Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches
Abstract
1. Introduction
2. Synthesis of Silver Nanoparticles
3. Characterisation Techniques
4. Interaction of AgNPs with Cancer Therapeutic Modalities
4.1. AgNPs and Radiotherapy
4.2. AgNPs and Phototherapy
4.3. AgNPs and Photoimmunotherapy
4.4. Synergistic Interactions Between AgNPs and Chemotherapeutic Medications
5. AgNPs as Drug Delivery Agents
6. Toxicity of Silver Nanoparticles
6.1. Cellular Uptake of AgNPs
6.2. Biodistribution of AgNPs
6.3. Principal Pathways of AgNPs’ Toxicity
6.4. Organ Specific and Cellular Toxicity
7. Standardized and Alternative Safety Assessment Methods for AgNPs
7.1. In Vitro Assays
7.2. Standard Regulatory Toxicology Tests
7.3. Alternative Models
8. Novel Methods and Approaches Evaluating AgNPs’ Toxicity
8.1. Multi-Omics and AgNPs Safety Profile Assessment
8.1.1. Genomics
8.1.2. Transcriptomics
8.1.3. Proteomics
8.1.4. Metabolomics
8.2. Organ-on-a-Chip (OoC) Platforms (Microfluidic Models/Microfluidic Chip)
Advantages, Limitations, and Future Perspectives of Organ-on-a-Chip for Nanotoxicology
8.3. Radiolabeling Techniques
Advantages, Limitations, and Future Perspectives of Radiolabelling
8.4. Lipidomics and Interactomics
8.5. High-Throughput Screening (HTS)
9. Prediction Models in AgNP Toxicity
9.1. Prediction of Dynamic Toxicity of Nanoparticles Using Machine Learning and AI
9.2. Biomarker Identification (e.g., Inflammatory Cytokines)
9.3. Pharmacokinetic (PBPK) Models
9.4. Quantitative Structure–Activity Relationships (QSAR)
10. Strategies to Mitigate the Potential Risk Associated with NP-Based Therapies
10.1. Surface Modifications
10.2. Other Strategies
11. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver Nanoparticles |
| AI | Artificial Intelligence |
| ADME | Absorption, Distribution, Metabolism, and Excretion |
| ATP | Adenosine Triphosphate |
| CAM | Chorioallantoic Membrane |
| DLS | Dynamic Light Scattering |
| DEG | Differentially Expressed Gene |
| DNA | Deoxyribonucleic Acid |
| ER | Endoplasmic Reticulum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HET-CAM | Hen’s Egg Test on the Chorioallantoic Membrane |
| IL | Interleukin |
| LDH | Lactate Dehydrogenase |
| ML | Machine Learning |
| mRNA | Messenger Ribonucleic Acid |
| MTT | “3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide” |
| NPs | Nanoparticles |
| OoC | Organ-on-a-Chip |
| PDT | Photodynamic Therapy |
| PIT | Photoimmunotherapy |
| PTT | Photothermal Therapy |
| ROS | Reactive Oxygen Species |
| RT | Radiotherapy |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SOD | Superoxide Dismutase |
| TEM | Transmission Electron Microscopy |
| TNF | Tumour Necrosis Factor |
| UV/Vis | Ultraviolet Visible Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| XRD | X-ray Diffraction |
| ZP | Zeta Potential |
References
- Salata, O. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef]
- Eifler, A.C.; Thaxton, C.S. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. Methods Mol. Biol. 2011, 726, 325–338. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mukherjee, S.; Mishra, M. Nanoparticles used in dentistry: A review. J. Oral Biol. Craniofac. Res. 2018, 8, 58–67. [Google Scholar] [CrossRef]
- Cadierno, V. Recent Advances in Organometallic Chemistry and Catalysis. Catalysts 2021, 11, 646. [Google Scholar] [CrossRef]
- Yan, N.; Xiao, C.; Kou, Y. Transition metal nanoparticle catalysis in green solvents. Coord. Chem. Rev. 2010, 254, 1179–1218. [Google Scholar] [CrossRef]
- Weiss, J.; Takhistov, P.; McClements, D.J. Functional Materials in Food Nanotechnology. J. Food Sci. 2006, 71, R107–R116. [Google Scholar] [CrossRef]
- Contado, C. Nanomaterials in consumer products: A challenging analytical problem. Front. Chem. 2015, 3, 48. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Karunakar, K.K.; Cheriyan, B.V.; R, K.; M, G.; B, A. Therapeutic advancements in nanomedicine: The multifaceted roles of silver nanoparticles. Biotechnol. Notes 2024, 5, 64–79. [Google Scholar] [CrossRef]
- Adamo, F.M.; Silva Barcelos, E.C.; De Falco, F.; Dorillo, E.; Rompietti, C.; Sorcini, D.; Stella, A.; Del Papa, B.; Baldoni, S.; Esposito, A.; et al. Therapeutic Targeting Potential of Novel Silver Nanoparticles Coated with Anti-CD20 Antibody against Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3618. [Google Scholar] [CrossRef]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Silver Nanoparticles (AgNPs) as an Antimycobacterial Agent: A Comprehensive Review. Antibiotics 2024, 13, 1106. [Google Scholar] [CrossRef]
- Takáč, P.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Takáčová, G. The Role of Silver Nanoparticles in the Diagnosis and Treatment of Cancer: Are There Any Perspectives for the Future? Life 2023, 13, 466. [Google Scholar] [CrossRef]
- Jagiello, K.; Ciura, K. In vitro to in vivo extrapolation to support the development of the next generation risk assessment (NGRA) strategy for nanomaterials. Nanoscale 2022, 14, 6735–6742. [Google Scholar] [CrossRef]
- Quintero-Quiroz, C.; Acevedo, N.; Zapata-Giraldo, J.; Botero, L.E.; Quintero, J.; Zárate-Triviño, D.; Saldarriaga, J.; Pérez, V.Z. Optimization of silver nanoparticle synthesis by chemical reduction and evaluation of its antimicrobial and toxic activity. Biomater. Res. 2019, 23, 27. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef]
- Chen, S.-F.; Zhang, H. Aggregation kinetics of nanosilver in different water conditions. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 035006. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef]
- Ghazali, S.; Jaafar, M.; Azizan, A. Synthesis of Silver Nanoparticles by Chemical Reduction Method: Effect of Reducing Agent and Surfactant Concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Ijaz, I.; Ezaz, G.; Ammara, N.; Bukhari, A. Detail review on chemical, physical and green synthesis, classification, characterizations and applications of nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- García-Barrasa, J.; López-de-Luzuriaga, J.M.; Monge, M. Silver nanoparticles: Synthesis through chemical methods in solution and biomedical applications. Cent. Eur. J. Chem. 2011, 9, 7–19. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, K.; Gondil, V.S.; Sharma, S.; Jain, D.V.S.; Chhibber, S.; Sharma, R.K.; Wangoo, N. Synthesis, characterization, mechanistic studies and antimicrobial efficacy of biomolecule capped and pH modulated silver nanoparticles. J. Mol. Liq. 2018, 249, 1145–1150. [Google Scholar] [CrossRef]
