Abstract
Over the past four decades, bivalves have become sentinel organisms in genotoxicity research due to their ecological relevance and sensitivity to environmental contaminants. This integrative review critically examines the evolution of genotoxicity in bivalves, from early cytogenetic assays to advanced transcriptomic approaches. It highlights key methodological developments, geographical research trends, and the recent integration of multi-endpoint analyses for a more robust, consistent environmental risk assessment. By synthesizing data from four decades of research, we provide a comprehensive overview of current knowledge while also critically identifying persistent challenges and suggesting directions for future research to allow better evaluation and mitigation of the genetic impacts of marine pollution.
Keywords:
genotoxicity; bivalves; cytogenetics; DNA; gene; transcriptomics; molecular responses; chemical stressor 1. Introduction
Organisms inhabiting coastal areas are exposed to a wide range of physical and chemical stressors originating from human activities. These stressors, which include heavy metals, pesticides and industrial chemicals, physical pollutants such as microplastics, or altered sediment dynamics, among other factors, leach into the marine environment, causing adverse effects on marine life [1,2,3]. Despite past and ongoing research, there remains a lack of comprehensive understanding of their full impact on marine ecosystems [1]. One main concern is the ingestion and bioaccumulation of chemical substances that are prevalent in the marine environment by marine organisms [4,5,6]. Some of these widespread substances can induce alterations and changes in genetic material at the DNA or chromosomal level; hence, they are called genotoxins [7,8]. The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) has defined genotoxins and genotoxicity as “agents or processes which alter the structure, information content, or segregation of DNA, including those which cause DNA damage by interfering with normal replication processes, or which in a non-physiological manner (temporarily) alter its replication” [9]. Genotoxins can initiate a cascading, delayed effect, beginning at low biological levels and causing modifications in the genetic material even at non-lethal, non-cytotoxic concentrations. These alterations often result in delayed consequences at the cellular level, which can extend to the organism and potentially lead to prolonged impacts at the population and community levels [10,11].
To fully understand and mitigate these potential risks, it is essential to employ tools with a wide application range that extends beyond the scope of classic toxicology. The growing concern over the increasing bioavailability of (geno)toxic agents in the marine environment has driven the development of more sophisticated approaches/advanced methodologies that better assess their biological impacts. This has led to the establishment of a new specialized field of toxicology—genetic toxicology, also known as genotoxicology or genotoxicity—dedicated to investigating the carcinogenicity and mutagenicity of these compounds at the genetic level [8,10].
In the early 1980s, the first reports on genotoxicity testing in bivalves were published (Figure 1). Since then, bivalves have become focal organisms in genotoxicity research, mainly due to their wide ecological distribution, sessile lifestyle, feeding mechanisms, and relatively short maturation cycle. Initial studies on bivalve genotoxicity employed experimental and monitoring approaches to examine a wide range of stressors. During the first decade of genotoxicity research, studies on bivalves were limited to Italy, the UK, and Croatia. Out of 470 studies performed between 1982 and 2025, 313 were carried out in Europe, with Italy taking the lead with 77 studies, followed by the UK and France with 55 and 47 studies, respectively (Figure 2a,b). Since 2004, there has been a significant increase in bivalve genotoxicity research in China, with 35 studies published and around half of them using omics endpoints (Figure 2a). Species-wise, the majority of studies carried out so far have used Mytilus sp. as the model species, representing 45% of the studies. However, the number of model species has been steadily increasing, with several other species currently being used in genotoxicity assays, such as Crassostrea sp., Dreissena sp., and Anodonta sp.
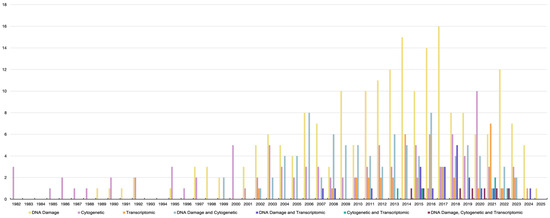
Figure 1.
Published genotoxic data by endpoint in chronological order from 1982 to 2025.
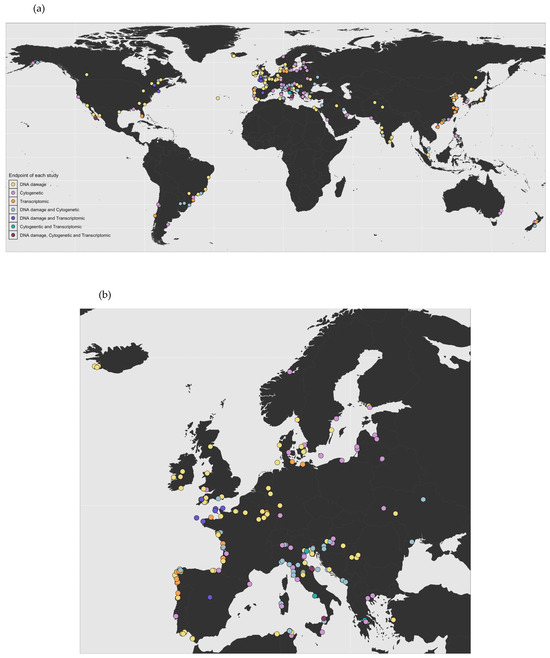
Figure 2.
Geographical distribution of published genotoxicity studies by endpoint: (a) worldwide; (b) Europe (R package version 1.0.0.9000. 2025).
This integrative review aims to provide a comprehensive summary of the published data on genotoxicity assays applied to bivalve tissue and analyze the different methodologies used to assess environmental genotoxicity in marine bivalves, examining their evolution over time and across geographic regions. Major scientific databases (including Web of Knowledge (Web of Science), Scopus, PubMed, Springer Link, and Google Scholar) were searched, primarily using a combination of the following keywords: “Genotoxic”, “bivalve”, “oyster”, “mussel”, “clam”, “cockle”, “scallop”, “contaminant”, and “pollution”. Studies were excluded if they did not involve genotoxicity investigations, did not utilize a bivalve model, or employed assays applied exclusively to mammalian cell lines or bacterial systems rather than directly to bivalve tissues. By synthesizing the results of the published studies, this review offers a clearer picture of the impact of environmental genotoxins on marine bivalves. This critical analysis of the research published so far aims to support future studies and contribute to the development of more effective strategies for monitoring and mitigating the adverse effects of chemical pollutants on marine life.
2. Endpoint Evolution over Time and Across Geographic Regions
2.1. Cytogenetic Endpoints
The earliest reports on bivalve genotoxicity focused on utilizing chromosome-level endpoints, such as chromosomal aberrations—both structural and numerical (aneuploidy)—and sister chromatid exchange (SCE) ([12,13,14]; Figure 1). These studies provided crucial information on the non-reversible impact on chromosomes that can be observed during cell division. Both aneuploidy and SCE levels displayed high sensitivity in detecting the genetic impact of elevated concentrations of heavy metals and hydrocarbons in mussel tissue (Supplementary Table S1). A consistent dose–response relationship between these endpoints and different toxicants was repeatedly observed, highlighting their importance in early environmental monitoring efforts.
During the first decade of genotoxicity studies on bivalves, two-thirds of the publications focused on cytogenetics and numerical chromosomal aberrations in particular, mainly in mussels and specifically Mytilus spp. (Figure 1). These direct chromosomal aberration and SCE tests require the capture of dividing cells in specific phases of the cell cycle.
In contrast, the micronucleus assay does not rely on cell division staging. Micronuclei can be observed in interphase cells and are formed by DNA fragmentation after stress, exposure, or chromosome mis-segregation during cell division. In the last case, a whole chromosome or part of it lags behind in anaphase, condenses, and then proceeds to the next division cycle independently of the main nucleus. This assay rose in popularity because it is a simple and inexpensive method with high sensitivity. From 1987 to 2025, the micronucleus assay was applied in 82.6% of genotoxicity studies with cytogenetic endpoints across various bivalve groups, including mussels, oysters, clams, cockles, and scallops, at different life stages. Compared to chromosomal structural and numerical aberration analysis, the micronucleus assay requires less specialized skills. This is particularly advantageous since chromosomal aberration assays cannot be used without prior knowledge of the diploid number (for numerical aberrations) and karyotype (for structural aberrations) of the tested species. However, chromosomal aberration analysis can provide additional information beyond what the micronucleus assay can offer, such as differentiating between clastogenic agents—which cause chromosome deletions, duplications, and translocation—and aneugenic agents—which cause aneuploidy. Applications of chromosomal structural and numerical aberration detection methodologies included various groups of bivalves but were geographically limited to Europe, with the exception of two studies recently performed in Qatar on the pearl oyster Pinctada radiata [15,16]. On the other hand, studies using the SCE assay were exclusively limited to mussels and ceased after Cornet’s publication [17]. The main concerns regarding this test remain unresolved, such as the preservation of intact labeled metaphases and the maintenance of a high-yield cell division rate for two or more cycles, both essential to ensuring the integrity and validity of the test [13,18]. Overall, research on bivalve genotoxicity utilizing cytogenetic endpoints continues to thrive, with a tendency to favor the micronucleus test over the others due to its simplicity and cost-effectiveness. Following is a brief summary of the main results of genotoxic studies for each cytogenetic endpoint technique, including recent methodological improvements.
2.1.1. Chromosomal Aberration(s)
In 1982 [12], Dixon assessed genotoxic damage in Mytilus edulis from polluted harbor sites. Through the examination of embryos, he noted a significantly higher aneuploidy level in cells from samples from contaminated sites. Shortly after, the use of Mytilus spp. mussels in environmental monitoring investigations was extended to sites that are not typically inhabited by mussels as part of the Mussel Watch Program in non-occupied sites [19]. The Mussel Watch Program by the National Centers for Coastal Ocean Science (NCCOS) initiated coastal monitoring projects to investigate the toxicity of a wide range of emerging contaminants. At the forefront of investigated toxicants was the anti-biofouling agent tributyltin (TBT), which was commonly used in the 1970s and was only effectively banned in 2008 [20]. In 1986, Dixon and Prosser published their work on how different concentrations of TBT ranging from 0.05 μg/L to 1 μg/L could cause cytotoxic effects on M. edulis embryos, but they concluded that this agent did not induce aneuploidy. However, Jha et al. [21] provided evidence for TBT’s involvement in inducing aneuploidy in 12-h-old mussel embryos, the same age as those used by Dixon and Prosser [22]. This difference was attributed to the duration of embryo exposure to the stressor, during which cell cycle dynamics must be taken into account [21]. Jha et al.’s team from Plymouth Research Center produced three publications—including the one just above—on the effect of dredging on the coastal line and the effect of TBT and its derivative TBTO (tributyltin oxide) on M. edulis larvae [21,23,24]. There was a positive correlation between induced chromosomal damage and contaminant concentration [21,23] and time [24]. Studies of numerical chromosomal aberrations were not always restricted to the traditional method of direct chromosomal counting with the use of the air-drying technique; the use of flow cytometry was introduced to this field in 2003 by Bihari et al. [25]. In this study, cell cycle alterations were observed to measure the influence of poor environmental conditions on mussel health, demonstrating the possibility of using flow cytometry as a sensitive tool to spot changes in the DNA cell cycle profile and ploidy levels throughout the different phases of the cell cycle.
The first study assessing genotoxicity in oysters was conducted by Bouilly et al. [26], who investigated the effects of the pesticide atrazine on adults and juveniles of the Pacific oyster Crassostrea gigas. Through a controlled laboratory experiment, the authors confirmed the genotoxic effects of atrazine, which showed a clear dose-dependent relationship. Embryos and larvae of the same species were tested by Cheung et al. [27], and the aneuploidy level was found to increase with the concentration of the alkylating agent methyl methane sulfonate (MMS) or the endocrine disturber benzo[a]pyrene (B[a]P). However, the number of aneuploid metaphases decreased at the highest concentration of B[a]b. The authors suggested that the dose-dependent relationship between a genotoxic agent and its effect is limited by the cell’s ability to metabolize that agent; once the toxicity threshold has exceeded that limit, observations of cytogenetic aberrations decrease due to a low cell division rate. The utilization of complementary techniques such as flow cytometry to unravel the cell proliferation state would be an advantage in these studies.
The previously mentioned studies provided strong cytogenetic evidence of genotoxicity in Mytilus sp. and C. gigas across different life stages. However, the long-term toxic effects remained unclear, particularly the potential genotoxic consequences of parental exposure to toxic agent(s) on bivalve offspring. To address this knowledge gap, a new cohort of genotoxic studies emerged. In 2004, the persistence of aneuploidy levels across generations was demonstrated in bivalves, C. gigas oyster, in particular. Studies confirmed that juveniles—although not directly exposed to a genotoxic agent—could inherit genetic damage from a parent previously exposed to atrazine [28,29] or diuron [29,30]. Unlike alterations in hemocyte parameters, chromosomal damage induced by exposure to diuron was irreversible [29]. Chromosome loss due to aneuploidy might result in the absence of genetic regions, including crucial genes, which can lead to severe physiological consequences such as disrupted sexual maturity and reduced embryo survival rates [29,30]. To assess such genetic impacts, Barranger et al. [30] pioneered the use of fluorescent in situ hybridization (FISH) for conducting genotoxic assays in bivalves. The experiment examined the progeny of C. gigas parents exposed to diuron during gonadal development. Aneuploidy in embryos affected the stability of DNA regions containing the 5S and 18-5.8-28S rRNA genes on chromosomes 4, 5, and 10. Notably, the selected doses of atrazine in this study were environmentally relevant. More recently, persisting aneuploidy has also been observed in other bivalve species. In 2017, persistent aneuploidy in Ruditapes philippinarum clams from a site highly contaminated with metals was found to be strongly correlated with sediment contamination rather than seasonal variations [31]. The authors concluded that aneuploidy was primarily influenced by contaminants in sediment rather than being a direct consequence of temporal fluctuations in bioavailable contaminants. The authors also observed vertical transmission of aneuploidy in R. philippinarum, which was attributed to long-term exposure to metal contaminants in the sediment.
Another case of season-independent persistent aneuploidy was reported by Leitão et al. [15] in the pearl oyster P. radiata in the Arabian Gulf. Aneuploidy levels were primarily associated with specific contaminants—mainly mercury and polycyclic aromatic hydrocarbons (PAHs)—accumulated in the oyster tissues rather than being a direct response to the seasonal fluctuations in bioavailable contaminants. The authors suggested that this persistent aneuploidy likely reflects chronic exposure to site-specific contaminants rather than a direct consequence of bioavailability. This raised an important question on the longevity of the genotoxic effects of contaminants on bivalves. In an attempt to answer that question, a translocation experiment investigated the potential for recovery in oysters translocated to sites with significantly different chemical compositions [16]. The authors observed a pattern of aneuploidy reduction in translocated oysters compared to controls, regardless of the contaminant levels in their tissues, although the change was not statistically significant. This pattern suggests that sediment composition may play a crucial role in influencing aneuploidy recovery in bivalves.
Most studies employing chromosomal abnormalities as endpoints have predominantly focused on numerical rather than structural alterations. This preference stems from the fact that detecting structural abnormalities is more technically demanding, as it requires the establishment of an optimal karyotype at the chromatin condensation level and the possibility of applying differential chromosomal banding techniques. Notably, Cheung et al. [27] reported chromatid breakages in metaphase chromosomes of C. gigas exposed to MMS and B[a]P. In a related context, Leitão et al. [32] hypothesized that chromosomal fission events could be triggered by environmental stressors. Their study investigated Cerastoderma edule cockles from Galicia, a region with a documented history of oil spill exposure.
2.1.2. Sister Chromatid Exchange (SCE)
SCE is a natural phenomenon in which the arms of chromosomes (sister chromatids) exchange genetic material to allow the genetic recombination and repair of genetic material/DNA damage. Arms labeled with bromodeoxyuridine (BrdU) can be traced in the following cell cycles, making observations of the exchange rate possible. Studies indicate that BrdU generates consistently low levels of SCE; thus, it has been used in control samples for labeling and as a genotoxic agent in some studies (e.g., [13,14]). Applications of the SCE test have focused on using embryos and larvae for two main reasons: the sensitivity of these developmental stages to chemical contamination and the brief experimental duration. The work of Dixon and Clarke [13] and Harrison and Johnes [14] unveiled a dose-dependent response relationship between BrdU, the alkylating agents mitomycin C (MMC) and MMS, and SCE in both the adults and larvae of M. edulis. Dixon and Prosser [22] tested the same species, and for the first time, both SCE and numerical chromosomal aberration assays were used together to measure the impact of TBTO and phenobarbital (PB) on larvae. In this study, TBTO did not cause cytogenetic damage, even in the presence of the known carcinogen phenobarbital (PB). On the other hand, in another study, the SCE level in M. galloprovincialis treated with carcinogenic mercury was two times higher than in those left untreated [33]. However, nitrilotriacetic acid—another known carcinogen for mammals—did not induce SCE and did not have synergetic effects on mercury genotoxicity [33]. A fact that cannot be overlooked is that the variability in results among SCE studies can be quite common due to several factors, including the time and duration of experimental exposure. Moreover, the random incorporation of the substance of interest without considering the cell cycle phase at the exposure time may produce misleading data [21,23]. Such information could be explored through in vitro tests of SCE, as demonstrated in the pilot study by Cornet [17], which involved BrdU incorporation into M. galloprovincialis mantle cell cultures. However, the data obtained were limited, and the methodological details were insufficient to ensure reproducibility, reflecting the broader challenges still faced in the field of bivalve cell culture.
2.1.3. Micronucleus Induction (MN)
A common finding in several studies utilizing assays based on micronuclei and other nuclear abnormalities in bivalves is a peak in the micronucleus rate shortly after exposure, which then declines over a few days of exposure and stabilizes at a level approximately twice the control for weeks (e.g., [34,35]). Recovery to initial baseline levels requires a longer depuration time. For example, in a study by Machado-Schiaffino et al. [36], mussels recovered after approximately six months. Siu et al. [37] noted a delayed increase in micronucleus formation after low doses of B[a]P exposure over the course of four weeks. Jaeschke et al. [38] noted the recovery of the micronucleus frequency after 21 days of depuration in M. edulis exposed to tritiated water. On the other hand, Politakis et al. [39] reported that a shorter depuration period of just 7 days was sufficient for M. galloprovincialis hemocytes to return to baseline micronucleus levels following exposure to paracetamol, with values no longer significantly different from those of the control specimens. Unlike the acute response that they had observed at higher concentrations of the same contaminant, this increase in genotoxicity persisted, highlighting the prolonged effects of lower, chronic exposures. Falfushynska et al. [40] found persistent nuclear abnormalities that lasted 14 days following a low dose of radiation exposure of 2 mGy in Anodonta anatina mussels. The micronucleus frequency is influenced by several factors, including the mitotic division index of cells [41,42], the cell type involved [43], and even abiotic aspects such as temperature [44]. The intraindividual variation in micronucleus frequencies in one sample also remains a challenge. Several cellular mechanisms, such as lower division index (e.g., [34,41]), cell death [45], and new cell turnover [46,47], may help to prevent further micronucleus formation. While valve closure was thought to contribute to reduced genotoxicity, experiments on embryos supported the prior hypothesis that other mechanisms play a role in limiting micronucleus production [42].
An interesting point raised by Falfushynska et al. [48] was that the origin of the tested species had a major influence on genotoxic responses rather than exposure conditions alone. It was demonstrated in A. anatina mussels that even after 14 days of exposure to copper, zinc, and cadmium, nuclear abnormalities were still correlated with the genotoxic contaminant levels found at their site of origin. To minimize such confounding effects, it is recommended to either prolong accumulation and depuration periods in clean conditions before starting to test individuals in pristine conditions or acquire test organisms from well-characterized, uncontaminated reference sites whenever possible. Tissue-specific toxicity has been addressed several times in the literature, such as in the work of Butrimavičienė et al. [49], where gill cells responded faster than hemocytes after laboratory exposure to metals in Anodonta cygnea. It is likely that Perumytilus purpuratus gills were more sensitive than hemocytes after copper exposure [50]. These findings were attributed to the fact that the gill is in direct contact with the outer environment, in contrast to hemocytes [50].
In the standard protocol of the micronucleus assay, nuclear abnormalities are typically expressed per 1000 cells to ensure consistency and comparability across studies. Reporting values as percentages of total observed cells, as seen in Abdulla et al.’s study [51], where frequencies as high as 45.57% were noted, may lead to misinterpretation and reduced accuracy, as it deviates from established reporting conventions.
Several recommendations have been proposed to improve micronucleus identification and scoring systems, including the use of automated systems and the standardization of the number of tested individuals (e.g., [46,52,53]). However, the majority of subsequent studies continued to rely on the previous classical methods. Micronucleus formation has been applied as a genotoxicity assessment technique in a large number of monitoring surveys (e.g., [54,55,56]), experimental approaches (e.g., [43,57,58]), and translocation studies [59,60] involving multiple bivalve species. In the majority of these studies, a consistent positive correlation was observed between site-specific pollution levels and micronuclei formation, reinforcing the assay’s reliability and sensitivity as a bioindicator of environmental genotoxicity.
2.2. DNA Damage Endpoints
Efforts to understand the immediate genotoxic impact that causes DNA alterations or adjustment have driven the advancement of the following assays in bivalves: DNA polymerase activity, DNA unwinding and alkaline elution, DNA adducts, and the comet assay [61,62,63,64,65]. DNA-molecule-based assays are not dependent on the cell division rate as cytogenetic assays are, but they can be more logistically demanding. The first DNA-molecule-based study in bivalves [61] evaluated the rate of DNA repair in isolated digestive gland and gill cells from M. galloprovincialis exposed to dimethyl sulfate (DMS). The results showed a significant inhibition of DNA polymerase activity in the gill cells but not in the digestive gland. In a subsequent study, the same methodological approach was applied to test the genotoxicity of heavy metals in the digestive gland cells. DNA repair was significantly inhibited in treatments with high concentrations of metals [66]. In the DNA unwinding assay, the mechanism and timing of double-stranded DNA unwinding are analyzed, where the smaller the molecular weight, the shorter the time required for DNA to unravel. This assay was used to assess the genotoxic impacts of agents that cause DNA strand breakage; hence, any DNA strand breakage can be a starting point for the unwinding process. One of the shortcomings of this test was the lack of a real quantitative measurement that reflects the generated level of DNA breakage [67]. Moreover, as in many other assays, DNA unwinding is subjected to physical and chemical artifacts that might contribute significantly to DNA breaks [67,68].
The alkaline single-cell gel electrophoresis/comet assay was first established in 1988 in an effort to develop an assay that measures damage and recovery in a single cell, quantifies DNA breakage in single and double strands, and eliminates RNA by alkalinization [68]. Consequently, since its first utilization in bivalve genotoxicity testing in 1997 by Sasaki et al. [65], the comet assay has become the most widely used method to assess DNA strand breakage in bivalves, accounting for 76% of such assays reported in the literature (Figure 3; Supplementary Table S2).
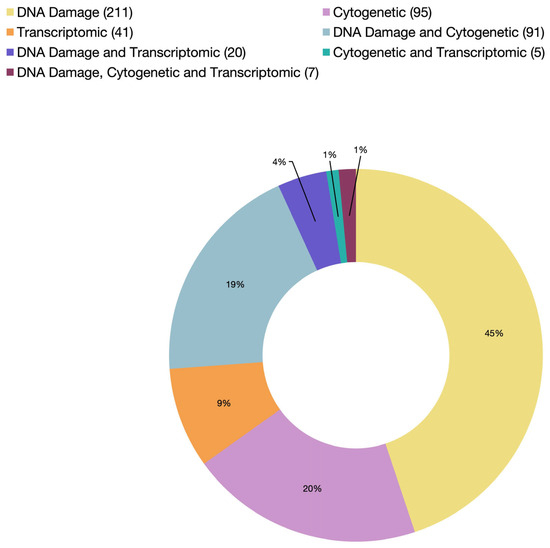
Figure 3.
A summary of cited articles by endpoint category from 1982 to 2025 (the exact number of articles in each category is indicated in parentheses in the legend).
