Gene Copy Number Dictates Extracellular Vesicle Cargo
Abstract
1. Introduction
2. Results and Discussion
2.1. Microfluidic Resistive Pulse Sensing (MRPS) Analysis of Copy Number Cell-Derived EVs Shows Variability in Count and Size
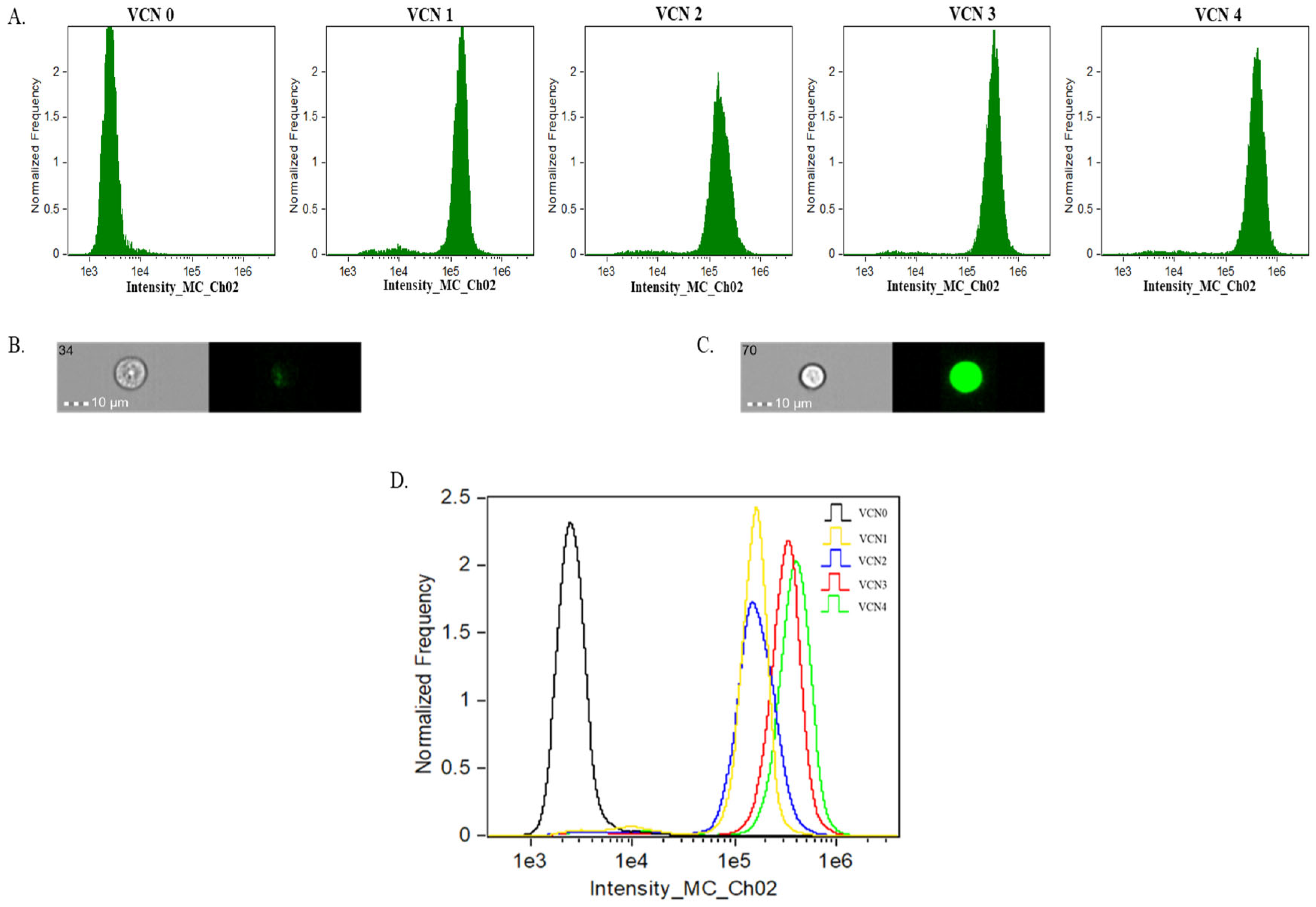
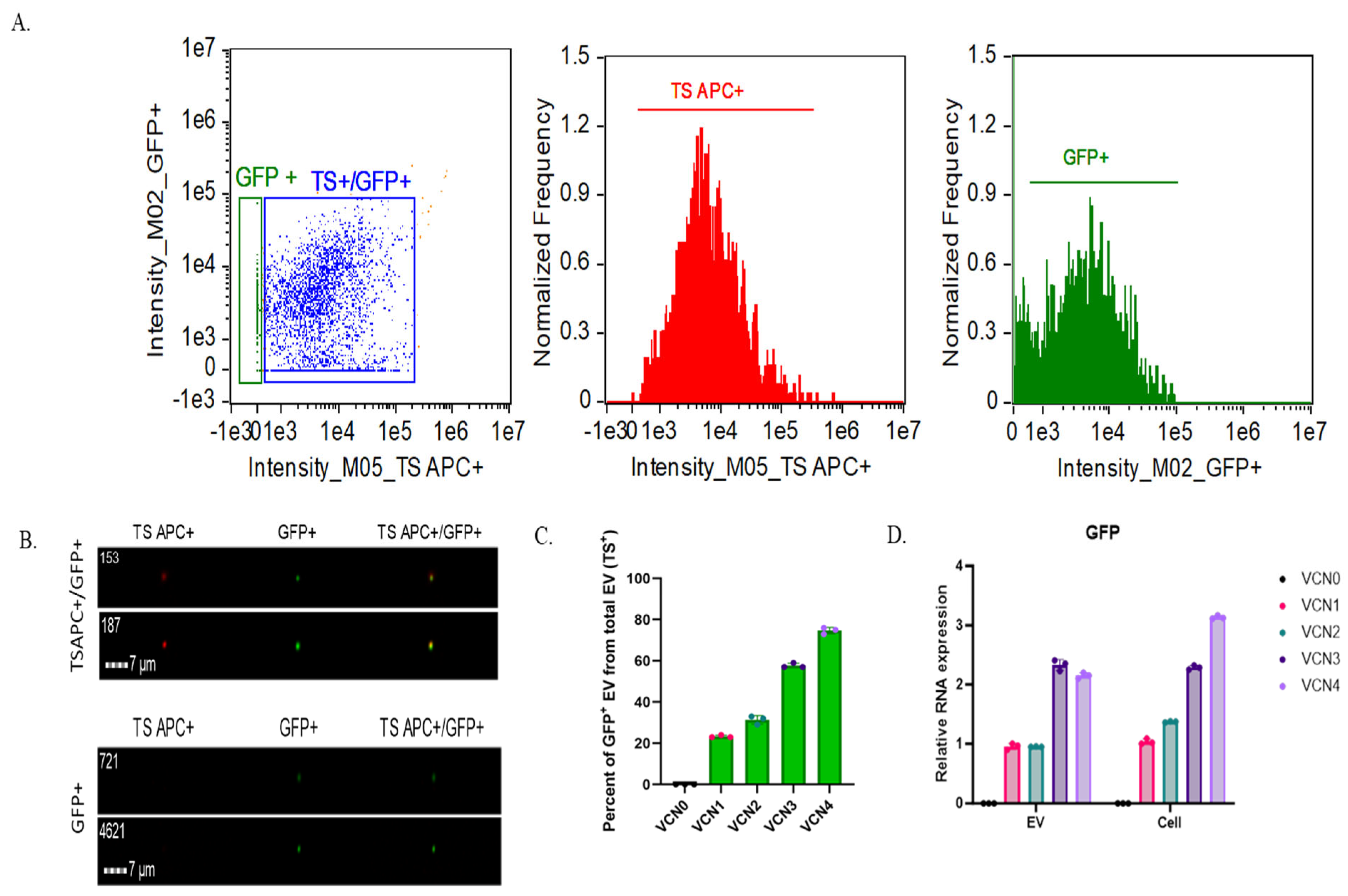
2.2. Stable GFP VCN Cell-Derived EVs Reveal Correlation with Protein Cargo but Not RNA Cargo
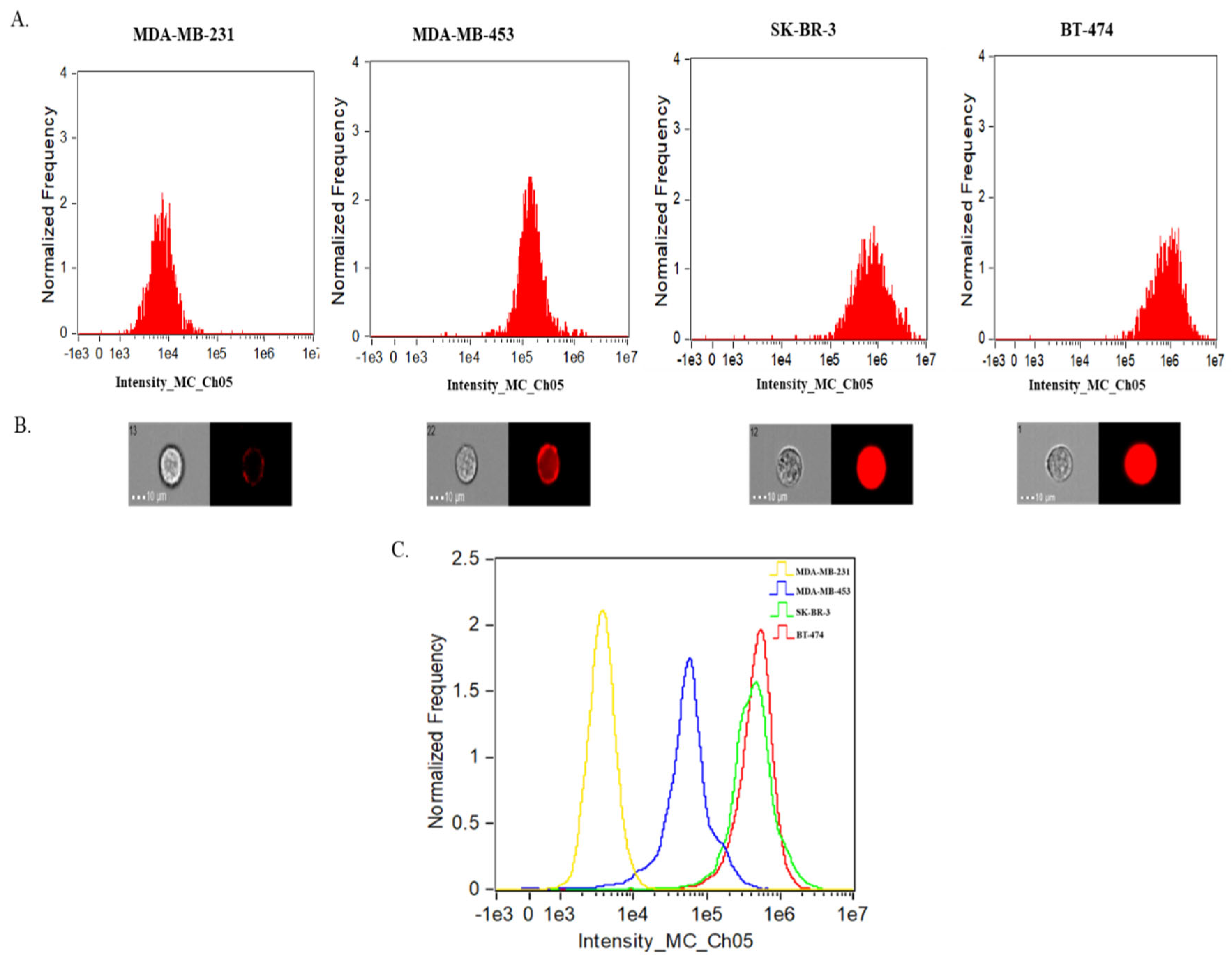
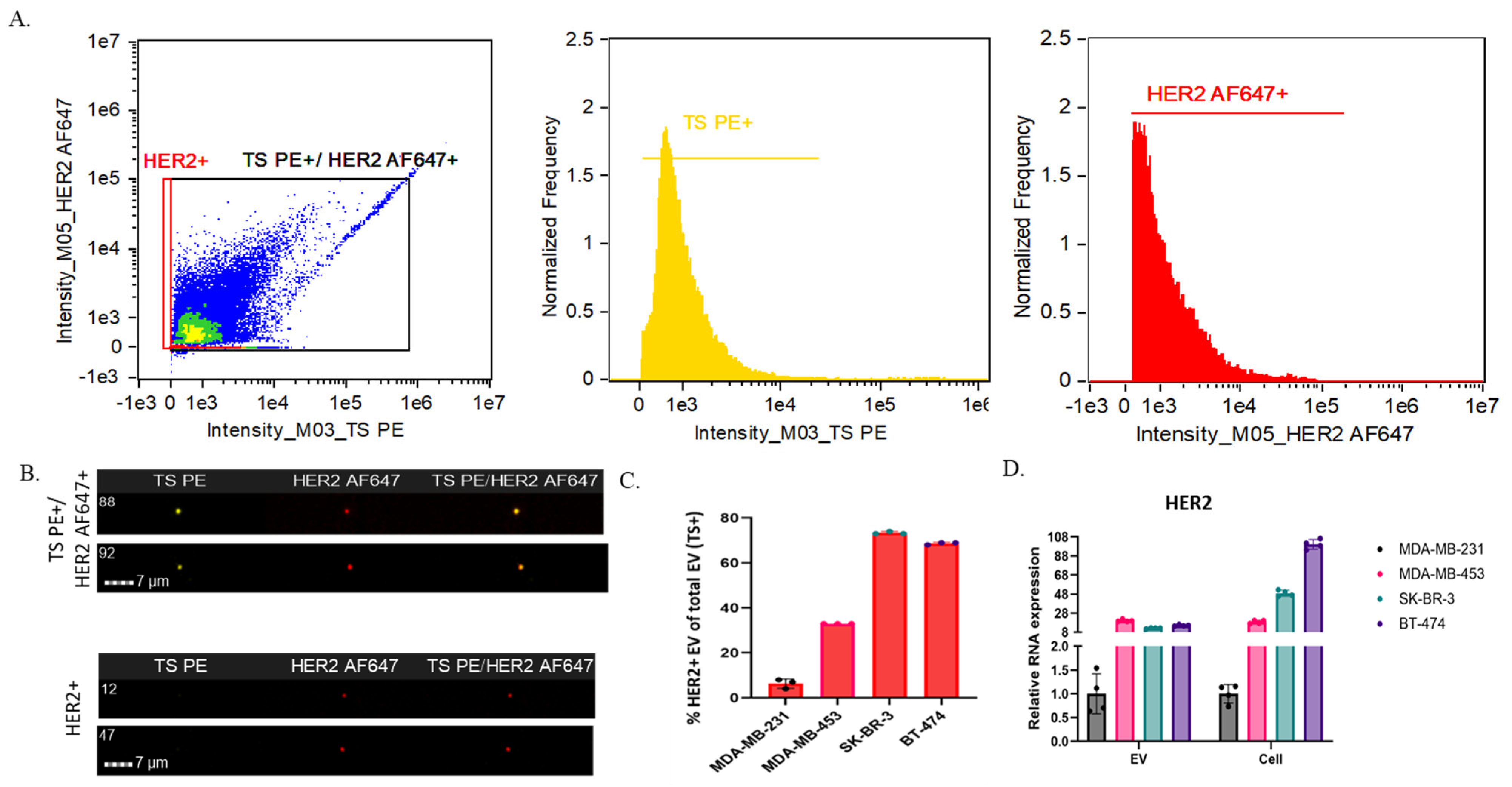
2.3. SRM 2373 Cell-Derived EVs Exhibit a Partial Correlation with Protein Cargo in the Context of HER2 Genes but Not with RNA Cargo
3. Materials and Methods
3.1. Cell Culture
3.2. EV Extraction
3.3. Microfluidic Resistive Pulse Sensing (MRPS)
3.4. EV Staining for Imaging Flow Cytometry (IFC)
3.5. IFC Measurements
3.6. Data Analysis with IDEAS Software (Version 6.3)
3.7. RNA Extraction
3.8. Relative RNA Quantification by Digital Droplet Polymerase Chain Reaction (ddPCR)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G. Exosomes: A Common Pathway for a Specialized Function. J. Biochem. 2006, 140, 13–21. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extra-cellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J. Control Release 2015, 219, 396–405. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, 6478. [Google Scholar] [CrossRef] [PubMed]