- Calderón-Jiménez, B.; Montoro Bustos, A.R.; Pereira Reyes, R.; Paniagua, S.A.; Vega-Baudrit, J.R. Novel pathway for the sonochemical synthesis of silver nanoparticles with near-spherical shape and high stability in aqueous media. Sci. Rep. 2022, 12, 882. [Google Scholar] [CrossRef]
- Jansirani, D.; Raja, N.; Hariprasanth, R.J.; Preethi, S.S.; Sorna Kumar, S.K. Synthesis of colloidal starched silver nanoparticles by sonochemical method and evaluation of its antibacterial activity. J. Chem. Pharm. Sci. 2016, 9, 177–179. [Google Scholar]
- Wang, N.; Ma, Z.; Zhou, S.; Liang, G. Facile fabrication of SERS substrate based on food residue eggshell membrane. Chem. Phys. Lett. 2016, 666, 45–50. [Google Scholar] [CrossRef]
- Xu, H.; Suslick, K.S. Sonochemical Synthesis of Highly Fluorescent Ag Nanoclusters. ACS Nano 2010, 4, 3209–3214. [Google Scholar] [CrossRef]
- Jara, N.; Milán, N.S.; Rahman, A.; Mouheb, L.; Boffito, D.C.; Jeffryes, C.; Dahoumane, S.A. Photochemical Synthesis of Gold and Silver Nanoparticles-A Review. Molecules 2021, 26, 4585. [Google Scholar] [CrossRef]
- Seku, K.R.; Gangapuram, B.; Pejjai, B.; Kadimpati, K.K.; Golla, N. Microwave-assisted synthesis of silver nanoparticles and their application in catalytic, antibacterial and antioxidant activities. J. Nanostructure Chem. 2018, 8, 179–188. [Google Scholar] [CrossRef]
- Starowicz, M.; Stypuła, B.; Banaś, J. Electrochemical synthesis of silver nanoparticles. Electrochem. Commun. 2006, 8, 227–230. [Google Scholar] [CrossRef]
- Sowani, H.M.; Mohite, P.; Munot, H.; Shouche, Y.; Bapat, T.; Kumar, A.; Kulkarni, M.; Zinjarde, S. Green synthesis of gold and silver nanoparticles by an Actinomycete Gordonia amicalis HS-11: Mechanistic aspects and biological application. Process Biochem. 2015, 51, 374–383. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid stabilized gold and silver nanoparticles derived from the Actinomycete Gordonia amicalis HS-11 as effective free radical scavengers. Enzym. Microb. Technol. 2016, 95, 164–173. [Google Scholar] [CrossRef]
- Baláž, M.; Balážová, Ľ.; Daneu, N.; Dutková, E.; Balážová, M.; Bujňáková, Z.; Shpotyuk, Y. Plant-Mediated Synthesis of Silver Nanoparticles and Their Stabilization by Wet Stirred Media Milling. Nanoscale Res. Lett. 2017, 12, 83. [Google Scholar] [CrossRef]
- Sastry, M.; Ahmad, A.; Khan, M.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef]
- Marchiol, L.; Mattiello, A.; Poscic, F.; Giordano, C.; Musetti, R. In vivo synthesis of nanomaterials in plants: Location of silver nanoparticles and plant metabolism. Nanoscale Res. Lett. 2014, 9, 101. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Synthesis of nanoparticles by microorganisms and their application in enhancing microbiological reaction rates. Chemosphere 2011, 82, 489–494. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Souza, G.I.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, Y.J.; Singh, P.; Mathiyalagan, R.; Jin, Y.; Yang, D.C. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1127–1132. [Google Scholar] [CrossRef]
- Dakhil, A.S. Biosynthesis of silver nanoparticle (AgNPs) using Lactobacillus and their effects on oxidative stress biomarkers in rats. J. King Saud. Univ.-Sci. 2017, 29, 462–467. [Google Scholar] [CrossRef]
- Sadowski, Z.; Maliszewska, I.; Grochowalska, B.; Polowczyk, I.; Koźlecki, T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci.-Pol. 2008, 26, 419–424. [Google Scholar]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A review of microbes mediated biosynthesis of silver nanoparticles and their enhanced antimicrobial activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef]
- El-Said, W.A.; Cho, H.-Y.; Yea, C.-H.; Choi, J.-W. Synthesis of Metal Nanoparticles Inside Living Human Cells Based on the Intracellular Formation Process. Adv. Mater. 2014, 26, 910–918. [Google Scholar] [CrossRef]
- Konował, E.; Sybis, M.; Modrzejewska-Sikorska, A.; Milczarek, G. Synthesis of dextrin-stabilized colloidal silver nanoparticles and their application as modifiers of cement mortar. Int. J. Biol. Macromol. 2017, 104, 165–172. [Google Scholar] [CrossRef]
- Chang, T.Y.; Chen, C.C.; Cheng, K.M.; Chin, C.Y.; Chen, Y.H.; Chen, X.A.; Sun, J.R.; Young, J.J.; Chiueh, T.S. Trimethyl chitosan-capped silver nanoparticles with positive surface charge: Their catalytic activity and antibacterial spectrum including multidrug-resistant strains of Acinetobacter baumannii. Colloids Surf. B Biointerfaces 2017, 155, 61–70. [Google Scholar] [CrossRef]
- Vasileva, P.; Donkova, B.; Karadjova, I.; Dushkin, C. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf. A Physicochem. Eng. Asp. 2011, 382, 203–210. [Google Scholar] [CrossRef]
- Venkatesan, J.; Lee, J.Y.; Kang, D.S.; Anil, S.; Kim, S.K.; Shim, M.S.; Kim, D.G. Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol. 2017, 98, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Koc, B.; Akyuz, L.; Cakmak, Y.S.; Sargin, I.; Salaberria, A.M.; Labidi, J.; Ilk, S.; Cekic, F.O.; Akata, I.; Kaya, M. Production and characterization of chitosan-fungal extract films. Food Biosci. 2020, 35, 100545. [Google Scholar] [CrossRef]
- Kwiczak-Yiğitbaşı, J.; Laçin, Ö.; Demir, M.; Ahan, R.E.; Şeker, U.Ö.Ş.; Baytekin, B. A sustainable preparation of catalytically active and antibacterial cellulose metal nanocomposites via ball milling of cellulose. Green Chem. 2020, 22, 455–464. [Google Scholar] [CrossRef]
- Płaza, G.A.; Chojniak, J.; Banat, I.M. Biosurfactant mediated biosynthesis of selected metallic nanoparticles. Int. J. Mol. Sci. 2014, 15, 13720–13737. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef]