Alkaline Single-Cell Gel Electrophoresis/Comet Assay
The earliest publications that introduced the comet assay in bivalve genotoxicity testing were centered on seawater monitoring, both in controlled laboratory set-ups and in field experiments [65,69,70,71]. Some of these studies investigated the effects of mutagens such as 3-chloro-4-dichloromethyl-5-hydroxy-2(H)-furanone (MX) and B[a]P on bivalve models. However, the exposure methods varied between studies. Sasaki et al. [65] conducted in vivo exposure experiments on Mizuhopecten yessoensis scallops and R. philippinarum clams, while Mitchelmore et al. [69] performed an in vitro exposure test on isolated digestive gland cells from M. edulis mussels. Interestingly, the two studies used the same exposure time of 4 h, which was enough to induce detectable genotoxic effects. This observation raised the question of the timeframe required for DNA damage to be reversible across different species and whether the extent of the genotoxic damage had been assessed fairly. In vivo exposure involves the organism’s full physiological and innate immune system response, whereas the in vitro assays isolate specific tissues, potentially limiting the complexity of the biological response and influencing the observed outcomes. Wilson et al. [71] tackled this issue by applying the comet assay on M. edulis both in vivo and in vitro. Although the in vivo exposure was as long as 14 days and the in vitro exposure lasted for only 1 h, the damage caused by the latter was significantly greater. Thus, the authors advised that the in vitro application of the comet assay on this species could be more useful. However, many subsequent studies that employed both in vivo and in vitro approaches did not provide sufficient evidence favoring one approach over the other, largely due to differences in the experimental design, species-specific response, cell type, and genotoxic agent. Rigonato et al. [72] investigated the recovery of the freshwater clam Corbicula fluminea following exposure to MMS and observed that the clams recovered within 9 days post-exposure. This experiment underlined the importance of incorporating an acclimation period prior to conducting the comet assay—a point also emphasized by Rigonato et al. [73], who recommended a 30-day acclimation period for bivalves before experimentation with the comet assay. Aligning with that, Nagarajappa et al. [74] observed a decline in genotoxic effects in mussel gonads 12 days after tobacco exposure in an experimental setting where DNA damage was evaluated using the comet assay every 48 h for 16 days. These findings highlight a common limitation in many studies: the comet assay is performed at a single time point, which can overlook important temporal patterns in DNA damage and recovery. Including multiple time points allows for a more accurate assessment of genotoxicity and recovery dynamics.
Among the factors that must be considered when standardizing doses between in vivo and in vitro approaches, the cell type plays a particularly critical role. It was observed that the toxicity of biotoxins was cell-dependent in R. decussatus, with hemocytes and gill cells responding differently under in vivo and in vitro conditions [75]. Similarly, Prego-Faraldo et al. [76] reported a similar cell-type-specific sensitivity to okadaic acid toxicity in M. galloprovincialis under in vitro conditions. Interestingly, this pattern of cell-dependent response was not observed in a comparative in vivo study involving hemocytes and hepatopancreas cells of M. galloprovincialis and the Pacific oyster C. gigas following biotoxin exposure [77]. That being said, higher sensitivity to in vivo exposure compared to in vitro assays using hemocytes was documented in mussels by Gačić et al. [78]. This difference was attributed to the absence of hemocyte proliferation during the 22-h in vitro exposure period, potentially limiting the manifestation of genotoxic effects.
Together, these studies highlight the strengths and limitations of each approach: while in vitro assays provide controlled conditions for assessing cell-specific responses, in vivo systems can reveal complex organism-level interactions, such as immune responses, metabolic activity, and tissue connectivity, that can either amplify or mitigate toxicity outcomes. These insights reinforce the need to consider the cell type and physiological context not only in interpreting genotoxicity results but also when establishing dose equivalency between experimental approaches and experimental models. Failure to do so may lead to misleading comparisons between in vivo and in vitro data, with the risk of overlooking critical biological differences that influence toxicological responses. The type of genotoxic agent is another important variable when comparing in vivo and in vitro responses. For example, Pruski and Dixon [79] exposed M. edulis to cadmium chloride (CdCl2) both in vivo (0.2 mg/L) for 4 weeks and in vitro (0.7–15 mg/L) for 5 h, which resulted in nuclear damage and the disruption of DNA repair mechanisms. However, their findings did not clearly favor one exposure route over the other, and CdCl2 was considered only weakly genotoxic in their system. In contrast, Banakou et al. [80] reported a direct genotoxic effect of CdCl2 in M. galloprovincialis hemocytes under in vitro conditions at both lower and higher concentrations than those used by Pruski and Dixon [79]. Similarly, Slobodskova et al. [81] observed cadmium-induced DNA damage in Corbicula japonica following in vivo exposure. These differing results may reflect differences not only in species and cell types but also in how cadmium chloride interacts with cellular systems under isolated (in vitro) versus integrated physiological conditions (in vivo). Notably, cadmium has been used as a positive control in several comet assay protocols (e.g., [78,82]), further supporting its relevance as a genotoxic agent in both exposure modes. Together, these studies highlight how the nature of the genotoxic agent, along with biological and methodological factors, can significantly influence the comparability and interpretation of in vivo and in vitro data.
Several studies have suggested that the comet assay is more suitable for detecting genotoxicity in germ cells than in somatic cells due to the limited DNA repair mechanisms in the former [70,83]. For example, Lewis and Galloway [84] observed that three days after discontinuing the exposure of M. edulis to B[a]P, recovery from DNA damage in hemocytes was significantly higher compared to that in sperm cells, highlighting the differential repair capabilities between these cell types. In addition, the authors demonstrated that exposure of male M. edulis to B[a]P for three days induced genotoxicity in larvae without affecting fertilization rates. In contrast, a study by Kadar et al. [85] indicated that exposing M. galloprovincialis sperm to zero-valent nano-iron (nZVI) for just 2 h significantly reduced fertilization success and impaired embryonic development. Similar developmental disruptions were reported in C. gigas embryos following exposure to various metals [86]. These studies underscore the varying sensitivities of different cell types to genotoxic agents and suggest that sperm cells may be more vulnerable to certain contaminants, which could have long-term effects on reproductive success. Although, as mentioned earlier, over two-thirds of the published genotoxicity assays in bivalves utilized the comet assay, its adequacy as a standalone tool for assessing genotoxicity is still currently questionable. Bellas et al. [87] highlighted several limitations in its application, suggesting that it may not fully capture the complexity of genotoxic effects in environmental settings. In their study, M. edulis mussels were caged at a site undergoing dredging and significant sediment mobilization, which may have contributed to the observed limitations of the comet assay in detecting genotoxicity under these conditions.
2.3. Transcriptomic Endpoints
Since its emergence in 2002, two decades after the first genotoxicity studies on bivalves and in parallel with the rapid development of next-generation sequencing technologies, the application of transcriptome-level analysis to genotoxicity assessment has significantly increased (Figure 1). Such growing interest has manifested in investigating the effects of various environmental stressors on gene expression profiles and associated functions. To date, a wide range of stressors have been investigated, including petrochemicals [88,89,90,91,92,93,94], metals [95,96,97,98,99], pesticides [100,101,102], biotoxins [103,104,105,106,107,108,109,110], plastics [111,112,113,114], nanomaterials [115], and abiotic parameters such as acidification [114] and UV radiation [90,116], among others (Table 1). Transcriptome analysis extends beyond observations of phenotypic changes by incorporating molecular techniques such as restriction fragment length polymorphism (RFLP), DNA fingerprinting, and gene amplification. One of the most powerful tools in transcriptomic studies is the reverse transcription polymerase chain reaction (RT-PCR), which enables the detection and quantification of gene expression changes using specific primers targeting genes of interest. Rodius et al. [88] used RNA arbitrarily primed PCR (RAP-PCR) to test the impact of heavily contaminated sediment containing a mix of organic and inorganic pollutants on the freshwater mussel Unio tumidus. The study provided evidence of the suitability of using this technique in the genotoxicity assessment of bivalves. The authors found a PCR product that was only present in mussels exposed to contaminants. However, the authors highlighted a key limitation of RAP-PCR: its inability to clearly distinguish between DNA damage and differential gene expression. Rodius et al. [88] suggested that this limitation could be overcome by combining RAP-PCR with other complementary genotoxicity assays that detect DNA damage, such as the micronucleus or comet assay. Moreover, bivalves have also played a role in the evolution of cancer research and the genotoxicity testing of cancer treatments [117]. For instance, bioactive extracts from the clams Donax variabilis, Donax incarnatus, and Donax cuneatus and the mussel Perna viridis, which are rich in polysaccharides, have shown antiproliferative effects on human cancer cell lines, highlighting their potential as candidates for cancer drug testing or development [118].

Table 1.
Published studies with transcriptomic endpoints since 2002.
The overall trends in cytogenetic, DNA damage, and transcriptomic assays in genotoxicity testing in bivalves show a shift from qualitative to quantitative methods, from manual scoring to automated analysis, and from single-tissue assessment to multiple-tissue investigations. A movement toward multi-assay integration for more comprehensive genotoxicity profiling is also noted. Additionally, efforts to standardize experimental protocols across laboratories have increased, ensuring better cross-study comparability and reproducibility. These advancements collectively reflect a broader move toward more reliable, objective, and mechanistically informed approaches in assessing genotoxicity in bivalves.
3. Discussion
3.1. The Use of Several Endpoints in a Single Study: Advantages and Key Findings
Integrating multiple genotoxicity assays within a single study provides a more comprehensive assessment of DNA damage at different biological levels and in different tissues. Among the various assay combinations, the micronucleus and comet assays are used together more frequently than any other tests (Figure 3). On the other hand, combining cytogenetic analysis (e.g., micronucleus or chromosomal aberration tests) and DNA-molecule-based analysis (e.g., comet assay) can help identify early, potentially repairable DNA damage caused by clastogenic agents before progressing to chromosomal alterations detectable at the cytogenetic level. Bolognesi et al. [163] were among the first to apply this integrative approach, combining at least two genotoxicity markers with distinct biological endpoints: the micronucleus assay (a cytogenetic endpoint) and alkaline elution (a DNA-molecule-base assay). The objective was to compare the sensitivity of these assays in detecting genotoxic effects in organisms exposed to heavy metals. Both assays successfully detected clastogenic effects induced by metals such as copper and mercury. However, discrepancies arose as to whether the observed DNA damage exhibited a clear dose–response relationship or a direct cause–effect interaction. Within the same year, Bresler et al. [164] monitored the health of several marine species along the Red Sea and Mediterranean coasts using a battery of genotoxicity biomarkers, including the micronucleus assay and DNA unwinding assay. A positive correlation was found between genotoxic effects and site-specific contamination levels. This pattern was particularly evident in the clam Donax trunculus, which exhibited greater sensitivity compared to other tested models, such as the clam Cyrenoida floridana and the mussel M. edulis.
A key distinction between cytogenetic tests and DNA-molecule-based assays lies in the persistence and detectability of DNA damage. For instance, the comet assay detects transient DNA damage that may be repaired over time, making it particularly useful for assessing short-term genotoxic effects and DNA repair mechanisms. In contrast, cytogenetic assessments are more suitable for detecting long-term, cumulative genotoxic effects, making them more appropriate for controlled laboratory experiments involving longer exposure durations.
While the genotoxic effects of chemical agents are well documented at the chromosomal and DNA strand levels, transcriptomic responses only started to be investigated in 2007, when studies started integrating gene expression analysis with traditional genotoxic endpoints. For instance, Di et al. [165] investigated the effects of B[a]P exposure in M. edulis using both the comet assay and transcriptomic analysis of the tumor-regulating genes p53 and ras. The study demonstrated that B[a]P exposure not only caused significant DNA strand breaks but also upregulated the expression of p53 and ras genes in hemocytes [133]. Another well-known genotoxic agent is mercury, which has been shown to cause chromosomal aberrations, DNA strand breaks, and micronuclei, among other effects, in humans [166,167,168], as well as in aquatic organisms such as Andinoacara rivulatus fish assessed with the micronucleus test [169] and Palaemon khori shrimp tested using aneuploidy assessment [170]. Yet, until recently, little was known regarding its molecular toxicity pathways. Pytharopoulou et al. [142] provided novel insights into mercury toxicity by finding its impact on the 40s ribosomal subunit, resulting in disturbed protein synthesis in mussels and contributing to micronucleus formation. Since then, studies combining multiple genotoxicity endpoints, including cytogenetic, molecular, and transcriptomic approaches, have expanded to investigate the effects of a wide range of chemical and physical stressors. The first two experimental studies integrating all three major genotoxicity assessment endpoints—cytogenetic, DNA strand break, and transcriptomic analysis—focused on the effects of nanoparticles and microplastics on M. galloprovincialis [144,148]. Canesi et al. [148] found that titanium oxide (TiO2) nanoparticles induced less genotoxicity than titanium oxide’s derivative 2,3,7,8-tetrachlorodibenzo-p-dioxins (2,3,7,8-TCDD), which is commonly known to be the most toxic form of TiO2. The three endpoints of the study showed evidence that TCCD is more toxic both in vivo and in vitro. In Avio et al.’s [144] study, a comprehensive battery of biomarkers, including DNA microarrays, micronuclei, and the comet assay, were utilized to assess the impact of microplastics. It was observed that pyrene-loaded polystyrene and polyethylene particles led to a higher micronucleus frequency in comparison to virgin microplastics. Interestingly, the comet assay showed the opposite pattern: mussels exposed to virgin microplastics exhibited higher levels of DNA breakage. The authors attributed this intriguing finding to the faster detectability of DNA strand breaks relative to micronucleus formation. Under more severe genotoxic stress, such as exposure to pyrene-loaded particles, the extent of DNA damage intensifies to the point of causing nuclear deformation, thereby increasing the frequency of micronuclei. Furthermore, transcriptomic analysis revealed both the upregulation and downregulation of various genes across treatments. Many of those genes are involved in vital molecular pathways, including detoxification, oxidative stress, immune responses, and cell cycle regulation.
Collectively, these findings highlight that the use of multiple genotoxicity endpoints, each targeting different biological levels, not only enhances detection sensitivity but also provides a comprehensive, multidimensional view of toxicological effects, thereby supporting more robust environmental risk assessments. For instance, the comet assay is particularly effective for detecting reversible DNA strand breaks and assessing DNA repair mechanisms, while the micronucleus assay detects more stable chromosomal alterations. Complementing these, transcriptomic analysis offers insights into gene expression pattern changes triggered by specific stressors, offering insights into affected molecular pathways. By integrating these complementary approaches, both transient and cumulative genotoxic effects can be detected—effects that might otherwise be missed when relying on a single assay.
3.2. Challenges in Genotoxicity Testing
As more efforts are being put into investigating the risks associated with exposure to genotoxic compounds, the number of published studies on this topic has increased significantly in the last four decades. Despite this progress, several challenges still persist in effectively utilizing existing knowledge, developing reliable testing assays, and drawing consistent, clear conclusions. One major difficulty in genotoxicity testing is the lack of standardized global guidelines for assay selection, data interpretation, and result validation. Some of these difficulties have been mitigated by the development of search engines, searchable databases, and data-sharing platforms, facilitating access to information and enhancing collaboration among researchers worldwide. However, the absence of universally standardized protocols continues to limit the comparability and reproducibility of the studies. Standardized guidelines are crucial for ensuring the selection of appropriate genotoxic approaches and bioassays, achieving statistically robust results, and providing realistic measures of genotoxic risk assessment. Addressing these elements comprehensively is essential for improving the consistency and reliability of genotoxicity testing. In 2015, in response to these challenges, the Organization for Economic Co-operation and Development (OECD) established the Genetic Toxicology Test Guidelines (TGs). These guidelines address many of the previous issues and aim to standardize genotoxicity testing by providing clear procedures for conducting assays across different study designs and laboratory settings. By establishing standardized methodologies, the OECD TGs enhance the consistency, reliability, and global comparability of genotoxicity assessments while also helping to reduce the variability in test outcomes and promote best practices in biological testing worldwide.
4. Concluding Remarks, Prospects, and Recommendations
Genotoxicity research in bivalves has made, as presented, considerable progress over recent decades, driven by growing environmental concerns and advances in molecular/omics tools. However, key methodological gaps highlight the need for more standardized, informed, and integrative approaches moving forward. To support the continued development and application of genotoxicity tools in bivalves, we recommend the following work areas to enhance their effectiveness.
The availability of genotoxic data in online public databases would increase the impact of existing research and pave the way for further advancements. This would improve the reproducibility of experimental work and support the integration of regional frameworks into more global applications of genotoxicity testing. We propose transforming the sources cited in this review and presented in the Supplementary Material into an open-access online database that can be continuously updated with future studies. This centralized resource would reduce duplicated efforts and offer inclusive support to researchers, policymakers, and other stakeholders. By making the data openly accessible, we aim to foster transparency, facilitate collaboration, and encourage broader engagement across the genotoxicity research community.
The lack of standardized methodologies remains a central challenge in genotoxicity testing. With the increasing number of genotoxicity studies, there is also growing recognition of diverse bivalve species as model organisms and an expanding variety of contaminants being investigated. However, despite this progress, there is still no consensus on quality assurance protocols, and quality control measures are inconsistently applied. Several experimental results are often influenced by the prior exposure history of collected organisms. Acclimation periods prior to experimentation are essential to mitigate this influence; however, their optimal duration can vary significantly depending on both the species and the type of assay used. Furthermore, it is now understood that genotoxic effects may be transmitted vertically across generations, reinforcing the need for more in-depth research into the persistence of DNA damage over time.
The standardization of bivalve genotoxicity testing could be advanced through the development of long-term cell lines from representative model species. However, this area of research remains underdeveloped in invertebrates due to the technical challenges associated with culturing and maintaining invertebrate cells over extended periods. While a few successful primary cell cultures have been established—such as in M. edulis for up to 22 months [171] and in Crassostrea madrasensis for 1 month [172]—their integration into genotoxicity testing frameworks has yet to be realized.
Assays such as SCE, chromosomal aberrations, and the micronucleus test are being refined and gaining recognition as reliable genotoxicity indicators. In vitro testing approaches also began to be incorporated into genotoxicity assessment frameworks during that period. In the late 1990s and early 2000s, advancements in genomics and molecular genetics ushered in a new era for genotoxicity testing. Techniques such as gene expression profiling emerged as powerful tools, enabling more mechanistic and predictive evaluations of genotoxic responses. Applying comprehensive omics tools—such as transcriptomics, proteomics, and metabolomics—holds great potential for providing deeper insights into the molecular pathways affected by genotoxins and can reveal tissue- or cell-specific sensitivities that traditional methods may overlook. However, despite the increasing use of transcriptomics in bivalve genotoxicity research—reported in at least 74 studies—only three have employed an in vitro approach. This highlights a critical gap and opportunity: integrating omics technologies with in vitro systems could yield more controlled, mechanistically insightful, and reproducible outcomes, significantly advancing the field.
When applying any of the aforementioned genotoxicity assays in environmental monitoring, it is essential to consider the nature of the contaminants and their associated molecular pathways. These assays typically reflect the cumulative impact of all environmental stressors, without identifying the specific agents responsible for the observed effects. As such, results must be interpreted with caution and within the context of known or suspected exposure scenarios. The genotoxic responses observed may be the outcome of complex interactions among multiple contaminants, as well as abiotic factors such as temperature, salinity, and pH. These interactions can either amplify or mitigate genotoxic effects, complicating the attribution of damage to a single causative agent. Therefore, a comprehensive understanding of the environmental context, including chemical analyses and ecological parameters, is critical for drawing meaningful conclusions from genotoxicity data.
The application of the above recommendations would be a step forward in the understanding of genotoxic impacts in bivalves, enabling more accurate risk assessments, improved environmental monitoring, and the development of globally relevant and aligned testing strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26115389/s1: References [22,37,48,54,163,164,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329,330,331,332,333,334,335,336,337,338,339,340,341,342,343,344,345,346,347,348,349,350,351,352,353,354,355,356,357,358,359,360,361,362,363,364,365,366,367,368,369,370,371,372,373,374,375,376,377,378,379,380,381,382,383,384,385,386,387,388,389,390,391,392,393,394,395,396,397,398,399,400,401,402,403,404,405,406,407,408,409,410,411,412,413,414,415,416,417,418,419,420,421,422,423,424,425,426,427,428,429,430,431,432,433,434,435,436,437,438,439,440,441,442,443,444,445,446,447,448,449,450,451,452,453,454,455,456,457,458,459,460,461,462,463,464,465,466,467,468,469,470,471,472,473,474,475,476,477,478,479,480,481,482,483,484,485,486,487,488,489] are cited in the supplementary materials.
Author Contributions
Conceptualization, Z.K. and A.L.; methodology, Z.K. and A.L.; validation, Z.K. and A.L.; formal analysis, Z.K. and A.L.; investigation, Z.K. and A.L.; resources, A.L.; data curation, Z.K. and A.L. writing—original draft preparation, Z.K.; writing—review and editing, Z.K. and A.L.; supervision, A.L.; project administration, A.L.; funding acquisition, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
Qatar Research Development and Innovation Council [ARG01-0528-230371].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Research reported in this publication was supported by the Qatar Research Development and Innovation Council [ARG01-0528-230371]. The content is solely the responsibility of the authors and does not necessarily represent the official views of Qatar Research Development and Innovation Council.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Halpern, B.S.; Frazier, M.; Potapenko, J.; Casey, K.S.; Koenig, K.; Longo, C.; Lowndes, J.S.; Rockwood, R.C.; Selig, E.R.; Selkoe, K.A.; et al. Spatial and Temporal Changes in Cumulative Human Impacts on the World’s Ocean. Nat. Commun. 2015, 6, 7615. [Google Scholar] [CrossRef] [PubMed]
- Mearns, A.J.; Reish, D.J.; Oshida, P.S.; Morrison, A.M.; Rempel-Hester, M.A.; Arthur, C.; Rutherford, N.; Pryor, R. Effects of Pollution on Marine Organisms. Water Environ. Res. 2016, 88, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Montesinos, F.; Molina, R.; Anfuso, G.; Manno, G.; Lo Re, C. Natural and Human Impacts on Coastal Areas. J. Mar. Sci. Eng. 2024, 12, 2017. [Google Scholar] [CrossRef]
- McDowell Capuzzo, J.; Moore, M.N.; Widdows, J. Effects of Toxic Chemicals in the Marine Environment: Predictions of Impacts from Laboratory Studies. Aquat. Toxicol. 1988, 11, 303–311. [Google Scholar] [CrossRef]
- Jakimska, A.; Konieczka, P.; Skóra, K.; Namieśnik, J. Bioaccumulation of Metals in Tissues of Marine Animals, Part I: The Role and Impact of Heavy Metals on Organisms. Pol. J. Environ. Stud. 2011, 20, 1117–1125. [Google Scholar]
- Weis, J.S.; Alava, J.J. (Micro)Plastics Are Toxic Pollutants. Toxics 2023, 11, 935. [Google Scholar] [CrossRef]
- Mansoori, A.N.; Gautam, R.K.; Tiwari, P.C. A Review on Genotoxicity. Asian J. Pharm. Res. 2014, 4, 162–165. Available online: https://asianjpr.com/AbstractView.aspx?PID=2014-4-3-7 (accessed on 15 January 2025).
- Marin-Morales, M.A.; Hoshina, M.M.; Hara, R.Q.; Sommaggio, L.R.D. Eco-Genotoxicity in Aquatic Systems. In Aquatic Toxicology; Polonini, H., Brayner, R., Eds.; OMICS Group eBooks: Foster City, CA, USA, 2016; pp. 1–19. [Google Scholar]
- United Nations. Globally Harmonized System of Classification and Labelling of Chemicals (GHS), 9th ed.; United Nations: New York, NY, USA; Geneva, Switzerland, 2021. [Google Scholar]
- Jha, A.N. Genotoxicological Studies in Aquatic Organisms: An Overview. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 552, 1–17. [Google Scholar] [CrossRef]
- WHO. Genotoxicity. In Principles and Methods for the Risk Assessment of Chemicals in Food; Environmental Health Criteria 240; World Health Organization: Geneva, Switzerland, 2020; Chapter 4.5; Available online: https://www.who.int/docs/default-source/food-safety/publications/section4-5-genotoxicity.pdf (accessed on 15 January 2025).
