Alpha1-Antitrypsin in Lung Diseases: A Cross-Sectional Observational Study
Abstract
1. Introduction
2. Results
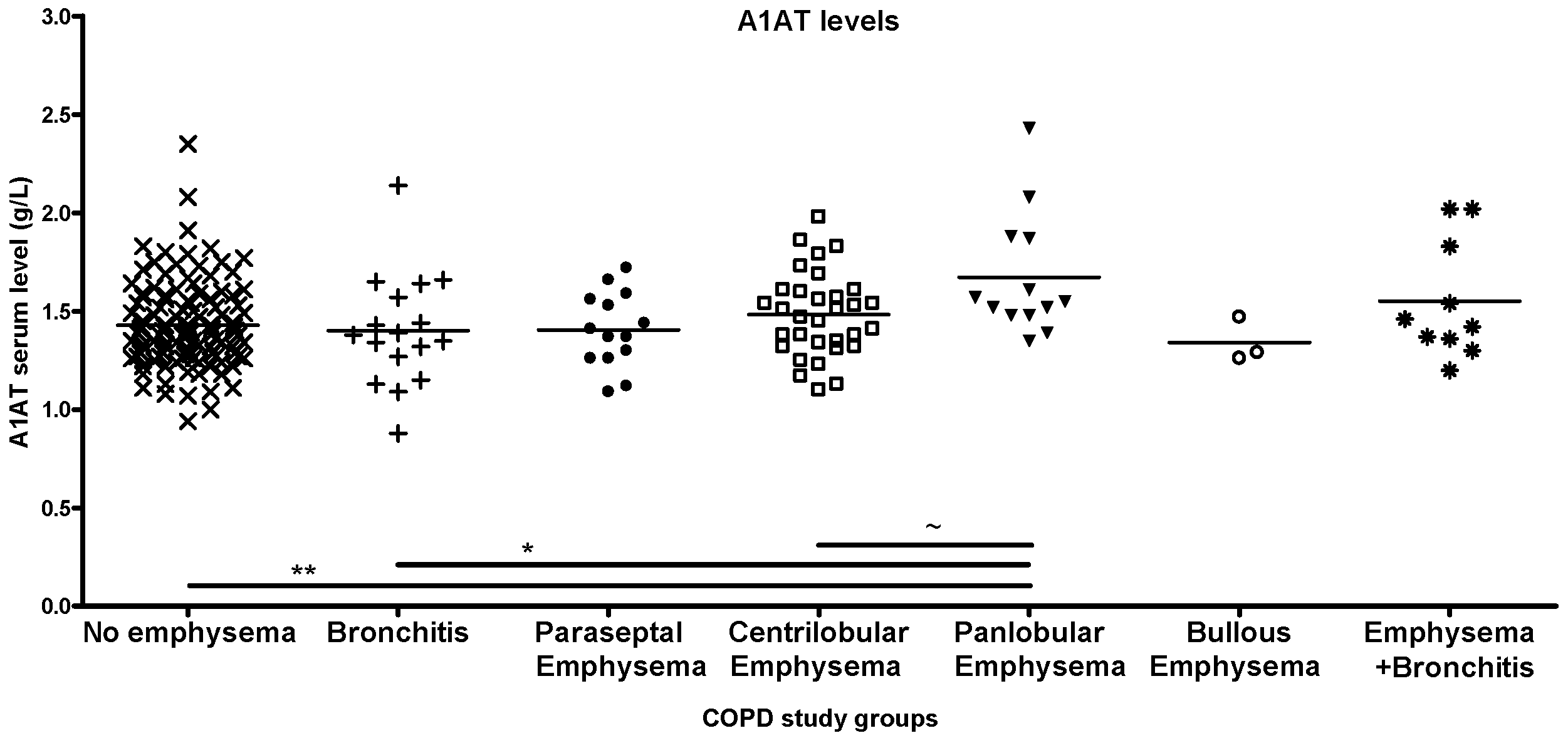
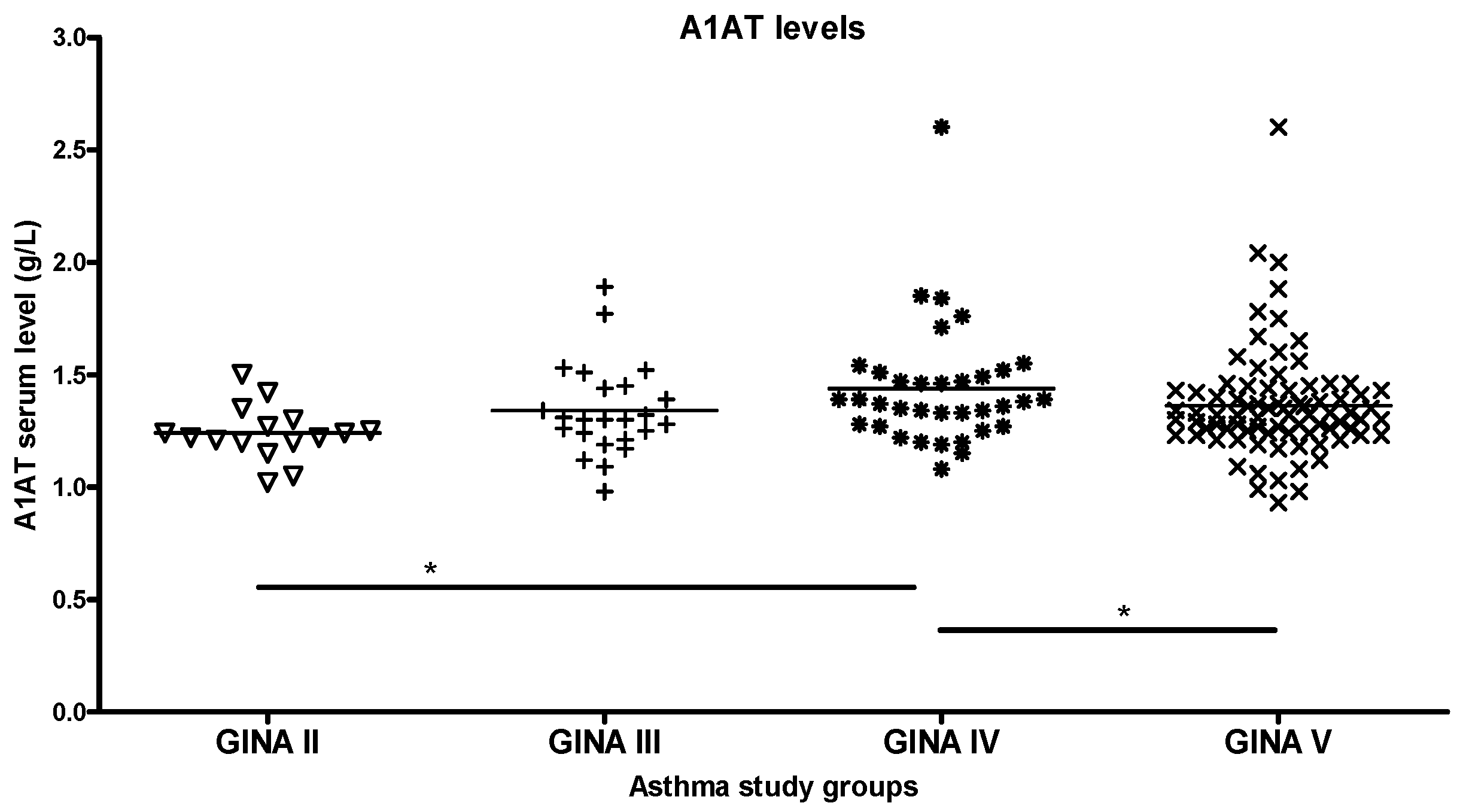
2.1. Groupwise Differences in Blood A1AT Levels
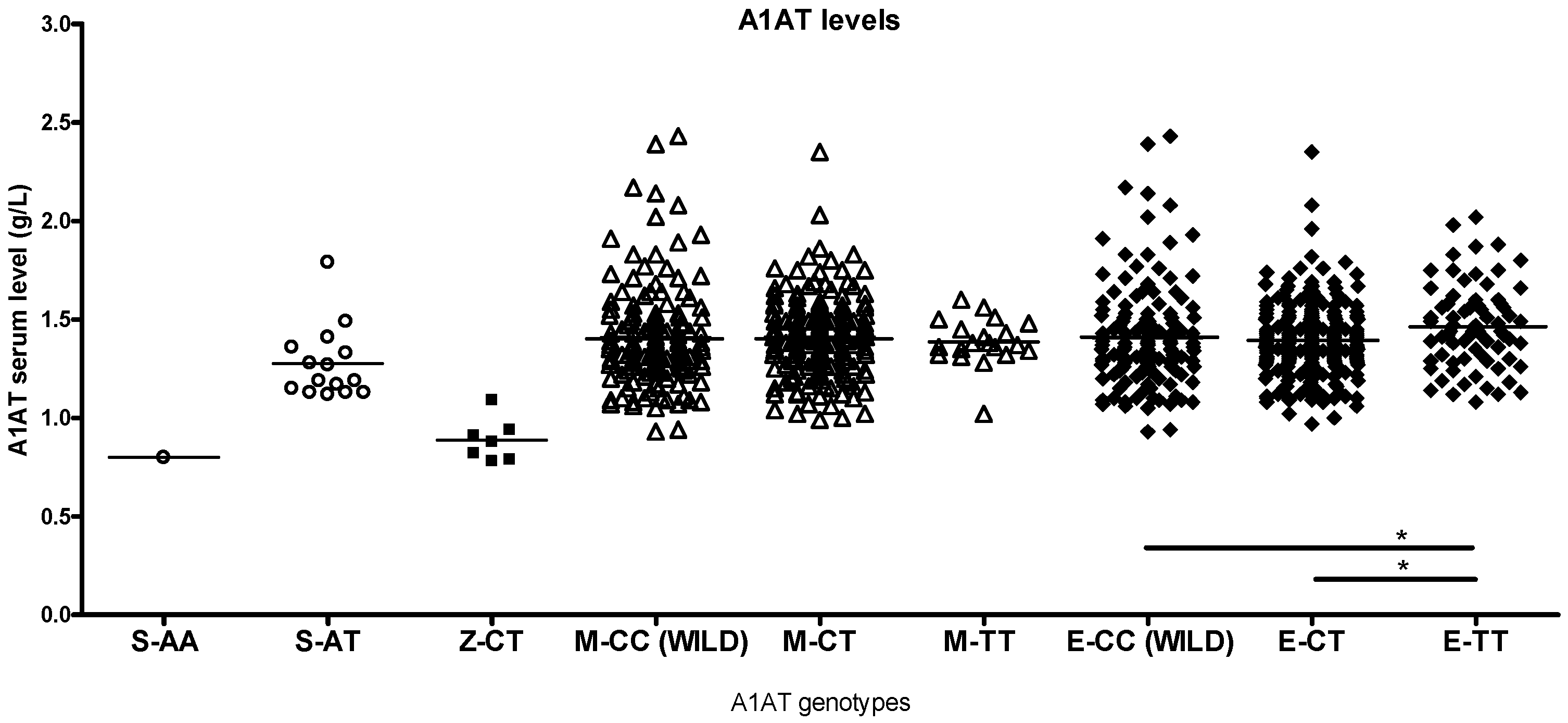
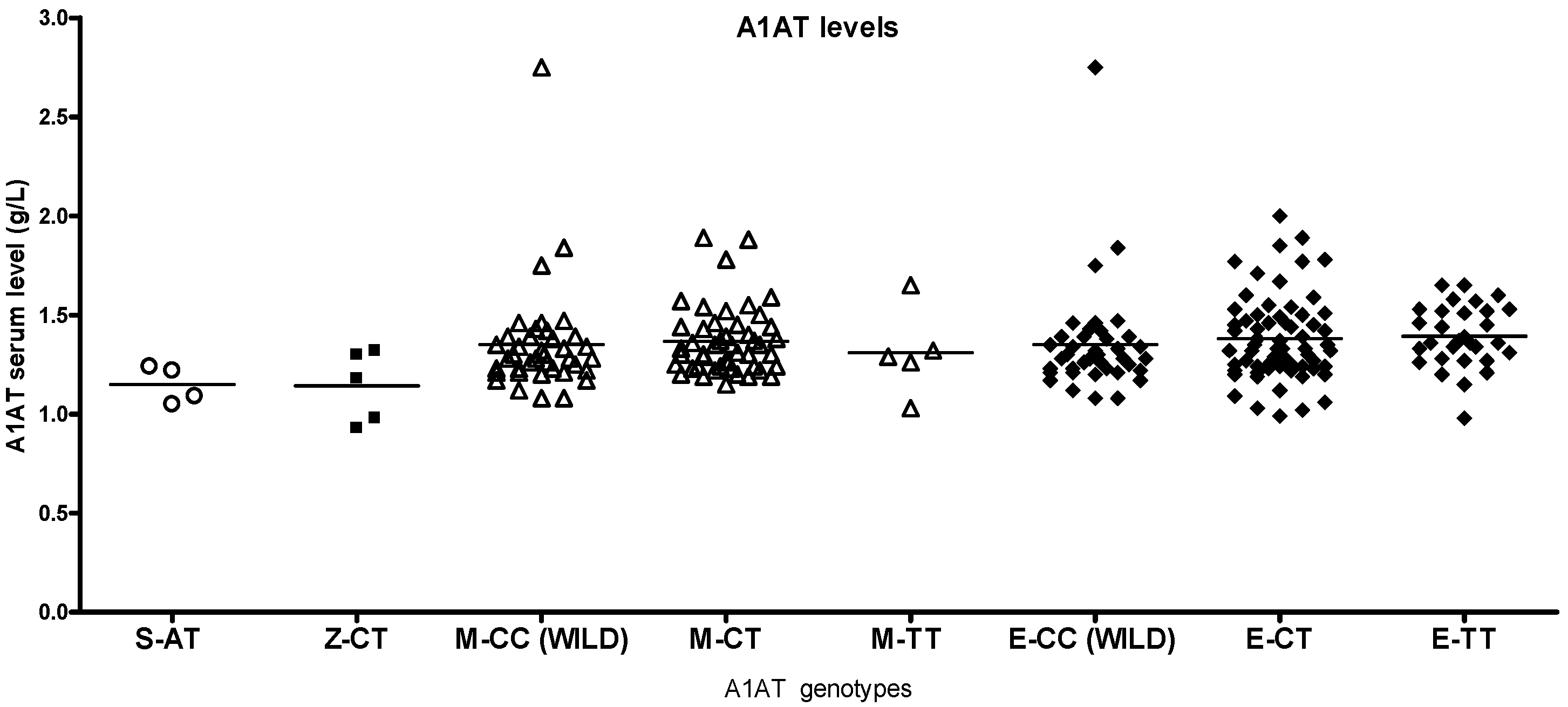
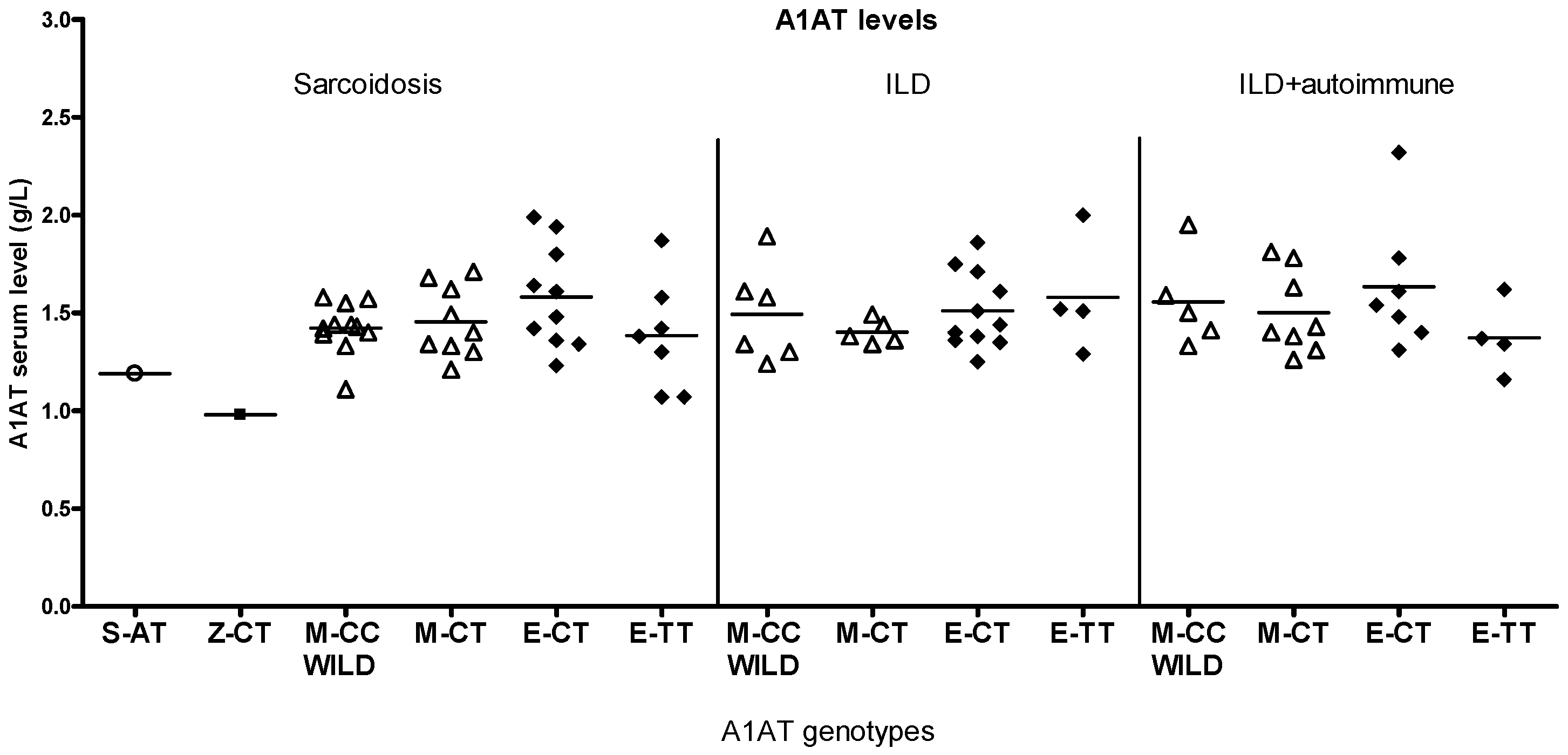
2.2. Differences by Genotype
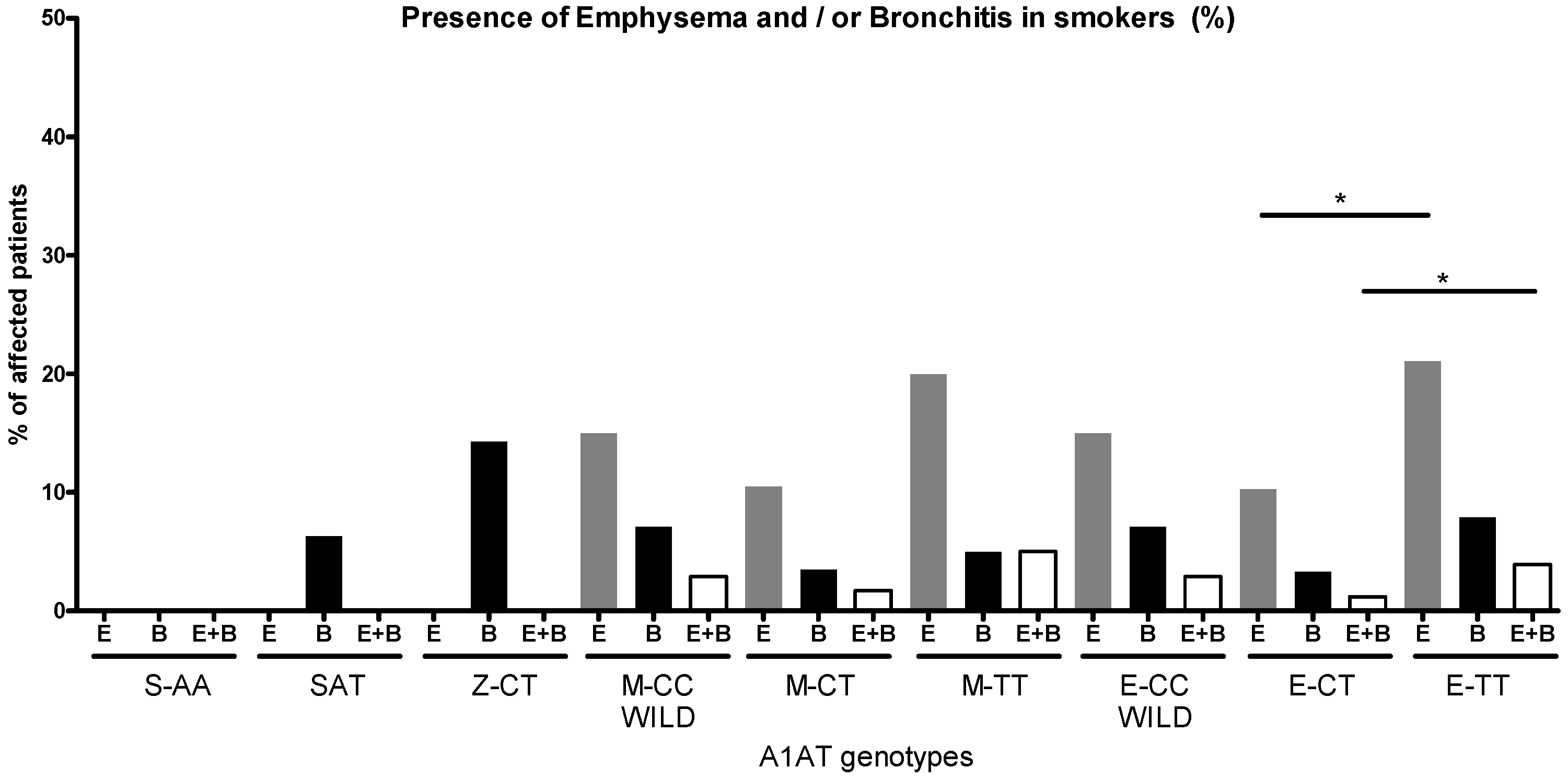
2.3. A1AT Activity
2.4. Correlations
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Low-Dose Computer Tomography
4.3. DNA Isolation
4.4. Real-Time PCR
4.5. A1AT Activity Assay
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Janciauskiene, S.; Welte, T. Well-Known and Less Well-Known Functions of Alpha-1 Antitrypsin. Its Role in Chronic Obstructive Pulmonary Disease and Other Disease Developments. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S4), S280–S288. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, U.; Rudzinski, S.; Jezela-Stanek, A.; Janciauskiene, S.; Chorostowska-Wynimko, J. Post-translational modifications of circulating alpha-1-antitrypsin protein. Int. J. Mol. Sci. 2020, 21, 9187. [Google Scholar] [CrossRef]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Muller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of alpha1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef]
- Voulgari, F.; Cummins, P.; Gardecki, T.I.; Beeching, N.J.; Stone, P.C.; Stuart, J. Serum levels of acute phase and cardiac proteins after myocardial infarction, surgery, and infection. Heart 1982, 48, 352–356. [Google Scholar] [CrossRef]
- Correale, M.; Totaro, A.; Abruzzese, S.; Di Biase, M.; Brunetti, N.D. Acute phase proteins in acute coronary syndrome: An up-to-date. Cardiovasc. Hematol. Agents Med. Chem. 2012, 10, 352–361. [Google Scholar] [CrossRef]
- Mansuy-Aubert, V.; Zhou, Q.L.; Xie, X.; Gong, Z.; Huang, J.Y.; Khan, A.R.; Aubert, G.; Candelaria, K.; Thomas, S.; Shin, D.J.; et al. Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 2013, 17, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Fijalkowska, I.; Zhen, L.; Medler, T.R.; Brown, E.; Cruz, P.; Choe, K.H.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Shapiro, L.; et al. A novel antiapoptotic role for a1-antitrypsin in the prevention of pulmonary emphysema. Am. J. Respir. Crit. Care Med. 2006, 173, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Petrache, I. Molecular multitasking in the airspace: A1-antitrypsin takes on thrombin and plasmin. Am. J. Respir. Cell Mol. Biol. 2007, 37, 130–134. [Google Scholar] [CrossRef]
- Janciauskiene, S. Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2001, 1535, 221–235. [Google Scholar] [CrossRef]
- McCarthy, C.; Saldova, R.; Wormald, M.R.; Rudd, P.M.; McElvaney, N.G.; Reeves, E.P. The role and importance of glycosylation of acute phase proteins with focus on alpha-1 antitrypsin in acute and chronic inflammatory conditions. J. Proteome Res. 2014, 13, 3131–3143. [Google Scholar] [CrossRef]
