In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions of Essential Oils
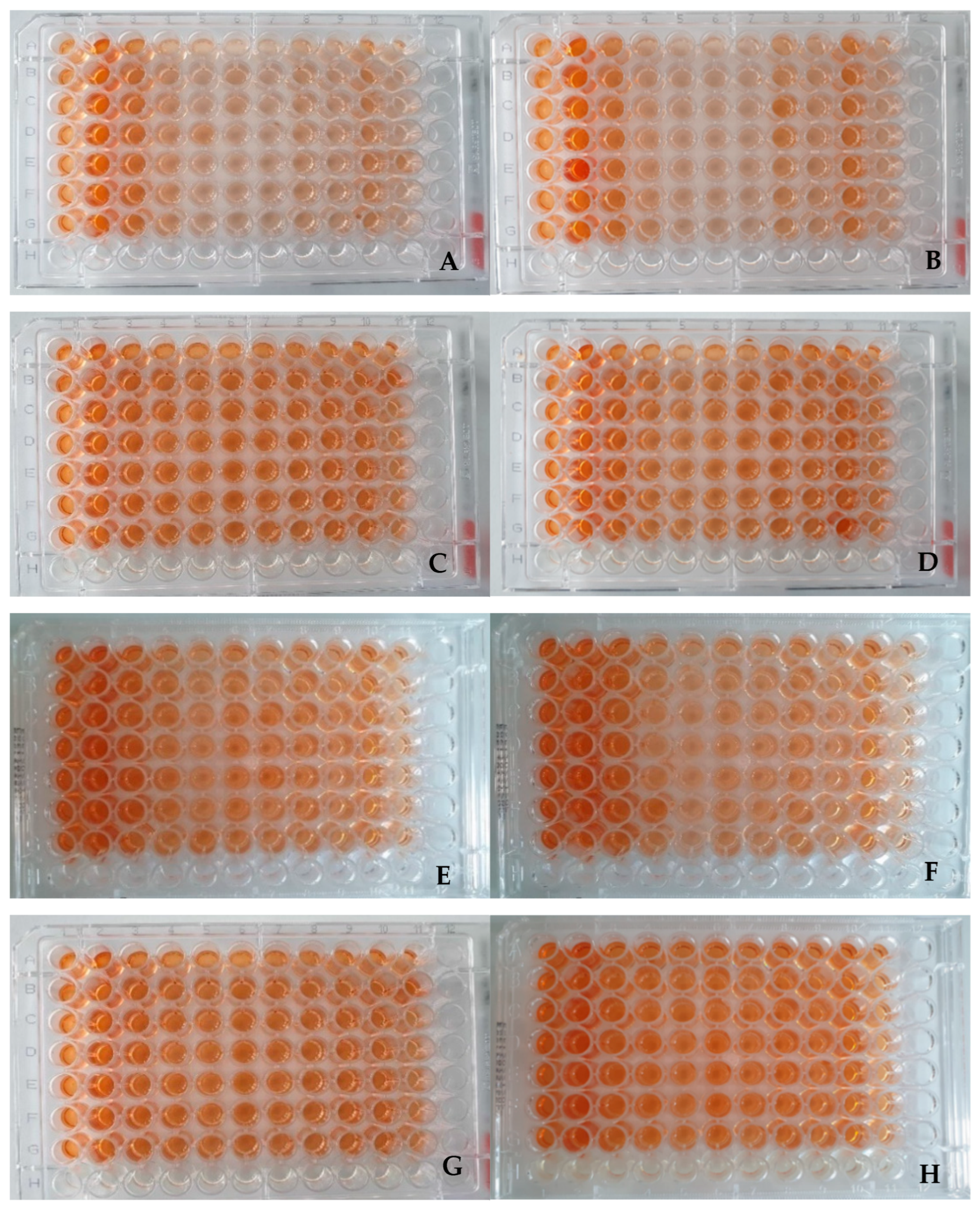
2.2. Visual Assessment of Plates
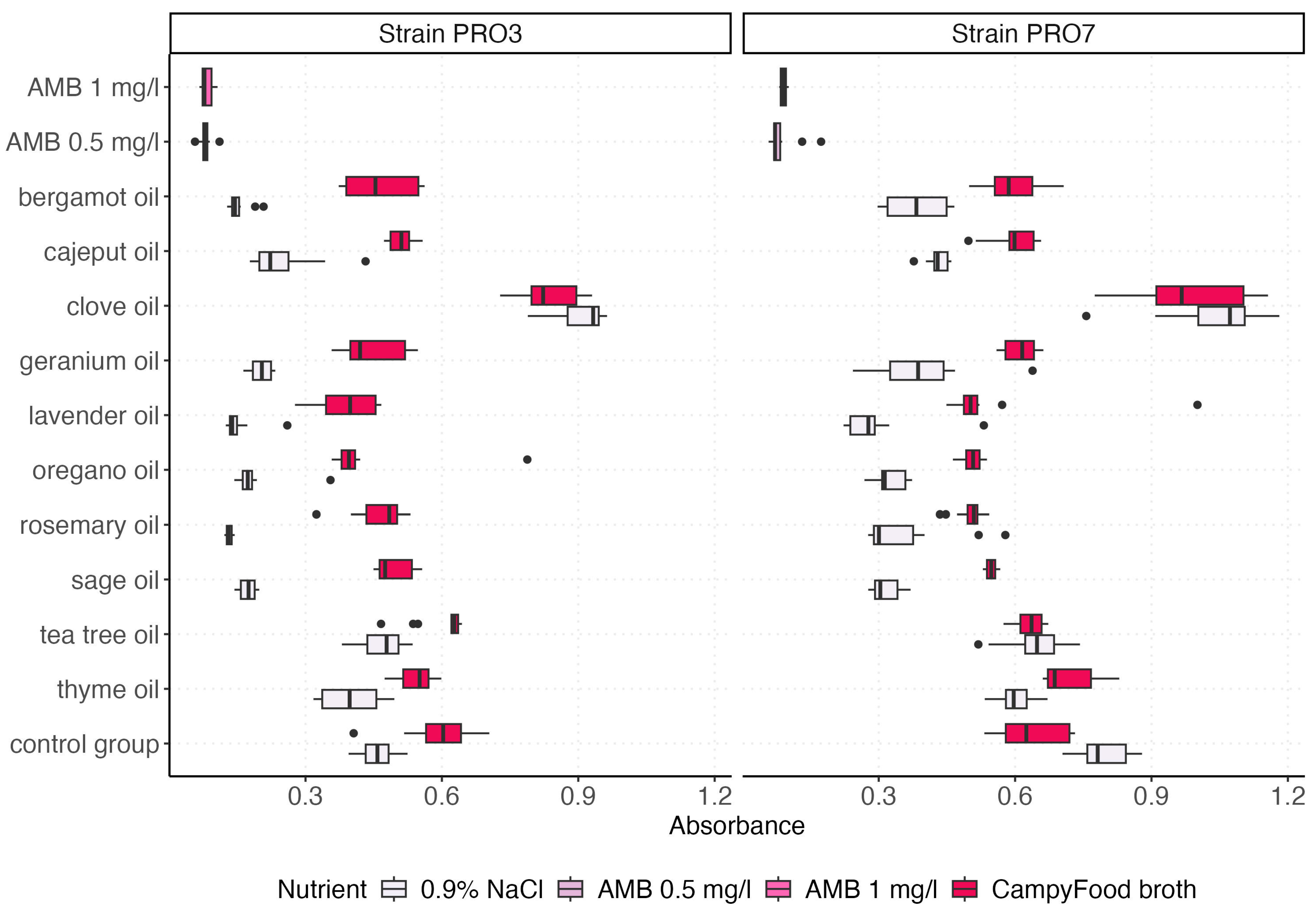
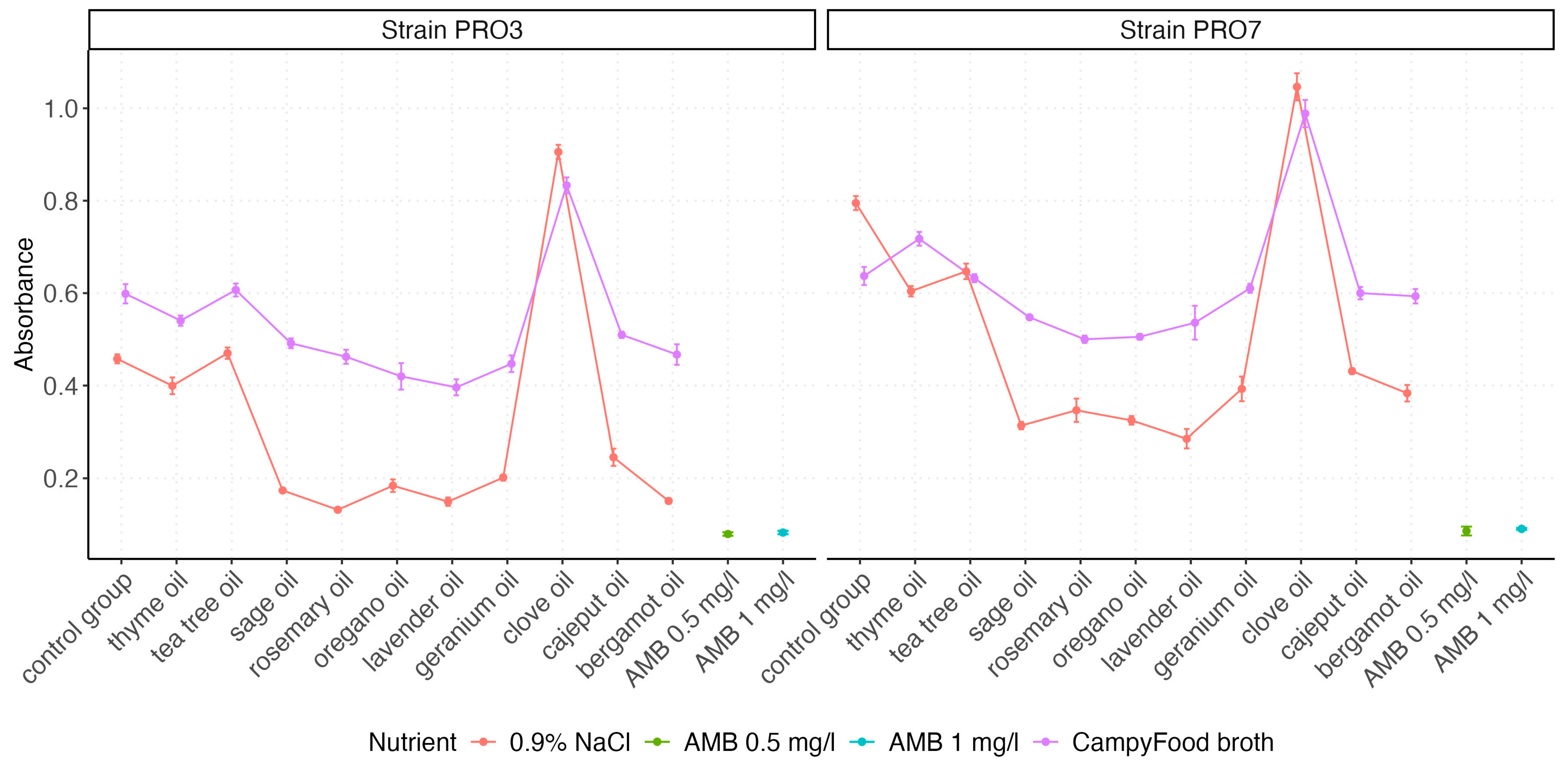
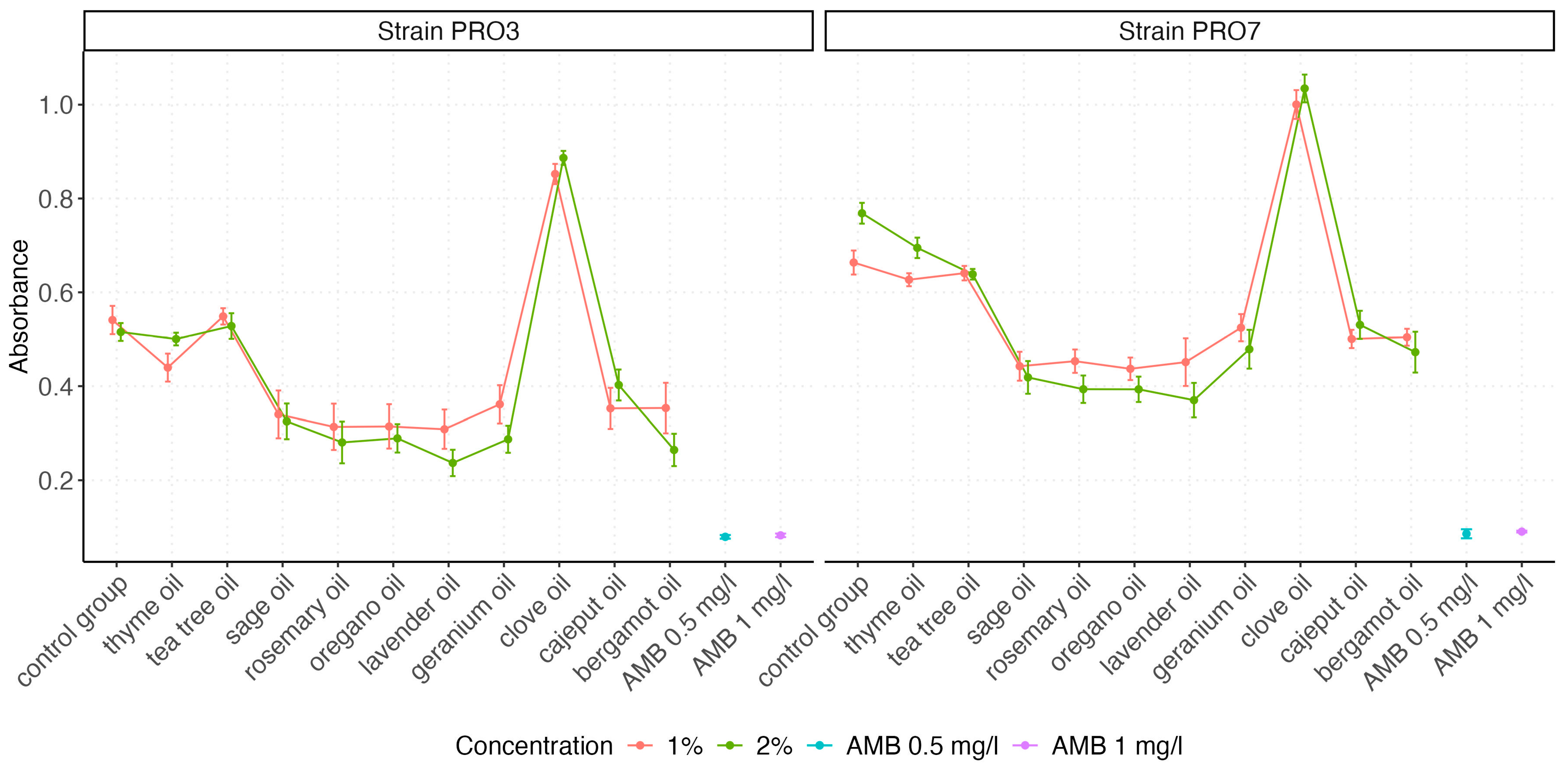
2.3. Absorbance Measurements
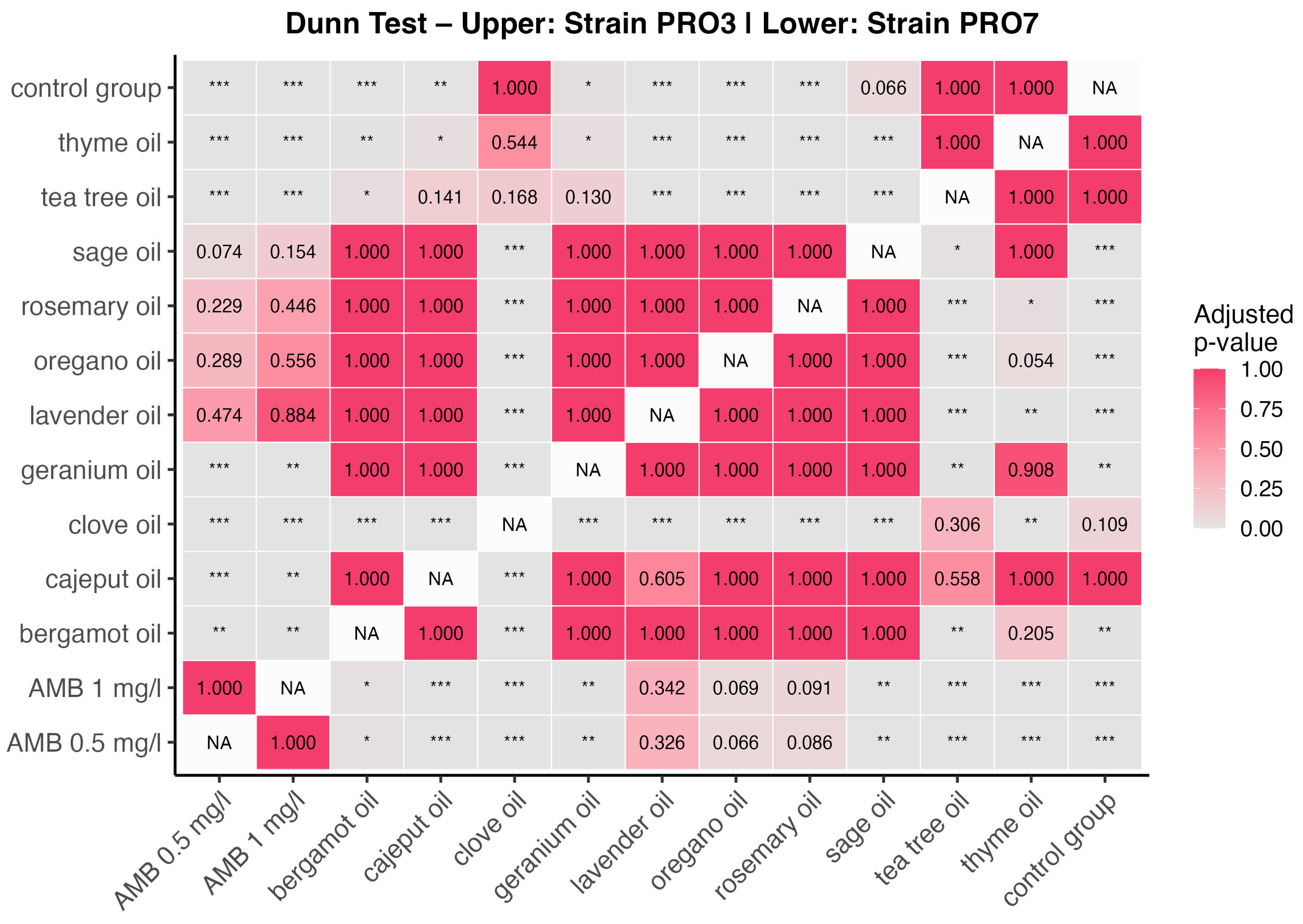
2.4. Group Comparison
2.5. Interactions
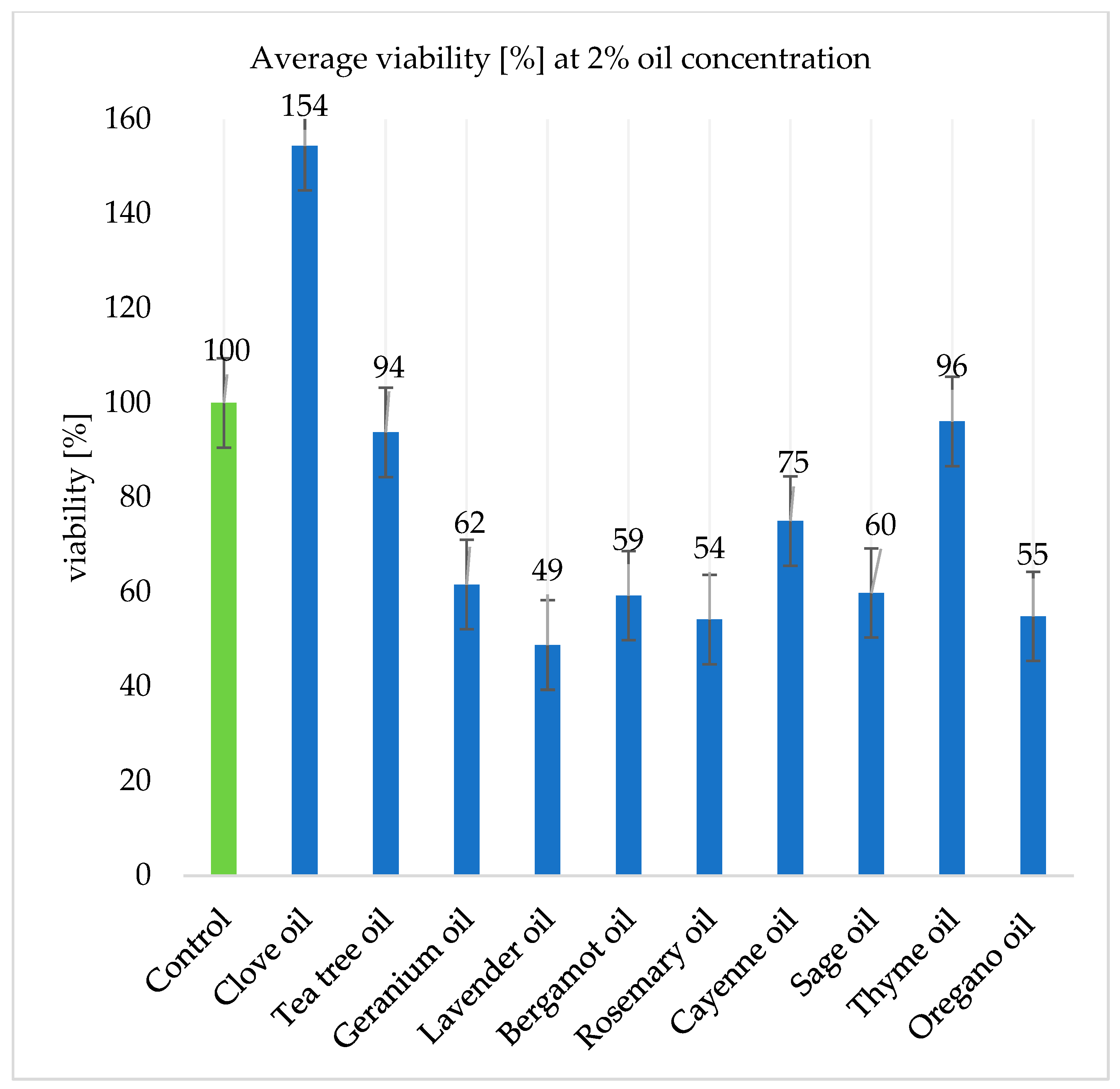
2.6. Algal Viability
3. Discussion
3.1. EO Characteristics
3.2. Prototheca Genus Taxonomy
3.3. Current Methods of Protothecosis Treatment Using EO and AMB
4. Materials and Methods
4.1. Biological Material
4.2. Tested Essential Oils
4.3. Analysis of Chemical Composition
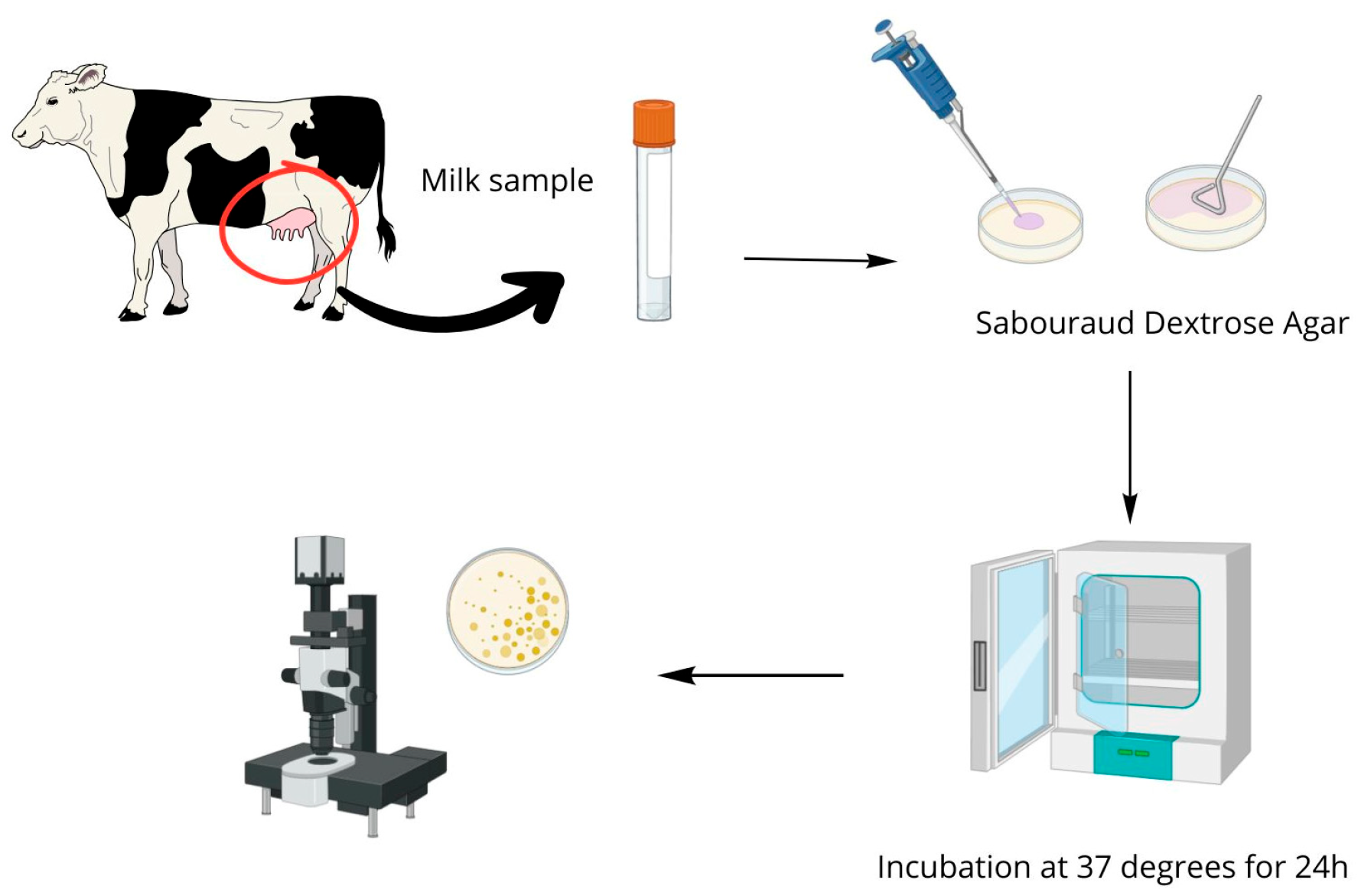
4.4. Isolation of Prototheca Species
4.5. Genetic Analysis
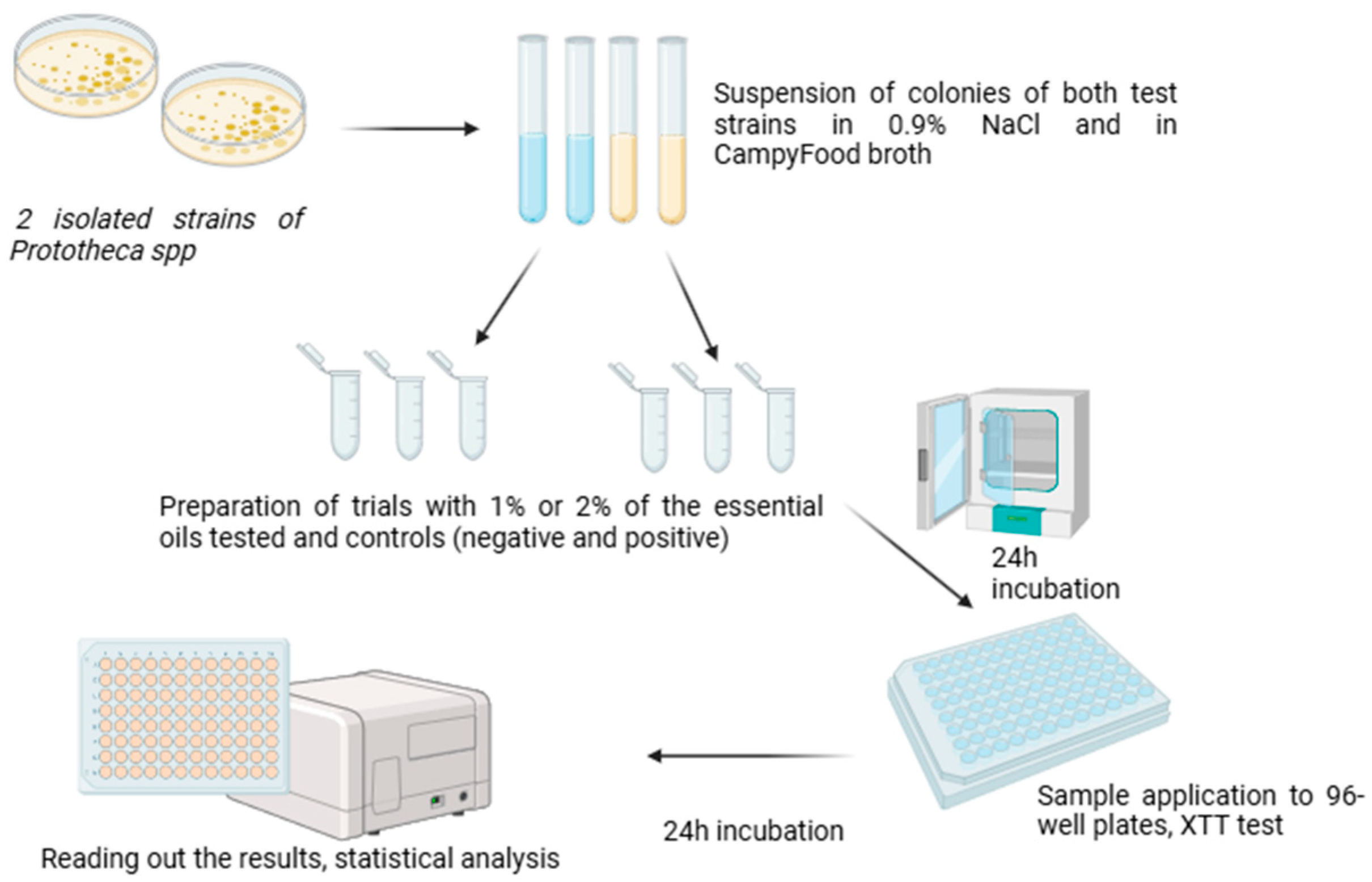
4.6. Toxicity Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagielski, T.; Krukowski, H.; Bochniarz, M.; Piech, T.; Roeske, K.; Bakuła, Z.; Wlazło, Ł.; Woch, P. Prevalence of Prototheca spp. on dairy farms in Poland—A cross-country study. Microb. Biotechnol. 2019, 12, 556–566. [Google Scholar] [CrossRef]
- Kalińska, A.; Gołębiewski, M.; Wójcik, A. Mastitis pathogens in dairy cattle—A review. World Sci. News 2017, 89, 22–31. [Google Scholar]
- McDougall, S.; Castle, R. Cow-level risk factors for clinical mastitis in the dry period in cows treated with an internal teat sealant alone at the end of lactation. N. Zeal. Vet. J. 2021, 69, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Heikkilä, A.M.; Nousiainen, J.I.; Pyörälä, S. Costs of clinical mastitis with special reference to premature culling. J. Dairy Sci. 2012, 95, 139–150. [Google Scholar] [CrossRef]
- Wawron, W.; Bochniarz, M.; Piech, T. Yeast mastitis in dairy cows in the middle-eastern part of Poland. Bull. Vet. Inst. Pulawy 2010, 54, 201–204. [Google Scholar]
- Park, H.-S.; Moon, D.C.; Hyun, B.-H.; Lim, S.-K. Occurrence and persistence of Prototheca zopfii in dairy herds of Korea. J. Dairy Sci. 2019, 10, 2539–2543. [Google Scholar] [CrossRef]
- Morandi, S.; Cremonesi, P.; Capra, E.; Silvetti, T.; Decimo, M.; Bianchini, V.; Alves, A.; Vargas, A.; Costa, G.; Ribeiro, M.; et al. Molecular typing and differences in biofilm formation and antibiotic susceptibilities among Prototheca strains isolated in Italy and Brazil. J. Dairy Sci. 2016, 99, 6436–6445. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Jin, E.; Wang, G.; Wu, L.; Shao, Z.; Wan, P.; Hu, C.; Li, J.; Chen, J.; et al. A survey of Prototheca bovis infection in dairy farms of the Hubei province China. J. Vet. Med. Sci. 2021, 83, 1248–1255. [Google Scholar] [CrossRef]
- Toyotome, T.; Matsui, S. Analysis of Prototheca and yeast species isolated from bulk tank milk collected in Tokachi District, Japan. J. Dairy Sci. 2022, 105, 8364–8370. [Google Scholar] [CrossRef]
- Jagielski, T.; Buzzini, P.; Lassa, H.; Malinowski, E.; Branda, E.; Turchetti, B.; Polleichtner, A.; Roesler, U.; Lagneau, P.E.; Marques, S.; et al. Multicentre Etest evaluation of in vitro activity of conventional antifungal drugs against European bovine Mastitis prototheca spp. isolates. J. Antimicrob. Chemother. 2012, 67, 1945–1947. [Google Scholar] [CrossRef]
- Lerche, M. Eine durch Algen (Prototheca) hervorgerufene Mastitis der Kuh. Berl. Munch. Tierarztl. Wochenschr. 1952, 4, 64–69. [Google Scholar]
