Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo
Abstract
1. Introduction
1.1. Photodynamic Chemotherapy for Triple-Negative Breast Cancer
1.2. Hydra as Model Organism
2. Results
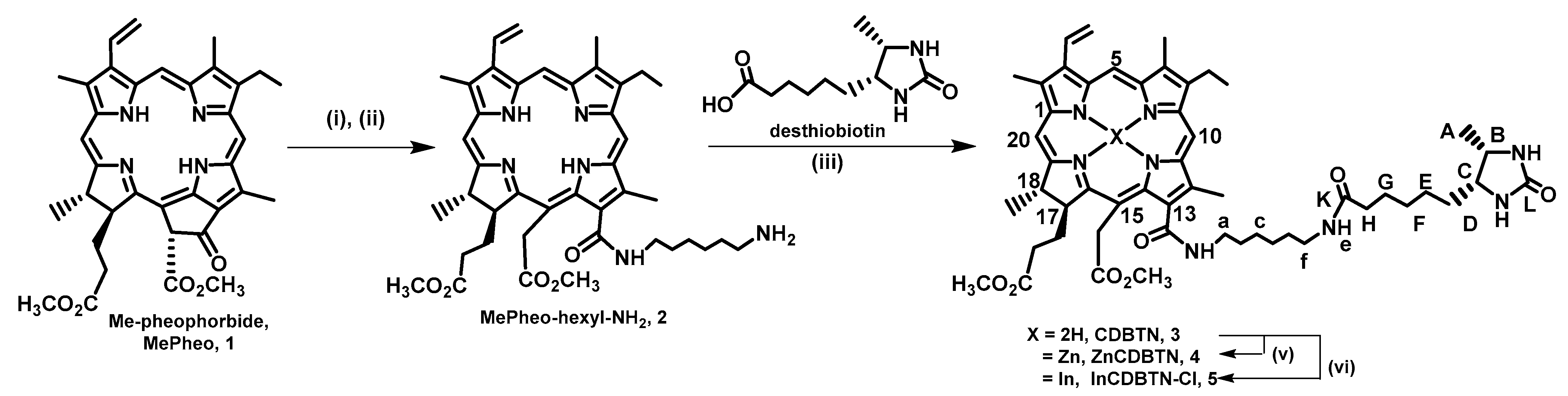
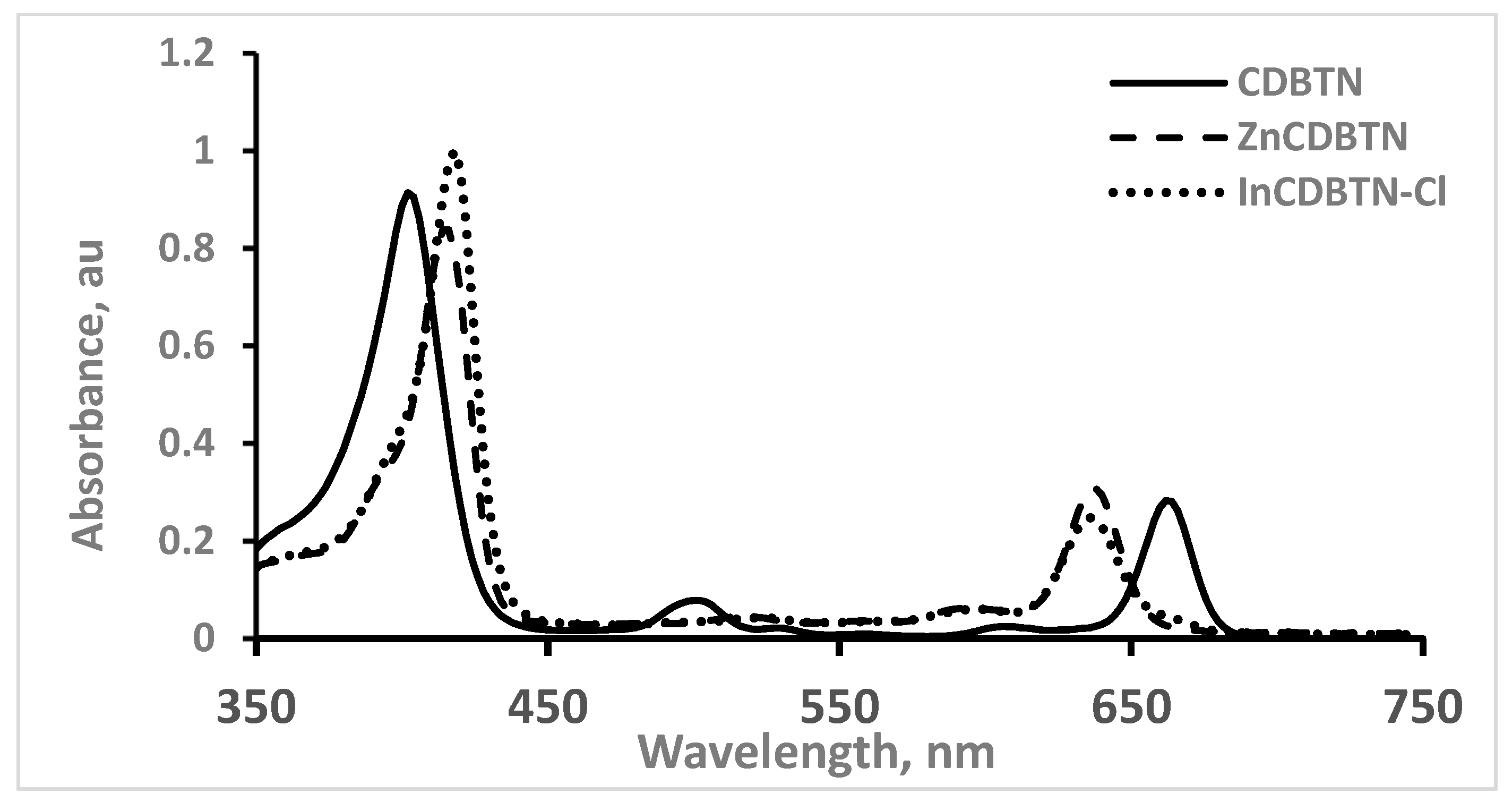
2.1. Chemical Synthesis of Photosensitizers
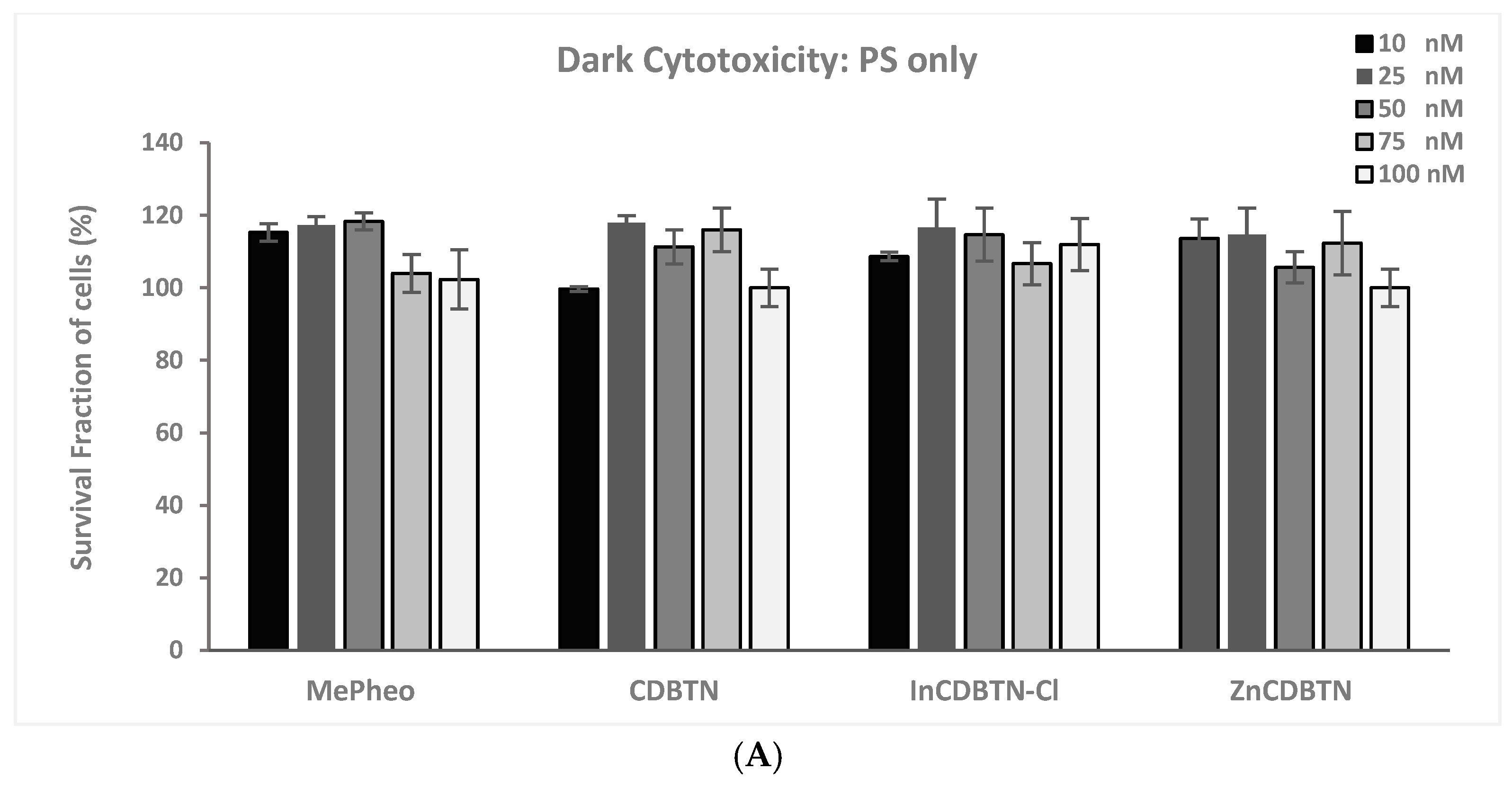
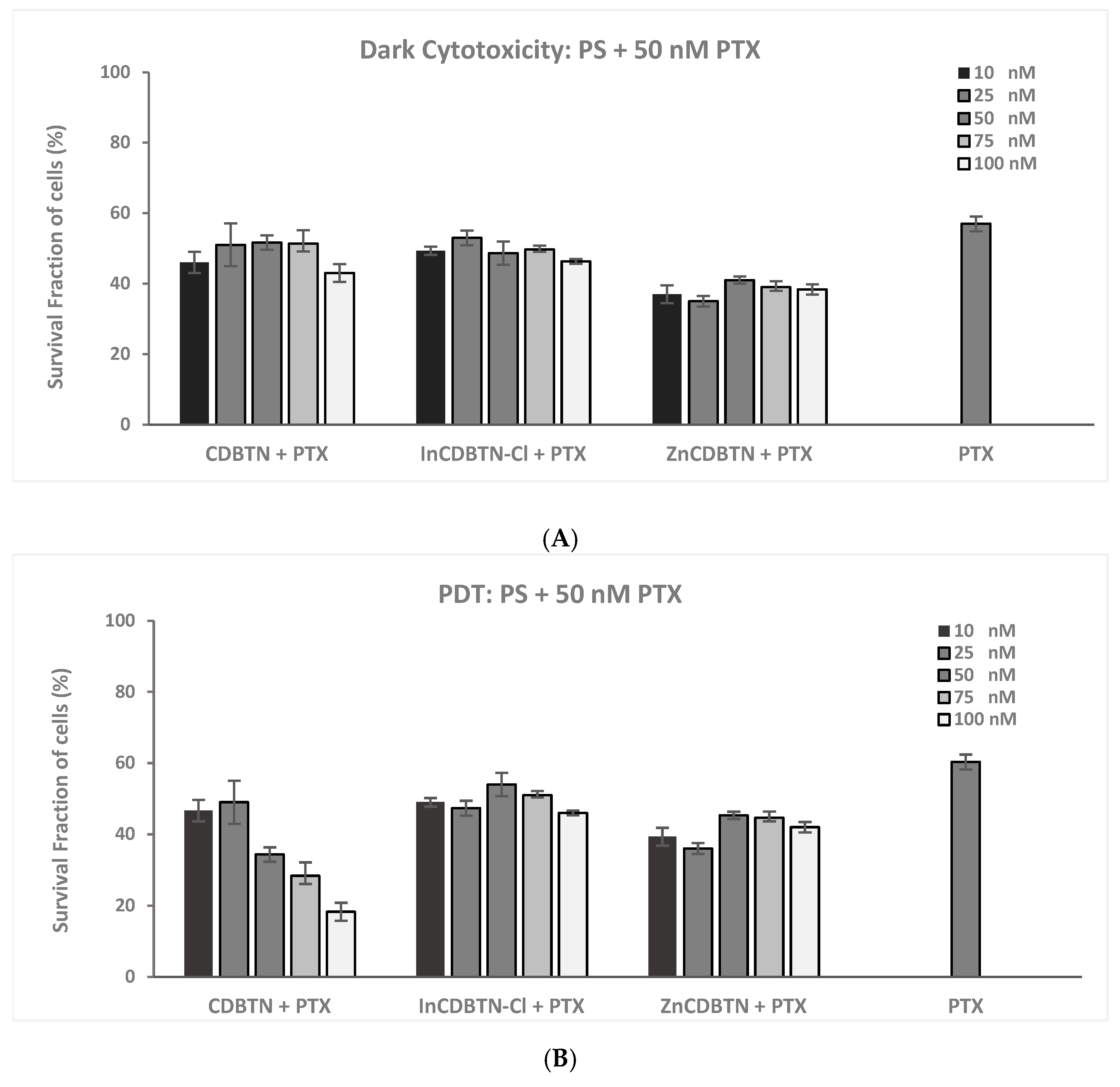
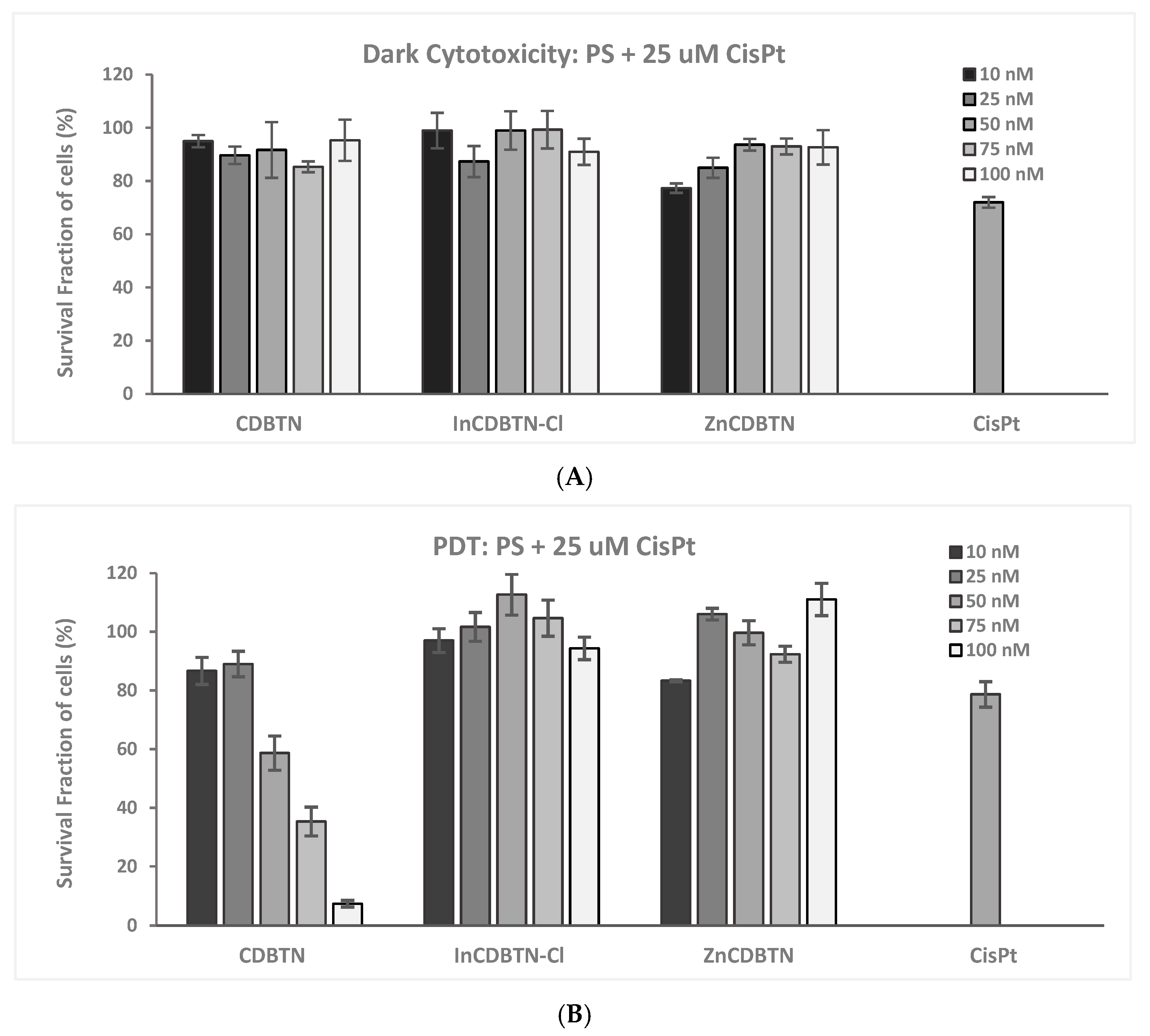
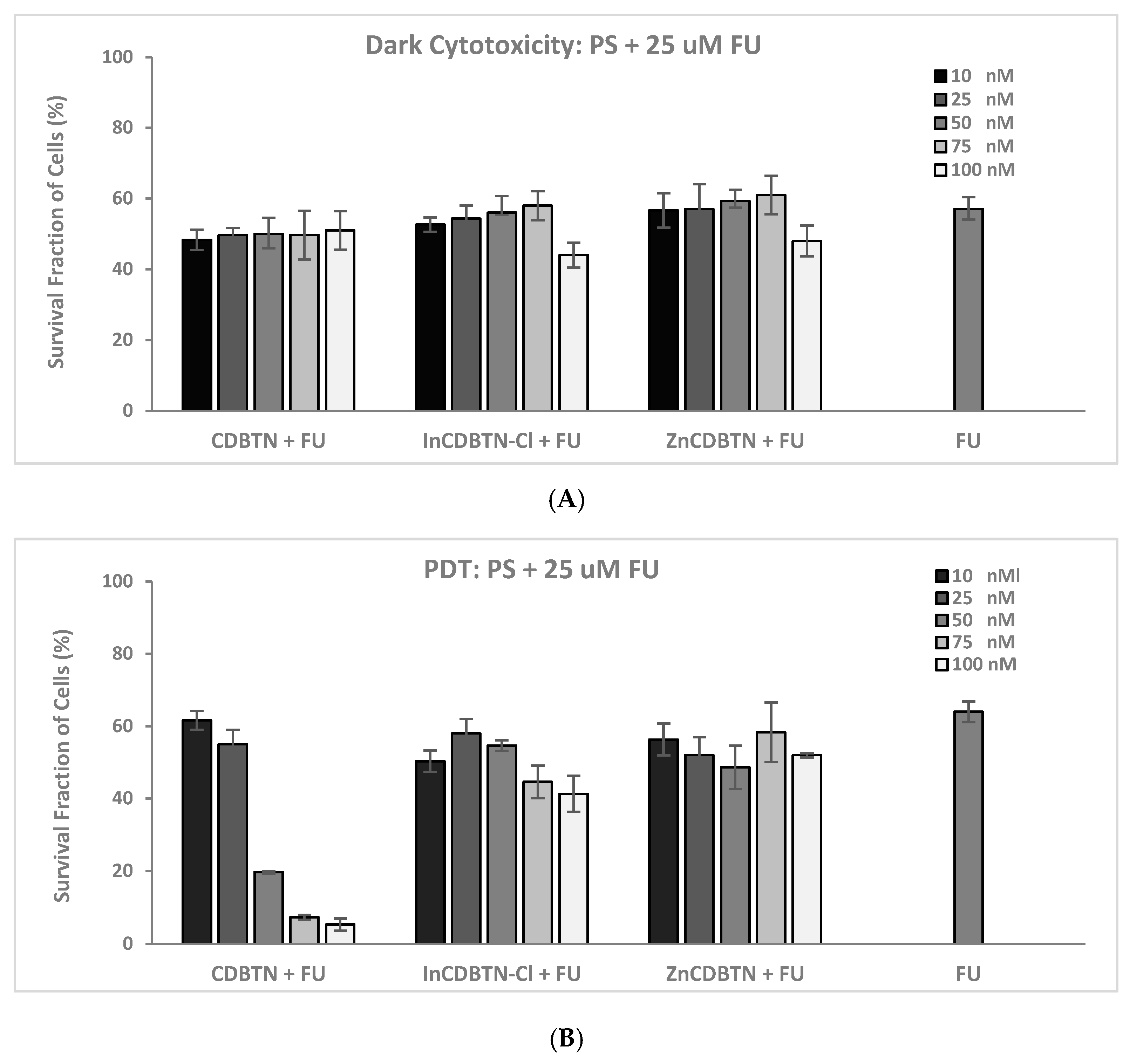
2.2. Binary Photodynamic Efficacy in Triple-Negative Breast Cancer Cells
2.3. Cell Viability Summary and Combination Index
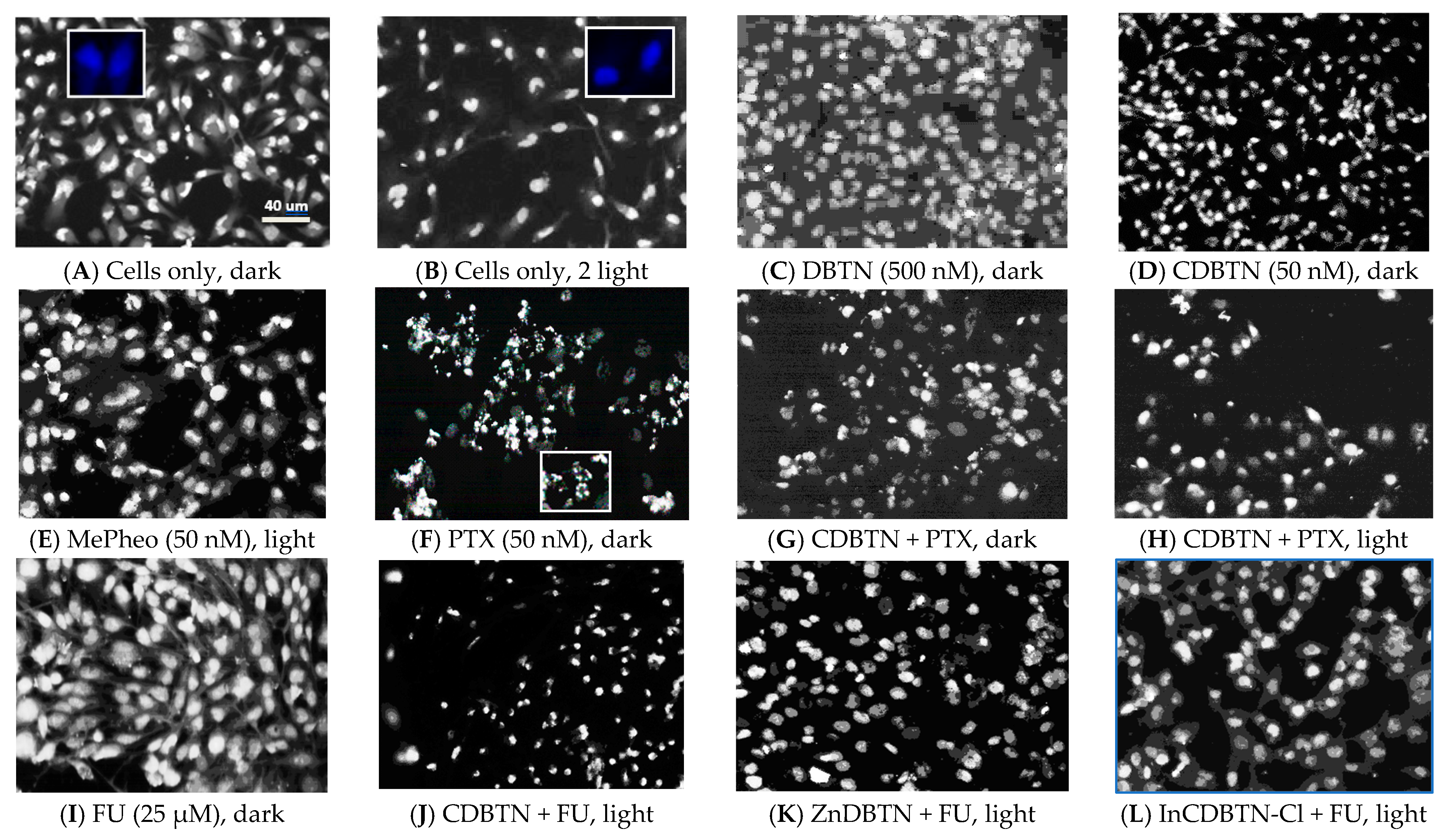
2.4. Fluorescence Microscopy
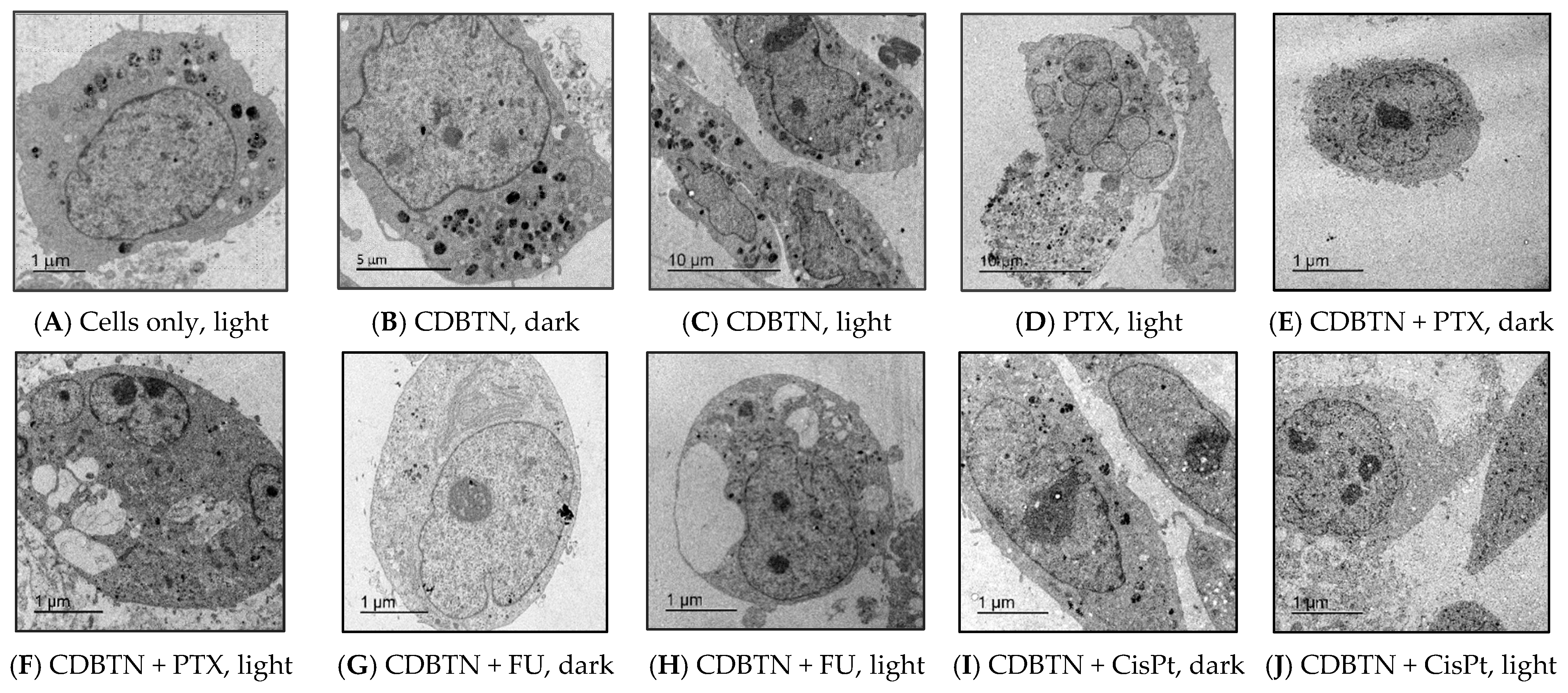
2.5. Transmission Electron Microscopy
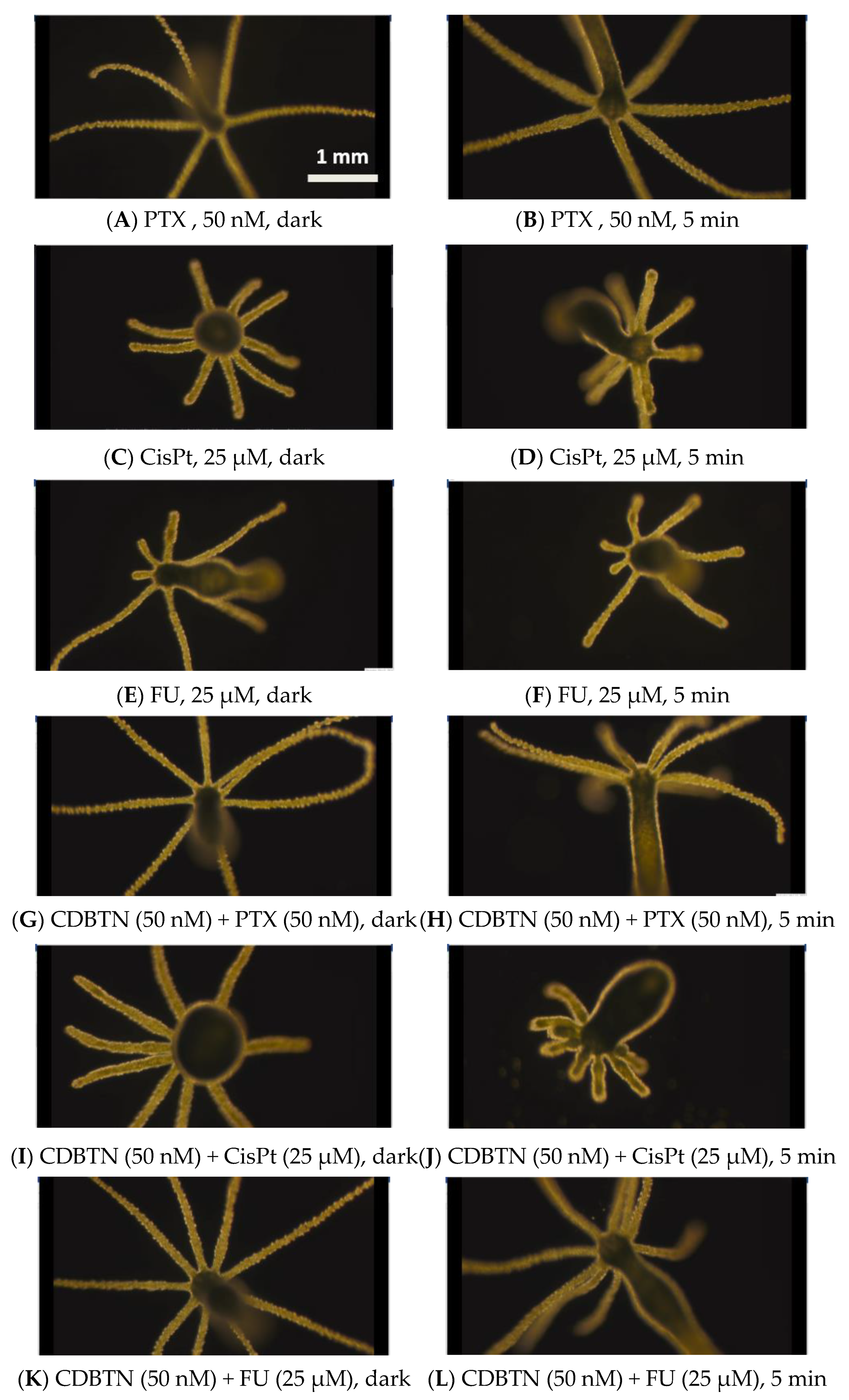
2.6. Hydra as a Model Organism for In Vivo Study
3. Discussion
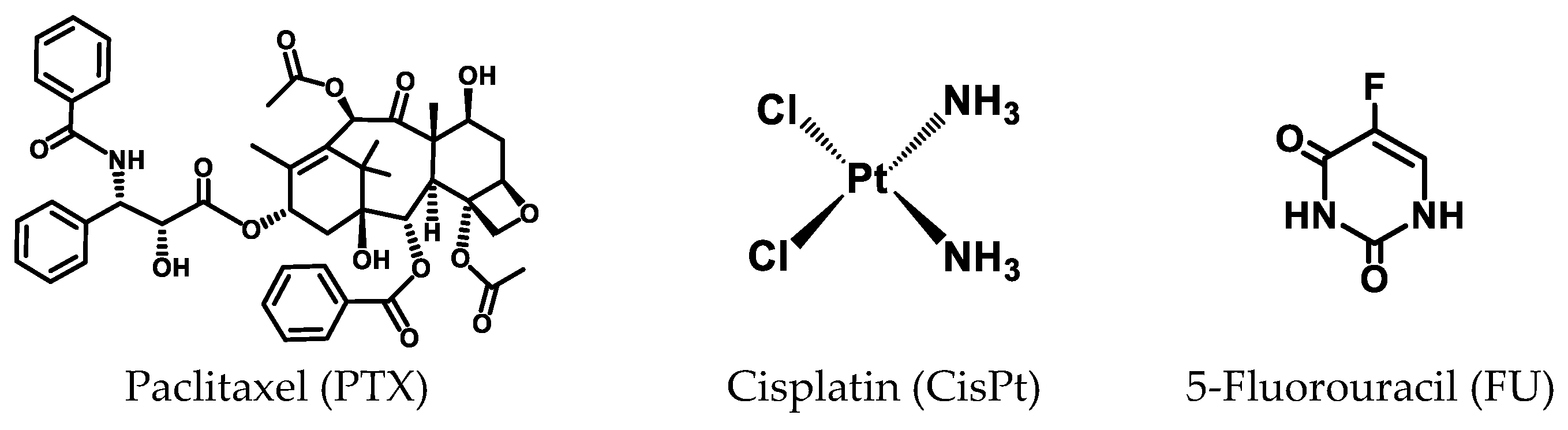
3.1. In Vitro Chemophotodynamic Treatment
3.1.1. PDT and PTX Combination
3.1.2. PDT and CisPt Combination
3.1.3. PDT and FU Combination
3.1.4. Cell Death Pathways in Photodynamic Chemotherapy
3.2. In Vivo Chemophotodynamic Treatment
4. Materials and Methods
4.1. Chemical Synthesis
4.2. In Vitro Cytotoxicity Assay