- Hosokawa, N.; Tremblay, L.O.; You, Z.; Herscovics, A.; Wada, I.; Nagata, K. Enhancement of endoplasmic reticulum (ER) degradation of misfolded Null Hong Kong alpha1-antitrypsin by human ER mannosidase. J. Biol. Chem. 2003, 278, 26287–26294. [Google Scholar] [CrossRef]
- Wu, Y.; Swulius, M.T.; Moremen, K.W.; Sifers, R.N. Elucidation of the molecular logic by which misfolded alpha 1-antitrypsin is preferentially selected for degradation. Proc. Natl. Acad. Sci. USA 2003, 100, 8229–8234. [Google Scholar] [CrossRef]
- Packer, N.H.; Wilkins, M.R.; Golaz, O.; Lawson, M.A.; Gooley, A.A.; Hochstrasser, D.F.; Redmond, J.W.; Williams, K.L. Characterization of human plasma glycoproteins separated by two-dimensional gel electrophoresis. Biotechnology 1996, 14, 66–70. [Google Scholar] [CrossRef]
- Marino, K.; Saldova, R.; Adamczyk, B.; Rudd, P.M. Changes in serum N-glycosylation profiles: Functional significance and potential for diagnostics. Carbohydr. Chem. 2011, 37, 57–93. [Google Scholar]
- Knezevic, A.; Polasek, O.; Gornik, O.; Rudan, I.; Campbell, H.; Hayward, C.; Wright, A.; Kolcic, I.; O’Donoghue, N.; Bones, J.; et al. Variability, heritability and environmental determinants of human plasma N-glycome. J. Proteome Res. 2009, 8, 694–701. [Google Scholar] [CrossRef]
- Komiyama, M.; Wada, H.; Ura, S.; Yamakage, H.; Satoh-Asahara, N.; Shimada, S.; Akao, M.; Koyama, H.; Kono, K.; Shimatsu, A.; et al. The effects of weight gain after smoking cessation on atherogenic a1-antitrypsin–low-density lipoprotein. Heart Vessel. 2015, 30, 734–739. [Google Scholar] [CrossRef]
- Kotani, K.; Yamada, T.; Taniguchi, N. The association between adiponectin, HDL-cholesterol and alpha1-antitrypsin-LDL in female subjects without metabolic syndrome. Lipids. Health. Dis. 2010, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.A.; Ortega-Gomez, A.; Rubio-Navarro, A.; Louedec, L.; Ho-Tin-Noe’, B.; Caligiuri, G.; Nicoletti, A.; Levoye, A.; Plantier, L.; Meilhac, O. High-density lipoproteins potentiate a1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am. J. Respir. Cell Mol. Biol. 2014, 51, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.; Cervantes-Laurean, D.; Kim, G.; McElvaney, N.G.; Wehr, N.; Moss, J.; Levine, R.L. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J. Biol. Chem. 2000, 275, 27258–27265. [Google Scholar] [CrossRef]
- Subramaniyam, D.; Virtala, R.; Pawłowski, K.; Clausen, I.G.; Warkentin, S.; Stevens, T.; Janciauskiene, S. TNF-alpha-induced selfexpression in human lung endothelial cells is inhibited by native and oxidized alpha1-antitrypsin. Int. J. Biochem. Cell Biol. 2008, 40, 258–271. [Google Scholar] [CrossRef]
