Pathophysiological Roles of the CX3CL1-CX3CR1 Axis in Renal Disease, Cardiovascular Disease, and Cancer
Abstract
1. Introduction
2. Renal Diseases
2.1. Renal Injury and Inflammation
2.2. Glomerulonephritis
2.3. Chronic Kidney Disease
2.4. Renal Allograft Rejection
2.5. Renal Infection Diseases
2.6. Diabetic Nephropathy
3. Cardiovascular Disease
3.1. Atherosclerosis
3.2. Coronary Artery Disease
4. Cancers
4.1. Prostate Cancer
4.2. Renal Cell Carcinoma
4.3. Lung Cancer
4.4. Colorectal Cancer
4.5. Hematological Cancer
5. Future Research
Author Contributions
Funding
Conflicts of Interest
References
- Julia, V.; Staumont-Salle, D.; Dombrowicz, D. Role of Fractalkine/CX3CL1 and Its Receptor CX3CR1 in Allergic Diseases. Med. Sci. 2016, 32, 260–266. [Google Scholar]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A New Class of Membrane-Bound Chemokine with a Cx3c Motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E. Chemokines and Their Receptors in Rheumatoid Arthritis: Future Targets? Arthritis Rheum. 2005, 52, 710–721. [Google Scholar] [CrossRef]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for Neuronally Derived Fractalkine in Mediating Interactions between Neurons and CX3CR1-Expressing Microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef]
- Umehara, H.; Bloom, E.T.; Okazaki, T.; Nagano, Y.; Yoshie, O.; Imai, T. Fractalkine in Vascular Biology: From Basic Research to Clinical Disease. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.R.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-Alpha-Converting Enzyme (Adam17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1). J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A Potential New Target for Inflammatory Diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The Disintegrin-like Metalloproteinase Adam10 Is Involved in Constitutive Cleavage of CX3CL1 (Fractalkine) and Regulates CX3CL1-Mediated Cell-Cell Adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef]
- Seegar, T.C.M.; Killingsworth, L.B.; Saha, N.; Meyer, P.A.; Patra, D.; Zimmerman, B.; Janes, P.W.; Rubinstein, E.; Nikolov, D.B.; Skiniotis, G.; et al. Structural Basis for Regulated Proteolysis by the A-Secretase Adam10. Cell 2017, 171, 1638–1648.E7. [Google Scholar] [CrossRef]
- Ley, K. Molecular Mechanisms of Leukocyte Recruitment in the Inflammatory Process. Cardiovasc. Res. 1996, 32, 733–742. [Google Scholar] [CrossRef]
- Christia, P.; Frangogiannis, N.G. Targeting Inflammatory Pathways in Myocardial Infarction. Eur. J. Clin. Investig. 2013, 43, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Umehara, H.; Bloom, E.; Okazaki, T.; Domae, N.; Imai, T. Fractalkine and Vascular Injury. Trends Immunol. 2001, 22, 602–607. [Google Scholar] [CrossRef]
- Fong, A.M.; Robinson, L.A.; Steeber, D.A.; Tedder, T.F.; Yoshie, O.; Imai, T.; Patel, D.D. Fractalkine and CX3CR1 Mediate a Novel Mechanism of Leukocyte Capture, Firm Adhesion, and Activation under Physiologic Flow. J. Exp. Med. 1998, 188, 1413–1419. [Google Scholar] [CrossRef]
- Luscinskas, F.W.; Gimbrone, M.A., Jr. Endothelial-Dependent Mechanisms in Chronic Inflammatory Leukocyte Recruitment. Annu. Rev. Med. 1996, 47, 413–421. [Google Scholar] [CrossRef]
- Skoda, M.; Stangret, A.; Szukiewicz, D. Fractalkine and Placental Growth Factor: A Duet of Inflammation and Angiogenesis in Cardiovascular Disorders. Cytokine Growth Factor Rev. 2018, 39, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Haskell, C.A.; Cleary, M.D.; Charo, I.F. Molecular Uncoupling of Fractalkine-Mediated Cell Adhesion and Signal Transduction. Rapid Flow Arrest of CX3CR1-Expressing Cells Is Independent of G-Protein Activation. J. Biol. Chem. 1999, 274, 10053–10058. [Google Scholar] [CrossRef]
- Rennert, K.; Heisig, K.; Groeger, M.; Wallert, M.; Funke, H.; Lorkowski, S.; Huber, O.; Mosig, A.S. Mosig. Recruitment of Cd16+ Monocytes to Endothelial Cells in Response to Lps-Treatment and Concomitant Tnf Release Is Regulated by CX3CR1 and Interfered by Soluble Fractalkine. Cytokine 2016, 83, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Cheng, K.; Ming, Y. CX3CL1/CX3CR1 Axis, as the Therapeutic Potential in Renal Diseases: Friend or Foe? Curr. Gene Ther. 2017, 17, 442–452. [Google Scholar] [CrossRef]
- D’Haese, J.G.; Friess, H.; Ceyhan, G.O. Therapeutic Potential of the Chemokine-Receptor Duo Fractalkine/CX3CR1: An Update. Expert Opin. Ther. Targets 2012, 16, 613–618. [Google Scholar] [CrossRef]
- Muraoka, S.; Nishio, J.; Kuboi, Y.; Imai, T.; Nanki, T. Rationale for and Clinical Development of Anti-Fractalkine Antibody in Rheumatic Diseases. Expert Opin. Biol. Ther. 2020, 20, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Cormican, S.; Griffin, M.D. Fractalkine (CX3CL1) and Its Receptor CX3CR1: A Promising Therapeutic Target in Chronic Kidney Disease? Front. Immunol. 2021, 12, 664202. [Google Scholar] [CrossRef]
- D’Haese, J.G.; Demir, I.E.; Friess, H.; Ceyhan, G.O. Fractalkine/CX3CR1: Why a Single Chemokine-Receptor Duo Bears a Major and Unique Therapeutic Potential. Expert Opin. Ther. Targets 2010, 14, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Chocarro, L.; Echaide, M.; Ausin, K.; Escors, D.; Kochan, G. Fractalkine in Health and Disease. Int. J. Mol. Sci. 2024, 25, 8007. [Google Scholar] [CrossRef]