- Bozzo, G.; Bonerba, E.; Di Pinto, A.; Bolzoni, G.; Ceci, E.; Mottola, A.; Tantillo, G.; Terio, V. Occurrence of Prototheca spp. in cow milk samples. New Microbiol. 2014, 37, 459–464. [Google Scholar]
- Libisch, B.; Picot, C.; Ceballos-Garzon, A.; Moravkova, M.; Klimesová, M.; Telkes, G.; Chuang, S.T.; Le Pape, P. Prototheca Infections and Ecology from a One Health Perspective. Microorganisms 2022, 10, 938. [Google Scholar] [CrossRef]
- Kano, R.; Kazuo Satoh Yaguchi, T.; Masuda, M.; Makimura, K.; de Hoog, G.S. Phenotypic Characteristics of Prototheca Species Occurring in Humans and Animals. Med. Mycol. J. 2022, 63, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mshana, S.E.; Sindato, C.; Matee, M.I.; Mboera, L.E.G. Antimicrobial Use and Resistance in Agriculture and Food Production Systems in Africa: A Systematic Review. Antibiotics 2021, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Ifedinezi, O.V.; Nnaji, N.D.; Anumudu, C.K.; Ekwueme, C.T.; Uhegwu, C.C.; Ihenetu, F.C.; Obioha, P.; Simon, B.O.; Ezechukwu, P.S.; Onyeaka, H. Environmental Antimicrobial Resistance: Implications for Food Safety and Public Health. Antibiotics 2024, 13, 1087. [Google Scholar] [CrossRef]
- Lambraki, I.A.; Cousins, M.; Graells, T.; Léger, A.; Henriksson, P.; Harbarth, S.; Troell, M.; Wernli, D.; Søgaard Jørgensen, P.; Desbois, A.P.; et al. Factors influencing antimicrobial resistance in the European food system and potential leverage points for intervention: A participatory, One Health study. PLoS ONE. 2022, 17, e0263914. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Alexa, V.T.; Galuscan, A.; Soica, C.M.; Cozma, A.; Coricovac, D.; Borcan, F.; Popescu, I.; Mioc, A.; Szuhanek, C.; Dehelean, C.A.; et al. In Vitro Assessment of the Cytotoxic and Antiproliferative Profile of Natural Preparations Containing Bergamot, Orange and Clove Essential Oils. Molecules 2022, 27, 990. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, H.; Ding, M.; Li, J.; Wang, D.; Li, H.; Sun, M.; Xia, F.; Bai, H.; Wang, M.; et al. Screening of Rosemary Essential Oils with Different Phytochemicals for Antioxidant Capacity, Keratinocyte Cytotoxicity, and Anti-Proliferative Activity. Molecules 2023, 28, 586. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Rolta, R.; Dev, K.; Sourirajan, A. Synergistic potential of essential oils with antibiotics to combat fungal pathogens: Present status and future perspectives. Phytother. Res. 2021, 35, 6089–6100. [Google Scholar] [CrossRef]
- Reichling, J.; Schnitzler, P.; Suschke, U.; Saller, R. Essential oils of aromatic plants with antibacterial, antifungal, antiviral, and cytotoxic properties—An overview. Complement. Med. Res. 2009, 16, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A.A. Comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Tkachev, A.V. Investigation of Plant’s Volatile Compounds; Offset-TM Publishing House: Novosibirsk, Russia, 2008; p. 969. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Kiki, M.J. In vitro antiviral potential, antioxidant, and chemical composition of clove (Syzygium aromaticum) essential oil. Molecules 2023, 28, 2421. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Hammer, K.A. Treatment of acne with tea tree oil (melaleuca) products: A review of efficacy, tolerability and potential modes of action. Int. J. Antimicrob. Agents 2015, 45, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Gokila Vani, M.; Wang, C.S.; Chen, C.C.; Chen, Y.C.; Lu, L.P.; Huang, C.H.; Lai, C.S.; Wang, S.Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhao, C.; Zhang, Z.; Nie, D.; Tang, W.; Li, Y. The Chemical Composition and Antibacterial and Antioxidant Activities of Five Citrus Essential Oils. Molecules 2022, 27, 7044. [Google Scholar] [CrossRef]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. J. Exp. Biol. 1999, 37, 124–130. [Google Scholar]
- Retnosari, S.; Retnosari, R.; Asmaningrum, H.P. Profile of the Indonesian essential oil from melaleuca cajuputi. Adv. Eng. Res. 2018, 171, 14–19. [Google Scholar]
- Sharifi-Rad, M.; Ozcelik, B.; Altı, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Borges Leal, A.L.A. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- De Martino, L.; De Feo, V.; Nazzaro, F. Chemical composition and in vitro antimicrobial and mutagenic activities of seven Lamiaceae essential oils. Molecules 2009, 14, 4213–4230. [Google Scholar] [CrossRef]
- Teles, A.M.; Rosa, T.D.D.S.; Mouchrek, A.N.; Abreu-Silva, A.L.; Calabrese, K.D.S.; Almeida-Souza, F. Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa Essential Oils: Chemical Composition, Antimicrobial and Antileishmanial Activity. Evid. Based Complement. Alternat. Med. 2019, 2421695, 12. [Google Scholar] [CrossRef]
- Jagielski, T.; Gawor, J.; Bakuła, Z.; Decewicz, P.; Maciszewski, K.; Karnkowska, A. Cytb as a New Genetic Marker for Differentiation of Prototheca Species. J. Clin. Microbiol. 2018, 56, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, B.; Głowacka, A.; Krukowski, H.; Lisowski, A.; Lassa, H.; Sienkiewicz, M. The in vitro efficacy of essential oils and antifungal drugs against Prototheca zopfii. Mycopathologia 2016, 181, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, B.; Kołodziej, B.; Głowacka, A.; Krukowski, H. The effect of some natural essential oils against bovine mastitis caused by Prototheca zopfii isolates in vitro. Mycopathologia 2018, 183, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Pisseri, F.; Pistelli, L.; Najar, B.; Luini, M.; Mancianti, F. In Vitro Activity of 30 Essential Oils against Bovine Clinical Isolates of Prototheca zopfii and Prototheca blaschkeae. Vet. Sci. 2018, 5, 45. [Google Scholar] [CrossRef]
- Catoi, C.; Bolfă, P.F.; Tabaran, F.A.; Borza, G. The inhibitory effect of some natural essential oils upon Prototheca algae in vitro growth. Bull. UASVM 2010, 67, 1. [Google Scholar]
- Abbasi Sani, B.; Eidi, S.; Ghodrati Azadi, H. In vitro activity of some native Iranian plants in Prototheca isolated from Clinical Bovine Mastitis. J. Vet. Lab. Res. 2023, 15, 97–107. [Google Scholar]
- Nojo, H.; Ishijima, S.A.; Morikawa, M.; Ito, T.; Kano, R. In vitro susceptibility testing of phytochemicals from essential oils against Prototheca species. J. Vet. Med. Sci. 2024, 86, 847–849. [Google Scholar] [CrossRef]
- Nelson, R.R. In-vitro activities of five plant essential oils against methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium. J. Antimicrob, Chemother. 1997, 40, 305–306. [Google Scholar] [CrossRef]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula x intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Luqman, S.; Dwivedi, G.R.; Darokar, M.P.; Kalra, A.; Khanuja, S.P. Potential of rosemary oil to be used in drug-resistant infections. Altern. Ther. Health Med. 2007, 13, 54–59. [Google Scholar] [PubMed]
