Analysis of the Causes of Split Pit in Peaches
Abstract
1. Introduction
2. Phenomenon of Split Pit in Peaches
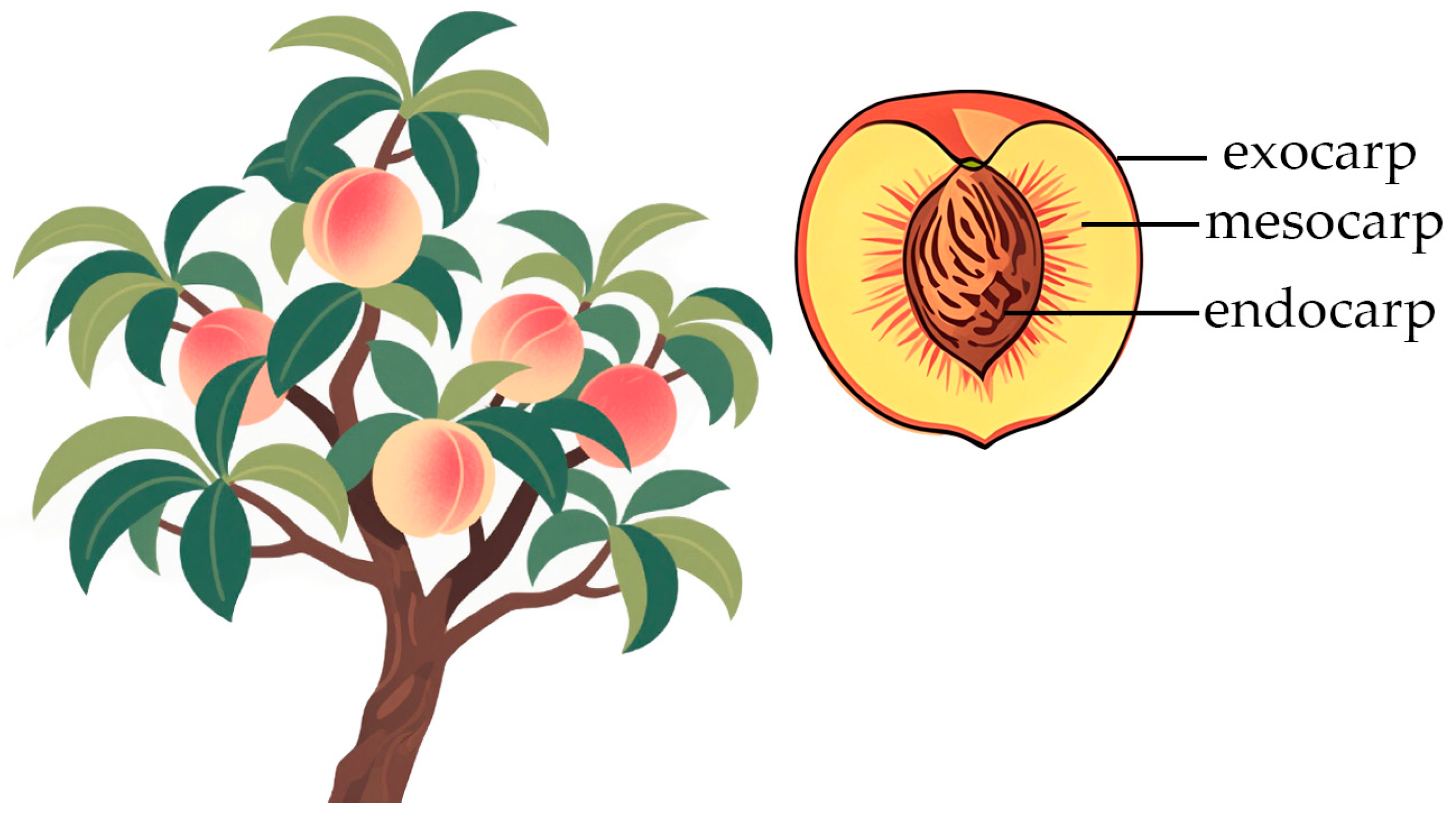
2.1. Fruit Structure of Peaches
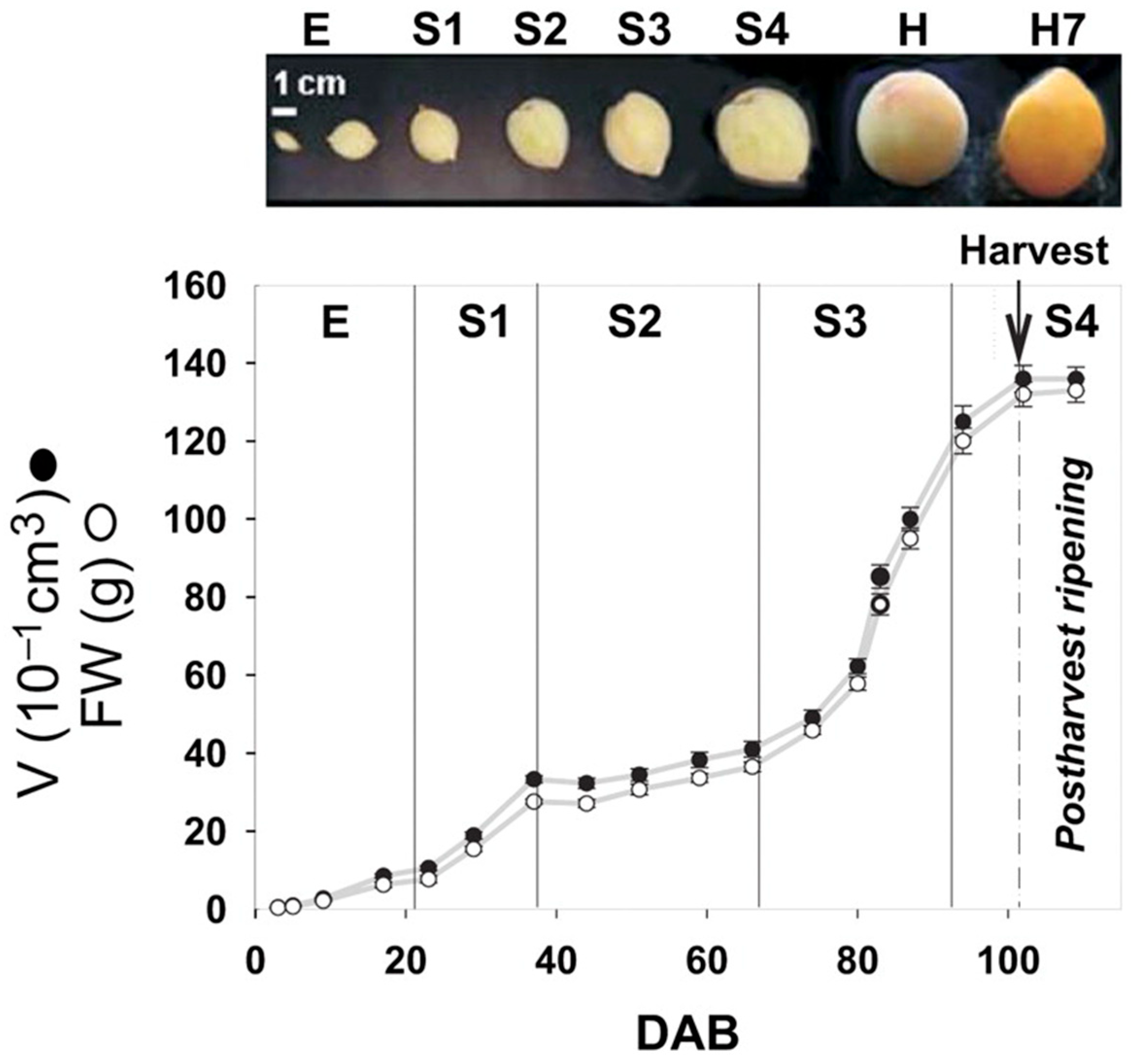
2.2. Period of Split Pit Occurrence in Peaches
2.3. Characteristics of Split-Pit Fruit
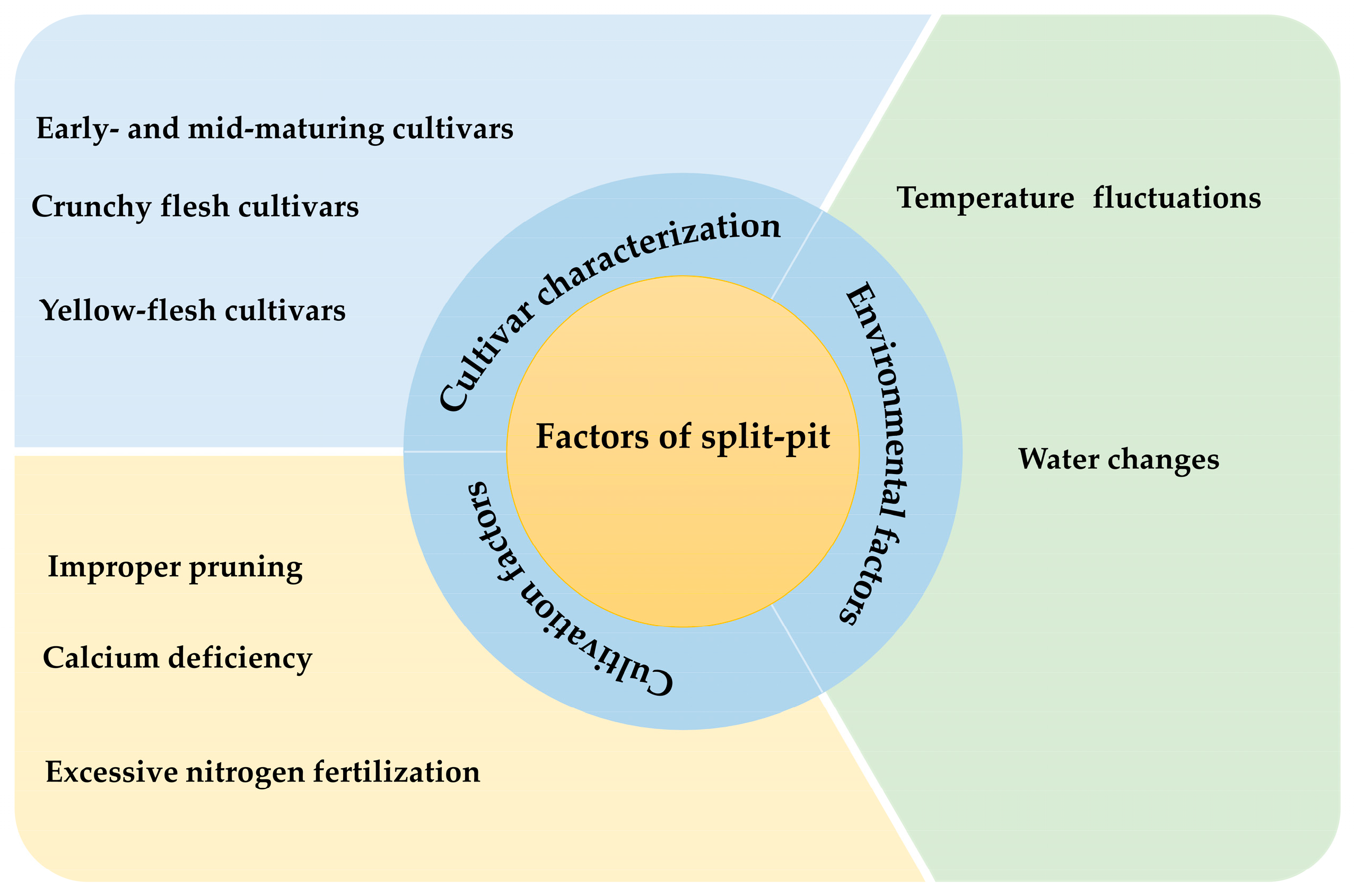
3. Factors Affecting Split Pit in Peaches
3.1. Germplasm Characterization
3.2. Environmental Factors
3.3. Cultivation and Management Factors
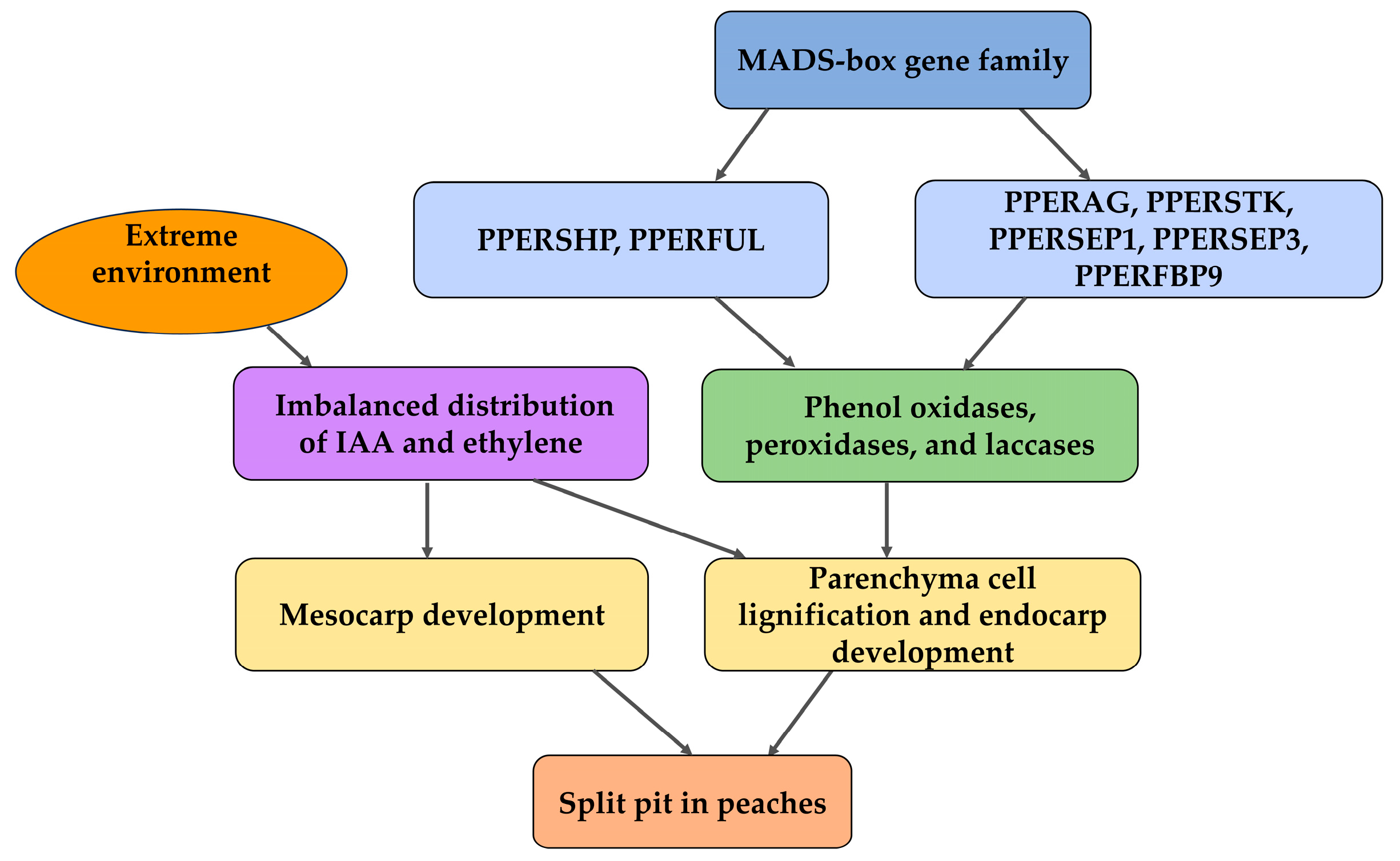
4. Molecular Mechanisms of Split Pit in Peaches
4.1. Endocarp Development and Lignification: Structural and Physiological Foundations in Peaches
4.2. Effect of Phytohormones on Split Pit in Peaches
4.3. Progress on Split-Pit Gene
4.4. Split-Pit Genes of Peaches
5. Prospects of Molecular Breeding Technologies for Split-Pit Resistance
5.1. Transcriptomics and Candidate Gene Mining
5.2. QTL Mapping and Multi-Trait Breeding
5.3. CRISPR Editing and Split-Pit Mechanism Research
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, T.; Wilf, P.; Huang, Y.; Zhang, S.; Zhou, Z. Peaches Preceded Humans: Fossil Evidence from SW China. Sci. Rep. 2015, 5, 16794. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. FAOStat Database; FAO: Rome, Italy, 2023. [Google Scholar]
- Davis, L.D. Split-pit of peaches, estimation of time when splitting occurs. Proc. Am. Soc. Hortic. Sci. 1941, 39, 183–189. [Google Scholar]
- Miki, T. Studies on the development of peach fruits with special reference to the cause of their split-pit. Bull. Chiba Coll. Agric. Hortic. 1932, 1, 1–118. [Google Scholar]
- Niu, L.; Cui, G.; Zeng, W.; Wang, Z. Causes and Prevention of Peach Fruit Pit Splitting Fruit. In Fruit Growers’ Friend; Zhengzhou Fruit Research Institute: Zhengzhou, China, 2021; pp. 45–46. [Google Scholar]