- Janas, A.M.; Sapoń, K.; Janas, T.; Stowell, M.H.; Janas, T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2016, 1858, 1139–1151. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extra-cellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Paugh, B.S.; Baranyi, L.; Roy, A.; He, H.-J.; Harris, L.; Cole, K.D.; Artlip, M.; Raimund, C.; Langan, P.S.; Jana, S.; et al. Reference standards for accurate validation and optimization of assays that determine integrated lentiviral vector copy number in transduced cells. Sci. Rep. 2021, 11, 389. [Google Scholar] [CrossRef]
- NIST HER2 Genomic DNA Standard Reference Material, SRM® 2373 | NIST. Available online: https://www.nist.gov/programs-projects/nist-her2-genomic-dna-standard-reference-material-srmr-2373 (accessed on 28 May 2025).
- He, H.-J.; Almeida, J.L.; Lund, S.P.; Steffen, C.R.; Choquette, S.; Cole, K.D. Development of NIST standard reference material 2373: Genomic DNA standards for HER2 measurements. Biomol. Detect. Quantif. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- van de Wakker, S.; van Oudheusden, J.; Mol, E.; Roefs, M.; Zheng, W.; Görgens, A.; El Andaloussi, S.; Sluijter, J.; Vader, P. Influence of short term storage conditions, concentration methods and excipients on extracellular vesicle recovery and function. Eur. J. Pharm. Biopharm. 2022, 170, 59–69. [Google Scholar] [CrossRef]
- Kawai-Harada, Y.; El Itawi, H.; Komuro, H.; Harada, M. Evaluation of EV Storage Buffer for Efficient Preservation of Engineered Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 12841. [Google Scholar] [CrossRef]
- Cimorelli, M.; Nieuwland, R.; Varga, Z.; van der Pol, E. Standardized procedure to measure the size distribution of extracellular vesicles together with other particles in biofluids with microfluidic resistive pulse sensing. PLoS ONE 2021, 16, e0249603. [Google Scholar] [CrossRef] [PubMed]
- Arab, T.; Mallick, E.R.; Huang, Y.; Dong, L.; Liao, Z.; Zhao, Z.; Gololobova, O.; Haughey, N.J.; Pienta, K.J.; Slusher, B.S.; et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles 2021, 10, e12079. [Google Scholar] [CrossRef]
- Shekari, F.; Alibhai, F.J.; Baharvand, H.; Börger, V.; Bruno, S.; Davies, O.; Giebel, B.; Gimona, M.; Salekdeh, G.H.; Martin-Jaular, L.; et al. Cell culture-derived extracellular vesicles: Considerations for reporting cell culturing parameters. J. Extracell. Biol. 2023, 2, e115. [Google Scholar] [CrossRef]
