Vat-Mediated Mucus Penetration Enables Genotoxic Activity of pks+ Escherichia coli
Abstract
1. Introduction
2. Results
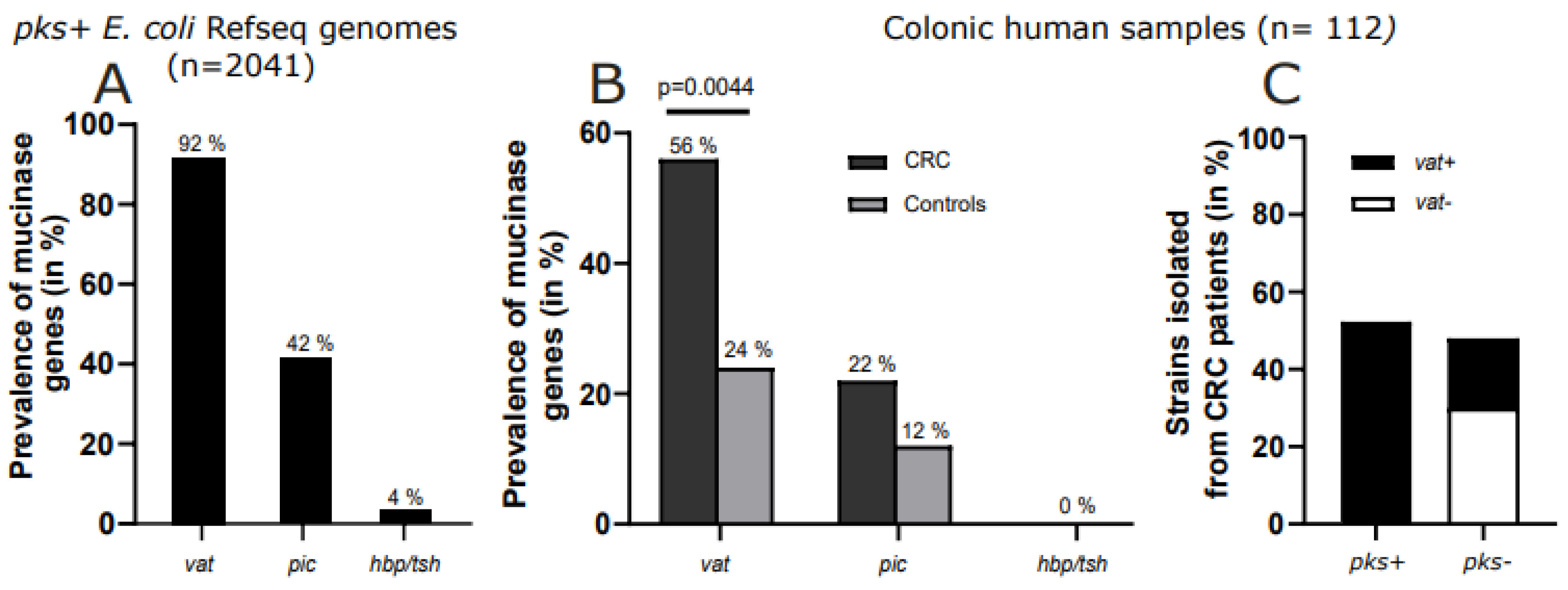
2.1. Vat Gene Predominates in pks+ E. coli
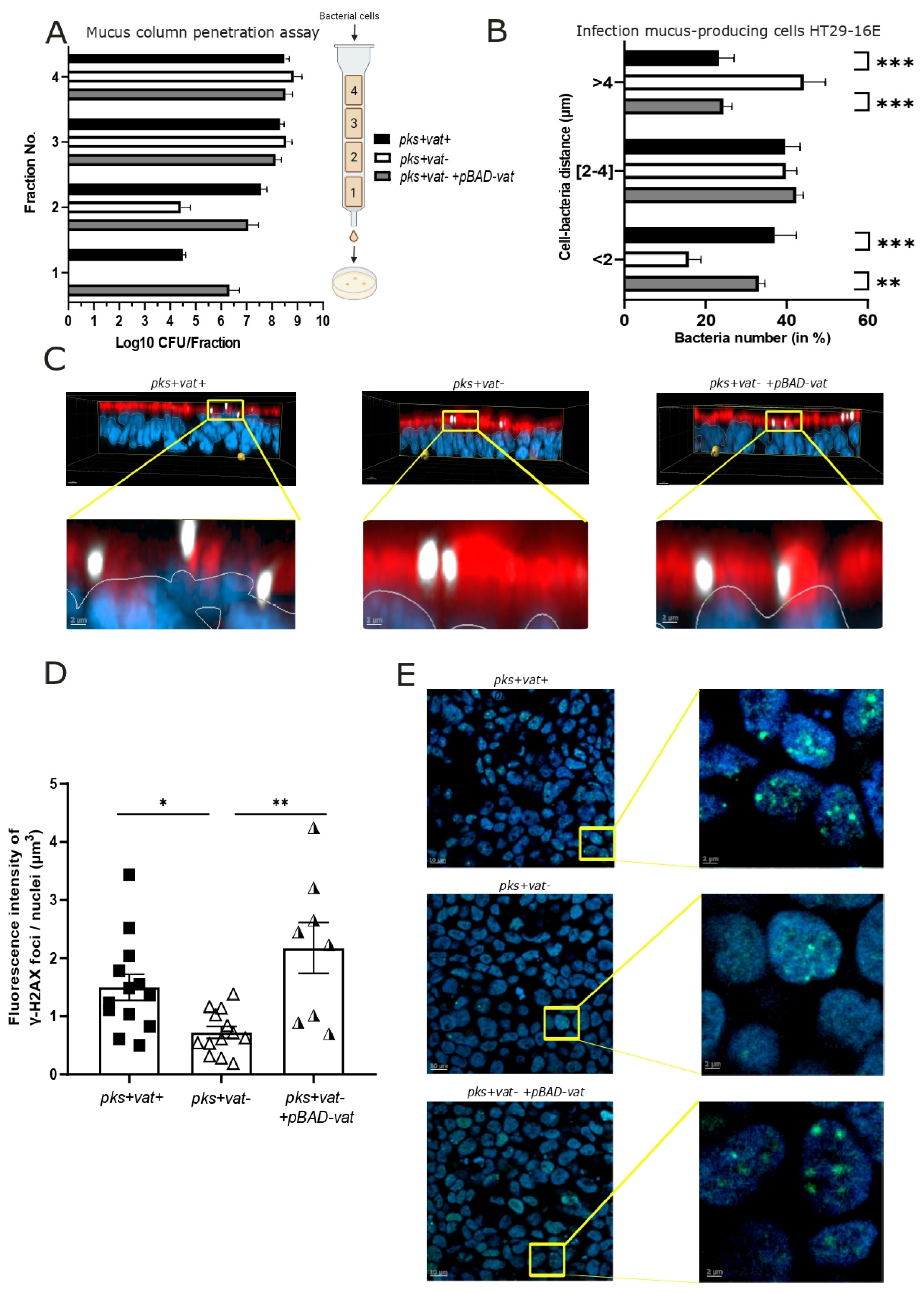
2.2. Vat Protease Promotes Mucosal Crossing of pks+ E. coli
2.3. Vat Protease Enhances the Genotoxic Effects of pks+ E. coli
2.4. Vat Protease Contributes to pks+ E. coli Colonization and Tumorigenesis in ApcMin/+ Mice
3. Discussion
4. Materials and Methods
4.1. Clinical E. coli Isolates
4.2. Bacterial Strains and Construction of Isogenic Mutants
4.3. Metagenomics Analysis and Screening of Mucin Proteases and pks Genes
4.4. Mucin Column Penetration Assay
4.5. Cell Culture
4.6. Fluorescence In Situ Hybridization (FISH)
4.7. Immunofluorescence
4.8. ApcMin/+ Mouse Model Information and Quantification of E. coli in Stools and Colonic Tissues
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | Colorectal cancer |
| CFU | Colony-forming unit |
| DNA | Deoxyribonucleic acid |
| DSS | Dextran sulfate sodium |
| FISH | Fluorescence in situ hybridization |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| SPATE | Serine protease autotransporters of enterobacteria |
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, M.W.; Jobin, C. Intestinal Bacteria and Colorectal Cancer: Etiology and Treatment. Gut Microbes 2023, 15, 2185028. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2021, 12, 807648. [Google Scholar] [CrossRef] [PubMed]
- Tjalsma, H.; Boleij, A.; Marchesi, J.R.; Dutilh, B.E. A Bacterial Driver-Passenger Model for Colorectal Cancer: Beyond the Usual Suspects. Nat. Rev. Microbiol. 2012, 10, 575–582. [Google Scholar] [CrossRef]
- Nougayrède, J.-P.; Homburg, S.; Taieb, F.; Boury, M.; Brzuszkiewicz, E.; Gottschalk, G.; Buchrieser, C.; Hacker, J.; Dobrindt, U.; Oswald, E. Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science 2006, 313, 848–851. [Google Scholar] [CrossRef]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef]
