The Profile of Selected Protein Markers of Senescence in the Placentas of Cows During Early–Mid-Pregnancy and Parturition with and Without the Retention of Fetal Membranes: A Preliminary Study
Abstract
1. Introduction
2. Results
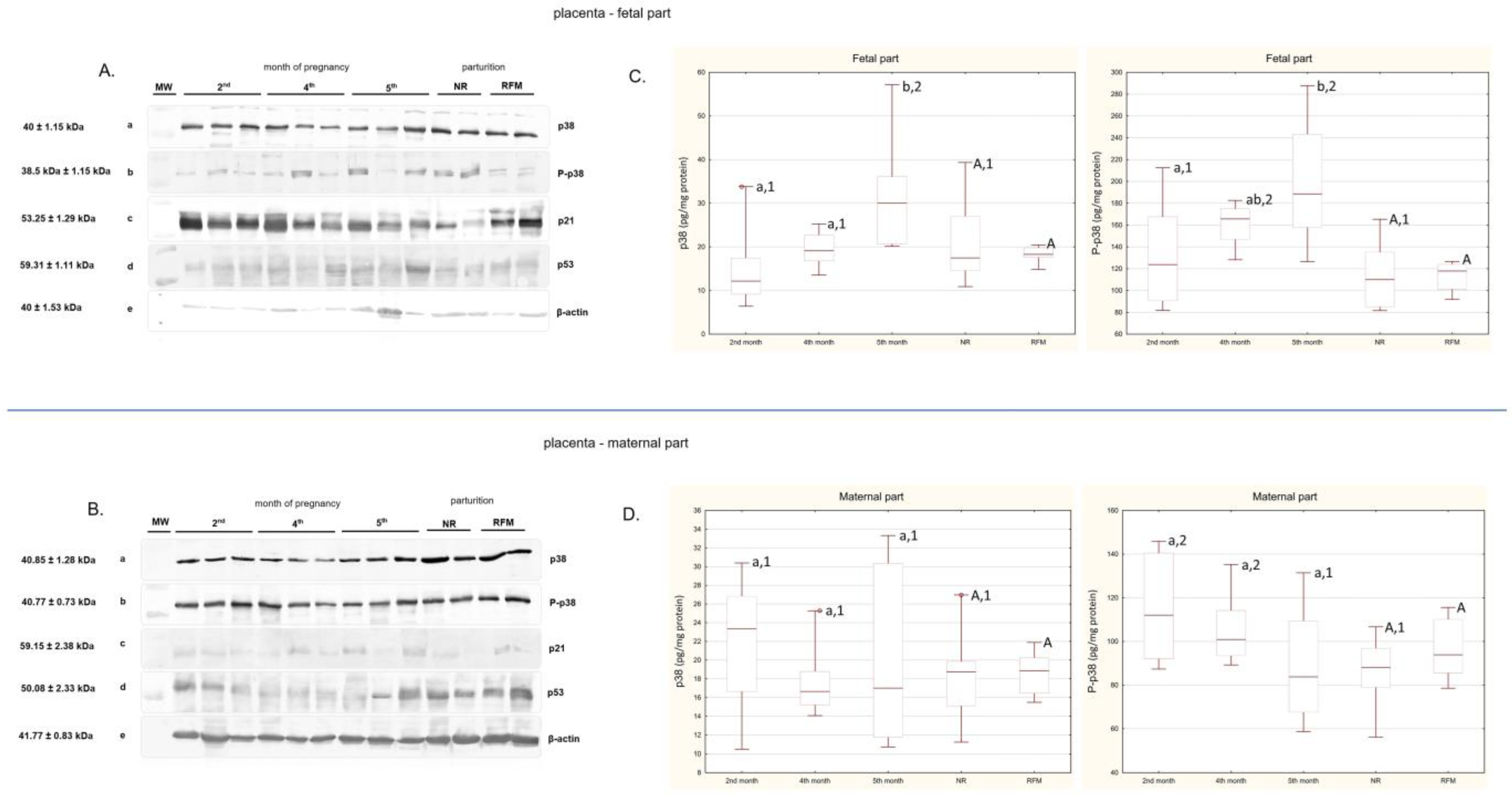
2.1. Western Blot
2.2. ELISA Determination of p38 and P-p38 Concentrations
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Homogenization
4.3. Western Blotting (WB) Analysis
4.4. ELISA
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RFM | Retained fetal membranes/retention of fetal membranes |
| NR | Not-retained fetal membranes |
| OS | Oxidative stress |
| MAPK | Mitogen-activated protein kinase |
| P-p38 | Phosphorylated p38 |
References
- Kajdy, A.; Modzelewski, J.; Cymbaluk-Płoska, A.; Kwiatkowska, E.; Bednarek-Jędrzejek, M.; Borowski, D.; Stefańska, K.; Rabijewski, M.; Torbé, A.; Kwiatkowski, S. Molecular Pathways of Cellular Senescence and Placental Aging in Late Fetal Growth Restriction and Stillbirth. Int. J. Mol. Sci. 2021, 22, 4186. [Google Scholar] [CrossRef] [PubMed]
- Cuenda, A.; Rousseau, S. P38 MAP-Kinases Pathway Regulation, Function and Role in Human Diseases. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2007, 1773, 1358–1375. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.S.; Redman, C. The Role of Cellular Senescence in Ageing of the Placenta. Placenta 2017, 52, 139–145. [Google Scholar] [CrossRef]
- Van Deursen, J.M. The Role of Senescent Cells in Ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef]
- Chuprin, A.; Gal, H.; Biron-Shental, T.; Biran, A.; Amiel, A.; Rozenblatt, S.; Krizhanovsky, V. Cell Fusion Induced by ERVWE1 or Measles Virus Causes Cellular Senescence. Genes Dev. 2013, 27, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Sultana, Z.; Maiti, K.; Dedman, L.; Smith, R. Is There a Role for Placental Senescence in the Genesis of Obstetric Complications and Fetal Growth Restriction? Am. J. Obstet. Gynecol. 2018, 218, S762–S773. [Google Scholar] [CrossRef]
- Goldman, B.; Radnaa, E.; Kechichian, T.; Menon, R. Silencing P38 MAPK Reduces Cellular Senescence in Human Fetal Chorion Trophoblast Cells. Am. J. Reprod. Immunol. 2023, 89, e13648. [Google Scholar] [CrossRef]
- Dutta, E.H.; Behnia, F.; Boldogh, I.; Saade, G.R.; Taylor, B.D.; Kacerovský, M.; Menon, R. Oxidative Stress Damage-Associated Molecular Signaling Pathways Differentiate Spontaneous Preterm Birth and Preterm Premature Rupture of the Membranes. Mol. Hum. Reprod. 2016, 22, 143–157. [Google Scholar] [CrossRef]
- Menon, R. Initiation of Human Parturition: Signaling from Senescent Fetal Tissues via Extracellular Vesicle Mediated Paracrine Mechanism. Obstet. Gynecol. Sci. 2019, 62, 199–211. [Google Scholar] [CrossRef]
- Menon, R.; Behnia, F.; Polettini, J.; Saade, G.R.; Campisi, J.; Velarde, M. Placental Membrane Aging and HMGB1 Signaling Associated with Human Parturition. Aging 2016, 8, 216–229. [Google Scholar] [CrossRef]
