Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics
Abstract
1. Introduction
1.1. VEGFR Family Role in Normal and Diseased Conditions
1.2. Molecular Targeting of the VEGFR System Using Imaging Probes
1.2.1. Radiolabeled Small Molecules
1.2.2. Radiolabeled Peptides
1.2.3. Radiolabeled Antibodies
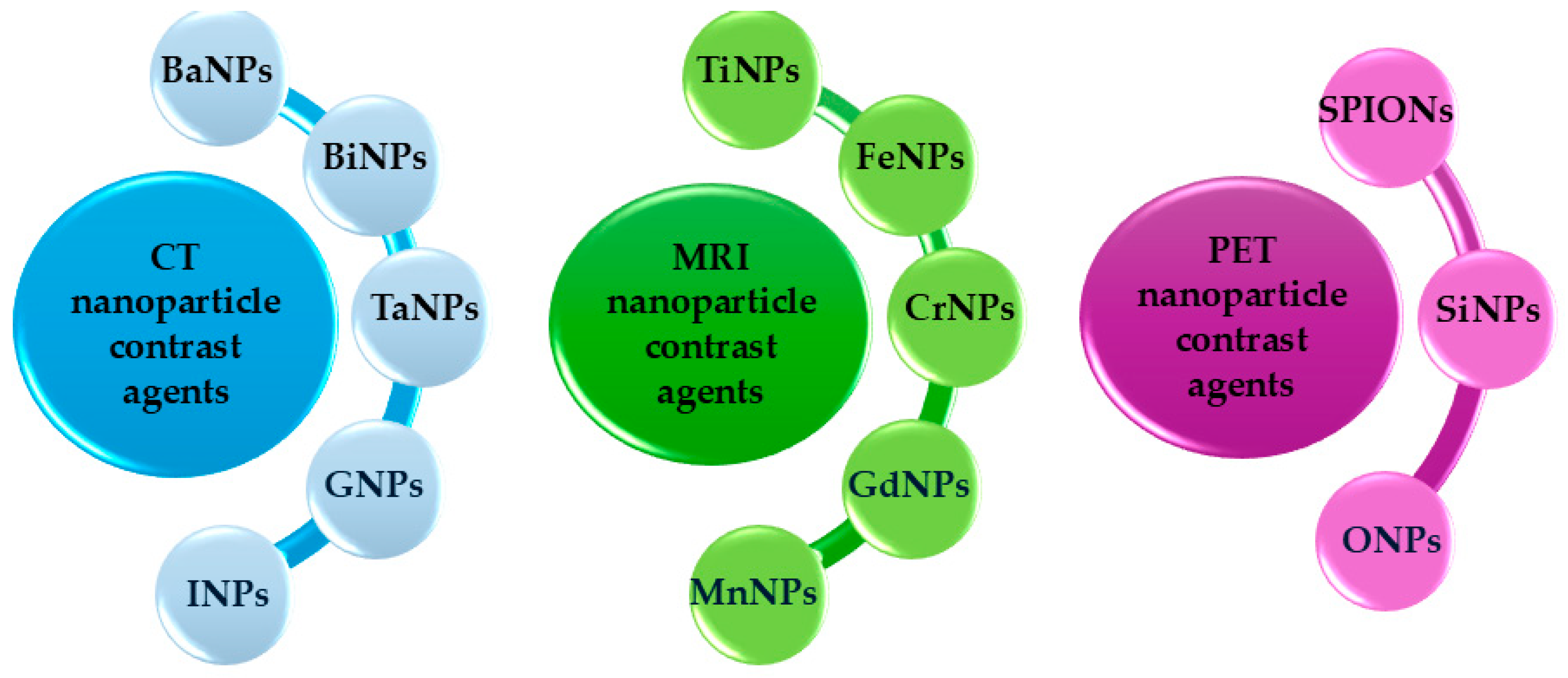
1.2.4. Nanoparticles and Novel Imaging Agents
2. Discussions and Perspective
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VEGFR | Vascular endothelial growth factor receptors |
| VEGF | Vascular endothelial growth factor |
| PlGF | Placental growth factor |
| PET | Positron emission tomography |
| SPECT | Single-photon emission computed tomography |
| MAPK | Mitogen-activated protein kinase |
| CSF | Cerebrospinal fluid |
| MRI | Magnetic resonance imaging |
| BBB | Blood–brain barrier |
| MW | Molecular weight |
| TKI | Tyrosine kinase inhibitors |
| HAECs | Human aortic endothelial cells |
| ID | Injected dose |
| PCR | Polymerase chain reaction |
| GMP | Good manufacturing practice |
| BFCA | Bifunctional chelating agent |
| DTPA-NCS | Isothiocyanatobenzyl-DTPA |
| DOTA | 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| NOTA | 1,4,7-triazacyclononane-1,4,7-triacetic acid |
| PEG | Polyethylene glycol |
| PBS | Phosphate-buffered saline |
| ELISA | Enzyme-linked immunosorbent assay |
| RIT | Radioimmunotherapy |
| WHO | World Health Organization |
| OS | Overall survival |
| IgG | Immunoglobulin |
| HUVECs | Human umbilical vein endothelial cells |
| NODAGA | 1,4,7-triazacyclononane-1-glutamic acid-4,7-diacetic acid |
| ITLC | Instant thin-layer chromatography |
| HPLC | High-performance liquid chromatography |
| NIRE | Near-infrared fluorescence |
| NSCLC | Non-small cell lung cancer |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| USMI | Ultrasound molecular imaging |
| RCC | Renal cell carcinoma |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| HCC | Hepatocellular carcinoma |
| RGD | Arginyl-glycyl-aspartic acid |
| AMD | Age-related macular degeneration |
| Da | Daltons |
| IC50 | Half-maximal inhibitory concentration |
| %ID/g | Percent injected dose per gram of tissue |
| MVD | Micro vessel density |
| CRC | Prostate cancer |
| SUVmax | Maximum standardized uptake value |
| CRC | Colorectal cancer |
| NIRF | Near-infrared fluorescence |
References
- Kaufman, N.E.M.; Dhingra, S.; Jois, S.D.; Vicente, M. Molecular Targeting of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor Receptor (VEGFR). Molecules 2021, 26, 1076. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M. Molecular biology of the VEGF and the VEGF receptor family. Semin. Thromb. Hemost. 2000, 26, 561–569. [Google Scholar] [CrossRef]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef]
- Monaghan, R.M.; Page, D.J.; Ostergaard, P.; Keavney, B.D. The physiological and pathological functions of VEGFR3 in cardiac and lymphatic development and related diseases. Cardiovasc. Res. 2021, 117, 1877–1890. [Google Scholar] [CrossRef]
- Masłowska, K.; Halik, P.K.; Tymecka, D.; Misicka, A.; Gniazdowska, E. The Role of VEGF Receptors as Molecular Target in Nuclear Medicine for Cancer Diagnosis and Combination Therapy. Cancers 2021, 13, 1072. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singhi, E.K.; Arroyo, J.P.; Ikizler, T.A.; Gould, E.R.; Brown, J.; Beckman, J.A.; Harrison, D.G.; Moslehi, J. Mechanisms of VEGF (Vascular Endothelial Growth Factor) Inhibitor-Associated Hypertension and Vascular Disease. Hypertension 2018, 71, e1–e8. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ma, H.; Li, F.; Cai, H.; Liang, R.; Chen, X.; Lan, T.; Yang, J.; Liao, J.; Yang, Y.; et al. PET imaging of VEGFR and integrins in glioma tumor xenografts using 89Zr labelled heterodimeric peptide. Bioorg. Med. Chem. 2022, 59, 116677. [Google Scholar] [CrossRef]
- Hu, K.; Shang, J.; Xie, L.; Hanyu, M.; Zhang, Y.; Yang, Z.; Xu, H.; Wang, L.; Zhang, M.R. PET Imaging of VEGFR with a Novel (64)Cu-Labeled Peptide. ACS Omega 2020, 5, 8508–8514. [Google Scholar] [CrossRef]
- Blankenberg, F.G.; Levashova, Z.; Goris, M.G.; Hamby, C.V.; Backer, M.V.; Backer, J.M. Targeted systemic radiotherapy with scVEGF/177Lu leads to sustained disruption of the tumor vasculature and intratumoral apoptosis. J. Nucl. Med. 2011, 52, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.J.; Sohn, R.; Lu, Z.H.; Arbeit, J.M.; Lapi, S.E. Detection of rapalog-mediated therapeutic response in renal cancer xenografts using 64Cu-bevacizumab immunoPET. PLoS ONE 2013, 8, e58949. [Google Scholar] [CrossRef] [PubMed]
- Abdlkadir, A.S.; Allouzi, S.; Obeidat, S.; Mikhail-Lette, M.; Shi, H.; Al-Ibraheem, A. Exploring utilities of [64Cu] Cu-DOTA-trastuzumab immunoPET in breast cancer: A systematic review and meta-analysis. Nucl. Med. Commun. 2025, 46, 503–514. [Google Scholar] [CrossRef]
- Badier, L.; Quelven, I. Zirconium 89 and Copper 64 for ImmunoPET: From Antibody Bioconjugation and Radiolabeling to Molecular Imaging. Pharmaceutics 2024, 16, 882. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front. Biosci. 2007, 12, 4267–4279. [Google Scholar] [CrossRef]
- Willmann, J.K.; Paulmurugan, R.; Chen, K.; Gheysens, O.; Rodriguez-Porcel, M.; Lutz, A.M.; Chen, I.Y.; Chen, X.; Gambhir, S.S. US imaging of tumor angiogenesis with microbubbles targeted to vascular endothelial growth factor receptor type 2 in mice. Radiology 2008, 246, 508–518. [Google Scholar] [CrossRef]
- Cai, W.; Rao, J.; Gambhir, S.S.; Chen, X. How molecular imaging is speeding up antiangiogenic drug development. Mol. Cancer Ther. 2006, 5, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Felmeden, D.C.; Blann, A.D.; Lip, G.Y. Angiogenesis: Basic pathophysiology and implications for disease. Eur. Heart J. 2003, 24, 586–603. [Google Scholar] [CrossRef] [PubMed]
- Kilarski, W.W. Physiological Perspective on Therapies of Lymphatic Vessels. Adv. Wound Care 2018, 7, 189–208. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Matsuda, A.; Yu, L. Beyond starving cancer: Anti-angiogenic therapy. J. Med. Ultrason 2024, 51, 605–610. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, X.; Xu, W.; Chen, J.; Yu, J.; Gamble, J.R.; McCaughan, G.W. Targeting the vasculature in hepatocellular carcinoma treatment: Starving versus normalizing blood supply. Clin. Transl. Gastroenterol. 2017, 8, e98. [Google Scholar] [CrossRef]
- Byrne, A.M.; Bouchier-Hayes, D.J.; Harmey, J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J. Cell. Mol. Med. 2005, 9, 777–794. [Google Scholar] [CrossRef]
