Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy
Abstract
1. Introduction
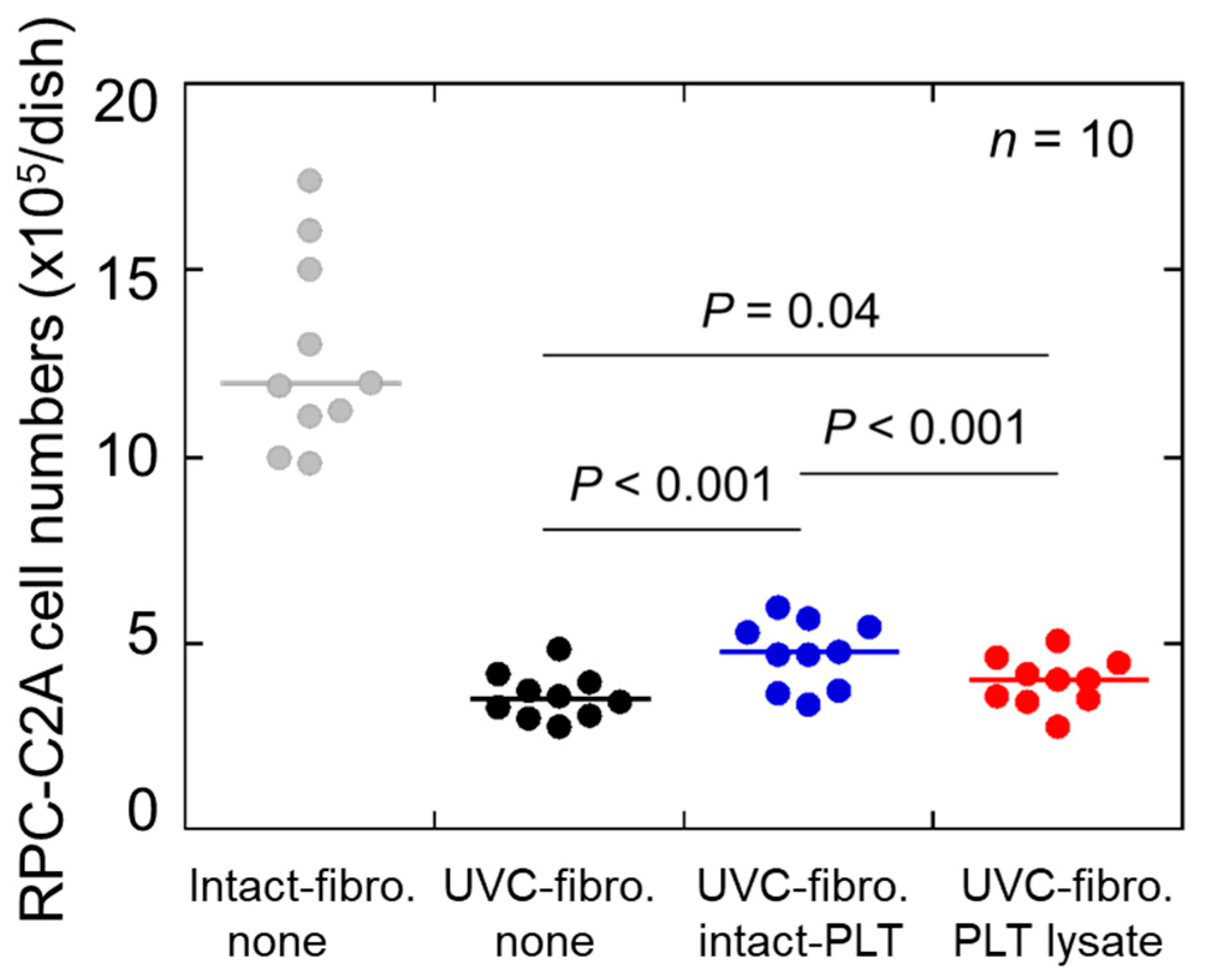
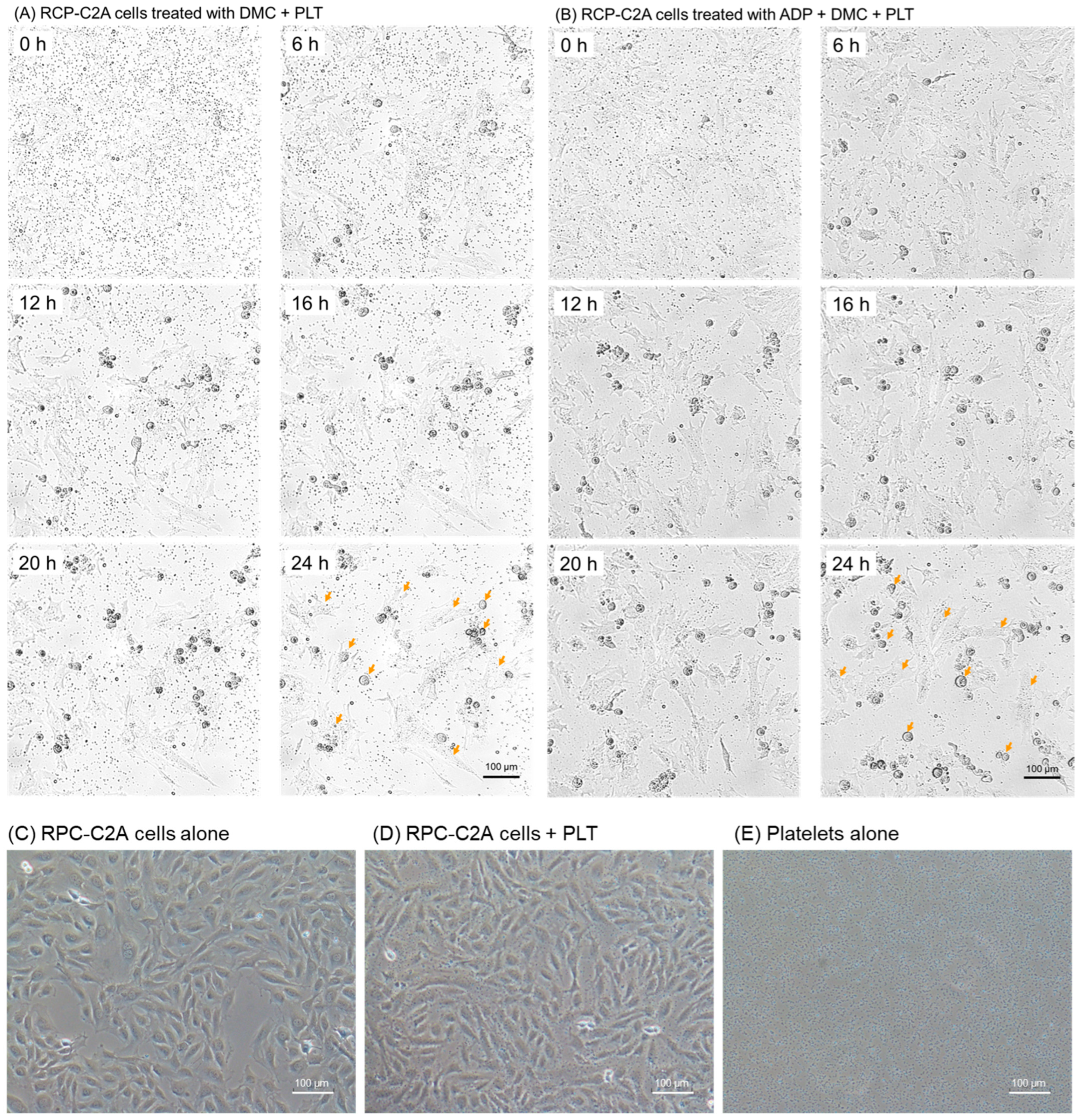
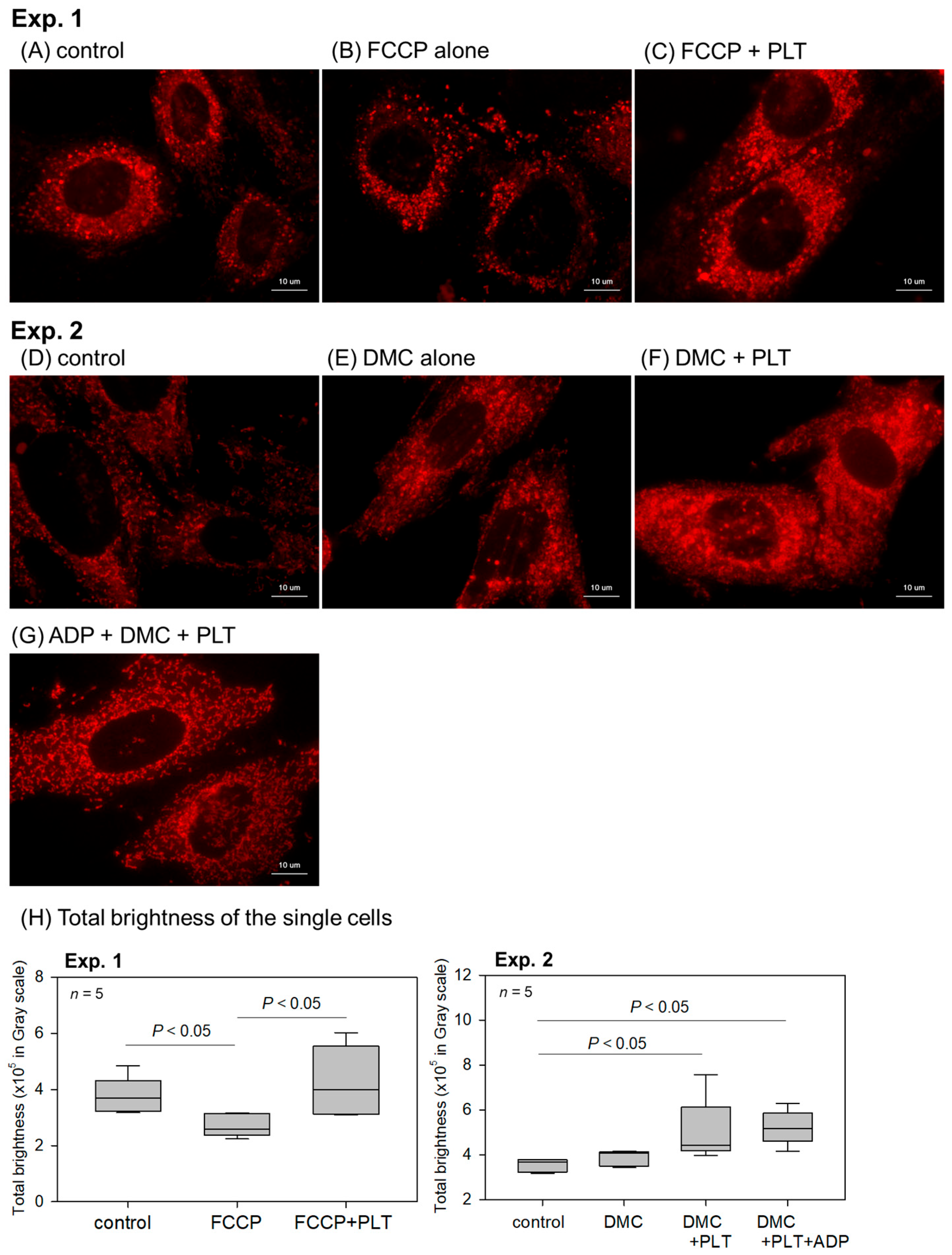
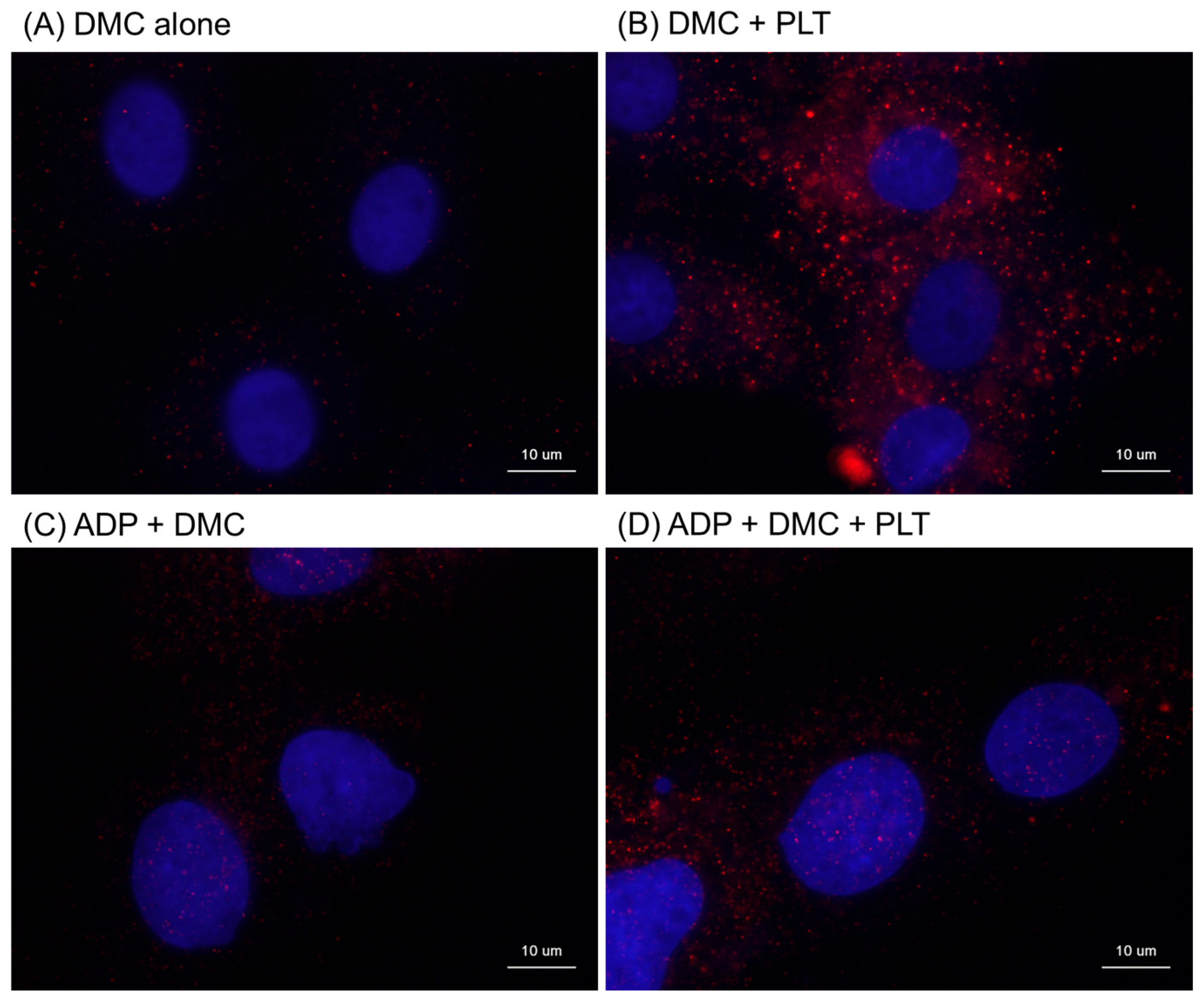
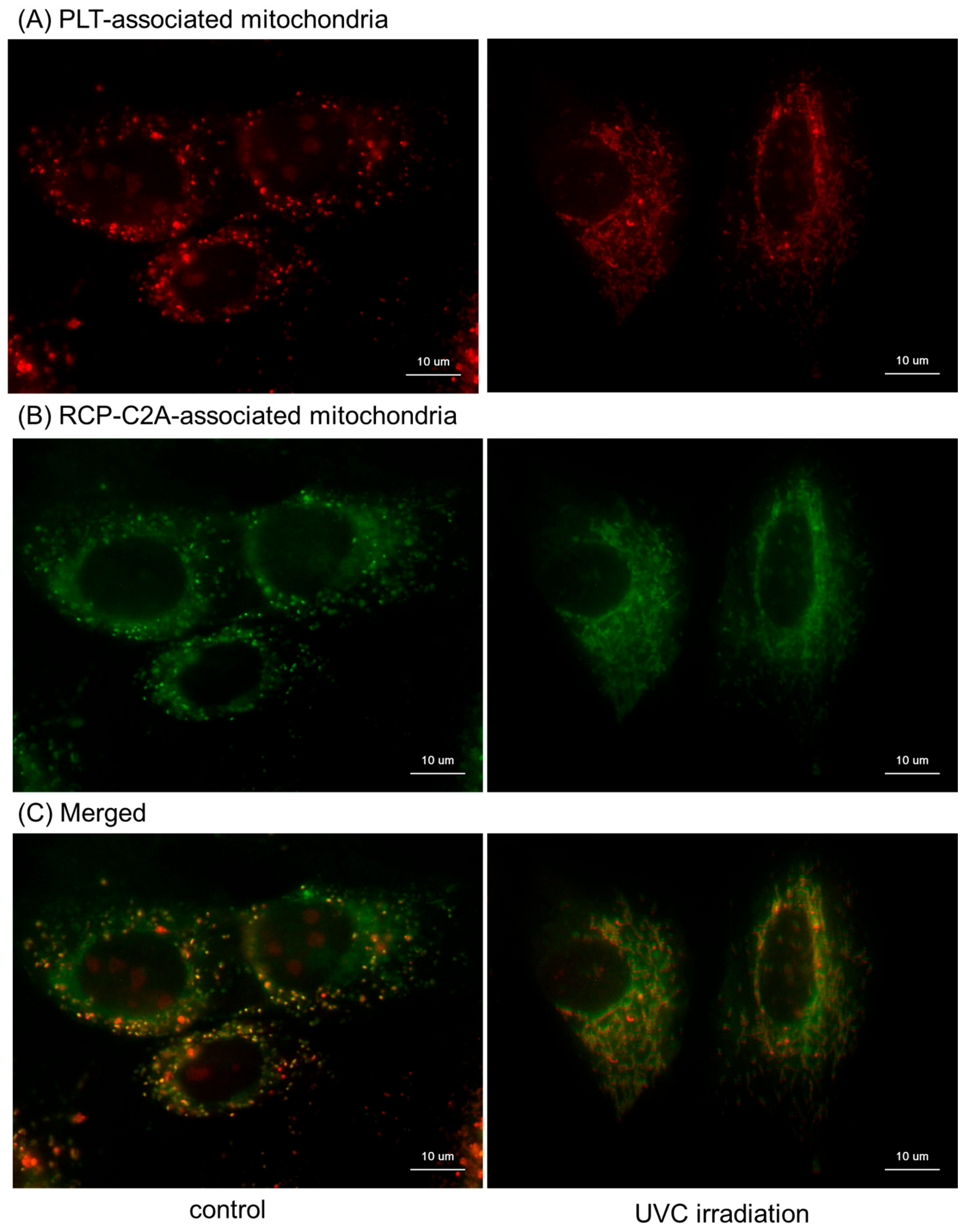
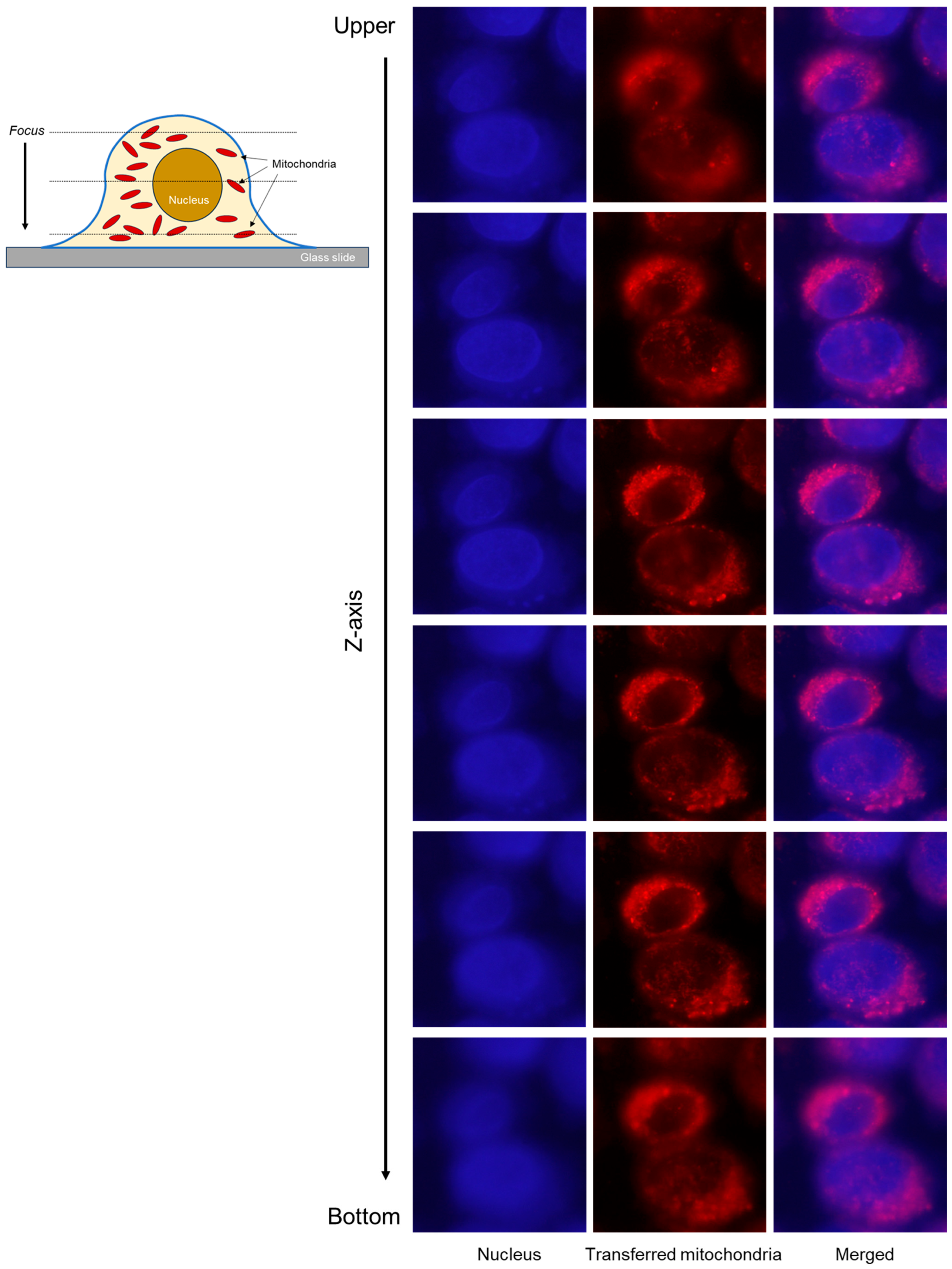
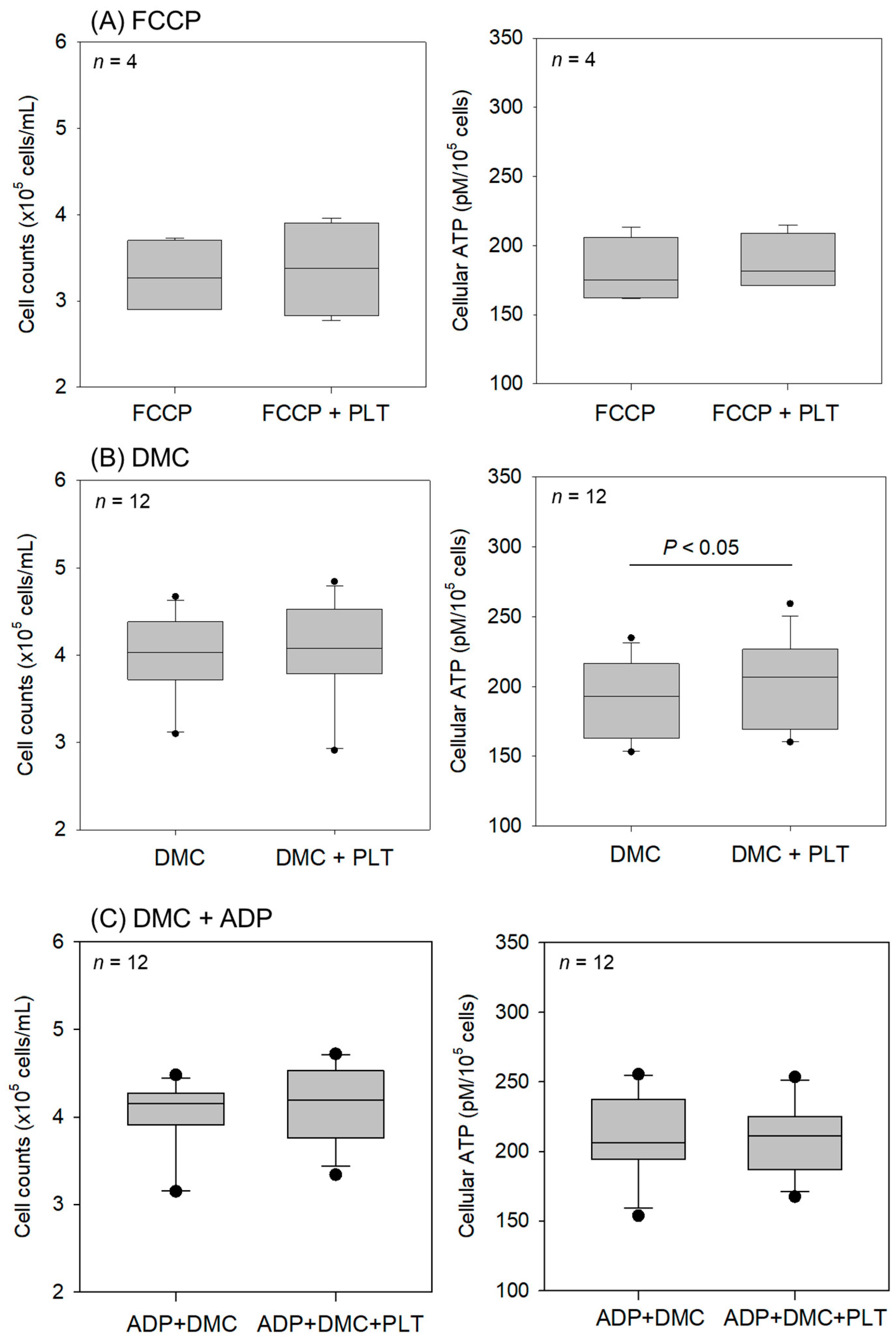
2. Results
3. Discussion
3.1. Proposal to Clinical Protocol
3.2. Limitations
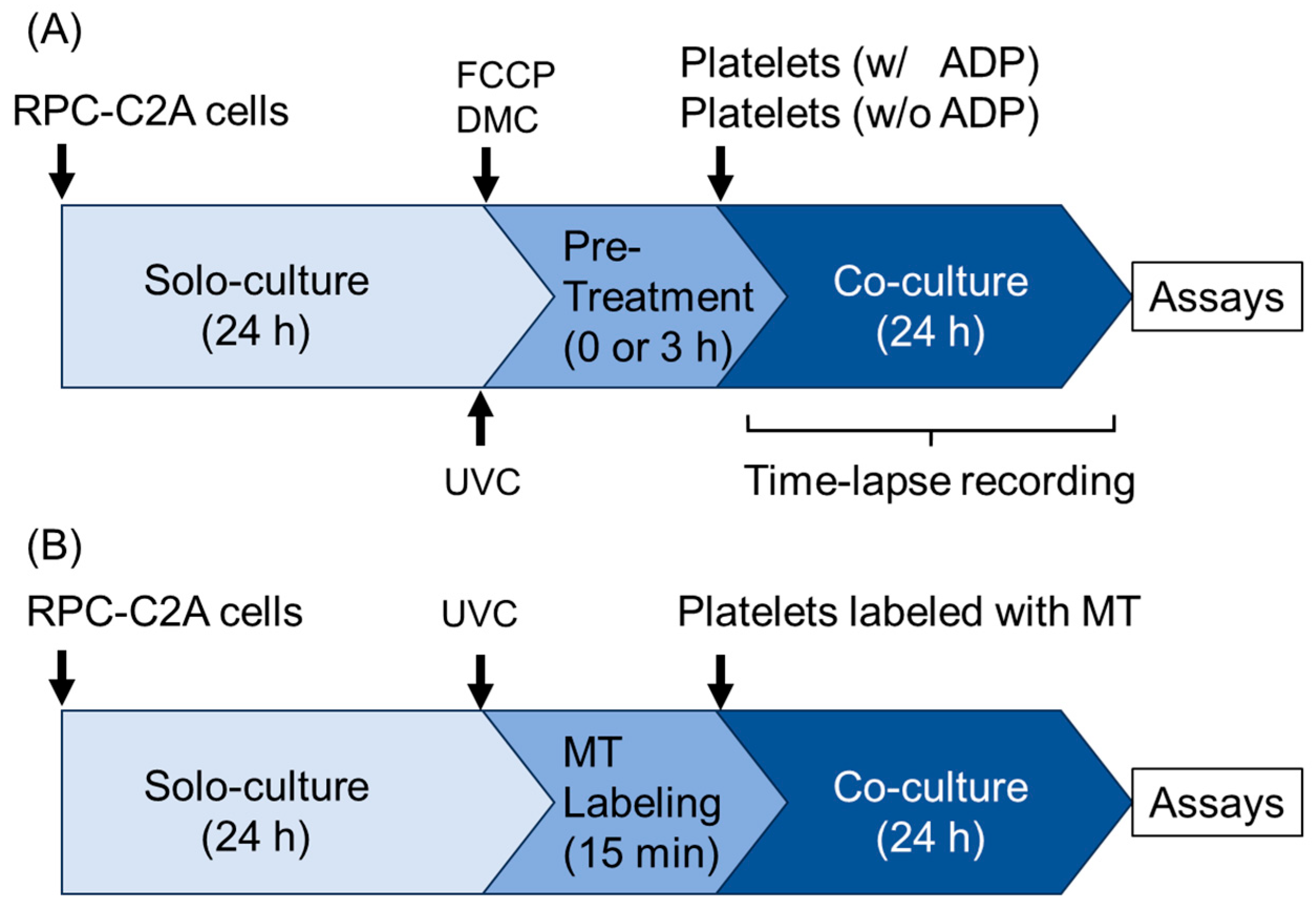
4. Materials and Methods
4.1. Preparation of PRP and Platelet Lysates
4.2. Rat Dental Pulp-Derived Fibroblasts and Ultraviolet Irradiation
4.3. Time-Lapse Videography
4.4. Visualization of Mitochondria Membrane Potential (Em)
4.5. Image Analyses of the Color Images Obtained from the Visualization of Em
4.6. Immunocytochemical Visualizations of Human Mitochondria Distribution
4.7. Visualization of Mitochondria Distribution Using MitoTracker
4.8. Scanning Electron Microscopy (SEM)
4.9. Determination of Fibroblast-Associated ATP Levels
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRP | Platelet-rich plasma |
| UVC | Ultraviolet C |
| ACD-A | A formulation of acid–citrate–dextrose |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FCCP | Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone |
| Em | Mitochondrial membrane potential |
| SEM | Scanning electron microscopy |
| ANOVA | Analysis of variance |
| ROS | Reactive oxygen species |
References
- Kawase, T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: Basic principles and concepts underlying recent advances. Odontology 2015, 103, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T. A Strategic and Worldwide Cooperative Challenge Required for the Next Generation of Platelet Concentrates. Int. J. Mol. Sci. 2022, 23, 3437. [Google Scholar] [CrossRef]