- Attupuram, N.M.; Kumaresan, A.; Narayanan, K.; Kumar, H. Cellular and Molecular Mechanisms Involved in Placental Separation in the Bovine: A Review. Mol. Reprod. Dev. 2016, 83, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Boos, A.; Janssen, V.; Mülling, C. Proliferation and Apoptosis in Bovine Placentomes during Pregnancy and around Induced and Spontaneous Parturition as Well as in Cows Retaining the Fetal Membranes. Reproduction 2003, 126, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Kamemori, Y.; Wakamiya, K.; Nishimura, R.; Hosaka, Y.; Ohtani, S.; Okuda, K. Expressions of Apoptosis-Regulating Factors in Bovine Retained Placenta. Placenta 2011, 32, 20–26. [Google Scholar] [CrossRef]
- Heazell, A.E.P.; Crocker, I.P. Live and Let Die—Regulation of Villous Trophoblast Apoptosis in Normal and Abnormal Pregnancies. Placenta 2008, 29, 772–783. [Google Scholar] [CrossRef]
- Scifres, C.M.; Nelson, D.M. Intrauterine Growth Restriction, Human Placental Development and Trophoblast Cell Death. In Proceedings of the Journal of Physiology, San Diego, CA, USA, 15 July 2009; Volume 587, pp. 3453–3458. [Google Scholar]
- Heazell, A.E.P.; Sharp, A.N.; Baker, P.N.; Crocker, I.P. Intra-Uterine Growth Restriction Is Associated with Increased Apoptosis and Altered Expression of Proteins in the P53 Pathway in Villous Trophoblast. Apoptosis 2011, 16, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Miyamoto, T.; Kobara, H.; Yamada, S.; Asaka, R.; Kikuchi, N.; Kashima, H.; Ohira, S.; Shiozawa, T. Trophoblast Type-Specific Expression of Senescence Markers in the Human Placenta. Placenta 2019, 85, 56–62. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-Classical P38 MAP Kinase Functions: Cell Cycle Checkpoints and Survival. Int. J. Biol. Sci. 2009, 5, 44–51. [Google Scholar] [CrossRef]
- Davenport, K.M.; Ortega, M.S.; Johnson, G.A.; Seo, H.; Spencer, T.E. Review: Implantation and Placentation in Ruminants. Animal 2023, 17, 100796. [Google Scholar] [CrossRef] [PubMed]
- Shenavai, S.; Preissing, S.; Hoffmann, B.; Dilly, M.; Pfarrer, C.; Özalp, G.R.; Caliskan, C.; Seyrek-Intas, K.; Schuler, G. Investigations into the Mechanisms Controlling Parturition in Cattle. Reproduction 2012, 144, 279–292. [Google Scholar] [CrossRef]
- Mitchell, B.F.; Taggart, M.J. Are Animal Models Relevant to Key Aspects of Human Parturition? Mitchell BF, Taggart MJ. Are Animal Models Relevant to Key Aspects of Human Parturition? Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 297, 525–545. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13713. [Google Scholar] [CrossRef] [PubMed]
- Wawrzykowski, J.; Jamioł, M.; Kankofer, M. The Role of Dermatopontin in Cell Adhesion in Bovine Placenta during Early-Mid Pregnancy and Parturition—Pilot Study. Theriogenology 2021, 171, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Bonney, E.A.; Krebs, K.; Saade, G.; Kechichian, T.; Trivedi, J.; Huaizhi, Y.; Menon, R. Differential Senescence in Feto-Maternal Tissues during Mouse Pregnancy. Placenta 2016, 43, 26–34. [Google Scholar] [CrossRef]
- Menon, R.; Boldogh, I.; Hawkins, H.K.; Woodson, M.; Polettini, J.; Syed, T.A.; Fortunato, S.J.; Saade, G.R.; Papaconstantinou, J.; Taylor, R.N. Histological Evidence of Oxidative Stress and Premature Senescence in Preterm Premature Rupture of the Human Fetal Membranes Recapitulated in Vitro. Am. J. Pathol. 2014, 184, 1740–1751. [Google Scholar] [CrossRef]