- Touyz, R.M.; Lang, N.N.; Herrmann, J.; van den Meiracker, A.H.; Danser, A.H.J. Recent Advances in Hypertension and Cardiovascular Toxicities With Vascular Endothelial Growth Factor Inhibition. Hypertension 2017, 70, 220–226. [Google Scholar] [CrossRef]
- Das, U.N. Diabetic macular edema, retinopathy and age-related macular degeneration as inflammatory conditions. Arch. Med. Sci. 2016, 12, 1142–1157. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Aragona, E.; Bandello, F. VEGF-targeting drugs for the treatment of retinal neovascularization in diabetic retinopathy. Ann. Med. 2022, 54, 1089–1111. [Google Scholar] [CrossRef]
- Tas, S.W.; Maracle, C.X.; Balogh, E.; Szekanecz, Z. Targeting of proangiogenic signalling pathways in chronic inflammation. Nat. Rev. Rheumatol. 2016, 12, 111–122. [Google Scholar] [CrossRef]
- Tanabe, K.; Wada, J.; Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 2020, 16, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Augustine, R.; Prasad, P.; Khalaf, I.M.N. Therapeutic angiogenesis: From conventional approaches to recent nanotechnology-based interventions. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 994–1008. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230; discussion 231. [Google Scholar] [CrossRef]
- Ziyad, S.; Iruela-Arispe, M.L. Molecular mechanisms of tumor angiogenesis. Genes Cancer 2011, 2, 1085–1096. [Google Scholar] [CrossRef]
- Modi, S.J.; Kulkarni, V.M. Vascular endothelial growth factor receptor (VEGFR-2)/KDR inhibitors: Medicinal chemistry perspective. Med. Drug Discov. 2019, 2, 100009. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef] [PubMed]
- Estrada, C.C.; Maldonado, A.; Mallipattu, S.K. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J. Am. Soc. Nephrol. 2019, 30, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Armaiz Pena, G.N.; Pradeep, S.; Cho, M.S.; Coleman, R.L.; Sood, A.K. Anti-vascular therapies in ovarian cancer: Moving beyond anti-VEGF approaches. Cancer Metastasis Rev. 2015, 34, 19–40. [Google Scholar] [CrossRef]
- Jussila, L.; Alitalo, K. Vascular growth factors and lymphangiogenesis. Physiol. Rev. 2002, 82, 673–700. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Simons, M. Molecular controls of lymphatic VEGFR3 signaling. Arter. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 421–429. [Google Scholar] [CrossRef]
- Antila, S.; Karaman, S.; Nurmi, H.; Airavaara, M.; Voutilainen, M.H.; Mathivet, T.; Chilov, D.; Li, Z.; Koppinen, T.; Park, J.H.; et al. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017, 214, 3645–3667. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Antila, S.; Nurmi, H.; Chilov, D.; Korhonen, E.A.; Fang, S.; Karaman, S.; Engelhardt, B.; Alitalo, K. Blockade of VEGFR3 signaling leads to functional impairment of dural lymphatic vessels without affecting autoimmune neuroinflammation. Sci. Immunol. 2023, 8, eabq0375. [Google Scholar] [CrossRef] [PubMed]
- Secker, G.A.; Harvey, N.L. VEGFR signaling during lymphatic vascular development: From progenitor cells to functional vessels. Dev. Dyn. 2015, 244, 323–331. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- García-Caballero, M.; Paupert, J.; Blacher, S.; Van de Velde, M.; Quesada, A.R.; Medina, M.A.; Noël, A. Targeting VEGFR-3/-2 signaling pathways with AD0157: A potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases. J. Hematol. Oncol. 2017, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Wang, X.L.; Zhao, J.; Qin, L.; Cao, J.L. VEGFR-3 blocking deteriorates inflammation with impaired lymphatic function and different changes in lymphatic vessels in acute and chronic colitis. Am. J. Transl. Res. 2016, 8, 827–841. [Google Scholar]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Wang, N.; Zhang, J.; Chen, M.; He, X.; Cui, Y.; Pang, S.; Yan, B. Genetic variants of VEGFR-1 gene promoter in acute myocardial infarction. Hum. Genom. 2019, 13, 56. [Google Scholar] [CrossRef]
- Aldebasi, Y.H.; Rahmani, A.H.; Khan, A.A.; Aly, S.M. The effect of vascular endothelial growth factor in the progression of bladder cancer and diabetic retinopathy. Int. J. Clin. Exp. Med. 2013, 6, 239–251. [Google Scholar] [PubMed]
- Xodjanazarovich, M.N. Assessment of the efficacy of VEGF drugs in the treatment of diabetic retinopathy. Eur. Int. J. Multidiscip. Res. Manag. Stud. 2024, 4, 20–24. [Google Scholar]
- Dai, T.; Li, B.; He, B.; Yan, L.; Gu, L.; Liu, X.; Qi, J.; Li, P.; Zhou, X. A novel mutation in the conserved sequence of vascular endothelial growth factor receptor 3 leads to primary lymphoedema. J. Int. Med. Res. 2018, 46, 3162–3171. [Google Scholar] [CrossRef]
- Wojciechowski, S.; Virenque, A.; Vihma, M.; Galbardi, B.; Rooney, E.J.; Keuters, M.H.; Antila, S.; Koistinaho, J.; Noe, F.M. Developmental dysfunction of the central nervous system lymphatics modulates the adaptive neuro-immune response in the perilesional cortex in a mouse model of traumatic brain injury. Front. Immunol. 2021, 11, 559810. [Google Scholar] [CrossRef] [PubMed]
- Braile, M.; Marcella, S.; Cristinziano, L.; Galdiero, M.R.; Modestino, L.; Ferrara, A.L.; Varricchi, G.; Marone, G.; Loffredo, S. VEGF-A in cardiomyocytes and heart diseases. Int. J. Mol. Sci. 2020, 21, 5294. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, X.; Cui, H.; Shi, J.; Yuan, G.; Shi, S.; Hu, Y. The role of the VEGF family in coronary heart disease. Front. Cardiovasc. Med. 2021, 8, 738325. [Google Scholar] [CrossRef]
- Farzaneh Behelgardi, M.; Zahri, S.; Mashayekhi, F.; Mansouri, K.; Asghari, S.M. A peptide mimicking the binding sites of VEGF-A and VEGF-B inhibits VEGFR-1/-2 driven angiogenesis, tumor growth and metastasis. Sci. Rep. 2018, 8, 17924. [Google Scholar] [CrossRef]
- Liu, X.; Cui, K.; Wu, H.; Li, K.S.; Peng, Q.; Wang, D.; Cowan, D.B.; Dixon, J.B.; Sathish Srinivasan, R.; Bielenberg, D.R. Promoting lymphangiogenesis and lymphatic growth and remodeling to treat cardiovascular and metabolic diseases. Arterioscler. Thromb. Vasc. Biol. 2023, 43, e1–e10. [Google Scholar] [CrossRef]
- Xu, W.; Harris, N.R.; Caron, K.M. Lymphatic vasculature: An emerging therapeutic target and drug delivery route. Annu. Rev. Med. 2021, 72, 167–182. [Google Scholar] [CrossRef]
- Zhou, H.; Lei, P.-j.; Padera, T.P. Progression of metastasis through lymphatic system. Cells 2021, 10, 627. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H661–H681. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Gonzalez, F.; Cabrillo-Estévez, L.; López-Valverde, G.; Cieza-Borrella, C.; Hernández-Galilea, E.; González-Sarmiento, R. Predictive value of VEGF A and VEGFR2 polymorphisms in the response to intravitreal ranibizumab treatment for wet AMD. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, Z. The expression of VEGF-C and it’s receptor VEGFR-3 correlates with lymph node metastasis in gastric cancer. Open J. Gastroenterol. 2014, 4, 357. [Google Scholar] [CrossRef]