- Adame-Gallegos, J.R.; Andrade-Ochoa, S.; Nevarez-Moorillon, G.V. Potential use of Mexican oregano essential oil against parasite, fungal and bacterial pathogens. J. Essent. Oil-Bear. Plants 2016, 19, 553–567. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramirez, L.A.; Leyva, J.M.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef]
- Massa, N.; Cantamessa, S.; Novello, G.; Ranzato, E.; Martinotti, S.; Pavan, M.; Rocchetti, A.; Berta, G.; Gamalero, E.; Bona, E. Antifungal activity of essential oils against azole-resistant and azole-susceptible vaginal Candida glabrata strains. Can. J. Microbiol. 2018, 64, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Philpot, W.N.; Nickerson, S.C. Mastitis: Counter Attack—A Strategy to Combat Mastitis; Babson Bros Company: Oak Brook, IL, USA, 1991. [Google Scholar]
- Stein, S.E. NIST 2020 Mass Spectral Library; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- McLafferty, F.W.; Stauffer, D.B. Wiley Science Solutions. In Wiley Registry of Mass Spectral Data, 12th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Pore, R.S. Selective medium for the isolation of Prototheca. Appl. Microbiol. 1973, 26, 648–649. [Google Scholar] [CrossRef]
- Jagielski, T.; Bakuła, Z.; Gawor, J.; Maciszewski, K.; Kusber, W.; Dyląg, M.; Nowakowska, J.; Gromadka, R.; Karnkowska, A. The genus Prototheca (Trebouxiophyceae, Chlorophyta) revisited: Implications from molecular taxonomic studies. Algal Res. 2019, 43, 101639. [Google Scholar] [CrossRef]
- Morello, L.; Tiroli, T.; Aretino, F.; Morandi, S.; Breviario, D. Preliminary Results, Perspectives, and Proposal for a Screening Method of In Vitro Susceptibility of Prototheca Species to Antimicrotubular Agents. Antimicrob Agents Chemother 2020, 64, e01392-19. [Google Scholar] [CrossRef]
- Dziurzyński, M.; Decewicz, P.; Iskra, M.; Bakuła, Z.; Jagielski, T. Prototheca-ID: A web-based application for molecular identification of Prototheca species. Database 2021, 2021, baab073. [Google Scholar] [CrossRef]
- Betlej, I.; Andres, B.; Cebulak, T.; Kapusta, I.; Balawejder, M.; Jaworski, S.; Lange, A.; Kutwin, M.; Pisulewska, E.; Kidacka, A.; et al. Antimicrobial Properties and Assessment of the Content of Bioactive Compounds Lavandula angustifolia Mill. Cultivated in Southern Poland. Molecules 2023, 28, 6416. [Google Scholar] [CrossRef]
- Kalińska, A.; Wawryło, C.; Tlatlik, W.; Gołębiewski, M.; Kot, M.; Lange, A.; Jaworski, S. Preliminary In Vitro Evaluation of Silver, Copper and Gold Nanoparticles as New Antimicrobials for Pathogens That Induce Bovine Locomotion Disorders. Int. J. Mol. Sci. 2024, 25, 9494. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Kot, M.; Radzikowski, D.; Smulski, S.; Gołębiewski, M. Silver and Copper Nanoparticles as the New Biocidal Agents Used in Pre- and Post-Milking Disinfectants with the Addition of Cosmetic Substrates in Dairy Cows. Int. J. Mol. Sci. 2023, 24, 1658. [Google Scholar] [CrossRef] [PubMed]
