Acute Downregulation of Zinc α2-Glycoprotein: Evidence from Human and HepG2 Cell Studies
Abstract
1. Introduction
2. Results
2.1. Clinical and Biochemical Features
2.2. ZAG Serum Levels After Three Insulin Stimulatory Dynamic Tests
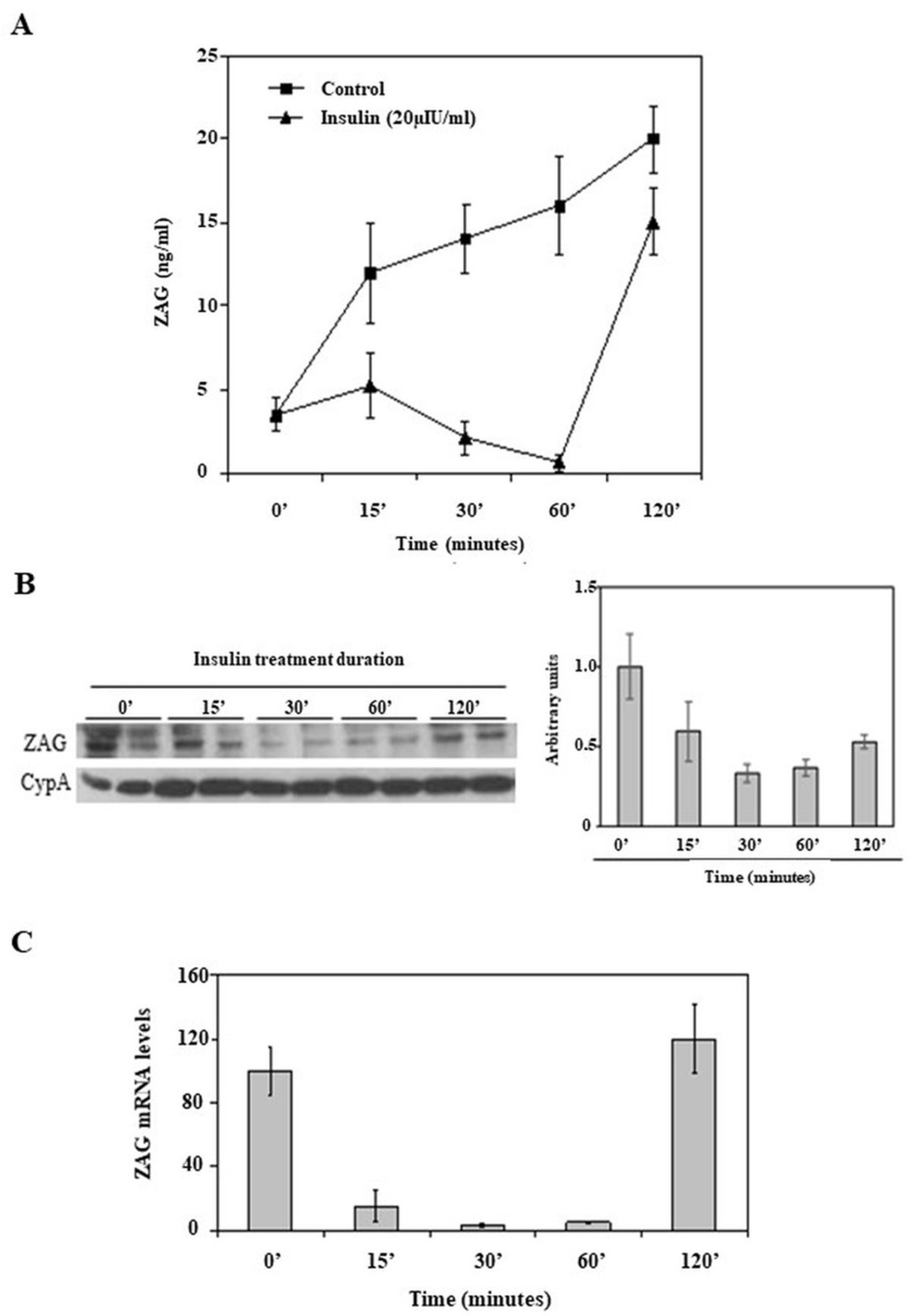
2.3. Effects of Acute Insulin Treatment on ZAG Production in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Laboratory Assessments
4.3. HepG2 Cultures
4.4. Total RNA Preparation and Real-Time PCR
4.5. Western Blot Analysis
4.6. ELISA Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZAG | Zinc-alpha2-glycoprotein |
| OGTT | Oral glucose tolerance test |
| ISDT | Insulin stimulatory dynamic tests |
| IR | Insulin receptor |
| MAPK | Mitogen-activated protein kinase |
| SDS | Sodium dodecyl sulfate |
References
- Sánchez, L.M.; Chirino, A.J.; Bjorkman, P. Crystal structure of human ZAG, a fat-depleting factor related to MHC molecules. Science 1999, 283, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Ohkubo, I.; Niwa, M.; Sasaki, M.; Tateyama, H.; Eimoto, T. Immunohistochemical localization of Zn-α2-glycoprotein in normal human tissues. J. Histochem. Cytochem. 1991, 39, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Selva, D.M.; Lecube, A.; Hernández, C.; Baena, J.A.; Fort, J.M.; Simó, R. Lower zinc-alpha2-glycoprotein production by adipose tissue and liver in obese patients unrelated to insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Liu, X.; Tan, C.; Mo, L.; Wang, H.; Peng, X.; Deng, F.; Chen, L. Expression and Function of Zinc-α2-Glycoprotein. Neurosci. Bull. 2019, 35, 540–550, Erratum in: Neurosci. Bull. 2019, 35, 778. [Google Scholar] [CrossRef]
- Hassan, M.I.; Waheed, A.; Yadav, S.; Singh, T.P.; Ahmad, F. Zinc α2-glycoproteins: A multidisciplinary protein. Mol. Cancer Res. 2008, 6, 892–906. [Google Scholar] [CrossRef]
- Mracek, T.; Stephens, N.A.; Gao, D.; Bao, Y.; Ross, J.A.; Rydén, M.; Arner, P.; Trayhurn, P.; Fearon, K.C.; Bing, C. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br. J. Cancer 2011, 104, 441–447. [Google Scholar] [CrossRef]
- Hansson, S.F.; Puchades, M.; Blennow, K.; Sjögren, M.; Davidsson, P. Validation of a prefractionation method followed by two-dimensional electrophoresis—Applied to cerebrospinal fluid proteins from frontotemporal dementia patients. Proteome Sci. 2004, 2, 7. [Google Scholar] [CrossRef][Green Version]
- García-Ramírez, M.; Canals, F.; Hernández, C.; Colomé, N.; Ferrer, C.; Carrasco, E.; García-Arumí, J.; Simó, R. Proteomic analysis of human vitreous fluid by fluorescence-based difference gel electrophoresis (DIGE): A new strategy for identifying potential candidates in the pathogenesis of proliferative diabetic retinopathy. Diabetologia 2007, 50, 1294–1303. [Google Scholar] [CrossRef]
