PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT)
Abstract
1. Introduction
2. Results
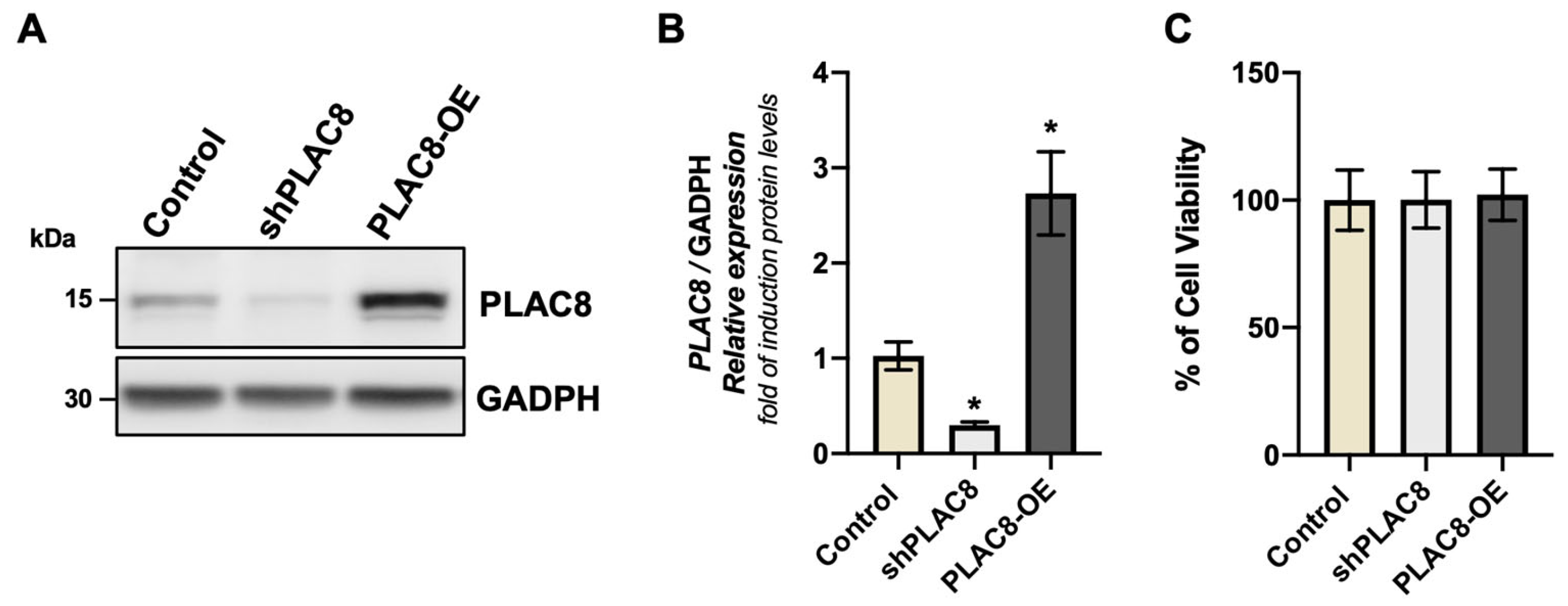
2.1. The Knockdown and Overexpression of PLAC8 Have No Effect on Cell Viability
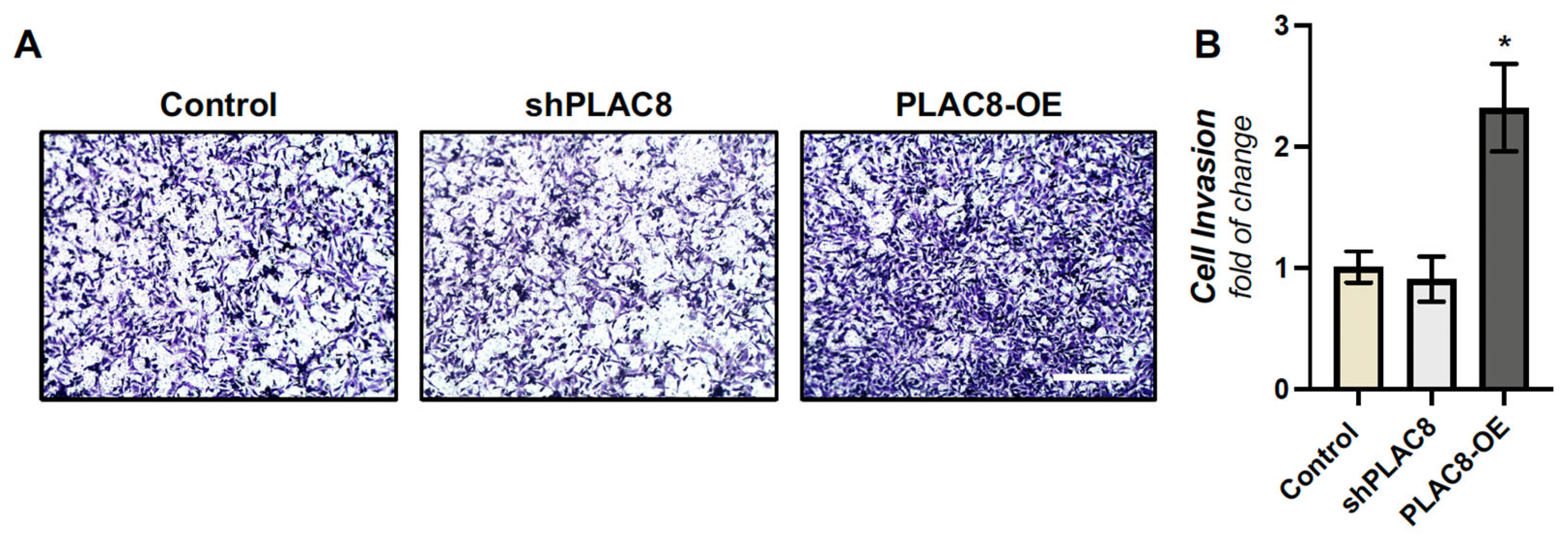
2.2. PLAC8 Promotes Invasion and Responds to Oxygen and TGF-β1 Signals in EVT Cells
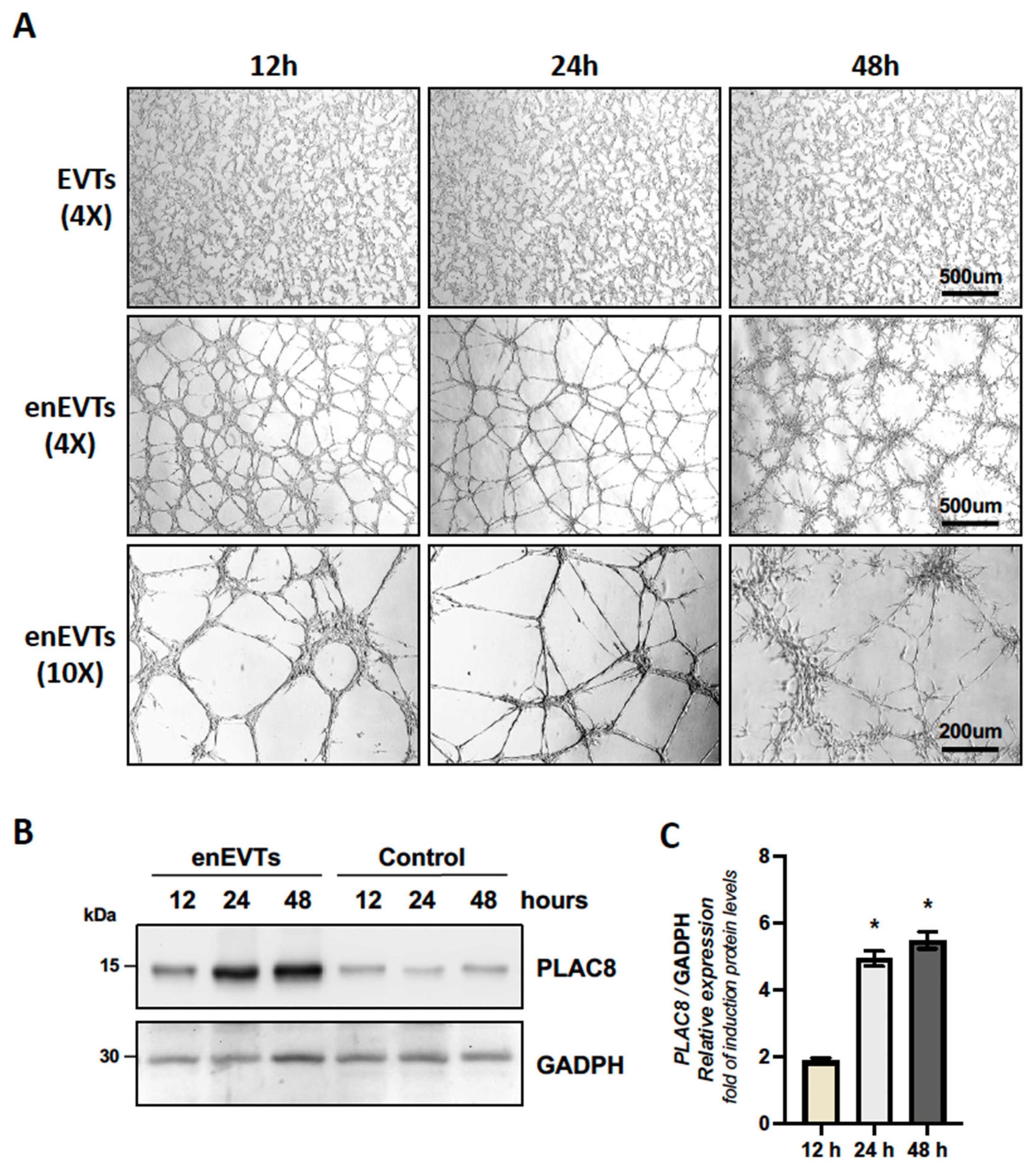
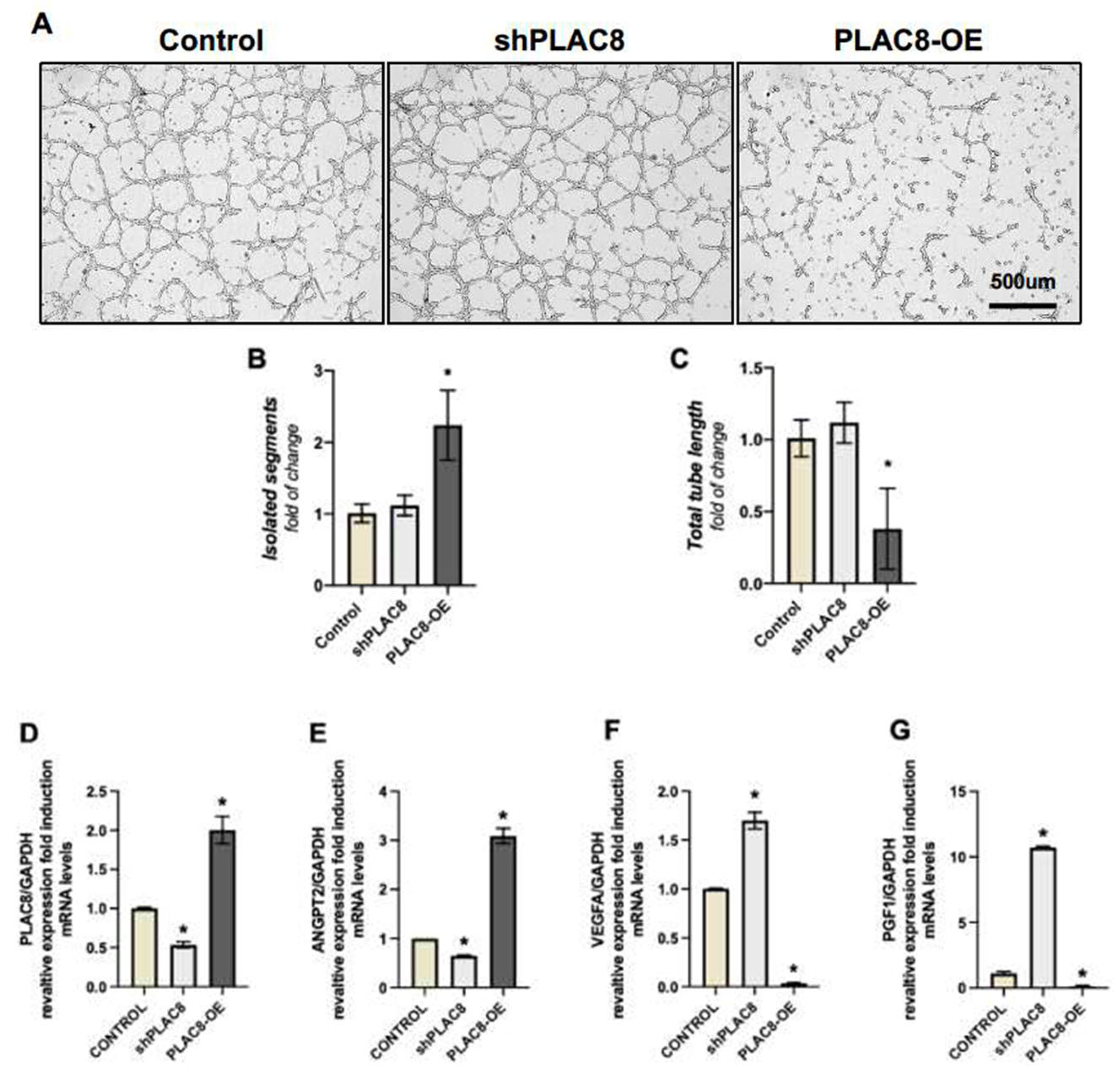
2.3. PLAC8 Expression Is Induced in Trophoblast Cells Stimulated to Acquire an Endothelial-like Phenotype, Acting as a Negative Regulator of This Process
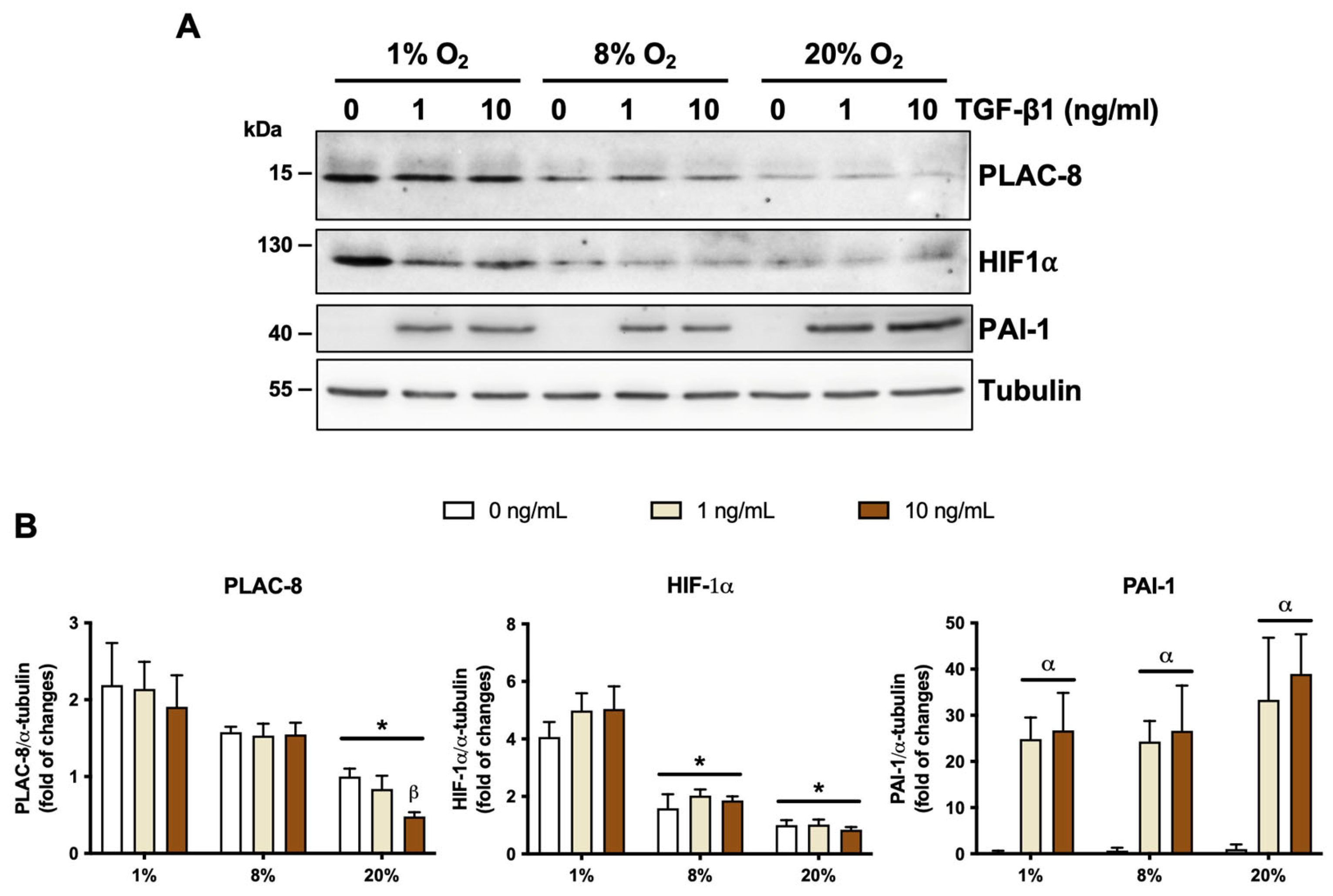
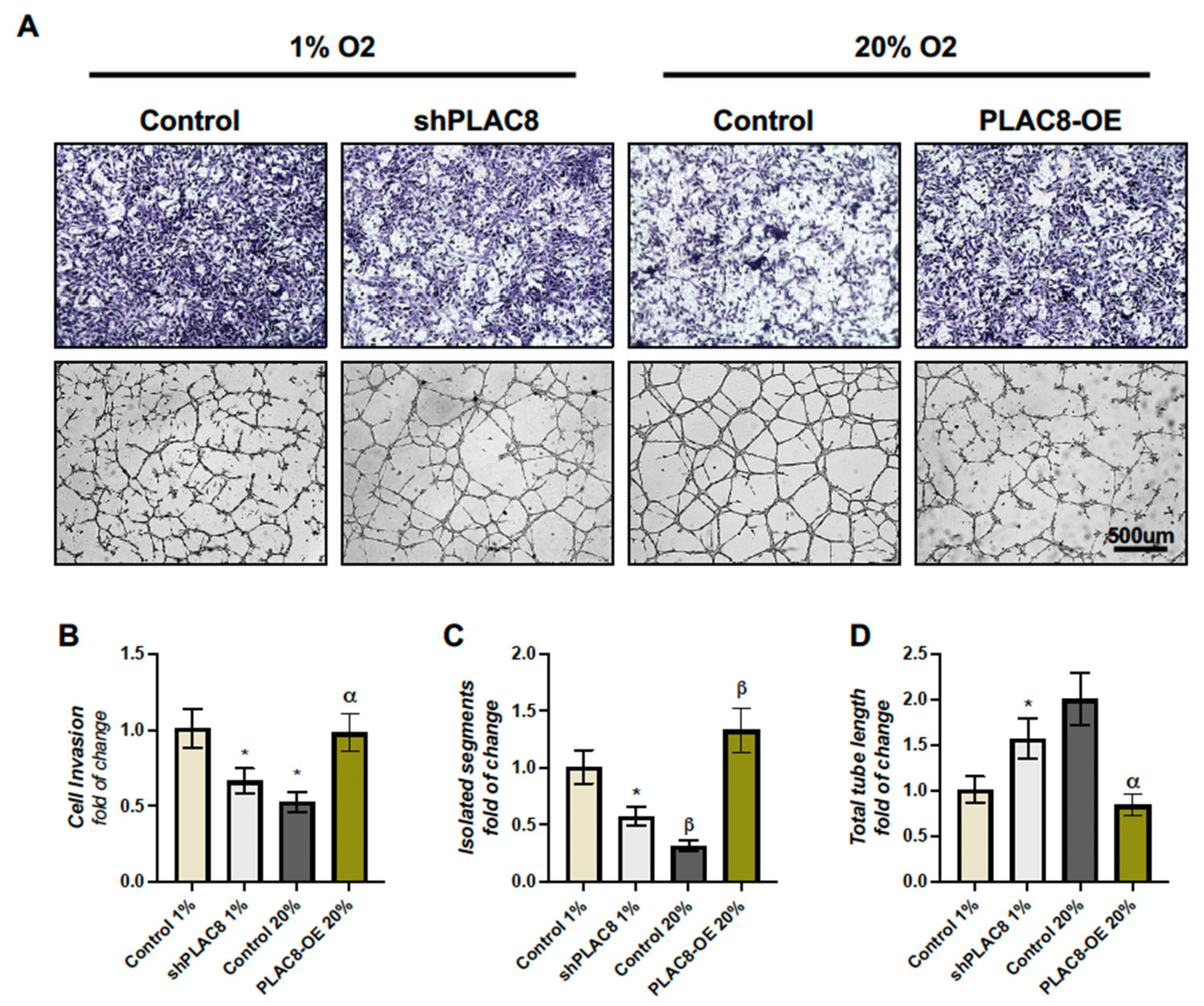
2.4. The Invasion and Differentiation of Trophoblasts Under Varying Oxygen Conditions Are Partially Regulated by the Differential Expression of PLAC8
3. Discussion
4. Materials and Methods
4.1. Reagents and Plasmid Constructs
4.2. Cell Culture
4.3. Cell Transfection
4.4. Cell Viability
4.5. Invasion Assay
4.6. Differentiation Assay
4.7. Effect of Oxygen and TGFb on Trophoblast Invasion
4.8. Protein Extraction and Western Blotting
4.9. RNA Extractions and Gene Expression RT-PCR
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PLAC8 | Placenta-Specific 8 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| ANGPT2 | Angiopoietin-2 |
| PGF | Placental growth factor |
| VEGF | Vascular endothelial growth factor |
References
