Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study
Abstract
1. Introduction
2. Results
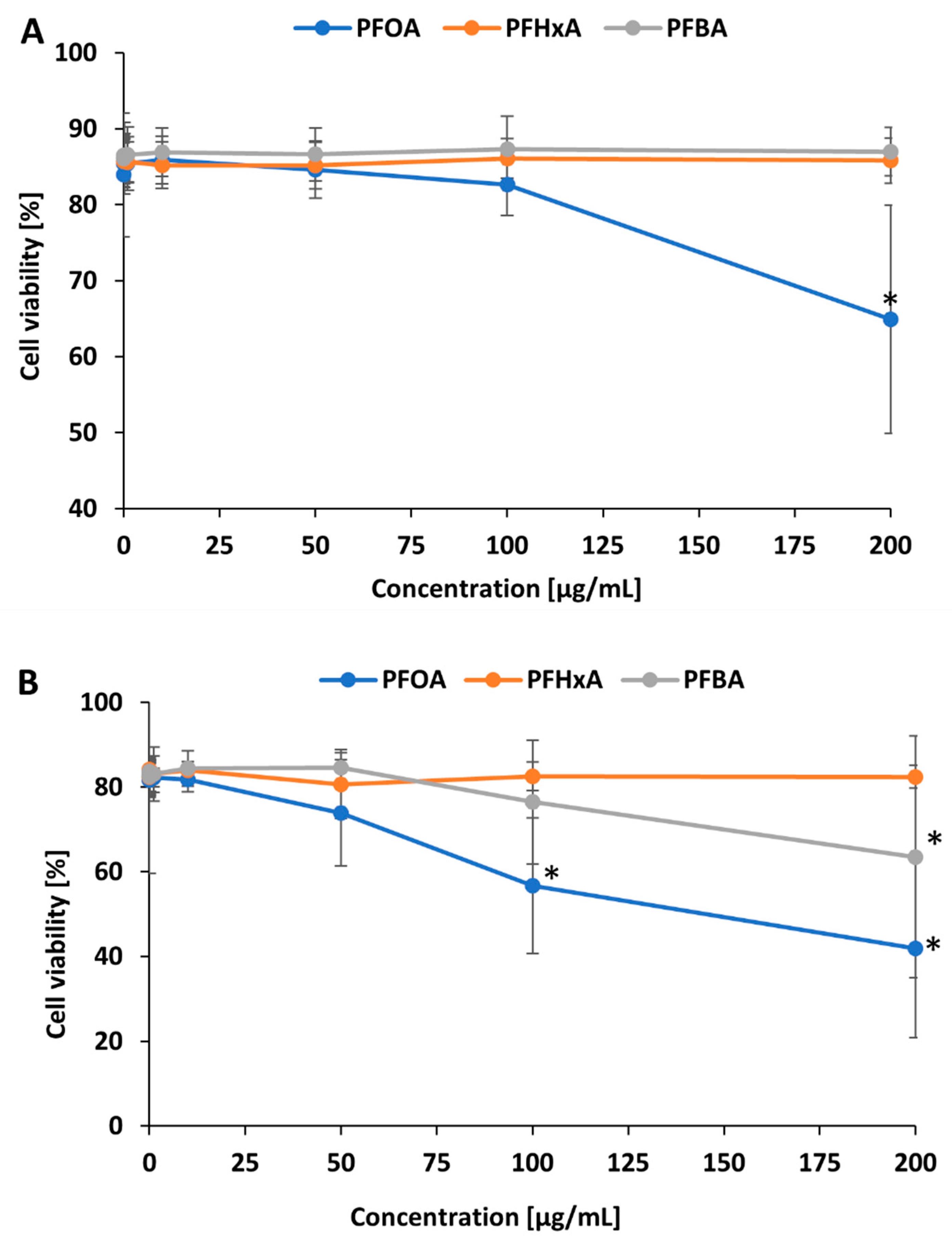
2.1. Cell Viability
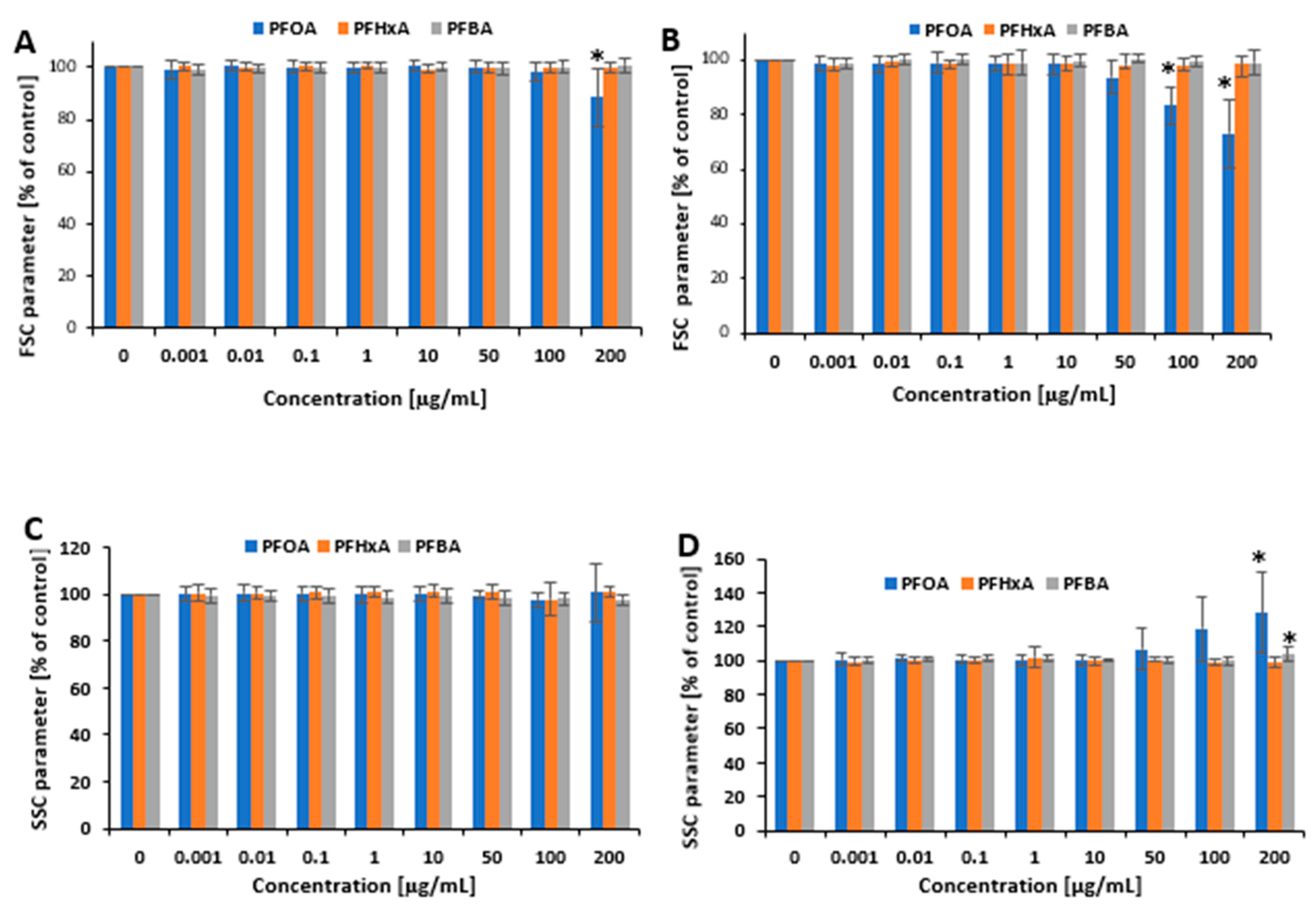
2.2. Cell Morphology
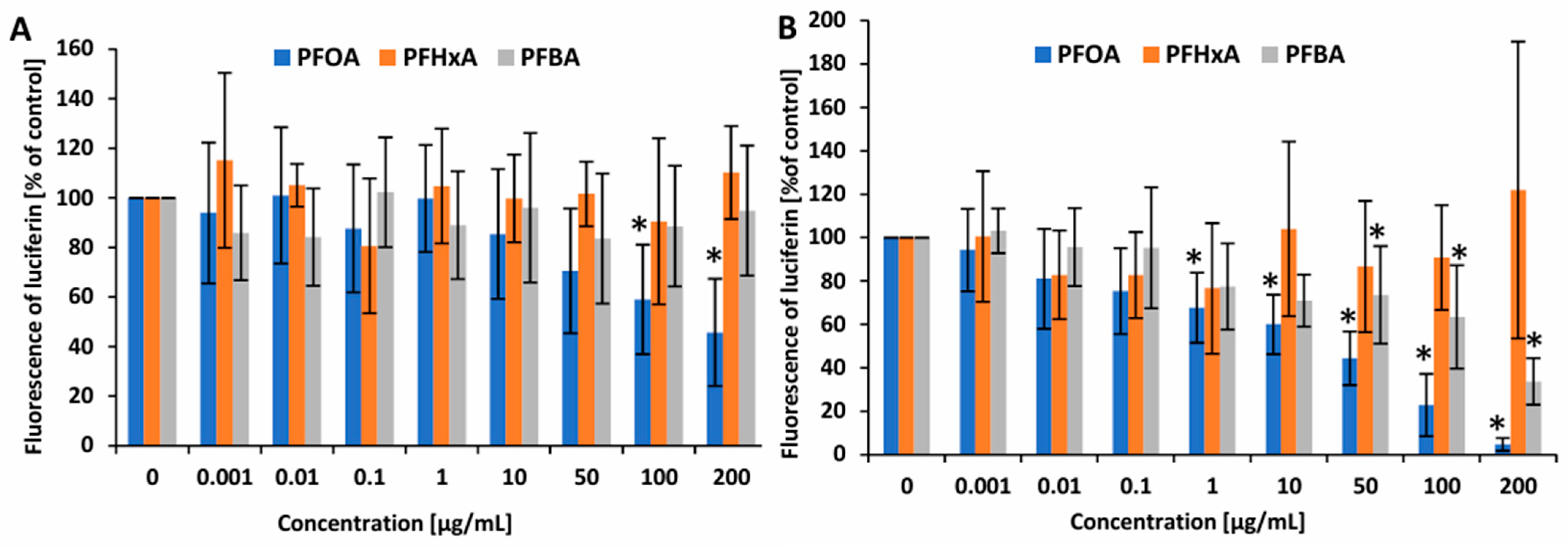
2.3. ATP Level
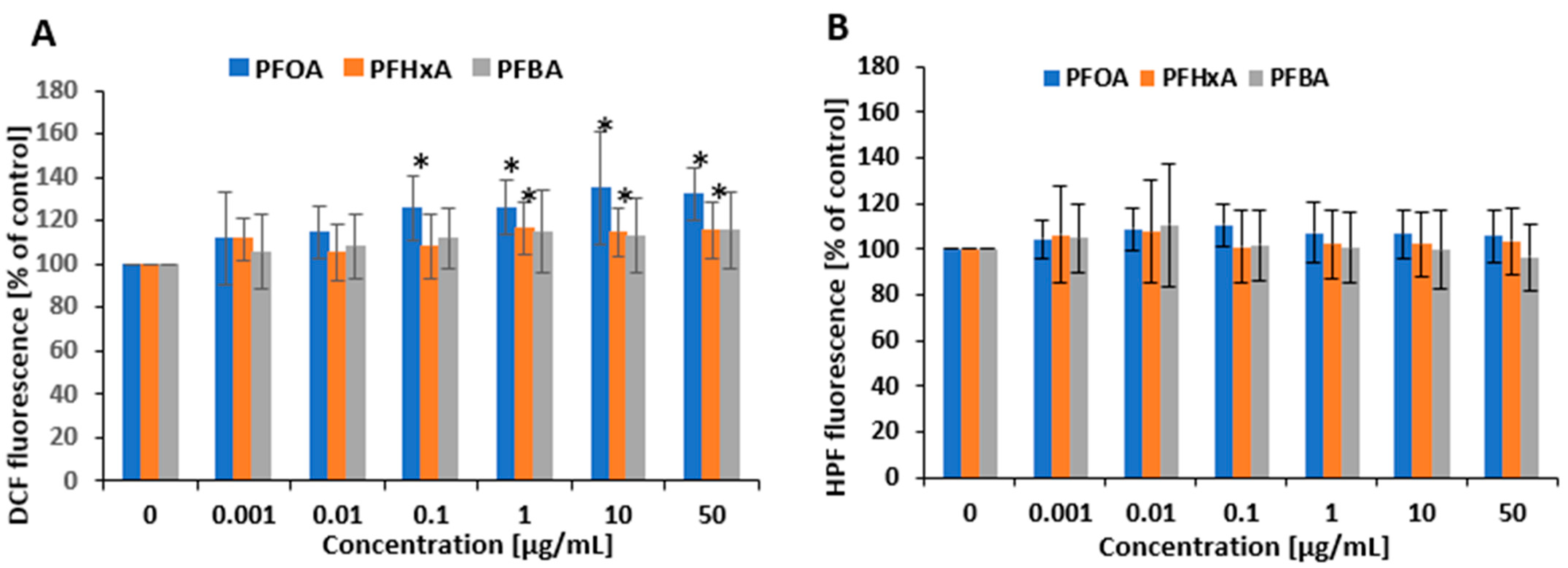
2.4. Oxidative Stress Induction
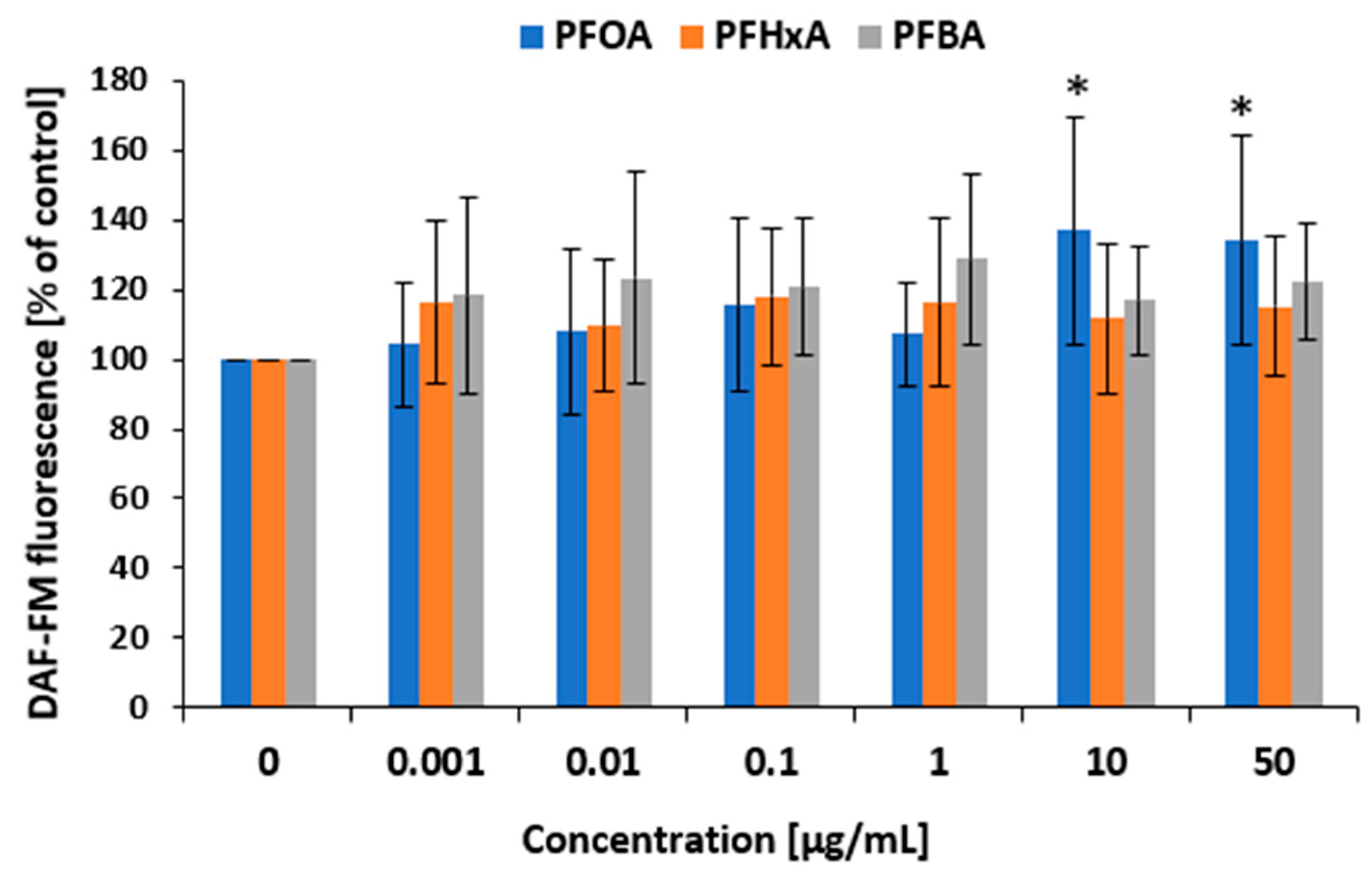
2.5. Nitrosative Stress Induction
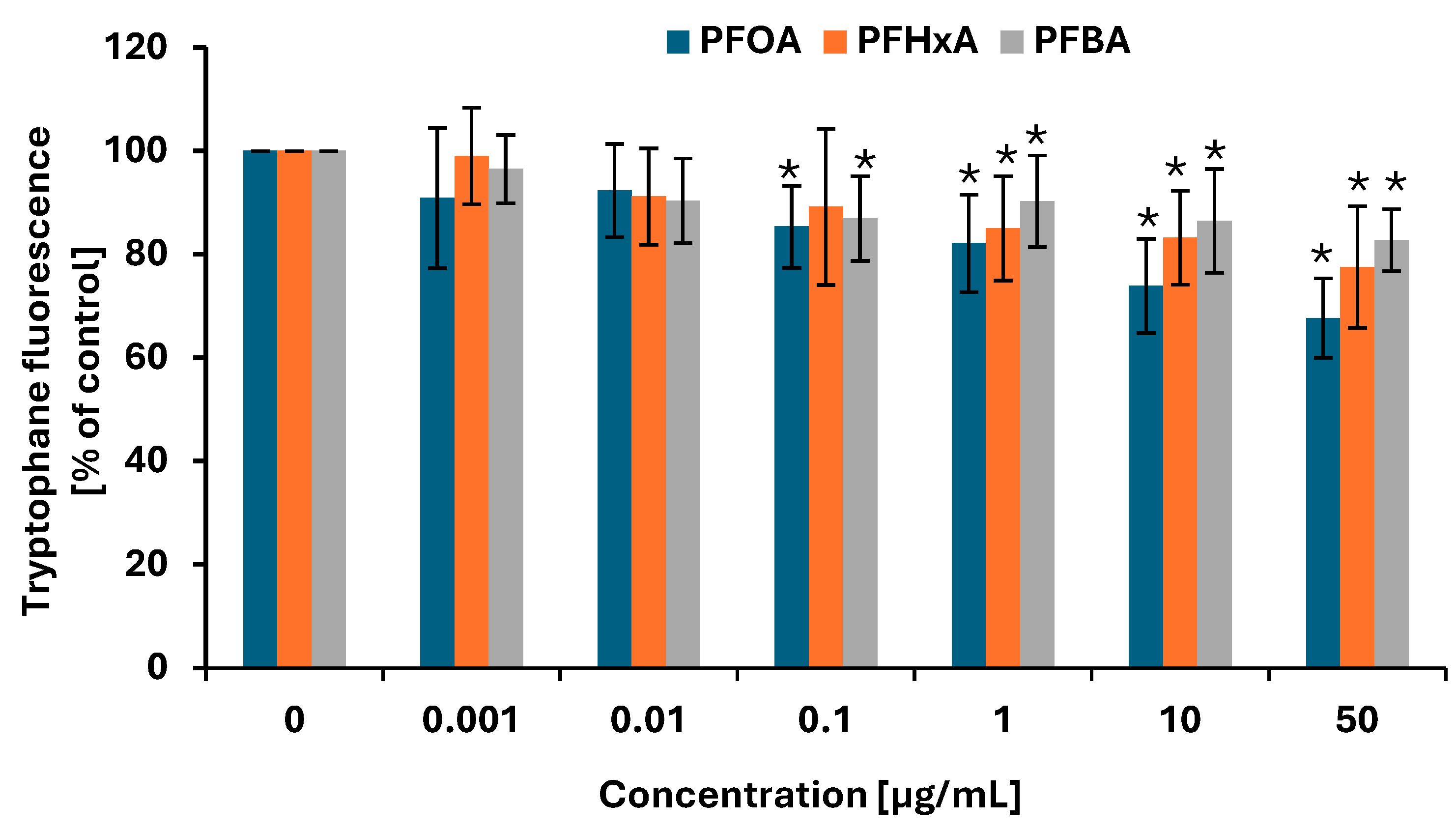
2.6. Protein Damage
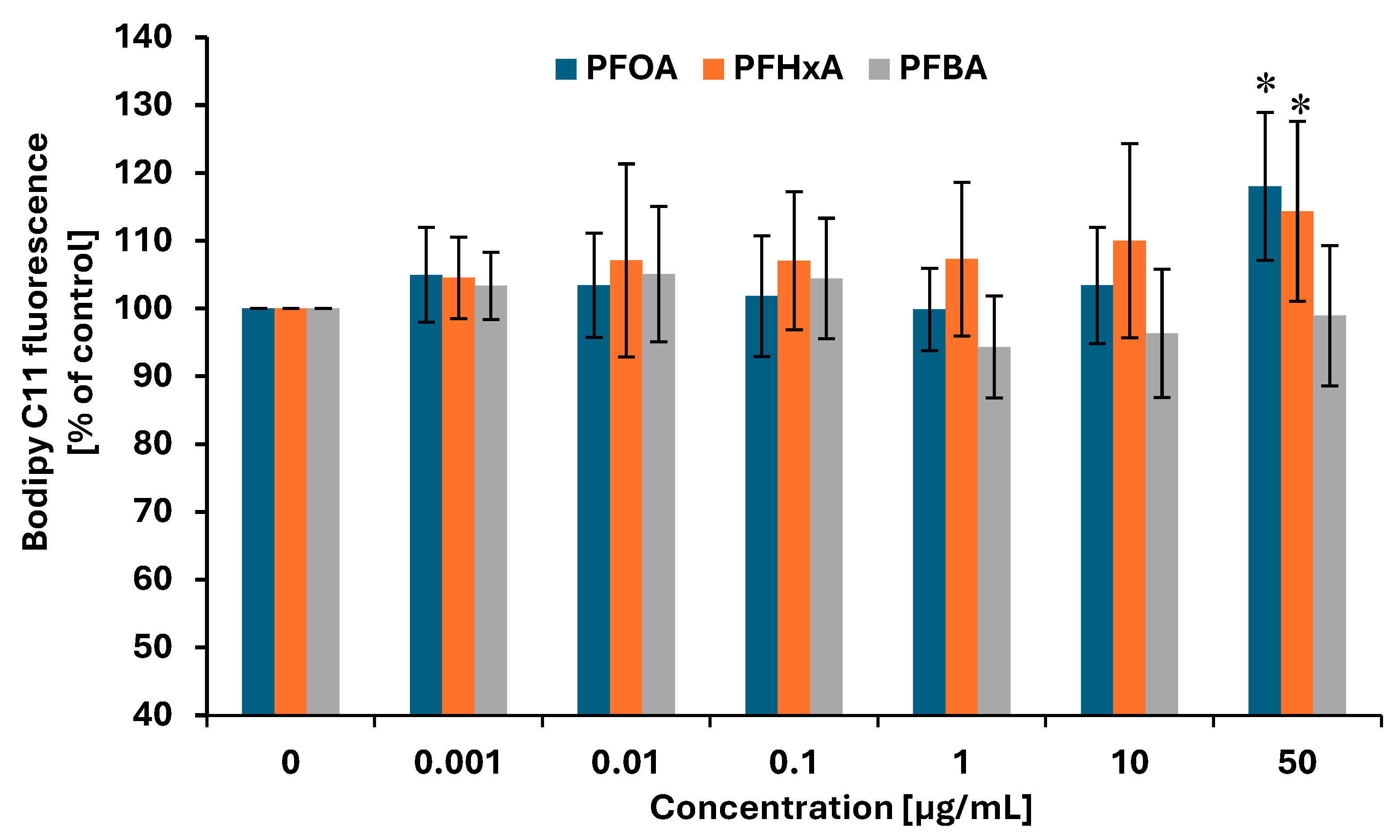
2.7. Lipid Peroxidation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Methods
4.2.1. Cell Isolation and Treatment
4.2.2. Cell Viability
4.2.3. Cell Morphology
4.2.4. ATP Level
4.2.5. ROS Measurement
4.2.6. hROS Measurement
4.2.7. RNS Measurement
4.2.8. Protein Damage
4.2.9. Lipid Peroxidation
4.2.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewapriya, P.; Chadwick, L.; Ghorbani Gorji, S.; Schulze, B.; Valsecchi, S.; Samanipour, S.; Thomas, K.V.; Kaserzon, S.L. Per- and polyfluoroalkyl substances (PFAS) in consumer products: Current knowledge and research gaps. J. Hazard. Mater. Lett. 2023, 4, 100086. [Google Scholar] [CrossRef]
- Trudel, D.; Horowitz, L.; Wormuth, M.; Scheringer, M.; Cousins, I.T.; Hungerbühler, K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008, 28, 251–269. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Gou, X.; Tian, M.; Yan, L.; Xia, P.; Ji, H.; Tan, H.; Shi, W.; Yu, H.; Zhang, X. A novel molecular pathway of lipid accumulation in human hepatocytes caused by PFOA and PFOS. Environ. Int. 2024, 191, 108962. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Bello, A.; Mangano, K.M.; Biswas, K.; Patel, P.P.; Pennoyer, E.H.; Wolever, T.M.S.; Heiger-Bernays, W.J.; Bello, D. Per- and poly-fluoroalkyl substances (PFAS) in circulation in a Canadian population: Their association with serum-liver enzyme biomarkers and piloting a novel method to reduce serum-PFAS. Environ. Health 2025, 24, 10. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Ma, X.; Griffin, N.; Liu, H. Perfluorooctanoic acid (PFOA) exposure induces renal filtration and reabsorption disorders via down-regulation of aquaporins. Toxicol. Lett. 2024, 392, 22–35. [Google Scholar] [CrossRef]