4.3. In Vivo Cytotoxicity Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, X.; Peng, J.; Meng, C.; Feng, F. Recent Advances for enhanced photodynamic therapy: From new mechanisms to innovative strategies. Chem. Sci. 2024, 15, 12234–12257. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Celli, J.P.; Broekgaarden, M.; Bulin, A.-L.; Uusimaa, P.; Pogue, B.; Hasan, T.; Huang, H.-C. Engineering photodynamics for treatment, priming and imaging. Nat. Rev. Bioeng. 2024, 2, 752–769. [Google Scholar] [CrossRef]
- Allamyradov, Y.; ben Yosef, J.; Annamuradov, B.; Ateyeh, M.; Street, C.; Whipple, H.; Er, A.O. Photodynamic Therapy Review: Past, Present, Future, Opportunities and Challenges. Photochem 2024, 4, 434–461. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef]
- Xiong, N.; Wu, H.; Yu, Z. Advancements and challenges in triple-negative breast cancer: A comprehensive review of therapeutic and diagnostic strategies. Front. Oncol. 2024, 14, 1405491. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Bekele, F.; Fekadu, G. Treatment Strategies Against Triple-Negative Breast Cancer: An Updated Review. Breast Cancer (Dove Med. Press) 2022, 14, 15–24. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Singh Chandel, A.K.