Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy
Abstract
1. Introduction
2. Results
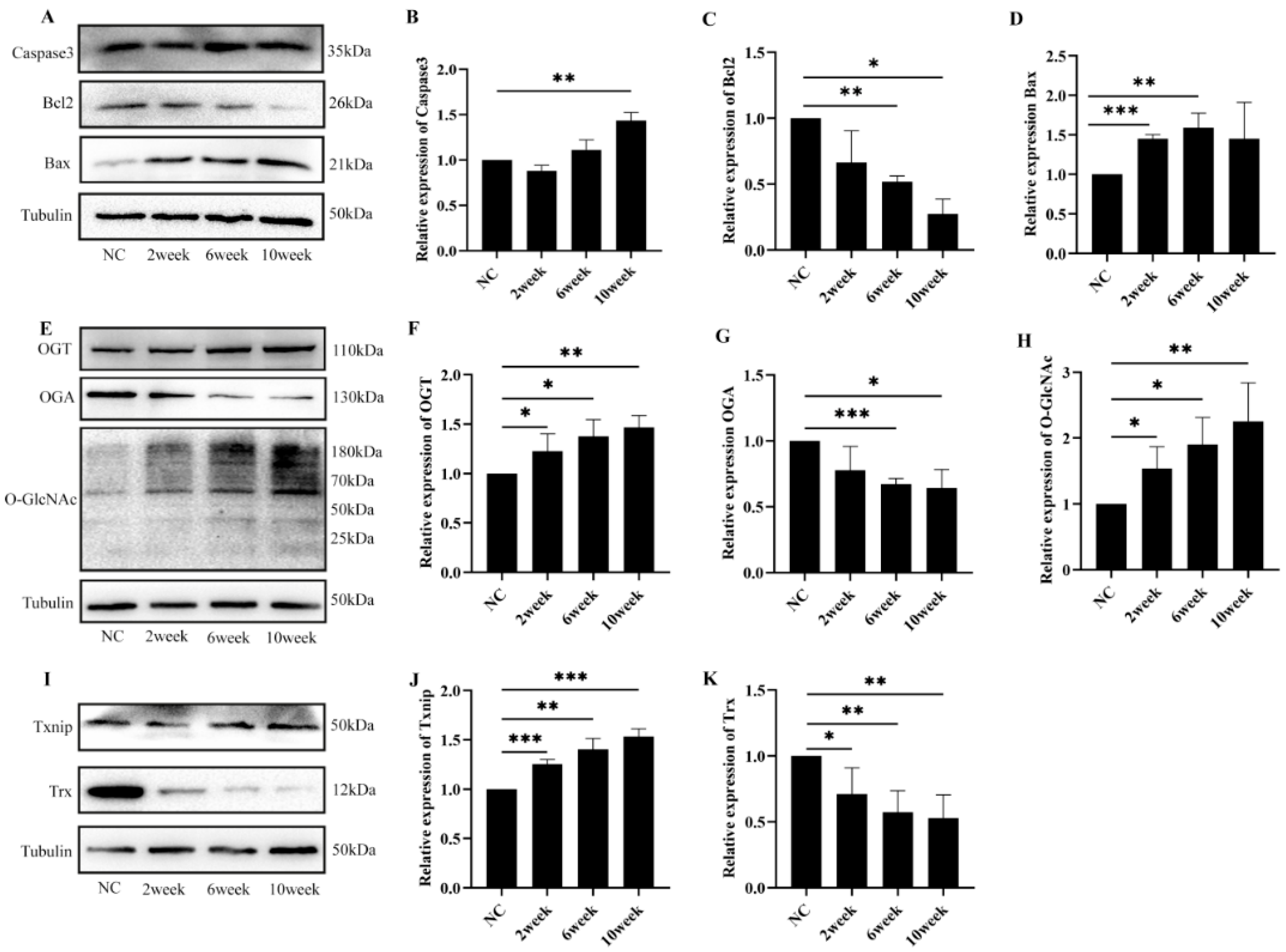
2.1. Diabetes-Induced Retinal Damage in Mice
2.2. Diabetes-Induced Retinal Cell Damage Is Associated with Alterations in O-GlcNAc Modification
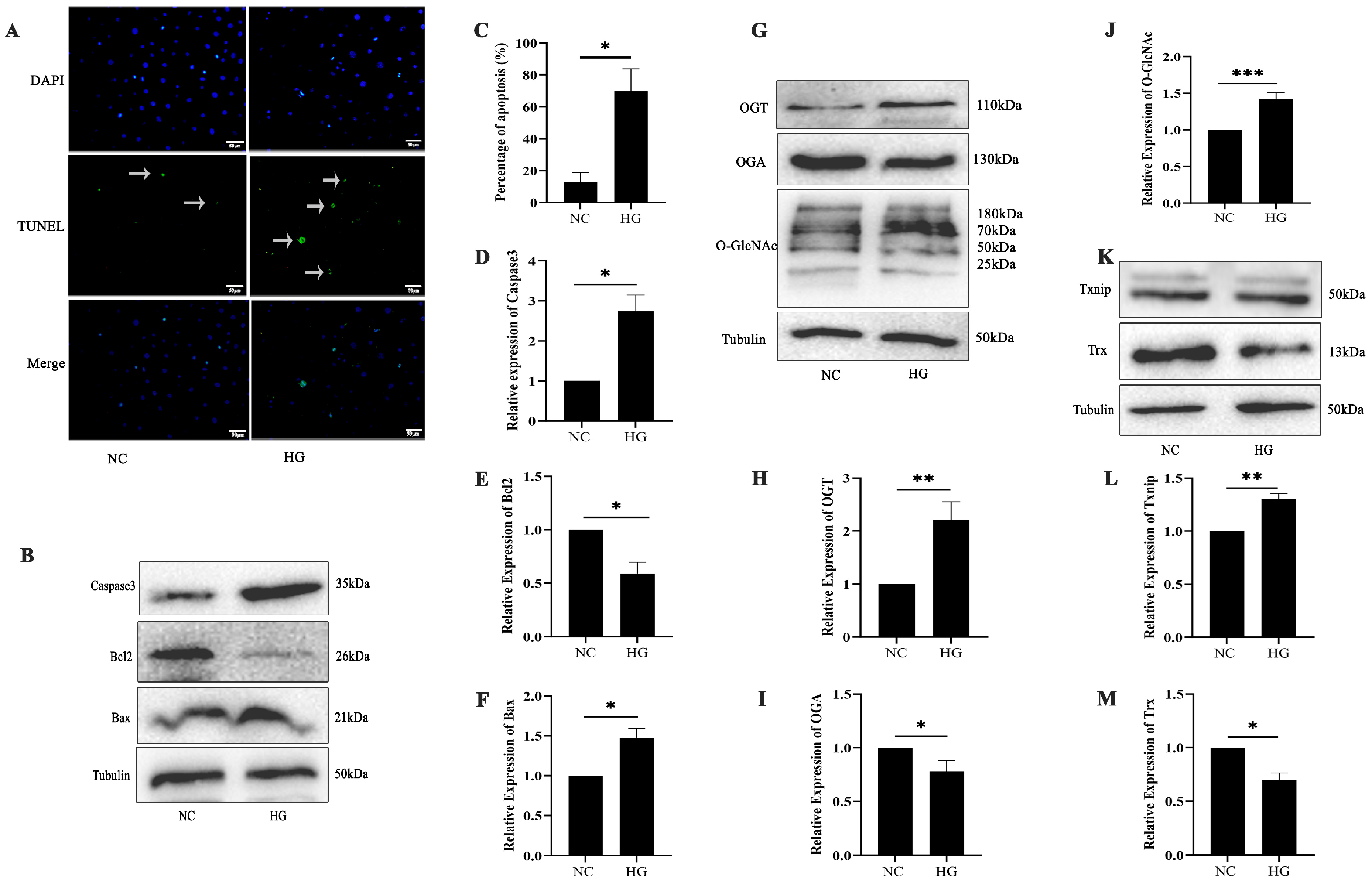
2.3. High-Glucose-Induced 661w Cell Apoptosis Is Related to O-GlcNAc Modification
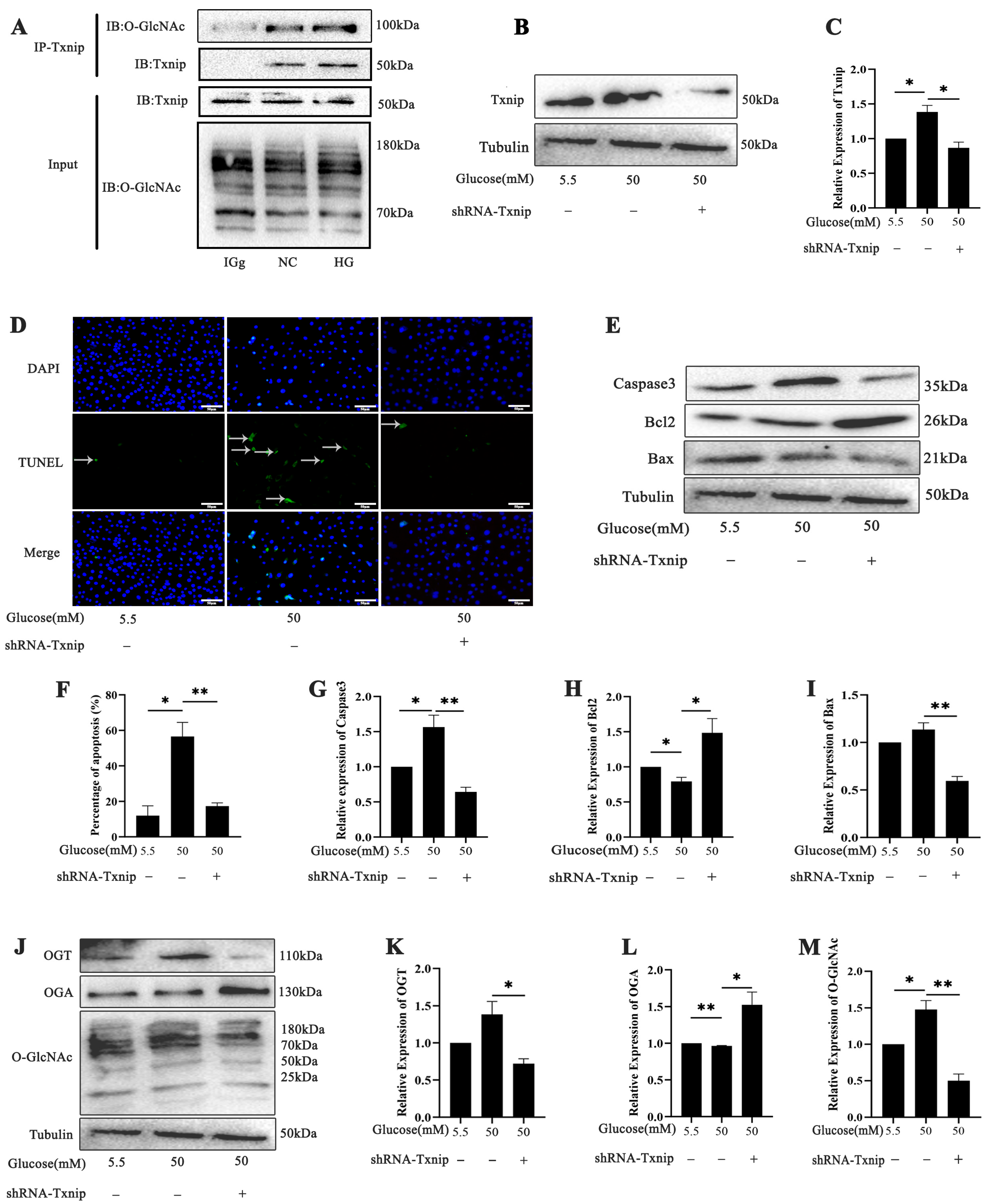
2.4. Effects of Txnip on High-Glucose-Induced Photoreceptor Cells’ Apoptosis with O-GlcNAc Modification Alterations In Vitro
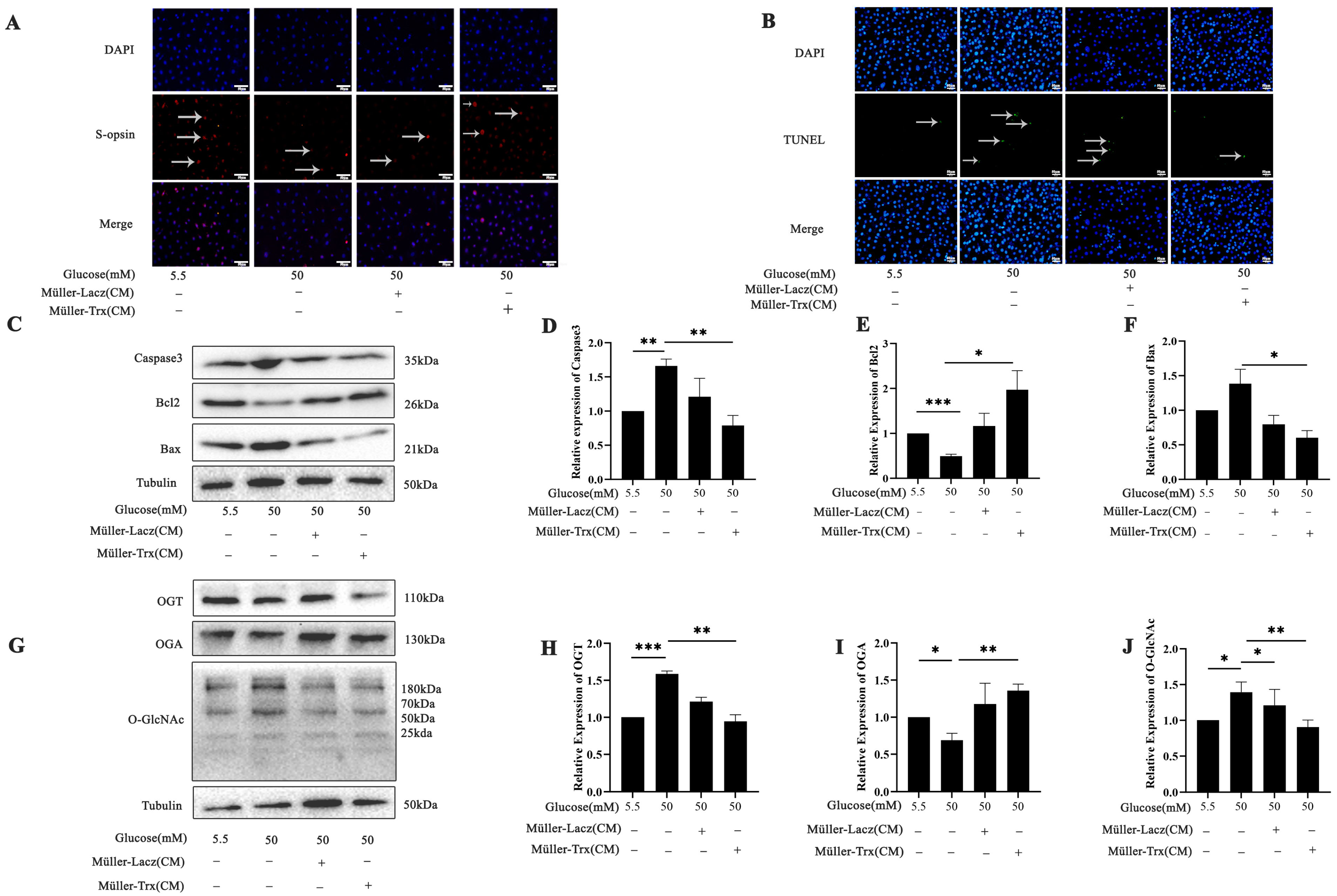
2.5. Effects of Trx Overexpression from Müller-Cell-Conditioned Media on O-GlcNAc Modification Change in 661w Cells in a Hyperglycemic State
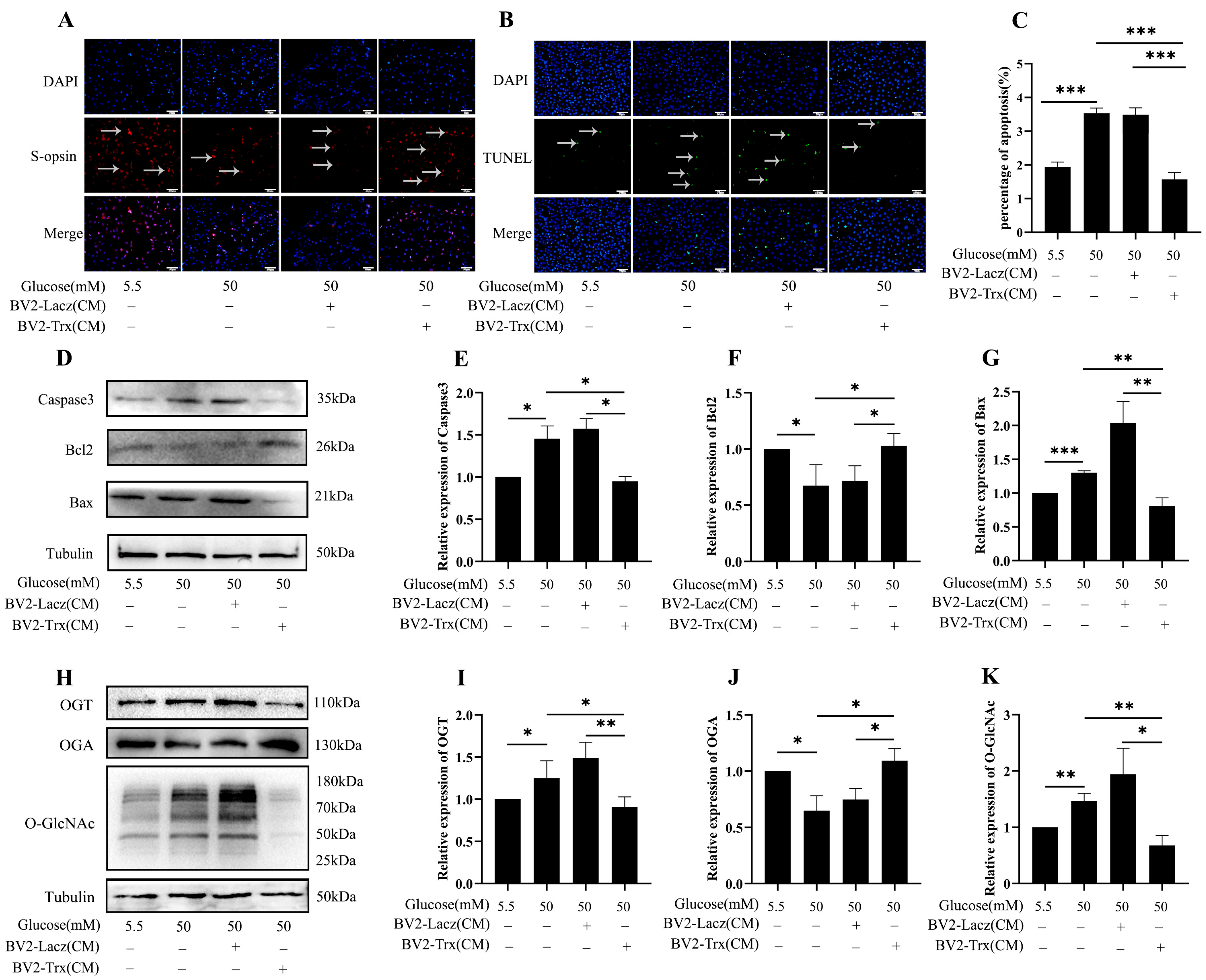
2.6. Effects of Trx Overexpression from BV2-Cell-Conditioned Media on O-GlcNAc Modification Change in 661w Cells in a Hyperglycemic State
3. Discussion
4. Materials and Methods
4.1. Diabetic Mouse Model Establishment
4.2. Electroretinogram (ERG)
4.3. Morphological Analysis
4.4. TdT-Mediated dUTP Nick-End Labeling
4.5. Fluorescent Immunohistochemical Staining
4.6. Cell Culture
4.7. Western Blot
4.8. CO-IP Assay
4.9. Transfection
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DR | Diabetic retinopathy |