- Chalmers, D.; Ende, B. A reappraisal of the growth and development of peach fruit. Funct. Plant Biol. 1975, 2, 623–634. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; Kim, W.S.; El-Kereamy, A.; Jayasankar, S.; Svircev, A.M.; Brown, D.C.W. Isolation and characterization of four ethylene signal transduction elements in plums (Prunus salicina L.). J. Exp. Bot. 2007, 58, 3631–3643. [Google Scholar] [CrossRef]
- Nakano, R.; Akimoto, H.; Fukuda, F.; Kawai, T.; Ushijima, K.; Fukamatsu, Y.; Kubo, Y.; Fujii, Y.; Hirano, K.; Morinaga, K.; et al. Nondestructive detection of split pit in peaches using an acoustic vibration method. Hortic. J. 2018, 87, 281–287. [Google Scholar] [CrossRef]
- Trainotti, L.; Zanin, D.; Casadoro, G. A cell wall-oriented genomic approach reveals a new and unexpected complexity of the softening in peaches. J. Exp. Bot. 2003, 54, 1821–1832. [Google Scholar] [CrossRef]
- Drogoudi, P.D.; Tsipouridis, C.G.; Pantelidis, G. Effects of crop load and time of thinning on the incidence of split pits, fruit yield, fruit quality, and leaf mineral contents in ‘Andross’ peach. J. Hortic. Sci. Biotechnol. 2009, 84, 505–509. [Google Scholar] [CrossRef]
- Tani, E.; Polidoros, A.N.; Flemetakis, E.; Stedel, C.; Kalloniati, C.; Demetriou, K.; Katinakis, P.; Tsaftaris, A.S. Characterization and expression analysis of AGAMOUS-like, SEEDSTICK-like, and SEPALLATA-like MADS-box genes in peach (Prunus persica) fruit. Plant Physiol. Biochem. 2009, 47, 690–700. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Liu, Y. Causes and control measures of fruit pit splitting in peach trees under protected cultivation. Hebei For. Sci. Technol. 2007, S1, 197. [Google Scholar] [CrossRef]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef]
- Kawai, T.; Ichioka, T.; Ikeda, A.; Ohashi, T.; Inohara, G.; Hirano, K.; Nakano, R.; Fukuda, F. Effect of split pit on maturation of ‘Shimizu Hakuto’ peach on trees. Hortic. J. 2021, 90, 365–373. [Google Scholar] [CrossRef]