- Dixon, D.R. Aneuploidy in Mussel Embryos (Mytilus edulis L.) Originating from a Polluted Dock. Mar. Biol. Lett. 1982, 3, 155–161. [Google Scholar]
- Dixon, D.R.; Clarke, K.R. Sister Chromatid Exchange: A Sensitive Method for Detecting Damage Caused by Exposure to Environmental Mutagens in the Chromosomes of Adult Mytilus edulis. Mar. Biol. Lett. 1982, 3, 163–172. [Google Scholar]
- Harrison, F.L.; Jones, I.M. An in vivo Sister-Chromatid Exchange Assay in the Larvae of the Mussel Mytilus edulis: Response to 3 Mutagens. Mutat. Res. Lett. 1982, 105, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.; Al-Shaikh, I.; Hassan, H.; Ben Hamadou, R.; Bach, S. First Genotoxicity Assessment of Marine Environment in Qatar Using the Local Pearl Oyster Pinctada radiata. Reg. Stud. Mar. Sci. 2017, 11, 23–31. [Google Scholar] [CrossRef]
- Khatir, Z.; Range, P.; Malik, M.; Al-Naimi, H.; Ben Hamadou, R.; Leitão, A. Is It Forever? Genotoxicological Impact of Marine Contaminants on Arabian/Persian Gulf Bivalves: An Experimental Approach. Reg. Stud. Mar. Sci. 2020, 34, 101054. [Google Scholar] [CrossRef]
- Cornet, M. Detection of Genotoxicity in the Marine Environment: A Preliminary Feasibility Study Using Primary Mussel Tissue Culture. Sci. Total Environ. 2007, 382, 22–29. [Google Scholar] [CrossRef]
- Dixon, D.R.; Wilson, J.T. Genetics and Marine Pollution. In Marine Genetics; Springer: Dordrecht, The Netherlands, 2000; pp. 29–43. [Google Scholar] [CrossRef]
- Al-Sabti, K.; Kurelec, B. Induction of Chromosomal Aberrations in the Mussel Mytilus galloprovincialis Watch. Bull. Environ. Contam. Toxicol. 1985, 35, 660–665. [Google Scholar] [CrossRef]
- Gipperth, L. The Legal Design of the International and European Union Ban on Tributyltin Antifouling Paint: Direct and Indirect Effects. J. Environ. Manag. 2009, 90, S86–S95. [Google Scholar] [CrossRef]
- Jha, A.N.; Hagger, J.A.; Hill, S.J. Tributyltin Induces Cytogenetic Damage in the Early Life Stages of the Marine Mussel, Mytilus edulis. Environ. Mol. Mutagen. 2000, 35, 343–350. [Google Scholar] [CrossRef]
- Dixon, D.R.; Prosser, H. An Investigation of the Genotoxic Effects of an Organotin Antifouling Compound (Bis(tributyltin) Oxide) on the Chromosomes of the Edible Mussel, Mytilus edulis. Aquat. Toxicol. 1986, 8, 185–195. [Google Scholar] [CrossRef]
- Jha, A.N.; Cheung, V.V.; Foulkes, M.E.; Hill, S.J.; Depledge, M.H. Detection of Genotoxins in the Marine Environment: Adoption and Evaluation of an Integrated Approach Using the Embryo–Larval Stages of the Marine Mussel, Mytilus edulis. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 464, 213–228. [Google Scholar] [CrossRef]
- Jha, A.N.; Hagger, J.A.; Hill, S.J.; Depledge, M.H. Genotoxic, cytotoxic and developmental effects of tributyltin oxide (TBTO): An integrated approach to the evaluation of the relative sensitivities of two marine species. Mar. Environ. Res. 2000, 50, 565–573. [Google Scholar] [CrossRef]
- Bihari, N.; Mičić, M.; Batel, R.; Zahn, R.K. Flow cytometric detection of DNA cell cycle alterations in hemocytes of mussels (Mytilus galloprovincialis) off the Adriatic coast, Croatia. Aquat. Toxicol. 2003, 64, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bouilly, K.; Leitão, A.; McCombie, H.; Lapègue, S. Impact of atrazine on aneuploidy in Pacific oysters, Crassostrea gigas. Environ. Toxicol. Chem. 2003, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Cheung, V.V.; Jha, A.; Owen, R.; Depledge, M.H.; Galloway, T.S. Development of the in vivo chromosome aberration assay in oyster (Crassostrea gigas) embryo–larvae for genotoxicity assessment. Mar. Environ. Res. 2006, 62, S278–S282. [Google Scholar] [CrossRef] [PubMed]
- Bouilly, K.; McCombie, H.; Leitão, A.; Lapègue, S. Persistence of atrazine impact on aneuploidy in Pacific oysters, Crassostrea gigas. Mar. Biol. 2004, 1, 1. [Google Scholar] [CrossRef]
- Bouilly, K.; Bonnard, M.; Gagnaire, B.; Renault, T.; Lapègue, S. Impact of diuron on aneuploidy and hemocyte parameters in Pacific oyster, Crassostrea gigas. Arch. Environ. Contam. Toxicol. 2007, 52, 58–63. [Google Scholar] [CrossRef]
- Barranger, A.; Benabdelmouna, A.; Dégremont, L.; Burgeot, T.; Akcha, F. Parental exposure to environmental concentrations of diuron leads to aneuploidy in embryos of the Pacific oyster, as evidenced by fluorescent in situ hybridization. Aquat. Toxicol. 2015, 159, 36–43. [Google Scholar] [CrossRef]
- Piló, D.; Carvalho, S.; Pereira, P.; Gaspar, M.B.; Leitão, A. Is metal contamination responsible for increasing aneuploidy levels in the Manila clam Ruditapes philippinarum? Sci. Total Environ. 2017, 577, 340–348. [Google Scholar] [CrossRef]
- Leitão, A.; Chaves, R.; Joaquim, S.; Matias, D.; Ruano, F.; Guedes-Pinto, H. Supernumerary Chromosomes on Southern European Populations of the Cockle Cerastoderma edule: Consequence of Environmental Pollution? Estuar. Coast. Shelf Sci. 2008, 79, 152–156. [Google Scholar] [CrossRef]
- Brunetti, R.; Gola, I.; Majone, F. Sister-chromatid exchange in developing eggs of Mytilus galloprovincialis LMK. (Bivalvia). Mutat. Res. Lett. 1986, 174, 207–211. [Google Scholar] [CrossRef]
- Majone, F.; Brunetti, R.; Gola, I.; Levis, A.G. Persistence of micronuclei in the marine mussel, Mytilus galloprovincialis, after treatment with mitomycin C. Mutat. Res. Lett. 1987, 191, 157–161. [Google Scholar] [CrossRef]
- Scarpato, R.; Migliore, L.; Barale, R. The micronucleus assay in Anodonta cygnea for the detection of drinking water mutagenicity. Mutat. Res. Lett. 1990, 245, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Machado-Schiaffino, G.; Bala, L.O.; Garcia-Vazquez, E. Recovery of normal cytogenetic records in mussels after cessation of pollutant effluents in Puerto Madryn (Patagonia, Argentina). Estuaries Coasts 2009, 32, 813–818. [Google Scholar] [CrossRef]
- Siu, W.H.; Mak, E.; Cao, J.; De Luca-Abbott, S.B.; Richardson, B.J.; Lam, P.K. Micronucleus induction in gill cells of green-lipped mussels (Perna viridis) exposed to mixtures of polycyclic aromatic hydrocarbons and chlorinated pesticides. Environ. Toxicol. Chem. 2004, 23, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, B.C.; Millward, G.E.; Moody, A.J.; Jha, A.N. Tissue-specific incorporation and genotoxicity of different forms of tritium in the marine mussel, Mytilus edulis. Environ. Pollut. 2011, 159, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Politakis, N.; Belavgeni, A.; Efthimiou, I.; Charalampous, N.; Kourkouta, C.; Dailianis, S. The impact of expired commercial drugs on non-target marine species: A case study with the use of a battery of biomarkers in hemocytes of mussels. Ecotoxicol. Environ. Saf. 2018, 148, 160–168. [Google Scholar] [CrossRef]
- Falfushynska, H.; Gnatyshyna, L.; Yurchak, I.; Stoliar, O.; Sokolova, I.M. Interpopulational variability of molecular responses to ionizing radiation in freshwater bivalves Anodonta anatina (Unionidae). Sci. Total Environ. 2016, 568, 444–456. [Google Scholar] [CrossRef]
- Brunetti, R.; Majone, F.; Gola, I.; Beltrame, C. The micronucleus test: Examples of application to marine ecology. Mar. Ecol. Prog. Ser. 1988, 44, 65–68. [Google Scholar] [CrossRef]
- Wrisberg, M.N.; Bilbo, C.M.; Spliid, H. Induction of micronuclei in hemocytes of Mytilus edulis and statistical analysis. Ecotoxicol. Environ. Saf. 1992, 23, 191–205. [Google Scholar] [CrossRef]
- Venier, P.; Maron, S.; Canova, S. Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo[a]pyrene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1997, 390, 33–44. [Google Scholar] [CrossRef]
- Fernández, B.; Campillo, J.A.; Martínez-Gómez, C.; Benedicto, J. Micronuclei and other nuclear abnormalities in mussels (Mytilus galloprovincialis) as biomarkers of cyto-genotoxic pollution in Mediterranean waters. Environ. Mol. Mutagen. 2011, 52, 479–491. [Google Scholar] [CrossRef]
- Majone, F.; Brunetti, R.; Fumagalli, O.; Gabriele, M.; Levis, A.G. Induction of micronuclei by mitomycin C and colchicine in the marine mussel Mytilus galloprovincialis. Mutat. Res. Lett. 1990, 244, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mersch, J.; Beauvais, M.-N.; Nagel, P. Induction of micronuclei in haemocytes and gill cells of zebra mussels, Dreissena polymorpha, exposed to clastogens. Mutat. Res. Genet. Toxicol. 1996, 371, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, K.; Berger, A. Long-term toxicity of hexabromocyclododecane (HBCDD) to the benthic clam Macoma balthica (L.) from the Baltic Sea. Aquat. Toxicol. 2009, 95, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Stoliar, O.B. Effect of in situ exposure history on the molecular responses of freshwater bivalve Anodonta anatina (Unionidae) to trace metals. Ecotoxicol. Environ. Saf. 2013, 89, 73–83. [Google Scholar] [CrossRef]
- Butrimavičienė, L.; Stankevičiūtė, M.; Kalcienė, V.; Jokšas, K.; Baršienė, J. Genotoxic, cytotoxic, and neurotoxic responses in Anodonta cygnea after complex metal mixture treatment. Environ. Sci. Pollut. Res. 2019, 26, 7627–7639. [Google Scholar] [CrossRef]
- Gaete, H.; Guerra, R.; Espinoza, P.; Fernández, D. Lysosomal membrane stability in hemocytes and micronuclei in gills of Perumytilus purpuratus Lamarck 1819 (Bivalvia: Mytilidae) exposed to copper. Bull. Environ. Contam. Toxicol. 2019, 103, 796–801. [Google Scholar] [CrossRef]
- Abdulla, A.A.R.; Naser, H.A.; Ayyad, G.J. Assessing genotoxic and cytotoxic effects in bivalves influenced by marine pollution in Bahrain, Arabian Gulf. Asian J. Water Environ. Pollut. 2019, 16, 35–42. [Google Scholar] [CrossRef]
- Burgeot, T.; His, E.; Galgani, F. The micronucleus assay in Crassostrea gigas for the detection of seawater genotoxicity. Mutat. Res. Genet. Toxicol. 1995, 342, 125–140. [Google Scholar] [CrossRef]
- Mersch, J.; Beauvais, M.-N. The micronucleus assay in the zebra mussel, Dreissena polymorpha, to in situ monitor genotoxicity in freshwater environments. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1997, 393, 141–149. [Google Scholar] [CrossRef]
- Dolcetti, L.; Venier, P. Susceptibility to genetic damage and cell types in Mediterranean mussels. Mar. Environ. Res. 2002, 54, 487–491. [Google Scholar] [CrossRef]
- Baršienė, J.; Lazutka, J.; Šyvokienė, J.; Dedonytė, V.; Rybakovas, A.; Bagdonas, E.; Bjornstad, A.; Andersen, O.K. Analysis of micronuclei in blue mussels and fish from the Baltic and North Seas. Environ. Toxicol. 2004, 19, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Çakal Arslan, Ö.; Parlak, H.; Katalay, S.; Boyacioglu, M.; Karaaslan, M.A.; Guner, H. Detecting micronuclei frequency in some aquatic organisms for monitoring pollution of Izmir Bay (western Turkey). Environ. Monit. Assess. 2009, 165, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Vailati, G.; Bacchetta, R. Histological Changes and Micronucleus Induction in the Zebra Mussel Dreissena polymorpha after Paraquat Exposure. Histol. Histopathol. 2006, 21, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, N.; Ali, A.M. Genotoxic effect of pesticides on Perna viridis. Pak. J. Zool. 2022, 54, 1322–1328. [Google Scholar] [CrossRef]
- Weis, P.; Weis, J.S.; Couch, J.; Daniels, C.; Chen, T. Pathological and genotoxicological observations in oysters (Crassostrea virginica) living on Chromated Copper Arsenate (CCA)-treated wood. Mar. Environ. Res. 1995, 39, 275–278. [Google Scholar] [CrossRef]
- Ausili, A.; Gabellini, M.; Cammarata, G.; Fattorini, D.; Benedetti, M.; Pisanelli, B.; Gorbi, S.; Regoli, F. Ecotoxicological and human health risk in a petrochemical district of Southern Italy. Mar. Environ. Res. 2008, 66, 215–217. [Google Scholar] [CrossRef]
- Accomando, R.; Viarengo, A.; Zonchedu, A.; Orunesu, M. Biochemical characterization of the DNA polymerase activity present in isolated nucleic from mussel tissues: A possible system for the evaluation of the rate of DNA repair. Comp. Biochem. Physiol. B 1989, 93, 747–751. [Google Scholar] [CrossRef]
- Accomando, R.; Viarengo, A.; Bordone, R.; Taningher, M.; Canesi, L.; Orunesu, M. A rapid method for detecting DNA strand breaks in Mytilus galloprovincialis lam. induced by genotoxic xenobiotic chemicals. Int. J. Biochem. 1991, 23, 227–229. [Google Scholar] [CrossRef]
- Bihari, N.; Batel, R.; Zahn, R.K. Fractionation of DNA from marine invertebrate (Maja Crispata, Mytilus galloprovincialis) haemolymph by alkaline elution. Comp. Biochem. Physiol. B 1992, 102, 419–424. [Google Scholar] [CrossRef]
- Harvey, J.S.; Parry, J.M. The detection of genotoxin-induced DNA adducts in the common mussel Mytilus edulis. Mutagenesis 1997, 12, 153–158. [Google Scholar] [CrossRef][Green Version]
- Sasaki, Y.F.; Izumiyama, F.; Nishidate, E.; Ishibashi, S.; Tsuda, S.; Matsusaka, N.; Asano, N.; Saotome, K.; Sofuni, T.; Hayashi, M. Detection of genotoxicity of polluted sea water using shellfish and the alkaline single-cell gel electrophoresis (SCE) assay: A preliminary study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1997, 393, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Accomando, R.; Viarengo, A.; Orunesu, M. In Vivo and in vitro effects of heavy metals on DNA polymerase activities in the digestive gland of Mytilus galloprovincialis lam. Comp. Biochem. Physiol. C 1990, 95, 271–274. [Google Scholar] [CrossRef]
- Nacci, D.; Nelson, S.; Nelson, W.; Jackim, E. Application of the DNA alkaline unwinding assay to detect DNA strand breaks in marine bivalves. Mar. Environ. Res. 1992, 33, 83–100. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Mitchelmore, C.L.; Birmelin, C.; Livingstone, D.R.; Chipman, J.K. Detection of DNA strand breaks in isolated mussel (Mytilus edulis) digestive gland cells using the comet assay. Ecotoxicol. Environ. Saf. 1998, 41, 51–58. [Google Scholar] [CrossRef]
- Steinert, S.A.; Streib-Montee, R.; Leather, J.M.; Chadwick, D.B. DNA damage in mussels at sites in San Diego Bay. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 399, 65–85. [Google Scholar] [CrossRef]
- Wilson, J.T.; Pascoe, P.L.; Parry, J.M.; Dixon, D.R. Evaluation of the comet assay as a method for the detection of DNA damage in the cells of a marine invertebrate, Mytilus edulis L. (Mollusca: Pelecypoda). Mutat. Res./Fundam. Mol. Mech. Mutagen. 1998, 399, 87–95. [Google Scholar] [CrossRef]
- Rigonato, J.; Mantovani, M.S.; Jordão, B.Q. Comet assay comparison of different Corbicula fluminea (Mollusca) tissues for the detection of genotoxicity. Genet. Mol. Biol. 2005, 28, 464–468. [Google Scholar] [CrossRef]
- Rigonato, J.; Mantovani, M.S.; Jordão, B.Q. Detection of genotoxicity of water from an urbanized stream, in Corbicula fluminea (Mollusca) (in vivo) and Cho-K1 cells (in vitro) using comet assay. Arch. Environ. Contam. Toxicol. 2010, 59, 31–38. [Google Scholar] [CrossRef]
- Nagarajappa, G.; Ganguly, A.; Goswami, U. DNA damage in male gonad cells of green mussel (Perna viridis) upon exposure to tobacco products. Ecotoxicology 2006, 15, 365–369. [Google Scholar] [CrossRef]
- Flórez-Barrós, F.; Prado-Álvarez, M.; Méndez, J.; Fernández-Tajes, J. Evaluation of genotoxicity in gills and hemolymph of clam Ruditapes decussatus fed with the toxic dinoflagellate Prorocentrum lima. J. Toxicol. Environ. Health A 2011, 74, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Valdiglesias, V.; Laffon, B.; Eirín-López, J.M.; Méndez, J. In vitro analysis of early genotoxic and cytotoxic effects of okadaic acid in different cell types of the mussel Mytilus galloprovincialis. J. Toxicol. Environ. Health A 2015, 78, 814–824. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.; O’Halloran, J.; O’Brien, N.M.; van Pelt, F.F.N.A.M. Does the marine biotoxin okadaic acid cause DNA fragmentation in the blue mussel and the Pacific oyster? Mar. Environ. Res. 2014, 101, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gačić, Z.; Kolarević, S.; Sunjog, K.; Kračun-Kolarević, M.; Paunović, M.; Knežević-Vukčević, J.; Vuković-Gačić, B. The impact of in vivo and in vitro exposure to base analogue 5-FU on the level of DNA damage in haemocytes of freshwater mussels Unio pictorum and Unio tumidus. Environ. Pollut. 2014, 191, 145–150. [Google Scholar] [CrossRef]
- Pruski, A.; Dixon, D. Effects of cadmium on nuclear integrity and DNA repair efficiency in the gill cells of Mytilus edulis L. Aquat. Toxicol. 2002, 57, 127–137. [Google Scholar] [CrossRef]
- Banakou, E.; Dailianis, S. Involvement of Na+/H+ exchanger and respiratory burst enzymes NADPH oxidase and NO synthase in Cd-induced lipid peroxidation and DNA damage in haemocytes of mussels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 346–352. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Solodova, E.E.; Slinko, E.N.; Chelomin, V.P. Evaluation of the genotoxicity of cadmium in gill cells of the clam Corbicula japonica using the comet assay. Russ. J. Mar. Biol. 2010, 36, 311–315. [Google Scholar] [CrossRef]
- Martinović, R.; Kolarević, S.; Kračun-Kolarević, M.; Kostić, J.; Marković, S.; Gačić, Z.; Kljajić, Z.; Vuković-Gačić, B. Genotoxic potential and heart rate disorders in the Mediterranean mussel Mytilus galloprovincialis exposed to superdispersant-25 and dispersed diesel oil. Mar. Environ. Res. 2015, 108, 83–90. [Google Scholar] [CrossRef]
- Juhel, G.; O’Halloran, J.; Culloty, S.C.; O’Riordan, R.M.; Davenport, J.; O’Brien, N.M.; James, K.F.; Furey, A.; Allis, O. In Vivo exposure to microcystins induces DNA damage in the haemocytes of the zebra mussel, Dreissena polymorpha, as measured with the comet assay. Environ. Mol. Mutagen. 2007, 48, 22–29. [Google Scholar] [CrossRef]
- Lewis, C.; Galloway, T. Reproductive consequences of paternal genotoxin exposure in marine invertebrates. Environ. Sci. Technol. 2009, 43, 928–933. [Google Scholar] [CrossRef]
- Kadar, E.; Tarran, G.A.; Jha, A.N.; Al-Subiai, S.N. Stabilization of engineered zero-valent nanoiron with Na-acrylic copolymer enhances spermiotoxicity. Environ. Sci. Technol. 2011, 45, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Cachot, J.; Brune, J.; Geffard, O.; Belles, A.; Budzinski, H.; Morin, B. Embryotoxic and genotoxic effects of heavy metals and pesticides on early life stages of Pacific oyster (Crassostrea gigas). Mar. Pollut. Bull. 2012, 64, 2663–2670. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Ekelund, R.; Halldórsson, H.P.; Berggren, M.; Granmo, Å. Monitoring of organic compounds and trace metals during a dredging episode in the Göta Älv estuary (SW Sweden) using caged mussels. Water Air Soil Pollut. 2007, 181, 265–279. [Google Scholar] [CrossRef]
- Rodius, F.; Hammer, C.; Vasseur, P. Use of RNA arbitrarily primed PCR to identify genomic alterations in the digestive gland of the freshwater bivalve Unio tumidus at a contaminated site. Environ. Toxicol. 2002, 17, 538–546. [Google Scholar] [CrossRef]
- Lima, I.; Peck, M.R.; Rendón-Von Osten, J.; Soares, A.M.V.M.; Guilhermino, L.; Rotchell, J.M. Ras gene in marine mussels: A molecular level response to petrochemical exposure. Mar. Pollut. Bull. 2008, 56, 633–640. [Google Scholar] [CrossRef]
- Romero, A.; Estévez-Calvar, N.; Dios, S.; Figueras, A.; Novoa, B. New insights into the apoptotic process in mollusks: Characterization of caspase genes in Mytilus galloprovincialis. PLoS ONE 2011, 6, e17003. [Google Scholar] [CrossRef]
- Liu, N.; Pan, L.; Gong, X.; Tao, Y.; Hu, Y.; Miao, J. Effects of benzo(a)pyrene on differentially expressed genes and haemocyte parameters of the clam Venerupis philippinarum. Ecotoxicology 2014, 23, 122–132. [Google Scholar] [CrossRef]
- Cai, Y.; Pan, L.; Hu, F.; Jin, Q.; Liu, T. Deep sequencing-based transcriptome profiling analysis of Chlamys farreri exposed to benzo[a]pyrene. Gene 2014, 551, 261–270. [Google Scholar] [CrossRef]
- Jiang, X.; Qiu, L.; Zhao, H.; Song, Q.; Zhou, H.; Han, Q.; Diao, X. Transcriptomic responses of Perna viridis embryo to benzo(a)pyrene exposure elucidated by RNA sequencing. Chemosphere 2016, 163, 125–132. [Google Scholar] [CrossRef]
- Deng, X.; Pan, L.; Cai, Y.; Jin, Q. Transcriptomic changes in the ovaries of scallop Chlamys farreri exposed to benzo[a]pyrene. Genes Genom. 2016, 38, 509–518. [Google Scholar] [CrossRef]
- Dedeh, A.; Ciutat, A.; Tran, D.; Bourdineaud, J.-P. DNA alterations triggered by environmentally relevant polymetallic concentrations in marine clams Ruditapes philippinarum and polychaete worms Hediste diversicolor. Arch. Environ. Contam. Toxicol. 2014, 67, 651–658. [Google Scholar] [CrossRef]
- Hanana, H.; Turcotte, P.; André, C.; Gagnon, C.; Gagné, F. Comparative study of the effects of gadolinium chloride and gadolinium-based magnetic resonance imaging contrast agent on freshwater mussel, Dreissena polymorpha. Chemosphere 2017, 181, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.L.; Bilbao, E.; Cardoso, C.; Soto, M.; Bebianno, M.J. Changes in metallothionein transcription levels in the mussel Mytilus galloprovincialis exposed to CdTe quantum dots. Ecotoxicology 2018, 27, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, W.-X.; Li, L.; Zhang, G. Tissue-specific molecular and cellular toxicity of Pb in the oyster (Crassostrea gigas): mRNA expression and physiological studies. Aquat. Toxicol. 2018, 198, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.-X. Integrated transcriptomics and proteomics revealed the distinct toxicological effects of multi-metal contamination on oysters. Environ. Pollut. 2021, 284, 117533. [Google Scholar] [CrossRef]