- Sandve, G.K.; Nekrutenko, A.; Taylor, J.; Hovig, E. Ten simple rules for reproducible computational research. PLoS Comput. Biol. 2013, 9, e1003285. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.D. Reproducible research in computational science. Science 2011, 334, 1226–1227. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y. Dynamic Documents with R and Knitr; Chapman and Hall/CRC: Boca Raton, FL, USA, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 1 March 2025).
- Kassambara, A. ggpubr: ‘ggplot2′ Based Publication Ready Plots, R package version 0.6.0; 2023; Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 1 March 2025).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R package version 1.0.7; 2020; Available online: https://CRAN.R-project.org/package=factoextra (accessed on 1 March 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 1 March 2025).
- Wickham, H.; Bryan, J. readxl: Read Excel Files, R package version 1.4.3; 2023; Available online: https://CRAN.R-project.org/package=readxl (accessed on 1 March 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation, R package version 1.1.4; 2023; Available online: https://CRAN.R-project.org/package=dplyr (accessed on 1 March 2025).



| Compound | RIexp. | RIlit. | Clove | Tea Tree | Geranium | Lavender | Bergamot | Rosemary | Cajeput | Sage | Thyme | Oregano |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | TIC (%) | |||
| Monoterpenes: | 0.2 | 93.2 | 95.7 | 92.9 | 100 | 94.9 | 99.0 | 98.3 | 95.7 | 94.2 | ||
| tricyclene | 924 | 921 a | - | - | - | - | - | - | - | 0.2 | - | - |
| 3-thujene | 929 | 926 b | - | - | - | - | 0.3 | 0.1 | 1.3 | - | - | - |
| α-pinene | 934 | 932 a | - | 3.7 | 0.9 | 0.2 | 1.6 | 20.5 | 5.2 | 8.2 | 2.3 | 2.8 |
| camphene | 949 | 946 a | - | - | - | - | - | 1.8 | - | 3.8 | - | - |
| sabinene | 974 | 973 b | - | 0.3 | - | - | 1.9 | - | - | 0.6 | - | - |
| β-pinene | 977 | 974 a | - | 0.3 | 0.7 | - | 9.2 | 12.3 | 2.0 | 2.3 | 2.1 | 2.2 |
| p-menth-3-ene | 984 | 984 a | - | 0.2 | - | - | - | - | - | - | - | - |
| myrcene | 991 | 988 a | - | - | - | 0.3 | 1.3 | 0.5 | 1.1 | 0.6 | 1.3 | 0.3 |
| p-mentha-1(7),8-diene | 1005 | 1003 a | - | - | - | - | - | - | - | - | 0.1 | - |
| α-phellandrene | 1005 | 1002 a | - | 0.2 | - | - | - | - | 0.9 | - | - | 0.2 |
| δ-3-carene | 1011 | 1008 a | - | - | - | - | - | - | 0.5 | - | - | - |
| 1,4-cineole | 1015 | 1012 a | - | - | - | - | - | - | 1.0 | - | - | - |
| α-terpinene | 1017 | 1017 b | - | 10.3 | - | - | - | 0.1 | 1.4 | - | - | 1.2 |
| p-menth-1-ene | 1022 | 1021 a | - | 0.2 | - | - | - | - | - | - | - | - |
| p-cymene | 1024 | 1020 a | - | 7.2 | 0.6 | 0.3 | 13.1 | 2.1 | 17.0 | 1.2 | 20.5 | 13.5 |
| limonene | 1028 | 1024 a | - | 3.6 | 0.6 | 8.5 | 30.6 | - | - | 8.8 | 3.5 | 1.0 |
| eucalyptol | 1030 | 1026 a | 0.2 | 3.7 | 0.4 | 4.8 | - | 24.0 | 44.4 | 12.4 | 2.5 | 1.2 |
| γ-terpinene | 1058 | 1054 a | - | 16.3 | - | - | 3.9 | 2.4 | 4.0 | - | - | 3.8 |
| trans-furanolinalool oxide | 1072 | 1072 b | - | 0.4 | 0.1 | - | 1.0 | - | - | - | - | - |
| cis-furanolinalool oxide | 1088 | 1088 b | - | - | 0.1 | - | 1.3 | - | - | - | - | - |
| α-terpinolene | 1089 | 1088 b | - | 3.9 | - | - | - | 0.1 | 3.4 | - | - | - |
| plinol a | 1091 | N/A | - | - | - | 0.1 | - | - | - | - | - | - |
| α-pinene epoxide | 1098 | 1099 a | - | - | - | - | - | - | - | 0.4 | - | - |
| linalool | 1102 | 1100 b | - | - | 9.6 | 30.9 | 18.7 | 2.8 | 0.9 | 2.1 | 11.4 | 4.0 |
| cis-rose oxide | 1111 | 1111 b | - | - | 1.2 | - | - | - | - | - | - | - |
| trans-2-pinanol | 1121 | 1119 a | - | - | - | 0.2 | - | - | - | - | - | - |