- Arthur, J.C.; Gharaibeh, R.Z.; Mühlbauer, M.; Perez-Chanona, E.; Uronis, J.M.; McCafferty, J.; Fodor, A.A.; Jobin, C. Microbial Genomic Analysis Reveals the Essential Role of Inflammation in Bacteria-Induced Colorectal Cancer. Nat. Commun. 2014, 5, 4724. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Cougnoux, A.; Dalmasso, G.; Martinez, R.; Buc, E.; Delmas, J.; Gibold, L.; Sauvanet, P.; Darcha, C.; Déchelotte, P.; Bonnet, M.; et al. Bacterial Genotoxin Colibactin Promotes Colon Tumour Growth by Inducing a Senescence-Associated Secretory Phenotype. Gut 2014, 63, 1932–1942. [Google Scholar] [CrossRef]
- Bossuet-Greif, N.; Vignard, J.; Taieb, F.; Mirey, G.; Dubois, D.; Petit, C.; Oswald, E.; Nougayrède, J.-P. The Colibactin Genotoxin Generates DNA Interstrand Cross-Links in Infected Cells. mBio 2018, 9, e02393-17. [Google Scholar] [CrossRef]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational Signature in Colorectal Cancer Caused by Genotoxic Pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Kim, C.S.; Healy, A.R.; Wernke, K.M.; Wang, Z.; Frischling, M.C.; Shine, E.E.; Wang, W.; Herzon, S.B.; Crawford, J.M. Structure Elucidation of Colibactin and Its DNA Cross-Links. Science 2019, 365, eaax2685. [Google Scholar] [CrossRef] [PubMed]
- Silpe, J.E.; Wong, J.W.H.; Owen, S.V.; Baym, M.; Balskus, E.P. The Bacterial Toxin Colibactin Triggers Prophage Induction. Nature 2022, 603, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Jans, M.; Kolata, M.; Blancke, G.; D’Hondt, A.; Gräf, C.; Ciers, M.; Sze, M.; Thiran, A.; Petta, I.; Andries, V.; et al. Colibactin-Driven Colon Cancer Requires Adhesin-Mediated Epithelial Binding. Nature 2024, 635, 472–480. [Google Scholar] [CrossRef]
- Reuter, C.; Alzheimer, M.; Walles, H.; Oelschlaeger, T.A. An Adherent Mucus Layer Attenuates the Genotoxic Effect of Colibactin. Cell. Microbiol. 2018, 20, e12812. [Google Scholar] [CrossRef]
- Payros, D.; Dobrindt, U.; Martin, P.; Secher, T.; Bracarense, A.P.F.L.; Boury, M.; Laffitte, J.; Pinton, P.; Oswald, E.; Oswald, I.P. The Food Contaminant Deoxynivalenol Exacerbates the Genotoxicity of Gut Microbiota. mBio 2017, 8, e00007-17. [Google Scholar] [CrossRef]