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Kaur, S.; Mayanglambam, P.; Bajwan, D.; Thakur, N. Chemotherapy and its Adverse Effects—A Systematic Review. Int. J. Nursing Ed. Res. 2022, 10, 399–402. [Google Scholar] [CrossRef]
- Akhtar, F.; Misba, L.; Khan, A.U. The dual role of photodynamic therapy to treat cancer and microbial infection. Drug Discov. Today 2024, 29, 104099. [Google Scholar] [CrossRef]
- Isaac-Lam, M.F.; Mee, A.D. Photodynamic Activity of Chlorin-Vitamin Conjugates at the Nanomolar Concentrations Against Triple-Negative Breast Cancer Cells. ACS Omega 2019, 4, 2907–2920. [Google Scholar] [CrossRef]
- Bossert, P.; Galliot, B. How to use Hydra as a model system to teach biology in the classroom. Int. J. Dev. Biol. 2012, 56, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Murugadas, A.; Zeeshan, M.; Akbarsha, M.A. Futuristic Approach to Alternative Model Organisms: Hydra Stakes Its Claim. In Alternatives to Animal Testing; Kojima, H., Seidle, T., Spielmann, H., Eds.; Springer: Singapore, 2019; pp. 110–123. [Google Scholar] [CrossRef]
- Wenger, Y.; Galliot, B. Punctuated emergences of genetic and phenotypic innovations in eumetazoan, bilaterian, eutelostome, and hominidae ancestors. Gen. Biol. Evol. 2013, 5, 1949–1968. [Google Scholar] [CrossRef]