| 3-pinanol | 1122 | 1121 c | - | - | - | 0.3 | - | - | - | - | 0.1 | - |
| plinol D | 1124 | 1125 b | - | - | - | 0.7 | - | - | - | - | 0.2 | - |
| trans-rose oxide | 1127 | 1127 b | - | - | 0.1 | - | - | - | - | - | - | - |
| cis-limonene oxide | 1133 | 1132 a | - | - | - | - | 1.0 | - | - | 0.1 | - | - |
| 1-terpineol | 1134 | 1130 a | - | - | - | 0.2 | - | - | - | - | - | - |
| trans-limonene oxide | 1137 | 1137 a | - | - | - | - | 0.6 | - | - | - | - | - |
| trans-pinocarveol | 1138 | 1135 a | - | 0.3 | - | - | - | - | - | - | - | - |
| camphor | 1143 | 1141 a | - | - | - | 6.9 | - | 21.9 | - | 22.1 | 0.2 | 0.1 |
| cis-β-terpineol | 1144 | 1140 a | - | 0.4 | 0.2 | - | - | - | - | - | - | - |
| epoxyterpinolene | 1145 | 1145 b | - | - | - | - | 0.2 | - | 0.3 | - | - | - |
| menthone | 1153 | 1154 b | - | - | 2.4 | - | - | - | - | - | - | - |
| trans-β-terpineol | 1163 | 1159 a | - | - | - | 0.2 | - | - | - | - | - | - |
| iso-menthone | 1164 | 1164 b | - | - | 7.1 | - | - | - | - | - | - | - |
| borneol | 1164 | 1165 a | - | - | - | 0.8 | - | 0.4 | - | 8.3 | 0.3 | - |
| terpinen-4-ol | 1176 | 1174 a | - | 32.1 | - | 0.7 | - | 2.9 | - | 0.3 | 0.1 | - |
| p-cymen-8-ol | 1184 | 1186 b | - | - | - | - | - | - | 0.5 | - | - | - |
| α-terpineol | 1190 | 1186 a | - | 9.3 | 1.3 | 5.2 | 0.2 | 3.1 | 11.6 | 4.3 | 6.2 | - |
| myrtenal | 1195 | 1195 a | - | - | - | - | - | - | - | 0.2 | - | - |
| dihydrocitronellol | 1196 | 1196 b | - | - | 0.6 | - | - | - | - | - | - | - |
| epoxylinalol | 1196 | 1198 c | - | - | - | - | 0.4 | - | - | - | - | - |
| γ-terpineol | 1196 | 1199 a | - | 0.3 | - | 1.8 | - | - | - | - | 0.9 | - |
| monoterpenoide | 1203 | - | - | 0.3 | - | - | - | - | - | - | - | - |
| citronellol | 1230 | 1229 b | - | - | 30.8 | - | - | - | - | - | 0.1 | - |
| monoterpenoide acetate | 1233 | - | - | - | - | 0.2 | - | - | - | - | - | - |
| neral | 1241 | 1242 b | - | - | 0.7 | - | - | - | - | - | - | - |
| carvone | 1243 | 1239 a | - | - | - | - | - | - | - | 0.1 | - | - |
| plinol d | 1248 | N/A | - | - | - | 0.1 | - | - | - | - | - | - |
| monoterpenoide | 1252 | - | - | 0.4 | - | - | - | - | - | - | - | - |
| linalyl acetate | 1257 | 1254 a | - | - | - | 29.1 | 14.6 | - | - | 10.8 | 0.1 | - |
| geraniol | 1258 | 1255 b | - | - | 15.6 | - | - | - | - | - | - | - |
| monoterpenoide formate | 1266 | - | - | - | 0.4 | - | - | - | - | - | - | - |
| 1,4-dihydroxy-p-menth-2-ene | 1268 | 1264 c | - | - | - | - | - | - | 0.3 | - | - | - |
| geranial | 1270 | 1272 b | - | - | 1.2 | - | - | - | - | - | - | - |
| citronellyl formate | 1277 | 1277 b | - | - | 10.6 | - | - | - | - | - | - | - |
| neryl formate | 1281 | 1280 a | - | - | 1.7 | - | - | - | - | - | - | - |
| iso-isopulegyl acetate | 1283 | 1283 b | - | - | - | 0.2 | - | - | 0.3 | - | - | - |
| bornyl acetate | 1285 | 1287 a | - | - | - | 0.6 | - | - | - | 7.8 | - | - |
| lavandulyl acetate | 1290 | 1288 a | - | - | - | 0.4 | - | - | - | - | - | - |
| trans-sabinyl acetate | 1292 | 1289 a | - | - | - | - | - | - | - | 1.8 | - | - |
| thymol | 1292 | 1289 a | - | - | - | - | - | - | 1.0 | - | 38.7 | 17.5 |
| carvacrol | 1297 | 1298 a | - | - | - | - | - | - | - | - | 5.2 | 46.4 |
| geranyl formate | 1302 | 1303 b | - | - | 5.1 | - | - | - | - | - | - | - |