- Kueppers, F. Alpha1-antitrypsin. Am. J. Hum. Genet. 1973, 25, 677–686. [Google Scholar] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Kohnlein, T.; Welte, T. The discovery of alpha1-antitrypsin and its role in health and disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Belmonte, I.; Nuñez, A.; Farago, G.; Barrecheguren, M.; Pons, M.; Orriols, G.; Gabriel-Medina, G.; Rodríguez-Frías, F.; Marc Miravitlles, M.; et al. New variants of alpha-1-antitrypsin: Structural simulations and clinical expression. Respir. Res. 2022, 23, 339. [Google Scholar] [CrossRef] [PubMed]
- Valiulis, A.; Utkus, A.; Stukas, R.; Valiulis, A.; Siderius, L. Introducing standards of the best medical practice for patients with inherited alpha-1-antitrypsin deficiency in Central Eastern Europe. Prilozi 2014, 35, 106–113. [Google Scholar]
- Laurell, C.B.; Eriksson, S. The electrophoretic α1-globulin pattern of serum in α1-antitrypsin deficiency. COPD J. Chronic Obstr. Pulm. Dis. 2013, 10, 3–8. [Google Scholar] [CrossRef]
- Gadek, J.E.; Klein, H.G.; Holland, P.V.; Crystal, R.G. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J. Clin. Investig. 1981, 68, 1158–1165. [Google Scholar]
- Pierce, J.A. α1-Antitrypsin Augmentation Therapy. Chest 1997, 112, 872–874. [Google Scholar] [CrossRef]
- McElvaney, N.G.; Birrer, P.; Crystal, R.G.; Chernick, M.S.; Frank, M.M.; Caplan, D.B. Aerosol α1 -antitrypsin treatment for cystic fibrosis. Lancet 1991, 337, 392–394. [Google Scholar] [CrossRef]
- Allen, E.D. Opportunities for the Use of Aerosolized α1-Antitrypsin for the Treatment of Cystic Fibrosis. Chest 1996, 110, 256S–260S. [Google Scholar] [CrossRef]
- Mulkareddy, V.; Roman, J. Pulmonary manifestations of alpha 1 antitrypsin deficiency. Am. J. Med. Sci. 2024, 368, 1–8. [Google Scholar] [CrossRef]
- Blanco, I.; Bueno, P.; Diego, I.; Perez-Holand, S.; Casas-Maldonado, F.; Esquinas, C.; Miravitlles, M. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: An update. Int. J. Chron. Obstr. Pulmon. Dis. 2017, 12, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; de Serres, F.J.; Fernandez-Bustillo, E.; Lara, B.; Miravitlles, M. Estimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countries. Eur. Respir. J. 2006, 27, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, D.C. Alpha 1-antitrypsin deficiency in Europe: Geographical distribution of Pi types S and Z. Respir. Med. 1998, 92, 367–377. [Google Scholar] [CrossRef]
- Stockley, R.A.; Parr, D.G. Antitrypsin deficiency: Still more to learn about the lung after 60 years. ERJ Open Res. 2024, 10, 00139–02024. [Google Scholar] [CrossRef]
- Hernandez, P.; Bossé, Y.; Bush, P.; Chapman, K.R.; Maltais, F.; Penz, E.D.; Walker, B.L.; Lal, A.; Marciniuk, D.D. Alpha-1-Antitrypsin Deficiency Targeted Testing and Augmentation Therapy: A Canadian Thoracic Society Meta-Analysis and Clinical Practice Guideline. Chest 2025, 167, 1044–1063. [Google Scholar] [CrossRef] [PubMed]