- Jang, S.I.; Jo, J.H.; Uwamahoro, C.; Jung, E.J.; Lee, W.J.; Bae, J.W.; Shin, S.; Lee, S.I.; Kim, M.O.; Moon, J.; et al. Role of Rab proteins in PFOA-induced changes in boar sperm motility and capacitation. Reprod. Toxicol. 2024, 130, 108745. [Google Scholar] [CrossRef]
- Ao, J.; Zhang, R.; Huo, X.; Zhu, W.; Zhang, J. Environmental exposure to legacy and emerging per- and polyfluoroalkyl substances and endometriosis in women of childbearing age. Sci. Total Environ. 2024, 10, 167838. [Google Scholar] [CrossRef]
- Zhao, N.; Zhang, X.; Li, Y.; Zhang, H.; Yang, E.; Ding, L.; Liu, Y. Associations between in utero exposure of per- and polyfluoroalkyl substances (PFAS) mixture and anthropometry measures at birth. Environ. Pollut. 2025, 373, 126093. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, L.; Wang, M.; Sun, Q. Associations between Per- and Polyfluoroalkyl Substances Exposures and Blood Lipid Levels among Adults—A Meta-Analysis. Environ. Health Perspect. 2023, 131, 56001. [Google Scholar] [CrossRef]
- Van der Veen, I.; Fiedler, H.; de Boer, J. Assessment of the per-and polyfluoroalkyl substances analysis under the Stockholm Convention—2018/2019. Chemosphere 2023, 313, 137549. [Google Scholar] [CrossRef]
- Vierke, L.; Staude, C.; Biegel-Engler, A.; Drost, W.; Schulte, C. Perfluorooctanoic acid (PFOA)—Main concerns and regulatory developments in Europe from an environmental point of view. Environ. Sci. Eur. 2012, 24, 16. [Google Scholar] [CrossRef]
- Antonopoulou, V.; Meyer, C.; Chadwick, P.; Gibson, B.; Sniehotta, F.F.; Vlaev, I.; Vassova, A.; Goffe, L.; Lorencatto, F.; McKinlay, A.; et al. Understanding healthcare professionals’ responses to patient complaints in secondary and tertiary care in the UK: A systematic review and behavioural analysis using the Theoretical Domains Framework. Health Res. Policy Syst. 2024, 22, 137. [Google Scholar] [CrossRef]