- Harnack, C.; Berger, H.; Liu, L.; Mollenkopf, H.-J.; Strowig, T.; Sigal, M. Short-Term Mucosal Disruption Enables Colibactin-Producing E. coli to Cause Long-Term Perturbation of Colonic Homeostasis. Gut Microbes 2023, 15, 2233689. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal Cancer in Mice Genetically Deficient in the Mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Parham, N.J.; Pollard, S.J.; Desvaux, M.; Scott-Tucker, A.; Liu, C.; Fivian, A.; Henderson, I.R. Distribution of the Serine Protease Autotransporters of the Enterobacteriaceae among Extraintestinal Clinical Isolates of Escherichia coli. J. Clin. Microbiol. 2005, 43, 4076–4082. [Google Scholar] [CrossRef]
- Hews, C.L.; Tran, S.-L.; Wegmann, U.; Brett, B.; Walsham, A.D.S.; Kavanaugh, D.; Ward, N.J.; Juge, N.; Schüller, S. The StcE Metalloprotease of Enterohaemorrhagic Escherichia coli Reduces the Inner Mucus Layer and Promotes Adherence to Human Colonic Epithelium Ex Vivo. Cell. Microbiol. 2017, 19, e12717. [Google Scholar] [CrossRef]
- Grys, T.E.; Siegel, M.B.; Lathem, W.W.; Welch, R.A. The StcE Protease Contributes to Intimate Adherence of Enterohemorrhagic Escherichia coli O157:H7 to Host Cells. Infect. Immun. 2005, 73, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Luo, Q.; Vickers, T.J.; Sheikh, A.; Lewis, W.G.; Fleckenstein, J.M. EatA, An Immunogenic Protective Antigen of Enterotoxigenic Escherichia coli, Degrades Intestinal Mucin. Infect. Immun. 2014, 82, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.M.; Sheikh, J.; Henderson, I.R.; Ruiz-Perez, F.; Cohen, P.S.; Nataro, J.P. The Pic Protease of Enteroaggregative Escherichia coli Promotes Intestinal Colonization and Growth in the Presence of Mucin. Infect. Immun. 2009, 77, 2465–2473. [Google Scholar] [CrossRef]
- Díaz, J.M.; Dozois, C.M.; Avelar-González, F.J.; Hernández-Cuellar, E.; Pokharel, P.; de Santiago, A.S.; Guerrero-Barrera, A.L. The Vacuolating Autotransporter Toxin (Vat) of Escherichia coli Causes Cell Cytoskeleton Changes and Produces Non-Lysosomal Vacuole Formation in Bladder Epithelial Cells. Front. Cell. Infect. Microbiol. 2020, 10, 299. [Google Scholar] [CrossRef]
