Abstract
In this study, we identified two β-hexosaminidase A isoforms (Mn-HexA1 and Mn-HexA2) in Macrobrachium nipponense through bioinformatics analysis and characterized their phylogenetic relationships. The open reading frames of Mn-HexA1 and Mn-HexA2 were 1641 bp (encoding 546 amino acids) and 1473 bp (encoding 490 amino acids), respectively. Both isoforms exhibited high conservation, sharing five identical functional domains, with 58.43% amino acid sequence similarity. Quantitative PCR analysis revealed that Mn-HexA1 expression was significantly higher than Mn-HexA2 across all developmental stages and tissues. During embryonic development, Mn-HexA1 showed elevated expression at the ZS, L15, and PL10, while Mn-HexA2 was upregulated only at L15 and PL10. In the breeding season and non-breeding season, Mn-HexA1 and Mn-HexA2 were predominantly expressed in the hepatopancreas at levels significantly higher than in other tissues. Moreover, their expression in most tissues was higher during the breeding season than in the non-breeding season. RNA interference experiments revealed that knockdown of both Mn-HexA isoforms significantly accelerated ovarian development in M. nipponense, with the Mn-HexA1-silenced group exhibiting faster progression than the Mn-HexA2-silenced group. These results demonstrate that Mn-HexA genes function as negative regulators of ovarian maturation, with Mn-HexA1 exerting a stronger inhibitory effect than Mn-HexA2.
1. Introduction
Macrobrachium nipponense is a commercially important freshwater crustacean species widely cultivated in Southeast Asia. As the only indigenous freshwater shrimp species that has achieved large-scale aquaculture production in China, M. nipponense plays a vital role in the regional aquaculture industry. According to recent statistics, the annual production of M. nipponense reached 226,392 tons in 2023 [1,2], demonstrating its significant economic value. The reproductive biology of M. nipponense presents unique challenges for aquaculture production. During the breeding season from April to October, female prawns exhibit accelerated gonadal maturation and shortened reproductive cycles. This reproductive strategy allows multiple generations to coexist in the same culture pond, which leads to a decline in the growth rate, resistance, and survival rate of shrimp seedlings, as well as increased risk of hypoxia due to higher stocking densities. These factors collectively contribute to substantial economic losses in M. nipponense aquaculture operations [3,4]. However, the molecular mechanism behind the precocious puberty of M. nipponense is still poorly understood, so it is of great significance to carry out relevant research.
The hepatopancreas has been well documented to play a pivotal role in ovarian maturation processes across various crustacean species [5]. As the primary metabolic organ, it facilitates nutrient digestion and absorption, subsequently transporting these essential dietary components to developing ovaries through hemolymph during oogenesis [6]. Building upon these established findings, our research team conducted a comprehensive transcriptomic comparison of hepatopancreatic tissue from female M. nipponense across ovarian developmental stages O1 to O5. Intriguingly, our analysis revealed two β-hexosaminidase A homologs (Mn-HexA1 and Mn-HexA2) that exhibited significant upregulation during the transition from stage O1 to O2 hepatopancreas [7]. Further bioinformatic investigation through KEGG pathway analysis identified substantial enrichment of the lysosome signaling pathway—a pathway demonstrating strong correlation with ovarian development [8]. Notably, Mn-HexA genes were prominently represented within this pathway. These collective findings strongly suggest that Mn-HexA1 and Mn-HexA2 may serve as functional regulators in the ovarian development of M. nipponense.
Hexosaminidases, members of the glycoside hydrolase (GH) 20 family, are primarily responsible for hydrolyzing terminal GalNAc residues from GM2 gangliosides within lysosomes. Reproductive studies have demonstrated remarkable hexosaminidase activity in spermatozoa across diverse species, including humans [9], canines [10], and insects [11]. It is involved in the combination and penetration with sperm–zona pellucida in vitro, which is very important for egg–sperm recognition [12,13]. Moreover, hexosaminidase has been shown to play an important role in primary binding between gametes in multiple species, including humans [14], hamsters [15], and Phallusia mammillata [16], in addition to modifying the sperm receptor glycoprotein on the egg envelope to block polyspermy. Notably, the regulatory function of hexosaminidase on the development of biological ovaries has not yet been studied, and there is also almost no research on it in crustaceans.
Building upon these findings, we conducted comprehensive bioinformatics analyses to characterize the sequence features and phylogenetic relationships of Mn-HexA1 and Mn-HexA2 in M. nipponense. Using quantitative real-time PCR (qPCR), we systematically investigated the expression profiles of these two isoforms across (1) different tissues during the breeding season and non-breeding season, (2) various embryonic developmental stages, (3) distinct ovarian developmental phases, and (4) the hepatopancreas at different maturation stages. The spatial distribution patterns of Mn-HexA1 and Mn-HexA2 transcripts in ovarian and hepatopancreatic tissues were further examined through in situ hybridization (ISH). To functionally validate their regulatory roles, RNA interference (RNAi) technology was employed to specifically knock down these genes and assess their impacts on ovarian development. This integrative approach provides novel insights into the molecular mechanisms underlying female precocious maturation in M. nipponense, potentially offering solutions to this persistent challenge in aquaculture practice.
2. Results
2.1. Full-Length Sequence Analysis of Mn-HexA1 and Mn-HexA2
Molecular characterization revealed that the HexA1 in M. nipponense contains an open reading frame (ORF) of 1641 bp, encoding a 546-amino-acid protein, which we designated as Mn-HexA1 (GenBank accession no. PV585537). The complete cDNA sequences and deduced amino acid sequences of Mn-HexA1 are presented in Figure S1. Bioinformatic analysis revealed that the Mn-HexA1 protein has a predicted molecular weight (Mw) of 62,136.46 Da and a theoretical isoelectric point (pI) of 5.28. Compositional analysis of the full-length Mn-HexA1 amino acid sequence demonstrated that L-leucine (L) was the most abundant residue (8.2%). Among all residues, the protein contains 51 positively charged amino acids (Arg + Lys) and 68 negatively charged amino acids (Asp + Glu), with a predicted molecular formula of C2813H4254N724O824S23. Secondary structure prediction indicated that Mn-HexA1 comprises 16 α-helices, 3 η-helices, 18 β-sheets, 1 α-turn, and 12 β-turns, as illustrated in Figure S2. Furthermore, a signal peptide (Sec/SPI type) was identified at positions 1–20 bp (amino acid sequence: MLGTKLLLVFVTVAVGPAIA).
Similarly, the HexA2 in M. nipponense was found to possess an ORF of 1473 bp, encoding a 490-amino-acid polypeptide, which we designated as Mn-HexA2 (GenBank accession no. PV585538). The complete cDNA sequences and deduced amino acid sequences of Mn-HexA2 are presented in Figure S1. The Mn-HexA2 protein was predicted to have a molecular weight (Mw) of 56,902.10 Da with a theoretical isoelectric point (pI) of 4.94. Amino acid composition analysis revealed that L-leucine (L) was the most abundant residue (8.8%) in the full-length Mn-HexA2 sequence. The protein contains 46 positively charged residues (Arg + Lys) and 73 negatively charged residues (Asp + Glu), with a predicted molecular formula of C2574H3858N658O765S20. Secondary structure prediction demonstrated that Mn-HexA2 consists of 13 α-helices, 17 β-sheets, and 12 β-turns, as illustrated in Figure S2. Additionally, a Sec/SPI-type signal peptide was identified at positions 1–17 bp, corresponding to the N-terminal amino acid sequence MLVLLVTLSMAVNLSAG.