- Akcha, F.; Barranger, A.; Bachère, E.; Berthelin, C.H.; Piquemal, D.; Alonso, P.; Sallan, R.R.; Dimastrogiovanni, G.; Porte, C.; Menard, D.; et al. Effects of an environmentally relevant concentration of diuron on oyster genitors during gametogenesis: Responses of early molecular and cellular markers and physiological impacts. Environ. Sci. Pollut. Res. 2016, 23, 8008–8020. [Google Scholar] [CrossRef]
- Rondon, R.; Akcha, F.; Alonso, P.; Menard, D.; Rouxel, J.; Montagnani, C.; Mitta, G.; Cosseau, C.; Grunau, C. Transcriptional changes in Crassostrea gigas oyster spat following a parental exposure to the herbicide diuron. Aquat. Toxicol. 2016, 175, 47–55. [Google Scholar] [CrossRef]
- Lee, D.-H.; Rhee, Y.-J.; Choi, K.-S.; Nam, S.-E.; Eom, H.-J.; Rhee, J.-S. Sublethal concentrations of atrazine promote molecular and biochemical changes in the digestive gland of the Pacific oyster Crassostrea gigas. Toxicol. Environ. Health Sci. 2017, 9, 50–58. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Aballay, A.E.; Hégaret, H.; Astuya, A.P.; Gallardo-Escárate, C. Transcriptional responses of Mytilus chilensis exposed in vivo to saxitoxin (STX). J. Molluscan Stud. 2013, 79, 323–331. [Google Scholar] [CrossRef]
- Suárez-Ulloa, V.; Fernández-Tajes, J.; Aguiar-Pulido, V.; Rivera-Casas, C.; González-Romero, R.; Ausió, J.; Méndez, J.; Dorado, J.; Eirín-López, J.M. The Chromevaloa database: A resource for the evaluation of okadaic acid contamination in the marine environment based on the chromatin-associated transcriptome of the mussel Mytilus galloprovincialis. Mar. Drugs 2013, 11, 830–841. [Google Scholar] [CrossRef]
- de Jesús Romero-Geraldo, R.; García-Lagunas, N.; Hernández-Saavedra, N.Y. Effects of in vitro exposure to diarrheic toxin producer Prorocentrum lima on gene expressions related to cell cycle regulation and immune response in Crassostrea gigas. PLoS ONE 2014, 9, e97181. [Google Scholar] [CrossRef]
- García-Lagunas, N.; de Jesús Romero-Geraldo, R.; Hernández-Saavedra, N.Y. Changes in gene expression and histological injuries as a result of exposure of Crassostrea gigas to the toxic dinoflagellate Gymnodinium catenatum. J. Molluscan Stud. 2015, 82, 193–200. [Google Scholar] [CrossRef][Green Version]
- Gonzalez-Romero, R.; Suarez-Ulloa, V.; Rodriguez-Casariego, J.; Garcia-Souto, D.; Diaz, G.; Smith, A.; Pasantes, J.J.; Rand, G.; Eirin-Lopez, J.M. Effects of Florida red tides on histone variant expression and DNA methylation in the eastern Oyster Crassostrea virginica. Aquat. Toxicol. 2017, 186, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Vieira, L.R.; Eirin-López, J.M.; Méndez, J.; Guilhermino, L. Transcriptional and biochemical analysis of antioxidant enzymes in the mussel Mytilus galloprovincialis during experimental exposures to the toxic dinoflagellate Prorocentrum lima. Mar. Environ. Res. 2017, 129, 304–315. [Google Scholar] [CrossRef]
- Huang, J.-H.; Jiao, Y.-H.; Li, L.; Li, D.-W.; Li, H.-Y.; Yang, W.-D. Small RNA analysis of Perna viridis after exposure to Prorocentrum lima, a DSP toxins-producing dinoflagellate. Aquat. Toxicol. 2021, 239, 105950. [Google Scholar] [CrossRef]
- Ventoso, P.; Pazos, A.J.; Blanco, J.; Pérez-Parallé, M.L.; Triviño, J.C.; Sánchez, J.L. Transcriptional Response in the Digestive Gland of the King Scallop (Pecten maximus) after the Injection of Domoic Acid. Toxins 2021, 13, 339. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Lacroix, C.; González Fernández, C.; Hégaret, H.; Lambert, C.; Le Goïc, N.; Frère, L.; Cassone, A.-L.; Sussarellu, R.; Fabioux, C.; et al. Exposure of marine mussels Mytilus spp. to polystyrene microplastics: Toxicity and influence on fluoranthene bioaccumulation. Environ. Pollut. 2016, 216, 724–737. [Google Scholar] [CrossRef]
- Li, Z.; Feng, C.; Pang, W.; Tian, C.; Zhao, Y. Nanoplastic-induced genotoxicity and intestinal damage in freshwater benthic clams (Corbicula fluminea): Comparison with microplastics. ACS Nano 2021, 15, 9469–9481. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, H.; Luan, L.; Luo, X.; Wang, X.; Lu, H.; Li, Y.; Wen, L.; Li, F.; Zhao, J. Functionalized polystyrene nanoplastic-induced energy homeostasis imbalance and the immunomodulation dysfunction of marine clams (Meretrix meretrix) at environmentally relevant concentrations. Environ. Sci. Nano 2021, 8, 2030–2048. [Google Scholar] [CrossRef]
- Yang, L.; Lv, L.; Liu, H.; Wang, M.; Sui, Y.; Wang, Y. Effects of ocean acidification and microplastics on microflora community composition in the digestive tract of the thick Shell Mussel Mytilus coruscus through 16s RNA gene sequencing. Bull. Environ. Contam. Toxicol. 2021, 107, 616–625. [Google Scholar] [CrossRef]
- Bebianno, M.J.; Gonzalez-Rey, M.; Gomes, T.; Mattos, J.J.; Flores-Nunes, F.; Bainy, A.C. Is gene transcription in mussel gills altered after exposure to Ag nanoparticles? Environ. Sci. Pollut. Res. 2015, 22, 17425–17433. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.; Sokolov, E.P.; Fisch, K.; Gazie, H.; Schulz-Bull, D.E.; Sokolova, I.M. Biomarker-based assessment of sublethal toxicity of organic UV filters (ensulizole and octocrylene) in a sentinel marine bivalve Mytilus edulis. Sci. Total Environ. 2021, 798, 149171. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.; Jerszyk, E.; Low, B.; Walker, C. Genotoxic stress–induced expression of p53 and apoptosis in leukemic clam hemocytes with cytoplasmically sequestered p53. Cancer Res. 2008, 68, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, D.; Samuel, A.; Sahayanathan, G.J.; Raja, K.; Chinnasamy, A. Anticancer Effect of Marine Bivalves Derived Polysaccharides against Human Cancer Cells. Biocatal. Agric. Biotechnol. 2022, 39, 102240. [Google Scholar] [CrossRef]
- An, L.-h.; Zheng, B.-h.; Liu, R.-z.; Fan, Q.; Wang, Q.-k.; Luo, Y.-f. Transcriptomic response to estrogen exposure in the male Zhikong scallop, Chlamys farreri. Mar. Pollut. Bull. 2014, 89, 59–66. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Wang, C.; Hu, B.; Wang, X.; Li, D. Benzotriazole UV stabilizer-induced genotoxicity in freshwater benthic clams: A survey on apoptosis, oxidative stress, histopathology and transcriptomics. Sci. Total. Environ. 2022, 857, 159055. [Google Scholar] [CrossRef]
- Lüchmann, K.H.; Mattos, J.J.; Siebert, M.N.; Dorrington, T.S.; Toledo-Silva, G.; Stoco, P.H.; Grisard, E.C.; Bainy, A.C.D. Suppressive subtractive hybridization libraries prepared from the digestive gland of the oyster Crassostrea brasiliana exposed to a diesel fuel water-accommodated fraction. Environ. Toxicol. Chem. 2012, 31, 1249–1253. [Google Scholar] [CrossRef]
- Wessel, N.; Rousseau, S.; Caisey, X.; Quiniou, F.; Akcha, F. Investigating the relationship between embryotoxic and genotoxic effects of benzo[a]pyrene, 17α-ethinylestradiol and endosulfan on Crassostrea gigas embryos. Aquat. Toxicol. 2007, 85, 133–142. [Google Scholar] [CrossRef]
- Estrada, N.; Núñez-Vázquez, E.J.; Palacios, A.; Ascencio, F.; Guzmán-Villanueva, L.; Contreras, R.G. In vitro Evaluation of Programmed Cell Death in the Immune System of Pacific Oyster Crassostrea gigas by the Effect of Marine Toxins. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Sussarellu, R.; Lebreton, M.; Rouxel, J.; Akcha, F.; Rivière, G. Copper induces expression and methylation changes of early development genes in Crassostrea gigas embryos. Aquat. Toxicol. 2018, 196, 70–78. [Google Scholar] [CrossRef]
- Akcha, F.; Barranger, A.; Bachère, E. Genotoxic and epigenetic effects of diuron in the Pacific oyster: In vitro evidence of interaction between DNA damage and DNA methylation. Environ. Sci. Pollut. Res. 2021, 28, 8266–8280. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Gonzalez, P.; Pardon, P.; Tapie, N.; Budzinski, H.; Cachot, J.; Morin, B. Comparative responses of sperm cells and embryos of Pacific oyster (Crassostrea gigas) to exposure to metolachlor and its degradation products. Aquat. Toxicol. 2014, 147, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Cachot, J.; Clérandeau, C.; Martin, C.; Mazzela, N.; Gonzalez, P.; Morin, B. An environmentally realistic pesticide and copper mixture impacts embryonic development and DNA integrity of the Pacific oyster, Crassostrea gigas. Environ. Sci. Pollut. Res. 2018, 27, 3600–3611. [Google Scholar] [CrossRef] [PubMed]
- Devos, A.; Dallas, L.J.; Voiseux, C.; Lecomte-Pradines, C.; Jha, A.N.; Fiévet, B. Assessment of growth, genotoxic responses and expression of stress related genes in the Pacific oyster Crassostrea gigas following chronic exposure to ionizing radiation. Mar. Pollut. Bull. 2015, 95, 688–698. [Google Scholar] [CrossRef]
- Hanana, H.; Turcotte, P.; Dubé, M.; Gagnon, C.; Gagné, F. Response of the freshwater mussel, Dreissena polymorpha to sub-lethal concentrations of samarium and yttrium after chronic exposure. Ecotoxicol. Environ. Saf. 2018, 165, 662–670. [Google Scholar] [CrossRef]
- Châtel, A.; Faucet-Marquis, V.; Gourlay-Francé, C.; Pfohl-Leszkowicz, A.; Vincent-Hubert, F. Genotoxicity and activation of cellular defenses in transplanted zebra mussels Dreissena polymorpha along the Seine river. Ecotoxicol. Environ. Saf. 2015, 114, 241–249. [Google Scholar] [CrossRef]
- Vernon, E.L.; Moore, M.N.; Bean, T.P.; Jha, A.N. Evaluation of interactive effects of phosphorus-32 and copper on marine and freshwater bivalve mollusks. Int. J. Radiat. Biol. 2020, 98, 1106–1119. [Google Scholar] [CrossRef]
- Xu, K.; Tang, Z.; Liu, S.; Liao, Z.; Xia, H.; Liu, L.; Wang, Z.; Qi, P. Effects of low concentrations copper on antioxidant responses, DNA damage and genotoxicity in thick shell mussel Mytilus coruscus. Fish Shellfish. Immunol. 2018, 82, 77–83. [Google Scholar] [CrossRef]
- Di, Y.; Schroeder, D.C.; Highfield, A.; Readman, J.W.; Jha, A.N. Tissue-specific expression of p53 and ras genes in response to the environmental genotoxicant benzo[α]pyrene in marine mussels. Environ. Sci. Technol. 2011, 45, 8974–8981. [Google Scholar] [CrossRef]
- Ruiz, P.; Orbea, A.; Rotchell, J.M.; Cajaraville, M.P. Transcriptional responses of cancer-related genes in turbot Scophthalmus maximus and mussels Mytilus edulis exposed to heavy fuel oil no. 6 and styrene. Ecotoxicology 2012, 21, 820–831. [Google Scholar] [CrossRef]
- Strehse, J.S.; Brenner, M.; Kisiela, M.; Maser, E. The explosive trinitrotoluene (TNT) induces gene expression of carbonyl reductase in the blue mussel (Mytilus spp.): A new promising biomarker for sea dumped war relicts? Arch. Toxicol. 2020, 94, 4043–4054. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yu, Z.; Song, X.; Yang, F. Effects of sodium dodecylbenzene sulfonate and sodium dodecyl sulfate on the Mytilus galloprovincialis biomarker system. Ecotoxicol. Environ. Saf. 2010, 73, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Aminot, Y.; Schroeder, D.C.; Readman, J.W.; Jha, A.N. Integrated biological responses and tissue-specific expression of p53 and ras genes in marine mussels following exposure to benzo(α)pyrene and C60 fullerenes, either alone or in combination. Mutagenesis 2017, 32, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Barranger, A.; Rance, G.A.; Aminot, Y.; Dallas, L.J.; Sforzini, S.; Weston, N.J.; Lodge, R.W.; Banni, M.; Arlt, V.M.; Moore, M.N.; et al. An integrated approach to determine interactive genotoxic and global gene expression effects of multiwalled carbon nanotubes (MWCNTs) and benzo[a]pyrene (BaP) on marine mussels: Evidence of reverse ‘Trojan Horse’ effects. Nanotoxicology 2019, 13, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Mollo, V.; Ambrosino, A.; Caccavale, F.; Troisi, J.; Febbraio, F.; Piscopo, M. Molecular effects of copper on the reproductive system of Mytilus galloprovincialis. Mol. Reprod. Dev. 2019, 86, 1357–1368. [Google Scholar] [CrossRef]
- Ferreira, M.F.; Turner, A.; Payet, M.; Grisolia, C.; Malard, V.; Moore, M.N.; Jha, A.N. Bioaccumulation and biological effects of hydrogenated cement particles in the marine bivalve, Mytilus galloprovincialis. Chemosphere 2024, 359, 142243. [Google Scholar] [CrossRef]
- Lettieri, G.; Notariale, R.; Ambrosino, A.; Di Bonito, A.; Giarra, A.; Trifuoggi, M.; Manna, C.; Piscopo, M. Spermatozoa Transcriptional Response and Alterations in PL Proteins Properties after Exposure of Mytilus galloprovincialis to Mercury. Int. J. Mol. Sci. 2021, 22, 1618. [Google Scholar] [CrossRef]
- Pytharopoulou, S.; Kournoutou, G.G.; Leotsinidis, M.; Georgiou, C.D.; Kalpaxis, D.L. Dysfunctions of the translational machinery in digestive glands of mussels exposed to mercury ions. Aquat. Toxicol. 2013, 134–135, 23–33. [Google Scholar] [CrossRef]
- Boukadida, K.; Cachot, J.; Morin, B.; Clerandeau, C.; Banni, M. Moderate temperature elevation increases susceptibility of early-life stage of the Mediterranean mussel, Mytilus galloprovincialis, to metal-induced genotoxicity. Sci. Total Environ. 2019, 663, 351–360. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Milan, M.; Benedetti, M.; Fattorini, D.; d’Errico, G.; Pauletto, M.; Bargelloni, L.; Regoli, F. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environ. Pollut. 2015, 198, 211–222. [Google Scholar] [CrossRef]
- Romdhani, I.; De Marco, G.; Cappello, T.; Ibala, S.; Zitouni, N.; Boughattas, I.; Banni, M. Impact of environmental microplastics alone and mixed with benzo[a]pyrene on cellular and molecular responses of Mytilus galloprovincialis. J. Hazard. Mater. 2022, 435, 128952. [Google Scholar] [CrossRef] [PubMed]
- Brandts, I.; Teles, M.; Gonçalves, A.P.; Barreto, A.; Franco-Martinez, L.; Tvarijonaviciute, A.; Martins, M.A.; Soares, A.M.V.M.; Tort, L.; Oliveira, M. Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine. Sci. Total Environ. 2018, 643, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Katsumiti, A.; Nieto, J.A.; Bori, J.; Jimeno-Romero, A.; Reip, P.; Arostegui, I.; Orbea, A.; Cajaraville, M.P. Short-term effects on antioxidant enzymes and long-term genotoxic and carcinogenic potential of CuO nanoparticles compared to bulk CuO and ionic copper in mussels Mytilus galloprovincialis. Mar. Environ. Res. 2015, 111, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Canesi, L.; Frenzilli, G.; Balbi, T.; Bernardeschi, M.; Ciacci, C.; Corsolini, S.; Della Torre, C.; Fabbri, R.; Faleri, C.; Focardi, S.; et al. Interactive effects of N-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis. Aquat. Toxicol. 2014, 153, 53–65. [Google Scholar] [CrossRef]
- Della Torre, C.; Balbi, T.; Grassi, G.; Frenzilli, G.; Bernardeschi, M.; Smerilli, A.; Guidi, P.; Canesi, L.; Nigro, M.; Monaci, F.; et al. Titanium dioxide nanoparticles modulate the toxicological response to cadmium in the gills of Mytilus galloprovincialis. J. Hazard. Mater. 2015, 297, 92–100. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; D’errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-term exposure of Mytilus galloprovincialis to diclofenac, Ibuprofen and Ketoprofen: Insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef]
- Lado-Insua, T.; Pérez, M.; Diz, A.P.; Presa, P. Temporal estimates of genetic diversity in some Mytilus galloprovincialis populations impacted by the Prestige oil-spill. Cont. Shelf Res. 2011, 31, 466–475. [Google Scholar] [CrossRef]
- Caliani, I.; De Marco, G.; Cappello, T.; Giannetto, A.; Mancini, G.; Ancora, S.; Maisano, M.; Parrino, V.; Cappello, S.; Bianchi, N.; et al. Assessment of the effectiveness of a novel BioFilm-Membrane BioReactor oil-polluted wastewater treatment technology by applying biomarkers in the mussel Mytilus galloprovincialis. Aquat. Toxicol. 2022, 243, 106059. [Google Scholar] [CrossRef]
- Mezzelani, M.; Nardi, A.; Bernardini, I.; Milan, M.; Peruzza, L.; D’Errico, G.; Fattorini, D.; Gorbi, S.; Patarnello, T.; Regoli, F. Environmental pharmaceuticals and climate change: The case study of carbamazepine in M. galloprovincialis under ocean acidification scenario. Environ. Int. 2021, 146, 106269. [Google Scholar] [CrossRef]
- Dallas, L.J.; Bean, T.P.; Turner, A.; Lyons, B.P.; Jha, A.N. Exposure to tritiated water at an elevated temperature: Genotoxic and transcriptomic effects in marine mussels (M. galloprovincialis). J. Environ. Radioact. 2016, 164, 325–336. [Google Scholar] [CrossRef]
- Dallas, L.J.; Turner, A.; Bean, T.P.; Lyons, B.P.; Jha, A.N. An integrated approach to assess the impacts of zinc pyrithione at different levels of biological organization in marine mussels. Chemosphere 2018, 196, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Sforzini, S.; Arlt, V.M.; Barranger, A.; Dallas, L.J.; Oliveri, C.; Aminot, Y.; Pacchioni, B.; Millino, C.; Lanfranchi, G.; et al. Assessing the impact of Benzo[a]pyrene on Marine Mussels: Application of a novel targeted low density microarray complementing classical biomarker responses. PLoS ONE 2017, 12, e0178460. [Google Scholar] [CrossRef] [PubMed]
- Baettig, C.G.; Barrick, A.; Zirngibl, M.; Lear, G.; Smith, K.F.; Northcott, G.L.; Tremblay, L.A. Development and validation of molecular biomarkers for the green-lipped mussel (Perna canaliculus). New Zealand J. Mar. Freshw. Res. 2023, 58, 364–383. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, W.; Jiang, H.; Chen, F.; Li, Y.; Gardea-Torresdey, J.L.; Zhou, X.-X.; Yan, B. Surface-Charge-Driven Ferroptosis and Mitochondrial Dysfunction Is Involved in Toxicity Diversity in the Marine Bivalve Exposed to Nanoplastics. ACS Nano 2024, 18, 2370–2383. [Google Scholar] [CrossRef]
- Stefani, F.; Casatta, N.; Ferrarin, C.; Izzotti, A.; Maicu, F.; Viganò, L. Gene expression and genotoxicity in Manila clam (Ruditapes philippinarum) modulated by sediment contamination and lagoon dynamics in the Po river delta. Mar. Environ. Res. 2018, 142, 257–274. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Dai, J.; Su, X.; Li, C.; Shen, L. Long-term effects of di-octyl phthalate on the expression of immune-related genes in Tegillarca granosa. Chin. J. Oceanol. Limnol. 2016, 34, 423–429. [Google Scholar] [CrossRef]
- Zha, S.; Tang, Y.; Shi, W.; Liu, H.; Sun, C.; Bao, Y.; Liu, G. Impacts of four commonly used nanoparticles on the metabolism of a marine bivalve species, Tegillarca granosa. Chemosphere 2022, 296, 134079. [Google Scholar] [CrossRef]
- Bigot, A.; Vasseur, P.; Rodius, F. SOD and CAT cDNA cloning, and expression pattern of detoxification genes in the freshwater bivalve Unio tumidus transplanted into the Moselle river. Ecotoxicology 2010, 19, 369–376. [Google Scholar] [CrossRef]
- Bolognesi, C.; Landini, E.; Roggieri, P.; Fabbri, R.; Viarengo, A. Genotoxicity biomarkers in the assessment of heavy metal effects in mussels: Experimental studies. Environ. Mol. Mutagen. 1999, 33, 287–292. [Google Scholar] [CrossRef]
- Bresler, V.; Abelson, A.; Dizer, H.; Sturm, A.; Kratke, R.; Fishelson, L.; Hansen, P.-D.; Bissinger, V. Marine molluscs and fish as biomarkers of pollution stress in littoral regions of the Red Sea, Mediterranean Sea and North Sea. Helgol. Mar. Res. 1999, 53, 219–243. [Google Scholar] [CrossRef]
- Di Pietro, A.; Visalli, G.; La Maestra, S.; Micale, R.; Baluce, B.; Matarese, G.; Cingano, L.; Scoglio, M.E. Biomonitoring of DNA Damage in Peripheral Blood Lymphocytes of Subjects with Dental Restorative Fillings. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 650, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, M.; Oshima, H.; Nakamura, M. Genotoxicity of Mercury Used in Chromosome Aberration Tests. Toxicol. Vitr. 2001, 15, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Crespo-López, M.E.; Macêdo, G.L.; Pereira, S.I.D.; Arrifano, G.P.F.; Picanço-Diniz, D.L.W.; Nascimento, J.L.; Herculano, A.M. Mercury and Human Genotoxicity: Critical Considerations and Possible Molecular Mechanisms. Pharmacol. Res. 2009, 60, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Osman, A.I.; Hosny, M.; Elgarahy, A.M.; Eltaweil, A.S.; Rooney, D.W.; Chen, Z.; Rahim, N.S.; Sekar, M.; Gopinath, S.C.; et al. The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review. ACS Omega 2024, 9, 5100–5126. [Google Scholar] [CrossRef]
- Nirchio, M.; Choco Ventimilla, O.J.; Quizhpe Cordero, P.F.; Hernández, J.G.; Oliveira, C. Genotoxic Effects of Mercury Chloride on the Neotropical Fish Andinoacara Rivulatus (Cichlidae: Cichlasomatini). Rev. Biol. Trop. 2019, 67, 745–754. [Google Scholar] [CrossRef]
- Hassan, H.; Benvenuto, C.; Al-Maslamani, I.; Chatting, M.; Mondal, D.; Leitão, A. Methylmercury, Trace Metals, Organotins and Their Effects on the Qatari Mangrove Shrimp, Palaemon khori. J. Mar. Sci. Eng. 2022, 10, 843. [Google Scholar] [CrossRef]
- Daugavet, M.A.; Blinova, M.I. Culture of Mussel (Mytilus edulis L.) Mantle Cells. Russ. J. Mar. Biol. 2015, 41, 63–67. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Shylaja, M.; Balasubramanian, T. An Optimized Protocol for Routine Development of Cell Culture from Adult Oyster, Crassostrea madrasensis. Cytotechnology 2024, 76, 177–187. [Google Scholar] [CrossRef]
- Wrisberg, M.; Vandergaag, M. In vivo detection of genotoxicity in waste water from a wheat and rye straw paper pulp factory. Sci. Total. Environ. 1992, 121, 95–108. [Google Scholar] [CrossRef]
- Wolfe, D.A.; Scott, K.J.; Clayton, J.R.; Lunz, J.; Payne, J.R.; Thompson, T.A. Comparative Toxicities of Polar and Non-Polar Organic Fractions from Sediments Affected by the Exxon Valdez Oil Spill in Prince William Sound, Alaska. Chem. Ecol. 1995, 10, 137–156. [Google Scholar] [CrossRef]