- Schenkelaars, Q.; Tomczyk, S.; Wenger, Y.; Ekundayo, K.; Girard, V.; Buzgariu, W.; Austad, S.; Galliot, B. Hydra, a Model System for Deciphering the Mechanisms of Aging and Resistance to Aging. In Conn’s Handbook of Models for Human Aging; Section III, Cellular Models and Invertebrates; Elsevier: Amsterdam, The Netherlands, 2018; Volume 38, pp. 507–520, Chapter 38. [Google Scholar] [CrossRef]
- Quinn, B.; Gagne, F.; Blaise, C. Hydra, a model system for environmental studies. Int. J. Dev. Biol. 2012, 56, 613–625. [Google Scholar] [CrossRef]
- Hua, Y.; Tian, X.; Zhang, X.; Song, G.; Liu, Y.; Zhao, Y.; Gao, Y.; Yin, F. Applications and challenges of photodynamic therapy in the treatment of skin malignancies. Front. Pharmacol. 2024, 15, 1476228. [Google Scholar] [CrossRef] [PubMed]
- Albericio, F.; El-Faham, A. Choosing the Right Coupling Reagent for Peptides: A Twenty-Five-Year Journey. Org. Proc. Res. Dev. 2018, 22, 760–772. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Biotin uptake by T47D breast cancer cells: Functional and molecular evidence of sodium-dependent multivitamin transporter (SMVT). Int. J. Pharm. 2013, 441, 535–543. [Google Scholar] [CrossRef]