- Kuonqui, K.; Campbell, A.-C.; Sarker, A.; Roberts, A.; Pollack, B.L.; Park, H.J.; Shin, J.; Brown, S.; Mehrara, B.J.; Kataru, R.P. Dysregulation of lymphatic endothelial VEGFR3 signaling in disease. Cells 2023, 13, 68. [Google Scholar] [CrossRef]
- Smith, E.S.; Whitty, E.; Yoo, B.; Moore, A.; Sempere, L.F.; Medarova, Z. Clinical Applications of Short Non-Coding RNA-Based Therapies in the Era of Precision Medicine. Cancers 2022, 14, 1588. [Google Scholar] [CrossRef]
- Li, T.; Wu, M.; Zhu, Y.Y.; Chen, J.; Chen, L. Development of RNA interference-based therapeutics and application of multi-target small interfering RNAs. Nucleic Acid. Ther. 2014, 24, 302–312. [Google Scholar] [CrossRef]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49 (Suppl. S2), 113s–128s. [Google Scholar] [CrossRef]
- Haubner, R.; Beer, A.J.; Wang, H.; Chen, X. Positron emission tomography tracers for imaging angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 2010, 37 (Suppl. S1), S86–S103. [Google Scholar] [CrossRef]
- Nagengast, W.B.; de Vries, E.G.; Hospers, G.A.; Mulder, N.H.; de Jong, J.R.; Hollema, H.; Brouwers, A.H.; van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Pirali Hamedani, M.; Mohammadzadeh, P.; Safari, M.; Esmaeil Sadat Ebrahimi, S.; Seyed Hamzeh, M.; Shafiee Ardestani, M.; Masoumeh Ghoreishi, S. 99mTc- Anionic dendrimer targeted vascular endothelial growth factor as a novel nano-radiotracer for in-vivo breast cancer imaging. Bioorg Chem. 2022, 128, 106085. [Google Scholar] [CrossRef]
- van Dam, M.A.; Vuijk, F.A.; Stibbe, J.A.; Houvast, R.D.; Luelmo, S.A.C.; Crobach, S.; Shahbazi Feshtali, S.; de Geus-Oei, L.F.; Bonsing, B.A.; Sier, C.F.M.; et al. Overview and Future Perspectives on Tumor-Targeted Positron Emission Tomography and Fluorescence Imaging of Pancreatic Cancer in the Era of Neoadjuvant Therapy. Cancers 2021, 13, 6088. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, M.; Zhu, Y.; Conti, P.S.; Chen, K. Development of PET probes for cancer imaging. Curr. Top. Med. Chem. 2015, 15, 795–819. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jiang, S.; Zu, Y.; Lee, D.Y.; Li, Z. A tyrosine kinase inhibitor-based high-affinity PET radiopharmaceutical targets vascular endothelial growth factor receptor. J. Nucl. Med. 2014, 55, 1525–1531. [Google Scholar] [CrossRef]
- Roskoski Jr, R. Rule of five violations among the FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2023, 191, 106774. [Google Scholar] [CrossRef]
- Xiong, B.; Wang, Y.; Chen, Y.; Xing, S.; Liao, Q.; Chen, Y.; Li, Q.; Li, W.; Sun, H. Strategies for structural modification of small molecules to improve blood–brain barrier penetration: A recent perspective. J. Med. Chem. 2021, 64, 13152–13173. [Google Scholar] [CrossRef]
- Mikitsh, J.L.; Chacko, A.-M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect. Med. Chem. 2014, 6, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Poot, A.J.; van der Wildt, B.; Stigter-van Walsum, M.; Rongen, M.; Schuit, R.C.; Hendrikse, N.H.; Eriksson, J.; van Dongen, G.A.; Windhorst, A.D. [11C] Sorafenib: Radiosynthesis and preclinical evaluation in tumor-bearing mice of a new TKI-PET tracer. Nucl. Med. Biol. 2013, 40, 488–497. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Miller, K.D.; Sledge, G.W.; Zheng, Q.-H. Synthesis of [18F] SU11248, a new potential PET tracer for imaging cancer tyrosine kinase. Bioorg. Med. Chem. Lett. 2005, 15, 4380–4384. [Google Scholar] [CrossRef]
- Kniess, T.; Bergmann, R.; Kuchar, M.; Steinbach, J.; Wuest, F. Synthesis and radiopharmacological investigation of 3-[4′-[18F] fluorobenzylidene] indolin-2-one as possible tyrosine kinase inhibitor. Bioorg. Med. Chem. 2009, 17, 7732–7742. [Google Scholar] [CrossRef]
- Ilovich, O.; Jacobson, O.; Aviv, Y.; Litchi, A.; Chisin, R.; Mishani, E. Formation of fluorine-18 labeled diaryl ureas—Labeled VEGFR-2/PDGFR dual inhibitors as molecular imaging agents for angiogenesis. Bioorg. Med. Chem. 2008, 16, 4242–4251. [Google Scholar] [CrossRef]
- Kuchar, M.; Oliveira, M.C.; Gano, L.; Santos, I.; Kniess, T. Radioiodinated sunitinib as a potential radiotracer for imaging angiogenesis—Radiosynthesis and first radiopharmacological evaluation of 5-[125I] Iodo-sunitinib. Bioorg. Med. Chem. lett. 2012, 22, 2850–2855. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Asano, A.; Magata, Y.; Ohmomo, Y.; Temma, T. Synthesis and evaluation of novel radioiodinated anthranilate derivatives for in vivo imaging of vascular endothelial growth factor receptor with single-photon emission computed tomography. Ann. Nucl. Med. 2020, 34, 486–495. [Google Scholar] [CrossRef]
- Samén, E.; Thorell, J.-O.; Lu, L.; Tegnebratt, T.; Holmgren, L.; Stone-Elander, S. Synthesis and preclinical evaluation of [11C] PAQ as a PET imaging tracer for VEGFR-2. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Lola, C.M.; Wang, M.; Miller, K.D.; Sledge, G.W.; Zheng, Q.-H. Radiosynthesis of [11C] Vandetanib and [11C] chloro-Vandetanib as new potential PET agents for imaging of VEGFR in cancer. Bioorg. Med. Chem. lett. 2011, 21, 3222–3226. [Google Scholar] [CrossRef] [PubMed]
- Kręcisz, P.; Czarnecka, K.; Królicki, L.; Mikiciuk-Olasik, E.; Szymański, P. Radiolabeled Peptides and Antibodies in Medicine. Bioconjugate Chem. 2021, 32, 25–42. [Google Scholar] [CrossRef]
- Kang, C.M.; Kim, S.M.; Koo, H.J.; Yim, M.S.; Lee, K.H.; Ryu, E.K.; Choe, Y.S. In vivo characterization of 68Ga-NOTA-VEGF 121 for the imaging of VEGF receptor expression in U87MG tumor xenograft models. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 198–206. [Google Scholar] [CrossRef]
- Hao, G.; Hajibeigi, A.; León-Rodríguez, L.M.; Oz, O.K.; Sun, X. Peptoid-based PET imaging of vascular endothelial growth factor receptor (VEGFR) expression. Am. J. Nucl. Med. Mol. Imaging 2011, 1, 65–75. [Google Scholar]
- Masłowska, K.; Witkowska, E.; Tymecka, D.; Halik, P.K.; Misicka, A.; Gniazdowska, E. Synthesis, physicochemical and biological study of gallium-68-and lutetium-177-labeled VEGF-A165/NRP-1 complex inhibitors based on peptide A7R and branched peptidomimetic. Pharmaceutics 2022, 14, 100. [Google Scholar] [CrossRef]
- Kim, E.G.; Kim, K.M. Strategies and Advancement in Antibody-Drug Conjugate Optimization for Targeted Cancer Therapeutics. Biomol. Ther. 2015, 23, 493–509. [Google Scholar] [CrossRef]
- Trencsényi, G.; Csikos, C.; Képes, Z. Targeted radium alpha therapy in the era of nanomedicine: In vivo results. Int. J. Mol. Sci. 2024, 25, 664. [Google Scholar] [CrossRef]
