Inosine, AMP, and Vidarabine: Network Pharmacology and LC-MS Reveal Key Bioactive Compounds in Periplaneta americana for Ulcerative Colitis Management
Abstract
1. Introduction
2. Results
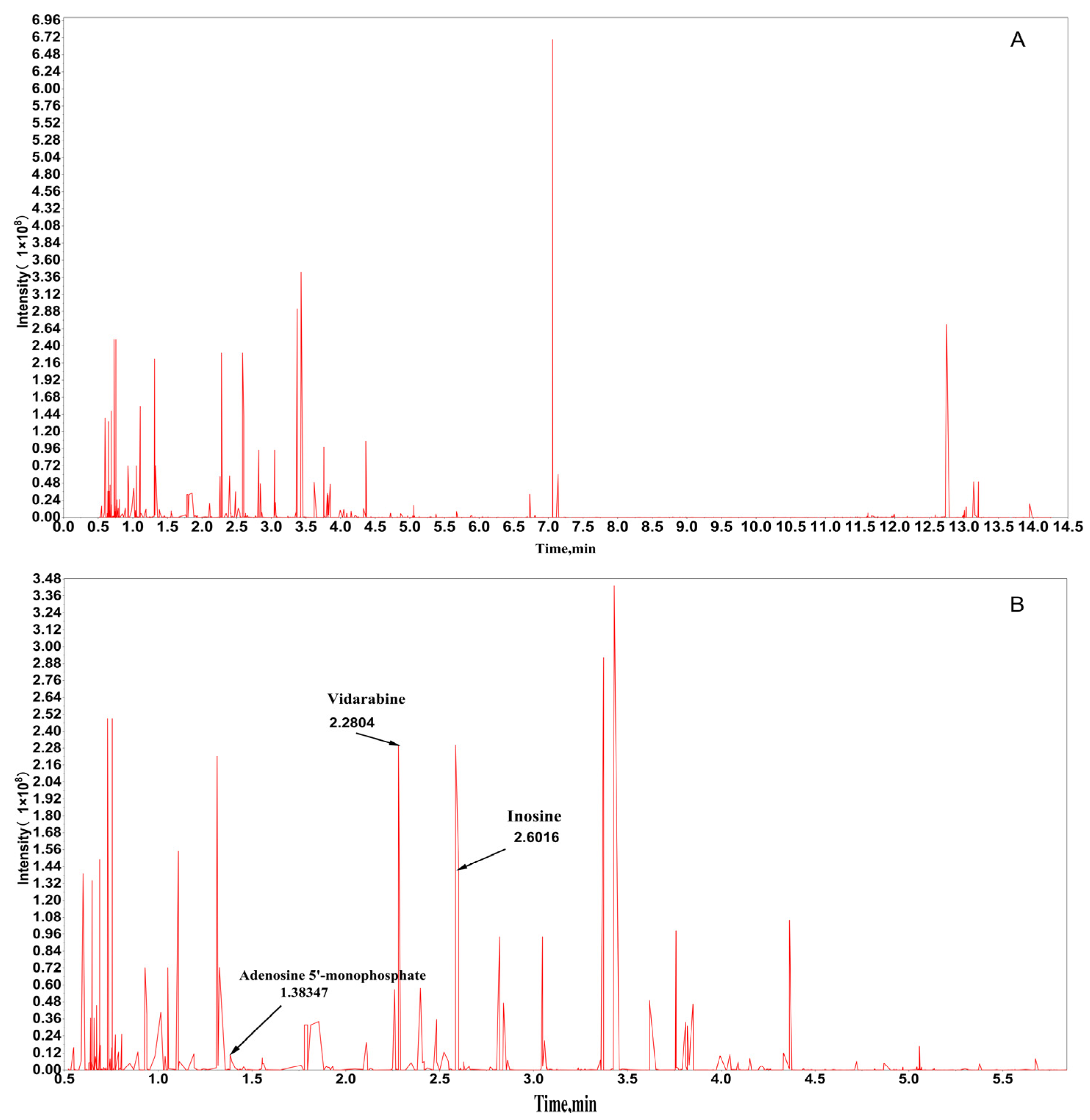
2.1. Chemical Composition Analysis of PAE
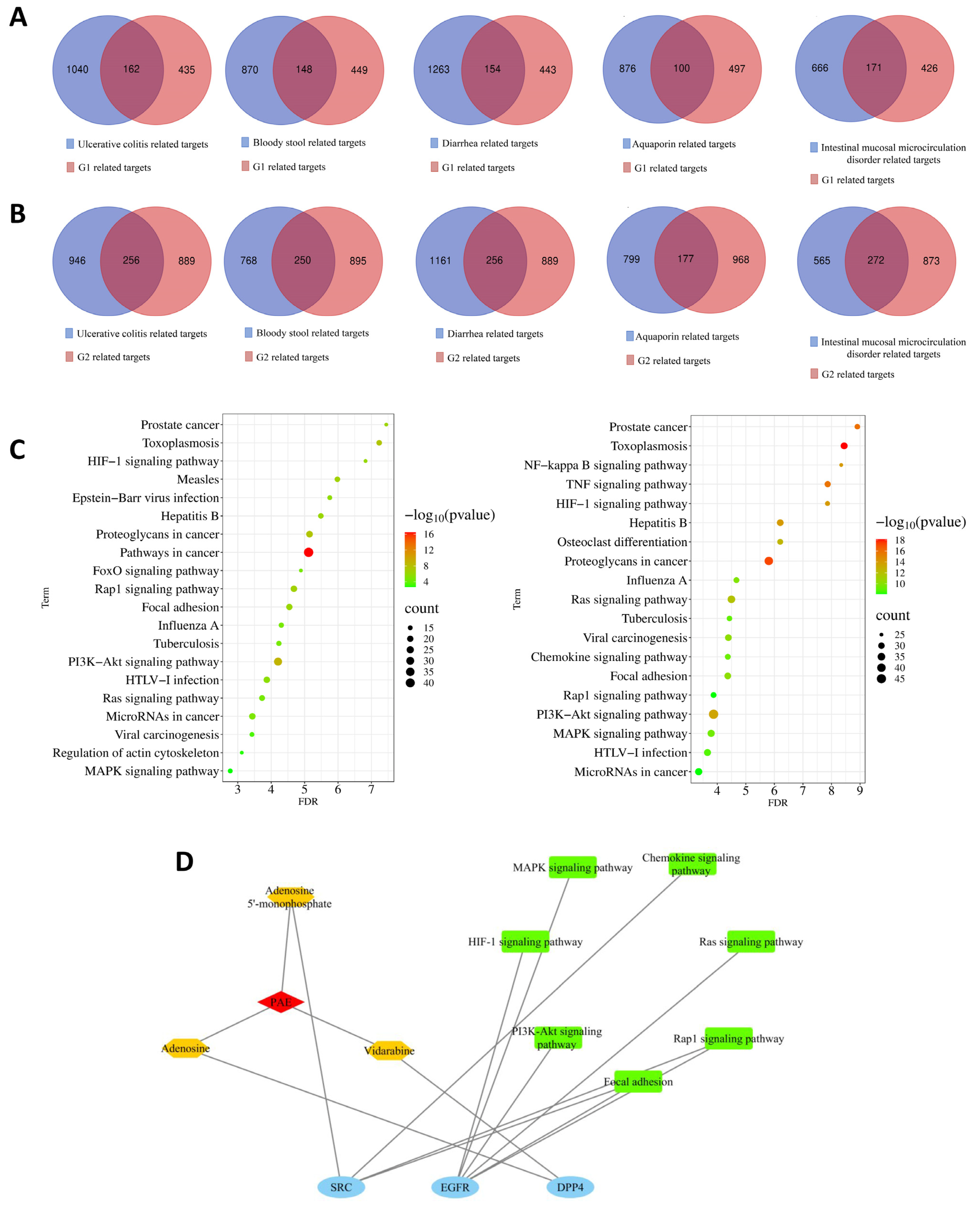
2.2. Network Pharmacological Analysis
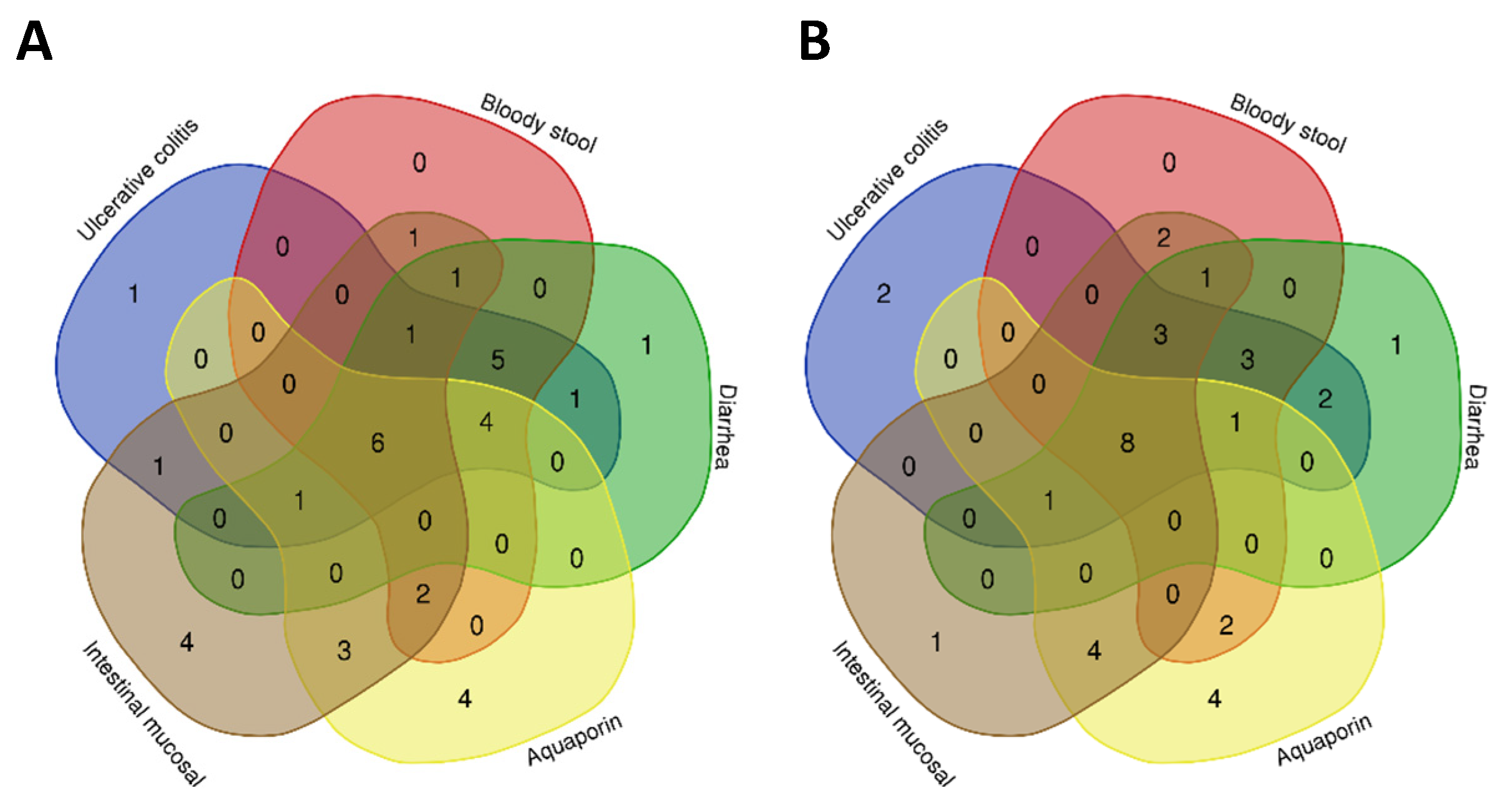
2.2.1. Screening of Targets of Compounds and Diseases and Construction of the PPI Network
2.2.2. Reverse Screening of Core Compounds
2.2.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
2.2.4. Compound-Target-Pathway Network Relationships
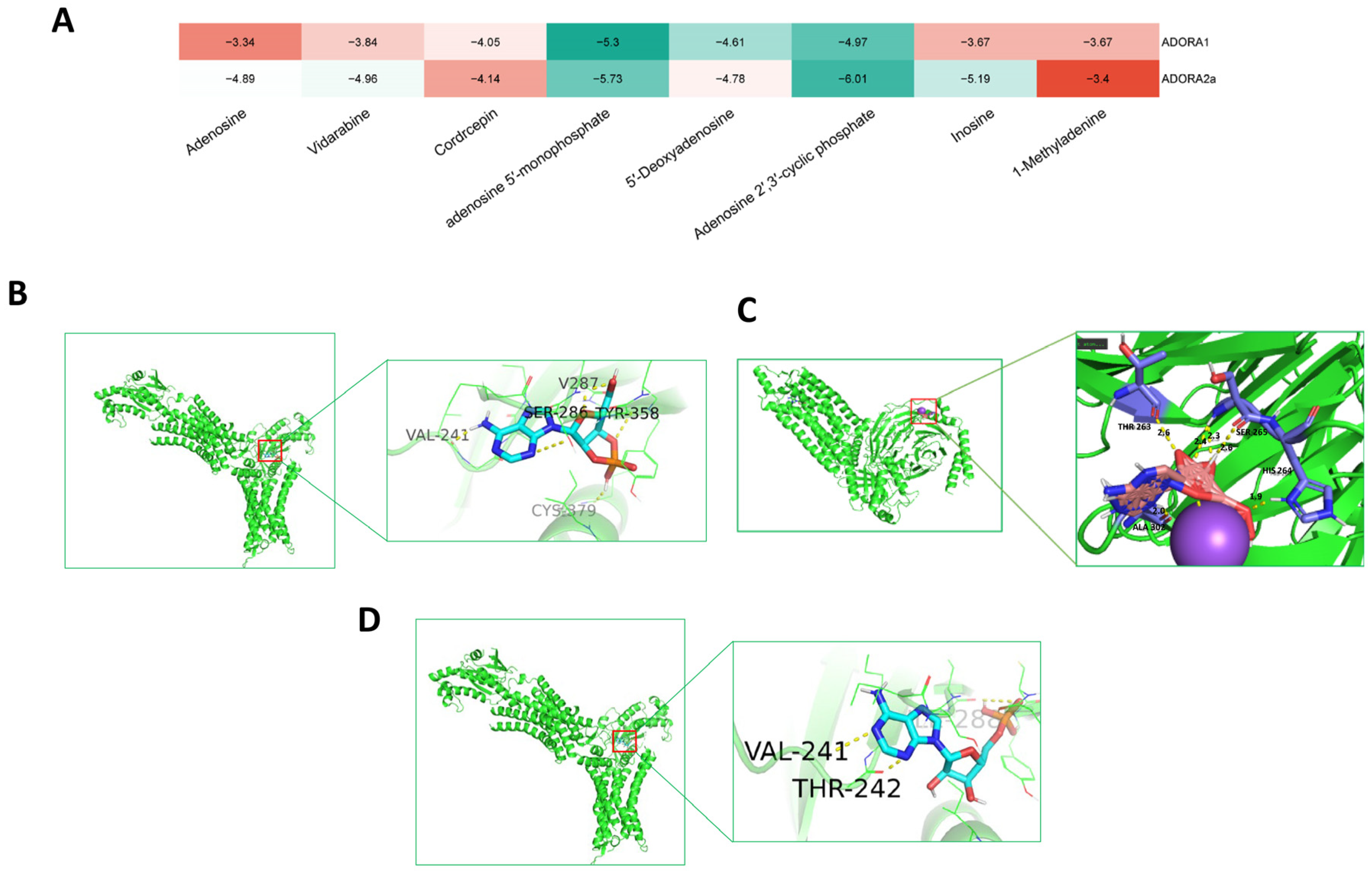
2.2.5. Molecular Docking
2.2.6. Quantitative and Qualitative Analysis by HPLC
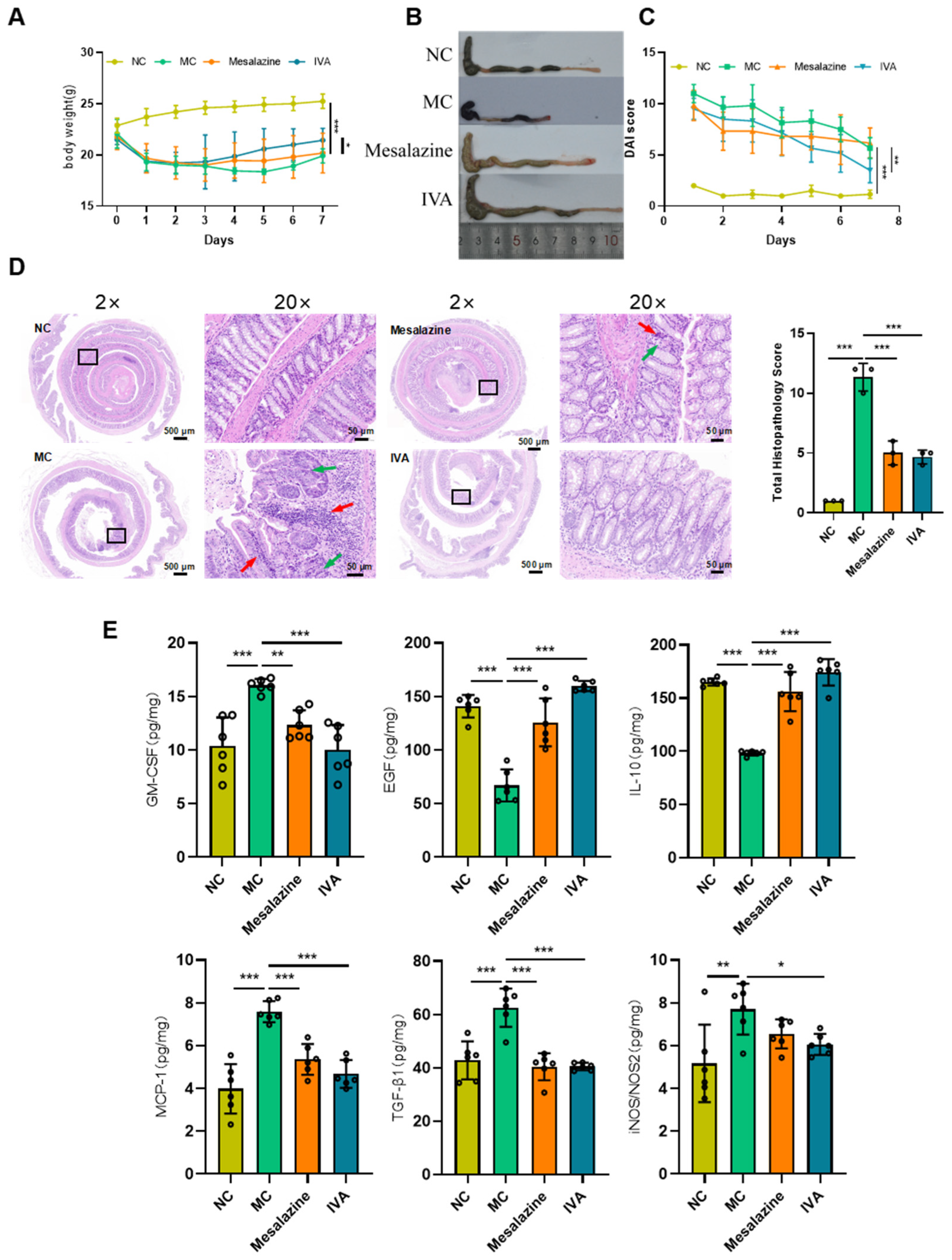
2.3. In Vivo Pharmacodynamic Validation of IVA in Treating Ulcerative Colitis
2.3.1. IVA Alleviates Dextran Sulfate Sodium (DSS)-Induced UC in Mice
2.3.2. IVA Reduces Colonic Mucosal Damage and Inflammatory Cell Infiltration
2.3.3. Cytokine Expression in Colonic Tissue
2.3.4. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Prepare and Chemical Composition Analysis of PAE
4.3. Network Pharmacology
4.4. In Vivo Pharmacodynamic Study of Active Compounds for UC
4.4.1. Establishment of Acute UC Model and Drug Treatment
4.4.2. Histological Evaluation of Colitis
4.4.3. Enzyme-Linked Immunosorbent Assay
4.4.4. Immunohistochemical Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Primers 2020, 6, 74. [Google Scholar] [CrossRef]
- Zhang, W.; Zou, M.; Fu, J.; Xu, Y.; Zhu, Y. Autophagy: A potential target for natural products in the treatment of ulcerative colitis. Biomed. Pharmacother. 2024, 176, 116891. [Google Scholar] [CrossRef]
- Colman, R.J.; Dhaliwal, J.; Rosen, M.J. Predicting therapeutic response in pediatric ulcerative colitis-A journey towards precision medicine. Front. Pediatr. 2021, 9, 634739. [Google Scholar] [CrossRef]
- Voelker, R. What is ulcerative colitis? Jama 2024, 331, 716. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, A.; Tu, P.; Hu, Z. Anti-tumor effects of the American cockroach, Periplaneta americana. Chin. Med. 2017, 12, 26. [Google Scholar] [CrossRef]
- Zhu, J.J.; Yao, S.; Guo, X.; Yue, B.S.; Ma, X.Y.; Li, J. Bioactivity-guided screening of wound-healing active cAdv Exp Med Biolonstituents from American Cockroach (Periplaneta americana). Molecules 2018, 23, 101. [Google Scholar] [CrossRef]