- Han, Z. Characteristics and control methods of peach fruit pit splitting. In Fruit Growers’ Friend; Zhengzhou Fruit Research Institute: Zhengzhou, China, 2021; pp. 35–37. [Google Scholar]
- Shi, G.L.; Hua, B.G.; Liu, Y.B.; Wang, Y.N.; Cao, A.J. The role of the plant hormones IAA and ethylene on split-pit formation of peach fruit. In Proceedings of the 2009 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 1–5. [Google Scholar]
- Han, Z.; You, Z.; Guan, W.; Ma, H.; Liu, Z. Relationship Between Peach Pit-Splitting and Specific Vascular Bundle Development and Nitrogen Application. Int. J. Fruit Sci. 2015, 15, 302–312. [Google Scholar] [CrossRef]
- Ge, S.; Wang, Y.; Yang, A. Effects of pit splitting on quality of peach fruit and preventive measures. North Hortic. 2007, 8, 20–21. [Google Scholar]
- Fukuda, F.; Yokoyama, N.; Yoshimura, R.; Kubota, N. Characteristic fruit development in ‘Shimizuhakuto’ peach in relation to physiological fruit drop. J. Jpn. Soc. Hortic. Sci. 2001, 70, 473–480. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E. Impact of genetic and climatic parameters on split-pit incidence in peach and nectarine. Sci. Hortic. 2022, 297, 110970. [Google Scholar] [CrossRef]
- Evert, D.R.; Gaines, T.P.; Mullinix, B.G. Effects of split-pit on elemental concentrations of peach fruit during pit hardening. Sci. Hortic. 1988, 34, 55–65. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Sun, S.; Xie, H.; Tu, M.; Jiang, G. Effects of foliage application of calcium on fruit quality related indexes and split-pit of peach. Southwest China J. Agric. Sci. 2014, 27, 896–898. [Google Scholar]
- Romanov, M.S.; Bobrov, A.V.F.C.; Wijesundara, D.S.A.; Romanova, E.S. Pericarp development and fruit structure in borassoid palms (Arecaceae–Coryphoideae–Borasseae). Ann. Bot. 2011, 108, 1489–1502. [Google Scholar] [CrossRef]