- Qin, R.; Ren, W.; Ya, G.; Wang, B.; He, J.; Ren, S.; Jiang, L.; Zhao, S. Role of Chemokines in the Crosstalk between Tumor and Tumor-Associated Macrophages. Clin. Exp. Med. 2023, 23, 1359–1373. [Google Scholar] [CrossRef]
- Zheng, L.; Gao, W.; Hu, C.; Yang, C.; Rong, R. Immune Cells in Ischemic Acute Kidney Injury. Curr. Protein Pept. Sci. 2019, 20, 770–776. [Google Scholar] [CrossRef] [PubMed]
- von Vietinghoff, S.; Kurts, C. Regulation and Function of CX3CR1 and Its Ligand CX3CL1 in Kidney Disease. Cell Tissue Res. 2021, 385, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Lin, S.L.; Chen, C.W.; Chiang, W.C.; Tsai, T.J.; Hsieh, B.S. Tumor Necrosis Factor-Alpha Stimulates Fractalkine Production by Mesangial Cells and Regulates Monocyte Transmigration: Down-Regulation by Camp. Kidney Int. 2003, 63, 474–486. [Google Scholar] [CrossRef]
- Wyatt, R.J.; Julian, B.A. IgA nephropathy. N. Engl. J. Med. 2013, 368, 2402–2414. [Google Scholar] [CrossRef]
- Luo, R.; Guo, S.M.; Li, Y.Q.; Yang, Y.; Li, M.L.; Han, M.; He, X.F.; Ge, S.W.; Xu, G. Plasma Fractalkine Levels Are Associated with Renal Inflammation and Outcomes in Immunoglobulin a Nephropathy. Nephrol. Dial. Transplant. 2019, 34, 1549–1558. [Google Scholar] [CrossRef]
- Cox, S.N.; Sallustio, F.; Serino, G.; Loverre, A.; Pesce, F.; Gigante, M.; Zaza, G.; Stifanelli, P.F.; Ancona, N.; Schena, F.P. Activated Innate Immunity and the Involvement of CX3CR1-Fractalkine in Promoting Hematuria in Patients with Iga Nephropathy. Kidney Int. 2012, 82, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Park, J.; Boo, H.J.; Yang, K.E.; Lee, C.J.; Lee, J.E.; Kim, K.; Kwon, G.Y.; Huh, W.; Kim, D.J.; et al. Clinical Value of Urinary Cytokines/Chemokines as Prognostic Markers in Patients with Crescentic Glomerulonephritis. Sci. Rep. 2022, 12, 10221. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Liao, Z.; Luo, L.; Deng, S.; Jiang, Y.; Wang, F.; Hu, X.; Yin, H.; Gong, G.; Feng, J.; et al. CX3CL1-Induced Cd16+ Monocytes Extravasation in Myeloperoxidase-Anca-Associated Vasculitis Correlates with Renal Damage. Front. Immunol. 2022, 13, 929244. [Google Scholar] [CrossRef]
- Cockwell, P.; Chakravorty, S.J.; Girdlestone, J.; Savage, C.O. Fractalkine Expression in Human Renal Inflammation. J. Pathol. 2002, 196, 85–90. [Google Scholar] [CrossRef]
- Andrews, B.S.; Eisenberg, R.A.; Theofilopoulos, A.N.; Izui, S.; Wilson, C.B.; McConahey, P.J.; Murphy, E.D.; Roths, J.B.; Dixon, F.J. Spontaneous Murine Lupus-Like Syndromes. Clinical and Immunopathological Manifestations in Several Strains. J. Exp. Med. 1978, 148, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Alajoleen, R.M.; Oakland, D.N.; Estaleen, R.; Shakeri, A.; Lu, R.; Appiah, M.; Sun, S.; Neumann, J.; Kawauchi, S.; Cecere, T.E.; et al. Tlr5 Deficiency Exacerbates Lupus-Like Disease in the Mrl/Lpr Mouse Model. Front. Immunol. 2024, 15, 1359534. [Google Scholar] [CrossRef]
- Fu, D.; Ma, J.; Gong, Q.; Senouthai, S.; Wang, J.; You, Y.; Pinhu, L. Fractalkine Mediates Lymphocyte Inflammation and Tubulointerstitial Lesions by Modifying the Treg/Th17 Balance in Lupus-Prone Mrl/Lpr Mice. Am. J. Transl. Res. 2020, 12, 6170–6186. [Google Scholar]
- Qing, X.; Zavadil, J.; Crosby, M.B.; Hogarth, M.P.; Hahn, B.H.; Mohan, C.; Gilkeson, G.S.; Bottinger, E.P.; Putterman, C. Nephritogenic Anti-DNA Antibodies Regulate Gene Expression in Mrl/Lpr Mouse Glomerular Mesangial Cells. Arthritis Rheum. 2006, 54, 2198–2210. [Google Scholar] [CrossRef]
- Nakatani, K.; Yoshimoto, S.; Iwano, M.; Asai, O.; Samejima, K.; Sakan, H.; Terada, M.; Hasegawa, H.; Nose, M.; Saito, Y. Fractalkine Expression and Cd16+ Monocyte Accumulation in Glomerular Lesions: Association with Their Severity and Diversity in Lupus Models. Am. J. Physiol. Renal Physiol. 2010, 299, F207–F216. [Google Scholar] [CrossRef]
- Cabana-Puig, X.; Lu, R.; Geng, S.; Michaelis, J.S.; Oakes, V.; Armstrong, C.; Testerman, J.C.; Liao, X.; Alajoleen, R.; Appiah, M.; et al. Cx(3)Cr1 Modulates Sle-Associated Glomerulonephritis and Cardiovascular Disease in Mrl/Lpr Mice. Inflamm. Res. 2023, 72, 1083–1097. [Google Scholar] [CrossRef]
- Schneider, K.M.; Bieghs, V.; Heymann, F.; Hu, W.; Dreymueller, D.; Liao, L.; Frissen, M.; Ludwig, A.; Gassler, N.; Pabst, O.; et al. CX3CR1 Is a Gatekeeper for Intestinal Barrier Integrity in Mice: Limiting Steatohepatitis by Maintaining Intestinal Homeostasis. Hepatology 2015, 62, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Cormican, S.; Negi, N.; Naicker, S.D.; Islam, M.N.; Fazekas, B.; Power, R.; Griffin, T.P.; Dennedy, M.C.; MacNeill, B.; Malone, A.F.; et al. Chronic Kidney Disease Is Characterized by Expansion of a Distinct Proinflammatory Intermediate Monocyte Subtype and by Increased Monocyte Adhesion to Endothelial Cells. J. Am. Soc. Nephrol. 2023, 34, 793–808. [Google Scholar] [CrossRef]
- Corken, A.; Ware, J.; Dai, J.; Arthur, J.M.; Smyth, S.; Davis, C.L.; Liu, J.; Harville, T.O.; Phadnis, M.A.; Mehta, J.L.; et al. Platelet-Dependent Inflammatory Dysregulation in Patients with Stages 4 or 5 Chronic Kidney Disease: A Mechanistic Clinical Study. Kidney360 2022, 3, 2036–2047. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Tgf-Β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Taduri, G.; O’Connell, J.; Teng, Y.; Cooke, V.G.; Woda, C.; Sugimoto, H.; Kalluri, R. Origin and Function of Myofibroblasts in Kidney Fibrosis. Nat. Med. 2013, 19, 1047–1053. [Google Scholar] [CrossRef]