- Csikos, C.; Vágner, A.; Nagy, G.; Kálmán-Szabó, I.; Szabó, J.P.; Ngo, M.T.; Szoboszlai, Z.; Szikra, D.; Krasznai, Z.T.; Trencsényi, G. In Vivo Preclinical Assessment of the VEGF Targeting Potential of the Newly Synthesized [52Mn] Mn-DOTAGA-Bevacizumab Using Experimental Cervix Carcinoma Mouse Model. Diagnostics 2023, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- van Es, S.C.; Brouwers, A.H.; Mahesh, S.V.K.; Leliveld-Kors, A.M.; de Jong, I.J.; Lub-de Hooge, M.N.; de Vries, E.G.E.; Gietema, J.A.; Oosting, S.F. 89Zr-Bevacizumab PET: Potential Early Indicator of Everolimus Efficacy in Patients with Metastatic Renal Cell Carcinoma. J. Nucl. Med. 2017, 58, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, B.; Paudyal, P.; Oriuchi, N.; Hanaoka, H.; Tominaga, H.; Endo, K. Positron emission tomography imaging and biodistribution of vascular endothelial growth factor with 64Cu-labeled bevacizumab in colorectal cancer xenografts. Cancer Sci. 2011, 102, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Oosterwijk-Wakka, J.C.; de Weijert, M.C.; Franssen, G.M.; Kolev, D.R.; de Haan, T.A.; Boerman, O.C.; Mulders, P.F.; Oosterwijk, E. Combination of sunitinib and 177Lu-labeled antibody cG250 targeted radioimmunotherapy: A promising new therapeutic strategy for patients with advanced renal cell cancer. Neoplasia 2022, 32, 100826. [Google Scholar] [CrossRef]
- Mitran, B.; Güler, R.; Roche, F.P.; Lindström, E.; Selvaraju, R.K.; Fleetwood, F.; Rinne, S.S.; Claesson-Welsh, L.; Tolmachev, V.; Ståhl, S. Radionuclide imaging of VEGFR2 in glioma vasculature using biparatopic affibody conjugate: Proof-of-principle in a murine model. Theranostics 2018, 8, 4462. [Google Scholar] [CrossRef]
- Harsini, S.; Alavi, A.; Rezaei, N. Introduction on Nuclear Medicine and Immunology; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Dammes, N.; Peer, D. Monoclonal antibody-based molecular imaging strategies and theranostic opportunities. Theranostics 2020, 10, 938. [Google Scholar] [CrossRef]
- Pedreáñez, A.; Mosquera-Sulbarán, J.; Muñóz, N.; Tene, D.; Robalino, J. Nanoantibodies: Small molecules, big possibilities. BioTechnologia 2021, 102, 321–336. [Google Scholar] [CrossRef]
- Sun, S.; Ding, Z.; Yang, X.; Zhao, X.; Zhao, M.; Gao, L.; Chen, Q.; Xie, S.; Liu, A.; Yin, S.; et al. Nanobody: A Small Antibody with Big Implications for Tumor Therapeutic Strategy. Int. J. Nanomed. 2021, 16, 2337–2356. [Google Scholar] [CrossRef]
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 6. [Google Scholar] [CrossRef]
- Alexander, E.; Leong, K.W. Discovery of nanobodies: A comprehensive review of their applications and potential over the past five years. J. Nanobiotechnol 2024, 22, 661. [Google Scholar] [CrossRef]
- Tan, H.; Zhou, J.; Yang, X.; Abudupataer, M.; Li, X.; Hu, Y.; Xiao, J.; Shi, H.; Cheng, D. 99mTc-labeled bevacizumab for detecting atherosclerotic plaque linked to plaque neovascularization and monitoring antiangiogenic effects of atorvastatin treatment in ApoE−/− mice. Sci. Rep. 2017, 7, 3504. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kim, E.M.; Cheong, S.J.; Kim, D.W.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Targeted molecular imaging of VEGF receptors overexpressed in ischemic microvasculature using chitosan-DC101 conjugates. J. Biomed. Mater. Res. A 2010, 92, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Camacho, X.; García, M.F.; Calzada, V.; Fernández, M.; Chabalgoity, J.A.; Moreno, M.; Barbosa de Aguiar, R.; Alonso, O.; Gambini, J.P.; Chammas, R. [99mTc (CO)3]-radiolabeled bevacizumab: In vitro and in vivo evaluation in a melanoma model. Oncology 2013, 84, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Camacho, X.; García, M.F.; Calzada, V.; Fernandez, M.; Alonso, O.; Gambini, J.P.; de Aguiar, R.B.; Machado, C.M.L.; Chammas, R.; Porcal, W. 99mTc-labeled Bevacizumab vía HYNIC for Imaging of Melanoma. J. Anal. Oncol. 2014, 3, 53–64. [Google Scholar]
- Kameswaran, M.; Pandey, U.; Sarma, H.D.; Samuel, G. Preparation of 99mTc carbonyl DTPA-bevacizumab and its bioevaluation in a melanoma model. Ann. Nucl. Med. 2014, 28, 911–916. [Google Scholar] [CrossRef]
- Janousek, J.; Barta, P.; Novy, Z.; Zilkova, K.; Trejtnar, F. Antiangiogenic human monoclonal antibody ramucirumab radiolabelling: In vitro evaluation on VEGFR2-positive cell lines. Anticancer. Res. 2019, 39, 735–744. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Engle, J.W.; Yang, Y.; Barnhart, T.E.; Cai, W. Positron emission tomography and near-infrared fluorescence imaging of vascular endothelial growth factor with dual-labeled bevacizumab. Am. J. Nucl. Med. Mol. Imaging 2011, 2, 1. [Google Scholar]
- Luo, H.; England, C.G.; Graves, S.A.; Sun, H.; Liu, G.; Nickles, R.J.; Cai, W. PET imaging of VEGFR-2 expression in lung cancer with 64Cu-labeled ramucirumab. J. Nucl. Med. 2016, 57, 285–290. [Google Scholar] [CrossRef]
- Gaykema, S.B.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J. 89Zr-bevacizumab PET imaging in primary breast cancer. J. Nuc. Med. 2013, 54, 1014–1018. [Google Scholar] [CrossRef]
- Bahce, I.; Huisman, M.C.; Verwer, E.E.; Ooijevaar, R.; Boutkourt, F.; Vugts, D.J.; van Dongen, G.A.; Boellaard, R.; Smit, E.F. Pilot study of 89Zr-bevacizumab positron emission tomography in patients with advanced non-small cell lung cancer. EJNMMI Res. 2014, 4, 35. [Google Scholar] [CrossRef]
- Cohen, R.; Stammes, M.A.; de Roos, I.H.; Stigter-van Walsum, M.; Visser, G.W.; van Dongen, G.A. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011, 1, 31. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Vugts, D.J.; Stigter-van Walsum, M.; Visser, G.W.; Van Dongen, G.A. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single-or dual-mode fluorescence and PET imaging. Nat. Protoc. 2013, 8, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, D.; Barnhart, T.E.; Cao, T.; Engle, J.W.; Chen, W.; Cai, W. Immuno-PET imaging of VEGFR-2 expression in prostate cancer with 89Zr-labeled ramucirumab. Am. J. Cancer Res. 2019, 9, 2037. [Google Scholar] [PubMed]
- Kameswaran, M.; Pandey, U.; Gamre, N.; Vimalnath, K.; Sarma, H.D.; Dash, A. Evaluation of 177Lu-CHX-A″-DTPA-Bevacizumab as a radioimmunotherapy agent targeting VEGF expressing cancers. Appl. Radiat. Isot. 2016, 114, 196–201. [Google Scholar] [CrossRef]
- Rainer, E.; Wang, H.; Traub-Weidinger, T.; Widhalm, G.; Fueger, B.; Chang, J.; Zhu, Z.; Marosi, C.; Haug, A.; Hacker, M. The prognostic value of [123I]-vascular endothelial growth factor ([123I]-VEGF) in glioma. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2396–2403. [Google Scholar] [CrossRef]