- Kawase, T.; Mubarak, S.; Mourão, C.F. The Platelet Concentrates Therapy: From the Biased Past to the Anticipated Future. Bioengineering 2020, 7, 82. [Google Scholar] [CrossRef]
- Knightly, N.; Lee, C.; O’Brien, L.; Qayyum, T.; Hurley, C.; Kelly, J. Role for platelet rich plasma as an adjuvant therapy in wound healing and burns. Eur. J. Plast. Surg. 2023, 46, 465–474. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Mariani, E.; Roffi, A.; Cattini, L.; Pulsatelli, L.; Assirelli, E.; Krishnakumar, G.S.; Cenacchi, A.; Kon, E.; Filardo, G. Release kinetic of pro- and anti-inflammatory biomolecules from platelet-rich plasma and functional study on osteoarthritis synovial fibroblasts. Cytotherapy 2020, 22, 344–353. [Google Scholar] [CrossRef]
- Cavallo, C.; Roffi, A.; Grigolo, B.; Mariani, E.; Pratelli, L.; Merli, G.; Kon, E.; Marcacci, M.; Filardo, G. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Res. Int. 2016, 2016, 6591717. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surgery. Am. Vol. 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Croisé, B.; Paré, A.; Joly, A.; Louisy, A.; Laure, B.; Goga, D. Optimized centrifugation preparation of the platelet rich plasma: Literature review. J. Stomatol. Oral. Maxillofac. Surg. 2020, 121, 150–154. [Google Scholar] [CrossRef]
- Gómez, L.A.; Escobar, M.; Peñuela, O. Standardization of a Protocol for Obtaining Platelet Rich Plasma from blood Donors; a Tool for Tissue Regeneration Procedures. Clin. Lab. 2015, 61, 973–980. [Google Scholar] [CrossRef]
- Muthu, S.; Krishnan, A.; Ramanathan, K.R. Standardization and validation of a conventional high yield platelet-rich plasma preparation protocol. Ann. Med. Surg. 2022, 82, 104593. [Google Scholar] [CrossRef] [PubMed]
- Pachito, D.V.; Bagattini, Â.M.; de Almeida, A.M.; Mendrone-Júnior, A.; Riera, R. Technical Procedures for Preparation and Administration of Platelet-Rich Plasma and Related Products: A Scoping Review. Front. Cell Dev. Biol. 2020, 8, 598816. [Google Scholar] [CrossRef]