- Wang, S.; Meng, X.M.; Ng, Y.Y.; Ma, F.Y.; Zhou, S.; Zhang, Y.; Yang, C.; Huang, X.R.; Xiao, J.; Wang, Y.Y.; et al. Tgf-Β/Smad3 Signalling Regulates the Transition of Bone Marrow-Derived Macrophages into Myofibroblasts During Tissue Fibrosis. Oncotarget 2016, 7, 8809–8822. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.J.; Schmid, H.; Cohen, C.D.; Blaschke, S.; Hemmerlein, B.; Zapf, A.; Müller, G.A.; Strutz, F. Potential Role of Fractalkine Receptor Expression in Human Renal Fibrogenesis. Kidney Int. 2007, 72, 599–607. [Google Scholar] [CrossRef]
- Kassianos, A.J.; Wang, X.; Sampangi, S.; Muczynski, K.; Healy, H.; Wilkinson, R. Increased Tubulointerstitial Recruitment of Human Cd141hi Clec9a+ and Cd1c+ Myeloid Dendritic Cell Subsets in Renal Fibrosis and Chronic Kidney Disease. Am. J. Physiol. Renal Physiol. 2013, 305, F1391–F1401. [Google Scholar] [CrossRef]
- Kassianos, A.J.; Wang, X.; Sampangi, S.; Afrin, S.; Wilkinson, R.; Healy, H. Fractalkine-CX3CR1-Dependent Recruitment and Retention of Human Cd1c+ Myeloid Dendritic Cells by In Vitro-Activated Proximal Tubular Epithelial Cells. Kidney Int. 2015, 87, 1153–1163. [Google Scholar] [CrossRef]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/Fractalkine Expression in Endothelial Cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef]
- Furuichi, K.; Gao, J.L.; Murphy, P.M. Chemokine Receptor CX3CR1 Regulates Renal Interstitial Fibrosis after Ischemia-Reperfusion Injury. Am. J. Pathol. 2006, 169, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Łyszkiewicz, M.; Witzlau, K.; Pommerencke, J.; Krueger, A. Chemokine Receptor CX3CR1 Promotes Dendritic Cell Development under Steady-State Conditions. Eur. J. Immunol. 2011, 41, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhang, J.; Xiao, Z.; Dong, Y.; Du, J. CX3CL1-CX3CR1 Interaction Increases the Population of Ly6c(-)CX3CR1(Hi) Macrophages Contributing to Unilateral Ureteral Obstruction-Induced Fibrosis. J. Immunol. 2015, 195, 2797–2805. [Google Scholar] [CrossRef]
- Engel, D.R.; Krause, T.A.; Snelgrove, S.L.; Thiebes, S.; Hickey, M.J.; Boor, P.; Kitching, A.R.; Kurts, C. CX3CR1 Reduces Kidney Fibrosis by Inhibiting Local Proliferation of Profibrotic Macrophages. J. Immunol. 2015, 194, 1628–1638. [Google Scholar] [CrossRef]
- Jang, H.R.; Kim, M.; Hong, S.; Lee, K.; Park, M.Y.; Yang, K.E.; Lee, C.J.; Jeon, J.; Lee, K.W.; Lee, J.E.; et al. Early Postoperative Urinary Mcp-1 as a Potential Biomarker Predicting Acute Rejection in Living Donor Kidney Transplantation: A Prospective Cohort Study. Sci. Rep. 2021, 11, 18832. [Google Scholar] [CrossRef]
- Xu, C.X.; Shi, B.Y.; Jin, Z.K.; Hao, J.J.; Duan, W.L.; Han, F.; Zhao, Y.L.; Ding, C.G.; Xue, W.J.; Ding, X.M.; et al. Multiple-Biomarkers Provide Powerful Prediction of Early Acute Renal Allograft Rejection by Combination of Serum Fractalkine, Ifn-Γ and Ip-10. Transpl. Immunol. 2018, 50, 68–74. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.F.; Su, Z.X.; Shi, L.P.; Chen, Y.H. Serum Fractalkine and Interferon-Gamma Inducible Protein-10 Concentrations Are Early Detection Markers for Acute Renal Allograft Rejection. Transplant. Proc. 2014, 46, 1420–1425. [Google Scholar] [CrossRef]
- Chakravorty, S.J.; Cockwell, P.; Girdlestone, J.; Brooks, C.J.; Savage, C.O. Fractalkine Expression on Human Renal Tubular Epithelial Cells: Potential Role in Mononuclear Cell Adhesion. Clin. Exp. Immunol. 2002, 129, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Bergler, T.; Segerer, S.; Rümmele, P.; Krüger, B.; Banas, M.C.; Reinhold, S.; Banas, B.; Krämer, B.K. Impact of Chemokine Receptor CX3CR1 in Human Renal Allograft Rejection. Transpl. Immunol. 2010, 23, 204–208. [Google Scholar] [CrossRef]
- Krupickova, L.; Fialova, M.; Novotny, M.; Svachova, V.; Mezerova, K.; Cecrdlova, E.; Viklicky, O.; Striz, I. Chemokine Profiles Are Affected in Serum of Patients with Acute Rejection of Kidney Allograft. Mediat. Inflamm. 2021, 2021, 5513690. [Google Scholar] [CrossRef]
- Robinson, L.A.; Nataraj, C.; Thomas, D.W.; Howell, D.N.; Griffiths, R.; Bautch, V.; Patel, D.D.; Feng, L.; Coffman, T.M. A Role for Fractalkine and Its Receptor (CX3CR1) in Cardiac Allograft Rejection. J. Immunol. 2000, 165, 6067–6072. [Google Scholar] [CrossRef] [PubMed]
- Chousterman, B.G.; Boissonnas, A.; Poupel, L.; Baudesson de Chanville, C.; Adam, J.; Tabibzadeh, N.; Licata, F.; Lukaszewicz, A.C.; Lombès, A.; Deterre, P.; et al. Ly6chigh Monocytes Protect against Kidney Damage During Sepsis via a CX3CR1-Dependent Adhesion Mechanism. J. Am. Soc. Nephrol. 2016, 27, 792–803. [Google Scholar] [CrossRef]
- Ishida, Y.; Hayashi, T.; Goto, T.; Kimura, A.; Akimoto, S.; Mukaida, N.; Kondo, T. Essential Involvement of CX3CR1-Mediated Signals in the Bactericidal Host Defense During Septic Peritonitis. J. Immunol. 2008, 181, 4208–4218. [Google Scholar] [CrossRef]
- Hoogendijk, A.J.; Wiewel, M.A.; van Vught, L.A.; Scicluna, B.P.; Belkasim-Bohoudi, H.; Horn, J.; Zwinderman, A.H.; Klein Klouwenberg, P.M.; Cremer, O.L.; Bonten, M.J.; et al. Plasma Fractalkine Is a Sustained Marker of Disease Severity and Outcome in Sepsis Patients. Crit. Care 2015, 19, 412. [Google Scholar] [CrossRef]
- Chen, X.; Wei, Q.; Hu, Y.; Wang, C. Role of Fractalkine in promoting inflammation in sepsis-induced multiple organ dysfunction. Infect. Genet. Evol. 2020, 85, 104569. [Google Scholar] [CrossRef]