- Collingridge, D.R.; Carroll, V.A.; Glaser, M.; Aboagye, E.O.; Osman, S.; Hutchinson, O.C.; Barthel, H.; Luthra, S.K.; Brady, F.; Bicknell, R. The development of [124I] iodinated-VG76e: A novel tracer for imaging vascular endothelial growth factor in vivo using positron emission tomography. Cancer Res. 2002, 62, 5912–5919. [Google Scholar]
- Jayson, G.C.; Zweit, J.; Jackson, A.; Mulatero, C.; Julyan, P.; Ranson, M.; Broughton, L.; Wagstaff, J.; Hakannson, L.; Groenewegen, G. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: Implications for trial design of antiangiogenic antibodies. J. Natl. Cancer Inst. 2002, 94, 1484–1493. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Carlton, M.M.; Knopp, M.V.; Hinkle, G.H. PET/CT imaging of I-124–radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5899–5903. [Google Scholar] [CrossRef]
- Christoforidis, J.B.; Briley, K.; Binzel, K.; Bhatia, P.; Wei, L.; Kumar, K.; Knopp, M.V. Systemic biodistribution and intravitreal pharmacokinetic properties of bevacizumab, ranibizumab, and aflibercept in a nonhuman primate model. Investig. Ophthalmol. Vis. Sci. IOVS 2017, 58, 5636–5645. [Google Scholar] [CrossRef]
- Shi, S.; Yang, K.; Hong, H.; Chen, F.; Valdovinos, H.F.; Goel, S.; Barnhart, T.E.; Liu, Z.; Cai, W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2015, 39, 39–46. [Google Scholar] [CrossRef]
- Zhong, J.; Su, M.; Jiang, Y.; Huang, L.; Chen, Y.; Huang, Z.; Zhang, X. VEGFR2 targeted microbubble-based ultrasound molecular imaging improving the diagnostic sensitivity of microinvasive cervical cancer. J. Nanobiotechnol. 2023, 21, 220. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Luo, Z.; Chen, L.; Wu, Z. Advances in nanotechnology-based targeted-contrast agents for computed tomography and magnetic resonance. Sci. Prog. Progress. 2024, 107, 00368504241228076. [Google Scholar] [CrossRef] [PubMed]
- Aziz, O.A.A.; Arafa, K.; Dena, A.S.A.; El-Sherbiny, I.M. Superparamagnetic iron oxide nanoparticles (SPIONs): Preparation and recent applications. J. Nanotech. Adv. Mat. 2020, 8, 21–29. [Google Scholar]
- Forte, E.; Fiorenza, D.; Torino, E.; Costagliola di Polidoro, A.; Cavaliere, C.; Netti, P.A.; Salvatore, M.; Aiello, M. Radiolabeled PET/MRI nanoparticles for tumor imaging. J. Clin. Med. 2019, 9, 89. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Cai, J.; Chen, X.; Zhou, Y.; Ma, X.; Dong, Q.; Li, F.; Xi, L. Primary Preclinical and Clinical Evaluation of 68Ga-DOTA-TMVP1 as a Novel VEGFR-3 PET Imaging Radiotracer in Gynecological Cancer. Clin. Cancer Res. 2020, 26, 1318–1326. [Google Scholar] [CrossRef]
- Yuan, Y.; Dong, X.; Chen, Y.; Xi, L.; Ma, D.; Dai, J.; Li, F. TMVP1448, a novel peptide improves detection of primary tumors and metastases by specifically targeting VEGFR-3. Biomed. Pharmacother. 2024, 177, 116980. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hong, Y.D.; Pyun, M.S.; Felipe, P.M.; Choi, S.J. Radiolabeling of monoclonal anti-vascular endothelial growth factor receptor 1 (VEGFR 1) with 177Lu for potential use in radioimmunotherapy. Appl. Radiat. Isot. 2009, 67, 1185–1189. [Google Scholar] [CrossRef]
- Bai, J.-W.; Qiu, S.-Q.; Zhang, G.-J. Molecular and functional imaging in cancer-targeted therapy: Current applications and future directions. Signal Transduct. Target. Ther. 2023, 8, 89. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.Q.; Broussy, S.; Han, B.; Fang, H. Recent advances of anti-angiogenic inhibitors targeting VEGF/VEGFR axis. Front. Pharmacol. 2023, 14, 1307860. [Google Scholar] [CrossRef]
- Ji, R.-C. The emerging importance of lymphangiogenesis in aging and aging-associated diseases. Mech. Ageing Dev. 2024, 221, 111975. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.; Bacskai, B.J.; Arbel-Ornath, M.; Aldea, R.; Bedussi, B.; Morris, A.W.; Weller, R.O.; Carare, R.O. Lymphatic clearance of the brain: Perivascular, paravascular and significance for neurodegenerative diseases. Cell. Mol. Neurobiol. 2016, 36, 181–194. [Google Scholar] [CrossRef]
- Martinac, A. Multidisciplinary Investigation into CSF Dynamics and Waste Clearance Mechanisms in the Human Central Nervous System; UNSW Sydney: Sydney, Australia, 2024. [Google Scholar]
- Wen, Y.-R.; Yang, J.-H.; Wang, X.; Yao, Z.-B. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 709–716. [Google Scholar] [PubMed]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef]
- Boisserand, L.S.B.; Geraldo, L.H.; Bouchart, J.; El Kamouh, M.-R.; Lee, S.; Sanganahalli, B.G.; Spajer, M.; Zhang, S.; Lee, S.; Parent, M. VEGF-C prophylaxis favors lymphatic drainage and modulates neuroinflammation in a stroke model. J. Exp. Med. 2024, 221, e20221983. [Google Scholar] [CrossRef]
- Miller, B.; Sewell-Loftin, M.K. Mechanoregulation of vascular endothelial growth factor receptor 2 in angiogenesis. Front. Cardiovasc. Med. 2022, 8, 804934. [Google Scholar] [CrossRef]
- Xu, J.-Q.; Liu, Q.-Q.; Huang, S.-Y.; Duan, C.-Y.; Lu, H.-B.; Cao, Y.; Hu, J.-Z. The lymphatic system: A therapeutic target for central nervous system disorders. Neural Regen. Res. 2023, 18, 1249–1256. [Google Scholar]
- das Neves, S.P.; Delivanoglou, N.; Da Mesquita, S. CNS-draining meningeal lymphatic vasculature: Roles, conundrums and future challenges. Front. Pharmacol. 2021, 12, 655052. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Roy, B. Decoding tumor angiogenesis for therapeutic advancements: Mechanistic insights. Biomedicines 2024, 12, 827. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Chunarkar-Patil, P.; Kaleem, M.; Mishra, R.; Ray, S.; Ahmad, A.; Verma, D.; Bhayye, S.; Dubey, R.; Singh, H.N.; Kumar, S. Anticancer drug discovery based on natural products: From computational approaches to clinical studies. Biomedicines 2024, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Huangfu, Z.; Yang, J.; Wang, G.; Hu, K.; Gao, M.; Zhong, Z. Imaging-guided targeted radionuclide tumor therapy: From concept to clinical translation. Adv. Drug Deliv. Rev. 2022, 190, 114538. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, G.A.; Beaino, W.; Windhorst, A.D.; Zwezerijnen, G.J.; Oprea-Lager, D.E.; Hendrikse, N.H.; van Kuijk, C.; Boellaard, R.; Huisman, M.C.; Vugts, D.J. The role of 89Zr-immuno-PET in navigating and derisking the development of biopharmaceuticals. J. Nucl. Med. 2021, 62, 438–445. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute, Clinical Trials Using VEGF-Targeting Agent. Available online: https://www.cancer.gov/research/participate/clinical-trials/intervention/vegf-targeting-agent?pn=1 (accessed on 7 May 2025).