- Kawase, T.; Mourão, C.F.; Fujioka-Kobayashi, M.; Mastrogiacomo, M. Editorial: Recent advances in platelet-concentrate therapy in regenerative medicine. Front. Bioeng. Biotechnol. 2024, 12, 1518095. [Google Scholar] [CrossRef]
- Bosco, F.; Giai Via, R.; Giustra, F.; Ghirri, A.; Cacciola, G.; Massè, A. Platelet-rich plasma for jumper’s knee: A comprehensive review of efficacy, protocols, and future directions. Eur. J. Orthop. Surg. Traumatol. 2024, 34, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, U.; Papaleontiou, A.; Imam, M.A.; Mahmood, A. Assessing Standardisation in Platelet-Rich Plasma (PRP) Injections for the Management of Greater Trochanteric Pain Syndrome (GTPS): A Systematic Review of the Available Literature. Cureus 2024, 16, e74690. [Google Scholar] [CrossRef]
- Maletic, A.; Dumic-Cule, I.; Brlek, P.; Zic, R.; Primorac, D. Autologous Platelet-Rich Plasma (PRP) for Treating Androgenetic Alopecia: A Novel Treatment Protocol Standardized on 2 Cases. J. Clin. Med. 2022, 11, 7327. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Evanson, J.R.; Guyton, M.K.; Oliver, D.L.; Hire, J.M.; Topolski, R.L.; Zumbrun, S.D.; McPherson, J.C.; Bojescul, J.A. Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Mil. Med. 2014, 179, 799–805. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Yoshioka, T.; Sugaya, H.; Gosho, M.; Aoto, K.; Kanamori, A.; Yamazaki, M. Growth factor levels in leukocyte-poor platelet-rich plasma and correlations with donor age, gender, and platelets in the Japanese population. J. Exp. Orthop. 2019, 6, 4. [Google Scholar] [CrossRef]
- Weibrich, G.; Kleis, W.K.G.; Hafner, G.; Hitzler, W.E. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J. Cranio-Maxillofac. Surg. 2002, 30, 97–102. [Google Scholar] [CrossRef]
