Emerging Immunotherapy Targets in Early Drug Development
Abstract
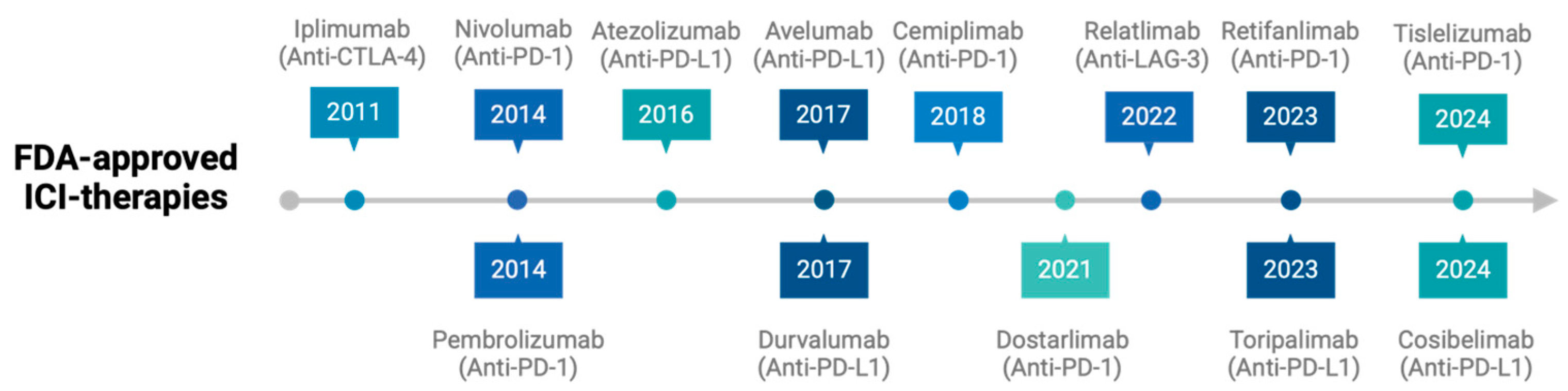
1. Introduction
2. Immune Checkpoint Inhibitors Beyond PD-1/PD-L1 and CTLA-4
2.1. Lymphocyte Activation Gene 3 (LAG-3 and CD223)
2.2. T-Cell Membrane Protein 3 (TIM-3)
2.3. T-Cell Immunoreceptor with Ig and ITIM Domains (TIGIT)
2.4. Other Inhibitory Checkpoints
3. Targeting Co-Stimulatory Pathways
3.1. OX40
3.2. 4-1BB (CD137)
3.3. Other Co-Stimulatory Receptors
4. Cytokine Modulation
4.1. Interleukin-2 (IL-2)
4.2. Interleukin-15 (IL-15)
4.3. Other Cytokines
5. Targeting the Tumor Microenvironment
5.1. Depleting Tregs with Anti-CCR8 Therapies
5.2. Blocking the “Don’t Eat Me” Signal: CD47
5.3. Inhibiting TGF-β Signaling
5.4. Targeting Metabolic Pathways
6. Exploring Novel Targets with Bispecific Antibodies
7. Current Challenges and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pehamberger, H.; Soyer, H.P.; Steiner, A.; Kofler, R.; Binder, M.; Mischer, P.; Pachinger, W.; Auböck, J.; Fritsch, P.; Kerl, H.; et al. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. Austrian Malignant Melanoma Cooperative Group. J. Clin. Oncol. 1998, 16, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: A retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Minasian, L.M.; Motzer, R.J.; Gluck, L.; Mazumdar, M.; Vlamis, V.; Krown, S.E. Interferon alfa-2a in advanced renal cell carcinoma: Treatment results and survival in 159 patients with long-term follow-up. J. Clin. Oncol. 1993, 11, 1368–1375. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- FDA Approval Timeline of Active Immunotherapies. CRI. Cancer Research Institute n.d. Available online: https://www.cancerresearch.org/regulatory-approval-timeline-of-active-immunotherapies (accessed on 27 February 2025).
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Pilard, C.; Ancion, M.; Delvenne, P.; Jerusalem, G.; Hubert, P.; Herfs, M. Cancer immunotherapy: It’s time to better predict patients’ response. Br. J. Cancer 2021, 125, 927–938. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Gettinger, S.; Choi, J.; Hastings, K.; Truini, A.; Datar, I.; Sowell, R.; Wurtz, A.; Dong, W.; Cai, G.; Melnick, M.A.; et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017, 7, 1420–1435. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, A.; Lei, Q.; Zhang, Y. Tumor-intrinsic signaling pathways: Key roles in the regulation of the immunosuppressive tumor microenvironment. J. Hematol. Oncol. 2019, 12, 125. [Google Scholar] [CrossRef]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.M.; Pauken, K.E.; Huang, A.C.; et al. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Taylor, N.A.; Vick, S.C.; Iglesia, M.D.; Brickey, W.J.; Midkiff, B.R.; McKinnon, K.P.; Reisdorf, S.; Anders, C.K.; Carey, L.A.; Parker, J.S.; et al. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J. Clin. Investig. 2017, 127, 3472–3483. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y. Tumor-associated macrophages: From basic research to clinical application. J. Hematol. Oncol. 2017, 10, 58. [Google Scholar] [CrossRef]
- Ni, Y.; Soliman, A.; Joehlin-Price, A.; Rose, P.G.; Vlad, A.; Edwards, R.P.; Mahdi, H. High TGF-β signature predicts immunotherapy resistance in gynecologic cancer patients treated with immune checkpoint inhibition. npj Precis. Oncol. 2021, 5, 101. [Google Scholar] [CrossRef]
- Tang, J.; Shalabi, A.; Hubbard-Lucey, V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018, 29, 84–91. [Google Scholar] [CrossRef]
- Saez-Ibanez, A.R.; Upadhaya, S.; Campbell, J. Immuno-oncology clinical trials take a turn beyond PD1/PDL1 inhibitors. Nat. Rev. Drug Discov. 2023, 22, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864. [Google Scholar] [CrossRef]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.-M.; Shimizu, K.; Maeda, T.K.; Ikubo, J.; Yoshikawa, H.; Maenaka, K.; Ishimaru, N.; Kosako, H.; et al. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity 2022, 55, 912–924.e8. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Nixon, M.J.; Wang, Y.; Wang, D.Y.; Castellanos, E.; Estrada, M.V.; Ericsson-Gonzalez, P.I.; Cote, C.H.; Salgado, R.; Sanchez, V.; et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight 2018, 3, e120360. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Woo, S.-R.; Turnis, M.E.; Goldberg, M.V.; Bankoti, J.; Selby, M.; Nirschl, C.J.; Bettini, M.L.; Gravano, D.M.; Vogel, P.; Liu, C.L.; et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012, 72, 917–927. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.; Lao, C.D.; et al. Three-Year Overall Survival With Nivolumab Plus Relatlimab in Advanced Melanoma From RELATIVITY-047. J. Clin. Oncol. 2025, 43, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Mendez, G.; Chao, J.; Nemecek, R.; Feeney, K.; Van Cutsem, E.; Al-Batran, S.-E.; Mansoor, W.; Maisey, N.; Pazo Cid, R.; et al. First-Line Nivolumab and Relatlimab Plus Chemotherapy for Gastric or Gastroesophageal Junction Adenocarcinoma: The Phase II RELATIVITY-060 Study. J. Clin. Oncol. 2024, 42, 2080–2093. [Google Scholar] [CrossRef]
- Feeney, K.; Joubert, W.L.; Bordoni, R.E.; Babu, S.; Marimuthu, S.; Hipkin, B.; Huang, L.; Tam, R.; Acosta Rivera, M. RELATIVITY-123: A phase 3, randomized, open-label study of nivolumab (NIVO) + relatlimab (RELA) fixed-dose combination (FDC) versus regorafenib or trifluridine + tipiracil (TAS-102) in later-line metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2023, 41, TPS278. [Google Scholar] [CrossRef]
- Segal, N.H.; Passhak, M.; Köse, F.; Kubala, E.; Elez, E.; Kawakami, H.; Guren, M.G.; Jonker, D.J.; Xu, R.-H.; Riera, J.; et al. Co-formulated favezelimab plus pembrolizumab versus standard-of-care in previously treated, PD-L1-positive metastatic colorectal cancer: The phase 3, randomized KEYFORM-007 study. J. Clin. Oncol. 2025, 43, LBA248. [Google Scholar] [CrossRef]
- Hernando-Calvo, A.; Vila-Casadesús, M.; Bareche, Y.; Gonzalez-Medina, A.; Abbas-Aghababazadeh, F.; Lo Giacco, D.; Martin, A.; Saavedra, O.; Brana, I.; Vieito, M.; et al. A pan-cancer clinical platform to predict immunotherapy outcomes and prioritize immuno-oncology combinations in early-phase trials. Med 2023, 4, 710–727.e5. [Google Scholar] [CrossRef] [PubMed]
- INSIGHT-003 Trial of Eftilagimod Alpha Combo in NSCLC Completes Enrollment. Available online: https://www.targetedonc.com/view/insight-003-trial-of-eftilagimod-alpha-combo-in-nsclc-completes-enrollment (accessed on 27 February 2025).