- Russell, S.T.; Zimmerman, T.P.; Domin, B.A.; Tisdale, M.J. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-α2-glycoprotein. Biochim. Biophys. Acta 2004, 1636, 59–68. [Google Scholar] [CrossRef]
- Bao, Y.; Bing, C.; Hunter, L.; Jenkins, J.R.; Wabitsch, M.; Trayhurn, P. Zinc-alpha2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 2005, 579, 41–47. [Google Scholar] [CrossRef]
- Bing, C.; Trayhurn, P. New insights into adipose tissue atrophy in cancer cachexia. Proc. Nutr. Soc. 2009, 68, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Mracek, T.; Gao, D.; Tzanavari, T.; Bao, Y.; Xiao, X.; Stocker, C.; Trayhurn, P.; Bing, C. Downregulation of zinc-α2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-α in adipocytes. J. Endocrinol. 2010, 204, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cabassi, A.; Tedeschi, S. Zinc-α2-glycoprotein as a marker of fat catabolism in humans. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Gohda, T.; Makita, Y.; Shike, T.; Tanimoto, M.; Funabiki, K.; Horikoshi, S.; Tomino, Y. Identification of epistatic interaction involved in obesity using KK/Ta mouse as a type 2 diabetes model: Is Zn-α2-glycoprotein-1 a candidate gene for obesity? Diabetes 2003, 52, 2175–2181. [Google Scholar] [CrossRef]
- Rolli, V.; Radosavljevic, M.; Astier, V.; Macquin, C.; Castan-Laurell, I.; Visentin, V.; Guigné, C.; Carpéné, C.; Valet, P.; Gilfillan, S.; et al. Lipolysis is altered in MHC class I zinc-(2)-glycoprotein deficient mice. FEBS Lett. 2007, 581, 394–400. [Google Scholar] [CrossRef]
- Russell, S.T.; Hirai, K.; Tisdale, M.J. Role of α3-adrenergic receptors in the action of a tumour lipid mobilizing factor. Br. J. Cancer 2002, 86, 424–428. [Google Scholar] [CrossRef]
- Bing, C.; Bao, Y.; Jenkins, J.; Sanders, P.; Manieri, M.; Cinti, S.; Tisdale, M.J.; Trayhurn, P. Zinc-α2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc. Natl. Acad. Sci. USA 2004, 101, 2500–2505. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Ning, D.; Zhu, H.; Shan, G.; Wang, D.; Ping, B.; Yu, Y.; Yang, H.; Yan, K.; et al. Low Serum ZAG Levels Correlate With Determinants of the Metabolic Syndrome in Chinese Subjects. Front. Endocrinol. 2020, 11, 154. [Google Scholar] [CrossRef]
- Sahin, R.B.; Kilic, F.A.; Hizli, P.; Baykan, O. Serum zinc-alpha-2 glycoprotein and insulin levels and their correlation with metabolic syndrome in patients with rosacea. J. Cosmet. Dermatol. 2023, 22, 645–650. [Google Scholar] [CrossRef]
- Banaszak, M.; Górna, I.; Przysławski, J. Zinc and the innovative Zinc-α2-Glycoprotein adipokine play an important role in lipid metabolism: A critical review. Nutrients 2021, 13, 2023. [Google Scholar] [CrossRef]