- Ghiyasiyan-Arani, M.; Salavati-Niasari, M.; Masjedi-Arani, M.; Mazloom, F. An easy sonochemical route for synthesis, characterization and photocatalytic performance of nanosized FeVO4 in the presence of aminoacids as green capping agents. J. Mater. Sci. Mater. Electron. 2018, 29, 474–485. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Dias, H.V.R.; Kharisov, B.I.; Pérez, B.O.; Pérez, V.M.J. The greener synthesis of nanoparticles. Trends Biotechnol. 2013, 31, 240–248. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green Synthesis of Silver Nanoparticles with Antibacterial Activity Using Various Medicinal Plant Extracts: Morphology and Antibacterial Efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Sutthanont, N.; Attrapadung, S.; Nuchprayoon, S. Larvicidal Activity of Synthesized Silver Nanoparticles from Curcuma zedoaria Essential Oil against Culex quinquefasciatus. Insects 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- AL-Shnani, F.; Al-Haddad, T.; Karabet, F.; Allaf, A.W. Chitosan Loaded with Silver Nanoparticles, CS-AgNPs, Using Thymus Syriacus, Wild Mint, and Rosemary Essential Oil Extracts as Reducing and Capping Agents; J. Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- de Melo, A.P.Z.; de Oliveira Brisola Maciel, M.V.; Sganzerla, W.G.; da Rosa Almeida, A.; de Armas, R.D.; Machado, M.H.; da Rosa, C.G.; Nunes, M.R.; Bertoldi, F.C.; Barreto, P.L.M. Antibacterial activity, morphology, and physicochemical stability of biosynthesized silver nanoparticles using thyme (Thymus vulgaris) essential oil. Mater. Res. Express 2020, 7, 015087. [Google Scholar] [CrossRef]
- Binupriya, A.R.; Sathishkumar, M.; Yun, S.-I. Myco-crystallization of Silver Ions to Nanosized Particles by Live and Dead Cell Filtrates of Aspergillus oryzae var. viridis and Its Bactericidal Activity toward Staphylococcus aureus KCCM 12256. Ind. Eng. Chem. Res. 2010, 49, 852–858. [Google Scholar] [CrossRef]
- Nanda, A.; Saravanan, M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine 2009, 5, 452–456. [Google Scholar] [CrossRef]
- Saifuddin, N.; Wong, C.W.; Yasumira, A.A.N. Rapid Biosynthesis of Silver Nanoparticles Using Culture Supernatant of Bacteria with Microwave Irradiation. J. Chem. 2009, 6, 734264. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Minaeian, S.; Shahverdi, H.R.; Jamalifar, H.; Nohi, A.-A. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: A novel biological approach. Process Biochem. 2007, 42, 919–923. [Google Scholar] [CrossRef]
- Al-Bahrani, R.; Raman, J.; Lakshmanan, H.; Hassan, A.A.; Sabaratnam, V. Green synthesis of silver nanoparticles using tree oyster mushroom Pleurotus ostreatus and its inhibitory activity against pathogenic bacteria. Mater. Lett. 2017, 186, 21–25. [Google Scholar] [CrossRef]
- Bhat, R.; Deshpande, R.; Ganachari, S.V.; Huh, D.S.; Venkataraman, A. Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom pleurotus Florida and their antibacterial activity studies. Bioinorg. Chem. Appl. 2011, 2011, 650979. [Google Scholar] [CrossRef]
- Philip, D. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 73, 374–381. [Google Scholar] [CrossRef]
- Madhanraj, R.; Eyini, M.; Balaji, P. Antioxidant Assay of Gold and Silver Nanoparticles from Edible Basidiomycetes Mushroom Fungi. Free Radic. Antioxid. 2017, 7, 137–142. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Baláž, M.; Bedlovičová, Z.; Daneu, N.; Siksa, P.; Sokoli, L.; Tkáčiková, Ľ.; Salayová, A.; Džunda, R.; Kováčová, M.; Bureš, R.; et al. Mechanochemistry as an Alternative Method of Green Synthesis of Silver Nanoparticles with Antibacterial Activity: A Comparative Study. Nanomaterials 2021, 11, 1139. [Google Scholar] [CrossRef]
- Amendola, V.; Polizzi, S.; Meneghetti, M. Free silver nanoparticles synthesized by laser ablation in organic solvents and their easy functionalization. Langmuir 2007, 23, 6766–6770. [Google Scholar] [CrossRef]
- Patil, V.; Murali Sastry, a. Electrostatically controlled diffusion of carboxylic acid derivatized Q-state CdS nanoparticles in thermally evaporated fatty amine films. J. Chem. Soc. Faraday Trans. 1997, 93, 4347–4353. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized Surface Plasmon Resonance Spectroscopy and Sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- van der Merwe, P.A.; Barclay, A.N. Analysis of cell-adhesion molecule interactions using surface plasmon resonance. Curr. Opin. Immunol. 1996, 8, 257–261. [Google Scholar] [CrossRef]
- Noginov, M.A.; Zhu, G.; Bahoura, M.; Adegoke, J.; Small, C.; Ritzo, B.A.; Drachev, V.P.; Shalaev, V.M. The effect of gain and absorption on surface plasmons in metal nanoparticles. Appl. Phys. B 2007, 86, 455–460. [Google Scholar] [CrossRef]
- Jiang, X.; Jiang, J.; Jin, Y.; Wang, E.; Dong, S. Effect of colloidal gold size on the conformational changes of adsorbed cytochrome c: Probing by circular dichroism, UV-visible, and infrared spectroscopy. Biomacromolecules 2005, 6, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Liu, H.; Webster, T.J. Nanomedicine for implants: A review of studies and necessary experimental tools. Biomaterials 2007, 28, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.K.; Pandey, D.K.; Yadav, R.R.; Singh, D. A study of ZnO nanoparticles and ZnO-EG nanofluid. J. Exp. Nanosci. 2013, 8, 731–741. [Google Scholar] [CrossRef]
- Macaluso, R.T. Introduction to Powder Diffraction and its Application to Nanoscale and Heterogeneous Materials. ACS Symp. Ser. 2010, 1010, 75–86. [Google Scholar]
- Vaia, R.A.; Liu, W. X-ray powder diffraction of polymer/layered silicate nanocomposites: Model and practice. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1590–1600. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, J.R.; Medintz, I.L. Analyzing Nanomaterial Bioconjugates: A Review of Current and Emerging Purification and Characterization Techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef]
- Baláž, M.; Bedlovicova, Z.; Kováčová, M.; Salayová, A.; Balážová, Ľ. Green and Bio-Mechanochemical Approach to Silver Nanoparticles Synthesis, Characterization and Antibacterial Potential. In Nanostructures for Antimicrobial and Antibiofilm Applications. Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 145–183. [Google Scholar] [CrossRef]