- Krebs, M.G.; Forster, M.; Majem, M.; Peguero, J.; Iams, W.; Clay, T.; Roxburgh, P.; Doger, B.; Bajaj, P.; Barba, A.; et al. Eftilagimod Alpha (a Soluble LAG-3 Protein) Combined with Pembrolizumab in Second-Line Metastatic NSCLC Refractory to Anti–Programmed Cell Death Protein 1/Programmed Death-Ligand 1-Based Therapy: Final Results from a Phase 2 Study. JTO Clin. Res. Rep. 2024, 5, 100725. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Ferris, R.L.; Gooding, W.E.; Chiosea, S.I.; Duvvuri, U.; Kim, S.; Kubik, M.; Sridharan, S.; Fenton, M.J.; Skinner, H.D.; Kelly, Z.R.; et al. Neoadjuvant nivolumab alone or in combination with relatlimab or ipilimumab in resectable head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2023, 41, 6018. [Google Scholar] [CrossRef]
- Kelly, R.J.; Landon, B.V.; Zaidi, A.H.; Singh, D.; Canzoniero, J.V.; Balan, A.; Hales, R.K.; Voong, K.R.; Battafarano, R.J.; Jobe, B.A.; et al. Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: A phase Ib trial and ctDNA analyses. Nat. Med. 2024, 30, 1023–1034. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Liang, T.; Wang, L.; Hu, L. TIM-3 is a potential prognostic marker for patients with solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 31705–31713. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti–TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti–PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Curigliano, G.; Santoro, A.; Kim, D.-W.; Tai, D.; Hodi, F.S.; Wilgenhof, S.; Doi, T.; Sabatos-Peyton, C.; Szpakowski, S.; et al. Sabatolimab in combination with spartalizumab in patients with non-small cell lung cancer or melanoma who received prior treatment with anti-PD-1/PD-L1 therapy: A phase 2 multicentre study. BMJ Open 2024, 14, e079132. [Google Scholar] [CrossRef]
- Harding, J.J.; Moreno, V.; Bang, Y.-J.; Hong, M.H.; Patnaik, A.; Trigo, J.; Szpurka, A.M.; Yamamoto, N.; Doi, T.; Fu, S.; et al. Blocking TIM-3 in Treatment-refractory Advanced Solid Tumors: A Phase Ia/b Study of LY3321367 with or without an Anti-PD-L1 Antibody. Clin. Cancer Res. 2021, 27, 2168–2178. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ribas, A.; Davar, D.; Eroglu, Z.; Wang, J.S.; Luke, J.J.; Hamilton, E.P.; Di Pace, B.; Wang, T.; Ghosh, S.; et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). J. Clin. Oncol. 2022, 40, 2504. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Chauvin, J.-M.; Zarour, H.M. TIGIT in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000957. [Google Scholar] [CrossRef]
- Joller, N.; Hafler, J.P.; Brynedal, B.; Kassam, N.; Spoerl, S.; Levin, S.D.; Sharpe, A.H.; Kuchroo, V.K. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J. Immunol. 2011, 186, 1338–1342. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8+ T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef]
- Joller, N.; Lozano, E.; Burkett, P.R.; Patel, B.; Xiao, S.; Zhu, C.; Xia, J.; Tan, T.G.; Sefik, E.; Yajnik, V.; et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity 2014, 40, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Annese, T.; Tamma, R.; Ribatti, D. Update in TIGIT Immune-Checkpoint Role in Cancer. Front. Oncol. 2022, 12, 871085. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Bedard, P.L.; LoRusso, P.; Gordon, M.S.; Bendell, J.; Oh, D.-Y.; Ahn, M.-J.; Garralda, E.; D’Angelo, S.P.; Desai, J.; et al. Anti-TIGIT Antibody Tiragolumab Alone or with Atezolizumab in Patients with Advanced Solid Tumors: A Phase 1a/1b Nonrandomized Controlled Trial. JAMA Oncol. 2023, 9, 1574–1582. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Roche Reports Update on Phase III SKYSCRAPER-01 Study Results. Available online: https://www.roche.com/media/releases/med-cor-2024-11-26 (accessed on 27 February 2025).
- Merck Provides Update on KeyVibe and KEYFORM Clinical Development Programs Evaluating Investigational Vibostolimab and Favezelimab Fixed-Dose Combinations with Pembrolizumab. MerckCom n.d. Available online: https://www.merck.com/news/merck-provides-update-on-keyvibe-and-keyform-clinical-development-programs-evaluating-investigational-vibostolimab-and-favezelimab-fixed-dose-combinations-with-pembrolizumab/ (accessed on 27 February 2025).
- Naidoo, J.; Peters, S.; Runglodvatana, Y.; Li, J.Y.-C.; Fong, C.H.; Ho, G.F.; How, S.H.; Juengsamarn, J.; Todd, T.; Marina, N.; et al. 1461|Phase 2 Randomized Study of Domvanalimab Combined with Zimberelimab in Front-Line, PD-(L)1 High, Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC): Results from ARC-10 Part 1. J. Immuno Ther. Cancer 2024, 12, A1690–A1691. Available online: https://jitc.bmj.com/content/12/Suppl_3/A1690 (accessed on 27 February 2025).
- Suh, W.-K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Miyatake, T.; Tringler, B.; Liu, W.; Liu, S.-H.; Papkoff, J.; Enomoto, T.; Torkko, K.C.; Dehn, D.L.; Swisher, A.; Shroyer, K.R. B7-H4 (DD-O110) is overexpressed in high risk uterine endometrioid adenocarcinomas and inversely correlated with tumor T-cell infiltration. Gynecol. Oncol. 2007, 106, 119–127. [Google Scholar] [CrossRef]
- Mugler, K.C.; Singh, M.; Tringler, B.; Torkko, K.C.; Liu, W.; Papkoff, J.; Shroyer, K.R. B7-h4 expression in a range of breast pathology: Correlation with tumor T-cell infiltration. Appl. Immunohistochem. Mol. Morphol. 2007, 15, 363–370. [Google Scholar] [CrossRef]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zheng, Z.; Li, X.; Zhu, Y.; Zhong, Z.; Peng, L.; Wu, Y. B7-H3 Overexpression Predicts Poor Survival of Cancer Patients: A Meta-Analysis. Cell Physiol. Biochem. 2016, 39, 1568–1580. [Google Scholar] [CrossRef]
- Zhou, W.-T.; Jin, W.-L. B7-H3/CD276: An Emerging Cancer Immunotherapy. Front. Immunol. 2021, 12, 701006. [Google Scholar] [CrossRef]
- Feustel, K.; Martin, J.; Falchook, G.S. B7-H3 Inhibitors in Oncology Clinical Trials: A Review. J. Immunother. Precis. Oncol. 2024, 7, 53–66. [Google Scholar] [CrossRef]
- Aggarwal, C.; Prawira, A.; Antonia, S.; Rahma, O.; Tolcher, A.; Cohen, R.B.; Lou, Y.; Hauke, R.; Vogelzang, N.; Zandberg, D.P.; et al. Dual checkpoint targeting of B7-H3 and PD-1 with enoblituzumab and pembrolizumab in advanced solid tumors: Interim results from a multicenter phase I/II trial. J. Immunother. Cancer 2022, 10, e004424. [Google Scholar] [CrossRef]
- MacroGenics Announces Closure of CP-MGA271-06 Study Evaluating Enoblituzumab Plus Checkpoint Inhibition in Head and Neck Cancer. MacroGenics, Inc. Available online: https://ir.macrogenics.com/news-releases/news-release-details/macrogenics-announces-closure-cp-mga271-06-study-evaluating (accessed on 28 February 2025).
- Salceda, S.; Tang, T.; Kmet, M.; Munteanu, A.; Ghosh, M.; Macina, R.; Liu, W.; Pilkington, G.; Papkoff, J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res. 2005, 306, 128–141. [Google Scholar] [CrossRef]
- Sachdev, J.C.; Bauer, T.M.; Chawla, S.P.; Pant, S.; Patnaik, A.; Wainberg, Z.A.; Inamdar, S.P.; Marina, N.; Sun, S.; Schmidt, M.; et al. Phase 1a/1b study of first-in-class B7-H4 antibody, FPA150, as monotherapy in patients with advanced solid tumors. J. Clin. Oncol. 2019, 37, 2529. [Google Scholar] [CrossRef]
- Lines, J.L.; Pantazi, E.; Mak, J.; Sempere, L.F.; Wang, L.; O’Connell, S.; Ceeraz, S.; Suriawinata, A.A.; Yan, S.; Ernstoff, M.S.; et al. VISTA is an immune checkpoint molecule for human T cells. Cancer Res. 2014, 74, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rubinstein, R.; Lines, J.L.; Wasiuk, A.; Ahonen, C.; Guo, Y.; Lu, L.-F.; Gondek, D.; Wang, Y.; Fava, R.A.; et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011, 208, 577–592. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Q.; Xia, H.; Zhu, G.; Feng, Y.; Wang, Q.; Zhang, Z.; He, W.; Lu, J.; Dong, C.; et al. Analysis of VISTA expression and function in renal cell carcinoma highlights VISTA as a potential target for immunotherapy. Protein Cell 2019, 10, 840–845. [Google Scholar] [CrossRef]
- Le Mercier, I.; Chen, W.; Lines, J.L.; Day, M.; Li, J.; Sergent, P.; Noelle, R.J.; Wang, L. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res. 2014, 74, 1933–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Kim, T.K. VISTA-mediated immune evasion in cancer. Exp. Mol. Med. 2024, 56, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Kakavand, H.; Jackett, L.A.; Menzies, A.M.; Gide, T.N.; Carlino, M.S.; Saw, R.P.M.; Thompson, J.F.; Wilmott, J.S.; Long, G.V.; Scolyer, R.A. Negative immune checkpoint regulation by VISTA: A mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod. Pathol. 2017, 30, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Rodon Ahnert, J.; Gruber, J.J.; Telli, M.L.; Mita, M.M.; Mita, A.C.; Kim, J.W.; Villalona-Calero, M.A.; Patel, M.; Yadav, S.S.; Sharma, P.; et al. A phase 1 first-in-human clinical trial of HMBD-002, an IgG4 monoclonal antibody targeting VISTA, in advanced solid tumors. J. Clin. Oncol. 2023, 41, TPS2664. [Google Scholar] [CrossRef]
- Sen, S.; Call, J.A.; Papadopoulos, K.P.; Smith, F.D.; McDermott, J.; van der Horst, E.; Weitzman, A. Initial results from a first-in-human phase 1 study of SNS-101 (pH-selective anti-VISTA antibody) alone or in combination with cemiplimab in patients with advanced solid tumors. J. Clin. Oncol. 2024, 42, 2600. [Google Scholar] [CrossRef]
- Lee, J.J.; Powderly, J.D.; Patel, M.R.; Brody, J.; Hamilton, E.P.; Infante, J.R.; Falchook, G.S.; Wang, H.; Adams, L.; Gong, L.; et al. Phase 1 trial of CA-170, a novel oral small molecule dual inhibitor of immune checkpoints PD-1 and VISTA, in patients (pts) with advanced solid tumor or lymphomas. J. Clin. Oncol. 2017, 35, TPS3099. [Google Scholar] [CrossRef]
- Kraehenbuehl, L.; Weng, C.-H.; Eghbali, S.; Wolchok, J.D.; Merghoub, T. Enhancing immunotherapy in cancer by targeting emerging immunomodulatory pathways. Nat. Rev. Clin. Oncol. 2022, 19, 37–50. [Google Scholar] [CrossRef]
- Leem, G.; Park, J.; Jeon, M.; Kim, E.-S.; Kim, S.W.; Lee, Y.J.; Choi, S.J.; Choi, B.; Park, S.; Ju, Y.S.; et al. 4-1BB co-stimulation further enhances anti-PD-1-mediated reinvigoration of exhausted CD39+ CD8 T cells from primary and metastatic sites of epithelial ovarian cancers. J. Immunother. Cancer 2020, 8, e001650. [Google Scholar] [CrossRef]
- Jeong, S.; Park, S.-H. Co-Stimulatory Receptors in Cancers and Their Implications for Cancer Immunotherapy. Immune Netw. 2020, 20, e3. [Google Scholar] [CrossRef]
- Sugamura, K.; Ishii, N.; Weinberg, A.D. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 2004, 4, 420–431. [Google Scholar] [CrossRef]
- Massarelli, E.; Lam, V.K.; Parra, E.R.; Rodriguez-Canales, J.; Behrens, C.; Diao, L.; Wang, J.; Blando, J.; Byers, L.A.; Yanamandra, N.; et al. High OX-40 expression in the tumor immune infiltrate is a favorable prognostic factor of overall survival in non-small cell lung cancer. J. Immunother. Cancer 2019, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, X.; Jia, K.; Dziadziuszko, R.; Zhao, S.; Deng, J.; Wang, H.; Hirsch, F.R.; Zhou, C. OX40 and OX40L protein expression of tumor infiltrating lymphocytes in non-small cell lung cancer and its role in clinical outcome and relationships with other immune biomarkers. Transl. Lung Cancer Res. 2019, 8, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xu, L.; Wu, H.; Liao, H.; Luo, L.; Liao, M.; Gong, J.; Deng, Y.; Yuan, K.; Wu, H.; et al. OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis. Oncoimmunology 2018, 7, e1404214. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.J.; Martin-Liberal, J.; Kristeleit, R.; Cho, D.C.; Blagden, S.P.; Berthold, D.; Cardin, D.B.; Vieito, M.; Miller, R.E.; Dass, P.H.; et al. First-in-human phase I/II, open-label study of the anti-OX40 agonist INCAGN01949 in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e004235. [Google Scholar] [CrossRef] [PubMed]