Sequence analysis revealed that Mn-HexA1 contains two conserved domains: Glycohydro_20b2 (positions 32–156) and GH20_HexA_HexB-like (positions 182–529). Similarly, Mn-HexA2 possesses two conserved domains: Glycohydro_20b2 (positions 29–182) and GH20_hexosaminidase superfamily (positions 203–481), demonstrating high sequence similarity between Mn-HexA1 and Mn-HexA2. Notably, both isoforms share five identical functional domains: (1) β-hexosaminidase A; (2) β-hexosaminidase A, eukaryotic type, N-terminal; (3) β-hexosaminidase A-like, domain 2; (4) glycoside hydrolase family 20, catalytic domain; and (5) glycoside hydrolase superfamily. Subcellular localization predictions indicated that residues 21–546 of Mn-HexA1 and 21–490 of Mn-HexA2 correspond to non-cytoplasmic domains. Furthermore, transmembrane domain analysis confirmed the absence of any transmembrane regions in either Mn-HexA1 or Mn-HexA2.
2.2. Similarity Comparison and Phylogenetic Analysis
Sequence alignment analysis using DNAMAN 9.0 revealed 58.43% similarity between Mn-HexA1 and Mn-HexA2. Comparative multiple sequence alignment with homologous genes from other species (listed in phylogenetic order: Macrobrachium rosenbergii, Palaemon carinicauda, Procambarus clarkii, Panulirus ornatus, Eriocheir sinensis, Halocaridina rubra, Petrolisthes cinctipes, and Penaeus vannamei) demonstrated that Mn-HexA1 exhibited sequence similarities of 91.76%, 81.17%, 73.57%, 71.56%, 70.56%, 70.45%, 69.26%, and 69.26%, respectively. Correspondingly, Mn-HexA2 showed sequence similarities of 83.40%, 71.40%, 63.68%, 63.71%, 62.84%, 64.02%, 61.29%, and 64.30% with these same species (Figure 1).
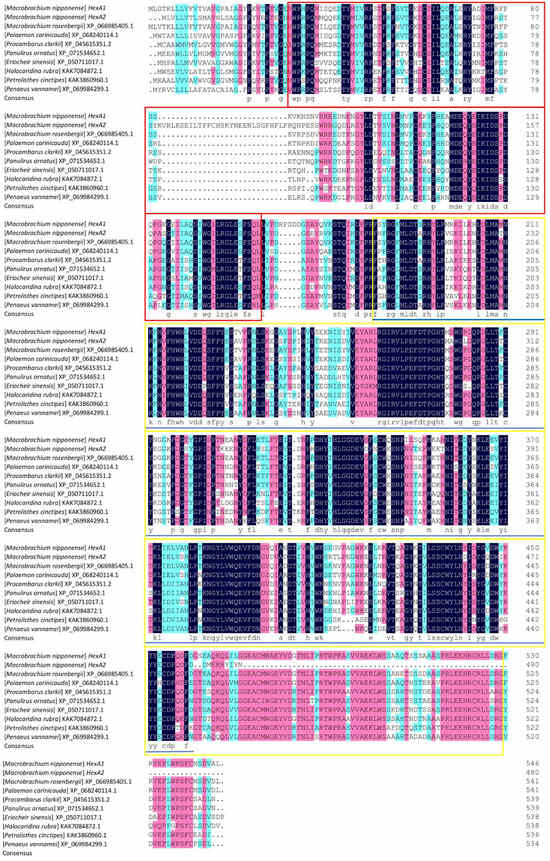
Figure 1.
Multiple-sequence alignment of amino acid sequences across various species. Conserved regions are highlighted in black, while conservative substitutions are indicated in pink. The red box denotes the Glycohydro_20b2 conserved domain in both Mn-HexA1 and Mn-HexA2, the yellow box marks the GH20_HexA_HexB-like conserved domain specific to Mn-HexA1, and the blue solid line delineates the GH20_hexosaminidase superfamily conserved domain unique to Mn-HexA2.
Phylogenetic analysis revealed limited research background for these genes, with homologous sequences from other species in NCBI being predominantly predicted genes. Using MEGA 11.0, we constructed a phylogenetic tree based on the amino acid sequences of Mn-HexA1, Mn-HexA2, and their homologs from other species. The phylogenetic tree demonstrated that Mn-HexA1 and Mn-HexA2 did not cluster with each other initially. Instead, each first grouped with distinct genes from Macrobrachium rosenbergii, then with Palaemon carinicauda from the same family Palaemonidae, before finally clustering together. Subsequently, they collectively branched toward other crustaceans, including Halocaridina rubra, Penaeus vannamei, and Eriocheir sinensis, ultimately converging with Insecta and Arachnida lineages (Figure 2).
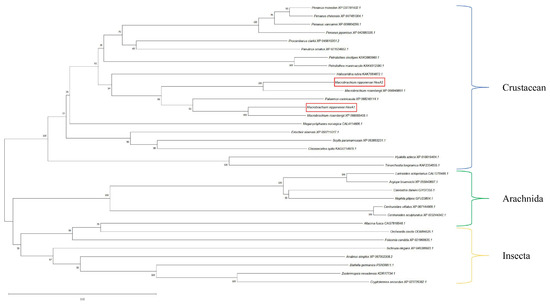
Figure 2.
Phylogenetic tree analysis of Mn-HexA1 and Mn-HexA2. The numbers on the branch represent the bootstrap percentages of the phylogenetic tree. Bootstrap copy to 1000. The terminal numbers correspond to GenBank accession numbers. The target genes is marked with the red boxes.
2.3. Expression Analysis of Mn-HexA1 and Mn-HexA2
2.3.1. Expression Analysis of Mn-HexA1 and Mn-HexA2 at Different Developmental Stages
The embryonic and developmental expression profiles of Mn-HexA1 and Mn-HexA2 are presented in Figure 3. During early embryonic development, Mn-HexA1 exhibited peak expression at the ZS (zoea stage), whereas Mn-HexA2 showed maximal expression during the PS (protozoea stage). In post-embryonic development, both isoforms demonstrated their highest expression levels at L15 (15-day-old larvae after hatching from the embryonic membrane). Throughout the planktonic larval stages, PL10 (the 10th day after metamorphosis) represented the developmental peak for both genes. Comparative analysis revealed that L15 was the overall stage of maximal expression for both Mn-HexA1 and Mn-HexA2. Importantly, Mn-HexA1 expression levels were significantly higher than Mn-HexA2 across all developmental stages (p < 0.01).
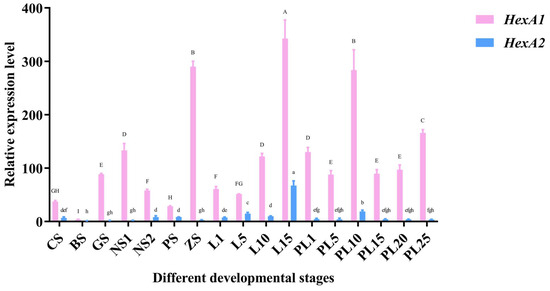
Figure 3.
Quantitative analysis of Mn-HexA1 and Mn-HexA2 expression patterns during various developmental stages by qPCR. Data are presented as mean ± SD (n = 6). Extremely significant differential expression was observed between Mn-HexA1 and Mn-HexA2 across all developmental stages (p < 0.01). Uppercase letters indicate statistically significant differences among Mn-HexA1 expression groups (p < 0.05), while lowercase letters denote significant differences among Mn-HexA2 expression groups (p < 0.05). Developmental stages from CS to PL25 are detailed in Table S1.