- Scheer, M.G.; Stollman, T.H.; Boerman, O.C.; Verrijp, K.; Sweep, F.C.; Leenders, W.P.; Ruers, T.J.; Oyen, W.J. Imaging liver metastases of colorectal cancer patients with radiolabelled bevacizumab: Lack of correlation with VEGF-A expression. Eur. J. Cancer 2008, 44, 1835–1840. [Google Scholar] [CrossRef]
- Cunha, L.; Horvath, I.; Ferreira, S.; Lemos, J.; Costa, P.; Vieira, D.; Veres, D.S.; Szigeti, K.; Summavielle, T.; Máthé, D. Preclinical imaging: An essential ally in modern biosciences. Mol. Diagn. Ther. 2014, 18, 153–173. [Google Scholar] [CrossRef]
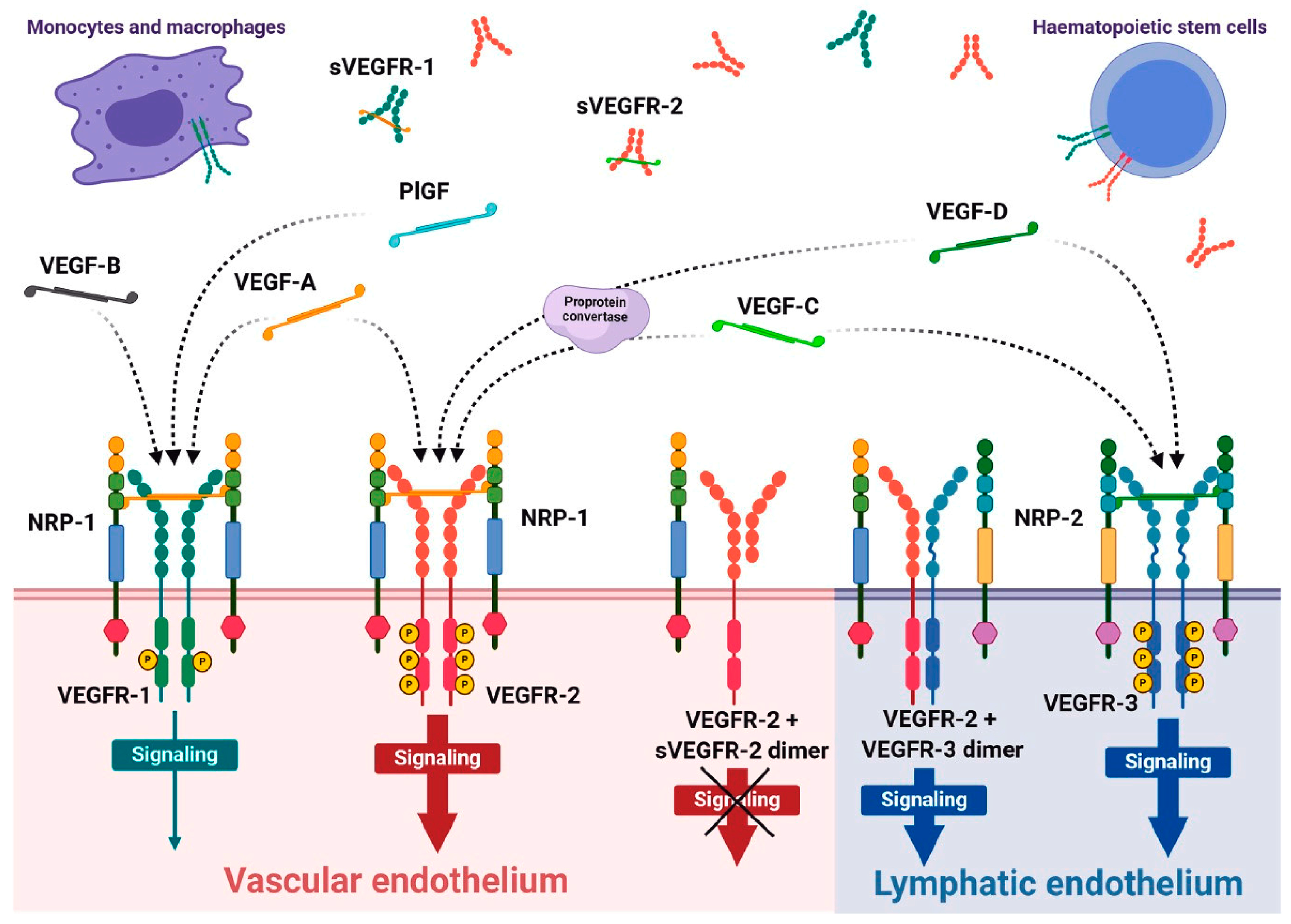
| VEGFR | Ligand (s) | Disease Conditions | Role in Disease | Broad or Specific Role * | Ref. |
|---|---|---|---|---|---|
| VEGFR-1 | VEGF-A, VEGF-B, PlGF | - Cancer: Tumor growth and metastasis - Cardiovascular diseases: Atherosclerosis, ischemia | Acts as a decoy receptor modulating VEGF-A availability; drives angiogenesis and inflammatory responses. | Broad | [49,50] |
| VEGFR-2 | VEGF-A, VEGF-C, VEGF-D | - Cancer: Solid tumors - Diabetic retinopathy | Central driver of angiogenesis, vascular permeability, and endothelial cell proliferation. | Broad | [8,51,52] |
| VEGFR-3 | VEGF-C, VEGF-D | - Lymphedema: Primary lymphedema, Milroy disease - Neurological diseases: Alzheimer’s disease, multiple sclerosis, traumatic brain injury, epilepsy | Regulates lymphangiogenesis and meningeal lymphatic function, influencing waste clearance, immune cell trafficking, and fluid drainage. | Specific to lymphatic and neuroimmune roles | [5,53,54] |
| VEGFR-1 and VEGFR-2 | VEGF-A | - Cancer: Tumor progression and metastasis - Ischemic diseases: Myocardial infarction, stroke | Orchestrated roles in pathological angiogenesis; VEGFR-1 modulates VEGFR-2 signaling to balance vascular growth. | Broad | [55,56,57] |
| VEGFR-3 | VEGF-C, VEGF-D | - Cancer: Lymphatic metastasis - Lymphatic disorders: Secondary lymphedema | Promote tumor lymphangiogenesis, immune modulation, and waste clearance; critical in metastasis and fluid homeostasis. | Broad | [58,59,60] |
| VEGFR-1 | PlGF | Preeclampsia - Cardiovascular diseases: Heart failure, hypertension | PlGF enhances VEGFR-1 signaling, exacerbating inflammation and vascular dysfunction in conditions like preeclampsia and ischemia. | Specific to vascular inflammation | [61] |
| VEGFR-2 | VEGF-A | Ocular diseases: Wet age-related macular degeneration (AMD), diabetic retinopathy | Overactivation induces pathological angiogenesis and vascular leakage, driving vision loss in retinal diseases. | Specific to ocular diseases | [62] |
| VEGFR-3 | VEGF-C, VEGF-D | - Cancer metastasis: Through lymphatic spread | Regulates lymphatic vessel growth and function; overexpression supports cancer cell dissemination and immune evasion. | Specific to lymphatic roles | [63,64] |
| Imaging /Therapy/Stage * | Compound | Targeting Agent | Application | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| SPECT/CT/Preclinical | Antibody | [99mTc]Tc MAG3-bevacizumab | Atherosclerotic cardiovascular | 1. Specific imaging of neovascularization in atherosclerotic plaques 2. Correlation with histopathology | 1. Repeated administration for imaging 2. Plaque heterogeneity not fully addressed | [102] |
| SPECT/Preclinical | Antibody | [99mTc]Tc-HYNIC-chtiosan-Cy5.5-DC101 | Ischemic microvasculature | High specificity, improved stability, potential for dual functionality | Complexity in synthesis, possible degradation in vivo | [103] |
| SPECT/Preclinical | Antibody | [99mTc]Tc-HYNIC-BV, [99mTc]Tc(CO)3-BV,[99mTc]Tc-DTPA-BV | Melanoma | Versatile radiolabeling, in vitro and in vivo stability, reduced immunogenicity | Complex synthesis and purification | [104,105,106] |
| SPECT/Preclinical | Antibody | [99mTc]Tc-Ram | VEGFR-2 receptor | High target specificity, theranostic potential, enhanced stability | Complex radiolabeling, non-specific uptake | [107] |
| SPECT/CT/Preclinical | Nanoparticle | [99mTc]Tc-dendrimer-anti-VEGF | Breast cancer | 1. High specificity for VEGF, which could enhance tumor targeting. 2.The 99mTc labeling makes it suitable for clinical imaging 3. Dendrimer-based probes can offer highly efficient drug delivery and reduced toxicity | 1. The specificity of the probe to VEGF in other tissues and tumors needs further investigation 2. Limited data on the long-term stability, circulation time, and biodistribution. 3. Challenges in large-scale clinical production | [70] |
| PET/CT/Preclinical, Clinical | Antibody | [111In]In-DTPA-BV | Colorectal cancer, ovarian tumor | Established radiochemistry, potential for monitoring therapy, safety of bevacizumab, clinical relevance | Poor correlation with VEGF-A expression, non-specific uptake | [69] |
| SPECT/CT/Preclinical | Affibody | [111In]In-NODAGA-ZVEGFR-2-Bp2 | GBM | High specificity, improved tumor penetration, rapid clearance, customizable affibody design, reduced immunogenicity | Limited tumor specificity for VEGFR-2 | [95] |
| PET/Preclinical | Small molecule | [18F]su11248 | Tyrosine kinase activity in cancer | High specificity, high resolution and sensitivity, favorable isotope properties, potential for early diagnosis | Complex synthesis, potential off-target effects, short half-life of 18F, competition with endogenous ligands | [78] |
| PET/Preclinical | Small molecule | [18F]3-[4′-Fluorobenzylidene]indolin-2-one | RTKs | Potential for broad cancer applications, facilitates personalized medicine | Complex synthesis, limited tumor specificity, off-target toxicity | [79] |
| PET/Preclinical | Small molecules | [18F]F-diaryl urea | Angiogenesis | Development of dual inhibitors, specificity for angiogenesis-related targets, potential for personalized medicine | Potential off-target effects, cost and technical barriers | [80] |
| PET/CT/Preclinical | Antibody | [64Cu]Cu-NOTA-BV | Renal carcinoma | Innovative use of immuno-PET, rapalog therapeutic monitoring, enhanced specificity | Potential for off-target effects | [12] |
| PET and NIRF/Preclinical | Antibody | [64Cu]Cu-NOTA-BV-800CW | GBM | Dual-modality imaging, real-time surgical guidance | High cost and limited accessibility, technical complexity | [108] |