- Vadlapudi, A.D.; Vadlapatla, R.K.; Pal, D.; Mitra, A.K. Functional and molecular aspects of biotin uptake via SMVT in human corneal epithelial (HCEC) and retinal pigment epithelial (D407) cells. AAPS J. 2012, 14, 832–842. [Google Scholar] [CrossRef]
- Russell-Jones, G.; McTavish, K.; McEwan, J.; Rice, J.; Nowotnik, D. Vitamin-mediated targeting as a potential mechanism to increase drug uptake by tumours. J. Inorg. Biochem. 2004, 98, 1625–1633. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Chen, J.; Chen, J.; Kuznetsova, L.; Wont, S.S.; Ojima, I. Mechanism-Based Tumor-Targeting Drug Delivery System. Validation of Efficient Vitamin Receptor-Mediated Endocytosis and Drug Release. Bioconj. Chem. 2010, 21, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Kornilova, A.Y.; Algayer, B.; Breslin, M.; Addona, G.H.; Uebele, V. Development of a fluorescence polarization binding assay for folate receptor. Anal. Biochem. 2013, 432, 59–62. [Google Scholar] [CrossRef]
- Rodal, G.H.; Rodal, S.K.; Moan, J.; Berg, K. Liposome-bound Zn(II)-phthalocyanine. Mechanisms for cellular uptake and photosensitization. J. Photochem. Photobiol. B Biol. 1998, 45, 150–159. [Google Scholar] [CrossRef]