- Lionakis, M.S.; Swamydas, M.; Fischer, B.G.; Plantinga, T.S.; Johnson, M.D.; Jaeger, M.; Green, N.M.; Masedunskas, A.; Weigert, R.; Mikelis, C.; et al. CX3CR1-Dependent Renal Macrophage Survival Promotes Candida Control and Host Survival. J. Clin. Investig. 2013, 123, 5035–5051. [Google Scholar] [CrossRef] [PubMed]
- Break, T.J.; Jaeger, M.; Solis, N.V.; Filler, S.G.; Rodriguez, C.A.; Lim, J.K.; Lee, C.C.; Sobel, J.D.; Netea, M.G.; Lionakis, M.S. CX3CR1 Is Dispensable for Control of Mucosal Candida Albicans Infections in Mice and Humans. Infect. Immun. 2015, 83, 958–965. [Google Scholar] [CrossRef]
- Selby, N.M.; Taal, M.W. An Updated Overview of Diabetic Nephropathy: Diagnosis, Prognosis, Treatment Goals and Latest Guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 3–15. [Google Scholar] [CrossRef]
- Hasegawa, G.; Nakano, K.; Sawada, M.; Uno, K.; Shibayama, Y.; Ienaga, K.; Kondo, M. Possible Role of Tumor Necrosis Factor and Interleukin-1 in the Development of Diabetic Nephropathy. Kidney Int. 1991, 40, 1007–1012. [Google Scholar] [CrossRef]
- Lim, A.K.; Tesch, G.H. Inflammation in Diabetic Nephropathy. Mediat. Inflamm. 2012, 2012, 146154. [Google Scholar] [CrossRef]
- Hickey, F.B.; Martin, F. Role of the Immune System in Diabetic Kidney Disease. Curr. Diabetes Rep. 2018, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, L.; Wang, B.; Wu, Z. Identification and Validation of Immune and Cuproptosis—Related Genes for Diabetic Nephropathy by Wgcna and Machine Learning. Front. Immunol. 2024, 15, 1332279. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.; Chen, B.; Pei, X.; Cao, Y.; Wei, Y.; Zhu, Y. Gene-Based Network Analysis Reveals Prognostic Biomarkers Implicated in Diabetic Tubulointerstitial Injury. Dis. Markers 2022, 2022, 2700392. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Matthews, G.J.; Shah, R.Y.; McLaughlin, C.; Chen, J.; Wolman, M.; Master, S.R.; Chai, B.; Xie, D.; Rader, D.J.; et al. Serum Fractalkine (CX3CL1) and Cardiovascular Outcomes and Diabetes: Findings from the Chronic Renal Insufficiency Cohort (Cric) Study. Am. J. Kidney Dis. 2015, 66, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Hinkle, C.C.; Ferguson, J.F.; Mehta, N.N.; Li, M.; Qu, L.; Lu, Y.; Putt, M.E.; Ahima, R.S.; Reilly, M.P. Fractalkine Is a Novel Human Adipochemokine Associated with Type 2 Diabetes. Diabetes 2011, 60, 1512–1518. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ikee, R.; Hemmi, N.; Hyodo, N.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Suzuki, S.; Miura, S. Fractalkine and Its Receptor, CX3CR1, Upregulation in Streptozotocin-Induced Diabetic Kidneys. Nephron Exp. Nephrol. 2004, 97, e17–e25. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. The Pathogenesis of Atherosclerosis (Second of Two Parts). N. Engl. J. Med. 1976, 295, 420–425. [Google Scholar] [CrossRef]
- Wong, B.W.; Wong, D.; McManus, B.M. Characterization of Fractalkine (CX3CL1) and CX3CR1 in Human Coronary Arteries with Native Atherosclerosis, Diabetes Mellitus, and Transplant Vascular Disease. Cardiovasc. Pathol. 2002, 11, 332–338. [Google Scholar] [CrossRef]
- Stolla, M.; Pelisek, J.; von Brühl, M.L.; Schäfer, A.; Barocke, V.; Heider, P.; Lorenz, M.; Tirniceriu, A.; Steinhart, A.; Bauersachs, J.; et al. Fractalkine Is Expressed in Early and Advanced Atherosclerotic Lesions and Supports Monocyte Recruitment via CX3CR1. PLoS ONE 2012, 7, e43572. [Google Scholar] [CrossRef]
- Saederup, N.; Chan, L.; Lira, S.A.; Charo, I.F. Fractalkine Deficiency Markedly Reduces Macrophage Accumulation and Atherosclerotic Lesion Formation in CCR2−/− Mice: Evidence for Independent Chemokine Functions in Atherogenesis. Circulation 2008, 117, 1642–1648. [Google Scholar] [CrossRef]
- Combadière, C.; Potteaux, S.; Rodero, M.; Simon, T.; Pezard, A.; Esposito, B.; Merval, R.; Proudfoot, A.; Tedgui, A.; Mallat, Z. Combined Inhibition of CCL2, CX3CR1, and CCR5 Abrogates Ly6cHi and Ly6cLo Monocytosis and Almost Abolishes Atherosclerosis in Hypercholesterolemic Mice. Circulation 2008, 117, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yin, R.X.; Lin, Q.Z.; Guo, T.; Shi, G.Y.; Sun, J.Q.; Shen, S.W.; Li, Q. Two Polymorphisms in the Fractalkine Receptor CX3CR1 Gene Influence the Development of Atherosclerosis: A Meta-Analysis. Dis. Markers 2014, 2014, 913678. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Kuninaka, Y.; Nosaka, M.; Kimura, A.; Taruya, A.; Furuta, M.; Mukaida, N.; Kondo, T. Prevention of Cacl2-Induced Aortic Inflammation and Subsequent Aneurysm Formation by the CCL3-CCR5 Axis. Nat. Commun. 2020, 11, 5994. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Ozaki, M.; Tanaka, A.; Mukaida, N.; Kondo, T. Crucial Involvement of IL-6 in Thrombus Resolution in Mice Via Macrophage Recruitment and the Induction of Proteolytic Enzymes. Front. Immunol. 2019, 10, 3150. [Google Scholar] [CrossRef]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Taruya, A.; Furuta, M.; Mukaida, N.; Kondo, T. Contribution of the Tnf-A (Tumor Necrosis Factor-A)-Tnf-Rp55 (Tumor Necrosis Factor Receptor P55) Axis in the Resolution of Venous Thrombus. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2638–2650. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, M.; Ishida, Y.; Kimura, A.; Kuninaka, Y.; Inui, M.; Mukaida, N.; Kondo, T. Absence of Ifn-Γ Accelerates Thrombus Resolution through Enhanced Mmp-9 and Vegf Expression in Mice. J. Clin. Investig. 2011, 121, 2911–2920. [Google Scholar] [CrossRef]