| DM | Diabetes mellitus |
| Trx | Thioredoxin |
| Txnip | Thioredoxin-interacting protein |
| H & E | Hematoxylin and eosin |
| ERG | Electroretinogram |
| FBS | Fetal bovine serum |
| GFAP | Glial fibrillary acidic protein |
| NVU | Neurovascular unit |
| ROS | Reactive oxygen species |
References
- Marciano, L.; Camerini, A.L.; Schulz, P.J. The Role of Health Literacy in Diabetes Knowledge, Self-Care, and Glycemic Control: A Meta-analysis. J. Gen. Intern. Med. 2019, 34, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, M.; Bryl, A.; Zorena, K. Retinal Vascular Endothelial Cell Dysfunction and Neuroretinal Degeneration in Diabetic Patients. J. Clin. Med. 2021, 10, 458. [Google Scholar] [CrossRef]
- Dong, W.; Imdad, L.; Xu, S.; Wang, Y.; Liu, C.; Song, S.; Li, Z.; Kong, Y.; Kong, L.; Ren, X. O-GlcNAc Modification Is a Promising Therapeutic Target for Diabetic Retinopathy. Int. J. Mol. Sci. 2024, 25, 6286. [Google Scholar] [CrossRef]
- Ren, X.; Sun, L.; Wei, L.; Liu, J.; Zhu, J.; Yu, Q.; Kong, H.; Kong, L. Liraglutide Up-regulation Thioredoxin Attenuated Müller Cells Apoptosis in High Glucose by Regulating Oxidative Stress and Endoplasmic Reticulum Stress. Curr. Eye Res. 2020, 45, 1283–1291. [Google Scholar] [CrossRef]