2.3.2. Expression Analysis of Mn-HexA1 and Mn-HexA2 in Different Tissue
As shown in Figure 4, both Mn-HexA1 and Mn-HexA2 exhibited predominant expression in the hepatopancreas, with significantly higher levels compared to other examined tissues (p < 0.01). Notably, gene expression in various tissues of both female and male prawns was generally elevated during the breeding season relative to the non-breeding season.
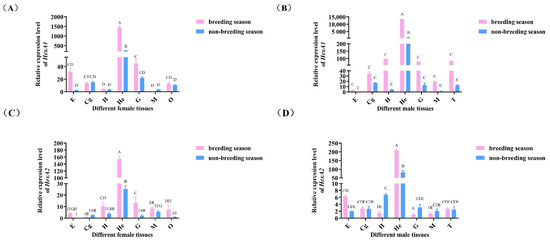
Figure 4.
Tissue expression analysis of (A) Mn-HexA1 in females, (B) Mn-HexA1 in males, (C) Mn-HexA2 in females, and (D) Mn-HexA2 in males during breeding season and non-breeding season quantified by qPCR. E: eyestalk; Cg: cerebral ganglion; H: heart; He: hepatopancreas; G: gill; M: muscle; O: ovary; T: testis. Data are presented as mean ± SD (n = 6). Different superscript letters indicate statistically significant differences (p < 0.05).
2.3.3. Expression Analysis of Mn-HexA1 and Mn-HexA2 in Hepatopancreas and Ovaries at Different Stages
In ovarian tissues (Figure 5A), both Mn-HexA1 and Mn-HexA2 exhibited significant upregulation from stage O1 (undeveloped stage of Ovary) to O2 (developing stage of Ovary) (p < 0.05), reaching peak expression levels at O2. The expression of Mn-HexA1 at O2 was significantly higher than all other developmental stages (p < 0.01), while its O3 (nearly ripe stage of the ovary) expression was markedly lower compared to other periods (p < 0.05). Similarly, Mn-HexA2 demonstrated significantly elevated expression at O2 relative to other ovarian stages (p < 0.05), though no significant differences were observed among remaining stages. Notably, Mn-HexA1 expression consistently surpassed Mn-HexA2 levels across all ovarian developmental periods (p < 0.05).
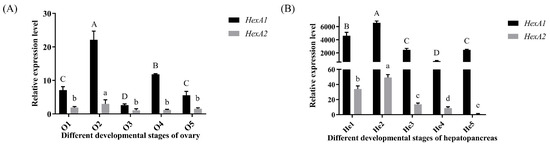
Figure 5.
qPCR analysis of Mn-HexA1 and Mn-HexA2 expression patterns during distinct developmental stages in (A) ovarian and (B) hepatopancreatic tissues. Data are presented as mean ± SD (n = 6). Significant differential expression was observed between Mn-HexA1 and Mn-HexA2 across all developmental stages (p < 0.05). Uppercase letters indicate statistically significant differences among Mn-HexA1 expression groups (p < 0.05), whereas lowercase letters denote significant variations among Mn-HexA2 expression groups (p < 0.05). The developmental stages from O1-O5 and He1-He5 are defined in Table S1.
In the hepatopancreas (Figure 5B), both Mn-HexA1 and Mn-HexA2 displayed a highly significant increase in expression from stage He1 (hepatopancreas in O1 stage) to He2 (hepatopancreas in O2 stage) (p < 0.01), reaching peak levels at He2 stage that were substantially higher than all other developmental stages (p < 0.01). In addition, Mn-HexA1 expression at He4 (hepatopancreas in O4 stage) was significantly lower compared to other periods (p < 0.01), while Mn-HexA2 exhibited its lowest expression at He5 (hepatopancreas in O5 stage) (p < 0.01). Furthermore, Mn-HexA1 expression consistently and significantly exceeded Mn-HexA2 levels across all hepatopancreatic developmental stages (p < 0.01).
2.4. Localization of Mn-HexA1 and Mn-HexA2 in Different Tissues
In situ hybridization (ISH) analysis demonstrated that both Mn-HexA1 and Mn-HexA2 exhibited strong hybridization signals in follicle cells (FCs), nurse cells (N), and the cytoplasmic membrane (CM) throughout all ovarian developmental stages. Notably, the hybridization signals of these two genes were generally weaker during the He2 stage (Figure S3).
2.5. Functional Analysis of Mn-HexA1 and Mn-HexA2
2.5.1. Interference Efficiency
To further investigate the roles of Mn-HexA1 and Mn-HexA2 in ovarian maturation, we performed RNA interference (RNAi) experiments. As demonstrated in Figure 6, the knockdown efficiency for Mn-HexA1 reached 39.96% on day 1 and 96.66% on day 4 post-injection (p < 0.05). Similarly, Mn-HexA2 showed knockdown efficiencies of 35.75% on day 1 and 89.06% on day 4 (p < 0.01), with both genes exhibiting statistically significant silencing effects.
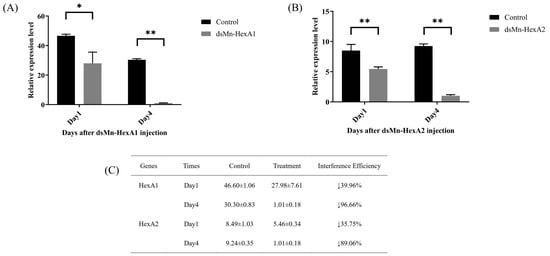
Figure 6.
Expression analysis of (A) Mn-HexA1 and (B) Mn-HexA2 in Macrobrachium nipponense hepatopancreas following dsRNA injection, with (C) corresponding interference test data. Values represent mean ± SD (n = 6). Asterisks indicate statistical significance: * (p < 0.05), ** (p < 0.01). Downward arrows (↓) denote expression suppression.
2.5.2. Effect of Mn-HexA1 and Mn-HexA2 Knockdown on Ovarian Development of M. nipponense
The comparative ovarian development between the experimental and control groups is presented in Figure 7. At trial initiation, all prawns were at O3. By day 5 after injection of dsRNA, less than 20% of shrimps in both experimental groups had ovarian development over O3, while over 80% entered the second reproductive cycle. In stark contrast, 85.31% of the control groups remained over O3 in the first cycle (p < 0.01), though no significant difference existed between the two experimental groups. On day 9, only 7.21% of prawns in control groups progressed over O3 versus approximately 50% in the two experimental groups (p < 0.01). Although Mn-HexA1-silenced groups showed marginally faster progression than Mn-HexA2-silenced group, the inter-group difference remained non-significant. On day 13, the proportion of shrimp ovaries over O3 in all groups gradually increased, with 36.91% in the control groups, compared to 67.49% and 55.49% in the Mn-HexA1-silenced and Mn-HexA2-silenced groups, respectively. There is still a highly significant difference between the control groups and the two experimental groups (p < 0.01). Notably, the Mn-HexA1-silenced group demonstrated significantly accelerated ovarian development relative to the Mn-HexA2-silenced group (p < 0.05).