- Liu, S.S.; Xu, Y.S.; Hilola, A.; Zhou, N.; He, M.; Zhang, C.G.; Liu, H. Study on the effect of the treatment of Periplaneta americana L. extract Ento-B by Dinitrochlorobenzene combined with acetic acid induced UC in rats. Acta Cir. Bras. 2021, 36, e360102. [Google Scholar] [CrossRef]
- Cao, T.; Wang, X.L.; Rao, J.Y.; Zhu, H.F.; Qi, H.Y.; Tian, Z. Periplaneta americana L. extract exerts neuroprotective effects by inhibiting endoplasmic reticulum stress via AKT-dependent pathway in experimental models of Parkinson’s disease. Chin. Med. 2024, 19, 157. [Google Scholar] [CrossRef]
- Rao, J.; Li, H.; Zhang, H.; Xiang, X.; Ding, X.; Li, L.; Geng, F.; Qi, H. Periplaneta americana (L.) extract activates the ERK/CREB/BDNF pathway to promote post-stroke neuroregeneration and recovery of neurological functions in rats. J. Ethnopharmacol. 2024, 321, 117400. [Google Scholar] [CrossRef]
- Li, X.; Pang, L.; Duan, J.; Huang, N.; Chen, X.; Huang, W.; Liu, Y.; Fu, C.; Zhang, C.; Tu, H.; et al. Eco-friendly antibacterial electrospinning nanofibrous film containing nano-silver green-synthesized by natural glycoprotein for infected wound healing. J. Colloid. Interface Sci. 2025, 683, 256–268. [Google Scholar] [CrossRef]
- Liao, Q.; Su, L.; Pang, L.; Li, J.; Li, H.; Li, J.; Liu, Y.; Zhang, J. Natural exosome-like nanoparticles derived from ancient medicinal insect Periplaneta americana L. as a novel diabetic wound healing accelerator. J. Nanobiotechnol. 2023, 21, 169. [Google Scholar] [CrossRef]
- Li, L.J.; Wang, M.Z.; Yuan, T.J.; Xu, X.H.; Dad, H.A.; Yu, C.L.; Hou, J.; Peng, L.H. The crude ethanol extract of Periplaneta americana L. stimulates wound healing in vitro & in vivo. Chin. Med. 2019, 14, 33. [Google Scholar]
- Tang, M.; Ni, L.L.; Xu, J.L.; Wang, Y.J.; Zhang, C.G.; Ali, T.; Wu, X.M.; Liu, H.; He, M. Kangfuxin liquid ameliorates dextran sulfate sodium (DSS)-induced acute ulcerative colitis in mice by modulating immune response and suppressing inflammation. Med. Sci. Monit. Basic. Res. 2021, 27, e930887. [Google Scholar] [CrossRef]
- Lin, X.; Chu, J.; Xiang, Y.; He, M.; Ma, Q.; Duan, J.; Wang, Y.; Sun, S. Kangfuxin liquid reduces the ultraviolet B-induced photodamage of HaCaT cells by regulating autophagy. Biosci. Biotechnol. Biochem. 2023, 87, 1485–1494. [Google Scholar] [CrossRef]