- Ikejima, H.; Imanishi, T.; Tsujioka, H.; Kashiwagi, M.; Kuroi, A.; Tanimoto, T.; Kitabata, H.; Ishibashi, K.; Komukai, K.; Takeshita, T.; et al. Upregulation of Fractalkine and Its Receptor, CX3CR1, Is Associated with Coronary Plaque Rupture in Patients with Unstable Angina Pectoris. Circ. J. 2010, 74, 337–345. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Luan, X.; Qi, T.; Li, D.; Chen, Y.; Ji, X.; Zhang, Y.; Chen, W. Independent Roles of Monocyte Chemoattractant Protein-1, Regulated on Activation, Normal T-Cell Expressed and Secreted and Fractalkine in the Vulnerability of Coronary Atherosclerotic Plaques. Circ. J. 2012, 76, 2167–2173. [Google Scholar] [CrossRef]
- Xu, B.; Qian, Y.; Zhao, Y.; Fang, Z.; Tang, K.; Zhou, N.; Li, D.; Wang, J. Prognostic Value of Fractalkine/CX3CL1 Concentration in Patients with Acute Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Cytokine 2019, 113, 365–370. [Google Scholar] [CrossRef]
- Gu, X.; Xu, J.; Yang, X.P.; Peterson, E.; Harding, P. Fractalkine Neutralization Improves Cardiac Function after Myocardial Infarction. Exp. Physiol. 2015, 100, 805–817. [Google Scholar] [CrossRef]
- Klapproth, E.; Witt, A.; Klose, P.; Wiedemann, J.; Vavilthota, N.; Künzel, S.R.; Kämmerer, S.; Günscht, M.; Sprott, D.; Lesche, M.; et al. Targeting Cardiomyocyte Adam10 Ectodomain Shedding Promotes Survival Early after Myocardial Infarction. Nat. Commun. 2022, 13, 7648. [Google Scholar] [CrossRef]
- Ali, M.T.; Martin, K.; Kumar, A.H.; Cavallin, E.; Pierrou, S.; Gleeson, B.M.; McPheat, W.L.; Turner, E.C.; Huang, C.L.; Khider, W.; et al. A Novel CX3CR1 Antagonist Eluting Stent Reduces Stenosis by Targeting Inflammation. Biomaterials 2015, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.X.; Ekinci, Y.; Spray, L.; Jeyalan, V.; Olin, T.; Richardson, G.; Austin, D.; Alkhalil, M.; Spyridopoulos, I. Fractalkine Signalling (Cx(3)Cl1/Cx(3)Cr1 Axis) as an Emerging Target in Coronary Artery Disease. J. Clin. Med. 2023, 12, 4821. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab Plus Ipilimumab Versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib Plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab Plus Axitinib Versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, M.; Mai, F.; Li, X.; Wang, W.; Huang, Q.; Du, X.; Ding, W.; Li, Y.; Barwick, B.G.; et al. Interruption of Klf5 Acetylation Promotes Pten-Deficient Prostate Cancer Progression by Reprogramming Cancer-Associated Fibroblasts. J. Clin. Investig. 2024, 134, e175949. [Google Scholar] [CrossRef]
- Shulby, S.A.; Dolloff, N.G.; Stearns, M.E.; Meucci, O.; Fatatis, A. CX3CR1-Fractalkine Expression Regulates Cellular Mechanisms Involved in Adhesion, Migration, and Survival of Human Prostate Cancer Cells. Cancer Res. 2004, 64, 4693–4698. [Google Scholar] [CrossRef]
- Liu, W.; Bian, C.; Liang, Y.; Jiang, L.; Qian, C.; Dong, J. CX3CL1: A Potential Chemokine Widely Involved in the Process Spinal Metastases. Oncotarget 2017, 8, 15213–15219. [Google Scholar] [CrossRef]
- Liu, P.; Liang, Y.; Jiang, L.; Wang, H.; Wang, S.; Dong, J. CX3CL1/Fractalkine Enhances Prostate Cancer Spinal Metastasis by Activating the Src/Fak Pathway. Int. J. Oncol. 2018, 53, 1544–1556. [Google Scholar] [CrossRef]
- Tang, J.; Xiao, L.; Cui, R.; Li, D.; Zheng, X.; Zhu, L.; Sun, H.; Pan, Y.; Du, Y.; Yu, X. CX3CL1 Increases Invasiveness and Metastasis by Promoting Epithelial-to-Mesenchymal Transition through the Tace/Tgf-A/Egfr Pathway in Hypoxic Androgen-Independent Prostate Cancer Cells. Oncol. Rep. 2016, 35, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.J.; Chen, Y.Y.; Lin, P.; Zou, H.F.; Lin, F.; Zhao, L.N.; Li, D.; Guo, L.; Tang, J.B.; Zheng, X.L.; et al. Hypoxia Increases CX3CR1 Expression Via Hif-1 and Nf-Κb in Androgen-Independent Prostate Cancer Cells. Int. J. Oncol. 2012, 41, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chen, Y.; Cui, R.; Li, D.; Xiao, L.; Lin, P.; Du, Y.; Sun, H.; Yu, X.; Zheng, X. Upregulation of Fractalkine Contributes to The proliferative Response of Prostate Cancer Cells to Hypoxia Via Promoting the G1/S Phase Transition. Mol. Med. Rep. 2015, 12, 7907–7914. [Google Scholar] [CrossRef]
- Blum, D.L.; Koyama, T.; M’Koma, A.E.; Iturregui, J.M.; Martinez-Ferrer, M.; Uwamariya, C.; Smith, J.A., Jr.; Clark, P.E.; Bhowmick, N.A. Chemokine Markers Predict Biochemical Recurrence of Prostate Cancer Following Prostatectomy. Clin. Cancer Res. 2008, 14, 7790–7797. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.M.; Madden, M.Z.; Arner, E.N.; Bader, J.E.; Ye, X.; Vlach, L.; Tigue, M.L.; Landis, M.D.; Jonker, P.B.; Hatem, Z.; et al. VHL Loss Reprograms the Immune Landscape to Promote an Inflammatory Myeloid Microenvironment in Renal Tumorigenesis. J. Clin. Investig. 2024, 134, e173934. [Google Scholar] [CrossRef]
- Hu, A.N.; Chen, F.C.; Wang, K.T.; Wang, Z.Q.; Liang, Y.; Dong, J. CX3CL1 in the Red Bone Marrow Promotes Renal Cell Carcinoma to Metastasize to the Spine by Involving the Src-Related Pathway. Neoplasma 2022, 69, 670–679. [Google Scholar] [CrossRef]
- Gong, Q.; Guo, Z.; Sun, W.; Du, X.; Jiang, Y.; Liu, F. CX3CL1 Promotes Cell Sensitivity to Ferroptosis and Is Associated with the Tumor Microenvironment in Clear Cell Renal Cell Carcinoma. BMC Cancer 2022, 22, 1184. [Google Scholar] [CrossRef]