- Seixas, S.; Marques, P.I. Known mutations at the cause of alpha-1 antitrypsin deficiency an updated overview of SERPINA1 variation spectrum. Appl. Clin. Genet. 2021, 14, 173–194. [Google Scholar] [CrossRef]
- Martín-González, E.; Hernández-Pérez, J.M.; Pérez, J.A.P.; Pérez-García, J.; Herrera-Luis, E.; González-Pérez, R.; González-González, O.; Mederos-Luis, E.; Sánchez-Machín, I.; Poza-Guedes, P.; et al. Alpha-1 antitrypsin deficiency and Pi*S and Pi*Z SERPINA1 variants are associated with asthma exacerbations. Pulmonology 2025, 31, 2416870. [Google Scholar] [CrossRef]
- Larsen, L.M.; Winther, S.V.; Kørvel-Hanquist, A.; Marott, S.C.W.; Landt, E.M.; Homøe, P.; Nordestgaard, B.G.; Dahl, M. Alpha-1 antitrypsin deficiency and risk of sleep apnea: A nationwide cohort study. Eur. Arch. Oto-Rhino-Laryngol. 2025, 282, 2679–2686. [Google Scholar] [CrossRef]
- Ortega, V.E.; Tejwani, V.; Shrivastav, A.K.; Pasha, S.; Zein, J.G.; Boorgula, M.; Castro, M.; Denlinger, L.; Erzurum, S.C.; Fahy, J.V.; et al. Alpha1-Antitrypsin Gene Variation Associates with Asthma Exacerbations and Related Health Care Utilization. J. Allergy Clin. Immunol. Pract. 2025, 13, S2213-2198(25)00164-3. [Google Scholar]
- Oriano, M.; Amati, F.; Gramegna, A.; De Soyza, A.; Mantero, M.; Sibila, O.; Chotirmall, S.H.; Voza, A.; Marchisio, P.; Blasi, F.; et al. Protease-Antiprotease Imbalance in Bronchiectasis. Int. J. Mol. Sci. 2021, 22, 5996. [Google Scholar] [CrossRef]
- Frangolias, D.D.; Ruan, J.; Wilcox, P.J.; Davidson, A.G.F.; Wong, L.T.K.; Berthiaume, Y.; Hennessey, R.; Freitag, A.; Pedder, L.; Corey, M.; et al. Alpha 1-antitrypsin deficiency alleles in cystic fibrosis lung disease. Am. J. Respir. Cell Mol. Biol. 2003, 29 Pt 1, 390–396. [Google Scholar] [CrossRef]
- Amati, F.; Gramegna, A.; Contarini, M.; Stainer, A.; Curcio, C.; Aliberti, S.; Corsico, A.G.; Blasi, F. Genetic and Serum Screening for Alpha-1-Antitrypsin Deficiency in Adult Patients with Cystic Fibrosis: A Single-Center Experience. Biomedicines 2022, 10, 3248. [Google Scholar] [CrossRef] [PubMed]
- Döring, G. Serine proteinase inhibitor therapy in alpha (1)-antitrypsin inhibitor deficiency and cystic fibrosis. Pediatr. Pulmonol. 1999, 28, 363–375. [Google Scholar] [CrossRef]
- Pini, L.; Paoletti, G.; Heffler, E.; Tantucci, E.; Puggioni, F. Asthma and alpha1-antitrypsin research group. Alpha1-antitrypsin deficiency and asthma. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Aiello, M.; Frizzelli, A.; Pisi, R.; Fantin, A.; Ghirardini, M.; Marchi, L.; Ferrarotti, I.; Bertorelli, G.; Percesepe, A.; Chetta, A. Alpha-1 antitrypsin deficiency is significantly associated with atopy in asthmatic patients. J. Asthma 2022, 59, 23–30. [Google Scholar] [CrossRef]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 1753465817750524. [Google Scholar] [CrossRef]
- Papaporfyriou, A.; Bartziokas, K.; Gompelmann, D.; Idzko, M.; Fouka, E.; Zaneli, S.; Bakakos, P.; Loukides, S.; Papaioannou, A.I. Cardiovascular diseases in COPD: From diagnosis and prevalence to therapy. Life 2023, 13, 1299. [Google Scholar] [CrossRef]