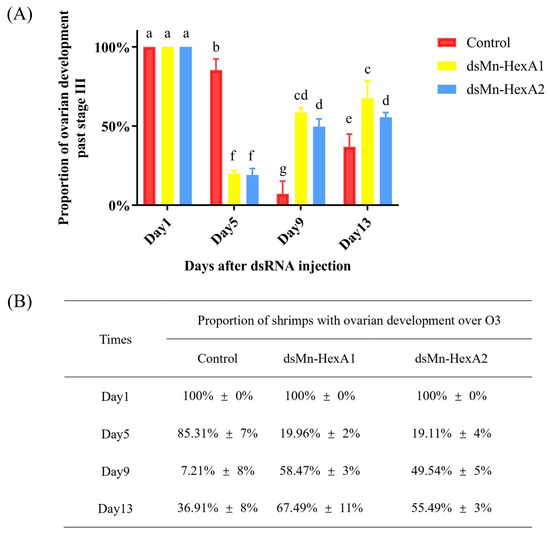
Figure 7.
(A) The percentage of female Macrobrachium nipponense ovaries past stage III after injection of dsRNA, with (B) corresponding interference test data. Different letters indicate significant differences. p < 0.05 was considered to be statistically significant.
3. Discussion
Hexosaminidases, as crucial members of glycosyl hydrolase family 20 (GH20) [17], exist in three primary isoforms: the major HexA and HexB isozymes, and the minor HexS variant. Structural studies reveal that HexA functions as a heterodimer of non-covalently linked α and β subunits, while HexB forms a homodimer of two β subunits, and HexS constitutes a homodimer of two α subunits [18]. Biochemically, their main functions include chitin degradation, protein N-glycan modification, glycoconjugates degradation, and sperm–egg recognition.
In humans, hexosaminidases is responsible for the hydrolysis of the terminal GalNAc residue from the GM2 ganglioside within the lysosome [19]. Specifically, mutations in the HEXA and HEXB genes lead to fatal neurodegenerative diseases: Tay–Sachs disease and Sandhoff disease, respectively [20,21,22]. Recent advances have revealed that a portion of cellular hexosaminidase activity may originate from extracellular vesicles (EVs) [23], while βGalNAc-Rhod-CM(Net2) has been identified as an effective molecular probe for monitoring hexosaminidase activity in live cells [24].
Altered hexosaminidase activity has been documented in various pathological conditions beyond lysosomal storage disorders, including several cancer types [25,26], asthma [27,28], and myeloproliferative disorders [29]. In addition, hexosanase is widely distributed in various organisms, such as plants [30,31], bacteria [32], and fungi [33].
In insects, emerging research has elucidated the functional roles of hexosaminidases, primarily involving two distinct mechanisms: modification of N-glycan structures in cellular glycoproteins [34] and chitin degradation during cuticle remodeling [35]. These enzymes exhibited complementary catalytic activities to chitinase: while chitinase cleaves internal bonds, hexosaminidases release terminal residues. Critical evidence comes from RNA interference studies demonstrating that hexosaminidase knockdown leads to lethal pupal entrapment within the exoskeleton [36]. This essential developmental function has positioned hexosaminidases as promising molecular targets for next-generation bioinsecticides [37].
The current understanding of hexosaminidase in crustaceans remains remarkably limited. To date, the only reported evidence comes from a transcriptomic study of Palaemon gravieri under saline stress conditions, where researchers identified putative HexA gene fragments but without subsequent functional validation [38]. This substantial knowledge gap highlights the need for systematic investigations into hexosaminidase’s physiological roles in crustacean species.
Extensive studies have demonstrated that hexosaminidase exhibits remarkable activity in spermatozoa and plays a crucial role in egg–sperm recognition [15]. Some researchers successfully purified and characterized β-hexosaminidase localized on the sperm membrane of Drosophila melanogaster [13]. Subsequent investigations confirmed its functional conservation with human orthologs, revealing direct involvement in sperm–zona pellucida binding and penetration in vitro [11]. In addition, hexosaminase can also modify the sperm receptor glycoprotein on the egg envelope, blocking polysperms and playing an important role in primary binding between gametes [14,15,16]. However, the potential regulatory effects of hexosaminidase on ovarian development remain unexplored, representing a significant knowledge gap in reproductive biology.
The current study identified Mn-HexA1 and Mn-HexA2 through comparative transcriptomic analysis of hepatopancreatic tissue at ovarian stages O1 and O2 in M. nipponense, with both isoforms showing significant upregulation during the transition from stage 1 to stage 2 [8]. KEGG enrichment reveals that the “lysosome” signaling pathway (map04142) is closely related to ovarian development, and Mn-HexA1 and Mn-HexA2 are located in this pathway. Several key lysosomal pathway components, Cathepsin D [8], Cathepsin L [39], and Niemann–Pick C1 protein [40] have been demonstrated to promote ovarian development, while lysosomal acid lipase plays a role in gonadal differentiation [41]. These findings collectively highlight the diverse functional roles of the lysosomal pathway in crustacean ovarian development.
Current evidence establishes that the lysosomal pathway containing HexA primarily facilitates macromolecule transport and catabolism. Of particular relevance to ovarian development, lysosomes directly participate in steroidogenesis through two key mechanisms: (1) generation of steroid precursors and (2) degradation of regulators governing ovarian steroid production, thereby maintaining cellular steroid homeostasis [42]. Lysosomes play a crucial role in steroidogenesis by releasing cholesterol from endocytosed LDL. This process involves lysosomal enzymes and cooperative action of NPC1 (a lysosomal membrane cholesterol transporter) and NPC2 (a soluble cholesterol-binding protein) [43]. Lysosomes also participate in degrading regulators of ovarian steroidogenesis, including LH-LHR complexes [44], FSH-FSHR complexes [45], and intrinsic PGF2α receptors [46]. Notably, our prior work confirmed that the lysosomal LIPA gene supports gonadal energy provision in M. nipponense through hydrolysis of triglycerides and cholesterol esters [41]. Some known functions of the HexA gene also focus on these aspects [16], so it may also participate in some part of the process, and the specific mechanism needs further study.
Bioinformatic analysis revealed that Mn-HexA1 and Mn-HexA2 share similar conserved domains and identical functional domains, suggesting overall functional similarity. However, sequence alignment showed limited homology at both nucleotide and amino acid levels, with only 58.43% amino acid identity. This substantial divergence indicates potential functional specialization between the two isoforms. Phylogenetic analysis provided further support for this hypothesis; although all identified orthologs from other species remain computationally predicted without experimental validation, the observation that Mn-HexA1 and Mn-HexA2 do not initially cluster together in the evolutionary tree reinforces the likelihood of functional divergence between Mn-HexA1 and Mn-HexA2.
The expression profiling revealed striking differences between the two isoforms, with Mn-HexA1 consistently exhibiting significantly higher expression levels than Mn-HexA2 across all examined tissues and developmental stages. This pronounced disparity suggests that Mn-HexA1 likely exerts more substantial physiological impacts on M. nipponense compared to its counterpart. The ubiquitous high expression of Mn-HexA1 throughout multiple developmental phases implies its potential multifunctional roles, possibly extending beyond ovarian development to include stress responses such as salinity adaptation [38]. In contrast, the stage-restricted expression pattern of Mn-HexA2 indicates a more specialized functional repertoire. The expression of Mn-HexA1 in ZS, L15, and PL10 stages was significantly higher than that in other stages, and the expression level of Mn-HexA2 did not fluctuate significantly in the early stage of embryonic development, while the expression of L15 and PL10 was significantly higher than that in other stages. ZS is the period when M. nipponense is about to emerge from the membrane, L15 is the critical period for metamorphosis and development of M. nipponense, and PL10 is the critical period for gonadal differentiation of M. nipponense [47]. These synchronized expression peaks during physiologically demanding developmental transitions strongly imply that Mn-HexA1 and Mn-HexA2 played a crucial role during the critical period. Based on the fundamental hydrolytic function of hexosaminidases, we speculate that Mn-HexA1 and Mn-HexA2 may be involved in energy regulation processes during these key developmental events.