- Liu, H.; Sun, S.; Wang, G.; Lu, M.; Zhang, X.; Wei, X.; Gao, X.; Huang, C.; Li, Z.; Zheng, J.; et al. Tyrosine Kinase Inhibitor Cabozantinib Inhibits Murine Renal Cancer by Activating Innate and Adaptive Immunity. Front. Oncol. 2021, 11, 663517. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Dong, T.; Yan, Y.; Sun, L.; Wang, W. Clinical Significance of Expression Level of CX3CL1-CX3CR1 Axis in Bone Metastasis of Lung Cancer. Clin. Transl. Oncol. 2021, 23, 378–388. [Google Scholar] [CrossRef]
- Li, S.; Liu, S.; Fang, S.; Zhao, F. Establishment and Verification of a Predictive Model for Bone Metastasis in Patients with Non-Small Cell Lung Cancer Based on Peripheral Blood Cx3cl and Ccl28. Am. J. Cancer Res. 2024, 14, 3059–3067. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Hu, A.; Sun, C.; Zhou, L.; Huang, Y.; Chen, Q.; Dong, J.; Zhou, X.; Zhang, F. Vertebral-Specific Activation of the CX3CL1/Icam-1 Signaling Network Mediates Non-Small-Cell Lung Cancer Spinal Metastasis by Engaging Tumor Cell-Vertebral Bone Marrow Endothelial Cell Interactions. Theranostics 2021, 11, 4770–4789. [Google Scholar] [CrossRef]
- Tang, W.; Jia, P.; Zuo, L.; Zhao, J. Suppression of CX3CL1 by Mir-497-5p Inhibits Cell Growth and Invasion through Inactivating the Erk/Akt Pathway in Nsclc Cells. Cell Cycle 2022, 21, 1697–1709. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhu, X.; Li, Q.; Liang, X.; Xie, J.; Hu, S.; Peng, W.; Li, C. Increased CX3CL1 Mrna Expression Level Is a Positive Prognostic Factor in Patients with Lung Adenocarcinoma. Oncol. Lett. 2019, 17, 4877–4890. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Chang, H.; Sun, S.J.; Liao, C.Y.; Wang, L.Y.; Ko, J.L.; Chang, J.T. Differential Impact of CX3CL1 on Lung Cancer Prognosis in Smokers and Non-Smokers. Mol. Carcinog. 2018, 57, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, X.; Liu, C.; Cheng, Y.; Li, B.; Zhang, W.; Zeng, R.; Chen, Q.; Zhang, Y.; Hu, S. Mesenchymal Stem Cells Elicits Anti-Pd1 Immunotherapy by Targeted Delivery of CX3CL1. Front. Pharmacol. 2023, 14, 1136614. [Google Scholar] [CrossRef] [PubMed]
- Erreni, M.; Siddiqui, I.; Marelli, G.; Grizzi, F.; Bianchi, P.; Morone, D.; Marchesi, F.; Celesti, G.; Pesce, S.; Doni, A.; et al. The Fractalkine-Receptor Axis Improves Human Colorectal Cancer Prognosis by Limiting Tumor Metastatic Dissemination. J. Immunol. 2016, 196, 902–914. [Google Scholar] [CrossRef]
- Olsen, R.S.; Nijm, J.; Andersson, R.E.; Dimberg, J.; Wågsäter, D. Circulating Inflammatory Factors Associated with Worse Long-Term Prognosis in Colorectal Cancer. World J. Gastroenterol. 2017, 23, 6212–6219. [Google Scholar] [CrossRef]
- Wada, A.; Ito, A.; Iitsuka, H.; Tsuneyama, K.; Miyazono, T.; Murakami, J.; Shibahara, N.; Sakurai, H.; Saiki, I.; Nakayama, T.; et al. Role of Chemokine CX3CL1 in Progression of Multiple Myeloma Via CX3CR1 in Bone Microenvironments. Oncol. Rep. 2015, 33, 2935–2939. [Google Scholar] [CrossRef]
- Sponaas, A.M.; Moen, S.H.; Liabakk, N.B.; Feyzi, E.; Holien, T.; Kvam, S.; Grøseth, L.A.; Størdal, B.; Buene, G.; Espevik, T.; et al. The Proportion of Cd16+Cd14Dim Monocytes Increases with Tumor Cell Load in Bone Marrow of Patients with Multiple Myeloma. Immun. Inflamm. Dis. 2015, 3, 94–102. [Google Scholar] [CrossRef]
- Marchica, V.; Toscani, D.; Corcione, A.; Bolzoni, M.; Storti, P.; Vescovini, R.; Ferretti, E.; Dalla Palma, B.; Vicario, E.; Accardi, F.; et al. Bone Marrow CX3CL1/Fractalkine Is a New Player of the Pro-Angiogenic Microenvironment in Multiple Myeloma Patients. Cancers 2019, 11, 321. [Google Scholar] [CrossRef]
- Mei, N.; Su, H.; Gong, S.; Du, H.; Zhang, X.; Wang, L.; Wang, H. High CX3CR1 Expression Predicts Poor Prognosis in Paediatric Acute Myeloid Leukaemia Undergoing Hyperleukocytosis. Int. J. Lab. Hematol. 2023, 45, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.; Adams, W.A.; Calescibetta, A.; Tu, N.; Dalton, R.; So, T.; Wei, M.; Ward, G.; Kostenko, E.; Christiansen, S.; et al. CX3CR1 Deficiency-Induced Til Tumor Restriction as a Novel Addition for Car-T Design in Solid Malignancies. iScience 2023, 26, 106443. [Google Scholar] [CrossRef] [PubMed]
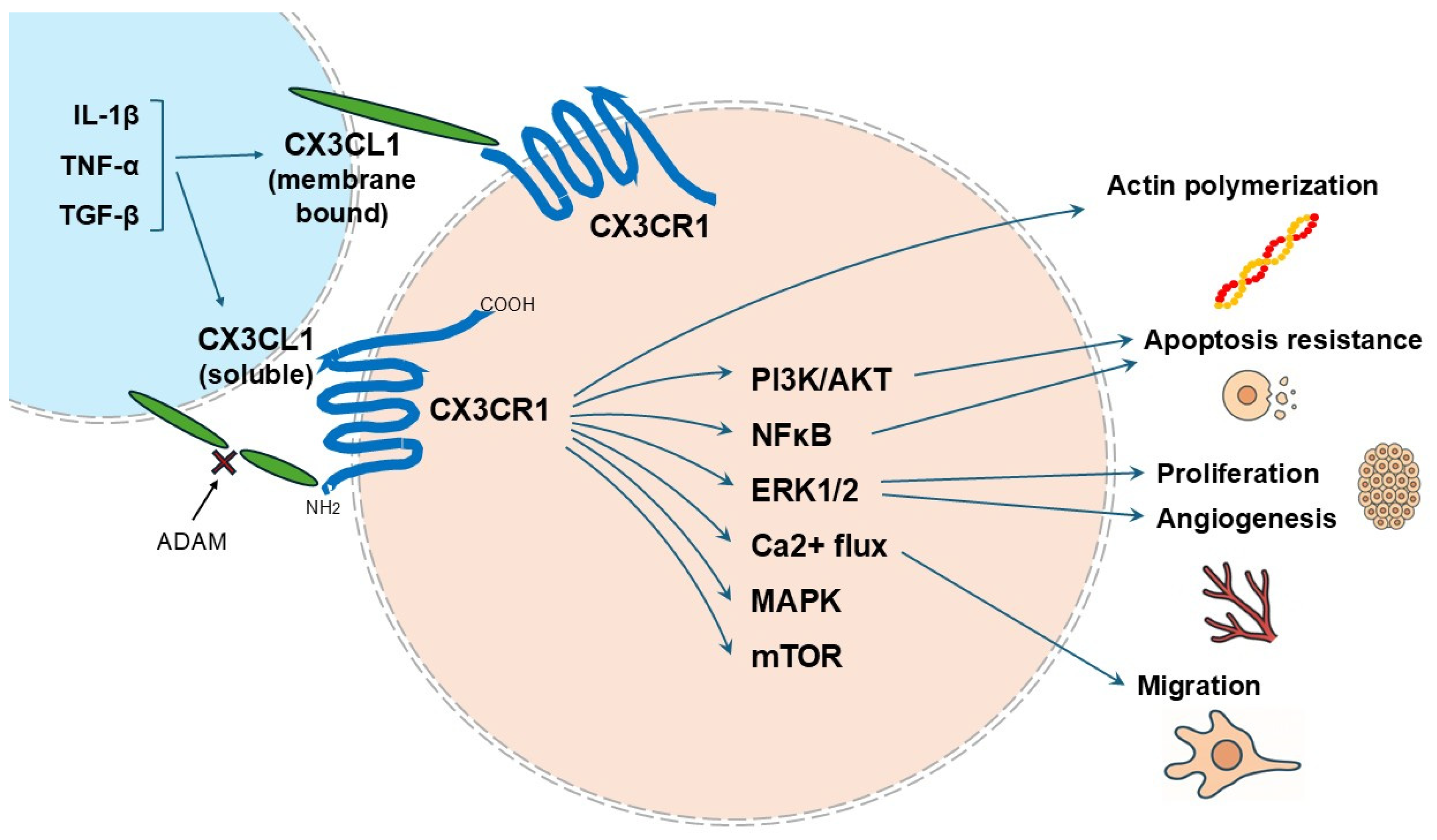

| Disease Type | Function | Prognosis with Increased Expression of CX3CL1 CX3CR1 Axis | Reference |
|---|---|---|---|
| Glomerulonephritis | In patients with IgA nephropathy, plasma levels of fractalkine were found to correlate with serum creatinine, 24 h urinary protein excretion, mesangial hypercellularity, infiltration of CD68-positive macrophages and CD20-positive B cells in renal tissue, as well as overall renal prognosis. | ↓ | [30] |
| Increased expression of fractalkine in the glomeruli and urine, associated with activation of the CX3CR1–fractalkine signaling pathway, may contribute to the worsening of gross hematuria. | ↓ | [31] | |
| Increased levels of urinary fractalkine tended to be associated with good prognosis with crescentic glomerulonephritis | ↑ | [32] | |
| MPO-ANCA increases CX3CL1 expression on human glomerular endothelial cells (HGECs) and promotes the recruitment of CD16+ monocytes to the kidney. | ↓ | [33] | |
| CX3CL1 mRNA was increased in the glomerular and tubular interstitium of patients with renal inflammation. | ↓ | [34] | |