| PET/Preclinical | Antibody | [64Cu]Cu-NOTA-RamAb | Lung cancer | Targeted imaging of VEGFR-2, high sensitivity and quantification, potential for therapy monitoring, clinical translation | Off-target accumulation | [109] |
| PET/Preclinical | Peptide | [64Cu]Cu-DOTA-GU40C4 | Prostate cancer | High stability, versatility of peptoid structure binding to VEGFR-2 and simplicity | Binding affinity concerns | [87] |
| PET/Preclinical | Nanographene | [64Cu]Cu-NOTA-GO-PEG-VEGF-121 | Brain Cancer | 1. GO’s large surface area enabled functionalization with targeting agents and therapeutic payloads, enhancing its versatility 2. Reducing off-target effects 3. Conducted in vivo studies using a xenograft model to confirm tumor accumulation 4. GO serves as a platform for both tumor imaging and therapeutic delivery, offering multimodal capabilities 5. VEGFR targeting improves tumor vascular interaction, enhancing therapeutic delivery | 1. Despite increased tumor specificity, non-specific high accumulation in the liver and spleen was noted, a common issue with nanoparticles 2. No evaluation of BBB permeability 3. The long-term stability of the functionalized GO in biological systems was not reported | [121] |
| PET/Preclinical | Peptide | [64Cu]Cu-DOTA-VEGF125-136 | Melanoma | 1. Demonstrated excellent binding affinity for VEGFR as shown by significant signal reduction in blocking studies (>90%), confirming target-mediated uptake 2. Rapid tumor accumulation and imaging 3. exhibited rapid clearance from blood (20 min) and low non-target tissue uptake, minimizing background signals and improving tumor-to-background contrast | 1. Rapid clearance reduced imaging windows and may necessitate precise timing. 2. Single receptor targeting may limit its utility in tumors with heterogeneous or low VEGFR expression | [10] |
| PET/Clinical | Antibody | [89Zr]Zr-N-suc-Df-BV | Breast cancer, lung cancer | Early detection, therapeutic monitoring, potential for combination therapies | Limited sample size, complexity of interpretation, high cost and limited accessibility | [110,111] |
| PET and NIRF/Preclinical | Antibody | [89Zr]Zr-N-suc-Df-BV/cetuximab-800CW, [89Zr]Zr-N-suc-Df-BV/cetuximab | Squamous cell carcinoma | Dual-mode imaging, inert coupling method, versatility for preclinical and clinical applications, improved sensitivity, enhanced targeting | Potential for high background signal in fluorescence | [112,113] |
| PET/CT/Preclinical | Antibody | [89Zr]Zr-N-suc-Df-Ram | Prostate cancer | Specific targeting of VEGFR-2, immuno-PET technology, clinical applicability, alignment of biological half life of intact Ab to 89Zr half life | Slow blood clearance, longer imaging window | [114] |
| PET/CT/Preclinical | Antibody | [89Zr]Zr-bevacizumab, [89Zr]-IgG | Ovarian Tumor | 1. High affinity for VEGF, ensuring precise imaging of VEGF-overexpressing tumors 2. Bevacizumab is an FDA-approved drug, facilitating potential clinical adaptation | 1. Stability of compound in vivo was not comprehensively assessed 2. Extended circulation time may increase non-specific background signal | [69] |
| PET/CT/Clinical | Antibody | [89Zr]Zr-bevacizumab | Renal Cell Carcinoma | 1.The use of a clinically available radiotracer (89Zr-bevacizumab) allows for translation into clinical practice 2. Early detection of therapy efficacy could lead to more personalized treatment plans, improving patient outcomes | 1. The study is limited by its small patient cohort 2. PET imaging using 89Zr-bevacizumab might not be suitable for all tumor types, limiting its applicability 3. Specificity and sensitivity in a heterogeneous patient population might vary, requiring further validation | [92] |
| SPECT/MRI/Clinical | Antibody | [123I]I-VEGF165 | Braintumor | 1. Non-invasively assess VEGF expression and angiogenesis in glioma patients 2. A significant correlation between the tumor-to-normal brain (T/N) uptake ratio and overall survival 3. Specificity for high-grade gliomas | 1. A total of 23 patients, with only 8 undergoing both imaging modalities. 2. Limited temporal imaging | [116] |
| SPECT/Clinical | Antibody | [123I]I-VEGF | Glioblastoma (GBM) | 1. 123I-VEGF provides an effective method for visualizing brain tumors, particularly those with significant angiogenesis. 2. Scintigraphy using 123I offers compatibility | 1. More studies are required to investigate the probe’s specificity for tumors and non-specific uptake in other organs/tissues 2. More information is needed on dosimetry, circulation time, and stability in clinical contexts, particularly for brain tumors where BBB disruption may play a role | [126] |
| PET/Preclinical | Antibody | [124I]I-VG76e | Fibrosarcoma | High specificity, potential for therapy monitoring, use of iodine-124, potential for therapy monitoring | Challenges with iodine-124 labeling, tracer stability and deiodination, biodistribution challenges | [117] |
| Therapy/PET/CT/MRI/Clinical | Antibody | [124I]I-HuMV833 | Tumorendothelial permeability | Comprehensive evaluation of HuMV833, promise for therapy monitoring, guidance for antiangiogenic trials, dual approaches of imaging and biology | Immunogenicity concerns, inadequate addressing of resistance mechanisms | [118] |
| PET/CT/Preclinical | Antibody | [124I]I-Ran, [124I]I-BV | Pharmacokinetic properties ofvitreous cavity | Innovative use of radiolabeled antibodies, relevant to ocular diseases, quantitative assessment, potential for clinical translation | High cost and complexity of radiolabeling, short follow-up period, lack of functional assessment | [119] |
| PET/CT/Preclinical | Peptide | [124I]I-aflibercept | Vitreous cavity | Comprehensive comparison of three anti-VEGF agents, use of a nonhuman primate model, relevant to ocular therapeutics, addresses regulatory and therapeutic concerns | Cost considerations, limited long-term data, variability in drug dosing, limited exploration of resistance mechanisms | [120] |
| Ultrasound/Preclinical | Antibody | [125I]MBs-I-Bt-Avas12a1 | Angiosarcoma tumor | Novel use of targeted microbubbles, high sensitivity and specificity for VEGFR-2, non-ionizing modality, potential for real-time imaging, economic and accessibility benefits | Limited depth of ultrasound, microbubble stability issues | [16] |