- Demathieu, C.; Chehimi, M.M.; Lipskier, J.-F.; Caminade, A.-M.; Majoral, J.-P. Characterization of Dendrimers by X-Ray Photoelectron Spectroscopy. Appl. Spectrosc. 1999, 53, 1277–1281. [Google Scholar] [CrossRef]
- Stevie, F.; Donley, C. Introduction to x-ray photoelectron spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 063204. [Google Scholar] [CrossRef]
- Pons, T.; Uyeda, H.T.; Medintz, I.L.; Mattoussi, H. Hydrodynamic dimensions, electrophoretic mobility, and stability of hydrophilic quantum dots. J. Phys. Chem. B 2006, 110, 20308–20316. [Google Scholar] [CrossRef]
- Fissan, H.; Ristig, S.; Kaminski, H.; Asbach, C.; Epple, M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods 2014, 6, 7324–7334. [Google Scholar] [CrossRef]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Sia, J.; Szmyd, R.; Hau, E.; Gee, H.E. Molecular Mechanisms of Radiation-Induced Cancer Cell Death: A Primer. Front. Cell Dev. Biol. 2020, 8, 41. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid. Med. Cell Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Carter, J.D.; Cheng, N.N.; Qu, Y.; Suarez, G.D.; Guo, T. Nanoscale energy deposition by X-ray absorbing nanostructures. J. Phys. Chem. B 2007, 111, 11622–11625. [Google Scholar] [CrossRef]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Biau, J.; Chautard, E.; Verrelle, P.; Dutreix, M. Altering DNA Repair to Improve Radiation Therapy: Specific and Multiple Pathway Targeting. Front. Oncol. 2019, 9, 1009. [Google Scholar] [CrossRef]
- Manivannan, K.; Cheng, C.C.; Anbazhagan, R.; Tsai, H.C.; Chen, J.K. Fabrication of silver seeds and nanoparticle on core-shell Ag@SiO(2) nanohybrids for combined photothermal therapy and bioimaging. J. Colloid. Interface Sci. 2019, 537, 604–614. [Google Scholar] [CrossRef]
- Thompson, E.A.; Graham, E.; MacNeill, C.M.; Young, M.; Donati, G.; Wailes, E.M.; Jones, B.T.; Levi-Polyachenko, N.H. Differential response of MCF7, MDA-MB-231, and MCF 10A cells to hyperthermia, silver nanoparticles and silver nanoparticle-induced photothermal therapy. Int. J. Hyperth. 2014, 30, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Diniz, F.R.; Maia, R.C.A.P.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2020, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Lee, S.; Amatya, R.; Cheong, H.; Moon, C.; Kwak, H.D.; Min, K.A.; Shin, M.C. ICG-Loaded PEGylated BSA-Silver Nanoparticles for Effective Photothermal Cancer Therapy. Int. J. Nanomed. 2020, 15, 5459–5471. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, X.Q.; An, J.; Cheng, K.; Hou, X.L.; Zhang, X.S.; Song, X.L.; Huang, K.C.; Chen, W.; Liu, B.; et al. A near-infrared light-controlled smart nanocarrier with reversible polypeptide-engineered valve for targeted fluorescence-photoacoustic bimodal imaging-guided chemo-photothermal therapy. Theranostics 2019, 9, 7666–7679. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Yang, Q.; Pan, M.; Hao, Y.; He, X.; Peng, J.; Qian, Z. Ag2S nanoparticle-mediated multiple ablations reinvigorates the immune response for enhanced cancer photo-immunotherapy. Biomaterials 2021, 264, 120451. [Google Scholar] [CrossRef]
- Amatya, R.; Hwang, S.; Park, T.; Chung, Y.J.; Ryu, S.; Lee, J.; Cheong, H.; Moon, C.; Min, K.A.; Shin, M.C. BSA/Silver Nanoparticle-Loaded Hydrogel Film for Local Photothermal Treatment of Skin Cancer. Pharm. Res. 2021, 38, 873–883. [Google Scholar] [CrossRef]
- Hou, X.L.; Dai, X.; Yang, J.; Zhang, B.; Zhao, D.H.; Li, C.Q.; Yin, Z.Y.; Zhao, Y.D.; Liu, B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B 2020, 8, 8623–8633. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy—Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef]
- Frew, A.J.; Johnstone, R.W.; Bolden, J.E. Enhancing the apoptotic and therapeutic effects of HDAC inhibitors. Cancer Lett. 2009, 280, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Igaz, N.; Kovács, D.; Rázga, Z.; Kónya, Z.; Boros, I.M.; Kiricsi, M. Modulating chromatin structure and DNA accessibility by deacetylase inhibition enhances the anti-cancer activity of silver nanoparticles. Colloids Surf. B Biointerfaces 2016, 146, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. Combination Effect of Silver Nanoparticles and Histone Deacetylases Inhibitor in Human Alveolar Basal Epithelial Cells. Molecules 2018, 23, 2046. [Google Scholar] [CrossRef] [PubMed]
- Sadat Shandiz, S.A.; Shafiee Ardestani, M.; Shahbazzadeh, D.; Assadi, A.; Ahangari Cohan, R.; Asgary, V.; Salehi, S. Novel imatinib-loaded silver nanoparticles for enhanced apoptosis of human breast cancer MCF-7 cells. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1–10. [Google Scholar] [CrossRef]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous Responses of Ovarian Cancer Cells to Silver Nanoparticles as a Single Agent and in Combination with Cisplatin. J. Nanomater. 2017, 2017, 5107485. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, S.; Wang, Y.; Dai, W.; Zou, H.; Wang, S.; Zhang, J.; Pan, J. Salinomycin effectively eliminates cancer stem-like cells and obviates hepatic metastasis in uveal melanoma. Mol. Cancer 2019, 18, 159. [Google Scholar] [CrossRef]
- Zhang, X.F.; Gurunathan, S. Combination of salinomycin and silver nanoparticles enhances apoptosis and autophagy in human ovarian cancer cells: An effective anticancer therapy. Int. J. Nanomed. 2016, 11, 3655–3675. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Nguyen-Tri, P.; Mohapatra, S.; Ma, D.; Rtimi, S.; Ghosh, S.; Basu, R.; Bera, S.; Vu, M.; Khater, A.; et al. Noble Metal-Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- van der Meel, R.; Sulheim, E.; Shi, Y.; Kiessling, F.; Mulder, W.J.M.; Lammers, T. Smart cancer nanomedicine. Nat. Nanotechnol. 2019, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- P.Velavan, C.K.; Palanivel, V. Nanoparticles as Drug Delivery Systems. J. Pharm. Sci. Res. 2015, 7, 1118–1122. [Google Scholar]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Henrich-Noack, P.; Nikitovic, D.; Neagu, M.; Docea, A.O.; Engin, A.B.; Gelperina, S.; Shtilman, M.; Mitsias, P.; Tzanakakis, G.; Gozes, I.; et al. The blood-brain barrier and beyond: Nano-based neuropharmacology and the role of extracellular matrix. Nanomedicine 2019, 17, 359–379. [Google Scholar] [CrossRef]