| CX3CL1 expression and CD16+ monocyte accumulation in glomeruli were associated with histopathological features of glomerular lesions in MRL/lpr mice | ↓ | [39] | |
| Glomerulonephritis in MRL/lpr mice is influenced by CX3CR1 through a mechanism dependent on gut microbial composition. | ↑ | [40] | |
| A significantly disrupted gut microbiota, along with a compromised intestinal barrier, was observed in the context of Cx3cr1 deficiency. | ↑ | [41] | |
| Chronic kidney disease | CKD patients exhibited higher plasma CX3CL1 levels, which were found to be negatively associated with eGFR. | ↓ | [42,43] |
| Fibrotic kidneys exhibited CX3CR1 presence in various cell populations, such as mononuclear cells, epithelial tubule cells, dendritic cells, and α-SMA/vimentin-positive interstitial myofibroblasts. | ↓ | [47] | |
| CD1c+ DCs as the predominant source of profibrotic TGF-β and highest expressors of the fractalkine receptor CX3CR1 within the renal DC compartment. | ↓ | [49] | |
| CX3CR1−/− mice showed significantly reduced collagen deposition and macrophage infiltration in kidney. | ↓ | [51] | |
| CX3CL1/CX3CR1 axis controls the survival of Ly6C−CX3CR1high macrophages | ↓ | [53] | |
| In the absence of CX3CR1, renal macrophage numbers were increased, along with enhanced production of TGF-β, a major profibrotic cytokine. This accumulation resulted from elevated local proliferation, even though monocyte recruitment was diminished and apoptotic activity was higher in renal tissue. | ↑ | [54] | |
| Renal allograft rejection | Pati Elevated serum CX3CL1 concentrations before transplantation were observed in patients with acute kidney allograft rejection, implying an increased proinflammatory status relative to individuals with favorable outcomes. | ↓ | [60] |
| The levels of fractalkine on day 0 of acute renal rejection group was significantly higher than that in no renal rejection group. | ↓ | [56] | |
| Acute renal rejection patients had significantly higher serum fractalkine levels compared to levels observed in the no renal rejection group and healthy controls. | ↓ | [57] | |
| CX3CL1 may have a functional role in leucocyte adhesion and retention, at selected tubular sites in acute renal inflammation. | ↓ | [58] | |
| Cells positive for CX3CR1 largely comprised CD68-expressing monocytes/macrophages and dendritic cells positive for CD209/DC-SIGN. The percentage area of CX3CR1 positivity was associated with steroid responsiveness and was predictive of worse clinical prognosis at 3 and 12 months post-transplantation. | ↓ | [59] | |
| Renal infection diseases | CX3CR1-dependent infiltration of Ly6C(high) inflammatory monocytes into the kidney was accompanied by altered cell motility and increased adhesion to the renal vascular wall. | ↑ | [62] |
| Renal macrophage deficiency in infected Cx3cr1−/− mice was due to reduced macrophage survival, not impaired proliferation, trafficking, or differentiation. | ↑ | [66] | |
| Diabetic Nephropathy | In patients with diabetic kidney disease, Gene Expression Omnibus data analysis revealed that CX3CR1 expression inversely correlated with estimated glomerular filtration rate. | ↓ | [72] |
| Higher CX3CL1 level also was associated with prevalent diabetes in adjusted models. | ↓ | [74] | |
| CX3CL1-CX3CR1 is a novel inflammatory adipose chemokine system that modulates monocyte adhesion to adipocytes and is associated with obesity, insulin resistance, and type 2 diabetes. | ↓ | [75] | |
| In the early phases of diabetic kidney disease, increased expression of CX3CL1 and CX3CR1 was observed. CX3CL1 was prominently stained in diabetic kidneys, notably within the glomerular capillary lumens and peritubular capillaries. Only a small number of CX3CR1-positive cells were detected infiltrating the diabetic glomeruli. | ↓ | [76] |
| Disease Type | Function | Prognosis with Increased Expression of CX3CL1 CX3CR1 Axis | Reference |
|---|---|---|---|
| Atherosclerosis | The number of CX3CL1-expressing cells positively correlates with the number of CX3CR1-positive cells in human carotid artery plaques. | ↓ | [78] |
| Soluble CX3CL1 levels in circulating blood were significantly elevated in the presence of severe stenosis of the carotid artery | ↓ | [79] | |
| CX3CL1−/− mice had significantly reduced macrophage accumulation and atherosclerotic lesion size compared to mice lacking each gene alone. | ↓ | [80] | |
| The joint inhibition of CCL2, CX3CR1, and CCR5 in ApoE-deficient mice led to the suppression of monocytosis in the bone marrow and lowered circulating monocyte levels, despite persistent hypercholesterolemia. | ↓ | [81] | |
| The 249I allele carriers of the CX3CR1 V249I polymorphism had a reduced risk of atherosclerosis and coronary artery disease in the heterozygous state. | ↓ | [82] | |
| Coronary artery disease | The plasma levels of soluble CX3CL1 were significantly increased in UAP patients with plaque rupture. | ↓ | [87] |
| The concentration of CX3CL1 was significantly elevated in acute myocardial infarction and unstable angina pectoris patients than in patients with unstable angina pectoris and without coronary heart disease | ↓ | [88] | |
| STEMI patients after primary percutaneous coronary intervention, CX3CL1 concentration on the day after PCI was inversely correlated with ventricle ejection fraction measurements one month later. | ↓ | [89] | |