- Doster, M.A.; Michailides, T.J. Relationship between shell discoloration of pistachio nuts and incidence of fungal decay and insect infestation. Plant Dis. 1999, 83, 259–264. [Google Scholar] [CrossRef]
- Colombo, L.; Franken, J.; VanderKrol, A.R.; Wittich, P.E.; Dons, H.J.M.; Angenent, G.C. Downregulation of ovule-specific MADS box genes from petunia results in maternally controlled defects in seed development. Plant Cell 1997, 9, 703–715. [Google Scholar] [CrossRef][Green Version]
- Anderson, N.A.; Tobimatsu, Y.; Ciesielski, P.N.; Ximenes, E.; Ralph, J.; Donohoe, B.S.; Ladisch, M.; Chapple, C. Manipulation of guaiacyl and syringyl monomer biosynthesis in an Arabidopsis Cinnamyl Alcohol dehydrogenase mutant results in atypical lignin biosynthesis and modified cell wall structure. Plant Cell 2015, 27, 2195–2209. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, Y.; Meng, H.; Wang, Y.; Guan, W.; Lu, P. Study on anatomy of peach fruit development and pit splitting. J. Beijing Univ. Agric. 2006, 4, 1–4. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Zhang, J.Y.; Liu, Y.X.; Zhao, Y.H.; Wu, G.L. Study on tissue structure and ultrastructural cytology of peach Fruit. North Hortic. 2018, 1, 29–34. [Google Scholar]
- Mendu, V.; Harman-Ware, A.E.; Crocker, M.; Jae, J.; Stork, J.; Morton, S.; Placido, A.; Huber, G.; DeBolt, S. Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels 2011, 4, 43. [Google Scholar] [CrossRef]
- Rapoport, H.F.; Pérez-López, D.; Hammami, S.B.M.; Agüera, J.; Moriana, A. Fruit pit hardening: Physical measurement during olive fruit growth. Ann. Appl. Biol. 2013, 163, 200–208. [Google Scholar] [CrossRef]
- McGrath, J.M.; Jancso, M.M.; Pichersky, E. Duplicate sequences with a similarity to expressed genes in the genome of Arabidopsis-Thaliana. Theor. Appl. Genet. 1993, 86, 880–888. [Google Scholar] [CrossRef]
- Kritzinger, I.; Lötze, E.; Jooste, M. Stone hardening and broken stones in Japanese plums (Prunus salicina Lindl.) evaluated by means of computed tomography scans. Sci. Hortic. 2017, 221, 1–9. [Google Scholar] [CrossRef]