- Schechter, A.L.; Stern, D.F.; Vaidyanathan, L.; Decker, S.J.; Drebin, J.A.; Greene, M.I.; Weinberg, R.A. The neu oncogene: An erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984, 312, 513–516. [Google Scholar] [CrossRef]
- Ross, J.S.; Slodkowska, E.A.; Symmans, W.F.; Pusztai, L.; Ravdin, P.M.; Hortobagyi, G.N. The HER-2 Receptor and Breast Cancer: Ten Years of Targeted Anti–HER-2 Therapy and Personalized Medicine. Oncologist 2009, 14, 320–368. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Broglio, K.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Prognosis of Women With Metastatic Breast Cancer by HER2 Status and Trastuzumab Treatment: An Institutional-Based Review. J. Clin. Oncol. 2010, 28, 92–98. [Google Scholar] [CrossRef]
- Stanley, A.; Ashrafi, G.H.; Seddon, A.M.; Modjtahedi, H. Synergistic effects of various Her inhibitors in combination with IGF-1R, C-MET and Src targeting agents in breast cancer cell lines. Sci. Rep. 2017, 7, 3964. [Google Scholar] [CrossRef]
- Gall, V.A.; Philips, A.V.; Qiao, N.A.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kulak, M.V.; Borcherding, N.; Maina, P.K.; Zhang, W.; Weigel, R.J.; Qi, H.H. A novel HER2 gene body enhancer contributes to HER2 expression. Oncogene 2018, 37, 687–694. [Google Scholar] [CrossRef]