- Mahmood, M.; Casciano, D.A.; Mocan, T.; Iancu, C.; Xu, Y.; Mocan, L.; Iancu, D.T.; Dervishi, E.; Li, Z.; Abdalmuhsen, M.; et al. Cytotoxicity and biological effects of functional nanomaterials delivered to various cell lines. J. Appl. Toxicol. 2010, 30, 74–83. [Google Scholar] [CrossRef]
- Varadharajaperumal, P.; Muthuswamy, S.; Pothagar, D.; Ganesan, M.; Santhanam, A. Adenia hondala-derived Biopolymer Nanoparticles Cause G2/M Cell Cycle Arrest in Breast Cancer Cells. Uttar Pradesh J. Zool. 2024, 45, 550–560. [Google Scholar] [CrossRef]
- Majd, M.H. Combination therapy of cisplatin and green silver nanoparticles enhances cytotoxicity and apoptosis in breast cancer cells. CP 2024, 6, 2770. [Google Scholar] [CrossRef]
- Ghobadi, M.; Salehi, S.; Ardestani, M.T.S.; Mousavi-Khattat, M.; Shakeran, Z.; Khosravi, A.; Cordani, M.; Zarrabi, A. Amine-functionalized mesoporous silica nanoparticles decorated by silver nanoparticles for delivery of doxorubicin in breast and cervical cancer cells. Eur. J. Pharm. Biopharm. 2024, 201, 114349. [Google Scholar] [CrossRef]
- Tunç, T. Synthesis and characterization of silver nanoparticles loaded with carboplatin as a potential antimicrobial and cancer therapy. Cancer Nanotechnol. 2024, 15, 2. [Google Scholar] [CrossRef]
- Tunç, T.; Hepokur, C.; Kari̇per, A. Synthesis and Characterization of Paclitaxel-Loaded Silver Nanoparticles: Evaluation of Cytotoxic Effects and Antimicrobial Activity. Bioinorg. Chem. Appl. 2024, 2024, 9916187. [Google Scholar] [CrossRef]
- Maher, S.; Kalil, H.; Liu, G.; Sossey-Alaoui, K.; Bayachou, M. Alginate-based hydrogel platform embedding silver nanoparticles and cisplatin: Characterization of the synergistic effect on a breast cancer cell line. Front. Mol. Biosci. 2023, 10, 1242838. [Google Scholar] [CrossRef] [PubMed]
- Al-Serwi, R.H.; Eladl, M.A.; El-Sherbiny, M.; Saleh, M.A.; Othman, G.; Alshahrani, S.M.; Alnefaie, R.; Jan, A.M.; Alnasser, S.M.; Albalawi, A.E.; et al. Targeted Drug Administration onto Cancer Cells Using Hyaluronic Acid–Quercetin-Conjugated Silver Nanoparticles. Molecules 2023, 28, 4146. [Google Scholar] [CrossRef]
- Malinga, T.; Kudanga, T.; Mbatha, L. Stealth doxorubicin conjugated bimetallic selenium/silver nanoparticles for targeted cervical cancer therapy. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 045006. [Google Scholar] [CrossRef]
- Muhammad, N.; Zhao, H.; Song, W.; Gu, M.; Li, Q.; Liu, Y.; Li, C.; Wang, J.; Zhan, H. Silver nanoparticles functionalized Paclitaxel nanocrystals enhance overall anti-cancer effect on human cancer cells. Nanotechnology 2021, 32, 085105. [Google Scholar] [CrossRef] [PubMed]
- Tobi, A.; Willmore, A.-M.A.; Kilk, K.; Sidorenko, V.; Braun, G.B.; Soomets, U.; Sugahara, K.N.; Ruoslahti, E.; Teesalu, T. Silver Nanocarriers Targeted with a CendR Peptide Potentiate the Cytotoxic Activity of an Anticancer Drug. Adv. Ther. 2021, 4, 2000097. [Google Scholar] [CrossRef]
- Li, F.; Yang, H.; Cao, Y.; Li, D.; Ma, J.; Liu, P. DOX-loaded silver nanotriangles and photothermal therapy exert a synergistic antibreast cancer effect via ROS/ERK1/2 signaling pathway. Nanotechnology 2022, 33, 075101. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Pham, D.P.; Kim, D. Oxidative Stress-Induced Silver Nano-Carriers for Chemotherapy. Pharmaceuticals 2022, 15, 1449. [Google Scholar] [CrossRef]
- Jabar, M.S.; Al- Shammaree, S.A.W. Doxorubicin Immobilization on chitosan-modified silver Nanoparticles as a drug delivery method for effective anticancer treatment. J. Contemp. Med. Sci. 2022, 8, 107. [Google Scholar] [CrossRef]
- Peruzynska, M.; Cendrowski, K.; Barylak, M.; Roginska, D.; Tarnowski, M.; Tkacz, M.; Kurzawski, M.; Machalinski, B.; Mijowska, E.; Drozdzik, M. Study on size effect of the silica nanospheres with solid core and mesoporous shell on cellular uptake. Biomed. Mater. 2015, 10, 065012. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Marchesano, V.; Hernandez, Y.; Salvenmoser, W.; Ambrosone, A.; Tino, A.; Hobmayer, B.; de la Fuente, J.M.; Tortiglione, C. Imaging inward and outward trafficking of gold nanoparticles in whole animals. ACS Nano 2013, 7, 2431–2442. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.J.; Hosseinian, F.; Willmore, W.G. A Current Overview of the Biological and Cellular Effects of Nanosilver. Int. J. Mol. Sci. 2018, 19, 2030. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of silver nanoparticles on human cells: Effect of particle size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1033–1043. [Google Scholar] [CrossRef]
- Lin, Z.; Monteiro-Riviere, N.A.; Riviere, J.E. Pharmacokinetics of metallic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 189–217. [Google Scholar] [CrossRef]
- Park, K.; Park, E.J.; Chun, I.K.; Choi, K.; Lee, S.H.; Yoon, J.; Lee, B.C. Bioavailability and toxicokinetics of citrate-coated silver nanoparticles in rats. Arch. Pharm. Res. 2011, 34, 153–158. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, P.; Yoon, J.; Lee, B.; Choi, K.; Kil, K.H.; Park, K. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology 2013, 7, 1120–1130. [Google Scholar] [CrossRef]
- Riviere, J.E. Of Mice, Men and Nanoparticle Biocoronas: Are In Vitro to In Vivo Correlations and Interspecies Extrapolations Realistic? Nanomedicine 2013, 8, 1357–1359. [Google Scholar] [CrossRef]
- Sahneh, F.D.; Scoglio, C.M.; Monteiro-Riviere, N.A.; Riviere, J.E. Predicting the impact of biocorona formation kinetics on interspecies extrapolations of nanoparticle biodistribution modeling. Nanomedicine 2015, 10, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, S.; Huang, Y.; Zhang, T.; Liu, X.; Hu, Y.; Zhang, Z.; Tang, M. Acute toxic effects and gender-related biokinetics of silver nanoparticles following an intravenous injection in mice. J. Appl. Toxicol. 2012, 32, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Recordati, C.; De Maglie, M.; Cella, C.; Argentiere, S.; Paltrinieri, S.; Bianchessi, S.; Losa, M.; Fiordaliso, F.; Corbelli, A.; Milite, G.; et al. Repeated oral administration of low doses of silver in mice: Tissue distribution and effects on central nervous system. Part. Fibre Toxicol. 2021, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, L.; Li, R.; Dan, M.; Liu, H.; Wang, X.; Wu, X.; Liu, Y.; Xu, L.; Xie, L. Bio-distribution and bio-availability of silver and gold in rat tissues with silver/gold nanorod administration. RSC Adv. 2018, 8, 12260–12268. [Google Scholar] [CrossRef]