- Diab, A.; Hamid, O.; Thompson, J.A.; Ros, W.; Eskens, F.A.L.M.; Doi, T.; Hu-Lieskovan, S.; Klempner, S.J.; Ganguly, B.; Fleener, C.; et al. A Phase I, Open-Label, Dose-Escalation Study of the OX40 Agonist Ivuxolimab in Patients with Locally Advanced or Metastatic Cancers. Clin. Cancer Res. 2022, 28, 71–83. [Google Scholar] [CrossRef]
- Glisson, B.S.; Leidner, R.S.; Ferris, R.L.; Powderly, J.; Rizvi, N.A.; Keam, B.; Schneider, R.; Goel, S.; Ohr, J.P.; Burton, J.; et al. Safety and Clinical Activity of MEDI0562, a Humanized OX40 Agonist Monoclonal Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 5358–5367. [Google Scholar] [CrossRef]
- Rowell, E.; Kinkead, H.; Torretti, E.; Becklund, B.; Sulzmaier, F.; Crago, W.; Jones, K.; Timmer, J.; Deveraux, Q.; Eckelman, B.; et al. 856 INBRX-106: A novel hexavalent anti-OX40 agonist for the treatment of solid tumors. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Garralda, E.; Cervantes, A.; Haddox, C.L.; Babiker, H.M.; Borad, M.J.; Leventakos, K.; Mehra, R.; Zugazagoitia, J.; Hanna, D.L.; et al. A phase I monotherapy dose escalation study of HFB301001, a novel next generation OX40 agonist monoclonal antibody, in adult patients with advanced solid tumors. J. Clin. Oncol. 2024, 42, 2531. [Google Scholar] [CrossRef]
- Kwon, B.S.; Kim, G.S.; Prystowsky, M.B.; Lancki, D.W.; Sabath, D.E.; Pan, J.L.; Weissman, S.M. Isolation and initial characterization of multiple species of T-lymphocyte subset cDNA clones. Proc. Natl. Acad. Sci. USA 1987, 84, 2896–2900. [Google Scholar] [CrossRef]
- Pollok, K.E.; Kim, Y.J.; Zhou, Z.; Hurtado, J.; Kim, K.K.; Pickard, R.T.; Kwon, B.S. Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol. 1993, 150, 771–781. [Google Scholar] [CrossRef]
- Schwarz, H.; Valbracht, J.; Tuckwell, J.; von Kempis, J.; Lotz, M. ILA, the human 4-1BB homologue, is inducible in lymphoid and other cell lineages. Blood 1995, 85, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kim, Y.-H.; Lee, S.-J.; Eom, H.-S.; Choi, B.K. 4-1BB immunotherapy: Advances and hurdles. Exp. Mol. Med. 2024, 56, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Logan, T.F.; Hodi, F.S.; McDermott, D.; Melero, I.; Hamid, O.; Schmidt, H.; Robert, C.; Chiarion-Sileni, V.; Ascierto, P.A.; et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017, 23, 1929–1936. [Google Scholar] [CrossRef]
- Segal, N.H.; He, A.R.; Doi, T.; Levy, R.; Bhatia, S.; Pishvaian, M.J.; Cesari, R.; Chen, Y.; Davis, C.B.; Huang, B.; et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 1816–1823. [Google Scholar] [CrossRef]
- Barve, M.A.; Tolcher, A.W.; Carvajal, R.D.; Izar, B.; El-Khoueiry, A.B.; Hanna, D.L.; Tarhini, A.A.; Whitman, E.D.; Bhatia, S.; Davar, D.; et al. A phase 1 study of AGEN2373, a novel CD137 agonist antibody designed to avoid hepatoxicity, in patients with advanced solid tumors. J. Clin. Oncol. 2023, 41, 2524. [Google Scholar] [CrossRef]
- Ma, Y.; Luo, F.; Zhang, Y.; Liu, Q.; Xue, J.; Huang, Y.; Zhao, Y.; Yang, Y.; Fang, W.; Zhou, T.; et al. Preclinical characterization and phase 1 results of ADG106 in patients with advanced solid tumors and non-Hodgkin’s lymphoma. Cell Rep. Med. 2024, 5, 101414. [Google Scholar] [CrossRef]
- Garralda, E.; Oberoi, A.; de Velasco, G.; Victoria, I.; Pesantez, D.; Eguren Santamaría, I.; Moreno, V.; Boni, V.; Cervantes, A.; Gambardella, V.; et al. First-in-human study (FIH) of FS222, a next-generation tetravalent PD-L1/CD137 bispecific antibody: Safety, pharmacodynamics (PD), and antitumor activity in patients (pts) with advanced solid tumors including PD-1 refractory melanoma. J. Clin. Oncol. 2024, 42, 2505. [Google Scholar] [CrossRef]
- McHugh, R.S.; Whitters, M.J.; Piccirillo, C.A.; Young, D.A.; Shevach, E.M.; Collins, M.; Byrne, M.C. CD4+CD25+ immunoregulatory T cells: Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 2002, 16, 311–323. [Google Scholar] [CrossRef]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef]
- Baltz, K.M.; Krusch, M.; Bringmann, A.; Brossart, P.; Mayer, F.; Kloss, M.; Baessler, T.; Kumbier, I.; Peterfi, A.; Kupka, S.; et al. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. 2007, 21, 2442–2454. [Google Scholar] [CrossRef]
- Davar, D.; Zappasodi, R. Targeting GITR in cancer immunotherapy – there is no perfect knowledge. Oncotarget 2023, 14, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Zappasodi, R.; Wang, H.; Naik, G.S.; Sato, T.; Bauer, T.; Bajor, D.; Rixe, O.; Newman, W.; Qi, J.; et al. Phase IB Study of GITR Agonist Antibody TRX518 Singly and in Combination with Gemcitabine, Pembrolizumab, or Nivolumab in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022, 28, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Borgeaud, M.; Sandoval, J.; Obeid, M.; Banna, G.; Michielin, O.; Addeo, A.; Friedlaender, A. Novel targets for immune-checkpoint inhibition in cancer. Cancer Treat. Rev. 2023, 120, 102614. [Google Scholar] [CrossRef]
- Patel, M.R.; Naing, A.; Burris III, H.A.; Lin, C.-C.; Curigliano, G.; Thistlethwaite, F.; Minchom, A.R.; Ascierto, P.A.; De Braud, F.G.; Cecchini, M.; et al. A phase 1/2 open-label study of KY1044, an anti-ICOS antibody with dual mechanism of action, as single agent and in combination with atezolizumab, in adult patients with advanced malignancies. J. Clin. Oncol. 2021, 39, 2624. [Google Scholar] [CrossRef]
- Yap, T.A.; Gainor, J.F.; Callahan, M.K.; Falchook, G.S.; Pachynski, R.K.; LoRusso, P.; Kummar, S.; Gibney, G.T.; Burris, H.A.; Tykodi, S.S.; et al. First-in-Human Phase I/II ICONIC Trial of the ICOS Agonist Vopratelimab Alone and with Nivolumab: ICOS-High CD4 T-Cell Populations and Predictors of Response. Clin. Cancer Res. 2022, 28, 3695–3708. [Google Scholar] [CrossRef]
- Hilton, J.F.; Ott, P.A.; Hansen, A.R.; Li, Z.; Mathew, M.; Messina, C.H.; Dave, V.; Ji, X.; Karpinich, N.O.; Hirschfeld, S.; et al. INDUCE-2: A Phase I/II, open-label, two-part study of feladilimab in combination with tremelimumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2024, 73, 44. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Legha, S.S.; Gianan, M.A.; Plager, C.; Eton, O.E.; Papadopoulous, N.E. Evaluation of interleukin-2 administered by continuous infusion in patients with metastatic melanoma. Cancer 1996, 77, 89–96. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; White, D.E.; Steinberg, S.M. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: Identification of the antigens mediating response. Ann. Surg. 1998, 228, 307–319. [Google Scholar] [CrossRef]
- Rosenberg, S.A. IL-2: The first effective immunotherapy for human cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Lin, J.-X.; Leonard, W.J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 2013, 38, 13–25. [Google Scholar] [CrossRef]
- Charych, D.H.; Hoch, U.; Langowski, J.L.; Lee, S.R.; Addepalli, M.K.; Kirk, P.B.; Sheng, D.; Liu, X.; Sims, P.W.; VanderVeen, L.A.; et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. 2016, 22, 680–690. [Google Scholar] [CrossRef]
- Diab, A.; Tannir, N.M.; Bentebibel, S.-E.; Hwu, P.; Papadimitrakopoulou, V.; Haymaker, C.; Kluger, H.M.; Gettinger, S.N.; Sznol, M.; Tykodi, S.S.; et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov. 2020, 10, 1158–1173. [Google Scholar] [CrossRef]
- Tannir, N.M.; Formiga, M.N.; Penkov, K.; Kislov, N.; Vasiliev, A.; Gunnar Skare, N.; Hong, W.; Dai, S.; Tang, L.; Qureshi, A.; et al. Bempegaldesleukin Plus Nivolumab Versus Sunitinib or Cabozantinib in Previously Untreated Advanced Clear Cell Renal Cell Carcinoma: A Phase III Randomized Study (PIVOT-09). J. Clin. Oncol. 2024, 42, 2800–2811. [Google Scholar] [CrossRef]
- Diab, A.; Gogas, H.; Sandhu, S.; Long, G.V.; Ascierto, P.A.; Larkin, J.; Sznol, M.; Franke, F.; Ciuleanu, T.E.; Pereira, C.; et al. Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results. J. Clin. Oncol. 2023, 41, 4756–4767. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, U.N.; Muzaffar, J.; Winer, I.; Rosen, S.D.; Hoimes, C.J.; Chauhan, A.; Spreafico, A.; Lewis, K.D.; Bruno, D.S.; Dumas, O.; et al. Nemvaleukin alfa, a modified interleukin-2 cytokine, as monotherapy and with pembrolizumab in patients with advanced solid tumors (ARTISTRY-1). J. Immunother. Cancer 2024, 12, e010143. [Google Scholar] [CrossRef]
- Frentzas, S.; Ahern, E.S.; Weickhardt, A.J.; Haydon, A.M.; de Souza, P.L.; Powderly, J.D.; Wyant, T.; Tang, J.; Richards, L.; Knickerbocker, A.; et al. A phase 1/2 study of AU-007, a monoclonal antibody (mAb) that binds to IL-2 and inhibits CD25 binding, in patients with advanced solid tumors: Interim results. J. Clin. Oncol. 2023, 41, e14507. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, R.; Zhao, X. Engineering cytokines for cancer immunotherapy: A systematic review. Front. Immunol. 2023, 14, 1218082. [Google Scholar] [CrossRef]
- Boersma, B.; Poinot, H.; Pommier, A. Stimulating the Antitumor Immune Response Using Immunocytokines: A Preclinical and Clinical Overview. Pharmaceutics 2024, 16, 974. [Google Scholar] [CrossRef]
- Codarri Deak, L.; Nicolini, V.; Hashimoto, M.; Karagianni, M.; Schwalie, P.C.; Lauener, L.; Varypataki, E.M.; Richard, M.; Bommer, E.; Sam, J.; et al. PD-1-cis IL-2R agonism yields better effectors from stem-like CD8+ T cells. Nature 2022, 610, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Murer, P.; Salazar, U.; Egli, N.; Petersen, L.; Neubert, P.; Stocker, C.; Rau, A.; Richter, K.; Katopodis, A.; Huber, C. 820 ANV600 is a novel PD-1 targeted IL-2Rβ/γ agonist that is combinable with therapeutic PD-1 inhibitors. J. Immunother. Cancer 2023, 11, A918. [Google Scholar] [CrossRef]
- Hsu, J.; Donahue, R.N.; Katragadda, M.; Lowry, J.; Huang, W.; Srinivasan, K.; Guntas, G.; Tang, J.; Servattalab, R.; Moisan, J.; et al. A T cell receptor β chain-directed antibody fusion molecule activates and expands subsets of T cells to promote antitumor activity. Sci. Transl. Med. 2023, 15, eadi0258. [Google Scholar] [CrossRef] [PubMed]
- Gulley, J.L.; Sullivan, R.J.; Friedman, C.F.; Sonpavde, G.P.; Tschernia, N.P.; Herrera, M.; Marabelle, A.; McCue, S.; Srinivasan, K.; Katragadda, M.; et al. 1470 START001: A phase 1/2 study of invikafusp alfa (STAR0602), a first-in-class TCR β chain-targeted bispecific antibody, as monotherapy in patients with antigen-rich solid tumors resistant to anti-PD(L)1. J. Immunother. Cancer 2024, 12. [Google Scholar] [CrossRef]
- Therapeutics, M. Marengo’s First-in-Class Invikafusp Alfa (STAR0602) Receives U.S. FDA Fast Track Designation for Treatment of Unresectable, Locally Advanced, or Metastatic Colorectal Cancers with High Tumor Mutational Burden (TMB-H). Available online: https://www.prnewswire.com/news-releases/marengos-first-in-class-invikafusp-alfa-star0602-receives-us-fda-fast-track-designation-for-treatment-of-unresectable-locally-advanced-or-metastatic-colorectal-cancers-with-high-tumor-mutational-burden-tmb-h-302344827.html (accessed on 28 February 2025).