| SPECT/Preclinical | Small molecule | [125I]5-I-sunitinib | Angiogenetic process | Integration of radiochemistry and pharmacology, efficient radiosynthesis protocol, exploration of VEGFR targeting, broad applicability | Limited clinical relevance of I-125, complexity of radiochemistry, no comparison with existing radiotracers | [81] |
| SPECT/CT/Preclinical | Small molecule | [125I]m-I-NPAE, [125I]p-I-NPAE[125I]m-I-NPAM[125I]p-I-NPAM | Prostate cancer | Insights into tumor angiogenesis, potential for multi-disease applications | Potential toxicity of derivatives, short-term evaluation | [82] |
| PET/Preclinical | Small molecule | [Methyl-11C]-sorafenib | Head and neck cancer | Focus on brain uptake, innovative use of knockout models, efficient radiosynthesis, potential clinical translation, contribution to drug transport studies | No functional imaging data, absence of comparison, radiochemical yield and stability concerns | [77] |
| PET/Preclinical | Small molecule | [N-Methyl-11C]-PAQ | Subcutaneous and intraperitoneal tumor models | Potential for cancer therapy monitoring, targeted imaging for VEGFR-2 | Potential for non-specific binding | [83] |
| Synthesis/Preclinical | Small molecule | [N-Methyl-11C]vandetanib, [N-methyl-11C]chloro-vandetanib,[O-methyl-11C]vandetanib[O-methyl-11C]chloro-vandetanib | Tumor angiogenesis | Efficient radiosynthesis protocols, dual tracer development, potential for multitarget imaging, broad applicability | No functional imaging data, lack of tumor models, no pharmacokinetic data | [84] |
| PET/Preclinical | Antibody | [68Ga]Ga-NOTA-VEGF-121 | Brain Cancer | 1. High affinity and specificity of NOTA-VEGF-121 for VEGFR-2 (IC50 = 1.66 nM), ensuring selective imaging of VEGFR-rich tumors 2. Readily accessible to 68Ga 3. High tumor-to-background contrast, facilitating accurate localization of VEGFR expression. 4. Rapid renal clearance minimized non-specific background signal, enhancing imaging quality | 1. The in vivo stability of radiolabeled compound was not extensively reported 2. No evaluation of probe uptake in CNS tumors with an intact BBB, limiting its application to glioblastomas with disrupted barriers 3. The rapid clearance of the probe may limit imaging windows and necessitate precise timing for imaging sessions | [86] |
| PET/CT/Preclinical/Clinical | Peptide | [68Ga]Ga –DOTA-TMVP1 | Ovarian, Cervical cancer | Improved tumor targeting for gynecological cancers due to high VEGFR-3 expressions | 1. Probe uptake in non-tumor tissues were not fully evaluated 2. The circulation time and dosimetry need further investigation | [127] |
| PET/CT/Preclinical | Pepetide | [68Ga]Ga –DOTA-TMVP1448 | Tumor metastatic lymph node | 1. TMVP1448 shows high specificity for VEGFR-3 (KD = 6.73 × 10−6 mol/L). 2. The peptide inhibitor allows for better tumor penetration and retention | More pre-clinical study, including biodistribution and dosimetry, is required to support its potential for clinical investigation | [128] |
| Therapy/Preclinical | Peptide | [177Lu]Lu -DOTA-Ahx-A7R,[177Lu]Lu Lys(hArg)-Dab(Ahx-DOTA-Lu)-Pro-Arg,[68 Ga]Ga -DOTA-Ahx-A7R,[68 Ga]Ga Lys(hArg)-Dab(Ahx-DOTA-Lu)-Pro-Arg | Cancer therapy | 1. Dual-isotope theranostic design. 2. The branched peptidomimetic exhibited stronger binding to NRP-1 than the linear A7R peptide | 1. Poor stability in human serum 2. Lack of in vivo evaluation | [88] |
| Therapy/Preclinical | Antibody | [177Lu]Lu-CHX-A”-DTPA-BV | Lymphoma and acute myeloid leukemia | Efficient radiolabeling with CHX-A’’-DTPA, demonstrated therapeutic potential | Pharmacokinetics of bevacizumab with limited extravascular distribution, immunogenicity concerns | [115] |
| Therapy/Preclinical | Antibody | [177Lu]Lu-DTPA-Anti- VEGFR-1 | Lung carcinoma | 1. Dual benefits of targeted radiotherapy and imaging due to β-particle emission and γ-photons 2. Minimizing off-target effects 3. Effective radiolabeling and binding specificity, supporting further in vivo evaluations | 1. In vivo xenograft or transgenic tumor models are not included 2. No analysis of BBB permeability 3. Non-specific uptake in non-target tissues was not thoroughly evaluated. 4. No exploration of the pharmacokinetics or circulation half-life | [129] |
| Therapy/Preclinical | Antibody | [177Lu]Lu-cG250 | Renal Cell Carcinoma | 1. Complementary mechanisms of action—anti-angiogenic effects of sunitinib and targeted cytotoxicity of 177Lu-cG250 radioimmunotherapy (RIT)—to improve therapeutic outcomes in RCC, particularly in resistant tumors 2. Proved effective in the sunitinib-resistant SK-RC-52 model, showing significant tumor growth delay and survival benefit (91% survival with two cycles) 3. Histopathological confirmation | 1. Lack of functional imaging 2. While survival and tumor response were analyzed, the study offers limited detailed evaluation of long-term radiotoxicity or potential damage to normal organs beyond basic survival and histology | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, H.; Lee, S.; Xu, W.; Langhans, S.A.; Johnson, D.K.; Stauff, E.; Kecskemethy, H.H.; Averill, L.W.; Yue, X. Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics. Int. J. Mol. Sci. 2025, 26, 5373. https://doi.org/10.3390/ijms26115373
Karimi H, Lee S, Xu W, Langhans SA, Johnson DK, Stauff E, Kecskemethy HH, Averill LW, Yue X. Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics. International Journal of Molecular Sciences. 2025; 26(11):5373. https://doi.org/10.3390/ijms26115373
Chicago/Turabian StyleKarimi, Hanieh, Sarah Lee, Wenqi Xu, Sigrid A. Langhans, David K. Johnson, Erik Stauff, Heidi H. Kecskemethy, Lauren W. Averill, and Xuyi Yue. 2025. "Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics" International Journal of Molecular Sciences 26, no. 11: 5373. https://doi.org/10.3390/ijms26115373
APA StyleKarimi, H., Lee, S., Xu, W., Langhans, S. A., Johnson, D. K., Stauff, E., Kecskemethy, H. H., Averill, L. W., & Yue, X. (2025). Advances in Molecular Imaging of VEGFRs: Innovations in Imaging and Therapeutics. International Journal of Molecular Sciences, 26(11), 5373. https://doi.org/10.3390/ijms26115373