- Altmann, C.; Schmidt, M.H.H. The Role of Microglia in Diabetic Retinopathy: Inflammation, Microvasculature Defects and Neurodegeneration. Int. J. Mol. Sci. 2018, 19, 110. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, T.; Ouyang, H.; Lu, B.; Wang, Z.; Ji, L. Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem. Pharmacol. 2019, 159, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Filhoulaud, G.; Benhamed, F.; Pagesy, P.; Bonner, C.; Fardini, Y.; Ilias, A.; Movassat, J.; Burnol, A.F.; Guilmeau, S.; Kerr-Conte, J.; et al. O-GlcNacylation Links TxNIP to Inflammasome Activation in Pancreatic β Cells. Front. Endocrinol. 2019, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.; Pavlou, S.; Harkin, K.; Stitt, A.W.; Xu, H.; Chen, M. IL-33 regulates Müller cell-mediated retinal inflammation and neurodegeneration in diabetic retinopathy. Dis. Models Mech. 2023, 16, dmm050174. [Google Scholar] [CrossRef]
- Fu, S.; Dong, S.; Zhu, M.; Sherry, D.M.; Wang, C.; You, Z.; Haigh, J.J.; Le, Y.Z. Müller Glia Are a Major Cellular Source of Survival Signals for Retinal Neurons in Diabetes. Diabetes 2015, 64, 3554–3563. [Google Scholar] [CrossRef]
- Liu, C.; Dong, W.; Li, J.; Kong, Y.; Ren, X. O-GlcNAc Modification and Its Role in Diabetic Retinopathy. Metabolites 2022, 12, 725. [Google Scholar] [CrossRef]