- Zhang, D.X.; Gutterman, D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2023–H2031. [Google Scholar] [CrossRef]
- Ketelhuth, D.F.J.; Hansson, G.K. Cellular immunity, low-density lipoprotein and atherosclerosis: Break of tolerance in the artery wall. Thromb. Haemost. 2011, 106, 779–786. [Google Scholar] [CrossRef]
- Jaimes, E.A.; DeMaster, E.G.; Tian, R.; Raij, L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arter. Thromb. Vasc. Biol. 2004, 24, 1031–1036. [Google Scholar] [CrossRef]
- Steffen, Y.; Vuillaume, G.; Stolle, K.; Roewer, K.; Lietz, M.; Schueller, J.; Lebrun, S.; Wallerath, T. Cigarette smoke and LDL cooperate in reducing nitric oxide bioavailability in endothelial cells via effects on both eNOS and NADPH oxidase. Nitric Oxide 2012, 27, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Dirksen, A.; Ferrarotti, I.; Koblizek, V.; Lange, P.; Mahadeva, R.; McElvaney, N.G.; Parr, D.; Piitulainen, E.; Roche, N.; et al. European Respiratory Society statement: Diagnosis and treatment of pulmonary disease in α1-antitrypsin deficiency. Eur. Respir. J. 2017, 50, 1700610. [Google Scholar] [CrossRef]
- Stoller, J.K.; Hupertz, V.; Aboussouan, L.S. Alpha-1 Antitrypsin Deficiency. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- North, T.L.; Ben-Shlomo, Y.; Cooper, C.; Deary, I.J.; Gallacher, J.; Kivimaki, M.; Kumari, M.; Martin, R.M.; Pattie, A.; Sayer, A.A.; et al. A study of common Mendelian disease carriers across ageing British cohorts: Meta-analyses reveal heterozygosity for alpha 1-antitrypsin deficiency increases respiratory capacity and height. J. Med. Genet. 2016, 53, 280–288. [Google Scholar] [CrossRef]
- Lobritto, S.J.; Lewin, B.; Behari, A.; Abrahams, J.; Decklebaum, R.J.; Sturley, S.L. In vitro and in vivo interaction of alpha-1-antitrypsin with low density lipoprotein, implications for atherosclerosis. Circulation 1998, 98, 108. [Google Scholar]
- Mashiba, S.; Wada, Y.; Takeya, M.; Sugiyama, A.; Hamakubo, T.; Nakamura, A.; Noguchi, N.; Niki, E.; Izumi, A.; Kobayashi, M.; et al. In vivo complex formation of oxidized alpha (1)-antitrypsin and LDL. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1801–1808. [Google Scholar] [CrossRef][Green Version]
- Syvanne, M.; Taskinen, M.R.; Nieminen, M.S.; Manninen, V.; Kesaniemi, Y.A.; Pasternack, A.; Nawrocki, J.W.; Haber, H.; Frick, M.H. A study to determine the response of coronary atherosclerosis to raising low high density lipoprotein cholesterol with a fibric acid derivative in men after coronary bypass surgery: The rationale, design, and baseline characteristics of the LOCAT Study. Control. Clin. Trials 1997, 18, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.; Stewart, D.; Hosking, J.D. Baseline characteristics of the study population in the Diabetes Atherosclerosis Intervention Study (DAIS). World Health Organization Collaborating Centre for the Study of Atherosclerosis in Diabetes. Am. J. Cardiol. 1999, 84, 1004–1010. [Google Scholar] [CrossRef]