| (1R,4R)-p-mentha-2,8-diene, 1-hydroperoxide | 1320 | N/A | - | - | - | - | 0.1 | - | - | 0.1 | - | - |
| p-mentha-1,4,-dien-7-ol | 1322 | 1325 a | - | - | - | - | - | - | - | 0.2 | - | - |
| citronellol epoxide | 1331 | N/A | - | - | 0.5 | - | - | - | - | - | - | - |
| α-terpinyl acetate | 1348 | 1346 a | - | - | - | - | - | - | 1.7 | 1.1 | - | - |
| (2R,4R)-p-mentha-6,8-diene, 2-hydroperoxide | 1362 | 1365 c | - | - | - | - | - | - | - | 0.2 | - | - |
| geranyl acetate | 1382 | 1379 a | - | - | - | - | - | - | - | 0.1 | - | - |
| geranyl propanoate | 1474 | 1476 a | - | - | 0.7 | - | - | - | - | 0.2 | - | - |
| geranyl isobutanoate | 1514 | 1514 a | - | - | 0.8 | - | - | - | - | - | - | - |
| geranyl butanoate | 1561 | 1562 a | - | - | 1.1 | - | - | - | - | - | - | - |
| geranyl tiglate | 1701 | 1703 b | - | - | 0.5 | - | - | - | - | - | - | - |
| Sesquiterpenes: | 21.6 | 6.8 | 3.2 | 7.1 | - | 5.1 | 1.0 | 1.7 | 4.3 | 5.8 | ||
| β-bourbonene | 1384 | 1387 a | - | - | 0.4 | - | - | - | - | - | - | - |
| (Z)-patchenol | 1314 | 1316 a | - | - | - | - | - | - | - | 0.3 | - | - |
| α-gurjunene | 1409 | 1409 a | - | 0.4 | - | - | - | - | - | - | - | - |
| β-caryophyllene | 1419 | 1417 a | 13.3 | - | 1.4 | 7.1 | - | 4.7 | 1.0 | 0.3 | 4.0 | 4.7 |
| aromandendrene | 1439 | 1439 b | - | 4.5 | - | - | - | - | - | - | - | - |
| (Z)-β-farnesene | 1443 | 1444 a | - | - | 0.1 | - | - | - | - | - | - | - |
| selina-5,11-diene | 1443 | 1442 b | - | 0.2 | - | - | - | - | - | - | - | - |
| α-humulene | 1454 | 1452 a | 5.1 | - | - | - | - | 0.3 | - | 0.1 | - | - |
| β-selinene | 1486 | 1489 a | - | 0.1 | - | - | - | - | - | - | - | - |
| allo-aromadendr-9-ene | 1489 | 1489 b | - | 0.2 | - | - | - | - | - | - | - | - |
| viridiflorene | 1496 | 1496 a | - | 0.9 | - | - | - | - | - | - | - | - |
| α-selinene | 1496 | 1498 a | - | - | - | - | - | - | - | - | - | - |
| δ-cadinene | 1524 | 1522 a | 0.8 | - | 0.1 | - | - | - | - | - | - | - |
| cyclocaryophyllane aldehyde | 1553 | 1555 b | 0.4 | - | - | - | - | - | - | - | - | - |
| dihydrocaryophyllene-5-one | 1559 | 1561 b | - | - | - | - | - | - | - | - | - | - |
| spathulenol | 1578 | 1577 a | - | - | - | - | - | - | - | 0.1 | - | - |
| caryophyllene oxide | 1583 | 1582 a | 1.6 | - | 0.8 | - | - | 0.1 | - | 0.6 | 0.2 | 1.1 |
| globulol | 1585 | 1587 b | - | 0.5 | - | - | - | - | - | - | - | - |
| humulene-6,7-epoxide | 1610 | 1612 b | 0.3 | - | - | - | - | - | - | 0.1 | - | - |
| 10-epi-γ-eudesmol | 1620 | 1622 a | - | - | 0.4 | - | - | - | - | - | - | - |
| Fenylopropanoids: | 78.2 | - | - | |||||||||
| eugenol | 1359 | 1356 a | 66.0 | - | - | - | - | - | - | - | - | - |
| eugenol acetate | 1529 | 1530 b | 12.3 | - | - | - | - | - | - | - | - | - |
| Other compounds: | - | - | 1.1 | - | - | - | - | - | - | - | ||
| 6-methyl-5-hepten-2-one | 986 | 986 b | - | - | 0.3 | - | - | - | - | - | - | - |
| phenyl ethyl alcohol | 1113 | 1112 b | - | - | 0.4 | - | - | - | - | - | - | - |
| 2-phenylethyl tiglate | 1585 | 1584 a | - | - | 0.4 | - | - | - | - | - | - | - |
| Oil | Concentration | N | Average | SE | Median | SD | Min | Max | IQR |
|---|---|---|---|---|---|---|---|---|---|
| Control group | 1% | 14 | 0.541 | 0.030 | 0.530 | 0.113 | 0.395 | 0.704 | 0.205 |
| 2% | 14 | 0.515 | 0.019 | 0.496 | 0.071 | 0.406 | 0.631 | 0.108 | |
| AMB | 0.5 mg/L | 12 | 0.079 | 0.004 | 0.078 | 0.013 | 0.057 | 0.111 | 0.009 |
