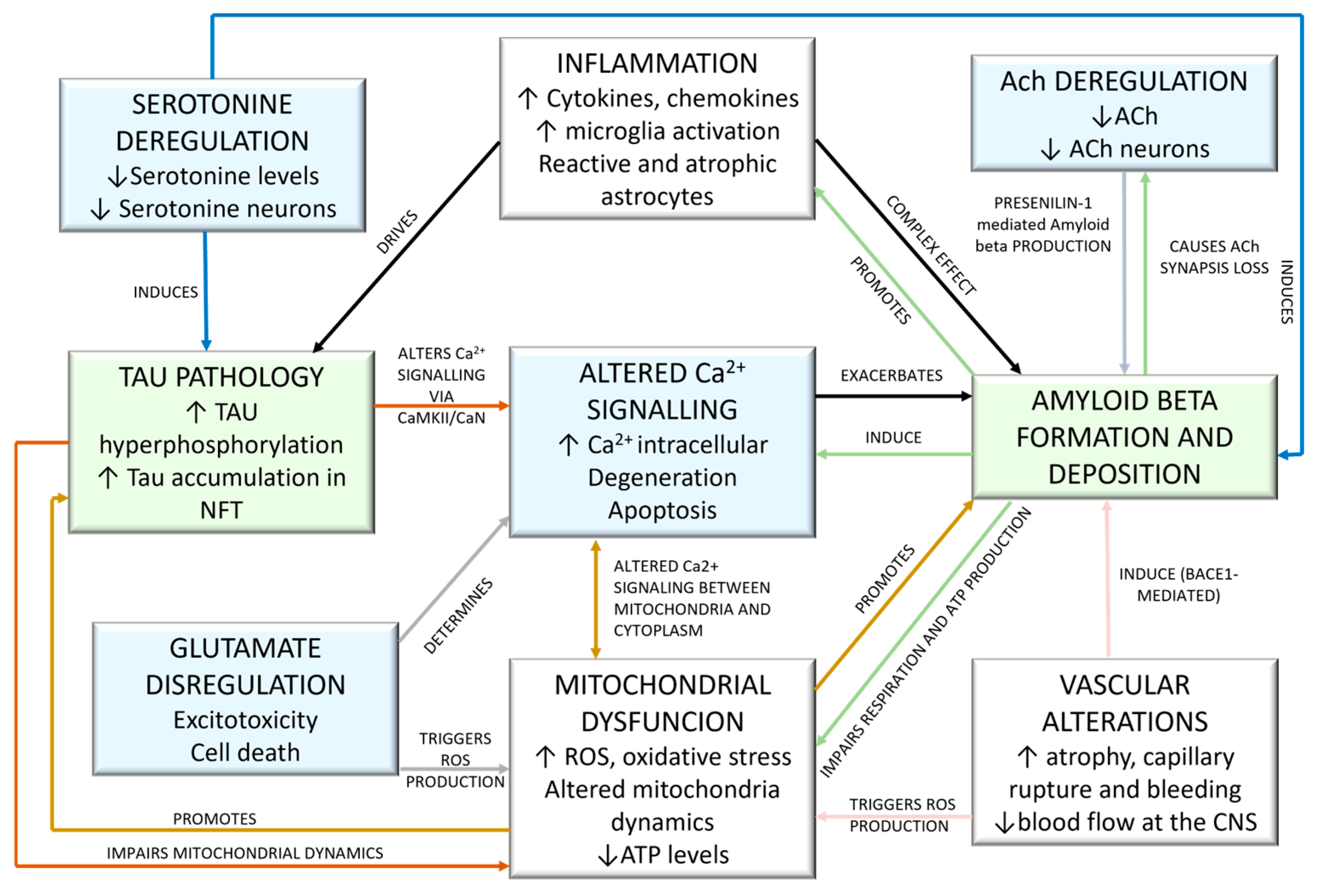

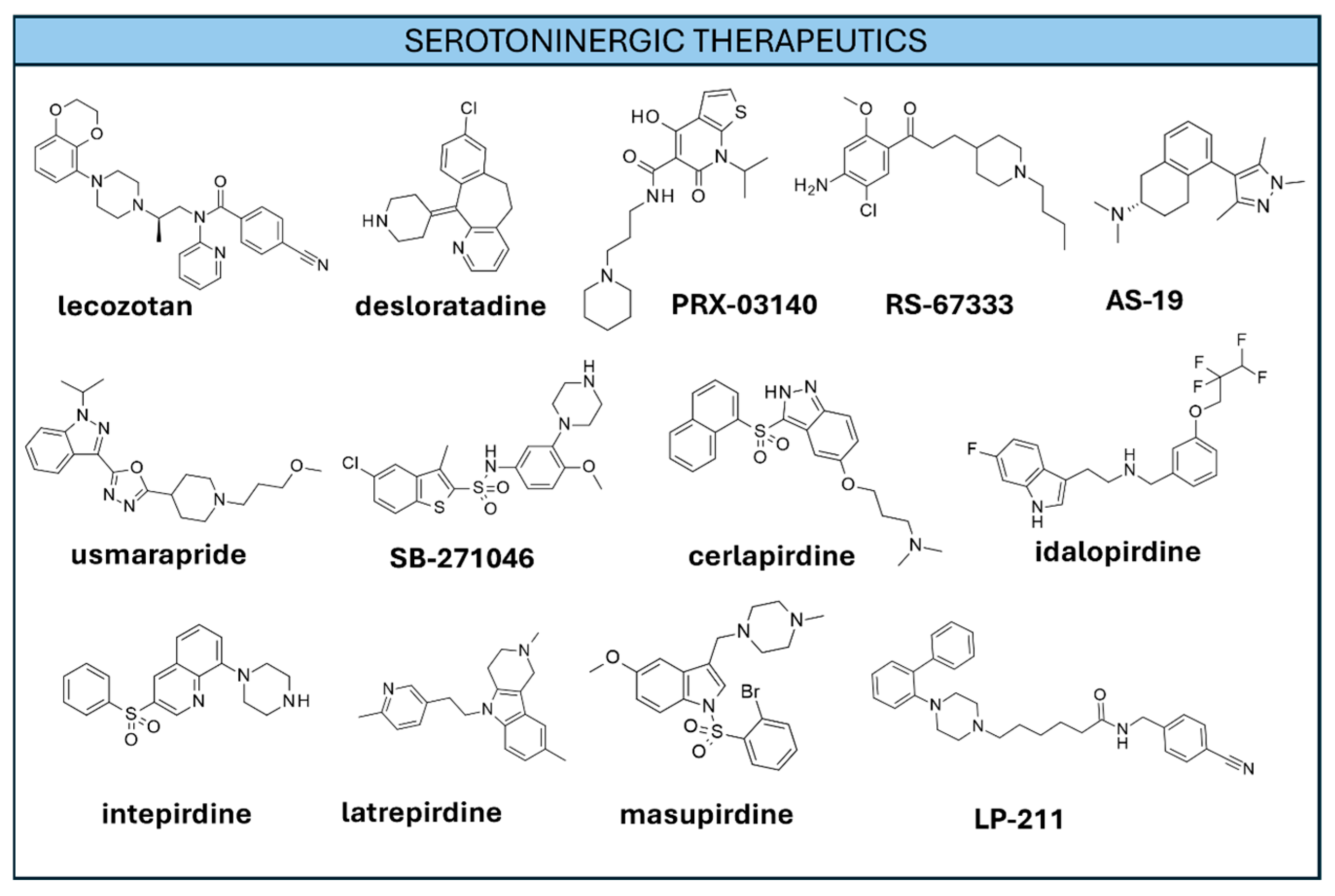

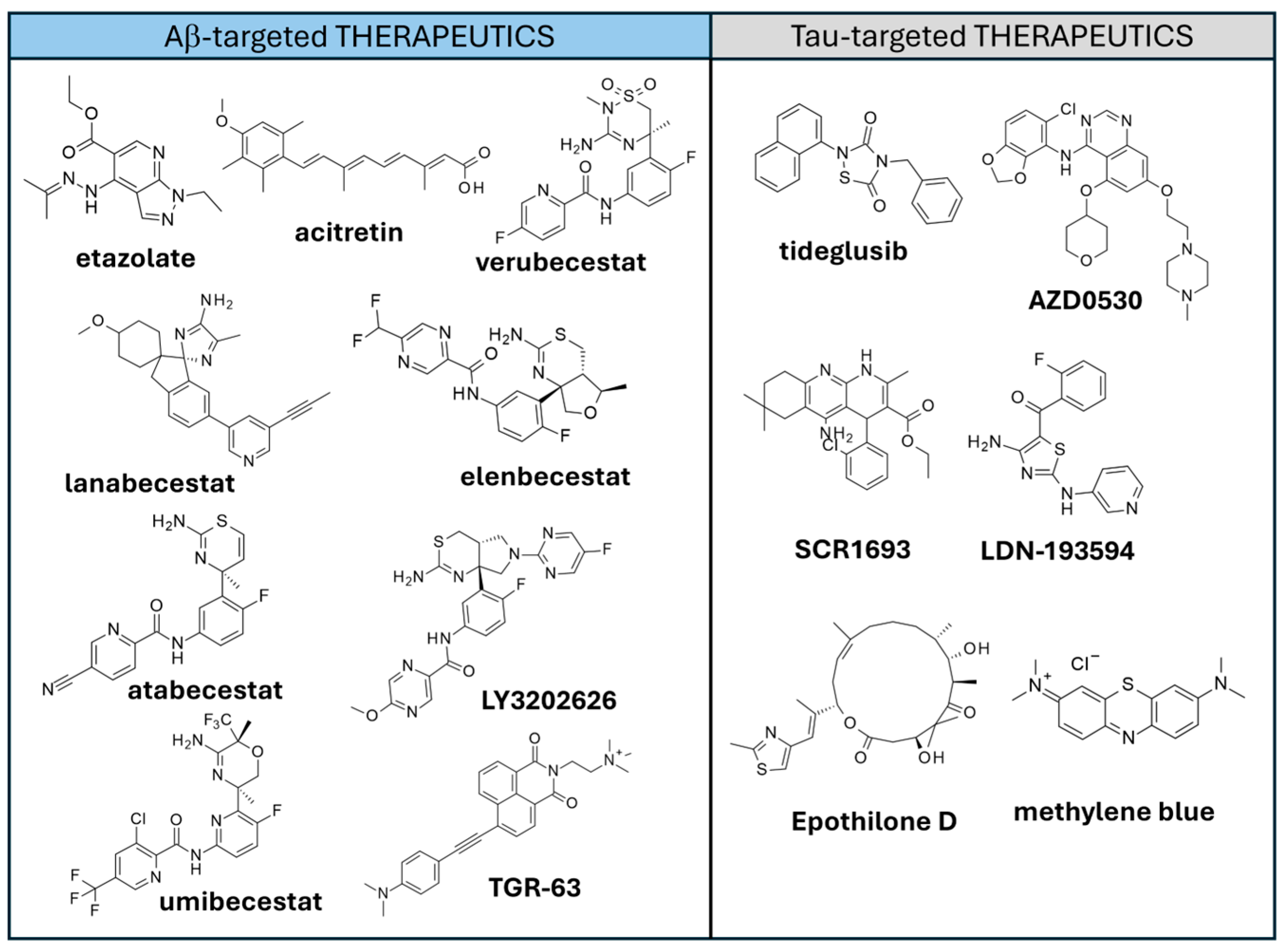
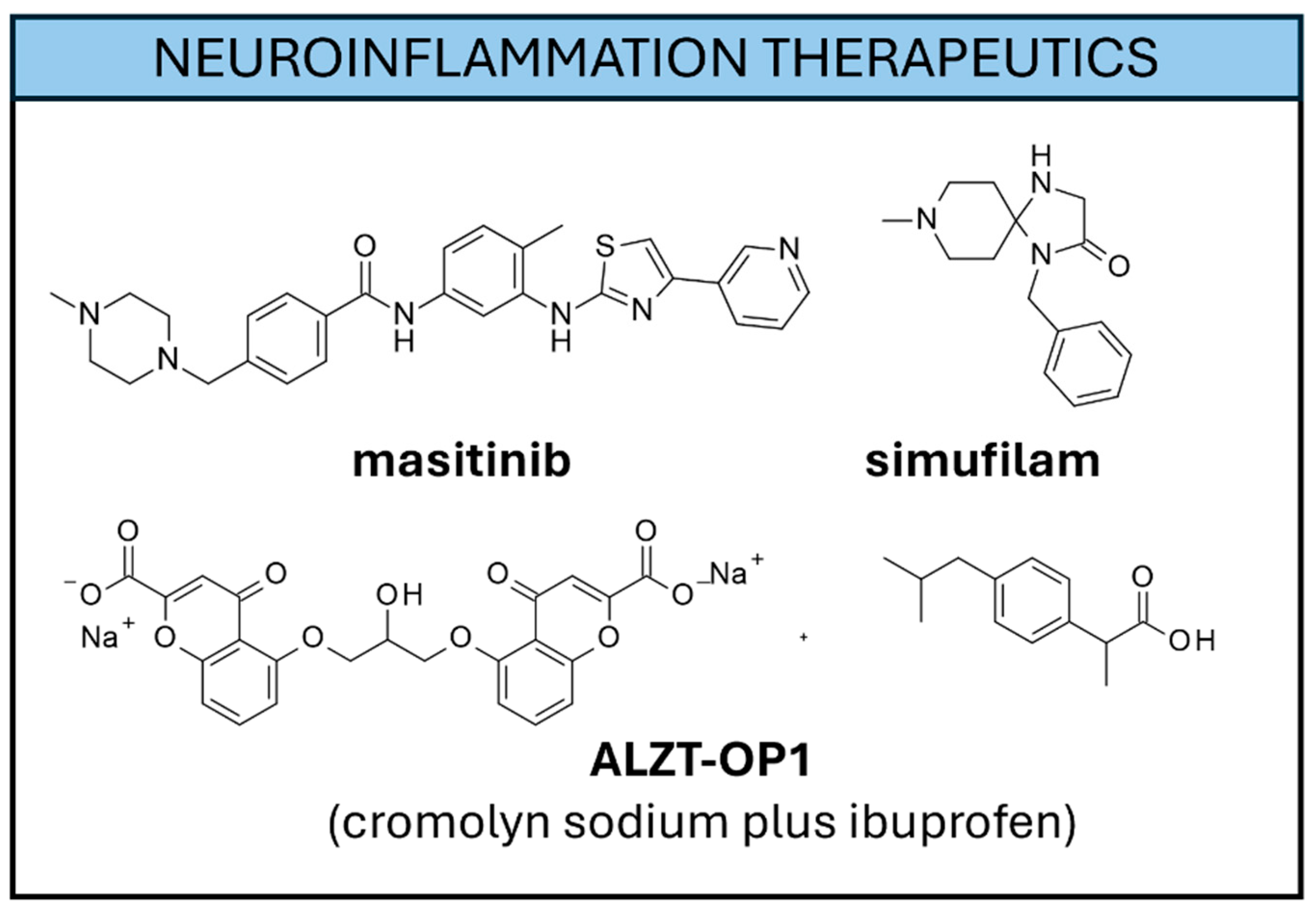

| Entry n. | Drug Name | Class | AD Stage | Appr. Year | Withdrawn | Side Effects | Administration | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Tacrine | AchEI | Mild-to-moderate | 1993 | Yes (2013) | Hepatotoxicity | oral | [308,309] |
| 2 | Donepezil | AchEI | Mild, moderate, severe AD | 1996 | No | Nausea, Vomiting, Diarrhea, Fatigue | Oral transdermal | [310] |
| 3 | Rivastigmine | AchEI | Mild-to-moderate | 2000 | No | Dizziness, Vertigo, Upper respiratory tract infection | Oral transdermal | [311,312] |