- Dixon, C.L.; Richardson, L.; Sheller-Miller, S.; Saade, G.; Menon, R. A Distinct Mechanism of Senescence Activation in Amnion Epithelial Cells by Infection, Inflammation, and Oxidative Stress. Am. J. Reprod. Immunol. 2018, 79, e12790. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.L.; Hempstock, J.; Bao, Y.P.; Skepper, J.N.; Burton, G.J. Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress: A Possible Factor in Human Early Pregnancy Failure. Am. J. Pathol. 2000, 157, 2111–2122. [Google Scholar] [CrossRef]
- Wawrzykowski, J.; Jamioł, M.; Mojsym, W.; Kankofer, M. The Comparison of Pro- and Antioxidative Parameters in Plasma and Placental Tissues during Early Phase of Placental Development in Cows. Mol. Biol. Rep. 2021, 48, 1291–1297. [Google Scholar] [CrossRef]
- Kankofer, M. Antioxidative Defence Mechanisms against Reactive Oxygen Species in Bovine Retained and Not-Retained Placenta: Activity of Glutathione Peroxidase, Glutathione Transferase, Catalase and Superoxide Dismutase. Placenta 2001, 22, 466–472. [Google Scholar] [CrossRef]
- Kankofer, M.; Lipko, J.; Zdunczyk, S. Total Antioxidant Capacity of Bovine Spontaneously Released and Retained Placenta. Pathophysiology 2005, 11, 215–219. [Google Scholar] [CrossRef]
- Jin, J.; Richardson, L.; Sheller-Miller, S.; Zhong, N.; Menon, R. Oxidative Stress Induces P38MAPK-Dependent Senescence in the Feto-Maternal Interface Cells. Placenta 2018, 67, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sheller-Miller, S.; Richardson, L.; Martin, L.; Jin, J.; Menon, R. Systematic Review of P38 Mitogen-Activated Kinase and Its Functional Role in Reproductive Tissues. Am. J. Reprod. Immunol. 2018, 80, e13047. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, N.; Gu, J.; Fu, S.; Peng, Z.; Zhao, C.; Zhang, Y.; Li, X.; Wang, Z.; Li, X.; et al. β-Hydroxybutyrate Induces Bovine Hepatocyte Apoptosis via an ROS-P38 Signaling Pathway. J. Dairy Sci. 2016, 99, 9184–9198. [Google Scholar] [CrossRef] [PubMed]
- Madan, P.; Calder, M.D.; Watson, A.J. Mitogen-Activated Protein Kinase (MAPK) Blockade of Bovine Preimplantation Embryogenesis Requires Inhibition of Both P38 and Extracellular Signal-Regulated Kinase (ERK) Pathways. Reproduction 2005, 130, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Doualla-Bell, F.; Koromilas, A.E. Induction of PG G/H Synthase-2 in Bovine Myometrial Cells by Interferon-τ Requires the Activation of the P38 MAPK Pathway. Endocrinology 2001, 142, 5107–5115. [Google Scholar] [CrossRef]
- Jung, Y.S.; Qian, Y.; Chen, X. Examination of the Expanding Pathways for the Regulation of P21 Expression and Activity. Cell Signal. 2010, 22, 1003–1012. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. P21 in Cancer: Intricate Networks and Multiple Activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Zhou, B.P.; Liao, Y.; Xia, W.; Spohn, B.; Lee, M.-H.; Hung, M.-C. Cytoplasmic Localization of P21 Cip1/WAF1 by Akt-Induced Phosphorylation in HER-2/Neu-Overexpressing Cells. Nat. Cell Biol. 2001, 3, 245–252. [Google Scholar] [CrossRef]
- Scott, M.T.; Morrice, N.; Ball, K.L. Reversible Phosphorylation at the C-Terminal Regulatory Domain of P21(Waf1/Cip1) Modulates Proliferating Cell Nuclear Antigen Binding. J. Biol. Chem. 2000, 275, 11529–11537. [Google Scholar] [CrossRef]