- KEMI (Swedish Chemicals Agency). Report 7/15: Occurrence and Use of Highly Fluorinated Substances and Alternatives. 2022. Available online: https://www.kemi.se/en/publications/reports/2015/report-7-15-occurrence-and-use-of-highly-fluorinated-substances-and-alternatives (accessed on 9 May 2025).
- Solan, M.E.; Lavado, R. The use of in vitro methods in assessing human health risks associated with short-chain perfluoroalkyl and polyfluoroalkyl substances (PFAS). J. Appl. Toxicol. 2022, 42, 1298–1309. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Lu, C.; Wang, X.; Du, Z.; Wang, J.; Li, B.; Wang, J.; Zhu, L. Comparison of the combined toxicity of PFOA and emerging alternatives: A comprehensive evaluation of oxidative damage, apoptosis and immunotoxicity in embryonic and adult zebrafish. Water Res. 2025, 273, 123028. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Li, Q.; Ding, X.; Tian, M.; Ma, Q.; Xu, D. Impacts of PFOS, PFOA and their alternatives on the gut, intestinal barriers and gut-organ axis. Chemosphere 2024, 361, 142461. [Google Scholar] [CrossRef]
- Teaf, C.M.; Garber, M.M.; Covert, D.J.; Tuovila, B.J. Perfluorooctanoic Acid (PFOA): Environmental Sources, Chemistry, Toxicology, and Potential Risks. Soil and Sediment Contamination. Int. J. 2019, 28, 258–273. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Tully, J.S.; Needham, L.L. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: Data from the national health and nutrition examination survey (NHANES). Environ. Sci. Technol. 2007, 41, 2237–2242. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Yang, Y.; Yang, L.; Zhu, Y.; Shan, G.; Zhu, L.; Zhang, S. External and internal human exposure to PFOA and HFPOs around a mega fluorochemical industrial park, China: Differences and implications. Environ. Int. 2021, 157, 106824. [Google Scholar] [CrossRef]
- Xu, Y.; Fletcher, T.; Pineda, D.; Lindh, C.H.; Nilsson, C.; Glynn, A.; Vogs, C.; Norström, K.; Lilja, K.; Jakobsson, K.; et al. Serum Half-Lives for Short-and Long-Chain Perfluoroalkyl Acids after Ceasing Exposure from Drinking Water Contaminated by Firefighting Foam. Environ. Health Perspect. 2020, 128, 77004. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Nilsson, H.; Cousins, I.T. Contribution of Direct and Indirect Exposure to Human Serum Concentrations of Perfluorooctanoic Acid in an Occupationally Exposed Group of Ski Waxers. Environ. Sci. Technol. 2016, 50, 7037–7046. [Google Scholar] [CrossRef]