- Gibold, L.; Garenaux, E.; Dalmasso, G.; Gallucci, C.; Cia, D.; Mottet-Auselo, B.; Faïs, T.; Darfeuille-Michaud, A.; Nguyen, H.T.T.; Barnich, N.; et al. The Vat-AIEC Protease Promotes Crossing of the Intestinal Mucus Layer by Crohn’s Disease-Associated Escherichia coli. Cell. Microbiol. 2016, 18, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.K.T.; Gaziri, L.C.J.; Venancio, E.J.; Vidotto, M.C. Detection of Tsh Protein Mucinolytic Activity by SDS-PAGE. J. Microbiol. Methods 2007, 68, 654–655. [Google Scholar] [CrossRef]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Augeron, C.; Laboisse, C.L. Emergence of Permanently Differentiated Cell Clones in a Human Colonic Cancer Cell Line in Culture After Treatment with Sodium Butyrate. Cancer Res. 1984, 44, 3961–3969. [Google Scholar]
- Raisch, J.; Buc, E.; Bonnet, M.; Sauvanet, P.; Vazeille, E.; de Vallée, A.; Déchelotte, P.; Darcha, C.; Pezet, D.; Bonnet, R.; et al. Colon Cancer-Associated B2 Escherichia coli Colonize Gut Mucosa and Promote Cell Proliferation. World J. Gastroenterol. 2014, 20, 6560–6572. [Google Scholar] [CrossRef]
- Chat, H.; Dalmasso, G.; Godfraind, C.; Bonnin, V.; Beyrouthy, R.; Bonnet, M.; Barnich, N.; Mettouchi, A.; Lemichez, E.; Bonnet, R.; et al. Cytotoxic Necrotizing Factor 1 Hinders Colon Tumorigenesis Induced by Colibactin-Producing Escherichia coli in ApcMin/+ Mice. Gut Microbes 2023, 15, 2229569. [Google Scholar] [CrossRef]
- Mah, L.-J.; El-Osta, A.; Karagiannis, T.C. gammaH2AX: A Sensitive Molecular Marker of DNA Damage and Repair. Leukemia 2010, 24, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The Application of ApcMin/+ Mouse Model in Colorectal Tumor Researches. J. Cancer Res. Clin. Oncol. 2019, 145, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Gagnière, J.; Bonnin, V.; Jarrousse, A.-S.; Cardamone, E.; Agus, A.; Uhrhammer, N.; Sauvanet, P.; Déchelotte, P.; Barnich, N.; Bonnet, R.; et al. Interactions between Microsatellite Instability and Human Gut Colonization by Escherichia coli in Colorectal cancer. Clin. Sci. 2017, 131, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step Inactivation of Chromosomal Genes in Escherichia coli K-12 Using PCR Products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Chaveroche, M.K.; Ghigo, J.M.; d’Enfert, C. A Rapid Method for Efficient Gene Replacement in the Filamentous Fungus Aspergillus nidulans. Nucleic Acids Res. 2000, 28, E97. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kiessling, S.; Alt, K.; Antáo, E.