Comparative analysis revealed significantly elevated expression of Mn-HexA1 and Mn-HexA2 across multiple tissues during the reproductive season compared to non-reproductive periods, suggesting enhanced metabolic activity potentially linked to gonadal development. Particularly noteworthy was the predominant expression in hepatopancreas, where transcript levels of Mn-HexA1 and Mn-HexA2 substantially exceeded those in other tissues (p < 0.01), indicating their crucial hepatic functions. Temporal expression profiling of ovarian and hepatopancreatic tissues demonstrated consistent upregulation of both Mn-HexA1 and Mn-HexA2 from stage I (O1, He1) to stage II (O2, He2), peaking at stage II. This developmental window (O1–O2) corresponds to the critical vitellogenic phase in M. nipponense, during which the hepatopancreas supplies essential glycoproteins and energy metabolites for ovarian development [48]. This result once again proves that Mn HexA may be involved in the energy regulation process of ovarian development.
The RNA interference experiment, conducted for the first time in M. nipponense under natural pond conditions, yielded significant findings. All experimental specimens were synchronized at ovarian stage III prior to dsRNA administration. By day 5 post-interference, both Mn-HexA1-silenced and Mn-HexA2-silenced groups had transitioned to the second reproductive cycle, whereas control specimens took until day 9 to achieve comparable progression (Figure 7). Notably, the Mn-HexA1 knockdown group exhibited progressively accelerated ovarian development compared to the Mn-HexA2 knockdown group, with statistically significant differences emerging by day 13 (p < 0.05). It is concluded that both subtypes of Mn-HexA genes inhibit ovarian development, and the effect of Mn-HexA1 is stronger than that of Mn-HexA2. This study represents the first functional characterization of HexA genes in crustacean ovarian development, providing novel insights into the molecular regulation of reproduction in M. nipponense. In the follow-up experiments, it is a good choice to enhance the effect of RNAi by feeding with feed, so as to explore more functions of the genes [49,50].
Current understanding of hexosaminidase’s reproductive functions remains remarkably limited, with existing studies primarily focused on protein purification [18]. As the first comprehensive study combining expression profiling and RNAi-based functional analysis of hexosaminidases in crustacean reproduction, our work provides significant mechanistic insights into this vital biological process.
Three key conclusions emerge with certainty: (1) Both Mn-HexA1 and Mn-HexA2 serve as critical negative regulators of ovarian development in M. nipponense, representing only the second identified class of such inhibitory factors in this species [51]. This deepens the understanding of the mechanistic study of crustacean ovarian development. (2) This work provides the first experimental evidence that hexosaminidases functionally regulate ovarian development. (3) This work is the first to explore the function of hexosaminidase in crustaceans. Intriguingly, emerging research in humans demonstrates that hepatic HexA directly modulates insulin-like growth factor signaling and glucose transport [52], a mechanism strikingly consistent with our hypothesis of Mn-HexA involvement in energy regulation, but it still needs to be strictly verified through targeted experiments.
4. Materials and Methods
4.1. Experimental Animals and Breeding Conditions
Healthy female Macrobrachium nipponense specimens (ovarian stage III; mean body weight ±0.5 g) were obtained from the Freshwater Fisheries Research Center of the Chinese Academy of Fishery Sciences (Wuxi, Jiangsu Province, China). The prawns were cultured in outdoor earthen ponds (70 m × 25 m × 0.7 m) containing cylindrical net cages (0.8 m diameter × 1 m height) under natural conditions. The ponds were supplemented with Hydrilla verticillata vegetation to simulate natural habitats. The water temperature was maintained at 25–30 °C throughout the experimental period. The specimens were fed twice daily (morning and evening) with commercial feed at 5% of total body weight.
4.2. Tissue Sample Collection
Various tissues including the eyestalk, cerebral ganglion, heart, hepatopancreas, gill, muscle, and gonads were dissected from M. nipponense specimens and immediately flash-frozen in liquid nitrogen. All collected tissues were subsequently stored at −80 °C until further analysis. Additionally, whole-body samples from different embryonic developmental stages and larval phases were systematically collected and preserved under identical conditions (−80 °C). The specific classification criteria for different developmental stages of ovaries, the hepatopancreas, and embryonic developmental periods are summarized in Table S1 [8,53].
4.3. Genes Cloning
The total RNA of M. nipponense at different developmental stages and different tissues was extracted using RNAiso Easy reagents (TakaRa, Dalian, China) according to the manufacturer’s instructions, and quality was assessed by 1.2% agarose gel electrophoresis. According to the manufacturer’s instructions, we converted single-stranded RNA to single-stranded cDNA using the M-MLV reverse transcriptase kit (TaKaRa). Then, the synthesized cDNA was kept at −20 °C for subsequent quantitative real-time PCR (qPCR) reaction to detect the expression pattern of the Mn-HexA1 and Mn-HexA2 in M. nipponense. EIF was used as the internal reference gene; this has been proved before. The content of Mn-CH7D mRNA was calculated by the 2−ΔΔCT method.
Total RNA was isolated from various tissues and developmental stages of M. nipponense using RNAiso Easy reagent (TakaRa) following the manufacturer’s protocol. RNA integrity was verified by 1.2% agarose gel electrophoresis. First-strand cDNA was synthesized from 1 μg of total RNA using the M-MLV Reverse Transcriptase Kit (Takara) according to the manufacturer’s instructions. The resulting cDNA products were stored at −20 °C for subsequent quantitative real-time PCR (qPCR) analysis.
The expression profiles of Mn-HexA1 and Mn-HexA2 in M. nipponense were analyzed by qPCR using EIF as the internal reference gene, as previously validated [54]. Relative mRNA levels were quantified using the 2−ΔΔCT method [55].
4.4. Bioinformatics Analysis
The cDNA fragments of the target genes Mn-HexA1 and Mn-HexA2 were obtained from our laboratory’s established cDNA library, which was previously constructed from hepatopancreatic transcriptomes of M. nipponense at different ovarian developmental stages [7]. Amino acid sequence alignment was analyzed by DNAMAN 9.0 software. The phylogenetic tree was constructed based on Mn-HexA1, Mn-HexA2, and their orthologs in other species by the neighbor-joining (NJ) method using MEGA 11.0 software. Potential open reading frames were identified using the ORF Finder tool (https://www.ncbi.nlm.nih.gov/orffinder/, accessed on 1 July 2024). The functional domains within the protein sequences were identified using the Conserved Domain Database (CDD) search tool available through NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 2 July 2024). Transmembrane helices in the amino acid sequences were predicted using DeepTMHMM, a deep-learning-based method for transmembrane topology prediction (https://dtu.biolib.com/DeepTMHMM, accessed on 2 July 2024). Protein functional domains were predicted using InterPro (https://www.ebi.ac.uk/interpro/, accessed on 2 July 2024). Signal peptide prediction was performed using SignalP-6.0 (https://services.healthtech.dtu.dk/services/SignalP-6.0/, accessed on 2 July 2024). Protein physicochemical characteristics including molecular weight (MW), isoelectric point (pI), and amino acid composition were calculated using ProtParam tool available on ExPASy server (https://web.expasy.org/protparam/, accessed on 2 July 2024). The three-dimensional protein structures were predicted using the SWISS-MODEL homology modeling server (https://swissmodel.expasy.org/, accessed on 3 July 2024) and subsequently analyzed with ESPript (https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi , accessed on 3 July 2024). Gene-specific primers were designed using the NCBI Primer-BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/, accessed on 3 July 2024). The primers used in this study are listed in Table S2.