- Nene, L.C.; Managa, M.; Nyokong, T. Photo-physicochemical properties and in vitro photodynamic therapy activity of morpholine-substituted Zinc(II)-Phthalocyanines π-π stacked on biotinylated graphene quantum dots. Dyes Pigments. 2019, 165, 488–498. [Google Scholar] [CrossRef]
- Luo, T.; Nash, G.T.; Xu, Z.; Jiang, X.; Liu, J.; Lin, W. Nanoscale Metal–Organic Framework Confines Zinc-Phthalocyanine Photosensitizers for Enhanced Photodynamic Therapy. J. Amer. Chem. Soc. 2021, 143, 13519–13524. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Amao, I.O.; Adeyemi, W.J.; Babalola, S.O.; Akinsuyi, O.S.; Ogunrombi, M.O.; Ogunlaja, A.S.; Mhlanga, S.D. Overview of Medical and Biological Applications of Indium(III) Complexes. Chem. A 2024, 7, 1729–1748. [Google Scholar] [CrossRef]
- Smith, C.B.; Days, L.C.; Alajroush, D.R.; Faye, K.; Khodour, Y.; Beebe, S.J.; Holder, A.A. Photodynamic Therapy of Inorganic Complexes for the Treatment of Cancer. Photochem. Photobiol. 2022, 98, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.R.; Inada, N.M.; Rettori, D.; Baratti, M.O.; Vercesi, A.E.; Jorge, R.A. In vitro photodynamic activity of chloro(5,10,15,20-tetraphenylporphyrinato)indium(III) loaded-poly(lactide-co-glycolide) nanoparticles in LNCaP prostate tumour cells. J. Photochem. Photobiol. B