- Lobo, S.E.; Leonel, L.C.P.; Miranda, C.M.; Coelho, T.M.; Ferreira, G.A.; Mess, A.; Abrão, M.S.; Miglino, M.A. The Placenta as an Organ and a Source of Stem Cells and Extracellular Matrix: A Review. Cells Tissues Organs 2016, 201, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Compendium on the Pathophysiology and Treatment of Hypertension Preeclampsia. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obs. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Anin, S.; Vince, G.; Quenby, S. Trophoblast invasion. Hum. Fertil. 2004, 7, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Red-Horse, K.; Zhou, Y.; Genbacev, O.; Prakobphol, A.; Foulk, R.; McMaster, M.; Fisher, S.J. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J. Clin. Investig. 2004, 114, 744–754. [Google Scholar] [CrossRef]
- Huppertz, B. The anatomy of the normal placenta. J. Clin. Pathol. 2008, 61, 1296–1302. [Google Scholar] [CrossRef]
- El-Sayed, A.A.F. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J. Obs. Gynecol. 2017, 56, 593–598. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Whitehead, C.; Li, J.; Thierry, B.; Le, T.D.; Winter, M. Identifying preeclampsia-associated genes using a control theory method. Brief. Funct. Genom. 2022, 21, 296–309. [Google Scholar] [CrossRef]
- Chang, W.L.; Liu, Y.W.; Dang, Y.L.; Jiang, X.X.; Xu, H.; Huang, X.; Wang, Y.L.; Wang, H.; Zhu, C.; Xue, L.Q.; et al. PLAC8, a new marker for human interstitial extravillous trophoblast cells, promotes their invasion and migration. Development 2018, 145, dev.148932. [Google Scholar] [CrossRef]
- Soncin, F.; Natale, D.; Parast, M.M. Signaling pathways in mouse and human trophoblast differentiation: A comparative review. Cell Mol. Life Sci. 2015, 72, 1291–1302. [Google Scholar] [CrossRef]