- Mirsepasi-Lauridsen, H.C. Therapy used to promote disease remission targeting gut dysbiosis, in UC patients with active disease. J. Clin. Med. 2022, 11, 7472. [Google Scholar] [CrossRef]
- Escudero-Hernández, C.; Münch, A.; Østvik, A.E.; Granlund, A.V.B.; Koch, S. The water channel aquaporin 8 is a critical regulator of intestinal fluid homeostasis in collagenous colitis. J. Crohns Colitis 2020, 14, 962–973. [Google Scholar] [CrossRef]
- Ning, H.; Liu, J.; Tan, J.; Yi, M.; Lin, X. The role of the Notch signalling pathway in the pathogenesis of ulcerative colitis: From the perspective of intestinal mucosal barrier. Front. Med. 2023, 10, 1333531. [Google Scholar] [CrossRef]
- Thomas, S.; Mercogliano, G.; Prendergast, G.C. Bin1 targeted immunotherapy alters the status of the enteric neurons and the microbiome during ulcerative colitis treatment. PLoS ONE 2022, 17, e0276910. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhang, X.; Tang, X.; Su, W.; Wang, Z.; Wang, H. Biochemical characterization of an arabinoside monophosphate specific 5′-nucleotidase-like enzyme from Streptomyces antibioticus. Biomolecules 2024, 14, 1368. [Google Scholar] [CrossRef]
- Simmons, S.R.; Herring, S.E.; Tchalla, E.Y.I.; Lenhard, A.P.; Bhalla, M.; Bou Ghanem, E.N. Activating A1 adenosine receptor signaling boosts early pulmonary neutrophil recruitment in aged mice in response to Streptococcus pneumoniae infection. Immun. Ageing 2024, 21, 34. [Google Scholar] [CrossRef]
- Uddin, J.; Sharma, A.; Wu, D.; Tomar, S.; Ganesan, V.; Reichel, P.E.; Thota, L.N.R.; Cabrera-Silva, R.I.; Marella, S.; Idelman, G.; et al. STARD7 maintains intestinal epithelial mitochondria architecture, barrier integrity, and protection from colitis. JCI Insight 2024, 9, e172978. [Google Scholar] [CrossRef]
- Ehikioya, C.O.; Osagie, A.M.; Omage, S.O.; Omage, K.; Azeke, M.A. Carbohydrate digestive enzyme inhibition, hepatoprotective, antioxidant and antidiabetic benefits of Persea americana. Sci. Rep. 2023, 13, 284. [Google Scholar] [CrossRef]
- He, M.; Yu, W.X.; Shen, Y.; Zhang, J.N.; Ni, L.L.; Li, Y.; Liu, H.; Zhao, Y.; Zhao, H.R.; Zhang, C.G. Kangfuxin alleviates ulcerative colitis in rats by inhibiting NF-κB p65 activation and regulating T lymphocyte subsets. Iran. J. Basic Med. Sci. 2023, 26, 882–890. [Google Scholar]