- Xiong, G.; Lingampalli, N.; Koltsov, J.C.B.; Leung, L.L.; Bhutani, N.; Robinson, W.H.; Chu, C.R. Men and Women Differ in the Biochemical Composition of Platelet-Rich Plasma. Am. J. Sports Med. 2018, 46, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.P.; Wider-Eberspächer, E.; Morton, L.; van Ham, M.; Pállinger, É.; Buzás, E.I.; Jänsch, L.; Dunay, I.R. Proteomic analysis of plasma-derived extracellular vesicles: Pre- and postprandial comparisons. Sci. Rep. 2024, 14, 23032. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, C.; Guo, E.; Zhu, X.; Li, N.; Huang, Y.; Wang, P.; Shan, H.; Yin, Y.; Wang, H.; et al. Platelet-Rich Plasma in Young and Elderly Humans Exhibits a Different Proteomic Profile. J. Proteome Res. 2024, 23, 1788–1800. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, K.H.; Kim, J.Y.; Kim, K.O.; Haotian, B.; Yuxuan, L.; Noh, K.C. Proteomic Classification and Identification of Proteins Related to Tissue Healing of Platelet-Rich Plasma. Clin. Orthop. Surg. 2020, 12, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Miroshnychenko, O.; Chalkley, R.J.; Leib, R.D.; Everts, P.A.; Dragoo, J.L. Proteomic analysis of platelet-rich and platelet-poor plasma. Regen. Ther. 2020, 15, 226–235. [Google Scholar] [CrossRef]
- Savastano, M.C.; Giannuzzi, F.; Savastano, A.; Cestrone, V.; Boselli, F.; Carlà, M.M.; D’Onofrio, N.C.; Biagini, I.; Rizzo, C.; Bianchi, M.; et al. Cord blood platelet-rich plasma: Proteomics analysis for ophthalmic applications. Clin. Proteom. 2025, 22, 1. [Google Scholar] [CrossRef]
- Mochizuki, T.; Ushiki, T.; Suzuki, K.; Sato, M.; Ishiguro, H.; Suwabe, T.; Edama, M.; Omori, G.; Yamamoto, N.; Kawase, T. Characterization of Leukocyte- and Platelet-Rich Plasma Derived from Female Collage Athletes: A Cross-Sectional Cohort Study Focusing on Growth Factor, Inflammatory Cytokines, and Anti-Inflammatory Cytokine Levels. Int. J. Mol. Sci. 2023, 24, 13592. [Google Scholar] [CrossRef] [PubMed]