- Huppertz, B. Oxygenation of the placenta and its role in pre-eclampsia. Pregnancy Hypertens. 2014, 4, 244–245. [Google Scholar] [CrossRef] [PubMed]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Lackner, A.I.; Dietrich, B.; Kunihs, V.; Haslinger, P.; Meinhardt, G.; Maxian, T.; Saleh, L.; Fiala, C.; Pollheimer, J.; et al. Transforming growth factor-β signaling governs the differentiation program of extravillous trophoblasts in the developing human placenta. Proc. Natl. Acad. Sci. USA 2022, 119, e2120667119. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.L.; Stoikos, C.; Findlay, J.K.; Salamonsen, L.A. TGF-β superfamily expression and actions in the endometrium and placenta. Reproduction 2006, 132, 217–232. [Google Scholar] [CrossRef]
- Cheng, J.C.; Chang, H.M.; Leung, P.C.K. Transforming Growth Factor-β1 Inhibits Trophoblast Cell Invasion by Inducing Snail-mediated Down-regulation of Vascular Endothelial-cadherin Protein. J. Biol. Chem. 2013, 288, 33181–33192. [Google Scholar] [CrossRef]
- Yoshihara, M.; Mizutani, S.; Matsumoto, K.; Kato, Y.; Masuo, Y.; Tano, S.; Mizutani, H.; Kotani, T.; Mizutani, E.; Shibata, K.; et al. Crosstalk between foetal vasoactive peptide hormones and placental aminopeptidases regulates placental blood flow: Its significance in preeclampsia. Placenta 2022, 121, 32–39. [Google Scholar] [CrossRef]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming growth factor-beta 1 (TGF-β1) induces angiogenesis through vascular endothelial growth factor (VEGF)-mediated apoptosis. J. Cell Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Lutz, S.; Feng, Y.; Kroll, J.; Hammes, H.; Wieland, T. Angiotensin II modulates VEGF-driven angiogenesis by opposing effects of type 1 and type 2 receptor stimulation in the microvascular endothelium. Cell Signal 2012, 24, 1261–1269. [Google Scholar] [CrossRef]