| 4 | Galantamine | AchEI | Mild-to-moderate | 2001 | No | Salivation, Bradycardia, Dizziness, Abdominal pain | Oral | [313] |
| 5 | Memantine | NMDAR antagonist | Moderate-to-severe | 2003 | No | Headache, Confusion, Dizziness, Urinary infections, Constipation, Somnolence, Agitation | Oral | [314] |
| 6 | Namzaric | AChEI NMDAR antagonist | Moderate-to-severe | 2014 | No | Dizziness, Agitation, Confusion, Diarrhea, Nasopharyngitis, Falls | Oral | [316] |
| 7 | Sodium oligomannate | Not known | Mild-to-moderate | 2019 | No | Infections, Gastrointestinal tract disorders, Nervous system problems | Oral | [317] |
| 8 | Aducanumab | Anti-Aβ mAbs | MCI, mild AD | 2021 | No (discontinued after approval) | ARIA a side effects | Intravenous | [318] |
| 9 | Lecanemab | Anti-Aβ mAbs | MCI, mild AD | 2023 | No | ARIA side effects | Intravenous | [319] |
| 10 | Donanemab | Anti-Aβ mAbs | MCI, mild AD | 2024 | No | ARIA side effects | Intravenous | [320] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, N.; Musumeci, F.; Casini, B.; Brullo, C.; D’Ursi, P.; Fossa, P.; Schenone, S.; Cichero, E. Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective. Int. J. Mol. Sci. 2025, 26, 6980. https://doi.org/10.3390/ijms26146980
Scarano N, Musumeci F, Casini B, Brullo C, D’Ursi P, Fossa P, Schenone S, Cichero E. Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective. International Journal of Molecular Sciences. 2025; 26(14):6980. https://doi.org/10.3390/ijms26146980
Chicago/Turabian StyleScarano, Naomi, Francesca Musumeci, Beatrice Casini, Chiara Brullo, Pasqualina D’Ursi, Paola Fossa, Silvia Schenone, and Elena Cichero. 2025. "Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective" International Journal of Molecular Sciences 26, no. 14: 6980. https://doi.org/10.3390/ijms26146980
APA StyleScarano, N., Musumeci, F., Casini, B., Brullo, C., D’Ursi, P., Fossa, P., Schenone, S., & Cichero, E. (2025). Alzheimer’s Disease Etiology Hypotheses and Therapeutic Strategies: A Perspective. International Journal of Molecular Sciences, 26(14), 6980. https://doi.org/10.3390/ijms26146980