- Ni, L.; Lu, Q.; Tang, M.; Tao, L.; Zhao, H.; Zhang, C.; Yu, Y.; Wu, X.; Liu, H.; Cui, R. Periplaneta americana extract ameliorates dextran sulfate sodium-induced ulcerative colitis via immunoregulatory and PI3K/AKT/NF-κB signaling pathways. Inflammopharmacology 2022, 30, 907–918. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, M.; Jin, J.; Chen, J.; Li, Z. Periplaneta americana (Insecta: Blattodea) and organ fibrosis: A mini review. Medicine 2022, 101, e32039. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Wu, Q.; Zhang, L.; Chen, Z.; Zhao, H.; Wu, X.; Zhao, Y.; Zhang, C.; Ge, J.; et al. Antioxidative effect of Periplaneta americana extract on dextran sulfate sodium-induced ulcerative colitis through activation of the Nrf2 signal. Pharm. Biol. 2023, 61, 949–962. [Google Scholar] [CrossRef]
- Herman-de-Sousa, C.; Pinheiro, A.R.; Paramos-de-Carvalho, D.; Costa, M.A.; Ferreirinha, F.; Magalhães-Cardoso, T.; Ribeiro, S.; Pelletier, J.; Sévigny, J.; Correia-de-Sá, P. Opposing effects of adenosine and inosine in human subcutaneous fibroblasts may be regulated by third party ADA cell providers. Cells 2020, 9, 651. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Tian, M.; Ji, J.; Hu, X.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H.; Cui, S. Mitigation of dextran-sodium-sulfate-induced colitis in mice through oral administration of microbiome-derived inosine and its underlying mechanisms. Int. J. Mol. Sci. 2023, 24, 13852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief. Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, Y.; Kang, C.; Zheng, Y.; Liu, X.; Liang, Z.; Yan, J. The pharmacological evidence of the chang-yan-ning formula in the treatment of colitis. Front. Pharmacol. 2022, 13, 1029088. [Google Scholar] [CrossRef]
- Cui, Y.; Hu, J.; Li, Y.; Au, R.; Fang, Y.; Cheng, C.; Xu, F.; Li, W.; Wu, Y.; Zhu, L.; et al. Integrated network pharmacology, molecular docking and animal experiment to explore the efficacy and potential mechanism of Baiyu Decoction against ulcerative colitis by enema. Drug Des. Dev. Ther. 2023, 17, 3453–3472. [Google Scholar] [CrossRef]
- Huang, J.Q.; Cheng, N.; Zhong, Y.B.; Zhang, Z.Y.; Huang, L.; Song, L.Z.; Li, M.D.; Deng, Y.F.; Zhou, W.; Zhao, H.M.; et al. Integrating network pharmacology and experimental verification to explore the mucosal protective effect of Chimonanthus nitens Oliv. Leaf Granule on ulcerative colitis. J. Ethnopharmacol. 2024, 321, 117540. [Google Scholar] [CrossRef]