- Li, H.; Chang, H.M.; Lin, Y.M.; Shi, Z.; Leung, P.C.K. TGF-β1 inhibits microvascular-like formation by decreasing VCAM1 and ICAM1 via the upregulation of SNAIL in human granulosa cells. Mol. Cell Endocrinol. 2021, 535, 111395. [Google Scholar] [CrossRef]
- Lash, G.E.; Otun, H.A.; Innes, B.A.; Bulmer, J.N.; Searle, R.F.; Robson, S.C. Inhibition of Trophoblast Cell Invasion by TGFB1, 2, and 3 Is Associated with a Decrease in Active Proteases1. Biol. Reprod. 2005, 73, 374–381. [Google Scholar] [CrossRef]
- Karmakar, S.; Das, C. Regulation of Trophoblast Invasion by IL-1β and TGF- β1: IL-1β AND TGF-β1 IN TROPHOBLAST INVASION. Am. J. Reprod. Immunol. 2002, 48, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Benian, A.; Madazlı, R.; Aksu, F.; Uzun, H.; Aydın, S. Plasma and Placental Levels of Interleukin-10, Transforming Growth Factor-1, and Epithelial- Cadherin in Preeclampsia. Obstet. Gynecol. 2002, 100, 327–331. [Google Scholar] [PubMed]
- Wang, X.; Wang, T.; Wang, J.; Niu, X.; Wang, K.; Hao, Z.; Gao, H. Circulating Transforming Growth Factor-β1 Levels in Preeclamptic Women: A Meta-analysis. Reprod. Sci. 2023, 30, 1952–1964. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zheng, J. Regulation of Placental Angiogenesis. Microcirculation 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Feng, X.; Wei, Z.; Tao, X.; Du, Y.; Wu, J.; Yu, Y.; Yu, H.; Zhao, H. PLAC8 promotes the autophagic activity and improves the growth priority of human trophoblast cells. FASEB J. 2021, 35, e21351. [Google Scholar] [CrossRef]