- Waldmann, P.; Pivcevic, B.; Müller, W.E.; Zahn, R.K.; Kurelec, B. Increased genotoxicity of acetylaminofluorene by modulators of multixenobiotic resistance mechanism: Studies with the fresh water clam Corbicula fluminea. Mutat. Res. Toxicol. 1995, 342, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Lyons, B.; Waldock, M.; Parry, J. The application of the 32P-postlabelling assay to aquatic biomonitoring. Mutat. Res. Mol. Mech. Mutagen. 1997, 378, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Lyons, B.; Page, T.; Stewart, C.; Parry, J. An assessment of the genotoxic impact of the Sea Empress oil spill by the measurement of DNA adduct levels in selected invertebrate and vertebrate species. Mutat. Res. Toxicol. Environ. Mutagen. 1999, 441, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Akcha, F.; Burgeot, T.; Venier, P.; Narbonne, J.F. Relationship Between Kinetics of Benzo[a]pyrene Bioaccumulation and DNA Binding in the Mussel Mytilus galloprovincialis. Bull. Environ. Contam. Toxicol. 1999, 62, 455–462. [Google Scholar] [CrossRef]
- Baršienė, J.; Lovejoy, D.B. Environmental Genotoxicity in Klaipėda Port Area. Int. Rev. Hydrobiol. 2000, 85, 663–672. [Google Scholar] [CrossRef]
- Pavlica, M.; Klobučar, G.I.; Vetma, N.; Erben, R.; Papeš, D. Detection of micronuclei in haemocytes of zebra mussel and great ramshorn snail exposed to pentachlorophenol. Mutat. Res. Toxicol. Environ. Mutagen. 2000, 465, 145–150. [Google Scholar] [CrossRef]
- Baršienė, J.; Bučinskienė, R. Cytogenetic Damage in the Tissues of Indigenous and Transferred Molluscs. Acta Zool. Litu. 2001, 11, 102–109. [Google Scholar] [CrossRef]
- Le Pennec, G.; Le Pennec, M. Evaluation of the toxicity of chemical compounds using digestive acini of the bivalve mollusc Pecten maximus L. maintained alive in vitro. Aquat. Toxicol. 2001, 53, 1–7. [Google Scholar] [CrossRef]
- Pavlica, M.; Klobučar, G.I.; Mojaš, N.; Erben, R.; Papeš, D. Detection of DNA damage in haemocytes of zebra mussel using comet assay. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 490, 209–214. [Google Scholar] [CrossRef]
- Dizer, H.; Fischer, B.; Harabawy, A.; Hennion, M.-C.; Hansen, P.-D. Toxicity of domoic acid in the marine mussel Mytilus edulis. Aquat. Toxicol. 2001, 55, 149–156. [Google Scholar] [CrossRef]
- Barsiene, J. Genotoxic impacts in Klaipeda Marine port and Bŭtinge oil terminal areas (Baltic Sea). Mar. Environ. Res. 2002, 54, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Bihari, N.; Hamer, B.; Jaksić, Z.; Fafandel, M.; Micić, M.; Batel, R. Application of alkaline elution, Fast Micromethod and flow cytometry in detection of marine contamination. Cell. Mol. Biol. 2002, 48, 373–377. [Google Scholar] [PubMed]
- Coughlan, B.; Hartl, M.; O’reilly, S.; Sheehan, D.; Morthersill, C.; van Pelt, F.; O’halloran, J.; O’brien, N. Detecting genotoxicity using the Comet assay following chronic exposure of Manila clam Tapes semidecussatus to polluted estuarine sediments. Mar. Pollut. Bull. 2002, 44, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Ericson, G.; Skarphéðinsdóttir, H.; Zuanna, L.; Svavarsson, J. DNA adducts as indicators of genotoxic exposure in indigenous and transplanted mussels, Mytilus edulis L. from Icelandic coastal sites. Mutat. Res. Toxicol. Environ. Mutagen. 2002, 516, 91–99. [Google Scholar] [CrossRef]
- Mičić, M.; Bihari, N.; Jakšić, Ž.; Müller, W.E.; Batel, R. DNA damage and apoptosis in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2002, 53, 243–262. [Google Scholar] [CrossRef]
- Mohamed, B.; Rym, B.D.; Amor, E.A.; Hamadi, B. Genotoxicity, Catalase, and Acetylcholinesterase in the Assessment of the Pollution Status of Some Sites on the Tunisian Littoral. Bull. Environ. Contam. Toxicol. 2003, 70, 854–860. [Google Scholar] [CrossRef]
- Pinto-Silva, C.C.; Ferreira, J.; Costa, R.; Filho, P.B.; Creppy, E.; Matias, W. Micronucleus induction in mussels exposed to okadaic acid. Toxicon 2003, 41, 93–97. [Google Scholar] [CrossRef]
- Dailianis, S.; Domouhtsidou, G.; Raftopoulou, E.; Kaloyianni, M.; Dimitriadis, V. Evaluation of neutral red retention assay, micronucleus test, acetylcholinesterase activity and a signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Mar. Environ. Res. 2003, 56, 443–470. [Google Scholar] [CrossRef]
- Pruski, A.M.; Dixon, D.R. Toxic vents and DNA damage: First evidence from a naturally contaminated deep-sea environment. Aquat. Toxicol. 2003, 64, 1–13. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Lupidi, G.; Villarini, M.; Falcioni, G. DNA Damage Induced by Copper on Erythrocytes of Gilthead Sea Bream Sparus aurata and Mollusk Scapharca inaequivalvis. Arch. Environ. Contam. Toxicol. 2003, 45, 350–356. [Google Scholar] [CrossRef]
- Gielazyn, M.L.; Ringwood, A.H.; Piegorsch, W.W.; Stancyk, S.E. Detection of oxidative DNA damage in isolated marine bivalve hemocytes using the comet assay and formamidopyrimidine glycosylase (Fpg). Mutat. Res. Toxicol. Environ. Mutagen. 2003, 542, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Buschini, A.; Carboni, P.; Martino, A.; Poli, P.; Rossi, C. Effects of temperature on baseline and genotoxicant-induced DNA damage in haemocytes of Dreissena polymorpha. Mutat. Res. Toxicol. Environ. Mutagen. 2003, 537, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Skarphéinsdóttir, H.; Ericson, G.; Dalla Zuanna, L.; Gilek, M. Tissue differences, dose-response relationship and persistence of DNA adducts in blue mussels (Mytilus edulis L.) exposed to benzo[a]pyrene. Aquat. Toxicol. 2003, 62, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Rank, J.; Jensen, K. Comet assay on gill cells and hemocytes from the blue mussel Mytilus edulis. Ecotoxicol. Environ. Saf. 2003, 54, 323–329. [Google Scholar] [CrossRef]
- Klobučar, G.I.; Pavlica, M.; Erben, R.; Papeš, D. Application of the micronucleus and comet assays to mussel Dreissena polymorpha haemocytes for genotoxicity monitoring of freshwater environments. Aquat. Toxicol. 2003, 64, 15–23. [Google Scholar] [CrossRef]
- Venier, P.; Tallandini, L.; Bisol, P.M. Characterization of Coastal Sites by Applying Genetic and Genotoxicity Markers in Mytilus galloprovincialis and Tapes Philippinarum. Chem. Ecol. 2003, 19, 113–128. [Google Scholar] [CrossRef]
- Bihari, N.; Fafanđel, M. Interspecies differences in DNA single strand breaks caused by benzo(a)pyrene and marine environment. Mutat. Res. Mol. Mech. Mutagen. 2004, 552, 209–217. [Google Scholar] [CrossRef]
- Conners, D.E.; Black, M.C. Evaluation of lethality and genotoxicity in the freshwater mussel Utterbackia imbecillis (Bivalvia: Unionidae) exposed singly and in combination to chemicals used in lawn care. Arch. Environ. Contam. Toxicol. 2004, 46, 362–371. [Google Scholar] [CrossRef]
- Pérez-Cadahía, B.; Laffon, B.; Pásaro, E.; Méndez, J. Evaluation of PAH bioaccumulation and DNA damage in mussels (Mytilus galloprovincialis) exposed to spilled Prestige crude oil. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 453–460. [Google Scholar] [CrossRef]
- Halldórsson, H.P.; Ericson, G.; Svavarsson, J. DNA strand breakage in mussels (Mytilus edulis L.) deployed in intertidal and subtidal zones in Reykjavík harbour. Mar. Environ. Res. 2004, 58, 763–767. [Google Scholar]
- Hartl, M.; Coughlan, B.; Sheehan, D.; Mothersill, C.; van Pelt, F.; O’Reilly, S.; Heffron, J.; O’Halloran, J.; O’Brien, N. Implications of seasonal priming and reproductive activity on the interpretation of Comet assay data derived from the clam, Tapes semidecussatus Reeves 1864, exposed to contaminated sediments. Mar. Environ. Res. 2004, 57, 295–310. [Google Scholar] [CrossRef]
- Bolognesi, C.; Frenzilli, G.; Lasagna, C.; Perrone, E.; Roggieri, P. Genotoxicity biomarkers in Mytilus galloprovincialis: Wild versus caged mussels. Mutat. Res. Mol. Mech. Mutagen. 2004, 552, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Lemiere, S.; Cossu-Leguille, C.; Bispo, A.; Jourdain, M.; Lanhers, M.; Burnel, D.; Vasseur, P. Genotoxicity related to transfer of oil spill pollutants from mussels to mammals via food. Environ. Toxicol. 2004, 19, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Buschini, A.; Branchi, E.; Carboni, P.; Furlini, M.; Martino, A.; Monteverde, M.; Poli, P.; Rossi, C. Comet and micronucleus assays in zebra mussel cells for genotoxicity assessment of surface drinking water treated with three different disinfectants. Sci. Total. Environ. 2004, 333, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Silva, C.C.; Creppy, E.E.; Matias, W.G. Micronucleus test in mussels Perna perna fed with the toxic dinoflagellate Prorocentrum lima. Arch. Toxicol. 2005, 79, 422–426. [Google Scholar] [CrossRef]
- Koukouzika, N.; Dimitriadis, V.K. Multiple biomarker comparison in Mytilus galloprovincialis from the Greece coast: “Lysosomal membrane stability, neutral red retention, micronucleus frequency and stress on stress”. Ecotoxicology 2005, 14, 449–463. [Google Scholar] [CrossRef]
- Lemiere, S.; Cossu-Leguille, C.; Charissou, A.-M.; Vasseur, P. DNA damage (comet assay) and 8-oxodGuo (HPLC-EC) in relation to oxidative stress in the freshwater bivalve Unio tumidus. Biomarkers 2005, 10, 41–57. [Google Scholar] [CrossRef]
- Rank, J.; Jensen, K.; Jespersen, P.H. Monitoring DNA damage in indigenous blue mussels (Mytilus edulis) sampled from coastal sites in Denmark. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 585, 33–42. [Google Scholar] [CrossRef]
- Gravato, C.; Oliveira, M.; Santos, M. Oxidative stress and genotoxic responses to resin acids in Mediterranean mussels. Ecotoxicol. Environ. Saf. 2005, 61, 221–229. [Google Scholar] [CrossRef]
- Hagger, J.A.; Depledge, M.H.; Galloway, T.S. Toxicity of tributyltin in the marine mollusc Mytilus edulis. Mar. Pollut. Bull. 2005, 51, 811–816. [Google Scholar] [CrossRef]
- Jha, A.N.; Dogra, Y.; Turner, A.; Millward, G.E. Impact of low doses of tritium on the marine mussel, Mytilus edulis: Genotoxic effects and tissue-specific bioconcentration. Mutat. Res. Toxicol. Environ. Mutagen. 2005, 586, 47–57. [Google Scholar] [CrossRef]
- Venier, P.; Zampieron, C. Evidence of genetic damage in grass gobies and mussels from the Venice lagoon. Environ. Int. 2005, 31, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Hagger, J.A.; Atienzar, F.A.; Jha, A.N. Genotoxic, cytotoxic, developmental and survival effects of tritiated water in the early life stages of the marine mollusc, Mytilus edulis. Aquat. Toxicol. 2005, 74, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Baršienė, J.; Lehtonen, K.K.; Koehler, A.; Broeg, K.; Vuorinen, P.J.; Lang, T.; Pempkowiak, J.; Šyvokienė, J.; Dedonyte, V.; Rybakovas, A.; et al. Biomarker responses in flounder (Platichthys flesus) and mussel (Mytilus edulis) in the Klaipėda-Būtingė area (Baltic Sea). Mar. Pollut. Bull. 2006, 53, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Bihari, N.; Fafand¯el, M.; Hamer, B.; Kralj-Bilen, B. PAH content, toxicity and genotoxicity of coastal marine sediments from the Rovinj area, Northern Adriatic, Croatia. Sci. Total. Environ. 2006, 366, 602–611. [Google Scholar] [CrossRef]
- Pellacani, C.; Buschini, A.; Furlini, M.; Poli, P.; Rossi, C. A battery of in vivo and in vitro tests useful for genotoxic pollutant detection in surface waters. Aquat. Toxicol. 2006, 77, 1–10. [Google Scholar] [CrossRef]
- Hartl, M.G.J.; Kilemade, M.; Coughlan, B.M.; O’Halloran, J.; VAN Pelt, F.N.A.M.; Sheehan, D.; Mothersill, C.; O’Brien, N.M. A Two-Species Biomarker Model for the Assessment of Sediment Toxicity in the Marine and Estuarine Environment Using the Comet Assay. J. Environ. Sci. Heal Part A 2006, 41, 939–953. [Google Scholar] [CrossRef]
- Cheung, V.V.; Depledge, M.H.; Jha, A.N. An evaluation of the relative sensitivity of two marine bivalve mollusc species using the Comet assay. Mar. Environ. Res. 2006, 62, S301–S305. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Moretti, M.; Carpenè, E.; Falcioni, G. Effect of different organotins on DNA of mollusk (Scapharca inaequivalvis) erythrocytes assessed by the comet assay. Sci. Total. Environ. 2006, 367, 163–169. [Google Scholar] [CrossRef]
- Le Goff, J.; Gallois, J.; Pelhuet, L.; Devier, M.H.; Budzinski, H.; Pottier, D.; André, V.; Cachot, J. DNA adduct measurements in zebra mussels, Dreissena polymorpha, Pallas. Potential use for genotoxicant biomonitoring of fresh water ecosystems. Aquat. Toxicol. 2006, 79, 55–64. [Google Scholar] [CrossRef]
- Rocher, B.; Le Goff, J.; Peluhet, L.; Briand, M.; Manduzio, H.; Gallois, J.; Devier, M.; Geffard, O.; Gricourt, L.; Augagneur, S.; et al. Genotoxicant accumulation and cellular defence activation in bivalves chronically exposed to waterborne contaminants from the Seine River. Aquat. Toxicol. 2006, 79, 65–77. [Google Scholar] [CrossRef]
- Machella, N.; Battino, M.; Pisanelli, B.; Regoli, F. Influence of the SCGE protocol on the amount of basal DNA damage detected in the Mediterranean mussel, Mytilus galloprovincialis. Environ. Mol. Mutagen. 2006, 47, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; De Falco, G.; Falugi, C.; Franzoni, M.; Monteverde, M.; Perrone, E.; Sgro, M.; Bolognesi, C. Genotoxicity biomarkers and acetylcholinesterase activity in natural populations of Mytilus galloprovincialis along a pollution gradient in the Gulf of Oristano (Sardinia, western Mediterranean). Environ. Pollut. 2006, 142, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, C.; Perrone, E.; Roggieri, P.; Sciutto, A. Bioindicators in monitoring long term genotoxic impact of oil spill: Haven case study. Mar. Environ. Res. 2006, 62, S287–S291. [Google Scholar] [CrossRef]
- Nigro, M.; Falleni, A.; Del Barga, I.; Scarcelli, V.; Lucchesi, P.; Regoli, F.; Frenzilli, G. Cellular biomarkers for monitoring estuarine environments: Transplanted versus native mussels. Aquat. Toxicol. 2006, 77, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-Y.; Hyun, C.-K. Comparative evaluation of the alkaline comet assay with the micronucleus test for genotoxicity monitoring using aquatic organisms. Ecotoxicol. Environ. Saf. 2006, 64, 288–297. [Google Scholar] [CrossRef]
- Krishnakumar, P.K.; Sasikumar, G.; Bhat, G.S.; Asokan, D.P.K. Biomarkers of environmental contaminants in field population of green mussel (Perna viridis) from Karnataka–Kerala coast (South West coast of India). Ecotoxicology 2006, 15, 347–352. [Google Scholar] [CrossRef]
- Jha, A.N.; Dogra, Y.; Turner, A.; Millward, G.E. Are low doses of tritium genotoxic to Mytilus edulis? Mar. Environ. Res. 2006, 62, S297–S300. [Google Scholar] [CrossRef]
- Villela, I.V.; de Oliveira, I.M.; da Silva, J.; Henriques, J.A. DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutat. Res. 2006, 605, 78–86. [Google Scholar]
- Francioni, E.; Wagener, A.L.; Scofield, A.L.; Depledge, M.H.; Cavalier, B.; Sette, C.B.; Carvalhosa, L.; Lozinsky, C.; Mariath, R. Polycyclic aromatic hydrocarbon in inter-tidal mussel Perna perna: Space-time observations, source investigation and genotoxicity. Sci. Total Environ. 2007, 372, 515–531. [Google Scholar]
- Rank, J.; Lehtonen, K.K.; Strand, J.; Laursen, M. DNA damage, acetylcholinesterase activity and lysosomal stability in native and transplanted mussels (Mytilus edulis) in areas close to coastal chemical dumping sites in Denmark. Aquat. Toxicol. 2007, 84, 50–61. [Google Scholar] [CrossRef]
- Anguiano, G.; Llera-Herrera, R.; Rojas, E.; Vazquez-Boucard, C. Subchronic organismal toxicity, cytotoxicity, genotoxicity, and feeding response of Pacific oyster (Crassostrea gigas) to lindane (gamma-HCH) exposure under experimental conditions. Environ. Toxicol. Chem. 2007, 26, 2192–2197. [Google Scholar] [CrossRef]
- Skarphéðinsdóttir, H.; Ericson, G.; Svavarsson, J.; Næs, K. DNA adducts and polycyclic aromatic hydrocarbon (PAH) tissue levels in blue mussels (Mytilus spp.) from Nordic coastal sites. Mar. Environ. Res. 2007, 64, 479–491. [Google Scholar] [CrossRef]
- Emmanouil, C.; Sheehan, T.; Chipman, J. Macromolecule oxidation and DNA repair in mussel (Mytilus edulis L.) gill following exposure to Cd and Cr(VI). Aquat. Toxicol. 2007, 82, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Halldórsson, H.P.; De Pirro, M.; Romano, C.; Svavarsson, J.; Sarà, G. Immediate biomarker responses to benzo[a]pyrene in polluted and unpolluted populations of the blue mussel (Mytilus edulis L.) at high-latitudes. Environ. Int. 2008, 34, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.; Binelli, A.; Cogni, D.; Provini, A. Evaluation of DNA damage induced by decabromodiphenyl ether (BDE-209) in hemocytes of Dreissena polymorpha using the comet and micronucleus assays. Environ. Mol. Mutagen. 2007, 48, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Villela, I.V.; de Oliveira, I.M.; Silveira, J.C.; Dias, J.F.; Henriques, J.A.P.; da Silva, J. Assessment of environmental stress by the micronucleus and comet assays on Limnoperna fortunei exposed to Guaíba hydrographic region samples (Brazil) under laboratory conditions. Mutat. Res. Toxicol. Environ. Mutagen. 2007, 628, 76–86. [Google Scholar] [CrossRef]
- Gagné, F.; Auclair, J.; Turcotte, P.; Fournier, M.; Gagnon, C.; Sauvé, S.; Blaise, C. Ecotoxicity of CdTe quantum dots to freshwater mussels: Impacts on immune system, oxidative stress and genotoxicity. Aquat. Toxicol. 2008, 86, 333–340. [Google Scholar] [CrossRef]
- Verlecar, X.; Jena, K.; Chainy, G. Modulation of antioxidant defences in digestive gland of Perna viridis (L.), on mercury exposures. Chemosphere 2008, 71, 1977–1985. [Google Scholar] [CrossRef]
- Pan, L.; Miao, J.; Wang, J.; Liu, J. AHH activity, tissue dose and DNA damage in different tissues of the scallop Chlamys farreri exposed to benzo[a]pyrene. Environ. Pollut. 2008, 153, 192–198. [Google Scholar] [CrossRef]
- Bocchetti, R.; Fattorini, D.; Pisanelli, B.; Macchia, S.; Oliviero, L.; Pilato, F.; Pellegrini, D.; Regoli, F. Contaminant accumulation and biomarker responses in caged mussels, Mytilus galloprovincialis, to evaluate bioavailability and toxicological effects of remobilized chemicals during dredging and disposal operations in harbour areas. Aquat. Toxicol. 2008, 89, 257–266. [Google Scholar] [CrossRef]
- Binelli, A.; Riva, C.; Cogni, D.; Provini, A. Genotoxic effects of p,p′-DDT (1,1,1-trichloro-2,2-bis-(chlorophenyl)ethane) and its metabolites in Zebra mussel (D. polymorpha) by SCGE assay and micronucleus test. Environ. Mol. Mutagen. 2008, 49, 406–415. [Google Scholar] [CrossRef]
- Štambuk, A.; Pavlica, M.; Malović, L.; Klobucšar, G.I.V. Persistence of DNA damage in the freshwater mussel Unio pictorum upon exposure to ethyl methanesulphonate and hydrogen peroxide. Environ. Mol. Mutagen. 2008, 49, 217–225. [Google Scholar] [CrossRef]
- Siu, S.Y.; Lam, P.K.; Martin, M.; Caldwell, C.W.; Richardson, B.J. The use of selected genotoxicity assays in green-lipped mussels (Perna viridis): A validation study in Hong Kong coastal waters. Mar. Pollut. Bull. 2008, 57, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Riva, C.; Cogni, D.; Provini, A. Assessment of the genotoxic potential of benzo(a)pyrene and p,p′-dichlorodiphenyldichloroethylene in Zebra mussel (Dreissena polymorpha). Mutat. Res. 2008, 649, 135–145. [Google Scholar]
- Klobučar, G.I.; Štambuk, A.; Hylland, K.; Pavlica, M. Detection of DNA damage in haemocytes of Mytilus galloprovincialis in the coastal ecosystems of Kaštela and Trogir bays, Croatia. Sci. Total. Environ. 2008, 405, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Petridis, P.; Jha, A.N.; Langston, W.J. Measurements of the genotoxic potential of (xeno-)oestrogens in the bivalve mollusc Scrobicularia plana, using the Comet assay. Aquat. Toxicol. 2009, 94, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Pisanelli, B.; Benedetti, M.; Fattorini, D.; Regoli, F. Seasonal and inter-annual variability of DNA integrity in mussels Mytilus galloprovincialis: A possible role for natural fluctuations of trace metal concentrations and oxidative biomarkers. Chemosphere 2009, 77, 1551–1557. [Google Scholar] [CrossRef]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. Cytotoxic and genotoxic effects of in vitro exposure to Triclosan and Trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 50–56. [Google Scholar] [CrossRef]
- Bissett, W.; Smith, L.; Thompson, J.A. Geostatistical analysis of DNA damage in oysters, Crassostrea virginica, in Lavaca Bay, Texas. Ecotoxicology 2008, 18, 69–74. [Google Scholar] [CrossRef]
- Rank, J. Intersex in Littorina littorea and DNA damage in Mytilus edulis as indicators of harbour pollution. Ecotoxicol. Environ. Saf. 2009, 72, 1271–1277. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Cogni, D.; Riva, C.; Provini, A. An in vitro biomarker approach for the evaluation of the ecotoxicity of non-steroidal anti-inflammatory drugs (NSAIDs). Toxicol. Vitr. 2009, 23, 935–942. [Google Scholar] [CrossRef]
- Bissett, W.; Smith, R.; Adams, L.G.; Field, R.; Moyer, W.; Phillips, T.; Scott, H.M.; Wade, T.; Sweet, S.; Thompson, J.A. An evaluation of the health status of the Lavaca Bay, Texas ecosystem using Crassostrea virginica as the sentinel species. Mar. Pollut. Bull. 2009, 58, 280–286. [Google Scholar] [CrossRef]
- Maria, V.L.; Santos, M.A.; Bebianno, M.J. Biomarkers of damage and protection in Mytilus galloprovincialis cross transplanted in Ria Formosa Lagoon (Portugal). Ecotoxicology 2009, 18, 1018–1028. [Google Scholar] [CrossRef]
- Ma, J.-J.; Pan, L.-Q.; Li, J.; Zhang, L. Effects of benzo[a]pyrene on DNA damage and histological alterations in gonad of scallop Chlamys farreri. Mar. Environ. Res. 2009, 67, 47–52. [Google Scholar]