- Ryugo, K. The rate of dry weight accumulation by the peach pit during the hardening process. Am. Soc. Hortic. Sci. 1961, 78, 132–137. [Google Scholar]
- Abeles, F.B.; Biles, C.L. Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiol. 1991, 95, 269–273. [Google Scholar] [CrossRef]
- Alba, C.M.; de Forchetti, S.M.; Tigier, H.A. Phenoloxidase of peach (Prunus persica) endocarp: Its relationship with peroxidases and lignification. Physiol. Plant. 2000, 109, 382–387. [Google Scholar] [CrossRef]
- Dardick, C.D.; Callahan, A.M.; Chiozzotto, R.; Schaffer, R.J.; Piagnani, M.C.; Scorza, R. Stone formation in peach fruit exhibits spatial coordination of the lignin and flavonoid pathways and similarity to Arabidopsis dehiscence. BMC Biol. 2010, 8, 17. [Google Scholar] [CrossRef]
- King, G.A.; Henderson, K.G.; Lill, R.E. Growth and anatomical and ultrastructural studies of nectarine fruit wall development. Bot. Gaz. 1987, 148, 443–455. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, G.; Li, H.; Wang, Y.; Gao, H.; Jemric, T.; Fu, D. Molecular and genetic events determining the softening of fleshy fruits: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 12482. [Google Scholar] [CrossRef]
- Chauvaux, N.; Child, R.; John, K.; Ulvskov, P.; Borkhardt, B.; Prinsen, E.; Van Onckelen, H.A. The role of auxin in cell separation in the dehiscence zone of oilseed rape pods. J. Exp. Bot. 1997, 48, 1423–1429. [Google Scholar] [CrossRef]
- González-Carranza, Z.H.; Lozoya-Gloria, E.; Roberts, J.A. Recent developments in abscission: Shedding light on the shedding process. Trends Plant Sci. 1998, 3, 10–14. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Z.; Sun, M.; An, X.; Qi, Y.; Zhao, S.; Zhang, Z.; Wang, H. Comparative transcriptome profiling provides insights into endocarp lignification of walnut (Juglans regia L.). Sci. Hortic. 2021, 282, 110030. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Fourquin, C. Role of the FUL–SHP network in the evolution of fruit morphology and function. J. Exp. Bot. 2014, 65, 4505–4513. [Google Scholar] [CrossRef]