- Chen, W.; He, L.; Zhong, L.; Sun, J.; Zhang, L.; Wei, D.; Wu, C. Identification of active compounds and mechanism of Huangtu Decoction for the treatment of ulcerative colitis by network pharmacology combined with experimental verification. Drug Des. Dev. Ther. 2021, 15, 4125–4140. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, M.; Wu, Y.; Jung, S.; Li, D.; He, N.; Lee, M.S. An efficient enzyme-triggered controlled release system for colon-targeted oral delivery to combat dextran sodium sulfate (DSS)-induced colitis in mice. Drug Deliv. 2021, 28, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Jena, G. Studies on the mechanism of local and extra-intestinal tissue manifestations in AOM-DSS-induced carcinogenesis in BALB/c mice: Role of PARP-1, NLRP3, and autophagy. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 4321–4337. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Bériou, G.; Josien, R. Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol. Biol. 2016, 1371, 197–203. [Google Scholar]
- Zhao, H.; Wang, Q.; Zhao, J.; Wang, D.; Liu, H.; Gao, P.; Shen, Y.; Wu, T.; Wu, X.; Zhao, Y.; et al. Ento-A alleviates DSS-induced experimental colitis in mice by remolding intestinal microbiota to regulate SCFAs metabolism and the Th17 signaling pathway. Biomed. Pharmacother. 2024, 170, 115985. [Google Scholar] [CrossRef]
- Chapuy, L.; Bsat, M.; Rubio, M.; Sarkizova, S.; Therrien, A.; Bouin, M.; Orlicka, K.; Weber, A.; Soucy, G.; Villani, A.C.; et al. IL-12 and mucosal CD14+ monocyte-Like cells induce IL-8 in colonic memory CD4+ T cells of patients with ulcerative colitis but not Crohn’s disease. J. Crohns Colitis 2020, 14, 79–95. [Google Scholar] [CrossRef]
- Geremia, A.; Arancibia-Cárcamo, C.V. Innate lymphoid cells in intestinal inflammation. Front. Immunol. 2017, 8, 1296. [Google Scholar] [CrossRef]
- Grimm, M.C.; Elsbury, S.K.; Pavli, P.; Doe, W.F. Enhanced expression and production of monocyte chemoattractant protein-1 in inflammatory bowel disease mucosa. J. Leukoc. Biol. 1996, 59, 804–812. [Google Scholar] [CrossRef]