- Waldmann, T.A.; Miljkovic, M.D.; Conlon, K.C. Interleukin-15 (dys)regulation of lymphoid homeostasis: Implications for therapy of autoimmunity and cancer. J. Exp. Med. 2020, 217, e20191062. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Chang, S.S.; Kramolowsky, E.; Gonzalgo, M.L.; Agarwal, P.K.; Bassett, J.C.; Bjurlin, M.; Cher, M.L.; Clark, W.; Cowan, B.E.; et al. IL-15 Superagonist NAI in BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer. NEJM Evid. 2023, 2, EVIDoa2200167. [Google Scholar] [CrossRef] [PubMed]
- Margolin, K.; Morishima, C.; Velcheti, V.; Miller, J.S.; Lee, S.M.; Silk, A.W.; Holtan, S.G.; Lacroix, A.M.; Fling, S.P.; Kaiser, J.C.; et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2018, 24, 5552–5561. [Google Scholar] [CrossRef]
- Garralda, E.; Naing, A.; Galvao, V.; LoRusso, P.; Grell, P.; Cassier, P.A.; Gomez-Roca, C.A.; Korakis, I.; Bechard, D.; Palova Jelinkova, L.; et al. Interim safety and efficacy results from AURELIO-03: A phase 1 dose escalation study of the IL-2/IL-15 receptor βγ superagonist SOT101 as a single agent and in combination with pembrolizumab in patients with advanced solid tumors. J. Clin. Oncol. 2022, 40, 2502. [Google Scholar] [CrossRef]
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Kornete, M.; Joyce, J.A. Re-education of macrophages as a therapeutic strategy in cancer. Immunotherapy 2019, 11, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef]
- Fearon, D.T. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2014, 2, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kim, M.-H.; Pyo, J.; Lee, S.-M.; Jang, J.-S.; Lee, D.-W.; Kim, K.W. CCR8 as a Therapeutic Novel Target: Omics-Integrated Comprehensive Analysis for Systematically Prioritizing Indications. Biomedicines 2023, 11, 2910. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Xiang, L.; Bullen, J.W.; Zhang, C.; Samanta, D.; Gilkes, D.M.; He, J.; Semenza, G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, E6215–E6223. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Mamai, O.; Akhurst, R.J. TGFβ: Signaling Blockade for Cancer Immunotherapy. Annu. Rev. Cancer Biol. 2022, 6, 123–146. [Google Scholar] [CrossRef]
- Qu, C.; Edwards, E.W.; Tacke, F.; Angeli, V.; Llodrá, J.; Sanchez-Schmitz, G.; Garin, A.; Haque, N.S.; Peters, W.; van Rooijen, N.; et al. Role of CCR8 and Other Chemokine Pathways in the Migration of Monocyte-derived Dendritic Cells to Lymph Nodes. J. Exp. Med. 2004, 200, 1231–1241. [Google Scholar] [CrossRef]
- Miller, M.D.; Hata, S.; De Waal Malefyt, R.; Krangel, M.S. A novel polypeptide secreted by activated human T lymphocytes. J. Immunol. 1989, 143, 2907–2916. [Google Scholar] [CrossRef]
- Whiteside, S.K.; Grant, F.M.; Gyori, D.S.; Conti, A.G.; Imianowski, C.J.; Kuo, P.; Nasrallah, R.; Sadiyah, F.; Lira, S.A.; Tacke, F.; et al. CCR8 marks highly suppressive Treg cells within tumours but is dispensable for their accumulation and suppressive function. Immunology 2021, 163, 512–520. [Google Scholar] [CrossRef]
- De Simone, M.; Arrigoni, A.; Rossetti, G.; Gruarin, P.; Ranzani, V.; Politano, C.; Bonnal, R.J.P.; Provasi, E.; Sarnicola, M.L.; Panzeri, I.; et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity 2016, 45, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, H.; Dombrecht, B.; Kiss, M.; Roose, H.; Allen, E.; Van Overmeire, E.; Kancheva, D.; Martens, L.; Murgaski, A.; Bardet, P.M.R.; et al. Therapeutic depletion of CCR8+ tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J. Immunother. Cancer 2021, 9, e001749. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.O.; L’Huillier, A.; Armington, S.; Mottershead, C.; Filippova, E.V.; Coder, B.D.; Petit, R.G.; Princiotta, M.F. Targeting CCR8 Induces Protective Antitumor Immunity and Enhances Vaccine-Induced Responses in Colon Cancer. Cancer Res. 2018, 78, 5340–5348. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, J.; Li, J.; Zhao, Y.; Zhang, T.; Yang, R.; Ma, X. Safety and efficacy profile of mogamulizumab (Poteligeo) in the treatment of cancers: An update evidence from 14 studies. BMC Cancer 2021, 21, 618. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Yao, J.; Xue, J.; Dai, J.; Ji, Y.; Markman, B.; Hiong, A.; Yang, X.; Zhao, R.; et al. Efficacy and safety of LM-108, an anti-CCR8 monoclonal antibody, in combination with an anti-PD-1 antibody in patients with gastric cancer: Results from phase 1/2 studies. J. Clin. Oncol. 2024, 42, 2504. [Google Scholar] [CrossRef]
- Feng, M.; Jiang, W.; Kim, B.Y.S.; Zhang, C.C.; Fu, Y.-X.; Weissman, I.L. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 2019, 19, 568–586. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.A.; Zheleznyak, A.; Fang, Y.F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a marker of self on red blood cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Logtenberg, M.E.W.; Scheeren, F.A.; Schumacher, T.N. The CD47-SIRPα Immune Checkpoint. Immunity 2020, 52, 742–752. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Li, C.; Liang, X.; Li, C.; Liu, Y.; Yi, Z.; Zhang, L.; Fu, S.; Zeng, Y. Role of CD47 in tumor immunity: A potential target for combination therapy. Sci. Rep. 2022, 12, 9803. [Google Scholar] [CrossRef] [PubMed]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Advani, R.; Flinn, I.; Popplewell, L.; Forero, A.; Bartlett, N.L.; Ghosh, N.; Kline, J.; Roschewski, M.; LaCasce, A.; Collins, G.P.; et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018, 379, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, N.J.; Patnaik, A.; Liao, J.B.; Moroney, J.W.; Miller, D.S.; Fleming, G.F.; Axt, M.; Wang, Y.V.; Agoram, B.; Volkmer, J.-P.; et al. A phase Ib study of the anti-CD47 antibody magrolimab with the PD-L1 inhibitor avelumab (A) in solid tumor (ST) and ovarian cancer (OC) patients. J. Clin. Oncol. 2020, 38, 18. [Google Scholar] [CrossRef]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N.; et al. Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study. J. Clin. Oncol. 2023, 41, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Maute, R.; Xu, J.; Weissman, I.L. CD47-SIRPα-targeted therapeutics: Status and prospects. Immunooncol Technol. 2022, 13, 100070. [Google Scholar] [CrossRef]
- Gilead Statement on Discontinuation of Phase 3 ENHANCE-3 Study in AML. Available online: https://www.gilead.com/company/company-statements/2024/gilead-statement-on-discontinuation-of-phase-3-enhance-3-study-in-aml (accessed on 28 February 2025).
- Huang, Z.; Pang, X.; Zhong, T.; Qu, T.; Jin, C.; Chen, N.A.; He, X.; Xia, D.; Jin, X.; Wang, Z.; et al. 266 AK117, a CD47 blocking antibody with robust macrophage activation without red blood cell hemagglutination. J. Immunother. Cancer 2021, 9. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Tian, C.; Shao, X.; Huang, J.; Chen, Z.-H.; Wang, Y.; Sun, T.; Yi, T.; Yu, X.; et al. 347MO The safety and efficacy of ivonescimab in combination with chemotherapy as first-line (1L) treatment for triple-negative breast cancer (TNBC). Ann. Oncol. 2024, 35, S360–S361. [Google Scholar] [CrossRef]
- Xia, Q.; Wu, T.; Wang, F.; Li, S.; Xia, Y.; Li, B.; Wang, Z.M.; Song, W.; Wang, L. The safety and efficacy of cadonilimab in combination with AK117 (anti-CD47 antibody) plus chemotherapy as first-line treatment for advanced gastric (G) or gastroesophageal junction (GEJ) cancer. J. Clin. Oncol. 2023, 41, e16050. [Google Scholar] [CrossRef]
- Akeso, Inc. ESMO 2024: Akeso Published Ivonescimab plus Ligufalimab as First-Line Treatment for PD-L1–Positive Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Available online: https://www.akesobio.com/en/media/akeso-news/240918-2/ (accessed on 25 March 2025).