- Kinsey, C.; Balakrishnan, V.; O’dell, M.R.; Huang, J.L.; Newman, L.; Whitney-Miller, C.L.; Hezel, A.F.; Land, H. Plac8 Links Oncogenic Mutations to Regulation of Autophagy and Is Critical to Pancreatic Cancer Progression. Cell Rep. 2014, 7, 1143–1155. [Google Scholar] [CrossRef]
- Mao, M.; Cheng, Y.; Yang, J.; Chen, Y.; Xu, L.; Zhang, X.; Li, Z.; Chen, C.; Ju, S.; Zhou, J.; et al. Multifaced roles of PLAC8 in cancer. Biomark. Res. 2021, 9, 73. [Google Scholar] [CrossRef]
- Roberts, J.M.; Hubel, C.A. The Two Stage Model of Preeclampsia: Variations on the Theme. Placenta 2009, 30, 32–37. [Google Scholar] [CrossRef]
- Treissman, J.; Yuan, V.; Baltayeva, J.; Le, H.T.; Castellana, B.; Robinson, W.P.; Beristain, A.G. Low oxygen enhances trophoblast column growth by potentiating differentiation of the extravillous lineage and promoting LOX activity. Development 2019, 147, dev.181263. [Google Scholar] [CrossRef]
- Lash, G.E.; Hornbuckle, J.; Brunt, A.; Kirkley, M.; Searle, R.F.; Robson, S.C.; Bulmer, J.N. Effect of Low Oxygen Concentrations on Trophoblast-Like Cell Line Invasion. Placenta 2007, 28, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, J.; Wang, W.; Yu, L.; Xu, X. PLAC8 Overexpression Promotes Lung Cancer Cell Growth via Wnt/β-Catenin Signaling. Zhang XL, editor. J. Immunol. Res. 2022, 2022, 8854196. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Environment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Burton, G. Placenta accreta spectrum: A need for more research on its aetiopathogenesis. BJOG Int. J. Obs. Gynaecol. 2018, 125, 1449–1450. [Google Scholar] [CrossRef]