- Kurtuluş, E.M.; Kariș, D.; Ercan, A.M.; Konukoğlu, D. Zinc alpha-2 glycoprotein, acylated ghrelin, and zinc levels in prediabetics. In Vivo 2024, 38, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Navarro, I.; Vilchis-Gil, J.; Cossío-Torres, P.E.; Hernández-Mendoza, H.; Klünder-Klünder, M.; Layseca-Espinosa, E.; Galicia-Cruz, O.G.; Rios-Lugo, M.J. Serum Zinc-Alpha-2 Glycoprotein and Zinc Levels and Their Relationship with Insulin Resistance and Biochemical Parameters in Overweight and Obese Children. Biol. Trace Elem. Res. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Gilani, A.; Stoll, L.; Homan, E.A.; Lo, J.C. Adipose Signals Regulating Distal Organ Health and Disease. Diabetes 2024, 73, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Pearsey, H.M.; Henson, J.; Sargeant, J.A.; Davies, M.J.; Khunti, K.; Suzuki, T.; Bowden-Davies, K.A.; Cuthbertson, D.J.; Yates, T.E. Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, W.; Yang, S.; Dai, H.; Xu, S.; Yang, G.; Li, L.; Tang, S.; Wang, Y. Association of metabolic syndrome components with circulating levels of cytokine clusters in young women. Endocr. Connect. 2021, 10, 66–75. [Google Scholar] [CrossRef]
- Elattar, S.; Dimri, M.; Satyanarayana, A. The tumor secretory factor ZAG promotes white adipose tissue browning and energy wasting. FASEB J. 2018, 32, 4727–4743. [Google Scholar] [CrossRef]
- Fan, G.; Dang, X.; Li, Y.; Chen, J.; Zhao, R.; Yang, X. Zinc-α2-glycoprotein promotes browning of white adipose tissue in cold-exposed male mice. Mol. Cell. Endocrinol. 2020, 501, 110669. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, H.; Dai, Y.; Pan, H.; Li, N.; Wang, L.; Yang, H.; Yan, K.; Gong, F. Zinc-α2-Glycoprotein Is Associated with Obesity in Chinese People and HFD-Induced Obese Mice. Front. Physiol. 2018, 9, 62. [Google Scholar] [CrossRef]
- Gong, F.Y.; Zhang, S.J.; Deng, J.Y.; Zhu, H.J.; Pan, H.; Li, N.S.; Shi, Y.F. Zinc-alpha2-glycoprotein is involved in regulation of body weight through inhibition of lipogenic enzymes in adipose tissue. Int. J. Obes. 2009, 33, 1023–1030. [Google Scholar] [CrossRef]
- Mracek, T.; Ding, Q.; Tzanavari, T.; Kos, K.; Pinkney, J.; Wilding, J.; Trayhurn, P.; Bing, C. The adipokine zinc-alpha2-glycoprotein (ZAG) is downregulated with fat mass expansion in obesity. Clin. Endocrinol. 2010, 72, 334–341. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafré, V.; Näf, S.; Escoté, X.; Caubet, E.; Gomez, J.M.; Miranda, M.; Chacon, M.R.; Gonzalez-Clemente, J.M.; Gallart, L.; Gutierrez, C.; et al. Circulating and adipose tissue gene expression of zinc-α2- glycoprotein in obesity: Its relationship with adipokine and lipolytic gene markers in subcutaneous and visceral fat. J. Clin. Endocrinol. Metab. 2009, 94, 5062–5069. [Google Scholar] [CrossRef]