- Binelli, A.; Cogni, D.; Parolini, M.; Riva, C.; Provini, A. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 2009, 91, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Canty, M.N.; Hutchinson, T.H.; Brown, R.J.; Jones, M.B.; Jha, A.N. Linking genotoxic responses with cytotoxic and behavioural or physiological consequences: Differential sensitivity of echinoderms (Asterias rubens) and marine molluscs (Mytilus edulis). Aquat. Toxicol. 2009, 94, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Parolini, M.; Cogni, D.; Pedriali, A.; Provini, A. A multi-biomarker assessment of the impact of the antibacterial trimethoprim on the non-target organism Zebra mussel (Dreissena polymorpha). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 329–336. [Google Scholar] [CrossRef]
- Stambuk, A.; Pavlica, M.; Vignjević, G.; Bolarić, B.; Klobucar, G.I. Assessment of genotoxicity in polluted freshwaters using caged painter’s mussel, Unio pictorum. Ecotoxicology 2009, 18, 430–439. [Google Scholar] [CrossRef]
- Revankar, P.R.; Shyama, S. Genotoxic effects of monocrotophos, an organophosphorous pesticide, on an estuarine bivalve, Meretrix ovum. Food Chem. Toxicol. 2009, 47, 1618–1623. [Google Scholar] [CrossRef]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Farkas, A.; Vehovszky, Á.; Gyori, J.; Stoliar, O.B. Vulnerability of biomarkers in the indigenous mollusk Anodonta cygnea to spontaneous pollution in a transition country. Chemosphere 2010, 81, 1342–1351. [Google Scholar] [CrossRef]
- Chatziargyriou, V.; Dailianis, S. The role of selenium-dependent glutathione peroxidase (Se-GPx) against oxidative and genotoxic effects of mercury in haemocytes of mussel Mytilus galloprovincialis (Lmk.). Toxicol. Vitr. 2010, 24, 1363–1372. [Google Scholar] [CrossRef]
- Stalter, D.; Magdeburg, A.; Oehlmann, J. Comparative toxicity assessment of ozone and activated carbon treated sewage effluents using an in vivo test battery. Water Res. 2010, 44, 2610–2620. [Google Scholar] [CrossRef]
- Guidi, P.; Frenzilli, G.; Benedetti, M.; Bernardeschi, M.; Falleni, A.; Fattorini, D.; Regoli, F.; Scarcelli, V.; Nigro, M. Antioxidant, genotoxic and lysosomal biomarkers in the freshwater bivalve (Unio pictorum) transplanted in a metal polluted river basin. Aquat. Toxicol. 2010, 100, 75–83. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Cogni, D.; Provini, A. Multi-biomarker approach for the evaluation of the cyto-genotoxicity of paracetamol on the zebra mussel (Dreissena polymorpha). Chemosphere 2010, 79, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Banni, M.; Negri, A.; Dagnino, A.; Jebali, J.; Ameur, S.; Boussetta, H. Acute effects of benzo[a]pyrene on digestive gland enzymatic biomarkers and DNA damage on mussel Mytilus galloprovincialis. Ecotoxicol. Environ. Saf. 2010, 73, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Bourgeault, A.; Gourlay-Francé, C.; Vincent-Hubert, F.; Palais, F.; Geffard, A.; Biagianti-Risbourg, S.; Pain-Devin, S.; Tusseau-Vuillemin, M. Lessons from a transplantation of zebra mussels into a small urban river: An integrated ecotoxicological assessment. Environ. Toxicol. 2010, 25, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Fedato, R.; Simonato, J.; Martinez, C.; Sofia, S. Genetic damage in the bivalve mollusk Corbicula fluminea induced by the water-soluble fraction of gasoline. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 700, 80–85. [Google Scholar] [CrossRef]
- Marcheselli, M.; Azzoni, P.; Mauri, M. Novel antifouling agent-zinc pyrithione: Stress induction and genotoxicity to the marine mussel Mytilus galloprovincialis. Aquat. Toxicol. 2011, 102, 39–47. [Google Scholar] [CrossRef]
- Quinn, B.; Schmidt, W.; O’rourke, K.; Hernan, R. Effects of the pharmaceuticals gemfibrozil and diclofenac on biomarker expression in the zebra mussel (Dreissena polymorpha) and their comparison with standardised toxicity tests. Chemosphere 2011, 84, 657–663. [Google Scholar] [CrossRef]
- Gagné, F.; André, C.; Cejka, P.; Hausler, R.; Fournier, M. Alterations in DNA metabolism in Elliptio complanata mussels after exposure to municipal effluents. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 100–107. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Moody, A.J.; Mustafa, S.A.; Jha, A.N. A multiple biomarker approach to investigate the effects of copper on the marine bivalve mollusc, Mytilus edulis. Ecotoxicol. Environ. Saf. 2011, 74, 1913–1920. [Google Scholar] [CrossRef]
- Fernández-Tajes, J.; Flórez, F.; Pereira, S.; Rábade, T.; Laffon, B.; Méndez, J. Use of three bivalve species for biomonitoring a polluted estuarine environment. Environ. Monit. Assess. 2010, 177, 289–300. [Google Scholar] [CrossRef]
- Almeida, C.; Pereira, C.; Gomes, T.; Bebianno, M.J.; Cravo, A. DNA damage as a biomarker of genotoxic contamination in Mytilus galloprovincialis from the south coast of Portugal. J. Environ. Monit. 2011, 13, 2559–2567. [Google Scholar] [CrossRef]
- Fernández-Tajes, J.; Rábade, T.; Laffon, B.; Méndez, J. Monitoring Follow Up of Two Areas Affected by the Prestige Oil Four Years After the Spillage. J. Toxicol. Environ. Health Part A 2011, 74, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Simon, O.; Floriani, M.; Cavalie, I.; Camilleri, V.; Adam, C.; Gilbin, R.; Garnier-Laplace, J. Internal distribution of uranium and associated genotoxic damages in the chronically exposed bivalve Corbicula fluminea. J. Environ. Radioact. 2011, 102, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.M.; Fernández-Tajes, J.; Rábade, T.; Flórez-Barrós, F.; Laffon, B.; Méndez, J. Comparison Between Two Bivalve Species as Tools for the Assessment of Pollution Levels in an Estuarian Environment. J. Toxicol. Environ. Health Part A 2011, 74, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A.; Provini, A. Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol. Environ. Saf. 2011, 74, 1586–1594. [Google Scholar] [CrossRef]
- Vincent-Hubert, F.; Arini, A.; Gourlay-Francé, C. Early genotoxic effects in gill cells and haemocytes of Dreissena polymorpha exposed to cadmium, B[a]P and a combination of B[a]P and Cd. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 723, 26–35. [Google Scholar] [CrossRef]
- Parolini, M.; Binelli, A.; Provini, A. Assessment of the Potential Cyto–Genotoxicity of the Nonsteroidal Anti-Inflammatory Drug (NSAID) Diclofenac on the Zebra Mussel (Dreissena polymorpha). Water Air Soil Pollut. 2010, 217, 589–601. [Google Scholar] [CrossRef]
- Farhadi, A.; Farahmand, H.; Mirvaghefi, A.; Khalili, B. A Genotoxicological Study in Persian Gulf on Rock Oyster (Soccostrea cucullata) using Micronuclei and RAPID Assays. Int. J. Environ. Res. 2011, 5, 567–572. [Google Scholar] [CrossRef]
- Taylor, A.M.; Maher, W.A. Exposure-dose-response of Anadara trapezia to metal contaminated estuarine sediments: Selenium spiked sediments. Aquat. Toxicol. 2012, 124–125, 152–162. [Google Scholar] [CrossRef]
- Kamel, N.; Attig, H.; Dagnino, A.; Boussetta, H.; Banni, M. Increased Temperatures Affect Oxidative Stress Markers and Detoxification Response to Benzo[a]Pyrene Exposure in Mussel Mytilus galloprovincialis. Arch. Environ. Contam. Toxicol. 2012, 63, 534–543. [Google Scholar] [CrossRef]
- Giannapas, M.; Karnis, L.; Dailianis, S. Generation of free radicals in haemocytes of mussels after exposure to low molecular weight PAH components: Immune activation, oxidative and genotoxic effects. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 155, 182–189. [Google Scholar] [CrossRef]
- Baršienė, J.; Rybakovas, A.; Garnaga, G.; Andreikėnaitė, L. Environmental genotoxicity and cytotoxicity studies in mussels before and after an oil spill at the marine oil terminal in the Baltic Sea. Environ. Monit. Assess. 2011, 184, 2067–2078. [Google Scholar] [CrossRef]
- Sures, B.; Zimmermann, S.; Messerschmidt, J.; von Bohlen, A. Relevance and Analysis of Traffic Related Platinum Group Metals(Pt, Pd, Rh) in the Aquatic Biosphere, with Emphasis on Palladium. Ecotoxicology 2002, 11, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.; Hamer, B.; Treursić, B.; Möhlenkamp, C.; Spira, D.; Korlević, M.; Reifferscheid, G.; Claus, E. Comparison of Bioaccumulation and Biomarker Responses in Dreissena polymorpha and D. bugensis After Exposure to Resuspended Sediments. Arch. Environ. Contam. Toxicol. 2012, 62, 614–627. [Google Scholar] [CrossRef]
- Martins, M.; Costa, P.M.; Raimundo, J.; Vale, C.; Ferreira, A.M.; Costa, M.H. Impact of remobilized contaminants in Mytilus edulis during dredging operations in a harbour area: Bioaccumulation and biomarker responses. Ecotoxicol. Environ. Saf. 2012, 85, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Hartl, M.G.J. Fluctuating estuarine conditions are not confounding factors for the Comet assay assessment of DNA damage in the mussel Mytilus edulis. Ecotoxicology 2012, 21, 1998–2003. [Google Scholar] [CrossRef] [PubMed]
- Millward, G.E.; Kadam, S.; Jha, A.N. Tissue-specific assimilation, depuration and toxicity of nickel in Mytilus edulis. Environ. Pollut. 2012, 162, 406–412. [Google Scholar] [CrossRef]
- Michel, C.; Vincent-Hubert, F. Detection of 8-oxodG in Dreissena polymorpha gill cells exposed to model contaminants. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 741, 1–6. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Zhukovskaya, A.F.; Chelomin, V.P. DNA damage in the gill cells of the marine scallop Mizuhopecten yessoensis during anoxic stress and aerobic recovery. Ocean Sci. J. 2012, 47, 95–100. [Google Scholar] [CrossRef]
- Rađa, B.; Šantić, M.; Kuprešanin, M. Monitoring DNA damage in Mytilus galloprovincialis from the Kaštela Bay in Croatia. Russ. J. Ecol. 2012, 43, 77–81. [Google Scholar] [CrossRef]
- Al-Subiai, S.N.; Arlt, V.M.; Frickers, P.E.; Readman, J.W.; Stolpe, B.; Lead, J.R.; Moody, A.J.; Jha, A.N. Merging nano-genotoxicology with eco-genotoxicology: An integrated approach to determine interactive genotoxic and sub-lethal toxic effects of C60 fullerenes and fluoranthene in marine mussels, Mytilus sp. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 92–103. [Google Scholar] [CrossRef][Green Version]
- Châtel, A.; Faucet-Marquis, V.; Perret, M.; Gourlay-Francé, C.; Uher, E.; Pfohl-Leszkowicz, A.; Vincent-Hubert, F. Genotoxicity assessment and detoxification induction in Dreissena polymorpha exposed to benzo[a]pyrene. Mutagenesis 2012, 27, 703–711. [Google Scholar] [CrossRef]
- Abdul-Aziz, K.K. The Health Status and Genetic Variations of the Bivalve, Pinctala radiata Affected by Environmental Pollution. J. Environ. Anal. Toxicol. 2012, 2, 138. [Google Scholar] [CrossRef]
- Binelli, A.; Pedriali, A.; Riva, C.; Parolini, M. Illicit drugs as new environmental pollutants: Cyto-genotoxic effects of cocaine on the biological model Dreissena polymorpha. Chemosphere 2012, 86, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A. Sub-lethal effects induced by a mixture of three non-steroidal anti-inflammatory drugs (NSAIDs) on the freshwater bivalve Dreissena polymorpha. Ecotoxicology 2011, 21, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A. Cyto-genotoxic effects induced by three brominated diphenyl ether congeners on the freshwater mussel Dreissena polymorpha. Ecotoxicol. Environ. Saf. 2012, 79, 247–255. [Google Scholar] [CrossRef]
- Taylor, A.M.; Maher, W.A. Exposure-dose-response of Tellina deltoidalis to metal-contaminated estuarine sediments. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 44–55. [Google Scholar] [CrossRef]
- Sacchi, A.; Mouneyrac, C.; Bolognesi, C.; Sciutto, A.; Roggieri, P.; Fusi, M.; Beone, G.M.; Capri, E. Biomonitoring study of an estuarine coastal ecosystem, the Sacca di Goro lagoon, using Ruditapes philippinarum (Mollusca: Bivalvia). Environ. Pollut. 2013, 177, 82–89. [Google Scholar] [CrossRef]
- Parolini, M.; Pedriali, A.; Binelli, A. Chemical and biomarker responses for site-specific quality assessment of the Lake Maggiore (Northern Italy). Environ. Sci. Pollut. Res. 2013, 20, 5545–5557. [Google Scholar] [CrossRef]
- Martins, M.; Costa, P.M.; Ferreira, A.M.; Costa, M.H. Comparative DNA damage and oxidative effects of carcinogenic and non-carcinogenic sediment-bound PAHs in the gills of a bivalve. Aquat. Toxicol. 2013, 142–143, 85–95. [Google Scholar] [CrossRef]
- JanakiDevi, V.; Nagarani, N.; YokeshBabu, M.; Kumaraguru, A.; Ramakritinan, C. A study of proteotoxicity and genotoxicity induced by the pesticide and fungicide on marine invertebrate (Donax faba). Chemosphere 2013, 90, 1158–1166. [Google Scholar] [CrossRef]
- Kolarević, S.; Knežević-Vukčević, J.; Paunović, M.; Kračun, M.; Vasiljević, B.; Tomović, J.; Vuković-Gačić, B.; Gačić, Z. Monitoring of DNA damage in haemocytes of freshwater mussel Sinanodonta woodiana sampled from the Velika Morava River in Serbia with the comet assay. Chemosphere 2013, 93, 243–251. [Google Scholar] [CrossRef]
- Buffet, P.-E.; Richard, M.; Caupos, F.; Vergnoux, A.; Perrein-Ettajani, H.; Luna-Acosta, A.; Akcha, F.; Amiard, J.-C.; Amiard-Triquet, C.; Guibbolini, M.; et al. A Mesocosm Study of Fate and Effects of CuO Nanoparticles on Endobenthic Species (Scrobicularia plana, Hediste diversicolor). Environ. Sci. Technol. 2013, 47, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Dallas, L.J.; Cheung, V.V.; Fisher, A.S.; Jha, A.N. Relative sensitivity of two marine bivalves for detection of genotoxic and cytotoxic effects: A field assessment in the Tamar Estuary, South West England. Environ. Monit. Assess. 2012, 185, 3397–3412. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaeri, M.; Ahmed, D.; McCluskey, F.; Turner, G.; Paterson, L.; Dyrynda, E.A.; Hartl, M.G. Potentiating toxicological interaction of single-walled carbon nanotubes with dissolved metals. Environ. Toxicol. Chem. 2013, 32, 2701–2710. [Google Scholar] [CrossRef]
- Almeida, C.; Pereira, C.G.; Gomes, T.; Cardoso, C.; Bebianno, M.J.; Cravo, A. Genotoxicity in two bivalve species from a coastal lagoon in the south of Portugal. Mar. Environ. Res. 2013, 89, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.; Araújo, O.; Pereira, R.; Almeida, A.C.; Cravo, A.; Bebianno, M.J. Genotoxicity of copper oxide and silver nanoparticles in the mussel Mytilus galloprovincialis. Mar. Environ. Res. 2013, 84, 51–59. [Google Scholar] [CrossRef]
- Vuković-Gačić, B.; Kolarević, S.; Sunjog, K.; Tomović, J.; Knežević-Vukčević, J.; Paunović, M.; Gačić, Z. Comparative study of the genotoxic response of freshwater mussels Unio tumidus and Unio pictorum to environmental stress. Hydrobiologia 2013, 735, 221–231. [Google Scholar] [CrossRef]
- Mustapha, N.; Zouiten, A.; Dridi, D.; Tahrani, L.; Zouiten, D.; Mosrati, R.; Cherif, A.; Chekir-Ghedira, L.; Mansour, H.B. Comet assay with gill cells of Mytilus galloprovincialis end point tools for biomonitoring of water antibiotic contamination: Biological treatment is a reliable process for detoxification. Toxicol. Ind. Health 2016, 32, 686–693. [Google Scholar] [CrossRef]
- Mat, A.M.; Haberkorn, H.; Bourdineaud, J.-P.; Massabuau, J.-C.; Tran, D. Genetic and genotoxic impacts in the oyster Crassostrea gigas exposed to the harmful alga Alexandrium minutum. Aquat. Toxicol. 2013, 140–141, 458–465. [Google Scholar] [CrossRef]
- Debenest, T.; Gagné, F.; Burgeot, T.; Blaise, C.; Pellerin, J. DNA integrity assessment in hemocytes of soft-shell clams (Mya arenaria) in the Saguenay Fjord (Québec, Canada). Environ. Sci. Pollut. Res. 2012, 20, 621–629. [Google Scholar] [CrossRef]
- Chandurvelan, R.; Marsden, I.D.; Gaw, S.; Glover, C.N. Waterborne cadmium impacts immunocytotoxic and cytogenotoxic endpoints in green-lipped mussel, Perna canaliculus. Aquat. Toxicol. 2013, 142–143, 283–293. [Google Scholar] [CrossRef]
- Michel, C.; Bourgeault, A.; Gourlay-Francé, C.; Palais, F.; Geffard, A.; Vincent-Hubert, F. Seasonal and PAH impact on DNA strand-break levels in gills of transplanted zebra mussels. Ecotoxicol. Environ. Saf. 2013, 92, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Toufexi, E.; Tsarpali, V.; Efthimiou, I.; Vidali, M.-S.; Vlastos, D.; Dailianis, S. Environmental and human risk assessment of landfill leachate: An integrated approach with the use of cytotoxic and genotoxic stress indices in mussel and human cells. J. Hazard. Mater. 2013, 260, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Dallas, L.J.; Bean, T.P.; Turner, A.; Lyons, B.P.; Jha, A.N. Oxidative DNA damage may not mediate Ni-induced genotoxicity in marine mussels: Assessment of genotoxic biomarkers and transcriptional responses of key stress genes. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 754, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Falfushynska, H.I.; Gnatyshyna, L.L.; Osadchuk, O.Y.; Farkas, A.; Vehovszky, A.; Carpenter, D.O.; Gyori, J.; Stoliar, O.B. Diversity of the molecular responses to separate wastewater effluents in freshwater mussels. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2014, 164, 51–58. [Google Scholar] [CrossRef]
- Dailianis, S.; Tsarpali, V.; Melas, K.; Karapanagioti, H.K.; Manariotis, I.D. Aqueous phenanthrene toxicity after high-frequency ultrasound degradation. Aquat. Toxicol. 2014, 147, 32–40. [Google Scholar] [CrossRef]
- Kumar, M.P.; Shyama, S.; Sonaye, B.; Naik, U.R.; Kadam, S.; Bipin, P.; D’costa, A.; Chaubey, R. Evaluation of γ-radiation-induced DNA damage in two species of bivalves and their relative sensitivity using comet assay. Aquat. Toxicol. 2014, 150, 1–8. [Google Scholar] [CrossRef]
- dos Santos, K.C.; Martinez, C.B. Genotoxic and biochemical effects of atrazine and Roundup(®), alone and in combination, on the Asian clam Corbicula fluminea. Ecotoxicol. Environ. Saf. 2014, 100, 7–14. [Google Scholar]
- Zhang, H.; Pan, L.; Tao, Y. Antioxidant responses in clam Venerupis philippinarum exposed to environmental pollutant hexabromocyclododecane. Environ. Sci. Pollut. Res. 2014, 21, 8206–8215. [Google Scholar] [CrossRef]
- Liu, C.; Gin, K.Y.H.; Chang, V.W.C. Multi-biomarker responses in green mussels exposed to PFCs: Effects at molecular, cellular, and physiological levels. Environ. Sci. Pollut. Res. 2013, 21, 2785–2794. [Google Scholar] [CrossRef]
- Goswami, P.; Hariharan, G.; Godhantaraman, N.; Munuswamy, N. An integrated use of multiple biomarkers to investigate the individual and combined effect of copper and cadmium on the marine green mussel (Perna viridis). J. Environ. Sci. Health A 2014, 49, 1564–1577. [Google Scholar] [CrossRef]
- Vázquez-Boucard, C.; Anguiano-Vega, G.; Mercier, L.; Rojas del Castillo, E. Pesticide residues, heavy metals, and DNA damage in sentinel oysters Crassostrea gigas from Sinaloa and Sonora, Mexico. J. Toxicol. Environ. Health A 2014, 77, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Héberger, K.; Kolarević, S.; Kračun-Kolarević, M.; Sunjog, K.; Gačić, Z.; Kljajić, Z.; Mitrić, M.; Vuković-Gačić, B. Evaluation of single-cell gel electrophoresis data: Combination of variance analysis with sum of ranking differences. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 771, 15–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buffet, P.-E.; Zalouk-Vergnoux, A.; Châtel, A.; Berthet, B.; Métais, I.; Perrein-Ettajani, H.; Poirier, L.; Luna-Acosta, A.; Thomas-Guyon, H.; Faverney, C.R.-D.; et al. A marine mesocosm study on the environmental fate of silver nanoparticles and toxicity effects on two endobenthic species: The ragworm Hediste diversicolor and the bivalve mollusc Scrobicularia plana. Sci. Total. Environ. 2014, 470–471, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Slobodskova, V.; Solodova, E.; Chelomin, V.P. Accumulation of Cadmium in the Marine Scallop Mizuhopecten yessoensis and Its Potential Consequences on DNA Damage. Environ. Res. J. 2012, 6, 131. [Google Scholar]
- Châtel, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Gourlay-Francé, C.; Vincent-Hubert, F. DNA adduct formation and induction of detoxification mechanisms in Dreissena polymorpha exposed to nitro-PAHs. Mutagenesis 2014, 29, 457–465. [Google Scholar] [CrossRef]
- Tian, S.; Pan, L.; Zhang, H. Identification of a CYP3A-like gene and CYPs mRNA expression modulation following exposure to benzo[a]pyrene in the bivalve mollusk Chlamys farreri. Mar. Environ. Res. 2014, 94, 7–15. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Binelli, A. Environmental concentrations of 3,4-methylenedioxymethamphetamine (MDMA)-induced cellular stress and modulated antioxidant enzyme activity in the zebra mussel. Environ. Sci. Pollut. Res. 2014, 21, 11099–11106. [Google Scholar] [CrossRef]
- D’agata, A.; Cappello, T.; Maisano, M.; Parrino, V.; Giannetto, A.; Brundo, M.V.; Ferrante, M.; Mauceri, A. Cellular biomarkers in the mussel Mytilus galloprovincialis(Bivalvia: Mytilidae) from Lake Faro (Sicily, Italy). Ital. J. Zool. 2014, 81, 43–54. [Google Scholar] [CrossRef]
- Rocha, T.L.; Gomes, T.; Cardoso, C.; Letendre, J.; Pinheiro, J.P.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Immunocytotoxicity, cytogenotoxicity and genotoxicity of cadmium-based quantum dots in the marine mussel Mytilus galloprovincialis. Mar. Environ. Res. 2014, 101, 29–37. [Google Scholar] [CrossRef]
- Liu, C.; Chang, V.W.; Gin, K.Y.; Nguyen, V.T. Genotoxicity of perfluorinated chemicals (PFCs) to the green mussel (Perna viridis). Sci. Total. Environ. 2014, 487, 117–122. [Google Scholar] [CrossRef]