- Illsley, N.P.; DaSilva-Arnold, S.C.; Zamudio, S.; Alvarez, M.; Al-Khan, A. Trophoblast invasion: Lessons from abnormally invasive placenta (placenta accreta). Placenta 2020, 102, 61–66. [Google Scholar] [CrossRef]
- Barragán-Zúñiga, L.J.; Marchat, L.A.; Carrasco-Wong, I.; Blanco-Castaneda, R.; Salas-Pacheco, J.M.; Simental-Mendia, L.E.; Correa-Ramírez, M.M.; Sosa-Macías, M.; Gutiérrez, J.; Galaviz-Hernandez, C. Evaluation of the PLAC8 Gene in Mexican Women With and Without Preeclampsia and Obesity. Front. Med. 2022, 9, 795309. [Google Scholar] [CrossRef]
- Soleymanlou, N.; Jurisica, I.; Nevo, O.; Ietta, F.; Zhang, X.; Zamudio, S.; Post, M.; Caniggia, I. Molecular Evidence of Placental Hypoxia in Preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4299–4308. [Google Scholar] [CrossRef]
- Hong, K.; Kim, S.H.; Cha, D.H.; Park, H.J. Defective Uteroplacental Vascular Remodeling in Preeclampsia: Key Molecular Factors Leading to Long Term Cardiovascular Disease. Int. J. Mol. Sci. 2021, 22, 11202. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primer 2023, 9, 8. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsueh, Y.W.; Chang, C.W.; Hsu, H.C.; Yang, T.C.; Lin, W.C.; Chang, H.M. Establishment of the fetal-maternal interface: Developmental events in human implantation and placentation. Front. Cell Dev. Biol. 2023, 11, 1200330. [Google Scholar] [CrossRef]