- Welsh, J.A.; van der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- He, Z.; Kwee, E.J.; Cleveland, M.H.; Cole, K.D.; Lin-Gibson, S.; He, H.-J. Quantitation and integrity evaluation of RNA genome in lentiviral vectors by direct reverse transcription-droplet digital PCR (direct RT-ddPCR). Sci. Rep. 2023, 13, 14470. [Google Scholar] [CrossRef] [PubMed]
- Spitzberg, J.D.; Ferguson, S.; Yang, K.S.; Peterson, H.M.; Carlson, J.C.T.; Weissleder, R. Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nat. Commun. 2023, 14, 1239. [Google Scholar] [CrossRef]
- Kosti, I.; Jain, N.; Aran, D.; Butte, A.J.; Sirota, M. Cross-tissue Analysis of Gene and Protein Expression in Normal and Cancer Tissues. Sci. Rep. 2016, 6, 24799. [Google Scholar] [CrossRef] [PubMed]
- Nicolet, B.P.; Wolkers, M.C. The relationship of mRNA with protein expression in CD8+ T cells associates with gene class and gene characteristics. PLoS ONE 2022, 17, e0276294. [Google Scholar] [CrossRef]
- O’grady, T.; Njock, M.-S.; Lion, M.; Bruyr, J.; Mariavelle, E.; Galvan, B.; Boeckx, A.; Struman, I.; Dequiedt, F. Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol. 2022, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Miceli, R.T.; Chen, T.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef]