- Muraglia, A.; Utyro, O.; Nardini, M.; Santolini, M.; Ceresa, D.; Agostini, V.; Nencioni, A.; Filaci, G.; Cancedda, R.; Mastrogiacomo, M. A simple cell proliferation assay and the inflammatory protein content show significant differences in human plasmas from young and old subjects. Front. Bioeng. Biotechnol. 2024, 12, 1408499. [Google Scholar] [CrossRef]
- Sánchez, M.; Mercader Ruiz, J.; Marijuán Pinel, D.; Sánchez, P.; Fiz, N.; Guadilla, J.; Azofra, J.; Beitia, M.; Delgado, D. Increasing the concentration of plasma molecules improves the biological activity of platelet-rich plasma for tissue regeneration. Sci. Rep. 2025, 15, 4523. [Google Scholar] [CrossRef]
- Jin, P.; Pan, Q.; Lin, Y.; Dong, Y.; Zhu, J.; Liu, T.; Zhu, W.; Cheng, B. Platelets Facilitate Wound Healing by Mitochondrial Transfer and Reducing Oxidative Stress in Endothelial Cells. Oxid. Med. Cell Longev. 2023, 2023, 2345279. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.; Yu, S.H.; Lee, S.E.; Park, J.H.; Cho, G.; Choi, C.; Han, K.; Kim, C.H.; Kang, Y.C. Platelet-derived mitochondria transfer facilitates wound-closure by modulating ROS levels in dermal fibroblasts. Platelets 2022, 34, 2151996. [Google Scholar] [CrossRef] [PubMed]
- Levoux, J.; Prola, A.; Lafuste, P.; Gervais, M.; Chevallier, N.; Koumaiha, Z.; Kefi, K.; Braud, L.; Schmitt, A.; Yacia, A.; et al. Platelets Facilitate the Wound-Healing Capability of Mesenchymal Stem Cells by Mitochondrial Transfer and Metabolic Reprogramming. Cell Metab. 2021, 33, 283–299.e9. [Google Scholar] [CrossRef]
- Chandel, N.S. Mitochondria: Back to the future. Nat. Rev. Mol. Cell Biol. 2018, 19, 76. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Coelho-Júnior, H.J.; Landi, F.; Picca, A. Mitochondrial Quantity and Quality in Age-Related Sarcopenia. Int. J. Mol. Sci. 2024, 25, 2052. [Google Scholar] [CrossRef]