- Derynck, R.; Turley, S.J.; Akhurst, R.J. TGFβ biology in cancer progression and immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 9–34. [Google Scholar] [CrossRef]
- Principe, D.R.; Doll, J.A.; Bauer, J.; Jung, B.; Munshi, H.G.; Bartholin, L.; Pasche, B.; Lee, C.; Grippo, P.J. TGF-β: Duality of function between tumor prevention and carcinogenesis. J. Natl. Cancer Inst. 2014, 106, djt369. [Google Scholar] [CrossRef]
- Calon, A.; Tauriello, D.V.F.; Batlle, E. TGF-beta in CAF-mediated tumor growth and metastasis. Semin. Cancer Biol. 2014, 25, 15–22. [Google Scholar] [CrossRef]
- Nixon, B.G.; Gao, S.; Wang, X.; Li, M.O. TGFβ control of immune responses in cancer: A holistic immuno-oncology perspective. Nat. Rev. Immunol. 2023, 23, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Danielpour, D. Advances and Challenges in Targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective. Pharmaceuticals 2024, 17, 533. [Google Scholar] [CrossRef] [PubMed]
- Melisi, D.; Oh, D.-Y.; Hollebecque, A.; Calvo, E.; Varghese, A.; Borazanci, E.; Macarulla, T.; Merz, V.; Zecchetto, C.; Zhao, Y.; et al. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J. Immunother. Cancer 2021, 9, e002068. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.J.; Do, R.K.; Yaqubie, A.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; Lahn, M.; Benhadji, K.A.; Kelley, R.K.; Abou-Alfa, G.K. Phase 1b study of galunisertib and ramucirumab in patients with advanced hepatocellular carcinoma. Cancer Med. 2021, 10, 3059–3067. [Google Scholar] [CrossRef]
- Jung, S.Y.; Hwang, S.; Clarke, J.M.; Bauer, T.M.; Keedy, V.L.; Lee, H.; Park, N.; Kim, S.-J.; Lee, J.I. Pharmacokinetic characteristics of vactosertib, a new activin receptor-like kinase 5 inhibitor, in patients with advanced solid tumors in a first-in-human phase 1 study. Investig. New Drugs 2020, 38, 812–820. [Google Scholar] [CrossRef]
- Yap, T.A.; Vieito, M.; Baldini, C.; Sepúlveda-Sánchez, J.M.; Kondo, S.; Simonelli, M.; Cosman, R.; van der Westhuizen, A.; Atkinson, V.; Carpentier, A.F.; et al. First-In-Human Phase I Study of a Next-Generation, Oral, TGFβ Receptor 1 Inhibitor, LY3200882, in Patients with Advanced Cancer. Clin. Cancer Res. 2021, 27, 6666–6676. [Google Scholar] [CrossRef]
- Shou, M.; Zhou, H.; Ma, L. New advances in cancer therapy targeting TGF-β signaling pathways. Mol. Ther. Oncolytics 2023, 31, 100755. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Morris, J.C.; Lawrence, D.P.; Tan, A.R.; Olencki, T.E.; Shapiro, G.I.; Dezube, B.J.; Berzofsky, J.A.; Hsu, F.J.; Guitart, J. Cutaneous keratoacanthomas/squamous cell carcinomas associated with neutralization of transforming growth factor β by the monoclonal antibody fresolimumab (GC1008). Cancer Immunol. Immunother. 2015, 64, 437–446. [Google Scholar] [CrossRef]
- Vaishampayan, U.N.; Sweis, R.F.; Kilari, D.; Tarhini, A.A.; Gainor, J.F.; Barve, M.A.; Sonpavde, G.P.; McKean, M.; Park, D.J.; Babu, S.; et al. Phase 1 study (DRAGON) of SRK-181 (linavonkibart), a latent TGFβ1 inhibitor, combined with pembrolizumab in patients with anti-PD1 resistant advanced solid tumors: Updated results of expansion part. J. Clin. Oncol. 2024, 42, 2507. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.-K.L.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Kim, T.M.; Vicente, D.; Felip, E.; Lee, D.H.; Lee, K.H.; Lin, C.-C.; Flor, M.J.; Di Nicola, M.; Alvarez, R.M.; et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J. Thorac. Oncol. 2020, 15, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Birrer, M.; Li, G.; Yunokawa, M.; Lee, J.-Y.; Kim, B.G.; Oppermann, C.P.; Zhou, Q.; Nishio, S.; Okamoto, A.; Wu, X.; et al. Bintrafusp Alfa for Recurrent or Metastatic Cervical Cancer After Platinum Failure: A Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Javle, M.M.; Verdaguer Mata, H.; de Braud, F.; Trojan, J.; Raoul, J.-L.; Kim, J.W.; Ueno, M.; Lee, C.-K.; Hijioka, S.; et al. Phase 2 trial of bintrafusp alfa as second-line therapy for patients with locally advanced/metastatic biliary tract cancers. Hepatology 2023, 78, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.C.; Lee, J.S.; Wu, Y.-L.; Cicin, I.; Dols, M.C.; Ahn, M.-J.; Cuppens, K.; Veillon, R.; Nadal, E.; Dias, J.M.; et al. Bintrafusp Alfa Versus Pembrolizumab in Patients With Treatment-Naive, Programmed Death-Ligand 1-High Advanced NSCLC: A Randomized, Open-Label, Phase 3 Trial. J. Thorac. Oncol. 2023, 18, 1731–1742. [Google Scholar] [CrossRef]
- Boreddy, S.R.; Nair, R.; Pandey, P.K.; Kuriakose, A.; Marigowda, S.B.; Dey, C.; Banerjee, A.; Kulkarni, H.; Sagar, M.; Krishn, S.R.; et al. BCA101 Is a Tumor-Targeted Bifunctional Fusion Antibody That Simultaneously Inhibits EGFR and TGFβ Signaling to Durably Suppress Tumor Growth. Cancer Res. 2023, 83, 1883–1904. [Google Scholar] [CrossRef]
- Hanna, G.; Kaczmar, J.; Zandberg, D.; Wong, D.; Yilmaz, E.; Sherman, E.; Hernando-Calvo, A.; Sacco, A.; Chung, C.; Bohr, D.; et al. Dose expansion results of the bifunctional EGFR/TGFβ inhibitor BCA101 with pembrolizumab in patients with recurrent, metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2023, 41, 6005. [Google Scholar] [CrossRef]
- Li, T.; Wang, X.; Niu, M.; Wang, M.; Zhou, J.; Wu, K.; Yi, M. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front. Immunol. 2023, 14, 1196970. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Wang, D.; Liu, S.; Xiao, T.; Gu, W.; Yang, H.; Wang, H.; Yang, M.; Chen, P. Metabolic reprogramming and immune evasion: The interplay in the tumor microenvironment. Biomark. Res. 2024, 12, 96. [Google Scholar] [CrossRef]
- Beatty, G.L.; O’Dwyer, P.J.; Clark, J.; Shi, J.G.; Bowman, K.J.; Scherle, P.A.; Newton, R.C.; Schaub, R.; Maleski, J.; Leopold, L.; et al. First-in-Human Phase 1 Study of the Oral Inhibitor of Indoleamine 2,3-Dioxygenase-1 Epacadostat (INCB024360) in Patients With Advanced Solid Malignancies. Clin. Cancer Res. 2017, 23, 3269–3276. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients With Advanced Solid Tumors: Phase I Results from a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018, 36, 3223–3230. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.-J.; Kim, T.M.; et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.-W.; Strickler, J.H.; Kim, S.-W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. CII 2023, 72, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Pretelli, G.; Desai, J.; Garralda, E.; Siu, L.L.; Steiner, T.M.; Au, L. Bispecific antibodies: Advancing precision oncology. Trends Cancer 2024, 10, 893–919. [Google Scholar] [CrossRef]
- Kellner, C.; Otte, A.; Cappuzzello, E.; Klausz, K.; Peipp, M. Modulating Cytotoxic Effector Functions by Fc Engineering to Improve Cancer Therapy. Transfus. Med. Hemother 2017, 44, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chames, P.; Van Regenmortel, M.; Weiss, E.; Baty, D. Therapeutic antibodies: Successes, limitations and hopes for the future. Br. J. Pharmacol. 2009, 157, 220–233. [Google Scholar] [CrossRef]
- Chu, W.; Xu, H.; Wang, Y.; Xie, Y.; Chen, Y.; Tan, X.; Huang, C.; Wang, G.; Wang, Q.; Luo, W.; et al. HER2/PD1 bispecific antibody in IgG4 subclass with superior anti-tumour activities. Clin. Transl. Med. 2022, 12, e791. [Google Scholar] [CrossRef]
- Wang, L.; Hoseini, S.S.; Xu, H.; Ponomarev, V.; Cheung, N.-K. Silencing Fc Domains in T cell-Engaging Bispecific Antibodies Improves T-cell Trafficking and Antitumor Potency. Cancer Immunol. Res. 2019, 7, 2013–2024. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.; Tian, W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 2020, 13, 45. [Google Scholar] [CrossRef]
- Brinkmann, U.; Kontermann, R.E. The making of bispecific antibodies. MAbs 2017, 9, 182–212. [Google Scholar] [CrossRef]
- Offner, S.; Hofmeister, R.; Romaniuk, A.; Kufer, P.; Baeuerle, P.A. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol. Immunol. 2006, 43, 763–771. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Kebenko, M.; Goebeler, M.-E.; Wolf, M.; Hasenburg, A.; Seggewiss-Bernhardt, R.; Ritter, B.; Rautenberg, B.; Atanackovic, D.; Kratzer, A.; Rottman, J.B.; et al. A multicenter phase 1 study of solitomab (MT110, AMG 110), a bispecific EpCAM/CD3 T-cell engager (BiTE®) antibody construct, in patients with refractory solid tumors. Oncoimmunology 2018, 7, e1450710. [Google Scholar] [CrossRef] [PubMed]
- Hummel, H.-D.; Kufer, P.; Grüllich, C.; Seggewiss-Bernhardt, R.; Deschler-Baier, B.; Chatterjee, M.; Goebeler, M.-E.; Miller, K.; de Santis, M.; Loidl, W.; et al. Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: Phase I, dose-escalation study findings. Immunotherapy 2021, 13, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Cattaruzza, F.; Nazeer, A.; To, M.; Hammond, M.; Koski, C.; Liu, L.Y.; Pete Yeung, V.; Rennerfeldt, D.A.; Henkensiefken, A.; Fox, M.; et al. Precision-activated T-cell engagers targeting HER2 or EGFR and CD3 mitigate on-target, off-tumor toxicity for immunotherapy in solid tumors. Nat. Cancer 2023, 4, 485–501. [Google Scholar] [CrossRef]
- Vir Biotechnology, Inc. Vir Biotechnology Announces Encouraging Safety and Efficacy Data in Ongoing Dose Escalation Trials for Dual Masked T-Cell Engagers VIR-5818 in Solid Tumors and VIR-5500 in mCRPC. Available online: https://investors.vir.bio/news/news-details/2025/Vir-Biotechnology-Announces-Encouraging-Safety-and-Efficacy-Data-in-Ongoing-Dose-Escalation-Trials-for-Dual-Masked-T-Cell-Engagers-VIR-5818-in-Solid-Tumors-and-VIR-5500-in-mCRPC/default.aspx (accessed on 28 February 2025).