Biol. 2009, 94, 101–112. [Google Scholar] [CrossRef]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Huang, S.; Li, A.; Zhang, Y.; Xue, Y.; Song, X.; Zhang, Y.; Hong, S.; Gao, H.; et al. Reusable and near-infrared light-activated Zinc(II) metalated porphyrin with synergetic PDT/PTT for eradicating bacterial pneumonia. Chem. Eng. J. 2023, 477, 146937. [Google Scholar] [CrossRef]
- Rychikhina, E.; Ivanova, S.S.; Romanenko, Y.V.; Koifman, O.I.; Stuzhin, P.A. Indium complexes of chlorin e6 trimethyl ester and methylpyropheophorbide a: Synthesis and photophysical characterization. Polyhedron 2022, 217, 115743. [Google Scholar] [CrossRef]
- Isaac-Lam, M.F. Chlorin Conjugates in Photodynamic Chemotherapy for Triple-Negative Breast Cancer. Pharmaceuticals 2024, 17, 576. [Google Scholar] [CrossRef]
- Nakai, M.; Maeda, Y.; Mashima, T.; Yano, S.; Sakuma, S.; Otake, E.; Morita, A.; Nakabayashi, Y. Syntheses and photodynamic properties of glucopyranoside-conjugated indium(III) porphyrins as a bifunctional agent. J. Porph. Phthal. 2013, 17, 1173–1182. [Google Scholar] [CrossRef]
- CompuSyn Software, Version 1.0; CompuSyn: Palo Alto, CA, USA, 2014. Available online: https://www.combosyn.com/ (accessed on 8 January 2025).