-M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian Pathogenic, Uropathogenic, and Newborn Meningitis-Causing Escherichia coli: How Closely Related Are They? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Macedonia, M.C.; Drewes, J.L.; Markham, N.O.; Simmons, A.J.; Roland, J.T.; Vega, P.N.; Scurrah, C.R.; Coffey, R.J.; Shrubsole, M.J.; Sears, C.L.; et al. Clinically Adaptable Polymer Enables Simultaneous Spatial Analysis of Colonic Tissues and Biofilms. NPJ Biofilms Microbiomes 2020, 6, 33. [Google Scholar] [CrossRef]
- Lee, M.S.; Hyun, H.; Park, I.; Kim, S.; Jang, D.-H.; Kim, S.; Im, J.-K.; Kim, H.; Lee, J.H.; Kwon, T.; et al. Quantitative Fluorescence In Situ Hybridization (FISH) of Magnetically Confined Bacteria Enables Early Detection of Human Bacteremia. Small Methods 2022, 6, 2101239. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chat, H.; Girondier, L.; Dalmasso, G.; Vachias, C.; Guillouard, L.; Bonnin, V.; Kavanaugh, D.; Birer, A.; Bonnet, M.; Barnich, N.; et al. Vat-Mediated Mucus Penetration Enables Genotoxic Activity of pks+ Escherichia coli. Int. J. Mol. Sci. 2025, 26, 5353. https://doi.org/10.3390/ijms26115353
Chat H, Girondier L, Dalmasso G, Vachias C, Guillouard L, Bonnin V, Kavanaugh D, Birer A, Bonnet M, Barnich N, et al. Vat-Mediated Mucus Penetration Enables Genotoxic Activity of pks+ Escherichia coli. International Journal of Molecular Sciences. 2025; 26(11):5353. https://doi.org/10.3390/ijms26115353
Chicago/Turabian StyleChat, Héloïse, Léa Girondier, Guillaume Dalmasso, Caroline Vachias, Laurent Guillouard, Virginie Bonnin, Devon Kavanaugh, Aurélien Birer, Mathilde Bonnet, Nicolas Barnich, and et al. 2025. "Vat-Mediated Mucus Penetration Enables Genotoxic Activity of pks+ Escherichia coli" International Journal of Molecular Sciences 26, no. 11: 5353. https://doi.org/10.3390/ijms26115353
APA StyleChat, H., Girondier, L., Dalmasso, G., Vachias, C., Guillouard, L., Bonnin, V., Kavanaugh, D., Birer, A., Bonnet, M., Barnich, N., Bonnet, R., & Delmas, J. (2025). Vat-Mediated Mucus Penetration Enables Genotoxic Activity of pks+ Escherichia coli. International Journal of Molecular Sciences, 26(11), 5353. https://doi.org/10.3390/ijms26115353