- Czajewski, I.; van Aalten, D.M.F. The role of O-GlcNAcylation in development. Development 2023, 150, dev201370. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Hu, X.; Wen, G.J.; Wang, Z.; Lin, W.J. O-GlcNAcylation in cancer development and immunotherapy. Cancer Lett. 2023, 566, 216258. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Liu, Q.; Chen, B.; Wu, F.; Li, Y.; Dong, X.; Ma, N.; Wu, Z.; Zhu, Y.; Wang, L.; et al. Protein O-GlcNAcylation coupled to Hippo signaling drives vascular dysfunction in diabetic retinopathy. Nat. Commun. 2024, 15, 9334. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.M.; Dias, M.C.; Alves, J.V.; Silva, J.L.M.; Rodrigues, D.; Silva, J.F.; Francescato, H.D.C.; Ramalho, L.N.Z.; Coimbra, T.M.; Tostes, R.C. Pharmacological activation of nuclear factor erythroid 2-related factor-2 prevents hyperglycemia-induced renal oxidative damage: Possible involvement of O-GlcNAcylation. Biochem. Pharmacol. 2024, 220, 115982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, X.; Shi, R.; He, T.; Chen, S.Y.; Cong, R.; He, B.; Liu, S.; Xu, H.; Gu, J.H. Hyperglycemia Aggravates the Cerebral Ischemia Injury via Protein O-GlcNAcylation. J. Alzheimer’s Dis. JAD 2023, 94, 651–668. [Google Scholar] [CrossRef]