- Balaz, M.; Vician, M.; Janakova, Z.; Kurdiova, T.; Surova, M.; Imrich, R.; Majercikova, Z.; Penesova, A.; Vlcek, M.; Kiss, A.; et al. Subcutaneous adipose tissue zinc-α2-glycoprotein is associated with adipose tissue and whole-body insulin sensitivity. Obesity 2014, 22, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Marrades, M.P.; Martínez, J.A.; Moreno-Aliaga, M.J. ZAG, a lipid mobilizing adipokine, is downregulated in human obesity. J. Physiol. Biochem. 2008, 64, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.X.; Li, G.; Ryan, A.S. Effects of Weight Loss and Aerobic Exercise Training on Adi-Pose Tissue Zinc α2-Glycoprotein and Associated Genes in Obesity. Cells 2023, 12, 2366. [Google Scholar] [CrossRef]
- Schiessel, D.L.; Baracos, V.E. Barriers to cancer nutrition therapy: Excess catabolism of muscle and adipose tissues induced by tumour products and chemotherapy. Proc. Nutr. Soc. 2018, 77, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, A.; Bik, W.; Kalisz, M.; Baranowska-Bik, A. Inflammasome NLRP3 Potentially Links Obesity-Associated Low-Grade Systemic Inflammation and Insulin Resistance with Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 5603. [Google Scholar] [CrossRef]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Iida, K.T.; Shimano, H.; Kawakami, Y.; Sone, H.; Toyoshima, H.; Suzuki, S.; Asano, T.; Okuda, Y.; Yamada, N. Insulin up-regulates tumor necrosis factor-alpha production in macrophages through an extracellular-regulated kinase-dependent pathway. J. Biol. Chem. 2001, 276, 32531–32537. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Patti, M.E.; Butte, A.J.; Crunkhorn, S.; Cusi, K.; Berria, R.; Kashyap, S.; Miyazaki, Y.; Kohane, I.; Costello, M.; Saccone, R.; et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc. Natl. Acad. Sci. USA 2003, 100, 8466–8471. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Dominy, J.E., Jr.; Blättler, S.M.; Jedrychowski, M.P.; Banks, A.S.; Lim, J.H.; Chim, H.; Gygi, S.P.; Puigserver, P. The cAMP/PKA pathway rapidly activates SIRT1 to promote fatty acid oxidation independently of changes in NAD(+). Mol. Cell 2011, 44, 851–863. [Google Scholar] [CrossRef]
- Ceperuelo-Mallafré, V.; Ejarque, M.; Duran, X.; Pachón, G.; Vázquez-Carballo, A.; Roche, K.; Núñez-Roa, C.; Garrido-Sánchez, L.; Tinahones, F.J.; Vendrell, J.; et al. Zinc-α2-Glycoprotein Modulates AKT-Dependent Insulin Signaling in Human Adipocytes by Activation of the PP2A Phosphatase. PLoS ONE 2015, 10, e0129644. [Google Scholar] [CrossRef]
| n | 24 |
| Age (years) | 53.0 ± 16.9 |