- Chuva De Sousa Lopes, S.M.; Alexdottir, M.S.; Valdimarsdottir, G. The TGFβ Family in Human Placental Development at the Fetal-Maternal Interface. Biomolecules 2020, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Blue, E.K.; Sheehan, B.M.; Nuss, Z.V.; Boyle, F.A.; Hocutt, C.M.; Gohn, C.R.; Varberg, K.M.; McClintick, J.N.; Haneline, L.S. Epigenetic Regulation of Placenta-Specific 8 Contributes to Altered Function of Endothelial Colony-Forming Cells Exposed to Intrauterine Gestational Diabetes Mellitus. Diabetes 2015, 64, 2664–2675. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Li, C.; Ma, H.; Wang, Y.; Cao, Z.; Graves-Deal, R.; Powell, A.E.; Starchenko, A.; Ayers, G.D.; Washington, M.K.; Kamath, V.; et al. Excess PLAC8 promotes an unconventional ERK2-dependent EMT in colon cancer. J. Clin. Investig. 2014, 124, 2172–2187. [Google Scholar] [CrossRef]
- Escalona-Rivano, R.; Chiarello, D.I.; Carvajal, L.; Carrasco-Wong, I.; Gutiérrez, J.A. Trophoblast Cell Recovery from Angiogenesis-Tube Formation Assay for Differentiation Marker Expression Analysis. J. Vis. Exp. 2024, e66454. [Google Scholar] [CrossRef]
- Carvajal, L.; Escalona, R.; Rivera, P.; Aguilera-Olguin, M.; Hernández-Cáceres, M.P.; Gutiérrez, J.; Morselli, E.; Leiva, A. The autophagy process and oxidized LDL independently modulate the invasion and differentiation of extravillous trophoblastic cells to an endothelial-like phenotype in normoxia. Placenta 2024, 158, 263–274. [Google Scholar] [CrossRef]
| Gene | Exon | Oligonucleotides | Sequence | Tm | Cycles |
|---|---|---|---|---|---|
| PLAC8 | 3–4 | PLAC8-F PLAC8-R | 5′-aactccaactggcagacagg-3′ 5′-gccatatcgggtcctgtaga-3′ | 60 °C | 40 |
| GAPDH | 1 | GAPDH-F GAPDH-R | 5′-aggtcggtgtgaacggatttg-3′ 5′-tgtagaccatgtagttgaggtca-3′ | 60 °C | 40 |