- Girardi, P.; Merler, E. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ. Res. 2019, 179, 108743. [Google Scholar] [CrossRef]
- Steenland, K.; Fletcher, T.; Savitz, D.A. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 2010, 118, 1100–1108. [Google Scholar] [CrossRef]
- Batzella, E.; Rosato, I.; Pitter, G.; Da Re, F.; Russo, F.; Canova, C.; Fletcher, T. Determinants of PFOA Serum Half-Life after End of Exposure: A Longitudinal Study on Highly Exposed Subjects in the Veneto Region. Environ. Health Perspect. 2024, 132, 27002. [Google Scholar] [CrossRef]
- Lucas, K.; Gaines, L.G.T.; Paris Davila, T.; Nylander French, L.A. Occupational exposure and serum levels of p er and polyfluoroalkyl substances (PFAS): A review. Am. J. Ind. Med. 2023, 66, 379–392. [Google Scholar] [CrossRef]
- Yao, J.; Dong, Z.; Jiang, L.; Pan, Y.; Zhao, M.; Bai, X.; Dai, J. Emerging and Legacy Perfluoroalkyl Substances in Breastfed Chinese Infants: Renal Clearance, Body Burden, and Implications. Environ. Health Perspect. 2023, 131, 37003. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substance s in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Peripheral Blood Mononuclear Cells. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; Chapter 15. [Google Scholar] [PubMed]
- Falcai, A.; Soeiro-Pereira, P.V.; Kubo, C.A.; Aranda, C.S.; Solé, D.; Condino-Neto, A. Peripheral blood mononuclear cells from severe asthmatic children release lower amounts of IL-12 and IL-4 after LPS stimulation. Allergol. Immunopathol. 2015, 43, 482–486. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef]
- Amstutz, V.H.; Sijm, D.T.H.M.; Vrolijk, M.F. Perfluoroalkyl substances and immunotoxicity: An in vitro structure-activity relationship study in THP-1-derived monocytes and macrophages. Chemosphere 2024, 364, 143075. [Google Scholar] [CrossRef]