- Grichine, A.; Jacob, S.; Eckly, A.; Villaret, J.; Joubert, C.; Appaix, F.; Pezet, M.; Ribba, A.S.; Denarier, E.; Mazzega, J.; et al. The fate of mitochondria during platelet activation. Blood Adv. 2023, 7, 6290–6302. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.P.; Ekhlak, M.; Dash, D. Energy metabolism in platelets fuels thrombus formation: Halting the thrombosis engine with small-molecule modulators of platelet metabolism. Metabolism 2023, 145, 155596. [Google Scholar] [CrossRef]
- Ushiki, T.; Mochizuki, T.; Suzuki, K.; Kamimura, M.; Ishiguro, H.; Suwabe, T.; Kawase, T. Modulation of ATP Production Influences Inorganic Polyphosphate Levels in Non-Athletes’ Platelets at the Resting State. Int. J. Mol. Sci. 2022, 23, 11293. [Google Scholar] [CrossRef]
- Ushiki, T.; Mochizuki, T.; Suzuki, K.; Kamimura, M.; Ishiguro, H.; Watanabe, S.; Omori, G.; Yamamoto, N.; Kawase, T. Platelet polyphosphate and energy metabolism in professional male athletes (soccer players): A cross-sectional pilot study. Physiol. Rep. 2022, 10, e15409. [Google Scholar] [CrossRef] [PubMed]
- Ushiki, T.; Mochizuki, T.; Suzuki, K.; Kamimura, M.I.H.; Suwabe, T.; Watanabe, S.; Omori, G.Y.; Kawase, T.N. Strategic Analysis of Body Composition Indices and Resting Platelet ATP Levels in Professional Soccer Players for Better Platelet-rich Plasma Therapy. Front. Bioeng. Biotechnol. 2023, 11, 1255860. [Google Scholar] [CrossRef]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-Rich Plasma: The PAW Classification System. Arthrosc. J. Arthrosc. Relat. Surg. 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, N.Z.; Perecin, F.; Méo, S.C.; Ferreira, C.R.; Tetzner, T.A.; Garcia, J.M. Demecolcine effects on microtubule kinetics and on chemically assisted enucleation of bovine oocytes. Cloning Stem Cells 2009, 11, 141–152. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS production by mitochondria: Function or dysfunction? Oncogene 2024, 43, 295–303. [Google Scholar] [CrossRef]