- Barranger, A.; Akcha, F.; Rouxel, J.; Brizard, R.; Maurouard, E.; Pallud, M.; Menard, D.; Tapie, N.; Budzinski, H.; Burgeot, T.; et al. Study of genetic damage in the Japanese oyster induced by an environmentally-relevant exposure to diuron: Evidence of vertical transmission of DNA damage. Aquat. Toxicol. 2014, 146, 93–104. [Google Scholar] [CrossRef]
- Eck-Varanka, B.; Kováts, N.; Hubai, K.; Paulovits, G.; Ferincz, Á.; Horváth, E. Genotoxic effect of Lythrum salicaria extract determined by the mussel micronucleus test. Acta Biol. Hung. 2015, 66, 460–463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zorita, I.; Larreta, J.; Montero, N.; Rodríguez, J.G.; Franco, J.; Borja, Á. Evaluation of the use of bioaccumulation and biological effects tools in caged mussels, within the European Water Framework Directive. Chem. Ecol. 2015, 31, 432–445. [Google Scholar] [CrossRef]
- Chandurvelan, R.; Marsden, I.D.; Glover, C.N.; Gaw, S. Assessment of a mussel as a metal bioindicator of coastal contamination: Relationships between metal bioaccumulation and multiple biomarker responses. Sci. Total. Environ. 2015, 511, 663–675. [Google Scholar] [CrossRef]
- Farkas, J.; Bergum, S.; Nilsen, E.; Olsen, A.; Salaberria, I.; Ciesielski, T.; Bączek, T.; Konieczna, L.; Salvenmoser, W.; Jenssen, B. The impact of TiO2 nanoparticles on uptake and toxicity of benzo(a)pyrene in the blue mussel (Mytilus edulis). Sci. Total. Environ. 2015, 511, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, X.; Jiang, M. Assessment of the toxicity of crude oil in Sinonovacula constricta clams. Aquat. Living Resour. 2015, 28, 119–126. [Google Scholar] [CrossRef]
- Michel, C.; Vincent-Hubert, F. DNA oxidation and DNA repair in gills of zebra mussels exposed to cadmium and benzo(a)pyrene. Ecotoxicology 2015, 24, 2009–2016. [Google Scholar] [CrossRef]
- Lacaze, E.; Pédelucq, J.; Fortier, M.; Brousseau, P.; Auffret, M.; Budzinski, H.; Fournier, M. Genotoxic and immunotoxic potential effects of selected psychotropic drugs and antibiotics on blue mussel (Mytilus edulis) hemocytes. Environ. Pollut. 2015, 202, 177–186. [Google Scholar] [CrossRef]
- Turja, R.; Lehtonen, K.K.; Meierjohann, A.; Brozinski, J.-M.; Vahtera, E.; Soirinsuo, A.; Sokolov, A.; Snoeijs, P.; Budzinski, H.; Devier, M.-H.; et al. The mussel caging approach in assessing biological effects of wastewater treatment plant discharges in the Gulf of Finland (Baltic Sea). Mar. Pollut. Bull. 2015, 97, 135–149. [Google Scholar] [CrossRef]
- Katsumiti, A.; Gilliland, D.; Arostegui, I.; Cajaraville, M.P. Mechanisms of Toxicity of Ag Nanoparticles in Comparison to Bulk and Ionic Ag on Mussel Hemocytes and Gill Cells. PLoS ONE 2015, 10, e0129039. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Kukla, S.P.; Chelomin, V.P. An analysis of the quality of the marine environment based on determination of the genotoxicity of DNA in the gill cells of the Yesso Scallop, Mizuhopecten yessoensis (Jay, 1856). Russ. J. Mar. Biol. 2015, 41, 495–498. [Google Scholar] [CrossRef]
- Hu, F.; Pan, L.; Xiu, M.; Jin, Q. Exposure of Chlamys farreri to tetrabromobisphenol A: Accumulation and multibiomarker responses. Environ. Sci. Pollut. Res. 2015, 22, 12224–12234. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Santonastaso, M.; Nigro, M.; Mottola, F.; Costagliola, D.; Bernardeschi, M.; Guidi, P.; Lucchesi, P.; Scarcelli, V.; Corsi, I.; et al. Genomic and chromosomal damage in the marine mussel Mytilus galloprovincialis: Effects of the combined exposure to titanium dioxide nanoparticles and cadmium chloride. Mar. Environ. Res. 2015, 111, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Belavgeni, A.; Dailianis, S. Investigation of toxic effects of imidazolium ionic liquids, [bmim][BF4] and [omim][BF4], on marine mussel Mytilus galloprovincialis with or without the presence of conventional solvents, such as acetone. Aquat. Toxicol. 2015, 164, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Binelli, A.; Magni, S.; Della Torre, C.; Parolini, M. Toxicity decrease in urban wastewaters treated by a new biofiltration process. Sci. Total. Environ. 2015, 537, 235–242. [Google Scholar] [CrossRef]
- Parolini, M.; Magni, S.; Traversi, I.; Villa, S.; Finizio, A.; Binelli, A. Environmentally relevant concentrations of galaxolide (HHCB) and tonalide (AHTN) induced oxidative and genetic damage in Dreissena polymorpha. J. Hazard. Mater. 2015, 285, 1–10. [Google Scholar] [CrossRef]
- Pérez-García, C.; Rouxel, J.; Akcha, F. Development of a comet-FISH assay for the detection of DNA damage in hemocytes of Crassostrea gigas. Aquat. Toxicol. 2015, 161, 189–195. [Google Scholar] [CrossRef]
- Aguirre-Martínez, G.V.; Del Valls, T.Á.; Martín-Díaz, M.L. General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol. Environ. Saf. 2016, 124, 18–31. [Google Scholar] [CrossRef]
- Marisa, I.; Matozzo, V.; Munari, M.; Binelli, A.; Parolini, M.; Martucci, A.; Franceschinis, E.; Brianese, N.; Marin, M.G. In vivo exposure of the marine clam Ruditapes philippinarum to zinc oxide nanoparticles: Responses in gills, digestive gland and haemolymph. Environ. Sci. Pollut. Res. 2016, 23, 15275–15293. [Google Scholar] [CrossRef]
- Toufexi, E.; Dailianis, S.; Vlastos, D.; Manariotis, I.D. Mediated effect of ultrasound treated Diclofenac on mussel hemocytes: First evidence for the involvement of respiratory burst enzymes in the induction of DCF-mediated unspecific mode of action. Aquat. Toxicol. 2016, 175, 144–153. [Google Scholar] [CrossRef]
- Behrens, D.; Rouxel, J.; Burgeot, T.; Akcha, F. Comparative embryotoxicity and genotoxicity of the herbicide diuron and its metabolites in early life stages of Crassostrea gigas: Implication of reactive oxygen species production. Aquat. Toxicol. 2016, 175, 249–259. [Google Scholar] [CrossRef]
- Pazi, I.; Kucuksezgin, F.; Kacar, A.; Gonul, L.T. Assessment of the effect of environmental pollution in a marina on caged mussels using chemical and genotoxic analysis. Chem. Ecol. 2016, 32, 756–773. [Google Scholar] [CrossRef]
- Kolarević, S.; Gačić, Z.; Kostić, J.; Sunjog, K.; Kračun-Kolarević, M.; Paunović, M.; Knežević-Vukčević, J.; Vuković-Gačić, B. Impact of Common Cytostatics on DNA Damage in Freshwater Mussels Uniopictorum and Unio tumidus. CLEAN—Soil Air Water 2016, 44, 1471–1476. [Google Scholar] [CrossRef]
- Chavan, P.; Kumar, R.; Kirubagaran, R.; Venugopalan, V.P. Chlorination-induced genotoxicity in the mussel Perna viridis: Assessment by single cell gel electrophoresis (comet) assay. Ecotoxicol. Environ. Saf. 2016, 130, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zouiten, A.; Beltifa, A.; Van Loco, J.; Mansour, H.B.; Reyns, T. Ecotoxicological potential of antibiotic pollution-industrial wastewater: Bioavailability, biomarkers, and occurrence in Mytilus galloprovincialis. Environ. Sci. Pollut. Res. Int. 2016, 23, 15343–15350. [Google Scholar] [CrossRef]
- Dissanayake, A.; Scarlett, A.G.; Jha, A.N. Diamondoid naphthenic acids cause in vivo genetic damage in gills and haemocytes of marine mussels. Environ. Sci. Pollut. Res. 2016, 23, 7060–7066. [Google Scholar] [CrossRef]
- Kacar, A.; Pazi, I.; Gonul, T.; Kucuksezgin, F. Marine pollution risk in a coastal city: Use of an eco-genotoxic tool as a stress indicator in mussels from the Eastern Aegean Sea. Environ. Sci. Pollut. Res. 2016, 23, 16067–16078. [Google Scholar] [CrossRef]
- Trombini, C.; da Fonseca, T.G.; Morais, M.; Rocha, T.L.; Blasco, J.; Bebianno, M.J. Toxic effects of cisplatin cytostatic drug in mussel Mytilus galloprovincialis. Mar. Environ. Res. 2016, 119, 12–21. [Google Scholar] [CrossRef]
- Kolarević, S.; Kračun-Kolarević, M.; Kostić, J.; Slobodnik, J.; Liška, I.; Gačić, Z.; Paunović, M.; Knežević-Vukčević, J.; Vuković-Gačić, B. Assessment of the genotoxic potential along the Danube River by application of the comet assay on haemocytes of freshwater mussels: The Joint Danube Survey. Sci. Total. Environ. 2016, 540, 377–385. [Google Scholar] [CrossRef]
- Aborgiba, M.; Kostić, J.; Kolarević, S.; Kračun-Kolarević, M.; Elbahi, S.; Knežević-Vukčević, J.; Lenhardt, M.; Paunović, M.; Gačić, Z.; Vuković-Gačić, B. Flooding modifies the genotoxic effects of pollution on a worm, a mussel and two fish species from the Sava River. Sci. Total. Environ. 2016, 540, 358–367. [Google Scholar] [CrossRef]
- Prego-Faraldo, M.V.; Valdiglesias, V.; Laffon, B.; Mendez, J.; Eirin-Lopez, J.M. Early Genotoxic and Cytotoxic Effects of the Toxic Dinoflagellate Prorocentrum lima in the Mussel Mytilus galloprovincialis. Toxins 2016, 8, 159. [Google Scholar] [CrossRef]
- Girardello, F.; Leite, C.C.; Villela, I.V.; Machado, M.d.S.; Juchem, A.L.M.; Roesch-Ely, M.; Fernandes, A.N.; Salvador, M.; Henriques, J.A.P. Titanium dioxide nanoparticles induce genotoxicity but not mutagenicity in golden mussel Limnoperna fortunei. Aquat. Toxicol. 2016, 170, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Magni, S.; Castiglioni, S.; Binelli, A. Amphetamine exposure imbalanced antioxidant activity in the bivalve Dreissena polymorpha causing oxidative and genetic damage. Chemosphere 2016, 144, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Magni, S.; Castiglioni, S.; Binelli, A. Genotoxic effects induced by the exposure to an environmental mixture of illicit drugs to the zebra mussel. Ecotoxicol. Environ. Saf. 2016, 132, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Martinović, R.; Kolarević, S.; Kračun-Kolarević, M.; Kostić, J.; Jokanović, S.; Gačić, Z.; Joksimović, D.; Đurović, M.; Kljajić, Z.; Vuković-Gačić, B. Comparative assessment of cardiac activity and DNA damage in haemocytes of the Mediterranean mussel Mytilus galloprovincialis in exposure to tributyltin chloride. Environ. Toxicol. Pharmacol. 2016, 47, 165–174. [Google Scholar] [CrossRef]
- Barón, E.; Dissanayake, A.; Vilà-Cano, J.; Crowther, C.; Readman, J.W.; Jha, A.N.; Eljarrat, E.; Barceló, D. Evaluation of the Genotoxic and Physiological Effects of Decabromodiphenyl Ether (BDE-209) and Dechlorane Plus (DP) Flame Retardants in Marine Mussels (Mytilus galloprovincialis). Environ. Sci. Technol. 2016, 50, 2700–2708. [Google Scholar] [CrossRef]
- Magni, S.; Parolini, M.; Binelli, A. Sublethal effects induced by morphine to the freshwater biological model Dreissena polymorpha. Environ. Toxicol. 2014, 31, 58–67. [Google Scholar] [CrossRef]
- Mundhe, A.Y.; Bhilwade, H.; Pandit, S.V. Genotoxicity and oxidative stress as biomarkers in freshwater mussel, Lamellidens marginalis (Lam.) exposed to monocrotophos. Indian J. Exp. Biol. 2016, 54, 822–828. [Google Scholar]
- Mezzelani, M.; Gorbi, S.; Da Ros, Z.; Fattorini, D.; D’Errico, G.; Milan, M.; Bargelloni, L.; Regoli, F. Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: Bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis. Mar. Environ. Res. 2016, 121, 31–39. [Google Scholar] [CrossRef]
- Tsarpali, V.; Goutas, A.; Karyda, A.; Efthimiou, I.; Antonopoulou, M.; Drosopoulou, E.; Vlastos, D.; Konstantinou, I.; Mavragani-Tsipidou, P.; Dailianis, S. The role of acetone in the [omim][BF4]-mediated adverse effects on tissues of mussels, human lymphocytes and the fruit fly Drosophila melanogaster. J. Hazard. Mater. 2017, 333, 339–347. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Kiwan, A.; Valbonesi, P.; Dinelli, E.; Pignotti, E.; Birke, M.; Fabbri, E. A comprehensive evaluation of the environmental quality of a coastal lagoon (Ravenna, Italy): Integrating chemical and physiological analyses in mussels as a biomonitoring strategy. Sci. Total. Environ. 2017, 598, 146–159. [Google Scholar] [CrossRef]
- Xie, J.; Yang, D.; Sun, X.; Cao, R.; Chen, L.; Wang, Q.; Li, F.; Wu, H.; Ji, C.; Cong, M.; et al. Individual and combined toxicities of benzo[a]pyrene and 2,2′,4,4′-tetrabromodiphenyl ether on early life stages of the Pacific oyster, Crassostrea gigas. Bull. Environ. Contam. Toxicol. 2017, 99, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Bhagat, J.; Sarker, M.S.; Gaitonde, D.C.S.; Sarker, S. Evaluation of the impact of bioaccumulation of PAH from the marine environment on DNA integrity and oxidative stress in marine rock oyster (Saccostrea cucullata) along the Arabian sea coast. Ecotoxicology 2017, 26, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Juhel, G.; Bayen, S.; Goh, C.; Lee, W.K.; Kelly, B.C. Use of a suite of biomarkers to assess the effects of carbamazepine, bisphenol A, atrazine, and their mixtures on green mussels, Perna viridis. Environ. Toxicol. Chem. 2016, 36, 429–441. [Google Scholar] [CrossRef] [PubMed]
- Belavgeni, A.; Dailianis, S. The role of phosphatidylinositol-3-OH-kinase (PI3-kinase) and respiratory burst enzymes in the [omim][BF4]-mediated toxic mode of action in mussel hemocytes. Fish Shellfish. Immunol. 2017, 68, 144–153. [Google Scholar] [CrossRef]
- Fathallah, S.; Medhioub, M.N.; Kraiem, M.M. Combined Toxicity of Lead and Cadmium on Embryogenesis and Early Larval Stages of the European Clam Ruditapes decussatus. Environ. Eng. Sci. 2013, 30, 357–364. [Google Scholar] [CrossRef]
- Chelomin, V.P.; Slobodskova, V.V.; Zakhartsev, M.; Kukla, S. Genotoxic potential of copper oxide nanoparticles in the bivalve mollusk Mytilus trossulus. J. Ocean Univ. China 2017, 16, 339–345. [Google Scholar] [CrossRef]
- Kameneva, P.A.; Krasheninina, E.A.; Slobodskova, V.V.; Kukla, S.P.; Orlova, T.Y. Accumulation and Tissue Distribution of Dinophysitoxin-1 and Dinophysitoxin-3 in the Mussel Crenomytilus grayanus Feeding on the Benthic Dinoflagellate Prorocentrum foraminosum. Mar. Drugs 2017, 15, 330. [Google Scholar] [CrossRef]
- Maranho, L.; Fontes, M.; Kamimura, A.; Nobre, C.; Moreno, B.; Pusceddu, F.; Cortez, F.; Lebre, D.; Marques, J.; Abessa, D.; et al. Exposure to crack cocaine causes adverse effects on marine mussels Perna perna. Mar. Pollut. Bull. 2017, 123, 410–414. [Google Scholar] [CrossRef]
- Ribeiro, F.; Garcia, A.R.; Pereira, B.P.; Fonseca, M.; Mestre, N.C.; Fonseca, T.G.; Ilharco, L.M.; Bebianno, M.J. Microplastics effects in Scrobicularia plana. Mar. Pollut. Bull. 2017, 122, 379–391. [Google Scholar] [CrossRef]
- Châtel, A.; Bruneau, M.; Lièvre, C.; Goupil, A.; Mouneyrac, C. Spermatozoa: A relevant biological target for genotoxicity assessment of contaminants in the estuarine bivalve Scrobicularia plana. Mar. Pollut. Bull. 2017, 116, 488–490. [Google Scholar] [CrossRef]
- Sohail, M.; Khan, M.N.; Qureshi, N.A.; Chaudhry, A.S. Monitoring DNA damage in gills of freshwater mussels (Anodonta anatina) exposed to heavy metals. Pak. J. Zool. 2017, 49, 321–328. [Google Scholar] [CrossRef]
- Kukla, S.P.; Slobodskova, V.V.; Chelomin, V.P. The genotoxicity of copper oxide nanoparticles to marine organisms based on the example of the Pacific mussel Mytilus trossulus gould, 1850 (Bivalvia: Mytilidae). Russ. J. Mar. Biol. 2017, 43, 171–175. [Google Scholar] [CrossRef]
- Fahmy, S.R.; A Sayed, D. Toxicological perturbations of zinc oxide nanoparticles in the Coelatura aegyptiaca mussel. Toxicol. Ind. Health 2017, 33, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, B.; Chen, B.; Sun, X.; Zhu, L.; Zhao, J.; Du, P.; Xing, B. Trophic transfer of TiO2 nanoparticles from marine microalga (Nitzschia closterium) to scallop (Chlamys farreri) and related toxicity. Environ. Sci. Nano 2016, 4, 415–424. [Google Scholar] [CrossRef]
- Jaruga, P.; Coskun, E.; Kimbrough, K.; Jacob, A.; Johnson, W.E.; Dizdaroglu, M. Biomarkers of oxidatively induced DNA damage in dreissenid mussels: A genotoxicity assessment tool for the Laurentian Great Lakes. Environ. Toxicol. 2017, 32, 2144–2153. [Google Scholar] [CrossRef]
- Guidi, P.; Bernardeschi, M.; Scarcelli, V.; Cantafora, E.; Benedetti, M.; Falleni, A.; Frenzilli, G. Lysosomal, genetic and chromosomal damage in haemocytes of the freshwater bivalve (Unio pictorum) exposed to polluted sediments from the River Cecina (Italy). Chem. Ecol. 2017, 33, 516–527. [Google Scholar] [CrossRef]
- Nardi, A.; Mincarelli, L.F.; Benedetti, M.; Fattorini, D.; D’Errico, G.; Regoli, F. Indirect effects of climate changes on cadmium bioavailability and biological effects in the Mediterranean mussel Mytilus galloprovincialis. Chemosphere 2017, 169, 493–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qu, M.; Adeleye, A.; Di, Y. Utilization of isolated marine mussel cells as an in vitro model to assess xenobiotics induced genotoxicity. Toxicol. Vitr. 2017, 44, 219–229. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; He, T.; Wang, J.; Wang, Z.; Li, P.; Lai, Y.; Sanganyado, E.; Liu, W. Integrated assessment of heavy metal pollution using transplanted mussels in eastern Guangdong, China. Environ. Pollut. 2018, 243, 601–609. [Google Scholar] [CrossRef]
- Butrimavičienė, L.; Baršienė, J.; Greiciūnaitė, J.; Stankevičiūtė, M.; Valskienė, R. Environmental genotoxicity and risk assessment in the Gulf of Riga (Baltic Sea) using fish, bivalves, and crustaceans. Environ. Sci. Pollut. Res. 2018, 25, 24818–24828. [Google Scholar] [CrossRef]
- Magni, S.; Gagné, F.; André, C.; Della Torre, C.; Auclair, J.; Hanana, H.; Parenti, C.C.; Bonasoro, F.; Binelli, A. Evaluation of uptake and chronic toxicity of virgin polystyrene microbeads in freshwater zebra mussel Dreissena polymorpha (Mollusca: Bivalvia). Sci. Total. Environ. 2018, 631–632, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Braga, M.A.; Brauko, K.M.; Vicentini, M.; Salgado, L.D.; de Assis, H.C.S.; Dolatto, R.G.; Grassi, M.T.; Sandrini-Neto, L.; Lana, P.C. Cytotoxicity and enzymatic biomarkers as early indicators of benthic responses to the soluble-fraction of diesel oil. Ecotoxicol. Environ. Saf. 2018, 164, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Cortez, F.S.; Souza, L.d.S.; Guimarães, L.L.; Almeida, J.E.; Pusceddu, F.H.; Maranho, L.A.; Mota, L.G.; Nobre, C.R.; Moreno, B.B.; Abessa, D.M.d.S.; et al. Ecotoxicological effects of losartan on the brown mussel Perna perna and its occurrence in seawater from Santos Bay (Brazil). Sci. Total. Environ. 2018, 637–638, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Dailianis, S. [omim][BF4]-mediated toxicity in mussel hemocytes includes its interaction with cellular membrane proteins. Aquat. Toxicol. 2018, 203, 88–94. [Google Scholar] [CrossRef]
- Koehlé-Divo, V.; Cossu-Leguille, C.; Pain-Devin, S.; Simonin, C.; Bertrand, C.; Sohm, B.; Mouneyrac, C.; Devin, S.; Giambérini, L. Genotoxicity and physiological effects of CeO2 NPs on a freshwater bivalve (Corbicula fluminea). Aquat. Toxicol. 2018, 198, 141–148. [Google Scholar] [CrossRef]
- Al-Fanharawi, A.A.; Rabee, A.M.; Al-Mamoori, A.M. Biochemical and molecular alterations in freshwater mollusks as biomarkers for petroleum product, domestic heating oil. Ecotoxicol. Environ. Saf. 2018, 158, 69–77. [Google Scholar] [CrossRef]
- Revel, M.; Fournier, M.; Robidoux, P.Y. Immunotoxicity and genotoxicity of single-walled carbon nanotubes co-exposed with cadmium in the freshwater mussel, Elliptio complanata. Environ. Toxicol. Pharmacol. 2018, 62, 177–180. [Google Scholar] [CrossRef]
- Pearson, H.B.; Dallas, L.J.; Comber, S.D.; Braungardt, C.B.; Worsfold, P.J.; Jha, A.N. Mixtures of tritiated water, zinc and dissolved organic carbon: Assessing interactive bioaccumulation and genotoxic effects in marine mussels, Mytilus galloprovincialis. J. Environ. Radioact. 2018, 187, 133–143. [Google Scholar] [CrossRef]
- Katsumiti, A.; Thorley, A.J.; Arostegui, I.; Reip, P.; Valsami-Jones, E.; Tetley, T.D.; Cajaraville, M.P. Cytotoxicity and cellular mechanisms of toxicity of CuO NPs in mussel cells in vitro and comparative sensitivity with human cells. Toxicol. Vitr. 2018, 48, 146–158. [Google Scholar] [CrossRef]
- Lepoutre, A.; Milliote, N.; Bonnard, M.; Palos-Ladeiro, M.; Rioult, D.; Bonnard, I.; Bastien, F.; Faassen, E.; Geffard, A.; Lance, E. Genotoxic and Cytotoxic Effects on the Immune Cells of the Freshwater Bivalve Dreissena polymorpha Exposed to the Environmental Neurotoxin BMAA. Toxins 2018, 10, 106. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; da Conceição, M.B.; Molisani, M.M.; Weber, L.I. Genotoxicity Biomonitoring Along a Coastal Zone Under Influence of Offshore Petroleum Exploration (Southeastern Brazil). Bull. Environ. Contam. Toxicol. 2018, 100, 338–343. [Google Scholar] [CrossRef] [PubMed]
- D’costa, A.H.; S.K., S.; M.K., P.K.; Furtado, S. The backwater clam (Meretrix casta) as a bioindicator species for monitoring the pollution of an estuarine environment by genotoxic agents. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 825, 8–14. [Google Scholar]
- Guidi, P.; Corsolini, S.; Bernardeschi, M.; Rocco, L.; Nigro, M.; Baroni, D.; Mottola, F.; Scarcelli, V.; Santonastaso, M.; Falleni, A.; et al. Dioxin-like compounds bioavailability and genotoxicity assessment in the Gulf of Follonica, Tuscany (Northern Tyrrhenian Sea). Mar. Pollut. Bull. 2018, 126, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Counihan, K.L. The physiological effects of oil, dispersant and dispersed oil on the bay mussel, Mytilus trossulus, in Arctic/Subarctic conditions. Aquat. Toxicol. 2018, 199, 220–231. [Google Scholar] [CrossRef]
- Stankevičiūtė, M.; Jakubowska, M.; Pažusienė, J.; Makaras, T.; Otremba, Z.; Urban-Malinga, B.; Fey, D.P.; Greszkiewicz, M.; Sauliutė, G.; Baršienė, J.; et al. Genotoxic and cytotoxic effects of 50 Hz 1 mT electromagnetic field on larval rainbow trout (Oncorhynchus mykiss), Baltic clam (Limecola balthica) and common ragworm (Hediste diversicolor). Aquat. Toxicol. 2019, 208, 109–117. [Google Scholar] [CrossRef]