- Hall, A.S.; Baynes, R.; Neumann, L.M.; Maibach, H.I.; Ormond, R.B. Skin Permeability of Perfluorocarboxylic Acids Using Flow-Through Diffusion on Porcine Skin. Toxics 2024, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, C.T.; Damayanti, N.P.; Guffey, S.C.; Serafin, J.S.; Irudayaraj, J.; Sepúlveda, M.S. Comparative in vitro toxicity assessment of perfluorinated carboxylic acids. J. Appl. Toxicol. 2017, 37, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Wasel, O.; Thompson, K.M.; Freeman, J.L. Assessment of unique behavioral, morphological, and molecular alterations in the comparative developmental toxicity profiles of PFOA, PFHxA, and PFBA using the zebrafish model system. Environ. Int. 2022, 170, 107642. [Google Scholar] [CrossRef]
- Guinn, M.; Bleris, L. Biological 2-input decoder circuit in human cells. ACS Synth. Biol. 2014, 3, 627–633. [Google Scholar] [CrossRef]
- Kang, T.; Moore, R.; Li, Y.; Sontag, E.; Bleris, L. Discriminating direct and indirect connectivities in biological networks. Proc. Natl. Acad. Sci. USA 2015, 112, 12893–12898. [Google Scholar] [CrossRef]
- Li, Y.; Nowak, C.M.; Pham, U.; Nguyen, K.; Bleris, L. Cell morphology-based machine learning models for human cell state classification. NPJ Syst. Biol. Appl. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Shimizu, S.; Tsujimoto, Y. Intracellular ATP levels determinecell death fate by apoptosis or necrosis. Cancer Res. 1997, 57, 1835–1840. [Google Scholar] [PubMed]
- Schütt, F.; Aretz, S.; Auffarth, G.U.; Kopitz, J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5354–5361. [Google Scholar] [CrossRef]
- Karimi, E.; Nikkhah, M.; Hosseinkhani, S. Label-Free and Bioluminescence-Based Nano-Biosensor for ATP Detection. Biosensors 2022, 12, 918. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Tehrani, K.H.M.E.; Hashemzaei, M.; Tabrizian, K.; Shahraki, J.; Hosseini, M.J. Mechanistic approach for the toxic effects of perfluorooctanoicacid on isolated rat liver and brain mitochondria. Hum. Exp. Toxicol. 2015, 34, 985–996. [Google Scholar] [CrossRef]