- Ao, H.; Li, H.; Zhao, X.; Liu, B.; Lu, L. TXNIP positively regulates the autophagy and apoptosis in the rat müller cell of diabetic retinopathy. Life Sci. 2021, 267, 118988. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Ting, D.S.; Cheung, G.C.; Wong, T.Y. Diabetic retinopathy: Global prevalence, major risk factors, screening practices and public health challenges: A review. Clin. Exp. Ophthalmol. 2016, 44, 260–277. [Google Scholar] [CrossRef]
- Ren, X.; Lv, J.; Wang, N.; Liu, J.; Gao, C.; Wu, X.; Yu, Y.; Teng, Q.; Dong, W.; Kong, H.; et al. Thioredoxin upregulation delays diabetes-induced photoreceptor cell degeneration via AMPK-mediated autophagy and exosome secretion. Diabetes Res. Clin. Pract. 2022, 185, 109788. [Google Scholar] [CrossRef]
- Hermans, M.P.; Valensi, P. Elevated triglycerides and low high-density lipoprotein cholesterol level as marker of very high risk in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 118–129. [Google Scholar] [CrossRef]
- Balsollier, C.; Pieters, R.J.; Anderluh, M. Overview of the Assays to Probe O-Linked β-N-Acetylglucosamine Transferase Binding and Activity. Molecules 2021, 26, 1037. [Google Scholar] [CrossRef] [PubMed]
- Bartolomé-Nebreda, J.M.; Trabanco, A.A.; Velter, A.I.; Buijnsters, P. O-GlcNAcase inhibitors as potential therapeutics for the treatment of Alzheimer’s disease and related tauopathies: Analysis of the patent literature. Expert Opin. Ther. Pat. 2021, 31, 1117–1154. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, E.A.; Burke-Conte, Z.; Rein, D.B.; Wittenborn, J.S.; Saaddine, J.; Lee, A.Y.; Flaxman, A.D. Prevalence of Diabetic Retinopathy in the US in 2021. JAMA Ophthalmol. 2023, 141, 747–754. [Google Scholar] [CrossRef]
- Hashemi, H.; Rezvan, F.; Pakzad, R.; Ansaripour, A.; Heydarian, S.; Yekta, A.; Ostadimoghaddam, H.; Pakbin, M.; Khabazkhoob, M. Global and Regional Prevalence of Diabetic Retinopathy; A Comprehensive Systematic Review and Meta-analysis. Semin. Ophthalmol. 2022, 37, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Simó, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front. Immunol. 2020, 11, 583687. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Church, K.A.; Rodriguez, D.; Vanegas, D.; Gutierrez, I.L.; Cardona, S.M.; Madrigal, J.L.M.; Kaur, T.; Cardona, A.E. Models of microglia depletion and replenishment elicit protective effects to alleviate vascular and neuronal damage in the diabetic murine retina. J. Neuroinflamm. 2022, 19, 300. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Vince, J.E.; De Nardo, D.; Gao, W.; Vince, A.J.; Hall, C.; McArthur, K.; Simpson, D.; Vijayaraj, S.; Lindqvist, L.M.; Bouillet, P.; et al. The Mitochondrial Apoptotic Effectors BAX/BAK Activate Caspase-3 and -7 to Trigger NLRP3 Inflammasome and Caspase-8 Driven IL-1β Activation. Cell Rep. 2018, 25, 2339–2353.e4. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imdad, L.; Xu, S.; Meng, Y.; Bao, K.; Dong, W.; Yin, X.; Tong, Y.; Zhang, W.; Ren, X.; Kong, L. Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy. Int. J. Mol. Sci. 2025, 26, 5369. https://doi.org/10.3390/ijms26115369
Imdad L, Xu S, Meng Y, Bao K, Dong W, Yin X, Tong Y, Zhang W, Ren X, Kong L. Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy. International Journal of Molecular Sciences. 2025; 26(11):5369. https://doi.org/10.3390/ijms26115369
Chicago/Turabian StyleImdad, Laraib, Shengnan Xu, Yulang Meng, Kaimin Bao, Wenkang Dong, Xuanya Yin, Yujie Tong, Wei Zhang, Xiang Ren, and Li Kong. 2025. "Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy" International Journal of Molecular Sciences 26, no. 11: 5369. https://doi.org/10.3390/ijms26115369
APA StyleImdad, L., Xu, S., Meng, Y., Bao, K., Dong, W., Yin, X., Tong, Y., Zhang, W., Ren, X., & Kong, L. (2025). Txnip/Trx Is a Potential Element in Regulating O-GlcNAc Modification in Photoreceptors to Alleviate Diabetic Retinopathy. International Journal of Molecular Sciences, 26(11), 5369. https://doi.org/10.3390/ijms26115369