- Yeri, A.; Courtright, A.; Reiman, R.; Carlson, E.; Beecroft, T.; Janss, A.; Siniard, A.; Richholt, R.; Balak, C.; Rozowsky, J.; et al. Total Extracellular Small RNA Profiles from Plasma, Saliva, and Urine of Healthy Subjects. Sci. Rep. 2017, 7, srep44061. [Google Scholar] [CrossRef]
- Chevillet, J.R.; Kang, Q.; Ruf, I.K.; Briggs, H.A.; Vojtech, L.N.; Hughes, S.M.; Cheng, H.H.; Arroyo, J.D.; Meredith, E.K.; Gallichotte, E.N.; et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl. Acad. Sci. USA 2014, 111, 14888–14893. [Google Scholar] [CrossRef]
- O’brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Gonzalez-Kozlova, E.; Soleymani, T.; La Salvia, S.; Kyprianou, N.; Sahoo, S.; Tewari, A.K.; Cordon-Cardo, C.; Stolovitzky, G.; Dogra, N. Extracellular vesicles carry distinct proteo-transcriptomic signatures that are different from their cancer cell of origin. iScience 2022, 25, 104414. [Google Scholar] [CrossRef]
- Lane, R.E.; Korbie, D.; Khanna, K.K.; Mohamed, A.; Hill, M.M.; Trau, M. Defining the relationship between cellular and extracellular vesicle (EV) content in breast cancer via an integrative multi-omic analysis. Proteomics 2024, 