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Escobar, M.L.; Echeverría, O.M.; Vázquez-Nin, G.H. Necrosis as Programmed Cell Death. In Cell Death—Autophagy, Apoptosis and Necrosis; Ntuli, T.M., Ed.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Liu, Z.G.; Jiao, D. Necroptosis, tumor necrosis and tumorigenesis. Cell Stress 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Godwin, W.C.; Hoffmann, G.F.; Gray, T.J.; Hughes, R.M. Imaging of morphological and biochemical hallmarks of apoptosis with optimized optogenetic tools. J. Biol. Chem. 2019, 294, 16918–16929. [Google Scholar] [CrossRef]
- Khing, T.M.; Choi, W.S.; Kim, D.M.; Po, W.W.; Thein, W.; Shin, C.Y.; Sohn, U.D. The effect of paclitaxel on apoptosis, autophagy and mitotic catastrophe in AGS cells. Sci. Rep. 2021, 11, 23490. [Google Scholar] [CrossRef]
- Chang, E.; Bu, J.; Ding, L.; Lou, J.W.H.; Valic, M.S.; Cheng, M.H.Y.; Rosilio, V.; Chen, J.; Zheng, G. Porphyrin-lipid stabilized paclitaxel nanoemulsion for combined photodynamic therapy and chemotherapy. J. Nanobiotechnol. 2021, 19, 154. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zhou, W.; Yu, R.; Rosenholm, J.M.; Tian, W.; Zhang, L.; Wang, D.; Zhang, H. Co-Delivery of Paclitaxel Prodrug, Gemcitabine and Porphine by Micelles for Pancreatic Cancer Treatment via Chemo-Photodynamic Combination Therapy. Pharmaceutics 2022, 14, 2280. [Google Scholar] [CrossRef] [PubMed]
- Effendi, W.I.; Nagano, T.; Tachihara, M.; Umezawa, K.; Kiriu, T.; Dokuni, R.; Katsurada, M.; Yamamoto, M.; Kobayashi, K.; Nishimura, Y. Synergistic interaction of gemcitabine and paclitaxel by modulating acetylation and polymerization of tubulin in non-small cell lung cancer cell lines. Cancer Manag. Res. 2019, 11, 3669–3679. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef]
- Mackeh, R.; Lorin, S.; Ratier, A.; Mejdoubi-Charef, N.; Baillet, A.; Bruneel, A.; Hamaï, A.; Codogno, P.; Poüs, C.; Perdiz, D. Reactive oxygen species, AMP-activated protein kinase, and the transcription cofactor p300 regulate α-tubulin acetyltransferase-1 (αTAT-1/MEC-17)-dependent microtubule hyperacetylation during cell stress. J. Biol Chem. 2014, 289, 11816–11828. [Google Scholar] [CrossRef]
- Erk, B.; Kamanli, A.F.; Guney Eskiler, G. The therapeutic efficacy of 5-ALA based photodynamic therapy and chemotherapy combination in triple negative breast cancer cells. Lasers Med. Sci. 2024, 39, 191. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rollakanti, K.R.; Brankov, N.; Brash, D.E.; Hasan, T.; Maytin, E.V. Fluorouracil Enhances Photodynamic Therapy of Squamous Cell Carcinoma via a p53-Independent Mechanism that Increases Protoporphyrin IX levels and Tumor Cell Death. Mol. Cancer Ther. 2017, 16, 1092–1101. [Google Scholar] [CrossRef]
- Anand, S.; Cheng, C.-E.; Shen, A.; Maytin, E.V. Combination of 5-fluorouracil with photodynamic therapy enhances immune responses in a murine model of actinic keratosis. Photodiagn. Photodyn. Ther. 2024, 46, 104124. [Google Scholar] [CrossRef]
- Tanaka, F.; Fukuse, T.; Wada, H.; Fukushima, M. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr. Pharm. Biotechnol. 2000, 1, 137–164. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ofengeim, D. A guide to cell death pathways. Nat. Rev. Mol. Cell Biol. 2024, 25, 379–395. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.V.; Vedunova, M.V.; Krysko, D.V. Targeting immunogenic cancer cell death by photodynamic therapy: Past, present and future. J. ImmunoTher. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef] [PubMed]
- Hajibabaie, F.; Abedpoor, N.; Mohamadynejad, P. Types of Cell Death from a Molecular Perspective. Biology 2023, 12, 1426. [Google Scholar] [CrossRef]
- Patergnani, S.; Missiroli, S.; Morciano, G.; Perrone, M.; Mantovani, C.M.; Anania, G.; Fiorica, F.; Pinton, P.; Giorgi, C. Understanding the Role of Autophagy in Cancer Formation and Progression Is a Real Opportunity to Treat and Cure Human Cancers. Cancers 2021, 13, 5622. [Google Scholar] [CrossRef]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Toso, R.J.; Thrower, D.; Wilson, L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc. Natl. Acad. Sci. USA 1993, 90, 9552–9556. [Google Scholar] [CrossRef] [PubMed]
- Cenklová, V. Photodynamic therapy with TMPyP—Porphyrine induces mitotic catastrophe and microtubule disorganization in HeLa and G361 cells, a comprehensive view of the action of the photosensitizer. J. Photochem. Photobiol. B Biol. 2017, 173, 522–537. [Google Scholar] [CrossRef]
- Kessel, D. Paraptosis and Photodynamic Therapy: A Progress Report. Photochem Photobiol. 2020, 96, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy. Photochem. Photobiol. 2019, 95, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Van der Meeren, L.; Verduijn, J.; Krysko, D.V.; Skirtach, A.G. AFM analysis enables differentiation between apoptosis, necroptosis, and ferroptosis in murine cancer cells. Iscience 2020, 23, 101816. [Google Scholar] [CrossRef]
- Sprooten, J.; De Wijngaert, P.; Vanmeerbeerk, I.; Martin, S.; Vangheluwe, P.; Schlenner, S.; Krysko, D.V.; Parys, J.B.; Bultynck, G.; Vandenabeele, P.; et al. Necroptosis in Immuno-Oncology and Cancer Immunotherapy. Cells 2020, 9, 1823. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Nagy, L.E.; Gautheron, J. Mediators of necroptosis: From cell death to metabolic regulation. EMBO Mol. Med. 2024, 16, 219–237. [Google Scholar] [CrossRef]
- Boutry, J.; Buysse, M.; Tissot, S.; Cazevielle, C.; Hamede, R.; Dujon, A.M.; Ujvari, B.; Giradeau, M.; Klimovich, A.; Thomas, F.; et al. Spontaneously occurring tumors in different wild-derived strains of hydra. Sci. Rep. 2023, 13, 7449. [Google Scholar] [CrossRef] [PubMed]
- Rathje, K.; Mortzfeld, B.; Hoeppner, M.P.; Taubenheim, J.; Bosch, T.C.G.; Klimovich, A. Dynamic interactions within the host-associated microbiota cause tumor formation in the basal metazoan Hydra. PLoS Pathog. 2020, 16, e1008375. [Google Scholar] [CrossRef] [PubMed]


| [CDBTN] Concentration | CDBTN Only | PTX 50 nM | CisPt 25 μM | FU 25 μM |
|---|---|---|---|---|
| 50 nM | 34% | 66% | 41% | 80% |
| 75 nM | 59% | 72% | 65% | 93% |
| 100 nM | 87% | 81% | 93% | 95% |
| CDBTN Concentration | Taxol 50 nM | CisPt 25 μM | FU 25 μM |
|---|---|---|---|
| 50 nM | 0.70 (S) | 1.37 (MA) | 0.55 (S) |
| 75 nM | 0.95 (NA) | 1.20 (SA) | 0.59 (S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutkowski, B.N.; Isaac-Lam, M.F. Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo. Int. J. Mol. Sci. 2025, 26, 5357. https://doi.org/10.3390/ijms26115357
Rutkowski BN, Isaac-Lam MF. Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo. International Journal of Molecular Sciences. 2025; 26(11):5357. https://doi.org/10.3390/ijms26115357
Chicago/Turabian StyleRutkowski, Bailey N., and Meden F. Isaac-Lam. 2025. "Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo" International Journal of Molecular Sciences 26, no. 11: 5357. https://doi.org/10.3390/ijms26115357
APA StyleRutkowski, B. N., & Isaac-Lam, M. F. (2025). Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo. International Journal of Molecular Sciences, 26(11), 5357. https://doi.org/10.3390/ijms26115357