- Talmud, P.J.; Martin, S.; Steiner, G.; Flavell, D.M.; Whitehouse, D.B.; Nagl, S.; Jackson, R.; Taskinen, M.R.; Frick, M.H.; Nieminen, M.S.; et al. Progression of atherosclerosis is associated with variation in the alpha1-antitrypsin gene. Arter. Thromb. Vasc. Biol. 2003, 23, 644–649. [Google Scholar] [CrossRef]
- Dahl, M.; Tybjaerg-Hansen, A.; Sillesen, H.; Jensen, G.; Steffensen, R.; Nordestgaard, B.G. Blood pressure, risk of ischemic cerebrovascular and ischemic heart disease, and longevity in alpha (1)-antitrypsin deficiency: The Copenhagen City Heart Study. Circulation 2003, 107, 747–752. [Google Scholar] [CrossRef]
- Nishioka, T.; Uchida, K.; Meno, K.; Ishii, T.; Aoki, T.; Imada, Y.; Makino, Y.; Hirata, K.; Matsumoto, Y.; Arinami, T.; et al. Alpha-1-antitrypsin and complement component C7 are involved in asthma exacerbation. Proteom. Clin. Appl. 2008, 2, 46–54. [Google Scholar] [CrossRef]
- Siri, D.; Farah, H.; Hogarth, D.K. Distinguishing alpha1-antitrypsin deficiency from asthma. Ann. Allergy Asthma Immunol. 2013, 111, 458–464. [Google Scholar] [CrossRef]
- Stirpe, E.; Bardaro, F. Alpha1-antitrypsin deficiency and asthma. Monaldi Arch. Chest Dis. 2022, 92, 4. [Google Scholar] [CrossRef]
- DeLuca, D.S.; Poluzioroviene, E.; Taminskiene, V.; Wrenger, S.; Utkus, A.; Valiulis, A.; Alasevičius, T.; Henderson, J.; Bush, A.; Welte, T.; et al. Pediatr Pulmonol. SERPINA1 gene polymorphisms in a population-based ALSPAC cohort. Pediatr. Pulmonol. 2019, 54, 1474–1478. [Google Scholar] [CrossRef]
- Li, L.; Chong, H.C.; Ng, S.Y.; Kwok, K.W.; Teo, Z.; Tan, E.H.; Choo, C.C.; Seet, J.E.; Choi, H.W.; Buist, M.L.; et al. Angiopoietin-like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep. 2015, 10, 654–663. [Google Scholar] [CrossRef]
- Alam, S.; Li, Z.; Janciauskiene, S.; Mahadeva, R. Oxidation of Z a1-antitrypsin by cigarette smoke induces polymerization: A novel mechanism of early-onset emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 45, 261–269. [Google Scholar] [CrossRef]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef]
- Brunetti, N.D.; Padalino, R.; De Gennaro, L.; Cuculo, A.; Ziccardi, L.; Pellegrino, P.L.; Di Biase, M. Acute phase proteins activation in subjects with coronary atherosclerosis and micro-vessel coronary circulation impairment. J. Thromb. Thrombolysis 2009, 28, 50–56. [Google Scholar] [CrossRef]
- Vila, N.; Millán, M.; Ferrer, X.; Riutort, N.; Escudero, D. Levels of alpha1-antitrypsin in plasma and risk of spontaneous cervical artery dissections: A case–control study. Stroke 2003, 34, E168–E169. [Google Scholar] [CrossRef]