- Khan, W.I.; Motomura, Y.; Wang, H.; El-Sharkawy, R.T.; Verdu, E.F.; Verma-Gandhu, M.; Rollins, B.J.; Collins, S.M. Critical role of MCP-1 in the pathogenesis of experimental colitis in the context of immune and enterochromaffin cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G803–G811. [Google Scholar] [CrossRef] [PubMed]
- Proost, P.; Wuyts, A.; Van Damme, J. Human monocyte chemotactic proteins-2 and -3: Structural and functional comparison with MCP-1. J. Leukoc. Biol. 1996, 59, 67–74. [Google Scholar] [CrossRef]
- Strieter, R.M.; Kunkel, S.L. The immunopathology of chemotactic cytokines. Adv. Exp. Med. Biol. 1993, 351, 19–28. [Google Scholar] [PubMed]
- Mohammadnia-Afrouzi, M.; Hosseini, A.Z.; Khalili, A.; Abediankenari, S.; Amari, A.; Aghili, B.; Nataj, H.H. Altered microRNA expression and immunosuppressive cytokine production by regulatory T cells of ulcerative colitis patients. Immunol. Invest. 2016, 45, 63–74. [Google Scholar] [CrossRef]
- Hume, G.E.; Fowler, E.V.; Lincoln, D.; Eri, R.; Templeton, D.; Florin, T.H.; Cavanaugh, J.A.; Radford-Smith, G.L. Angiotensinogen and transforming growth factor beta1: Novel genes in the pathogenesis of Crohn’s disease. J. Med. Genet. 2006, 43, e51. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Wey, J.; Stoeltzing, O.; Liu, W.; Fan, F.; Bucana, C.; Mansfield, P.F.; Ryan, A.J.; Ellis, L.M. ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor with additional activity against epidermal growth factor receptor tyrosine kinase, inhibits orthotopic growth and angiogenesis of gastric cancer. Mol. Cancer Ther. 2004, 3, 1041–1048. [Google Scholar] [CrossRef]
- Hao, P.; Huang, Y.; Peng, J.; Yu, J.; Guo, X.; Bao, F.; Dian, Z.; An, S.; Xu, T.R. IRS4 promotes the progression of non-small cell lung cancer and confers resistance to EGFR-TKI through the activation of PI3K/Akt and Ras-MAPK pathways. Exp. Cell. Res. 2021, 403, 112615. [Google Scholar] [CrossRef]
- Zhang, J.N.; Sun, M.Z.; Liu, H.; Zhang, H.C.; Xiao, H.; Zhao, Y.; Zhang, C.; Zhao, H.R. The ethanol extract of Periplaneta Americana L. improves ulcerative colitis induced by a combination of chronic stress and TNBS in rats. Acta Cir. Bras. 2022, 37, e370505. [Google Scholar] [CrossRef]
- Sham, H.P.; Bazett, M.; Bosiljcic, M.; Yang, H.; Luk, B.; Law, H.T.; Morampudi, V.; Yu, H.B.; Pankovich, J.; Sutcliffe, S.; et al. Immune stimulation using a gut microbe-based immunotherapy reduces disease pathology and improves barrier function in ulcerative colitis. Front. Immunol. 2018, 9, 2211. [Google Scholar] [CrossRef] [PubMed]