- Inupakutika, M.A.; Sengupta, S.; Devireddy, A.R.; Azad, R.K.; Mittler, R. The evolution of reactive oxygen species metabolism. J. Exp. Bot. 2016, 67, 5933–5943. [Google Scholar] [CrossRef] [PubMed]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Solan, M.E.; Koperski, C.P.; Senthilkumar, S.; Lavado, R. Short-chain per-and polyfluoralkyl substances (PFAS) effects on oxidative stress biomarkers in human liver, kidney, muscle, and microglia cell lines. Environ. Res. 2023, 223, 115424. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Yamashita, S.; Okamoto, M.; Cooley, M.A.; Ozaki, K.; Everett, E.T.; Suzuki, M. Perfluorooctanoic acid-induced cell death via the dual roles of ROS-MAPK/ERK signaling in ameloblast-lineage cells. Ecotoxicol. Environ. Saf. 2023, 260, 115089. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef]
- Phelps, D.W.; Palekar, A.I.; Conley, H.E.; Ferrero, G.; Driggers, J.H.; Linder, K.E.; Kullman, S.W.; Reif, D.M.; Sheats, M.K.; DeWitt, J.C.; et al. Legacy and emerging per-and polyfluoroalkyl substances suppress the neutrophil respiratory burst. J. Immunotoxicol. 2023, 20, 2176953. [Google Scholar] [CrossRef]
- Obiako, P.C.; Ayisire, S.O.; Sayes, C.M. Impact of perfluorooctanoic acid (PFOA) and perfluorobutanoic acid (PFBA) on oxidative stress and metabolic biomarkers in human neuronal cells (SH-SY5Y). Environ Int. 2024, 190, 108864. [Google Scholar] [CrossRef]
- Haider, M.S.; Jaskani, M.J.; Fang, J. 14—Overproductionof ROS: Underlying molecular mechanism of scavenging and redox signaling. In Biocontrol Agents and Secondary Metabolites; Woodhead Publishing: Cambridge, UK, 2021; pp. 347–382. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J.; Wang, X.; Chen, S.; Zhao, Y.; Gu, F.; Xu, A.; Wu, L. Mutagenicity of PFOA in mammalian cells: Role of mitochondria-dependent reactive oxygen species. Environ. Sci. Technol. 2011, 45, 1638–1644. [Google Scholar] [CrossRef]
- Adedara, I.A.; Abioye, O.O.; Oyedele, G.T.; Ikeji, C.N.; Afolabi, B.A.; Rocha, J.B.T.; Farombi, E.O. Perfluorooctanoic acid induces behavioral impairment and oxidative injury in Nauphoeta cinerea nymphs. Environ. Sci. Pollut. Res. Int. 2023, 30, 110340–110351. [Google Scholar] [CrossRef]