- Sandström, C.S.; Ohlsson, B.; Melander, O.; Westin, U.; Mahadeva, R.; Janciauskiene, S. An association between type 2 diabetes and alpha-antitrypsin deficiency. Diabet. Med. 2008, 25, 1370–1373. [Google Scholar] [CrossRef]
- Schachner, T.; Golderer, G.; Sarg, B.; Lindner, B.; Bonaros, N.; Mikuz, G.; Laufer, G.; Werner, E.R. The amounts of alpha 1antitrypsin protein are reduced in the vascular wall of the acutely dissected human ascending aorta. Eur. J. Cardio-Thoracic Surg. 2010, 37, 684–690. [Google Scholar] [CrossRef]
- Madar, T.; Shahaf, G.; Sheiner, E.; Brazg, J.; Levinson, J.; Yaniv Salem, S.; Twina, G.; Baron, J.; Mazor, M.; Holcberg, G.; et al. Low levels of circulating alpha-1 antitrypsin are associated with spontaneous abortions. J. Matern.-Fetal Neonatal Med. 2013, 26, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Ragnoli, B.; Chiazza, F.; Tarsi, G.; Malerba, M. Biological pathways and mechanisms linking COPD and cardiovascular disease. Ther. Adv. Chronic Dis. 2025, 16, 20406223251314286. [Google Scholar] [CrossRef] [PubMed]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. 2013, 22, 454–475. [Google Scholar] [CrossRef]
- Hughes, J.R. Comorbidity and smoking. Nicotine Tob. Res. 1999, 1, S149–S152. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.J.; Bhatt, S.P.; Anzueto, A.; Bowler, R.P.; DeMeo, D.L.; Diaz, A.A.; Dransfield, M.T.; Fawzy, A.; Foreman, M.G.; Hanania, N.A.; et al. Clinical Epidemiology of COPD: Insights from 10 Years of the COPDGene Study. Chest 2019, 156, 228–238. [Google Scholar] [CrossRef]
- Inouye, M.; Ripatti, S.; Kettunen, J.; Lyytikäinen, L.P.; Oksala, N.; Laurila, P.P.; Kangas, A.J.; Soininen, P.; Savolainen, M.J.; Viikari, J.; et al. Novel loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 2012, 8, e1002907. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, V.; Topic, A.; Milinkovic, N.; Lazic, Z.; Ivosevic, A.; Radojkovic, D.; Rankov, A.D. Association of the methionine sulfoxide reductase A rs10903323 gene polymorphism with functional activity and oxidative modification of alpha-1-antitrypsin in COPD patients. Pulmonology 2024, 30, 122–129. [Google Scholar] [CrossRef]
- Kerpel-Fronius, A.; Megyesfalvi, Z.; Markóczy, Z.; Solymosi, D.; Csányi, P.; Tisza, J.; Kecskés, A.; Baranyi, B.; Csánky, E.; Dóka, A.; et al. HUNCHEST-II contributes to a shift to earlier-stage lung cancer detection: Final results of a nationwide screening program. Eur. Radiol. 2024, 34, 3462–3470. [Google Scholar] [CrossRef]
- Hatabu, H.; Hunninghake, G.M.; Richeldi, L.; Brown, K.K.; Wells, A.U.; Remy-Jardin, M.; Verschakelen, J.; Nicholson, A.G.; Beasley, M.B.; Christiani, D.C.; et al. Interstitial lung abnormalities detected incidentally on CT: A position paper from the Fleischner Society. Lancet Respir. Med. 2020, 8, 726–737. [Google Scholar] [CrossRef]