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef]
- Klebanoff, C.A.; Chandran, S.S.; Baker, B.M.; Quezada, S.A.; Ribas, A. T cell receptor therapeutics: Immunological targeting of the intracellular cancer proteome. Nat. Rev. Drug Discov. 2023, 22, 996–1017. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Piperno-Neumann, S.; Rutkowski, P.; Baurain, J.-F.; Schlaak, M.; Butler, M.O.; Sullivan, R.J.; Dummer, R.; Kirkwood, J.M.; Orloff, M.; et al. Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2023, 389, 2256–2266. [Google Scholar] [CrossRef]
- Fenis, A.; Demaria, O.; Gauthier, L.; Vivier, E.; Narni-Mancinelli, E. New immune cell engagers for cancer immunotherapy. Nat. Rev. Immunol. 2024, 24, 471–486. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Kim, H.R.; Saavedra, O.; Cervantes, A.; Lugowska, I.A.; Oberoi, A.; El-Khoueiry, A.B.; Thomas, J.S.; Rogowski, W.; Lopez, J.S.; Shim, B.Y.; et al. Preliminary results from the phase 2 study of AFM24 in combination with atezolizumab in patients with EGFR wild-type (EGFR-WT) non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2024, 42, 2522. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, Y.; Yang, H.; Wang, J.; Lou, H.; Li, D.; Wang, K.; Zhang, H.; Wu, T.; Li, Y.; et al. Cadonilimab plus platinum-based chemotherapy with or without bevacizumab as first-line treatment for persistent, recurrent, or metastatic cervical cancer (COMPASSION-16): A randomised, double-blind, placebo-controlled phase 3 trial in China. Lancet 2024, 404, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, Y.; Li, Z.; Zhang, X.; Gao, X.; Liu, B.; Wang, Y.; Ba, Y.; Li, N.; Zhang, R.; et al. First-line cadonilimab plus chemotherapy in HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma: A randomized, double-blind, phase 3 trial. Nat. Med. 2025, 31, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.; Pravin, J.; Rodrigues, L.; Uhlenbroich, S.; Everett, K.L.; Wollerton, F.; Morrow, M.; Tuna, M.; Brewis, N. CD137/OX40 Bispecific Antibody Induces Potent Antitumor Activity that Is Dependent on Target Coengagement. Cancer Immunol. Res. 2020, 8, 781–793. [Google Scholar] [CrossRef]
- Liu, B.; Guo, H.; Xu, J.; Qin, T.; Guo, Q.; Gu, N.; Zhang, D.; Qian, W.; Dai, J.; Hou, S.; et al. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs 2018, 10, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Dominik, P.K.; Stanfield, J.; Ding, S.; Yang, W.; Kurd, N.; Llewellyn, R.; Heyen, J.; Wang, C.; Melton, Z.; et al. Dual checkpoint blockade of CD47 and PD-L1 using an affinity-tuned bispecific antibody maximizes antitumor immunity. J. Immunother. Cancer 2021, 9, e003464. [Google Scholar] [CrossRef]
- Wu, T.; Dai, Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2017, 387, 61–68. [Google Scholar] [CrossRef]
- Sun, Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett. 2016, 380, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, Y.; Li, J.-K.; Du, W.-X.; Li, R.-G.; Yang, J.; Li, J.; Li, F.; Tan, H.-B. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020, 470, 126–133. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, X.; Wang, L.; Liu, K.; Liu, W.; Bo, L.; Wang, L. Identifying tumour microenvironment-related signature that correlates with prognosis and immunotherapy response in breast cancer. Sci. Data 2023, 10, 119. [Google Scholar] [CrossRef]
- Jacquemin, V.; Antoine, M.; Dom, G.; Detours, V.; Maenhaut, C.; Dumont, J.E. Dynamic Cancer Cell Heterogeneity: Diagnostic and Therapeutic Implications. Cancers 2022, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.X.; Loree, J.M.; Titmuss, E.; Jonker, D.J.; Kennecke, H.F.; Berry, S.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; et al. Liver Metastases and Immune Checkpoint Inhibitor Efficacy in Patients with Refractory Metastatic Colorectal Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2346094. [Google Scholar] [CrossRef]
- Guven, D.C.; Kavgaci, G.; Erul, E.; Syed, M.P.; Magge, T.; Saeed, A.; Yalcin, S.; Sahin, I.H. The Efficacy of Immune Checkpoint Inhibitors in Microsatellite Stable Colorectal Cancer: A Systematic Review. Oncologist 2024, 29, e580–e600. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism insights and therapeutic intervention of tumor metastasis: Latest developments and perspectives. Signal Transduct. Target. Ther. 2024, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Day, D.; Monjazeb, A.M.; Sharon, E.; Ivy, S.P.; Rubin, E.H.; Rosner, G.L.; Butler, M.O. From Famine to Feast: Developing Early-Phase Combination Immunotherapy Trials Wisely. Clin. Cancer Res. 2017, 23, 4980–4991. [Google Scholar] [CrossRef]
- Walsh, L.A.; Quail, D.F. Decoding the tumor microenvironment with spatial technologies. Nat. Immunol. 2023, 24, 1982–1993. [Google Scholar] [CrossRef]
- LaFleur, M.W.; Sharpe, A.H. CRISPR Screens to Identify Regulators of Tumor Immunity. Annu. Rev. Cancer Biol. 2022, 6, 103–122. [Google Scholar] [CrossRef]
- Arnold, C. Inside the nascent industry of AI-designed drugs. Nat. Med. 2023, 29, 1292–1295. [Google Scholar] [CrossRef]
- Paul, D.; Sanap, G.; Shenoy, S.; Kalyane, D.; Kalia, K.; Tekade, R.K. Artificial intelligence in drug discovery and development. Drug Discov. Today 2021, 26, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Cardeña-Gutiérrez, A.; López Barahona, M. Predictive Biomarkers of Severe Immune-Related Adverse Events with Immune Checkpoint Inhibitors: Prevention, Underlying Causes, Intensity, and Consequences. Front. Med. 2022, 9, 908752. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Hajjar, J.; Gulley, J.L.; Atkins, M.B.; Ciliberto, G.; Meric-Bernstam, F.; Hwu, P. Strategies for improving the management of immune-related adverse events. J. Immunother. Cancer 2020, 8, e001754. [Google Scholar] [CrossRef]
- Watson, G.A.; Doi, J.; Hansen, A.R.; Spreafico, A. Novel strategies in immune checkpoint inhibitor drug development: How far are we from the paradigm shift? Br. J. Clin. Pharmacol. 2020, 86, 1753–1768. [Google Scholar] [CrossRef]

| Drug Type | Mechanism | Trial ID | Drugs Combination | Phase | Tumors | Status | Results |
|---|---|---|---|---|---|---|---|
| LAG-3 | |||||||
| Relatlimab (BMS-986016) | Antagonist mAb | NCT01968109 | Single therapy, anti-PD-1 (nivolumab) | 1/2a | Advanced malignancies | Active, not recruiting | Cohort C: ORR = 16%; Cohort D: ORR = 9.2–12.1% |
| NCT03044613 | Anti-PD-1 (nivolumab) and CRT | 1 | Neoadjuvant esophageal/GEJ carcinoma | Active, not recruiting | Arm B: pCR = 21.4% | ||
| NCT04658147 | Anti-PD-1 (nivolumab) | 1 | Resectable HCC | Recruiting | n/a | ||
| NCT05337137 | Anti-PD-1 (nivolumab), bevacizumab | 1 | Advanced HCC | Active, not recruiting | n/a | ||
| NCT06683755 | Anti-PD-1 (nivolumab), anti-CTLA-4 (ipilimumab) | 1/2 | Advanced Melanoma | Not yet recruiting | n/a | ||
| INCAGN02385 | Antagonist mAb | NCT04370704 | Anti-PD-1 (retinfalimab), anti-TIM-3 (INCAGN02390) | 1/2 | Advanced malignancies | Active, not recruiting | n/a |
| LBL-007 | Antagonist mAb | NCT03744468 | Anti-PD-1 (tislelizumab), anti-TIM-3 (BGB-A425) | 1/2 | Advanced malignancies | Active, not recruiting | n/a |
| NCT05102006 | Anti-PD-1 (toripalimab) | 1/2 | Advanced malignancies | Recruiting | ORR = 13.3%; DCR = 48.0% | ||
| TQB2223 | Antagonist mAb | NCT05894421 | Anti-PD-1 (penpulimab) | 1 | Advances malignancies | Recruiting | n/a |
| NCT06320080 | Anti-PD-1 (penpulimab) | 1 | Advanced HCC | Recruiting | n/a | ||
| IBI110 | Antagonist mAb | NCT06494943 | Anti-PD-1 (sintilimab) | 1 | LA HNSCC | Active, not recruiting | n/a |
| Eftilagimod alpha (IMP321) | Soluble fusion protein | NCT03252938 | Single therapy, CT, or avelumab | 1 | Advanced malignancies | Recruiting | n/a |
| TIM-3 | |||||||
| BC4302 | Antagonist mAb | NCT06608940 | Anti-PD-L1 (durvalumab), anti-CTLA-4 (tremelimumab) | 1/2 | Advanced HCC | Not yet recruiting | n/a |
| Cobolimab (TSR-022) | Antagonist mAb | NCT02817633 | Single therapy, anti-PD-1 (nivolumab/dostarlimab) | 1 | Advanced malignancies | Recruiting | n/a |
| Sabatolimab (MBG453) | Antagonist mAb | NCT03961971 | Anti-PD-1 (spartalizumab) | 1 | Recurrent GBM | Active, not recruiting | n/a |
| INCAGN02390 | Antagonist mAb | NCT04370704 | Anti-PD-1 (retifanlimab), anti-LAG-3 (INCAGN02385) | 1/2 | Advanced melanoma | Active, not recruiting | n/a |
| TIGIT | |||||||
| COM902 | Antagonist mAb | NCT04354246 | Single therapy, anti-PVRIG (COM701), anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Recruiting | MSS-CRC cohort: ORR 5%; DCR 40%. |
| Tiragolumab | Antagonist mAb | NCT05394337 | Anti-PD-L1 (atezolizumab) | 1/2 | Neoadjuvant UC | Recruiting | n/a |
| Domvanalimab | Antagonist mAb | NCT04656535 | Anti-PD-1 (zimberelimab) | 0/1 | GBM | Recruiting | n/a |