- Akkerman, J.W.; Holmsen, H. Interrelationships among platelet responses: Studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood 1981, 57, 956–966. [Google Scholar] [CrossRef] [PubMed]
- George, M.J.; Bynum, J.; Nair, P.; Cap, A.P.; Wade, C.E.; Cox, C.S., Jr.; Gill, B.S. Platelet biomechanics, platelet bioenergetics, and applications to clinical practice and translational research. Platelets 2018, 29, 431–439. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T.; Kamiya, M.; Hayama, K.; Nagata, M.; Okuda, K.; Yoshie, H.; Burns, D.M.; Tsuchimochi, M.; Nakata, K. X-ray and ultraviolet C irradiation-induced γ-H2AX and p53 formation in normal human periosteal cells in vitro: Markers for quality control in cell therapy. Cytotherapy 2015, 17, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Kasugai, S.; Hasegawa, N.; Ogura, H. Application of the MTT colorimetric assay to measure cytotoxic effects of phenolic compounds on established rat dental pulp cells. J. Dent. Res. 1991, 70, 127–130. [Google Scholar] [CrossRef]
- Li, Z.; Chen, X.; Sun, X.; Zhou, Q.; Chen, J.; Leno, G.H.; Engelhardt, J.F. Nuclear transfer of M-phase ferret fibroblasts synchronized with the microtubule inhibitor demecolcine. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 1126–1134. [Google Scholar] [CrossRef]
- Puri, R.N.; Colman, R.W. ADP-induced platelet activation. Crit. Rev. Biochem. Mol. Biol. 1997, 32, 437–502. [Google Scholar] [CrossRef]
- Maro, B.; Marty, M.C.; Bornens, M. In vivo and in vitro effects of the mitochondrial uncoupler FCCP on microtubules. EMBO J. 1982, 1, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Kasahara, T.; Kawabata, H.; Watanabe, T.; Nishiyama, K.; Kitamura, Y.; Watanabe, T.; Okudera, H.; Mochizuki, T.; Ushiki, T.; et al. Inhibitory effects of NaF on mitochondrial energy generation in human platelets in vitro. Front. Toxicol. 2024, 6, 1421184. [Google Scholar] [CrossRef] [PubMed]