- Menon, R.; Fortunato, S.J. Distinct Pathophysiologic Pathways Induced by in Vitro Infection and Cigarette Smoke in Normal Human Fetal Membranes. Am. J. Obstet. Gynecol. 2009, 200, 334.e1–334.e8. [Google Scholar] [CrossRef]
- Yang, W.H.; Kim, J.E.; Nam, H.W.; Ju, J.W.; Kim, H.S.; Kim, Y.S.; Cho, J.W. Modification of P53 with O-Linked N-Acetylglucosamine Regulates P53 Activity and Stability. Nat. Cell Biol. 2006, 8, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Favetta, L.A.; Robert, C.; King, W.A.; Betts, D.H. Expression Profiles of P53 and P66shc during Oxidative Stress-Induced Senescence in Fetal Bovine Fibroblasts. Exp. Cell Res. 2004, 299, 36–48. [Google Scholar] [CrossRef]
- Botta, C.; Pellegrini, G.; Hässig, M.; Pesch, T.; Prähauser, B.; Wunderlin, S.; Guscetti, F.; Schneeberger, M.; Schmitt, S.; Basso, W.; et al. Bovine Fetal Placenta During Pregnancy and the Postpartum Period. Vet. Pathol. 2019, 56, 248–258. [Google Scholar] [CrossRef]
- Grunert, E. Zurückbleiben der Nachgeburt (Retentio secundinarum, Ret.sec.). In Tiergeburtshilfe, 4th ed.; Grunert, E., Arbeiter, K., Eds.; Verlag Paul Parey: Berlin, Germany, 1993; pp. 390–401. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Levene, H. Robust tests for equality of variances. In Contributions to Probability and Statistics; Stanford University Press: Palo Alto, CA, USA, 1960; pp. 278–292. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Welch, B.L. The generalization of ‘STUDENT’S’ problem when several different population variances are involved. Biometrika 1947, 34, 28–35. [Google Scholar] [CrossRef]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosztowny, E.; Wawrzykowski, J.T.; Jamioł, M.A.; Kankofer, M. The Profile of Selected Protein Markers of Senescence in the Placentas of Cows During Early–Mid-Pregnancy and Parturition with and Without the Retention of Fetal Membranes: A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 5475. https://doi.org/10.3390/ijms26125475
Kosztowny E, Wawrzykowski JT, Jamioł MA, Kankofer M. The Profile of Selected Protein Markers of Senescence in the Placentas of Cows During Early–Mid-Pregnancy and Parturition with and Without the Retention of Fetal Membranes: A Preliminary Study. International Journal of Molecular Sciences. 2025; 26(12):5475. https://doi.org/10.3390/ijms26125475
Chicago/Turabian StyleKosztowny, Ewelina, Jacek T. Wawrzykowski, Monika A. Jamioł, and Marta Kankofer. 2025. "The Profile of Selected Protein Markers of Senescence in the Placentas of Cows During Early–Mid-Pregnancy and Parturition with and Without the Retention of Fetal Membranes: A Preliminary Study" International Journal of Molecular Sciences 26, no. 12: 5475. https://doi.org/10.3390/ijms26125475
APA StyleKosztowny, E., Wawrzykowski, J. T., Jamioł, M. A., & Kankofer, M. (2025). The Profile of Selected Protein Markers of Senescence in the Placentas of Cows During Early–Mid-Pregnancy and Parturition with and Without the Retention of Fetal Membranes: A Preliminary Study. International Journal of Molecular Sciences, 26(12), 5475. https://doi.org/10.3390/ijms26125475