- Pabón-Mora, N.; Wong, G.K.S.; Ambrose, B.A. Evolution of fruit development genes in flowering plants. Front. Plant Sci. 2014, 5, 300. [Google Scholar] [CrossRef]
- Irish, V.F.; Litt, A. Flower development and evolution: Gene duplication, diversification and redeployment. Curr. Opin. Genet. Dev. 2005, 15, 454–460. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, H.Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Savidge, B.; Rounsley, S.; Yanofsky, M. Temporal relationship between the transcription of two arabidopsis MADS box genes and the floral organ identity genes. Plant Cell 1995, 7, 721–733. [Google Scholar] [CrossRef]
- Mandel, M.A.; Yanofsky, M.F. The Arabidopsis AGL8 MADS box gene is expressed in inflorescence meristems and is negatively regulated by APETALA1. Plant Cell 1995, 7, 1763–1771. [Google Scholar] [CrossRef]
- Ferrándiz, C.; Liljegren, S.J.; Yanofsky, M.F. Negative regulation of the SHATTERPROOF genes by FRUITFULL during Arabidopsis fruit development. Science 2000, 289, 436–438. [Google Scholar] [CrossRef]
- Tani, E.; Polidoros, A.N.; Tsaftaris, A.S. Characterization and expression analysis of FRUITFULL- and SHATTERPROOF-like genes from peach (Prunus persica) and their role in split-pit formation. Tree Physiol. 2007, 27, 649–659. [Google Scholar] [CrossRef]
- Dinneny, J.R.; Yanofsky, M.F. Drawing lines and borders: How the dehiscent fruit of Arabidopsis is patterned. Bioessays 2005, 27, 42–49. [Google Scholar] [CrossRef]