- Bing, X.; Xuelei, L.; Wanwei, D.; Linlang, L.; Keyan, C. EGCG maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-κB signaling pathway in rats. Can. J. Gastroenterol. Hepatol. 2017, 2017, 3057268. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Peña, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed]

| U-B-D-A-I | U-B-I | U-D-A | |||
|---|---|---|---|---|---|
| G1 | G2 | G1 | G2 | G1 | G2 |
| EGFR * | EGFR | ESR2 * | MAPK14 * | SLC6A4 * | MAPK14 |
| PTGS2 * | - | EGFR | PIK3CA * | EGFR | PIK3CA |
| - | - | FYN * | MTOR * | DPP4 * | SLC6A4 |
| - | - | IDO1 * | EGFR | PTGS1 * | MTOR |
| - | - | PTGS2 | AKT1 * | PTGS2 | EGFR |
| - | - | ESR1 * | JAK2 * | NOS2 * | AKT1 |
| - | - | - | JAK3 * | - | JAK2 |
| - | - | - | PARP1 * | - | PARP1 |
| - | - | - | JAK1 * | - | DPP4 |
| - | - | - | SRC * | - | SRC |
| - | - | - | CDK2 * | - | - |
| G1-Uc-Bloody Stool-Diarrhea-Mucosal Microcirculation Disorders-Aquaporin | G2-Uc-Bloody Stool-Diarrhea-Mucosal Microcirculation Disorders-Aquaporin |
|---|---|
| PI3K-Akt signaling pathway | Ras signaling pathway |
| Pathways in cancer | Pathways in cancer |
| Rap1 signaling pathway | Rap1 signaling pathway |
| Focal adhesion | Focal adhesion |
| Proteoglycans in cancer | HIF-1 signaling pathway |
| Ras signaling pathway | MAPK signaling pathway |
| Proteoglycans in cancer | |
| PI3K-Akt signaling pathway |
| Name | Structural Formula | Percentage of Peak Area |
|---|---|---|
| Inosine |  | 0.03180 |
| Adenosine 5′-monophosphate | 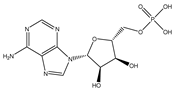 | 0.01004 |
| Vidarabine | 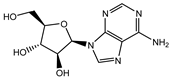 | 0.001655 |
| Adenosine | 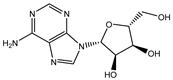 | 0.001655 |
| 5′-Deoxyadenosine | 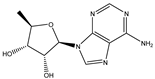 | 0.0004593 |
| Cordycepin | 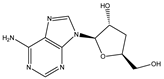 | 0.0004593 |
| Adenosine 2′,3′-cyclic phosphate | 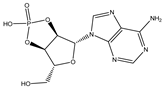 | 0.0004106 |
| 1-Methyladenine | 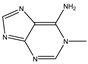 | 0.0001255 |
| N6-(delta 2-Isopentenyl)-adenine |  | 7.0262 × 10−5 |
| N6-isopentenyladenosine | 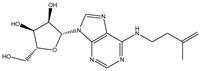 | 4.9368 × 10−5 |
| 5′-S-Methyl-5′-thioadenosine | 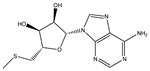 | 1.5003 × 10−5 |
| Time (min) | A% (0.1% FA in Water) | B% (Acetonitrile) |