| PM1021 | Antagonist mAb | NCT05537051 | Single therapy, PD-1 × TGF-β (PM8001) | 1 | Advanced malignancies | Not yet recruiting | n/a |
| Tamgiblimab (IBI939) | Antagonist mAb | NCT04353830 | Single therapy, anti-PD-1 (sintilimab) | 1 | Advanced malignancies | Not yet recruiting | n/a |
| Belrestotug (EOS-448) | Antagonist mAb | NCT05060432 | Single therapy, anti-PD-1 (pembrolizumab or dostarlimab), A2AR inhibitor (inupnant), chemotherapy | 1/2 | Advanced malignancies | Active, not recruiting | n/a |
| AB308 | Antagonist mAb | NCT04772989 | Anti-PD-1 (zimberelimab) | 1 | Advanced malignancies | Active, not recruiting | n/a |
| AK127 | Antagonist mAb | NCT05868876 | AK104 (PD-1 × CTLA-4) | 1 | Advanced malignancies | Recruiting | n/a |
| HLX53 | Fc fusion protein | NCT05394168 | Single therapy, anti-PD-1 (nivolumab) | 1 | Advanced malignancies | Active, not recruiting | n/a |
| B7-H5 | |||||||
| HMBD-002 | Antagonist mAb | NCT05082610 | Anti-PD-1 (pembrolizumab) | 1 | Advances malignancies | Recruiting | n/a |
| SNS-101 | Antagonist mAb | NCT05864144 | Anti-PD-1 (cemiplimab) | 1/2 | Advances malignancies | Recruiting | ORR = 3%; DCR = 35% |
| PMC-309 | Antagonist mAb | NCT05957081 | Anti-PD-1 (pembrolizumab) | 1 | Advances malignancies | Not yet recruiting | n/a |
| Drug Type | Mechanism | Trial ID | Drugs Combination | Phase | Tumors | Status | Results |
|---|---|---|---|---|---|---|---|
| OX40 | |||||||
| INBRX-106 | Agonistic mAb | NCT04198766 | Anti-PD-1 (pembrolizumab) | 1/2a | Advanced malignancies | Recruiting | n/a |
| BGB-A445 | Agonistic mAb | NCT04215978 | Anti-PD-1 (tislelizumab) | 1/2 | Advanced malignancies | Active, not recruiting | ORR = 23%; DCR 66.7% |
| Agonistic mAb | NCT05661955 | Anti-PD-1 (tislelizumab) | 1 | Advanced UC, RCC, melanoma | Recruiting | n/a | |
| ES102 | Agonistic mAb | NCT04730843 | Anti-PD-1 (toripalimab) | 1 | Advanced malignancies | Recruiting | n/a |
| HFB301001 | Agonistic mAb | NCT06623136 | Single therapy | 1 | Advanced malignancies | Active, not recruiting | DCR > 60% |
| HLX51 | Agonistic mAb | NCT05788107 | Single therapy | 1 | Advanced malignancies | Not yet recruiting | n/a |
| GEN1055 | Agonistic mAb | NCT06391775 | Anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Recruiting | n/a |
| 4-1BB | (CD137) | ||||||
| YH004 | Agonistic mAb | NCT05564806 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| EU101 | Agonistic mAb | NCT04903873 | Single therapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| ADG206 | Agonistic mAb | NCT05614258 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| LVGN6051 | Agonistic mAb | NCT05301764 | TKI (anlotinib) | 1/2 | Soft-tissue sarcomas | Recruiting | ORR = 6.9%; DCR 86.2%; G3–4 TRAEs: 61.54% |
| GITR | |||||||
| REGN6569 | Agonistic mAb | NCT04465487 | Anti-PD-1 (cemiplimab) | 1 | Advanced malignancies | Active, not recruiting | ORR = 6.9% |
| Drug Type | Mechanism | Trial ID | Drugs Combination | Phase | Tumors | Status | Results |
|---|---|---|---|---|---|---|---|
| IL-2 | |||||||
| THOR-747 | Non-alfa IL-2 | NCT04009681 | Anti-PD-1 (pembrolizumab), anti-EGFR (cetuximab) | 1/2 | Advanced malignancies | Active, not recruiting | ORR = 5.9% |
| MDNA11 | Non-alfa IL-2 | NCT05086692 | Anti-PD-1 (pembrolizumab) | 1/2 | Advanced malignancies | Recruiting | ORR = 7.7% |
| ALKS-4230 (Nevmaleukin alfa) | Fusion protein (IL-2Ra) | NCT04592653 | Anti-PD-1 (pembrolizumab) | 1/2 | Advanced malignancies | Active, not recruiting | n/a |
| AU007 | Agonistic mAb | NCT05267626 | Anti-PD-1 (avelumab), aldesleukin | 1/2 | Advanced malignancies | Recruiting | n/a |
| XTX202 | IL-2 tumor-activated | NCT05052268 | Single therapy | 1/2 | Advanced malignancies | Active, not recruiting | DCR = 31% |
| WTX-124 | IL-2 prodrug | NCT05479812 | Anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Recruiting | Monotherapy: ORR = 30% |
| ODC-IL2 | IL-2 prodrug | NCT06770764 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| RO7284755 (Eciskafusp alfa) | Immunocytokine PD1-IL2v | NCT04303858 | Anti-PD-L1 (atezolizumab) | 1/2 | Advanced malignancies | Recruiting | n/a |
| ANV600 | Immunocytokine PD1-IL2v | NCT06470763 | Anti-PD-1 (pembrolizumab) | 1/2 | Advanced malignancies | Recruiting | n/a |
| STAR0602 (invikafusp alfa) | Bifunctional Antibody-fusion (TCR/IL2) | NCT05592626 | Single therapy | 1/2 | Advanced malignancies | Recruiting | DCR = 60% |
| IL-15 | |||||||
| N-803 | IL-15 superagonist (intravesical) | NCT02138734 | Intravesical BCG | 1/2 | NMIBC | Recruiting | n/a |
| IL-15 superagonist (subcutaneous) | NCT06253494 | Anti-PD-1 (pembrolizumab), lenvatinib, and HER2 Autologous DC vaccine | 1/2 | Endometrial cancer | Recruiting | n/a | |
| IL-15 superagonist (subcutaneous) | NCT06149481 | SX-682, TriAdeno vaccine, and Retifanlimab | 1/2 | Advanced melanoma | Recruiting | n/a | |
| SOT201 | Immunocytokine PD-L1/IL-15 | NCT06163391 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| SAR445877 | Immunocytokine PD-L1/IL-15 | NCT05584670 | Anti-EGFR (cetuximab) | 1/2 | Advanced malignancies | Recruiting | n/a |
| Drug Type | Mechanism | Trial ID | Drugs Combination | Phase | Tumors | Status | Results |
|---|---|---|---|---|---|---|---|
| Anti-CCR8 | |||||||
| CHS-114 | Anti-CCR8 mAb | NCT06657144 | anti-PD-1 (toripalimab) | 1 | Advanced malignancies | Not yet recruiting | n/a |
| NCT05635643 | single therapy | 1 | Advanced malignancies | Recruiting | n/a | ||
| LM-108 | Anti-CCR8 mAb | NCT05199753 | anti-PD-1 | 1/2 | Advanced malignancies | Recruiting | Pooled analysis (gastric): ORR = 36.1%; G3/4 TRAEs = 37.5% |
| BAY 3375968 | Anti-CCR8 mAb | NCT05537740 | anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Recruiting | n/a |
| S-531011 | Anti-CCR8 mAb | NCT05101070 | anti-PD-1 (pembrolizumab) | 1/2 | Advanced malignancies | Recruiting | n/a |
| GS-1811 | Anti-CCR8 mAb | NCT05007782 | anti-PD-1 (zimberelimab) | 1 | Advanced malignancies | Recruiting | n/a |
| BMS-986340 | Anti-CCR8 mAb | NCT04895709 | anti-PD-1 (nivolumab), chemotherapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| AMG-355 | Anti-CCR8 mAb | NCT06131398 | anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Recruiting | n/a |
| RO7502175 | Anti-CCR8 mAb | NCT05581004 | anti-PD-1 (pembrolizumab, anti-PD-L1 (atezolizumab) | 1 | Advanced malignancies | Recruiting | n/a |
| BGB-A3055 | Anti-CCR8 mAb | NCT05935098 | anti-PD-1 (tislellizumab) | 1 | Advanced malignancies | Recruiting | n/a |
| ABBV-514 | Anti-CCR8 mAb | NCT05005403 | anti-PD-1 (budigalimab) | 1 | Advanced malignancies | Recruiting | n/a |
| IPG7236 | Anti-CCR8 mAb | NCT05142592 | single therapy | 1 | Advances malignancies | Recruiting | n/a |
| QLP2117 | Anti-CCR8 mAb | NCT05830045 | single therapy | 1 | Advances malignancies | Recruiting | n/a |
| CM369 | Anti-CCR8 mAb | NCT05690581 | single therapy | 1 | Advances malignancies | Recruiting | n/a |
| HC006 | Anti-CCR8 mAb | NCT06304571 | single therapy | 1 | Advances malignancies | Recruiting | n/a |
| ZL-1218 | Anti-CCR8 mAb | NCT05859464 | anti-PD-1 (pembrolizumab) | 1 | Advances malignancies | Recruiting | n/a |
| Anti-CD47 | |||||||
| Evorpacept (ALX148) | Anti-CD47 fusion protein | NCT03013218 | Anti-PD-1 (pembrolizumab), anti-HER2 (Trastuzumab), CT | 1 | Advanced solid tumors | Active, not recruiting | HNSCC ORR = 20%; NSCLC ORR = 5%; G/GEJ ORR = 21.1% |
| NCT05524545 | Enfortumab-vedotin | 1 | Advanced UC | Recruiting | ORR = 63% | ||
| HCB101 | Anti-CD47 fusion protein | NCT05892718 | Single therapy | 1 | Advanced solid tumors | Recruiting | |
| STI-6643 | Anti-CD47 mAb | NCT04900519 | Single therapy | 1 | Advanced solid tumors | Recruiting | n/a |
| IMC-002 | Anti-CD47 mAb | NCT05276310 | Single therapy | 1 | Advanced solid tumors | Recruiting | DCR = 45.5% |
| AUR103 | Small molecule | NCT05607199 | Single therapy | 1 | Advanced solid tumors | Recruiting | n/a |
| DS-1103a | Anti-SIRPα mAb | NCT05765851 | Trastuzumab-deruxtecan | 1 | Advanced solid tumors | Recruiting | n/a |
| HMPL-A83 | Anti-CD47 mAb | NCT05429008 | Single therapy | 1 | Advanced solid tumors | Recruiting | n/a |
| TGF-β | |||||||
| Galunisertib | Oral TGF-βR1 inhibitor | NCT05700656 | CT | 1/2 | Advanced CRC | Recruiting | n/a |
| Vactovasertib | Oral TGF-βR1 inhibitor | NCT05588648 | Single therapy | 1/2 | Osteosarcoma | Recruiting | n/a |
| NCT03732274 | Anti-PD-L1 (durvalumab) | 1/2 | Advanced NSCLC | Active, not recruiting | ORR = 30.8% | ||
| NCT03724851 | Anti-PD-1 (pembrolizumab) | 1/2 | CRC and G/GEJ adenocarcinoma | Active, not recruiting | ORR = 13.3% | ||
| LY3200882 | Oral TGF-βR1 inhibitor | NCT02937272 | CT, anti-PD-L1 (LY3300054) | 1 | Advanced malignancies | Active, not recruiting | Pancreatic: DCR = 75% |