- Østergaard, L. Don’t ‘leaf’ now. The making of a fruit. Curr. Opin. Plant Biol. 2009, 12, 36–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, T.; Wang, Y.Q.; Yang, R.; Li, W.Q.; Liu, K.W.; Sun, N.R.; Hussian, I.; Ma, X.Y.; Yu, H.R.; et al. Expression characterization of ABCDE class MADS-box genes in Brassica rapa with different pistil types. Plants 2023, 12, 2218. [Google Scholar] [CrossRef]
- Hussain, S. Native RNA-sequencing throws its hat into the transcriptomics ring. Trends Biochem. Sci. 2018, 43, 225–227. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.-J. Advances in genomic, transcriptomic, and metabolomic analyses of fruit quality in fruit crops. Hortic. Plant J. 2020, 6, 361–371. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wen, B.; Fu, X.; Li, D.; Chen, X.; Gao, D.; Li, L.; Xiao, W. Transcriptome analysis of peach (Prunus persica) fruit skin and differential expression of related pigment genes. Sci. Hortic. 2019, 250, 271–277. [Google Scholar] [CrossRef]
- Bie, H.; Wang, H.; Wang, L.; Li, Y.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; Cao, K. Mining genes related to single fruit weight of peach (Prunus persica) based on WGCNA and GSEA. Horticulturae 2023, 9, 1335. [Google Scholar] [CrossRef]
- Hayat, U.; Ke, C.; Wang, L.; Zhu, G.; Fang, W.; Wang, X.; Chen, C.; Li, Y.; Wu, J. Using quantitative trait locus mapping and genomic resources to improve breeding precision in peaches: Current insights and future prospects. Plants 2025, 14, 175. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Fei, J.; Lu, J.; Jiang, Q.; Liu, Z.; Yao, D.; Qu, J.; Liu, S.; Guan, S.; Ma, Y. Maize plant architecture trait QTL mapping and candidate gene identification based on multiple environments and double populations. BMC Plant Biol. 2022, 22, 110. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Dondini, L.; Martínez-Gómez, P.; Ruiz, D. Identification of QTLs linked to fruit quality traits in apricot (Prunus armeniaca L.) and biological validation through gene expression analysis using qPCR. Mol. Breed. 2019, 39, 28. [Google Scholar] [CrossRef]