| 1 mg/L | 12 | 0.083 | 0.004 | 0.078 | 0.013 | 0.067 | 0.107 | 0.020 | |
| Bergamot oil | 1% | 14 | 0.354 | 0.054 | 0.358 | 0.201 | 0.137 | 0.562 | 0.397 |
| 2% | 14 | 0.265 | 0.034 | 0.264 | 0.129 | 0.127 | 0.400 | 0.245 | |
| Cajeput oil | 1% | 14 | 0.353 | 0.044 | 0.344 | 0.163 | 0.177 | 0.544 | 0.306 |
| 2% | 14 | 0.403 | 0.033 | 0.452 | 0.123 | 0.239 | 0.558 | 0.241 | |
| Clove oil | 1% | 14 | 0.852 | 0.022 | 0.831 | 0.081 | 0.728 | 0.959 | 0.137 |
| 2% | 14 | 0.886 | 0.015 | 0.911 | 0.056 | 0.789 | 0.963 | 0.098 | |
| Geranium oil | 1% | 14 | 0.362 | 0.041 | 0.334 | 0.152 | 0.193 | 0.547 | 0.299 |
| 2% | 14 | 0.287 | 0.029 | 0.292 | 0.108 | 0.163 | 0.405 | 0.211 | |
| Lavender oil | 1% | 14 | 0.309 | 0.042 | 0.349 | 0.156 | 0.129 | 0.467 | 0.306 |
| 2% | 14 | 0.237 | 0.028 | 0.213 | 0.105 | 0.125 | 0.358 | 0.206 | |
| Oregano oil | 1% | 14 | 0.315 | 0.047 | 0.356 | 0.176 | 0.143 | 0.788 | 0.226 |
| 2% | 14 | 0.289 | 0.030 | 0.282 | 0.113 | 0.171 | 0.420 | 0.215 | |
| Rosemary oil | 1% | 14 | 0.314 | 0.049 | 0.302 | 0.184 | 0.123 | 0.513 | 0.351 |
| 2% | 14 | 0.281 | 0.044 | 0.228 | 0.166 | 0.122 | 0.531 | 0.296 | |
| Sage oil | 1% | 14 | 0.340 | 0.051 | 0.319 | 0.190 | 0.144 | 0.557 | 0.376 |
| 2% | 14 | 0.325 | 0.038 | 0.324 | 0.142 | 0.176 | 0.476 | 0.271 | |
| Tea tree oil | 1% | 14 | 0.549 | 0.017 | 0.536 | 0.065 | 0.432 | 0.629 | 0.113 |
| 2% | 14 | 0.528 | 0.027 | 0.493 | 0.102 | 0.380 | 0.644 | 0.195 | |
| Thyme oil | 1% | 14 | 0.440 | 0.030 | 0.414 | 0.112 | 0.317 | 0.599 | 0.203 |
| 2% | 14 | 0.500 | 0.014 | 0.478 | 0.051 | 0.447 | 0.575 | 0.098 |
| Group | Code |
|---|---|
| Clove oil | 1 |
| Tea tree oil | 2 |
| Geranium oil | 3 |
| Lavender oil | 4 |
| Bergamot oil | 5 |
| Rosemary oil | 6 |
| Cajeput oil | 7 |
| Sage oil | 8 |
| Thyme oil | 9 |
| Oregano oil | 10 |
| Control | 11 |
| Strain | Species | Herd Location | Material | Preliminary Identification | GenBank |
|---|---|---|---|---|---|
| PRO3 | Prototheca bovis | Kuyavian- Pomerania | Quarter milk | Phenotypic characteristics | PQ151373 |
| PRO7 | Prototheca bovis | West Pomerania | Quarter milk | Phenotypic characteristics | PQ151374 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuczyńska, M.; Kot, M.; Stocki, M.; Zapora, E.; Jagielski, T.; Perlińska-Teresiak, M.; Kalińska, A. In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. Int. J. Mol. Sci. 2025, 26, 5451. https://doi.org/10.3390/ijms26125451
Kuczyńska M, Kot M, Stocki M, Zapora E, Jagielski T, Perlińska-Teresiak M, Kalińska A. In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. International Journal of Molecular Sciences. 2025; 26(12):5451. https://doi.org/10.3390/ijms26125451
Chicago/Turabian StyleKuczyńska, Maria, Magdalena Kot, Marcin Stocki, Ewa Zapora, Tomasz Jagielski, Magdalena Perlińska-Teresiak, and Aleksandra Kalińska. 2025. "In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows" International Journal of Molecular Sciences 26, no. 12: 5451. https://doi.org/10.3390/ijms26125451
APA StyleKuczyńska, M., Kot, M., Stocki, M., Zapora, E., Jagielski, T., Perlińska-Teresiak, M., & Kalińska, A. (2025). In Vitro Determination of Cytotoxic Effects of Ten Essential Oils on Prototheca bovis, Which Causes Mastitis in Dairy Cows. International Journal of Molecular Sciences, 26(12), 5451. https://doi.org/10.3390/ijms26125451