- Hida, T.; Nishino, M.; Hino, T.; Lu, J.; Putman, R.K.; Gudmundsson, E.F.; Araki, T.; Valtchinov, V.I.; Honda, O.; Yanagawa, M.; et al. Traction bronchiectasis/bronchiolectasis is associated with interstitial lung abnormality mortality. Eur. J. Radiol. 2020, 129, 109073. [Google Scholar] [CrossRef]
- Putman, R.K.; Gudmundsson, G.; Axelsson, G.T.; Hida, T.; Honda, O.; Araki, T.; Yanagawa, M.; Nishino, M.; Miller, E.R.; Eiriksdottir, G.; et al. Imaging patterns are associated with interstitial lung abnormality progression and mortality. Am. J. Respir. Crit. Care Med. 2019, 200, 175–18312. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15.e1, Erratum in J. Pediatr. 2017, 184, 243. [Google Scholar] [CrossRef] [PubMed]
- Mascalchi, M.; Camiciottoli, G.; Diciotti, S. Lung densitometry: Why, how and when. J. Thorac. Dis. 2017, 9, 3319–3345. [Google Scholar] [CrossRef] [PubMed]





| Study Groups | Patient Number | Male/Female Ratio | Age (Year ± SD) |
|---|---|---|---|
| Controls/healthy non-smokers | 85 | 26/49 | 64.6 ± 10.7 |
| Smokers (no COPD) | 291 | 97/128 | 60.6 ± 7.2 |
| Ex-smokers (no COPD) | 127 | 61/45 | 63.4 ± 7.6 |
| Smokers with COPD | 187 | 81/70 | 63.4 ± 6.2 |
| Ex-smokers with COPD | 64 | 24/24 | 66.9 ± 8.4 |
| Not categorized | 85 | 42/29 | 62.6 ± 7.3 |
| Asthma | 128 | 49/79 | 54.3 ± 14.3 |
| Smokers with asthma | 66 | 34/32 | 58.5 ± 11.6 |
| Sarcoidosis | 17 | 9/8 | 49.6 ± 10.6 |
| Sarcoidosis + F | 13 | 9/4 | 51.4 ± 10.4 |
| ILD + autoimmune | 22 | 4/18 | 62.4 ± 9.4 |
| ILD + AI + bronchiectasis | 7 | 2/5 | 66.4 ± 13.2 |
| ILD + AI + emphysema | 7 | 2/5 | 67.5 ± 9.7 |
| ILD + AI + asthma | 4 | 1/3 | 48.5 ± 19.3 |
| ILD | 19 | 11/8 | 65.2 ± 15.8 |
| ILD + bronchiectasis | 10 | 4/6 | 71.5 ± 6.5 |
| Cystic fibrosis | 26 | 13/13 | 33.6 ± 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Páska, C.; Barta, I.; Csoma, Z.; Gajdócsi, R.; Szél, V.; Kerpel-Fronius, A.; Solymosi, D.; Örlős, Z.; Antus, B. Alpha1-Antitrypsin in Lung Diseases: A Cross-Sectional Observational Study. Int. J. Mol. Sci. 2025, 26, 5400. https://doi.org/10.3390/ijms26115400
Páska C, Barta I, Csoma Z, Gajdócsi R, Szél V, Kerpel-Fronius A, Solymosi D, Örlős Z, Antus B. Alpha1-Antitrypsin in Lung Diseases: A Cross-Sectional Observational Study. International Journal of Molecular Sciences. 2025; 26(11):5400. https://doi.org/10.3390/ijms26115400
Chicago/Turabian StylePáska, Csilla, Imre Barta, Zsuzsanna Csoma, Réka Gajdócsi, Viktória Szél, Anna Kerpel-Fronius, Diána Solymosi, Zoltán Örlős, and Balázs Antus. 2025. "Alpha1-Antitrypsin in Lung Diseases: A Cross-Sectional Observational Study" International Journal of Molecular Sciences 26, no. 11: 5400. https://doi.org/10.3390/ijms26115400
APA StylePáska, C., Barta, I., Csoma, Z., Gajdócsi, R., Szél, V., Kerpel-Fronius, A., Solymosi, D., Örlős, Z., & Antus, B. (2025). Alpha1-Antitrypsin in Lung Diseases: A Cross-Sectional Observational Study. International Journal of Molecular Sciences, 26(11), 5400. https://doi.org/10.3390/ijms26115400