- Masuki, H.; Isobe, K.; Kawabata, H.; Tsujino, T.; Yamaguchi, S.; Watanabe, T.; Sato, A.; Aizawa, H.; Mourao, C.F.; Kawase, T. Acute cytotoxic effects of silica microparticles used for coating of plastic blood-collection tubes on human periosteal cells. Odontology 2020, 108, 545–552. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, K.; Kasahara, T.; Kawabata, H.; Tsujino, T.; Kitamura, Y.; Watanabe, T.; Nakamura, M.; Mochizuki, T.; Ushiki, T.; Kawase, T. Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy. Int. J. Mol. Sci. 2025, 26, 5504. https://doi.org/10.3390/ijms26125504
Nishiyama K, Kasahara T, Kawabata H, Tsujino T, Kitamura Y, Watanabe T, Nakamura M, Mochizuki T, Ushiki T, Kawase T. Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy. International Journal of Molecular Sciences. 2025; 26(12):5504. https://doi.org/10.3390/ijms26125504
Chicago/Turabian StyleNishiyama, Koji, Tomoni Kasahara, Hideo Kawabata, Tetsuhiro Tsujino, Yutaka Kitamura, Taisuke Watanabe, Masayuki Nakamura, Tomoharu Mochizuki, Takashi Ushiki, and Tomoyuki Kawase. 2025. "Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy" International Journal of Molecular Sciences 26, no. 12: 5504. https://doi.org/10.3390/ijms26125504
APA StyleNishiyama, K., Kasahara, T., Kawabata, H., Tsujino, T., Kitamura, Y., Watanabe, T., Nakamura, M., Mochizuki, T., Ushiki, T., & Kawase, T. (2025). Mitochondrial Transfer from Human Platelets to Rat Dental Pulp-Derived Fibroblasts in the 2D In Vitro System: Additional Implication in PRP Therapy. International Journal of Molecular Sciences, 26(12), 5504. https://doi.org/10.3390/ijms26125504