| Survival and cardiac function were significantly improved in the group treated with anti-CX3CL1 antibody after myocardial infarction. | ↓ | [90] | |
| When ADAM10-mediated cleavage of CX3CL1 is abolished, IL-1β-driven inflammation is suppressed, neutrophil release from the bone marrow is decreased, and infiltration into myocardial tissue is limited. | ↓ | [91] | |
| CX3CR1 represents a promising therapeutic target to inhibit monocyte adhesion and inflammation as well as in-stent neointimal hyperplasia, while preserving stent re-endothelialization. | ↓ | [92] |
| Cancer Type | Function | Prognosis with Increased Expression of CX3CL1 CX3CR1 Axis | Reference |
|---|---|---|---|
| Prostate cancer | CX3CR1 was required for FGF9 to activate FGF receptor 1 (FGFR1) signaling. | ↓ | [97] |
| Targeting CX3CL1 and its influence on the EGFR, Src, and FAK signaling pathways could provide new avenues for early intervention against spinal metastases in prostate cancer. | ↓ | [100] | |
| CX3CL1/CX3CR1 induces EMT and migration and invasion of androgen-independent prostate cancer cells through TACE/TGF-α/EGFR pathway activation. | ↓ | [101] | |
| Hypoxia-induced CX3CR1 expression requires both HIF-1 and NF-κB and is linked to increased movement and invasiveness of prostate cancer cells. | ↓ | [102] | |
| The activation of CX3CR1 in prostate cancer cells recruits an important antiapoptotic signaling pathways such as Akt/GSK3. | ↓ | [98] | |
| Under hypoxic conditions, CX3CL1 secretion and expression were increased, resulting in enhanced proliferation of prostate cancer cells through stimulation of the cell cycle. | ↓ | [103] | |
| Renal cancer | The chemokine CX3CL1 was highly expressed in human ccRCC tumors and was associated with Vhl deficiency | ↓ | [105] |
| Elevated levels of CX3CL1 suppressed both proliferation and metastatic potential of tumor cells while enhancing their sensitivity to ferroptosis in ccRCC | ↑ | [107] | |
| CX3CL1 in the red bone marrow of spinal cancellous bone enhances migration and invasion abilities of RCC cells | ↓ | [106] | |
| Cabozantinib treatment induced expression of CX3CL1 as T cell-related chemokines in the tumor microenvironment | ↑ | [108] | |
| Lung cancer | CX3CL1 was elevated in patients with bone metastases in NSCLC patients. | ↓ | [109,110] |
| MiR-497-5p expression is reduced in both NSCLC tissues and cell lines, where it suppresses tumor proliferation and invasion by downregulating CX3CL1 and subsequently inhibiting the ERK/AKT signaling pathway. | ↓ | [112] | |
| Elevated CX3CL1 mRNA expression in lung adenocarcinoma tissues was associated with better patient outcomes, potentially through mechanisms involving cell adhesion molecules, leukocyte transendothelial migration, and NK cell cytotoxic activity. | ↑ | [113] | |
| Colorectal cancer | Mesenchymal stem cells recruits CX3CR1high macrophages and promotes M1 polarization to inhibit tumor growth via highly secretion of CX3CL1. | ↑ | [115] |
| The CX3CL1-CX3CR1 chemokine axis expressed by tumors acts to retain cells by enhancing adhesion between similar cells, thereby restricting tumor dissemination to distant metastatic locations. | ↑ | [116] | |
| High plasma CX3CL1 level was an independent poor prognostic factor. | ↓ | [117] | |
| Hematological cancer | CX3CR1 is expressed in certain multiple myeloma cell lines, where stimulation with CX3CL1 activates the Akt and ERK1/2 signaling pathways and enhances cell adhesion. The differentiation of osteoclast precursor cells was inhibited by a neutralizing anti-CX3CL1 antibody. | ↓ | [118] |
| In multiple myeloma, the proportion of CX3CR1-high non-classical monocytes (CD16+CD14dim) increases with tumor burden, and these cells produce tumor-promoting cytokines such as TNF-α, IL-6, and CCL3 in response to stimuli derived from apoptotic tumor cells. | ↓ | [119] | |
| The CX3CL1/CX3CR1 axis promotes bone marrow angiogenesis in multiple myeloma by mobilizing CX3CR1-positive monocytes and endothelial cells in response to TNF-α–dependent CX3CL1 production. | ↓ | [120] | |
| In pediatric AML, hyperleukocytosis is associated with poor prognosis, and high CX3CR1 expression is linked to reduced survival through its interaction with CX3CL1 and involvement in immune response pathways, as well as strong associations with immune cell infiltration. | ↓ | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwahashi, Y.; Ishida, Y.; Mukaida, N.; Kondo, T. Pathophysiological Roles of the CX3CL1-CX3CR1 Axis in Renal Disease, Cardiovascular Disease, and Cancer. Int. J. Mol. Sci. 2025, 26, 5352. https://doi.org/10.3390/ijms26115352
Iwahashi Y, Ishida Y, Mukaida N, Kondo T. Pathophysiological Roles of the CX3CL1-CX3CR1 Axis in Renal Disease, Cardiovascular Disease, and Cancer. International Journal of Molecular Sciences. 2025; 26(11):5352. https://doi.org/10.3390/ijms26115352
Chicago/Turabian StyleIwahashi, Yuya, Yuko Ishida, Naofumi Mukaida, and Toshikazu Kondo. 2025. "Pathophysiological Roles of the CX3CL1-CX3CR1 Axis in Renal Disease, Cardiovascular Disease, and Cancer" International Journal of Molecular Sciences 26, no. 11: 5352. https://doi.org/10.3390/ijms26115352
APA StyleIwahashi, Y., Ishida, Y., Mukaida, N., & Kondo, T. (2025). Pathophysiological Roles of the CX3CL1-CX3CR1 Axis in Renal Disease, Cardiovascular Disease, and Cancer. International Journal of Molecular Sciences, 26(11), 5352. https://doi.org/10.3390/ijms26115352