24, e2300089. [Google Scholar] [CrossRef] [PubMed]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.; Yang, K.S.; Zelga, P.; Liss, A.S.; Carlson, J.C.T.; del Castillo, C.F.; Weissleder, R. Single-EV analysis (sEVA) of mutated proteins allows detection of stage 1 pancreatic cancer. Sci. Adv. 2022, 8, eabm3453. [Google Scholar] [CrossRef] [PubMed]
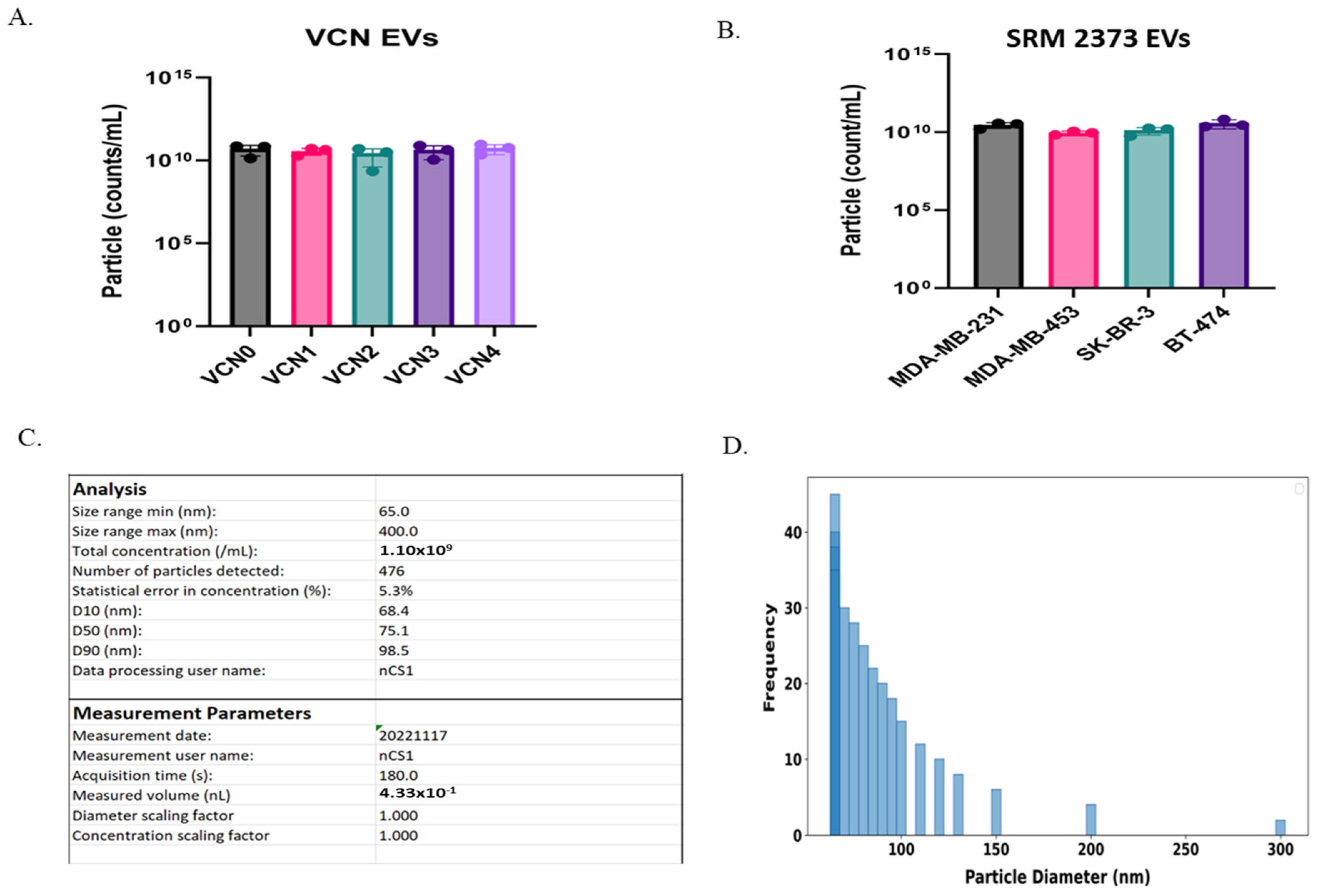
| EV Sample | Particles (1/mL) |
|---|---|
| VCN 0 | 5.2 × 1010 (±3.3 × 1010) |
| VCN 1 | 3.8 × 1010 (±1.7 × 1010) |
| VCN 2 | 2.8 × 1010 (±2.4 × 1010) |
| VCN 3 | 4.5 × 1010 (±3.4 × 1010) |
| VCN 4 | 5.7 × 1010 (±3.5 × 1010) |
| EV Sample | Particles (1/mL) |
|---|---|
| MDA-MB-231 | 3.0 × 1010 (±1.2 × 1010) |
| MDA-MB-453 | 9.3 × 1010 (±2.5 × 109) |
| SK-BR-3 | 1.3 × 1010 (±6.7 × 109) |
| BT-474 | 3.9 × 1010 (±2.3 × 1010) |
| EV Sample | TS+ Particles (1/mL) | GFP+ Particles (1/mL) | % of GFP+ EVs of Total EVs (TS+) |
|---|---|---|---|
| VCN0 | 1.6 × 109 (±1.9 × 105) | 4.4 × 106 (±1.4 × 103) | 0% |
| VCN1 | 2.3 × 109 (±2.0 × 105) | 5.3 × 108 (±6.3 × 104) | 23% |
| VCN2 | 3.7 × 109 (±3.4 × 105) | 1.2 × 109 (±7.8 × 104) | 31% |
| VCN3 | 1.5 × 109 (±7.7 × 104) | 8.4 × 108 (±4.0 × 104) | 57% |
| VCN4 | 2.7 × 109 (±3.4 × 105) | 2.00 × 109 (±2.1 × 105) | 74% |