| ANGPT2 | 7–8 | ANGPT2-F ANGPT2-R | 5′-ccccactgttgctaaagaaga-3′ 5′-catcctcacgtcgctgaata-3′ | 60 °C | 40 |
| PGF | 3–4 | PGF-F PGF-R | 5′-gtggacgtcgtgtccgagta-3′ 5′-aacgtgctgagagaacgtca-3′ | 60 °C | 40 |
| VEGF | 2–3 | VEGF-F VEGF-R | 5′-aaggaggagggcagaatcat-3′ 5′-aagatgtccaccagggtctc-3′ | 60 °C | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán-Zúñiga, L.J.; Escalona-Rivano, R.; Cordero-Tirado, C.; Sosa-Macías, M.; Carrasco-Wong, I.; Gutiérrez, J.; Galaviz-Hernandez, C. PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT). Int. J. Mol. Sci. 2025, 26, 5371. https://doi.org/10.3390/ijms26115371
Barragán-Zúñiga LJ, Escalona-Rivano R, Cordero-Tirado C, Sosa-Macías M, Carrasco-Wong I, Gutiérrez J, Galaviz-Hernandez C. PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT). International Journal of Molecular Sciences. 2025; 26(11):5371. https://doi.org/10.3390/ijms26115371
Chicago/Turabian StyleBarragán-Zúñiga, Laura J., Rodrigo Escalona-Rivano, Catalina Cordero-Tirado, Martha Sosa-Macías, Ivo Carrasco-Wong, Jaime Gutiérrez, and Carlos Galaviz-Hernandez. 2025. "PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT)" International Journal of Molecular Sciences 26, no. 11: 5371. https://doi.org/10.3390/ijms26115371
APA StyleBarragán-Zúñiga, L. J., Escalona-Rivano, R., Cordero-Tirado, C., Sosa-Macías, M., Carrasco-Wong, I., Gutiérrez, J., & Galaviz-Hernandez, C. (2025). PLAC8 Expression Regulates Trophoblast Invasion and Conversion into an Endothelial Phenotype (eEVT). International Journal of Molecular Sciences, 26(11), 5371. https://doi.org/10.3390/ijms26115371