- Walker, M.; Parsons, D. The biological fate of silver ions following the use of silver-containing wound care products—A review. Int. Wound J. 2014, 11, 496–504. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Andonova, V.; Ivanova, N.; Gugleva, V.; Dobreva, M.; Stefanov, S.R.; Pehlivanov, I. Silver Nanoparticles as Multi-Functional Drug Delivery Systems. In Nanomedicines; Farrukh, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Borrego, B.; Juárez-Moreno, K.; García-García, M.; Mota Morales, J.D.; Bogdanchikova, N.; Huerta-Saquero, A. Toxicity of silver nanoparticles in biological systems: Does the complexity of biological systems matter? Toxicol. Lett. 2017, 276, 11–20. [Google Scholar] [CrossRef]
- Hante, N.K.; Medina, C.; Santos-Martinez, M.J. Effect on Platelet Function of Metal-Based Nanoparticles Developed for Medical Applications. Front. Cardiovasc. Med. 2019, 6, 139. [Google Scholar] [CrossRef]
- De Matteis, V.; Malvindi, M.A.; Galeone, A.; Brunetti, V.; De Luca, E.; Kote, S.; Kshirsagar, P.; Sabella, S.; Bardi, G.; Pompa, P.P. Negligible particle-specific toxicity mechanism of silver nanoparticles: The role of Ag+ ion release in the cytosol. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 731–739. [Google Scholar] [CrossRef]
- Tardillo Suárez, V.; Karepina, E.; Chevallet, M.; Gallet, B.; Cottet-Rousselle, C.; Charbonnier, P.; Moriscot, C.; Michaud-Soret, I.; Bal, W.; Fuchs, A.; et al. Nuclear translocation of silver ions and hepatocyte nuclear receptor impairment upon exposure to silver nanoparticles. Environ. Sci. Nano 2020, 7, 1373–1387. [Google Scholar] [CrossRef]
- Wāng, Y.; Han, Y.; Xu, D.X. Developmental impacts and toxicological hallmarks of silver nanoparticles across diverse biological models. Environ. Sci. Ecotechnol. 2024, 19, 100325. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.D.; Sposito, J.C.V.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.C.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Amooaghaie, R.; Saeri, M.R.; Azizi, M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol. Environ. Saf. 2015, 120, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Che, B.; Zhang, L.W.; Dong, G.; Luo, Q.; Xin, L. Comparative genotoxicity of silver nanoparticles in human liver HepG2 and lung epithelial A549 cells. J. Appl. Toxicol. 2017, 37, 495–501. [Google Scholar] [CrossRef]
- Kora, A.J.; Sashidhar, R.B. Biogenic silver nanoparticles synthesized with rhamnogalacturonan gum: Antibacterial activity, cytotoxicity and its mode of action. Arab. J. Chem. 2018, 11, 313–323. [Google Scholar] [CrossRef]
- Galbiati, V.; Cornaghi, L.; Gianazza, E.; Potenza, M.A.; Donetti, E.; Marinovich, M.; Corsini, E. In vitro assessment of silver nanoparticles immunotoxicity. Food Chem. Toxicol. 2018, 112, 363–374. [Google Scholar] [CrossRef]
- Zhao, Q.; Sun, X.Y.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Construction of biomimetic silver nanoparticles in the treatment of lymphoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111648. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S. Silver nanoparticle protein corona and toxicity: A mini-review. J. Nanobiotechnol. 2015, 13, 55. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Podila, R.; Aldossari, A.A.; Emerson, H.; Powell, B.A.; Ke, P.C.; Rao, A.M.; Brown, J.M. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol. Sci. 2015, 143, 136–146. [Google Scholar] [CrossRef]
- Park, J.W.; Henry, T.B.; Ard, S.; Menn, F.M.; Compton, R.N.; Sayler, G.S. The association between nC60 and 17α-ethinylestradiol (EE2) decreases EE2 bioavailability in zebrafish and alters nanoaggregate characteristics. Nanotoxicology 2011, 5, 406–416. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, C.; Ni, J. Effect of inorganic nanoparticles on 17β-estradiol and 17α-ethynylestradiol adsorption by multi-walled carbon nanotubes. Environ. Pollut. 2015, 205, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Ferroni, L.; Gardin, C.; Rigo, C.; Stocchero, M.; Vindigni, V.; Cairns, W.; Zavan, B. Silver nanoparticles and mitochondrial interaction. Int. J. Dent. 2013, 2013, 312747. [Google Scholar] [CrossRef] [PubMed]
- El-Habit, O.; Moussa, E.; Hassan, B. Cytotoxicity of Silver Nanoparticles in Mice Liver Cells: An Ultrastructure Study. Egypt. J. Hosp. Med. 2014, 57, 554–564. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.; Ingle, T.; Yan, J.; He, W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef]
- Blanco, J.; Tomás-Hernández, S.; García, T.; Mulero, M.; Gómez, M.; Domingo, J.L.; Sánchez, D.J. Oral exposure to silver nanoparticles increases oxidative stress markers in the liver of male rats and deregulates the insulin signalling pathway and p53 and cleaved caspase 3 protein expression. Food Chem. Toxicol. 2018, 115, 398–404. [Google Scholar] [CrossRef]
- Mao, B.-H.; Chen, Z.-Y.; Wang, Y.-J.; Yan, S.-J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci. Rep. 2018, 8, 2445. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef]
- Bhandary, B.; Marahatta, A.; Kim, H.R.; Chae, H.J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2012, 14, 434–456. [Google Scholar] [CrossRef]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef]
- Mao, B.H.; Tsai, J.C.; Chen, C.W.; Yan, S.J.; Wang, Y.J. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology 2016, 10, 1021–1040. [Google Scholar] [CrossRef]
- Samutrtai, P.; Krobthong, S.; Roytrakul, S. Proteomics for Toxicological Pathways Screening: A Case Comparison of Low-concentration Ionic and Nanoparticulate Silver. Anal. Sci. 2020, 36, 981–987. [Google Scholar] [CrossRef]