- Wang, G.; Sun, J.; Li, L.; Li, J.; Li, P. Perfluorobutanoic acid triggers metabolic and transcriptional reprogramming in wheat seedlings. Sci. Total Environ. 2024, 927, 172343. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Endirlik, B.Ü.; Eken, A.; Canpınar, H.; Öztürk, F.; Gürbay, A. Perfluorooctanoic acid affects mouse brain and liver tissue through oxidative stress. Arh. Hig. Rada Toksikol. 2022, 73, 148–157. [Google Scholar] [CrossRef]
- Alamo, A.; La Vignera, S.; Mogioì, L.M.; Crafa, A.; Barbagallo, F.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. In-Vitro Effects of Perfluorooctanoic Acid on Human Sperm Function: What Are the Clinical Consequences? J. Clin. Med. 2024, 13, 2201. [Google Scholar] [CrossRef]
- Crebelli, R.; Caiola, S.; Conti, L.; Cordelli, E.; De Luca, G.; Dellatte, E.; Eleuteri, P.; Iacovella, N.; Leopardi, P.; Marcon, F.; et al. Can sustained exposure to PFAS trigger a genotoxic response? A comprehensive genotoxicity assessment in mice after subacute oral administration of PFOA and PFBA. Regul. Toxicol. Pharmacol. 2019, 106, 169–177. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R.; Yin, N.; Faiola, F. The short-chain perfluorinated compounds PFBS, PFHxS, PFBA and PFHxA, disrupt human mesenchymal stem cell self-renewal and adipogenic differentiation. J. Environ. Sci. 2020, 88, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Hu, S.; Xu, T.; Yang, Y.; Cao, M.; Wei, S.; Song, Y.; Han, J.; Yin, D. Insight into the differential toxicity of PFOA and PFBA based on a 3D-cultured MDA-MB-231 cell model. J. Hazard. Mater. 2024, 465, 133499. [Google Scholar] [CrossRef]
- Sobolewski, T.N.; Trousdale, R.C.; Gauvin, C.L.; Lawrence, C.M.; Walker, R.A. Nanomolar PFOA Concentrations Affect Lipid Membrane Structure: Consequences for Bioconcentration Mechanisms. Environ. Sci. Technol. 2025, 59, 709–718. [Google Scholar] [CrossRef]
- Boyd, V.; Cholewa, O.M.; Papas, K.K. Limitations in the Use of Fluorescein Diacetate/Propidium Iodide (FDA/PI) and Cell Permeable Nucleic Acid Stains for Viability Measurements of Isolated Islets of Langerhans. Curr. Trends Biotechnol. Pharm. 2008, 2, 66–84. [Google Scholar] [PubMed]