- Slobodskova, V.V.; Zhuravel, E.V.; Kukla, S.P.; Chelomin, V.P. Evaluation of DNA Damage in the Marine Mussel Crenomytilus grayanus as a Genotoxic Biomarker of Pollution. J. Ocean Univ. China 2019, 18, 159–164. [Google Scholar] [CrossRef]
- Ortega, A.d.S.B.; Maranho, L.A.; Nobre, C.R.; Moreno, B.B.; Guimarães, R.S.; Lebre, D.T.; Abessa, D.M.d.S.; Ribeiro, D.A.; Pereira, C.D.S. Detoxification, oxidative stress, and cytogenotoxicity of crack cocaine in the brown mussel Perna perna. Environ. Sci. Pollut. Res. 2018, 26, 27569–27578. [Google Scholar] [CrossRef]
- Cortez, F.S.; Souza, L.d.S.; Guimarães, L.L.; Pusceddu, F.H.; Maranho, L.A.; Fontes, M.K.; Moreno, B.B.; Nobre, C.R.; Abessa, D.M.d.S.; Cesar, A.; et al. Marine contamination and cytogenotoxic effects of fluoxetine in the tropical brown mussel Perna perna. Mar. Pollut. Bull. 2019, 141, 366–372. [Google Scholar] [CrossRef]
- Khan, M.I.; Zahoor, M.; Khan, A.; Gulfam, N. Bioaccumulation of Heavy Metals and their Genotoxic Effect on Freshwater Mussel. Bull. Environ. Contam. Toxicol. 2018, 102, 52–58. [Google Scholar] [CrossRef]
- Drif, F.; Abdennour, C.; Ciğerci, İ.H.; Ali, M.M.; Mansouri, O.; Messarah, M. Preliminary Assessment of Stress and Genotoxicity Biomarkers in Bivalve Molluscs from the Gulf of Annaba, Algeria. Bull. Environ. Contam. Toxicol. 2019, 102, 555–559. [Google Scholar] [CrossRef]
- Rodrigues, F.P.; Carvalho, S.d.C.e.S.; Martinez, C.B.d.R.; Malafaia, G.; Guedes, C.L.B.; Jordão, B.Q. Are the damaging effects of oil refinery effluents on Corbicula fluminea (mollusca) reversible after its transfer to clean water? Ecol. Indic. 2019, 101, 1045–1054. [Google Scholar] [CrossRef]
- Fonseca, T.; Carriço, T.; Fernandes, E.; Abessa, D.; Tavares, A.; Bebianno, M. Impacts of in vivo and in vitro exposures to tamoxifen: Comparative effects on human cells and marine organisms. Environ. Int. 2019, 129, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.E.; Accoroni, S.; Mezzelani, M.; Lugarini, F.; Bacchiocchi, S.; Siracusa, M.; Tavoloni, T.; Piersanti, A.; Totti, C.; Regoli, F.; et al. Biological Effects of the Azaspiracid-Producing Dinoflagellate Azadinium dexteroporum in Mytilus galloprovincialis from the Mediterranean Sea. Mar. Drugs 2019, 17, 595. [Google Scholar] [CrossRef] [PubMed]
- Strubbia, S.; Lyons, B.; Lee, R. Spatial and temporal variation of three biomarkers in Mytilus edulis. Mar. Pollut. Bull. 2019, 138, 322–327. [Google Scholar] [CrossRef]
- Vernon, E.L.; Jha, A.N. Assessing relative sensitivity of marine and freshwater bivalves following exposure to copper: Application of classical and novel genotoxicological biomarkers. Mutat. Res. Toxicol. Environ. Mutagen. 2019, 842, 60–71. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Molisani, M.M.; da Conceição, M.B.; Weber, L.I. Characterisation of coastal aquaculture sites in the state of Rio de Janeiro, Brazil, using genotoxicity biomarkers. Reg. Stud. Mar. Sci. 2019, 32. [Google Scholar] [CrossRef]
- Amaral, Q.D.F.D.; Da Rosa, E.; Wronski, J.G.; Zuravski, L.; Querol, M.V.M.; dos Anjos, B.; de Andrade, C.F.F.; Machado, M.M.; de Oliveira, L.F.S. Golden mussel (Limnoperna fortunei) as a bioindicator in aquatic environments contaminated with mercury: Cytotoxic and genotoxic aspects. Sci. Total. Environ. 2019, 675, 343–353. [Google Scholar] [CrossRef]
- Qu, M.; Ding, J.; Wang, Y.; Chen, S.; Zhang, Y.; Di, Y. Genetic impacts induced by BaP and Pb in Mytilus coruscus: Can RAPD be a validated tool in genotoxicity evaluation both in vivo and in vitro? Ecotoxicol. Environ. Saf. 2019, 169, 529–538. [Google Scholar] [CrossRef]
- Markich, S.J. Dataset of genotoxic and cytotoxic effects on the pygmy mussel, Xenostrobus securis, from the highly urbanised Sydney Estuary, Australia: Relationships with metal bioaccumulation. Data Brief 2020, 30, 105460. [Google Scholar] [CrossRef]
- Rybakovas, A.; Arbačiauskas, K.; Markovskienė, V.; Jokšas, K. Contamination and genotoxicity biomarker responses in bivalve mussels from the major Lithuanian rivers. Environ. Mol. Mutagen. 2019, 61, 338–354. [Google Scholar] [CrossRef]
- Turja, R.; Sanni, S.; Stankevičiūtė, M.; Butrimavičienė, L.; Devier, M.-H.; Budzinski, H.; Lehtonen, K.K. Biomarker responses and accumulation of polycyclic aromatic hydrocarbons in Mytilus trossulus and Gammarus oceanicus during exposure to crude oil. Environ. Sci. Pollut. Res. 2020, 27, 15498–15514. [Google Scholar] [CrossRef] [PubMed]
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246. [Google Scholar] [CrossRef]
- Lastumäki, A.; Turja, R.; Brenner, M.; Vanninen, P.; Niemikoski, H.; Butrimavičienė, L.; Stankevičiūtė, M.; Lehtonen, K.K. Biological effects of dumped chemical weapons in the Baltic Sea: A multi-biomarker study using caged mussels at the Bornholm main dumping site. Mar. Environ. Res. 2020, 161, 105036. [Google Scholar] [CrossRef] [PubMed]
- Kloukinioti, M.; Politi, A.; Kalamaras, G.; Dailianis, S. Feeding regimes modulate biomarkers responsiveness in mussels treated with diclofenac. Mar. Environ. Res. 2020, 156, 104919. [Google Scholar] [CrossRef]
- Al-Howiti, N.S.; Ben Othmen, Z.O.; Ben Othmane, A.; Chaffai, A.H. Use of Tridacna maxima, a bivalve in the biomonitoring of the Saudi Arabian Red Sea coast. Mar. Pollut. Bull. 2020, 150, 110766. [Google Scholar] [CrossRef]
- Magni, S.; Bonasoro, F.; Della Torre, C.; Parenti, C.C.; Maggioni, D.; Binelli, A. Plastics and biodegradable plastics: Ecotoxicity comparison between polyvinylchloride and Mater-Bi® micro-debris in a freshwater biological model. Sci. Total. Environ. 2020, 720, 137602. [Google Scholar] [CrossRef]
- Binelli, A.; Pietrelli, L.; Di Vito, S.; Coscia, L.; Sighicelli, M.; Della Torre, C.; Parenti, C.C.; Magni, S. Hazard evaluation of plastic mixtures from four Italian subalpine great lakes on the basis of laboratory exposures of zebra mussels. Sci. Total. Environ. 2019, 699, 134366. [Google Scholar] [CrossRef]
- Braga, A.C.; Pereira, V.; Marçal, R.; Marques, A.; Guilherme, S.; Costa, P.R.; Pacheco, M. DNA damage and oxidative stress responses of mussels Mytilus galloprovincialis to paralytic shellfish toxins under warming and acidification conditions—Elucidation on the organ-specificity. Aquat. Toxicol. 2020, 228, 105619. [Google Scholar] [CrossRef]
- Fernandes, E.; Fonseca, T.G.; Carriço, T.; Mestre, N.; Tavares, Á.; Bebianno, M.J. Cytotoxic responses of the anticancer drug cyclophosphamide in the mussel Mytilus galloprovincialis and comparative sensitivity with human cells lines. Chemosphere 2020, 261, 127678. [Google Scholar] [CrossRef]
- Xia, B.; Zhang, J.; Zhao, X.; Feng, J.; Teng, Y.; Chen, B.; Sun, X.; Zhu, L.; Sun, X.; Qu, K. Polystyrene microplastics increase uptake, elimination and cytotoxicity of decabromodiphenyl ether (BDE-209) in the marine scallop Chlamys farreri. Environ. Pollut. 2020, 258, 113657. [Google Scholar] [CrossRef]
- Revel, M.; Châtel, A.; Perrein-Ettajani, H.; Bruneau, M.; Akcha, F.; Sussarellu, R.; Rouxel, J.; Costil, K.; Decottignies, P.; Cognie, B.; et al. Realistic environmental exposure to microplastics does not induce biological effects in the Pacific oyster Crassostrea gigas. Mar. Pollut. Bull. 2020, 150, 110627. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.; Mestre, N.C.; Abel, S.; Fonseca, T.G.; Carteny, C.C.; Willems, T.; Prinsen, E.; Cormier, B.; Keiter, S.S.; Bebianno, M.J. Effects of the UV filter, oxybenzone, adsorbed to microplastics in the clam Scrobicularia plana. Sci. Total. Environ. 2020, 733, 139102. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, S.; Rabeh, I.; Telahigue, K.; Tir, M.; Chetoui, I.; Fouzai, C.; Nechi, S.; Chelbi, E.; El Cafsi, M.; Soudani, N. Assessment of oxidative stress, genotoxicity and histopathological responses in the digestive gland of Ruditapes decussatus collected from northern Tunisian lagoons. Sci. Mar. 2020, 84, 403–420. [Google Scholar] [CrossRef]
- Perovic, S.; Sljukic, B.; Šrut, M.; Perovic, A.; Klobučar, G.I.V. Evaluation of DNA damage in haemolymph of freshwater mussels Unio pictorum from Lake Skadar. Biologia 2019, 75, 431–436. [Google Scholar] [CrossRef]
- D’costa, A.H.; Furtado, S.; Greeshma, S.S.; Shyama, S.K. Integrated response of the toxicity of environmentally relevant concentrations of copper in the backwater clam Meretrix casta. Chem. Ecol. 2020, 36, 982–995. [Google Scholar] [CrossRef]
- Vernon, E.L.; Bean, T.P.; Jha, A.N. Assessing relative biomarker responses in marine and freshwater bivalve molluscs following exposure to phosphorus 32 (32P): Application of genotoxicological and molecular biomarkers. J. Environ. Radioact. 2020, 213, 106120. [Google Scholar] [CrossRef]
- Cole, M.; Liddle, C.; Consolandi, G.; Drago, C.; Hird, C.; Lindeque, P.K.; Galloway, T.S. Microplastics, microfibres and nanoplastics cause variable sub-lethal responses in mussels (Mytilus spp.). Mar. Pollut. Bull. 2020, 160, 111552. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, K.; Hong, S.H.; Park, K.-I.; Park, J.-W. Impact of polyethylene terephthalate microfiber length on cellular responses in the Mediterranean mussel Mytilus galloprovincialis. Mar. Environ. Res. 2021, 168, 105320. [Google Scholar] [CrossRef]
- Mitrić, M.; Ramšak, A. Sampling Site Specific Biomarker Responses in Mediterranean Mussels from the Adriatic Sea. Bull. Environ. Contam. Toxicol. 2021, 106, 310–317. [Google Scholar] [CrossRef]
- Musrri, C.A.; Palma-Rojas, C.; von Brand, E.; Abessa, D.M.S. Environmental Genotoxicity Assessment Using Micronucleus (and Nuclear Abnormalities) Test on Intertidal Mussel Perumytilus purpuratus: A Tool for Biomonitoring the Chilean Coast. Bull. Environ. Contam. Toxicol. 2021, 107, 77–83. [Google Scholar] [CrossRef]
- Kleinert, C.; Poirier-Larabie, S.; Gagnon, C.; André, C.; Gagné, F. Occurrence and ecotoxicity of cytostatic drugs 5-fluorouracil and methotrexate in the freshwater unionid Elliptio complanata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 244, 109027. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.d.S.; Bonnail, E.; Maranho, L.A.; Pusceddu, F.H.; Cortez, F.S.; Cesar, A.; Ribeiro, D.A.; Riba, I.; Abessa, D.M.d.S.; DelValls, Á.; et al. Sub-lethal combined effects of illicit drug and decreased pH on marine mussels: A short-time exposure to crack cocaine in CO2 enrichment scenarios. Mar. Pollut. Bull. 2021, 171, 112735. [Google Scholar] [CrossRef] [PubMed]
- Joachim, S.; Beaudouin, R.; Daniele, G.; Geffard, A.; Bado-Nilles, A.; Tebby, C.; Palluel, O.; Dedourge-Geffard, O.; Fieu, M.; Bonnard, M.; et al. Effects of diclofenac on sentinel species and aquatic communities in semi-natural conditions. Ecotoxicol. Environ. Saf. 2021, 211, 111812. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; D’costa, A.; Kumar, M.P.; Shyama, S. DNA damage and biochemical responses in estuarine bivalve Donax incarnatus (Gmelin, 1791) exposed to sub-lethal concentrations of an organophosphate pesticide monocrotophos. Environ. Monit. Assess. 2021, 193, 1–12. [Google Scholar] [CrossRef]
- Katsumiti, A.; Losada-Carrillo, M.P.; Barros, M.; Cajaraville, M.P. Polystyrene nanoplastics and microplastics can act as Trojan horse carriers of benzo(a)pyrene to mussel hemocytes in vitro. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Braga, A.C.; Marçal, R.; Marques, A.; Guilherme, S.; Vilariño, Ó.; Martins, J.M.L.; Gago-Martínez, A.; Costa, P.R.; Pacheco, M. Invasive clams (Ruditapes philippinarum) are better equipped to deal with harmful algal blooms toxins than native species (R. decussatus): Evidence of species-specific toxicokinetics and DNA vulnerability. Sci. Total. Environ. 2021, 767, 144887. [Google Scholar] [CrossRef]
- Duroudier, N.; Katsumiti, A.; Mikolaczyk, M.; Schäfer, J.; Bilbao, E.; Cajaraville, M.P. Cell and tissue level responses in mussels Mytilus galloprovincialis dietarily exposed to PVP/PEI coated Ag nanoparticles at two seasons. Sci. Total. Environ. 2021, 750, 141303. [Google Scholar] [CrossRef]
- André, C.; Pilote, M.; Gagnon, C.; Gagné, F. Ecotoxicological impacts of oil sand mining activity to endemic caged mussels Pyganodon grandis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 251, 109193. [Google Scholar] [CrossRef]
- Vernon, E.L.; Jha, A.N.; Ferreira, M.F.; Slomberg, D.L.; Malard, V.; Grisolia, C.; Payet, M.; Turner, A. Bioaccumulation, release and genotoxicity of stainless steel particles in marine bivalve molluscs. Chemosphere 2022, 303, 134914. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Sousa, V.S.; Teixeira, M.R.; Bebianno, M.J. Chronic toxicity of polystyrene nanoparticles in the marine mussel Mytilus galloprovincialis. Chemosphere 2022, 287, 132356. [Google Scholar] [CrossRef]
- Connolly, M.; Little, S.; Hartl, M.G.J.; Fernandes, T.F. An Integrated Testing Strategy for Ecotoxicity (ITS-ECO) Assessment in the Marine Environmental Compartment using Mytilus spp.: A Case Study using Pristine and Coated CuO and TiO2 Nanomaterials. Environ. Toxicol. Chem. 2022, 41, 1390–1406. [Google Scholar] [CrossRef] [PubMed]
- Essawy, A.E.; El Sherif, S.S.; Osman, G.Y.; El Morshedy, R.M.; Al-Nasser, A.S.; Sheir, S.K. Immune responses, DNA damage and ultrastructural alterations of gills in the marine mussel Lithophaga lithophaga exposed to CuO nanoparticles. Environ. Sci. Pollut. Res. 2021, 29, 15800–15815. [Google Scholar] [CrossRef] [PubMed]
- Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Kukla, S.P.; Dovzhenko, N.V. Genotoxic Properties of Polystyrene (PS) Microspheres in the Filter-Feeder Mollusk Mytilus trossulus (Gould, 1850). J. Mar. Sci. Eng. 2022, 10, 273. [Google Scholar] [CrossRef]
- Alam, R.; Ehiguese, F.O.; Vitale, D.; Martín-Díaz, M.L. Oxidative stress response to hydrogen peroxide exposure of Mytilus galloprovincialis and Ruditapes philippinarum: Reduced embryogenesis success and altered biochemical response of sentinel marine bivalve species. Environ. Chem. Ecotoxicol. 2022, 4, 97–105. [Google Scholar] [CrossRef]
- Dovzhenko, N.V.; Chelomin, V.P.; Mazur, A.A.; Kukla, S.P.; Slobodskova, V.V.; Istomina, A.A.; Zhukovskaya, A.F. Oxidative Stress in Far Eastern Mussel Mytilus trossulus (Gould, 1850) Exposed to Combined Polystyrene Microspheres (µPSs) and CuO-Nanoparticles (CuO-NPs). J. Mar. Sci. Eng. 2022, 10, 707. [Google Scholar] [CrossRef]
- Mundhe, A.; Pandit, S. EVALUATION OF STRESS OF ENVIRONMENTAL RELEVANT CONCENTRATION OF GLYPHOSATE PESTICIDE ON LAMELLIDENS MARGINALIS. Pollut. Res. 2022, 1022–1028. [Google Scholar] [CrossRef]
- Cabral, D.S.; Medeiros, L.C.C.; Alves, B.V.B.; Passos, L.S.; Pereira, T.M.; Merçon, J.; Castheloge, V.D.; Chippari-Gomes, A.R. Do iron and manganese affect the health of the estuarine oyster Crassostrea rhizophorae? Estuar. Coast. Shelf Sci. 2022, 268. [Google Scholar] [CrossRef]
- Latchere, O.; Roman, C.; Métais, I.; Perrein-Ettajani, H.; Mouloud, M.; Georges, D.; Feurtet-Mazel, A.; Gigault, J.; Catrouillet, C.; Baudrimont, M.; et al. Using Environmental Micro and Nanoplastics for a Better Risk Assessment of Plastic Particles in the Bivalve Corbicula Fluminea. SSRN 2022. Available online: https://ssrn.com/abstract=4261562 (accessed on 15 January 2025). [CrossRef]
- Slobodskova, V.V.; Chelomin, V.P.; Kukla, S.P.; Mazur, A.A. Copper Induced DNA Damage in the Gills of the Mussel Mytilus trossulus and Reversibility after Depuration. J. Mar. Sci. Eng. 2022, 10, 1570. [Google Scholar] [CrossRef]
- Ivorra, L.; Cruzeiro, C.; Ramos, A.; Tagulao, K.; Cardoso, P.G. How can environmental conditions influence dicofol genotoxicity on the edible Asiatic clam, Meretrix meretrix? Environ. Pollut. 2022, 293, 118467. [Google Scholar] [CrossRef]
- Sussarellu, R.; Chouvelon, T.; Aminot, Y.; Couteau, J.; Loppion, G.; Dégremont, L.; Lamy, J.-B.; Akcha, F.; Rouxel, J.; Berthelin, C.; et al. Differences in chemical contaminants bioaccumulation and ecotoxicology biomarkers in Mytilus edulis and Mytilus galloprovincialis and their hybrids. Environ. Pollut. 2022, 292, 118328. [Google Scholar] [CrossRef]
- Marzougui, Z.; Huet, S.; Blier, A.-L.; Le Hégarat, L.; Tounsi-Kettiti, H.; Kharrat, R.; Marrouchi, R.; Fessard, V. Investigation of the Genotoxic Potential of the Marine Toxin C17-SAMT Using the In Vivo Comet and Micronucleus Assays. Mar. Drugs 2022, 20, 619. [Google Scholar] [CrossRef] [PubMed]
- Calisi, A.; Giordano, M.E.; Dondero, F.; Maisano, M.; Fasulo, S.; Lionetto, M.G. Morphological and functional alterations in hemocytes of Mytilus galloprovincialis exposed in high-impact anthropogenic sites. Mar. Environ. Res. 2023, 188, 105988. [Google Scholar] [CrossRef] [PubMed]
- Sia Su, G.L.; Del Mundo, M.L.L.; Barredo, E.K.D.; Sia Su, M.L.L.; Ramos, G.B. Assessment of copper total bioaccumulation and genotoxicity in Boac River, Marinduque Island, Philippines two decades post-mining disaster: Pseudodon sp. as aquatic fauna indicator. Banwa B 2023, 18, art071. [Google Scholar]
- Crowther, C.; Turner, A.; Moore, M.N.; Jha, A.N. Assessing the effects of single and binary exposures of copper and lead on Mytilus galloprovincialis: Physiological and genotoxic approaches. Aquat. Toxicol. 2023, 265, 106741. [Google Scholar] [CrossRef]
- Kukla, S.P.; Chelomin, V.P.; Mazur, A.A.; Slobodskova, V.V.; Mazur, M.A. SiO2 Nanoparticles Suspension Exposures with Marine Invertebrates: Genotoxicity Response. Water 2022, 15, 162. [Google Scholar] [CrossRef]
- Métais, I.; Latchere, O.; Roman, C.; Perrein-Ettajani, H.; Mouloud, M.; Georges, D.; Audroin, T.; Catrouillet, C.; Gigault, J.; Mazel, A.F.; et al. Continuum from microplastics to nanoplastics: Effects of size and source on the estuarine bivalve Scrobicularia plana. Environ. Sci. Pollut. Res. 2023, 30, 45725–45739. [Google Scholar] [CrossRef]
- Roman, C.; Mahé, P.; Latchere, O.; Catrouillet, C.; Gigault, J.; Métais, I.; Châtel, A. Effect of size continuum from nanoplastics to microplastics on marine mussel Mytilus edulis: Comparison in vitro/in vivo exposure scenarios. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 264, 109512. [Google Scholar] [CrossRef]
- Latchere, O.; Roman, C.; Métais, I.; Perrein-Ettajani, H.; Mouloud, M.; Georges, D.; Feurtet-Mazel, A.; Gigault, J.; Catrouillet, C.; Baudrimont, M.; et al. Toxicity assessment of environmental MPs and NPs and polystyrene NPs on the bivalve Corbicula fluminea using a multi-marker approach. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 273, 109714. [Google Scholar] [CrossRef]
- Oliveira, I.B.; Carvalhais, A.; Guilherme, S.; Oliveira, H.; Oliveira, C.C.; Ferrão, L.; Cabrita, E.; Pacheco, M.; Mieiro, C. Shipping can be achieved but cargo arrives with damage—Titanium dioxide nanoparticles affect the DNA of Pacific oyster sperm. Aquat. Toxicol. 2023, 258, 106446. [Google Scholar] [CrossRef]
- Duarte, L.F.d.A.; Ortega, A.d.S.B.; Paço, M.d.S.; Sadauskas-Henrique, H.; Cesar-Ribeiro, C.; Souza, I.C.; Monteiro, R.; Monferrán, M.V.; Wunderlin, D.A.; Fernandes, M.N.; et al. Settleable atmospheric particulate matter harms a marine invertebrate: Integrating chemical and biological damage in a bivalve model. Sci. Total. Environ. 2023, 881, 163380. [Google Scholar] [CrossRef]
- Kolarević, S.; Kračun-Kolarević, M.; Marić, J.J.; Djordjević, J.; Vuković-Gačić, B.; Joksimović, D.; Martinović, R.; Bajt, O.; Ramšak, A. Single and combined potential of polystyrene microparticles and fluoranthene in the induction of DNA damage in haemocytes of Mediterranean mussel (Mytilus galloprovincialis). Mutagenesis 2022, 38, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Guidi, P.; Bernardeschi, M.; Palumbo, M.; Buttino, I.; Vitiello, V.; Scarcelli, V.; Chiaretti, G.; Fiorati, A.; Pellegrini, D.; Pontorno, L.; et al. Eco-Friendly Engineered Nanomaterials Coupled with Filtering Fine-Mesh Net as a Promising Tool to Remediate Contaminated Freshwater Sludges: An Ecotoxicity Investigation. Nanomaterials 2023, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Nour, O.M.; El-Saidy, S.A.; Ghoneim, A.Z. Multiple-biomarker approach in the assessment of bisphenol A effect on the grooved carpet clam Ruditapes decussatus (Linnaeus, 1758). BMC Zool. 2024, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Ventura, E.; Gonçalves, J.M.; Vilke, J.M.; D’Errico, G.; Benedetti, M.; Regoli, F.; Bebianno, M.J. Are mixtures of micro/nanoplastics more toxic than individual micro or nanoplastic contamination in the clam Ruditapes decussatus? Mar. Pollut. Bull. 2024, 206, 116697. [Google Scholar] [CrossRef]
- Xing, Y.-Y.; Pu, X.-M.; Pan, J.-F.; Xu, J.-Y.; Liu, C.; Lu, D.-C. From antioxidant defense to genotoxicity: Deciphering the tissue-specific impact of AgNPs on marine clam Ruditapes philippinarum. Aquat. Toxicol. 2024, 270, 106883. [Google Scholar] [CrossRef]
- Slaby, S.; Geffard, A.; Fisson, C.; Bonnevalle-Normand, M.; Allonier-Fernandes, A.-S.; Amara, R.; Bado-Nilles, A.; Bonnard, I.; Bonnard, M.; Burlion-Giorgi, M.; et al. Advancing environmental monitoring across the water continuum combining biomarker analysis in multiple sentinel species: A case study in the Seine-Normandie Basin (France). J. Environ. Manag. 2024, 358, 120784. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; Aguirre-Martínez, G.V.; Martin-Díaz, L.M.; Blasco, J.; Lara-Martín, P.A.; Sendra, M. Personal care products: An emerging threat to the marine bivalve Ruditapes philippinarum. Environ. Sci. Pollut. Res. 2024, 31, 20461–20476. [Google Scholar] [CrossRef]
- Fol, M.F.; Zahyan, S.K.; Sayed, D.A. Toxicity of copper oxide nanoparticles on the Asian clam Corbicula fluminea using multiple biomarkers. J. Nanoparticle Res. 2024, 27, 7. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).