- Hernández Mora, J.R.; Micheletti, D.; Bink, M.; Van de Weg, E.; Cantín, C.; Nazzicari, N.; Caprera, A.; Dettori, M.T.; Micali, S.; Banchi, E.; et al. Integrated QTL detection for key breeding traits in multiple peach progenies. BMC Genom. 2017, 18, 404. [Google Scholar] [CrossRef]
- Xu, X.; Yuan, Y.; Feng, B.; Deng, W. CRISPR/Cas9-mediated gene-editing technology in fruit quality improvement. Food Qual. Saf. 2020, 4, 159–166. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, S.; Tian, H.; Fu, D.; Zhu, B.; Luo, Y.; Zhu, H. Lycopene Is enriched in tomato fruit by CRISPR/Cas9-mediated multiplex genome editing. Front. Plant Sci. 2018, 9, 559. [Google Scholar] [CrossRef]
- Uluisik, S.; Chapman, N.H.; Smith, R.; Poole, M.; Adams, G.; Gillis, R.B.; Besong, T.M.D.; Sheldon, J.; Stiegelmeyer, S.; Perez, L.; et al. Genetic improvement of tomato by targeted control of fruit softening. Nat. Biotechnol. 2016, 34, 950–952. [Google Scholar] [CrossRef]
- Uluisik, S.; Seymour, G.B. Pectate lyases: Their role in plants and importance in fruit ripening. Food Chem. 2020, 309, 125559. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Huang, H.; Cao, S.; Wang, Q. Analysis of the Causes of Split Pit in Peaches. Int. J. Mol. Sci. 2025, 26, 5460. https://doi.org/10.3390/ijms26125460
Yu Z, Huang H, Cao S, Wang Q. Analysis of the Causes of Split Pit in Peaches. International Journal of Molecular Sciences. 2025; 26(12):5460. https://doi.org/10.3390/ijms26125460
Chicago/Turabian StyleYu, Zhibo, Honghao Huang, Shuangxin Cao, and Qi Wang. 2025. "Analysis of the Causes of Split Pit in Peaches" International Journal of Molecular Sciences 26, no. 12: 5460. https://doi.org/10.3390/ijms26125460
APA StyleYu, Z., Huang, H., Cao, S., & Wang, Q. (2025). Analysis of the Causes of Split Pit in Peaches. International Journal of Molecular Sciences, 26(12), 5460. https://doi.org/10.3390/ijms26125460