| EV Sample | TS+ Particles (1/mL) | HER2+ Particles (1/mL) | % of HER2+ EVs of Total EVs (TS+) |
|---|---|---|---|
| MDA-MB-231 | 3.6 × 109 (±1.9 × 106) | 2.1 × 108 (±7.4 × 105) | 6% |
| MDA-MB-453 | 1.1 × 109 (±6.1 × 106) | 3.7 × 108 (±2.2 × 106) | 33% |
| SK-BR-3 | 1.3 × 109 (±9.0 × 105) | 9.2 × 108 (±6.2 × 105) | 73% |
| BT-474 | 5.6 × 109 (±7.0 × 106) | 3.9 × 109 (±4.7 × 106) | 69% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poudel, S.; He, Z.; Izac, J.; Wang, L. Gene Copy Number Dictates Extracellular Vesicle Cargo. Int. J. Mol. Sci. 2025, 26, 5496. https://doi.org/10.3390/ijms26125496
Poudel S, He Z, Izac J, Wang L. Gene Copy Number Dictates Extracellular Vesicle Cargo. International Journal of Molecular Sciences. 2025; 26(12):5496. https://doi.org/10.3390/ijms26125496
Chicago/Turabian StylePoudel, Sumeet, Zhiyong He, Jerilyn Izac, and Lili Wang. 2025. "Gene Copy Number Dictates Extracellular Vesicle Cargo" International Journal of Molecular Sciences 26, no. 12: 5496. https://doi.org/10.3390/ijms26125496
APA StylePoudel, S., He, Z., Izac, J., & Wang, L. (2025). Gene Copy Number Dictates Extracellular Vesicle Cargo. International Journal of Molecular Sciences, 26(12), 5496. https://doi.org/10.3390/ijms26125496