4.5. In Situ Hybridization
Ovarian samples (stages 1–5) and stage 2 hepatopancreatic tissues fixed in 4% paraformaldehyde solution were used for ISH studies. DIG-labeled antisense and sense RNA probes were designed based on Mn-HexA1 and Mn-HexA2 cDNA sequences using Primer5 software. The experimental procedures followed previously established protocols [56]. HE groups represent the blank control of routine hematoxylin–eosin staining, negative indicates the control group hybridized with sense probe, and positive indicates the experimental group hybridized with antisense probe. The probe sequences are shown in Table S2.
4.6. RNAi Experiment
The primers for double-stranded RNA (dsRNA) synthesis were designed using SnapDragon online software (https://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl , accessed on 5 July 2024). The dsRNAs targeting Mn-HexA1 and Mn-HexA2 were synthesized using the TranscriptAid™ T7 High Yield Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA), with specific primers listed in Table S2. The concentration of synthesized dsRNA was measured at 260 nm using a BioPhotometer (Eppendorf, Hamburg, Germany), with purity verified by A260/A280 ratio (1.8–2.0).
To evaluate the interference efficiency, a short-term RNA interference test was conducted separately, and samples of hepatopancreas were collected on the 1st and 4th day after injection of dsRNA. After total RNA was extracted and reverse-transcribed into cDNA, the expression level of the target gene was detected by qPCR to determine the interference efficiency. Total RNA was extracted from collected samples and reverse-transcribed into cDNA. The knockdown efficiency was quantitatively assessed by measuring target gene expression levels using qPCR.
A long-term RNA interference experiment was conducted to randomly allocate 270 healthy female M. nipponense (1.46 ± 0.19 g) from phase 3 to 9 aquaculture ponds in equal proportions, namely the HexA1 experimental group, HexA2 experimental group, and control group, with 3 parallel groups in each group (n = 30). The experimental groups was injected with dsRNA at a dose of 4 μg/g (calculated per gram of body weight) through the pericardial cavity of M. nipponense, while the control groups were injected with the same unit dose of dsGFP. The injection frequency was once every four days. The ovarian development stages of each shrimp were observed and recorded every day, and the proportion of shrimps whose ovarian development exceeds the third stage was determined.
4.7. Data Analysis
All quantitative data are presented as mean ± standard deviation (mean ± SD). Statistical analyses were performed using SPSS Statistics 24.0 (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison test was employed to determine significant differences between control and treatment groups. Differences were considered statistically significant at p < 0.05 and highly significant at p < 0.01. Relative gene expression levels were calculated using the comparative 2−ΔΔCT method.
5. Conclusions
This study is the first discovery of gonadal development function of β-hexosaminidase A in crustaceans. The RNAi test of Macrobrachium nipponense was carried out in a mud pond for the first time. The synchronous interference test of two subtypes of the same gene in M. nipponense was carried out for the first time. It is also the second ovarian development inhibitory gene found in M. nipponense. This study provides the first comprehensive functional characterization of Mn-HexA1 and Mn-HexA2 in crustacean reproduction through integrated bioinformatic, molecular, and physiological approaches, confirming the inhibitory effect of Mn HexA on ovarian development. It also provides some new insights and ideas to solve the production problem of rapid sexual maturation and advance our understanding of crustacean reproductive endocrinology while identifying potential molecular targets for aquaculture management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26125459/s1.
Author Contributions
Investigation, methodology, and writing—original draft, Z.W.; conceptualization, H.Q., S.J. (Shubo Jin), and W.Z.; software, Z.G. and M.X. (Mingjia Xu); formal analysis and data curation, H.Q. and W.Z.; resources and investigation, S.J. (Sufei Jiang), Y.X. and M.X. (Ming Xu); writing—review and editing, H.Q.; visualization and supervision, H.Q.; funding acquisition, H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from Central Public-interest Scientific Institution Basal Research Fund CAFS (2025JBFZ03, 2023TD39), the earmarked fund for CARS-48-07, and the seed industry revitalization project of Jiangsu province (JBGS [2021]118). We would like to thank the Jiangsu Province Platform for the Conservation and Utilization of Agricultural Germplasm.
Institutional Review Board Statement
All experimental procedures were conducted with approval from the Institutional Animal Care and Use Ethics Committee of the Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences (Wuxi, China) (authorization no. 20240520008, 13 May 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author for scientific purposes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiang, G.; Xue, Y.; Huang, X. Temperature-Induced Sex Differentiation in River Prawn (Macrobrachium nipponense): Mechanisms and Effects. Int. J. Mol. Sci. 2024, 25, 1207. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Li, Y.; Zhang, R.; Liu, H.; Ma, X.; Wu, L.; Qiao, Z.; Li, X. Identification of Ribosomal Protein L24 (RPL24) from the Oriental River Prawn, Macrobrachium nipponense, and Its Roles in Ovarian Development. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 266, 111154. [Google Scholar] [CrossRef]
- Qiao, H.; Fu, H.; Xiong, Y.; Jiang, S.; Zhang, W.; Sun, S.; Jin, S.; Gong, Y.; Wang, Y.; Shan, D.; et al. Molecular Insights into Reproduction Regulation of Female Oriental River Prawns Macrobrachium nipponense through Comparative Transcriptomic Analysis. Sci. Rep. 2017, 7, 12161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, X.; Li, Y.; Liu, X.; Zhang, S.; Li, H.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z. Molecular and Functional Characterization of Ribosome Protein S24 in Ovarian Development of Macrobrachium nipponense. Int. J. Biol. Macromol. 2024, 254, 127934. [Google Scholar] [CrossRef] [PubMed]
- Fatima, H.; Ayub, Z.; Ali, S.; Siddiqui, G. Biochemical Composition of the Hemolymph, Hepatopancreas, Ovary, and Muscle during Ovarian Maturation in the Penaeid Shrimps Fenneropenaeus Merguiensis and F. penicillatus (Crustacea: Decapoda). Turk. J. Zool. 2013, 37, 334–347. [Google Scholar] [CrossRef]