- Das, N.C.; Roy, B.; Patra, R.; Choudhury, A.; Ghosh, M.; Mukherjee, S. Surface-Modified Noble Metal Nanoparticles as Antimicrobial Agents: Biochemical, Molecular and Therapeutic Perspectives. In Nanotechnology for Advances in Medical Microbiology; Maddela, N.R., Chakraborty, S., Prasad, R., Eds.; Springer: Singapore, 2021; pp. 165–205. [Google Scholar] [CrossRef]
- Grzelak, A.; Wojewódzka, M.; Meczynska-Wielgosz, S.; Zuberek, M.; Wojciechowska, D.; Kruszewski, M. Crucial role of chelatable iron in silver nanoparticles induced DNA damage and cytotoxicity. Redox Biol. 2018, 15, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Jiravova, J.; Tomankova, K.B.; Harvanova, M.; Malina, L.; Malohlava, J.; Luhova, L.; Panacek, A.; Manisova, B.; Kolarova, H. The effect of silver nanoparticles and silver ions on mammalian and plant cells in vitro. Food Chem. Toxicol. 2016, 96, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Iwakoshi, N.N.; Glimcher, L.H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell Biol. 2003, 23, 7448–7459. [Google Scholar] [CrossRef]
- Asselah, T.; Bièche, I.; Mansouri, A.; Laurendeau, I.; Cazals-Hatem, D.; Feldmann, G.; Bedossa, P.; Paradis, V.; Martinot-Peignoux, M.; Lebrec, D.; et al. In vivo hepatic endoplasmic reticulum stress in patients with chronic hepatitis C. J. Pathol. 2010, 221, 264–274. [Google Scholar] [CrossRef]
- Chichova, M.; Shkodrova, M.; Vasileva, P.; Kirilova, K.; Doncheva-Stoimenova, D. Influence of silver nanoparticles on the activity of rat liver mitochondrial ATPase. J. Nanopart. Res. 2014, 16, 2243. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Silva, R.; Varela, A.T.; Duarte, F.V.; Rolo, A.P.; Hussain, S.; Palmeira, C.M. Low-dose, subchronic exposure to silver nanoparticles causes mitochondrial alterations in Sprague-Dawley rats. Nanomedicine 2016, 11, 1359–1375. [Google Scholar] [CrossRef]
- Cascione, M.; Rizzello, L.; Manno, D.; Serra, A.; De Matteis, V. Green Silver Nanoparticles Promote Inflammation Shutdown in Human Leukemic Monocytes. Materials 2022, 15, 775. [Google Scholar] [CrossRef]
- Al-Doaiss, A.; Jarrar, Q.; Moshawih, S. Hepatic histopathological and ultrastructural alterations induced by 10 nm silver nanoparticles. IET Nanobiotechnol. 2020, 14, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Garcés, M.; Magnani, N.D.; Pecorelli, A.; Calabró, V.; Marchini, T.; Cáceres, L.; Pambianchi, E.; Galdoporpora, J.; Vico, T.; Salgueiro, J.; et al. Alterations in oxygen metabolism are associated to lung toxicity triggered by silver nanoparticles exposure. Free Radic. Biol. Med. 2021, 166, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Yang, S.Y.; Gu, J.L.; Meng, J.; Xu, H.Y.; Cao, J.M. The acute toxic effects of silver nanoparticles on myocardial transmembrane potential, I(Na) and I(K1) channels and heart rhythm in mice. Nanotoxicology 2017, 11, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, Z. Tissue toxicity following the vaginal administration of nanosilver particles in rabbits. Regen. Biomater. 2015, 2, 261–265. [Google Scholar] [CrossRef]
- Raj, A.; Shah, P.; Agrawal, N. Sedentary behavior and altered metabolic activity by AgNPs ingestion in Drosophila melanogaster. Sci. Rep. 2017, 7, 15617. [Google Scholar] [CrossRef]
- Shati, A.A.; Elsaid, F.G. Biosynthesized silver nanoparticles and their genotoxicity. J. Biochem. Mol. Toxicol. 2020, 34, e22418. [Google Scholar] [CrossRef]
- Liu, F.; Mahmood, M.; Xu, Y.; Watanabe, F.; Biris, A.S.; Hansen, D.K.; Inselman, A.; Casciano, D.; Patterson, T.A.; Paule, M.G.; et al. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front. Neurosci. 2015, 9, 115. [Google Scholar] [CrossRef]
- Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Medina, C.; Radomski, M.W. Pharmacological and toxicological effects of co-exposure of human gingival fibroblasts to silver nanoparticles and sodium fluoride. Int. J. Nanomed. 2014, 9, 1677–1687. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Milić, M.; Leitinger, G.; Pavičić, I.; Zebić Avdičević, M.; Dobrović, S.; Goessler, W.; Vinković Vrček, I. Cellular uptake and toxicity effects of silver nanoparticles in mammalian kidney cells. J. Appl. Toxicol. 2015, 35, 581–592. [Google Scholar] [CrossRef]
- Liu, X.; Shan, K.; Shao, X.; Shi, X.; He, Y.; Liu, Z.; Jacob, J.A.; Deng, L. Nanotoxic Effects of Silver Nanoparticles on Normal HEK-293 Cells in Comparison to Cancerous HeLa Cell Line. Int. J. Nanomed. 2021, 16, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Huang, C.C.; Pranata, R.; Lee, Y.H.; Chen, Y.Y.; Wu, Y.H.; Wang, Y.J. Modulation of Innate Immune Toxicity by Silver Nanoparticle Exposure and the Preventive Effects of Pterostilbene. Int. J. Mol. Sci. 2021, 22, 2536. [Google Scholar] [CrossRef] [PubMed]
- Zhornik, A.; Baranova, L.; Volotovski, I.; Chizhik, S.; Drozd, L.; Sudas, M.; Ngo, Q.; Hoai Chau, N.; Huynh, T.; Dao, T. Interaction of nanosilver particles with human lymphocyte cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025003. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Bao, D.; Oh, Z.G.; Chen, Z. Characterization of Silver Nanoparticles Internalized by Arabidopsis Plants Using Single Particle ICP-MS Analysis. Front. Plant Sci. 2016, 7, 32. [Google Scholar] [CrossRef]
- Chen, H.H.; Chien, C.C.; Petibois, C.; Wang, C.L.; Chu, Y.S.; Lai, S.F.; Hua, T.E.; Chen, Y.Y.; Cai, X.; Kempson, I.M.; et al. Quantitative analysis of nanoparticle internalization in mammalian cells by high resolution X-ray microscopy. J. Nanobiotechnol. 2011, 9, 14. [Google Scholar] [CrossRef]
- Gottstein, C.; Wu, G.; Wong, B.J.; Zasadzinski, J.A. Precise Quantification of Nanoparticle Internalization. ACS Nano 2013, 7, 4933–4945. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, Y.J.; Shin, D.Y.; Oh, S.M.; Chung, K.H. Appropriate in vitro methods for genotoxicity testing of silver nanoparticles. Environ. Health Toxicol. 2013, 28, e2013003. [Google Scholar] [CrossRef]
- de Mello Silva Oliveira, N.; Reis Resende, M.; Alexandre Morales, D.; de ragão Umbuzeiro, G.; Boriollo, M.F.G. In vitro mutagenicity assay (Ames test) and phytochemical characterization of seeds oil of Helianthus annuus Linné (sunflower). Toxicol. Rep. 2016, 3, 733–739. [Google Scholar] [CrossRef]