| SRK-181 | Anti-TGF-β1 | NCT04291079 | Anti-PD-1 or anti-PD-L1 | 1 | Advanced malignancies | Active, not recruiting | ORR = 16.2%; DCR = 51.5% |
| Drug Type | Mechanism | Trial ID | Drugs Combination | Phase | Tumors | Status | Results |
|---|---|---|---|---|---|---|---|
| T-cell engagers | |||||||
| BA1202 | CEA × CD3 | NCT05909241 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| TAK-186 (MVC-101 | EGFR × CD3 | NCT04844073 | Single therapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| CX-904 | EGFR × CD3 | NCT05387265 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| KM257 | HER2 × CD3 | NCT05320874 | Single therapy, CT | 1 | Advanced malignancies | Not yet recruiting | n/a |
| IMM2902 | HER2 × CD3 | NCT05805956 | Single therapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| Tarlatamab | DLL3 × CD3 | NCT03319940 | Single therapy | 1 | SCLC | Active, not recruiting | ORR = 23.4%; |
| Ubamatamab (REGN4018) | MUC16 × CD3 | NCT03564340 | Single therapy, anti-PD-1 (cemiplimab) | 1/2 | Ovarian cancer | Active, not recruiting | ORR = 18.2% |
| CC-1 | PSMA × CD3 | NCT04104607 | Single therapy | 1 | CRPC | Recruiting | n/a |
| NCT05646550 | Single therapy | 1 | Prostate cancer | Recruiting | n/a | ||
| REGN4336 | PSMA × CD3 | NCT05125016 | REGN5678 (PSMA × CD28), anti-PD-1 (cemiplimab) | 1/2 | CRPC | Recruiting | n/a |
| Xaluritamig (AMG-509) | STEAP1 × CD3 | NCT04221542 | Single therapy | 1 | CRPC | Recruiting | ORR = 22.7% |
| Cabotamig (ARB202) | CDH17 × CD3 | NCT05411133 | Single therapy | 1/2 | Advanced GI | Recruiting | n/a |
| XmAb819 | ENNP3 × CD3 | NCT05433142 | Single therapy | 1 | Advanced RCC | Recruiting | n/a |
| JNJ-87890387 | ENNP3 × CD3 | NCT06178614 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| EMB-07 | ROR1 × CD3 | NCT05607498 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| BA1202 | CEA × CD3 | NCT05909241 | Single therapy | 1 | Advances malignancies | Recruiting | n/a |
| AZD5863 | Claudin18.2 × CD3 | NCT06005493 | Single therapy | 1/2 | Advanced GI | Recruiting | n/a |
| XmAb541 | Claudin6 × CD3 | NCT06276491 | Single therapy | 1 | Advances malignancies | Recruiting | n/a |
| BNT-142 | Claudin6 × CD3 | NCT05262530 | Single therapy | 1 | Advances malignancies | Recruiting | n/a |
| CC-3 | B7-H3 × CD3 | NCT05999396 | Monotherapy | 1 | Advanced CRC | Recruiting | n/a |
| GEN1047 | B7-H4 × CD3 | NCT05180474 | Monotherapy | 1/2 | Advanced solid tumors | Active, not recruiting | n/a |
| BA3182 | CAB-EPCAM × CAB-CD3 | NCT05808634 | Single therapy | 1/2 | Advances adenocarcinoma | Recruiting | n/a |
| IM401 TCER® | MAGEA4/8 × TCR | NCT05359445 | Single therapy, pembrolizumab | 1 | Advanced malignancies (restricted to HLA A*02:01) | Recruiting | DCR = 55% |
| IM402 TCER® | PRAME × TCR | NCT05958121 | Single therapy | 1/2 | Advanced malignancies (restricted to HLA-A*02:01) | Recruiting | n/a |
| γδ T-cell engagers | |||||||
| LAVA-1207 | PSMA × CD3 | NCT05369000 | Single therapy, IL-2, pembrolizumab | 1/2 | CRPC | Active, not recruiting | n/a |
| NK-cell engagers | |||||||
| AFM24 | EGFR × CD16 | NCT05109442 | Anti-PD-L1 (atezolizumab) | 1 | Advanced malignancies | Recruiting | EGFR wt: ORR = 26.7% |
| Immunomodulators | |||||||
| Dual inhibitory checkpoints | |||||||
| IBI318 | PD-1 × CTLA-4 | NCT04777084 | Single therapy | 1 | NSCLC | Recruiting | n/a |
| SSGJ-706 | PD-1 × CTLA-4 | NCT06533605 | Single therapy | 1 | Advances malignancies | Not yet recruiting | n/a |
| Lorigerlimab (MDG019) | PD-1 × CTLA-4 | NCT03761017 | Single therapy | 1 | Advances malignancies | Active, not recruiting | CPRC cohort: ORR = 25.7% |
| Cadonilimab (AK104) | PD-1 × CTLA-4 | NCT05426005 | Single therapy | 1 | Advanced dMMR CRC | Recruiting | n/a |
| PD-1 × CTLA-4 | NCT05994001 | LM-302 (Claudin18.2 × CD3) | 1/2 | Billiary tract cancer | Recruiting | ORR = 50% | |
| SI-B003 | PD-1 × CTLA-4 | NCT04606472 | Single therapy | 1 | Advances malignancies | Recruiting | ORR = 16.1%; DCR = 50% |
| Tobemstomig (RO7245669) | PD-1 × LAG-3 | NCT04140500 | Single therapy | 1 | Advances malignancies | Active, not recruiting | ORR = 17.1%; DCR = 51.4% |
| INCA32459 | PD-1 × LAG-3 | NCT05577182 | Single therapy | 1 | Advances malignancies | Active, not recruiting | n/a |
| AK129 | PD-1 × LAG-3 | NCT05645276 | Single therapy | 1/2 | Advances malignancies | Recruiting | n/a |
| AZD7789 | PD-1 × TIM-3 | NCT04931654 | Single therapy | 1/2 | Advanced malignancies | Active, not recruiting | NSCLC: DCR = 47%; G3/4 TRAEs = 23% |
| LB1410 | PD-1 × TIM-3 | NCT05357651 | Single therapy | 1 | Advanced malignancies | Recruiting | DCR = 35% |
| Rilvegostomig (AZD2936) | PD-1 × TIGIT | NCT04995523 | Single therapy | 1/2 | Advanced NSCLC | Recruiting | ORR = 3,9%; DCR = 43% |
| BC008-1A | PD-1 × TIGIT | NCT06773481 | Single therapy | 1 | Recurrent glioma | Not yet recruiting | n/a |
| HLX301 | PD-L1 × TIGIT | NCT05102214 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| Co-stimulatory bsAbs | |||||||
| FS120 | OX40 × 4-1BB | NCT05263180 | Anti-PD-1 (pembrolizumab) | 1 | Advanced malignancies | Active, not recruiting | n/a |
| EMB-09 | OX40 × PD-L1 | NCT05263180 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| XmAb®808 | CD28 × B7-H3 | NCT05585034 | Pembrolizumab | 1 | Advanced malignancies | Recruiting | n/a |
| BNA035 | 4-1BB × EGFR | NCT05150457 | Monotherapy | 1 | Advanced malignancies | Recruiting | n/a |
| ABL103 | 4-1BB × B7-H4 | NCT06126666 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| YH3267 | 4-1BB × HER2 | NCT05523947 | Monotherapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| HLX35 | 4-1BB × EGFR | NCT05360381 | Single therapy | 1 | Advanced malignancies | Active, not recruiting | n/a |
| PRS-344/S095012 | 4-1BB × PD-L1 | NCT05159388 | Single therapy | 1 | Advanced malignancies | Active, not recruiting | n/a |
| CB307 | 4-1BB × PSMA | NCT04839991 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| ABL503 | 4-1BB × PD-L1 | NCT04762641 | Single therapy | 1 | Advanced malignancies | Recruiting | ORR = 15.3%; DCR = 61.5% |
| FS222 | 4-1BB × PD-L1 | NCT05924906 | Single therapy | 1 | Advanced malignancies | Recruiting | n/a |
| ALG.APV-527 | 4-1BB × 5T4 | NCT05934539 | Single therapy | 1/2 | Advanced malignancies | Recruiting | n/a |
| PM1032 | 4-1BB × Claudin18.2 | NCT05839106 | Single therapy | 1/2 | Advanced malignancies | Recruiting | ORR = 12.5%; DCR = 56.2%; G3/4 TRAEs: 10% |
| Anti-CD47 bsAbs | |||||||
| Peluntamig (PT217) | DLL3 × CD47 | NCT05652686 | anti-PD-L1 (atezolizumab), CT | 1/2 | Neuroendocrine tumors/carcinoma | Recruiting | n/a |
| BAT7104 | PD-L1 × CD47 | NCT05767060 | Single therapy | 1 | Advanced solid tumors | Recruiting | n/a |
| IMM2520 | PD-L1 × CD47 | NCT05780307 | Single therapy | 1 | Advanced solid tumors | Recruiting | DCR = 54.5%; G3/4 TRAEs 56% |
| IMM2902 | HER-2 × CD47 | NCT05805956 | Single therapy | 1 | Advanced solid tumors | Recruiting | DCR = 30.8%; G3/4 TRAEs 25% |
| IBC0966 | PD-L1 × CD47 | NCT04980690 | Single therapy | 1/2 | Advanced solid tumors | Recruiting | n/a |
| HX009 | PD-1 × CD47 | NCT05731752 | Single therapy | 1 | Advanced solid tumors | Active, not recruiting | n/a |
| TGF-β bsAbs | |||||||
| YM101 | PD-L1 × TGF-β | NCT05028556 | Single therapy | 1 | Advanced malignancies | Active, not recruiting | n/a |
| BCA101 | EGFR × TGF-β | NCT04429542 | Anti-PD-1 (pembrolizumab) | 1 | Advances malignancies | Recruiting | ORR = 23.3%; DCR 81.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morchón-Araujo, D.; Catani, G.; Mirallas, O.; Pretelli, G.; Sánchez-Pérez, V.; Vieito, M.; Braña, I.; Pujol-Borrell, R.; Garralda, E.; Hernando-Calvo, A. Emerging Immunotherapy Targets in Early Drug Development. Int. J. Mol. Sci. 2025, 26, 5394. https://doi.org/10.3390/ijms26115394
Morchón-Araujo D, Catani G, Mirallas O, Pretelli G, Sánchez-Pérez V, Vieito M, Braña I, Pujol-Borrell R, Garralda E, Hernando-Calvo A. Emerging Immunotherapy Targets in Early Drug Development. International Journal of Molecular Sciences. 2025; 26(11):5394. https://doi.org/10.3390/ijms26115394
Chicago/Turabian StyleMorchón-Araujo, Daniel, Greta Catani, Oriol Mirallas, Giulia Pretelli, Vicky Sánchez-Pérez, María Vieito, Irene Braña, Ricardo Pujol-Borrell, Elena Garralda, and Alberto Hernando-Calvo. 2025. "Emerging Immunotherapy Targets in Early Drug Development" International Journal of Molecular Sciences 26, no. 11: 5394. https://doi.org/10.3390/ijms26115394
APA StyleMorchón-Araujo, D., Catani, G., Mirallas, O., Pretelli, G., Sánchez-Pérez, V., Vieito, M., Braña, I., Pujol-Borrell, R., Garralda, E., & Hernando-Calvo, A. (2025). Emerging Immunotherapy Targets in Early Drug Development. International Journal of Molecular Sciences, 26(11), 5394. https://doi.org/10.3390/ijms26115394