- Feng, W.; Zhao, Z.; Wang, J.; Han, T. Nutrient Composition of Ovary, Hepatopancreas and Muscle Tissues in Relation to Ovarian Development Stage of Female Swimming Crab, Portunus Trituberculatus. Animals 2023, 13, 3220. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Xiong, Y.; Cheng, D.; Wang, J.; Jin, S.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Hepatopancreas Transcriptome Analyses Provide New Insights into the Molecular Regulatory Mechanism of Fast Ovary Maturation in Macrobrachium nipponense. BMC Genom. 2022, 23, 625. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, W.; Jiang, S.; Xiong, Y.; Jin, S.; Pan, F.; Zhu, J.; Gong, Y.; Wu, Y.; Qiao, H.; et al. Cathepsin D Plays a Vital Role in Macrobrachium nipponense of Ovary Maturation: Identification, Characterization, and Function Analysis. Genes 2022, 13, 1495. [Google Scholar] [CrossRef]
- Menendez-Helman, R.J.; Sanjurjo, C.; Miranda, P.V. Seminal Plasma Hexosaminidase in Patients with Normal and Abnormal Spermograms. Iran. J. Reprod. Med. 2015, 13, 541–548. [Google Scholar]
- Szczubiał, M.; Kankofer, M.; Wawrzykowski, J.; Dąbrowski, R.; Bochniarz, M.; Brodzki, P. Activity of the Glycosidases β-Galactosidase, α-l-Fucosidase, β-N-Acetyl-Hexosaminidase, and Sialidase in Uterine Tissues from Female Dogs in Diestrus with and without Pyometra. Theriogenology 2022, 177, 133–139. [Google Scholar] [CrossRef]
- Intra, J.; Cenni, F.; Pavesi, G.; Pasini, M.; Perotti, M.-E. Interspecific Analysis of the Glycosidases of the Sperm Plasma Membrane in Drosophila. Mol. Reprod. Dev. 2009, 76, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Pasini, M.E.; Intra, J.; Matsumoto, M.; Briani, F.; Hoshi, M.; Perotti, M.E. Identification and Expression Analysis of Drosophilamelanogaster Genes Encoding β-Hexosaminidases of the Sperm Plasma Membrane. Glycobiology 2006, 16, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Ogiso, M.; Hoshi, M.; Perotti, M.-E.; Pasini, M.E. Purification and Characterization of the Plasma Membrane Glycosidases of Drosophila melanogaster Spermatozoa. Insect Biochem. Mol. Biol. 2002, 32, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Perez Martinez, S.L.; Menendez Helman, R.J.; Zitta, K.S.; Brandelli, A.; Miranda, P.V. Characterization of Human Sperm N-Acetylglucosaminidase. Int. J. Androl. 2008, 31, 315–324. [Google Scholar] [CrossRef]
- Zitta, K.; Wertheimer, E.V.; Miranda, P.V. Sperm N-Acetylglucosaminidase Is Involved in Primary Binding to the Zona Pellucida. Mol. Hum. Reprod. 2006, 12, 557–563. [Google Scholar] [CrossRef]
- Koyanagi, R.; Honegger, T.G. Molecular Cloning and Sequence Analysis of an Ascidian Egg β-N-Acetylhexosaminidase with a Potential Role in Fertilization. Dev. Growth Differ. 2003, 45, 209–218. [Google Scholar] [CrossRef]
- Intra, J.; Pavesi, G.; Horner, D.S. Phylogenetic Analyses Suggest Multiple Changes of Substrate Specificity within the Glycosyl Hydrolase 20 Family. BMC Evol. Biol. 2008, 8, 214. [Google Scholar] [CrossRef]
- Venugopal, A.; Mondal, S.; Ranganatha, K.S.; Datta, D.; Kumar, N.S.; Swamy, M.J. Purification and Biochemical/Biophysical Characterization of Two Hexosaminidases from the Fresh Water Mussel, Lamellidens Corrianus. Int. J. Biol. Macromol. 2020, 149, 754–766. [Google Scholar] [CrossRef]
- Wendeler, M.; Sandhoff, K. Hexosaminidase Assays. Glycoconj. J. 2009, 26, 945–952. [Google Scholar] [CrossRef]
- Mark, B.L.; Mahuran, D.J.; Cherney, M.M.; Zhao, D.; Knapp, S.; James, M.N.G. Crystal Structure of Human β-Hexosaminidase B: Understanding the Molecular Basis of Sandhoff and Tay–Sachs Disease. J. Mol. Biol. 2003, 327, 1093–1109. [Google Scholar] [CrossRef]
- Mahuran, D.J. Biochemical Consequences of Mutations Causing the GM2 Gangliosidoses. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 1999, 1455, 105–138. [Google Scholar] [CrossRef]
- Lemieux, M.J.; Mark, B.L.; Cherney, M.M.; Withers, S.G.; Mahuran, D.J.; James, M.N.G. Crystallographic Structure of Human β-Hexosaminidase A: Interpretation of Tay-Sachs Mutations and Loss of GM2 Ganglioside Hydrolysis. J. Mol. Biol. 2006, 359, 913–929. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cerrotti, G.; Sagini, K.; Delo, F.; Buratta, S.; Pellegrino, R.M.; Alabed, H.B.R.; Fratini, F.; Emiliani, C.; Urbanelli, L. Evidence of Lysosomal β-Hexosaminidase Enzymatic Activity Associated with Extracellular Vesicles: Potential Applications for the Correction of Sandhoff Disease. J. Funct. Biomater. 2024, 15, 153. [Google Scholar] [CrossRef]
- Lee, J.; Boo, J.; Kim, Y.-H.; Roh, J.; Ko, S.-K.; Shin, I. A Fluorescent Probe for Selective Detection of Lysosomal β-Hexosaminidase in Live Cells. Talanta 2024, 271, 125715. [Google Scholar] [CrossRef]
- Olszewska, E.; Borzym-Kluczyk, M.; Rzewnicki, I.; Rutkowska, J.; Knas, M.; Rogowski, M.; Waniewska, E.; Wielgosz, R. Hexosaminidase as a New Potential Marker for Larynx Cancer. Clin. Biochem. 2009, 42, 1187–1189. [Google Scholar] [CrossRef]
- Plucinsky, M.C.; Prorok, J.J.; Alhadeff, J.A. β-Hexosaminidase From Colon and Sera of Dukes-Classified Colorectal Cancer Patients: Activity Levels, Isozyme Patterns, and Kinetic Properties2. JNCI J. Natl. Cancer Inst. 1986, 77, 57–62. [Google Scholar] [CrossRef]
- Samarasinghe, A.E.; Penkert, R.R.; Hurwitz, J.L.; Sealy, R.E.; LeMessurier, K.S.; Hammond, C.; Dubin, P.J.; Lew, D.B. Questioning Cause and Effect: Children with Severe Asthma Exhibit High Levels of Inflammatory Biomarkers Including Beta-Hexosaminidase, but Low Levels of Vitamin A and Immunoglobulins. Biomedicines 2020, 8, 393. [Google Scholar] [CrossRef]
- Fukuishi, N.; Murakami, S.; Ohno, A.; Yamanaka, N.; Matsui, N.; Fukutsuji, K.; Yamada, S.; Itoh, K.; Akagi, M. Does β-Hexosaminidase Function Only as a Degranulation Indicator in Mast Cells? The Primary Role of β-Hexosaminidase in Mast Cell Granules. J. Immunol. 2014, 193, 1886–1894. [Google Scholar] [CrossRef]
- Emiliani, C.; Ciferri, S.; Mencarelli, S.; Mezzasoma, A.M.; Momi, S.; Orlacchio, A.; Gresele, P. Defective Platelet β-N-Acetyl Hexosaminidase Content and Release in Chronic Myeloproliferative Disorders. Platelets 2006, 17, 20–29. [Google Scholar] [CrossRef]
- Strasser, R.; Bondili, J.S.; Schoberer, J.; Svoboda, B.; Liebminger, E.; Glössl, J.; Altmann, F.; Steinkellner, H.; Mach, L. Enzymatic Properties and Subcellular Localization of Arabidopsis β-N-Acetylhexosaminidases. Plant Physiol. 2007, 145, 5–16. [Google Scholar] [CrossRef]