|---|---|---|
| 0 | 98 | 2 |
| 0.5 | 98 | 2 |
| 10 | 50 | 50 |
| 11 | 5 | 95 |
| 13 | 5 | 95 |
| 13.1 | 98 | 2 |
| 15 | 98 | 2 |
| Weight Loss | Stool Consistency | Bleeding | Score |
|---|---|---|---|
| None | Normal | Normal | 0 |
| 1–5% | Normal | Occult blood (+) | 1 |
| 5–10% | Loose stool | Occult blood (++) | 2 |
| 10–15% | Loose stool | Occult blood (+++) | 3 |
| More than 15% | Diarrhea | Gross bleeding | 4 |
| Infiltration | Epithelium | Score |
|---|---|---|
| No infiltrate | Normal morphology | 0 |
| Infiltrate around crypt basis | Loss of goblet cells | 1 |
| Infiltrate reaching to lamina muscularis mucosae | Loss of goblet cells in large areas | 2 |
| Extensive infiltration reaching the lamina muscularis mucosae and thickening of the mucosa with abundant edema | Loss of crypts | 3 |
| Infiltration of the lamina submucosa | Loss of crypts in large areas | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Shi, Z.-M.; He, Y.; Xi, Z.-W.; Che, Y.-H.; Zhao, H.-R.; Zhang, C.-G.; Liu, H.; Hu, K.-F. Inosine, AMP, and Vidarabine: Network Pharmacology and LC-MS Reveal Key Bioactive Compounds in Periplaneta americana for Ulcerative Colitis Management. Int. J. Mol. Sci. 2025, 26, 5446. https://doi.org/10.3390/ijms26125446
Li Y, Shi Z-M, He Y, Xi Z-W, Che Y-H, Zhao H-R, Zhang C-G, Liu H, Hu K-F. Inosine, AMP, and Vidarabine: Network Pharmacology and LC-MS Reveal Key Bioactive Compounds in Periplaneta americana for Ulcerative Colitis Management. International Journal of Molecular Sciences. 2025; 26(12):5446. https://doi.org/10.3390/ijms26125446
Chicago/Turabian StyleLi, Yue, Zheng-Mei Shi, Yong He, Zu-Wei Xi, Yi-Hao Che, Hai-Rong Zhao, Cheng-Gui Zhang, Heng Liu, and Kong-Fa Hu. 2025. "Inosine, AMP, and Vidarabine: Network Pharmacology and LC-MS Reveal Key Bioactive Compounds in Periplaneta americana for Ulcerative Colitis Management" International Journal of Molecular Sciences 26, no. 12: 5446. https://doi.org/10.3390/ijms26125446
APA StyleLi, Y., Shi, Z.-M., He, Y., Xi, Z.-W., Che, Y.-H., Zhao, H.-R., Zhang, C.-G., Liu, H., & Hu, K.-F. (2025). Inosine, AMP, and Vidarabine: Network Pharmacology and LC-MS Reveal Key Bioactive Compounds in Periplaneta americana for Ulcerative Colitis Management. International Journal of Molecular Sciences, 26(12), 5446. https://doi.org/10.3390/ijms26125446