- Yang, N.-C.; Ho, W.-M.; Chen, Y.-H.; Hu, M.-L. A convenient one step extraction of cellular ATP using boiling water for the luciferin-luciferase assay of ATP. Anal. Biochem. 2012, 306, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Setsukinai, K.I.; Urano, Y.; Katsuko, K.; Majima, H.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2002, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef]
- Vivian, J.T.; Callis, P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001, 80, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J.; Mokra, K.; Bąk, A. Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol. In Vitro 2015, 29, 1464–1472. [Google Scholar] [CrossRef]
- Xiao, L.; Yi, Q. Isolation of adoptively transferred CD8+ T cells in mouse tumor tissues for lipid peroxidation detection. STAR Protoc. 2023, 4, 101945. [Google Scholar] [CrossRef]
- Regulation (EU) 2016/679; General Data Protection Regulation (GDPR). European Parliament and Council: Brussels, Belgium, 2016.
| PFOA | PFHxA | PFBA |
|---|---|---|
| CAS: 335-67-1 | CAS: 307-24-4 | CAS: 375-22-4 |
 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarska, I.; Mokra, K.; Michałowicz, J. Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study. Int. J. Mol. Sci. 2025, 26, 5408. https://doi.org/10.3390/ijms26115408
Kaczmarska I, Mokra K, Michałowicz J. Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study. International Journal of Molecular Sciences. 2025; 26(11):5408. https://doi.org/10.3390/ijms26115408
Chicago/Turabian StyleKaczmarska, Izabela, Katarzyna Mokra, and Jaromir Michałowicz. 2025. "Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study" International Journal of Molecular Sciences 26, no. 11: 5408. https://doi.org/10.3390/ijms26115408
APA StyleKaczmarska, I., Mokra, K., & Michałowicz, J. (2025). Perfluorooctanoic Acid and Its Short-Chain Substitutes Induce Cytotoxic and Prooxidative Changes in Human Peripheral Blood Mononuclear Cells: A Comparative Study. International Journal of Molecular Sciences, 26(11), 5408. https://doi.org/10.3390/ijms26115408