- Oikawa, A.; Itoh, E.; Ishihara, A.; Iwamura, H. Purification and Characterization of β-N-Acetylhexosaminidase from Maize Seedlings. J. Plant Physiol. 2003, 160, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Tsujibo, H.; Miyamoto, K.; Yoshimura, M.; Takata, M.; Miyamoto, J.; Inamori, Y. Molecular Cloning of the Gene Encoding a Novel β-N-Acetylhexosaminidase from a Marine Bacterium, Alteromonas sp. Strain O-7, and Characterization of the Cloned Enzyme. Biosci. Biotechnol. Biochem. 2002, 66, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Horsch, M.; Mayer, C.; Sennhauser, U.; Rast, D.M. β-N-Acetylhexosaminidase: A Target for the Design of Antifungal Agents. Pharmacol. Ther. 1997, 76, 187–218. [Google Scholar] [CrossRef] [PubMed]
- Tomiya, N.; Narang, S.; Park, J.; Abdul-Rahman, B.; Choi, O.; Singh, S.; Hiratake, J.; Sakata, K.; Betenbaugh, M.J.; Palter, K.B.; et al. Purification, Characterization, and Cloning of a Spodoptera Frugiperda Sf9 β-N-Acetylhexosaminidase That Hydrolyzes Terminal N-Acetylglucosamine on the N-Glycan Core*. J. Biol. Chem. 2006, 281, 19545–19560. [Google Scholar] [CrossRef]
- Hogenkamp, D.G.; Arakane, Y.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Characterization and Expression of the β-N-Acetylhexosaminidase Gene Family of Tribolium Castaneum. Insect Biochem. Mol. Biol. 2008, 38, 478–489. [Google Scholar] [CrossRef]
- Guo, P.-P.; Yang, X.-B.; Yang, H.; Zhou, C.; Long, G.-Y.; Jin, D.-C. Knockdown of the β-N-Acetylhexosaminidase Genes by RNA Interference Inhibited the Molting and Increased the Mortality of the White-Backed Planthopper, Sogatella furcifera. Pestic. Biochem. Physiol. 2025, 207, 106216. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Q. Recent Advances in Glycoside Hydrolase Family 20 and 84 Inhibitors: Structures, Inhibitory Mechanisms and Biological Activities. Bioorganic Chem. 2024, 142, 106870. [Google Scholar] [CrossRef]
- Shi, W.; Hu, R.; Zhao, R.; Zhu, J.; Shen, H.; Li, H.; Wang, L.; Yang, Z.; Jiang, Q.; Qiao, Y.; et al. Transcriptome Analysis of Hepatopancreas and Gills of Palaemon gravieri under Salinity Stress. Gene 2023, 851, 147013. [Google Scholar] [CrossRef]
- Jiang, S.; Xiong, Y.; Zhang, W.; Zhu, J.; Cheng, D.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Molecular Characterization of a Novel Cathepsin L in Macrobrachium nipponense and Its Function in Ovary Maturation. Front. Endocrinol. 2022, 12, 816813. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Xiong, Y.; Zhang, M.; Yuan, H.; Niu, Y.; Qiao, H.; Fu, H. NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium nipponense. Int. J. Mol. Sci. 2024, 25, 6049. [Google Scholar] [CrossRef]
- Cai, P.; Zhang, W.; Jiang, S.; Xiong, Y.; Qiao, H.; Yuan, H.; Gao, Z.; Zhou, Y.; Jin, S.; Fu, H. Role of Mn-LIPA in Sex Hormone Regulation and Gonadal Development in the Oriental River Prawn, Macrobrachium nipponense. Int. J. Mol. Sci. 2024, 25, 1399. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Andersen, C.L.; Ye, X. Functions of Lysosomes in Mammalian Female Reproductive System. Reprod. Dev. Med. 2020, 4, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Esmaeilian, Y.; Hela, F.; Bildik, G.; İltumur, E.; Yusufoglu, S.; Yakin, K.; Oktem, O. Discovery of Autophagy as a Universal Mechanism for Sex Steroid Synthesis in Human Ovary and Testis. Autophagy Rep. 2023, 2, 2251804. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D. Response of the Corpus Luteum to Luteinizing Hormone. Environ. Health Perspect. 1981, 38, 47–50. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Kishi, H.; Shi, M.; Galet, C.; Bhaskaran, R.S.; Hirakawa, T.; Ascoli, M. Postendocytotic Trafficking of the Follicle-Stimulating Hormone (FSH)-FSH Receptor Complex. Mol. Endocrinol. 2003, 17, 2162–2176. [Google Scholar] [CrossRef]
- Mitra, S.; Rao, C. Receptors for Gonadotropins and Prostaglandins in Lysosomes of Bovine Corpora Lutea. Arch. Biochem. Biophys. 1978, 185, 126–133. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, W.; Xiong, Y.; Jiang, S.; Qiao, H.; Gong, Y.; Wu, Y.; Fu, H. Identification of Important Genes Involved in the Sex-Differentiation Mechanism of Oriental River Prawn, Macrobrachium nipponense, During the Gonad Differentiation and Development Period. Front. Genet. 2022, 13, 797796. [Google Scholar] [CrossRef]
- Jiang, S.; Qiao, H.; Fu, H.; Gu, Z. Hepatopancreas Proteomic Analysis Reveals Key Proteins and Pathways in Regulatory of Ovary Maturation of Macrobrachium nipponense. Animals 2023, 13, 977. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, M.; Xiong, J.; Zhang, M.; Wu, X. Sequence Characteristics, Evolutionary History and Expression Pattern of BCO2 in Chinese Mitten Crab Eriocheir Sinensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 56, 101524. [Google Scholar] [CrossRef]
- Zhang, Y.; Kan, D.; Zhou, Y.; Lian, H.; Ge, L.; Shen, J.; Dai, Z.; Shi, Y.; Han, C.; Liu, X.; et al. Efficient RNA Interference Method by Feeding in Brachionus Plicatilis (Rotifera). Biotechnol. Lett. 2024, 46, 961–971. [Google Scholar] [CrossRef]
- Qiao, H.; Xiong, Y.; Zhang, W.; Fu, H.; Jiang, S.; Sun, S.; Bai, H.; Jin, S.; Gong, Y. Characterization, Expression, and Function Analysis of Gonad-Inhibiting Hormone in Oriental River Prawn, Macrobrachium nipponense and Its Induced Expression by Temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.K.; Bayliss, J.; Nie, S.; de Nardo, W.; Keenan, S.N.; Anari, M.; Taddese, A.Z.; Williamson, N.A.; Ooi, G.J.; Brown, W.A.; et al. Liver-Secreted Hexosaminidase A Regulates Insulin-Like Growth Factor Signaling and Glucose Transport in Skeletal Muscle. Diabetes 2022, 72, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, L.; Wu, P.; Zhao, W.; Li, E.; Qin, J. cDNA Cloning and Expression of Ubc9 in the Developing Embryo and Ovary of Oriental River Prawn, Macrobrachium nipponense. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Fu, Y.; Jin, S.; Fu, H.; Qiao, H.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y.; et al. Comparative Transcriptome Analysis of Lethality in Response to RNA Interference of the Oriental River Prawn (Macrobrachium nipponense). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 38, 100802. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jin, S.; Fu, H.; Jiang, S.; Xiong, Y.; Sun, S.; Qiao, H.; Zhang, W.; Gong, Y.; Wu, Y. Molecular Cloning, Expression, and In Situ Hybridization Analysis of Forkhead Box Protein L2 during Development in Macrobrachium nipponense. J. World Aquac. Soc. 2018, 49, 429–440. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).