| Sex (male/female) | 12/12 |
| BMI (kg/m2) | 24.8 ± 4.9 |
| Fasting glucose (mg/dL) | 100.4 ± 10.8 |
| Insulin (pmol/L) | 11.7 ± 5.5 |
| C-peptide (pmol/L) | 4.2 ± 6.1 |
| 0 min | 30 min | 60 min | 90 min | 120 min | ||
| OGTT | ZAG (µg/mL) | 42.1 ± 18.3 | 38.3 ± 15.8 | 36.5 ± 20.2 | 35.2 ± 19.7 a | 40.4 ± 21.9 |
| Insulin (pmol/L) | 13.3 ± 7.7 | 73.7 ± 43.2 b | 84.3 ± 50.5 b | 118.3 ± 80.7 b | 104.2 ± 70.3 b | |
| C-peptide (pmol/L) | 2.9 ± 0.8 | 6.5 ± 2.7 b | 9.8 ± 4.6 b | 12.2 ± 4.6 b | 12.5 ± 4.3 b | |
| Glucose (mmol/L) | 94.1 ± 7.6 | 167.5 ± 31.0 b | 187.5 ± 44.9 b | 176.0 ± 53.2 b | 150.4 ± 46.0 b | |
| 0 min | 60 min | 90 min | 120 min | 180 min | ||
| Standardized liquid nutritional supplement | ZAG (µg/mL) | 42.3 ± 20.3 | 38.1 ± 18.8 | 37.4 ± 16.5 | 31.5 ± 14.9 a | 38.8 ± 25.6 |
| Insulin (pmol/L) | 13.3 ± 8.1 | 68.2 ± 39.6 b | 51.7 ± 36.1 b | 44.1 ± 37.1 b | 18.3 ± 10.8 a | |
| C-peptide (pmol/L) | 3.3 ± 1.2 | 8.6 ± 3.6 b | 7.5 ± 2.8 b | 7.0 ± 2.9 b | 4.7 ± 1.6 b | |
| Glucose (mmol/L) | 102.4 ± 10.2 | 129.4 ± 27.4 b | 115.2 ± 28.5 a | 104.3 ± 23.8 | 90.0 ± 10.8 b | |
| 0 min | 5 min | 15 min | ||||
| 1 mg Glucagon | ZAG (µg/mL) | 42.2 ± 26.7 | 34.4 ± 17.0 a | 38.2 ± 20.1 | ||
| Insulin (pmol/L) | 11.7 ± 5.5 | 74.4 ± 34.8 b | 50.0 ± 23.4 b | |||
| C-peptide (pmol/L) | 4.2 ± 6.1 | 6.7 ± 3.2 | 5.8 ± 2.6 | |||
| Glucose (mmol/L) | 100.4 ± 10.8 | 113.8 ± 10.0 b | 131.9 ± 16.3 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Masip, È.; Selva, D.M.; Hernández, C.; Ciudin, A.; Salinas-Roca, B.; Cabrera-Serra, J.; Simó, R.; Lecube, A. Acute Downregulation of Zinc α2-Glycoprotein: Evidence from Human and HepG2 Cell Studies. Int. J. Mol. Sci. 2025, 26, 5438. https://doi.org/10.3390/ijms26125438
Navarro-Masip È, Selva DM, Hernández C, Ciudin A, Salinas-Roca B, Cabrera-Serra J, Simó R, Lecube A. Acute Downregulation of Zinc α2-Glycoprotein: Evidence from Human and HepG2 Cell Studies. International Journal of Molecular Sciences. 2025; 26(12):5438. https://doi.org/10.3390/ijms26125438
Chicago/Turabian StyleNavarro-Masip, Èlia, David M. Selva, Cristina Hernández, Andreea Ciudin, Blanca Salinas-Roca, Julia Cabrera-Serra, Rafael Simó, and Albert Lecube. 2025. "Acute Downregulation of Zinc α2-Glycoprotein: Evidence from Human and HepG2 Cell Studies" International Journal of Molecular Sciences 26, no. 12: 5438. https://doi.org/10.3390/ijms26125438
APA StyleNavarro-Masip, È., Selva, D. M., Hernández, C., Ciudin, A., Salinas-Roca, B., Cabrera-Serra, J., Simó, R., & Lecube, A. (2025). Acute Downregulation of Zinc α2-Glycoprotein: Evidence from Human and HepG2 Cell Studies. International Journal of Molecular Sciences, 26(12), 5438. https://doi.org/10.3390/ijms26125438