- Žegura, B.; Filipič, M. Application of In Vitro Comet Assay for Genotoxicity Testing. In Optimization in Drug Discovery: In Vitro Methods; Yan, Z., Caldwell, G.W., Eds.; Humana Press: Totowa, NJ, USA, 2004; pp. 301–313. [Google Scholar]
- Flower, N.A.; Brabu, B.; Revathy, M.; Gopalakrishnan, C.; Raja, S.V.; Murugan, S.S.; Kumaravel, T.S. Characterization of synthesized silver nanoparticles and assessment of its genotoxicity potentials using the alkaline comet assay. Mutat. Res. 2012, 742, 61–65. [Google Scholar] [CrossRef]
- Doherty, A.T. The in vitro micronucleus assay. Methods Mol. Biol. 2012, 817, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, D.H.; Yan, J.; Chen, Y.; Mittelstaedt, R.A.; Zhang, Y.; Biris, A.S.; Heflich, R.H.; Chen, T. Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay. Mutat. Res. 2012, 745, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Dan, M.; Yang, Y.; Lyu, J.; Shao, A.; Cheng, X.; Chen, L.; Xu, L. Acute toxicity and genotoxicity of silver nanoparticle in rats. PLoS ONE 2017, 12, e0185554. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Yousof, S.; Erfan, H.; Mohamed Hosny, M.; Shehata, S.A.; El-Sayed, K. Subacute toxic effects of silver nanoparticles oral administration and withdrawal on the structure and function of adult Albino Rats’ hepatic tissue. Saudi J. Biol. Sci. 2022, 29, 3890–3898. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Wu, M.; Lv, J.; Yang, Y.; Dan, M.; Liu, L.; Chen, L.; Wu, X.; Fan, C.; et al. In vivo carcinogenicity study of silver nanoparticles in transgenic rasH2 mice by one single-dose intravenous administration. J. Nanopart. Res. 2020, 22, 146. [Google Scholar] [CrossRef]
- Mohamed, Y.; Abdel-Wahab, E.G.; Ali, A.F.; and Abd El-Rahman, H.A. Estimation of silver nanoparticles effect on the reproductive health of female Wistar rats. Egypt. J. Basic Appl. Sci. 2022, 9, 340–358. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- Tirpakova, Z.; Demcisakova, Z.; Luptakova, L.; Hurnikova, J.; Coma, M.; Urban, L.; Gal, P.; Medvecky, L.; Petrovova, E. Novel approach for biomaterial assessment: Utilizing the Ex Ovo quail cam assay for biocompatibility pre-screening. Vet. Res. Commun. 2024, 49, 24. [Google Scholar] [CrossRef]
- Pomraenke, M.; Bolney, R.; Winkens, T.; Perkas, O.; Pretzel, D.; Theis, B.; Greiser, J.; Freesmeyer, M. A Novel Breast Cancer Xenograft Model Using the Ostrich Chorioallantoic Membrane-A Proof of Concept. Vet. Sci. 2023, 10, 349. [Google Scholar] [CrossRef]
- Peterovová, E.; Sedmera, D.; Mísek, I.; Lesník, F.; Luptáková, L. Bendiocarbamate toxicity in the chick embryo. Folia Biol. 2009, 55, 61–65. [Google Scholar] [CrossRef]
- Kollmansperger, S.; Anders, M.; Werner, J.; Saller, A.M.; Weiss, L.; Süß, S.C.; Reiser, J.; Schneider, G.; Schusser, B.; Baumgartner, C.; et al. Nociception in Chicken Embryos, Part II: Embryonal Development of Electroencephalic Neuronal Activity In Ovo as a Prerequisite for Nociception. Animals 2023, 13, 2839. [Google Scholar] [CrossRef] [PubMed]
- Kundeková, B.; Máčajová, M.; Meta, M.; Čavarga, I.; Bilčík, B. Chorioallantoic Membrane Models of Various Avian Species: Differences and Applications. Biology 2021, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Bresee, K.L.; Michaelson, C.L.; Tyrell, D.A. Domains of differential cell proliferation and formation of amnion folds in chick embryo ectoderm. Anat. Rec. 1994, 238, 225–236. [Google Scholar] [CrossRef]
- Kuzderová, G.; Sovová, S.; Rendošová, M.; Gyepes, R.; Sabolová, D.; Kožárová, I.; Balážová, Ľ.; Vilková, M.; Kello, M.; Liška, A.; et al. Influence of proline and hydroxyproline as antimicrobial and anticancer peptide components on the silver(i) ion activity: Structural and biological evaluation with a new theoretical and experimental SAR approach. Dalton Trans. 2024, 53, 10834–10850. [Google Scholar] [CrossRef]
- Balážová, Ľ.; Kurhajec, S.; Kello, M.; Bedlovičová, Z.; Zigová, M.; Petrovová, E.; Beňová, K.; Mojžiš, J.; Eftimová, J. Antiproliferative Effect of Phellodendron amurense Rupr. Based on Angiogenesis. Life 2022, 12, 767. [Google Scholar] [CrossRef]
- TUMOR IMPLANTATIONS IN THE DEVELOPING EMBRYO. J. Am. Med. Assoc. 1911, LVI, 741–742. [CrossRef]
- Buhr, C.R.; Wiesmann, N.; Tanner, R.C.; Brieger, J.; Eckrich, J. The Chorioallantoic Membrane Assay in Nanotoxicological Research-An Alternative for In Vivo Experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef]
- Mangir, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using ex Ovo Chick Chorioallantoic Membrane (CAM) Assay To Evaluate the Biocompatibility and Angiogenic Response to Biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef]
- Spielmann, H.; Liebsch, M.; Kalweit, S.; Moldenhauer, F.; Wirnsberger, T.; Holzhütter, H.-G.; Schneider, B.; Glaser, S.; Gerner, I.; Pape, W.J.W.; et al. Results of a Validation Study in Germany on Two in Vitro Alternatives to the Draize Eye Irritation Test, the HET-CAM Test and the 3T3 NRU Cytotoxicity Test. Altern. Lab. Anim. 1996, 24, 741–858. [Google Scholar] [CrossRef]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Sarau, O.; Tăculescu, E.; Semenescu, A.-D.; Dumitru, R.; Alex-Robert, J.; Poenaru, M.; Dehelean, C.-A.; Chevereşan, A. Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract. Inorganics 2024, 12, 160. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Mu, H. Microenvironmental pH Modification in Buccal/Sublingual Dosage Forms for Systemic Drug Delivery. Pharmaceutics 2023, 15, 637. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Monteiro Machado, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Testing vaginal irritation with the Hen’s Egg Test-Chorioallantoic Membrane assay. Altex 2018, 35, 495–503. [Google Scholar] [CrossRef]
- Dahl, J.E. Potential of dental adhesives to induce mucosal irritation evaluated by the HET-CAM method. Acta Odontol. Scand. 2007, 65, 275–283. [Google Scholar] [CrossRef]
- Osmari, B.F.; Medeiros, G.A.; Reolon, J.B.; Prado, V.C.; Brucker, N.; Cruz, L. Cationic nanocapsule suspension as an alternative to the sublingual delivery of nifedipine. Pharm. Dev. Technol. 2023, 28, 403–413. [Google Scholar] [CrossRef]
- Ortega, A.; da Silva, A.B.; da Costa, L.M.; Zatta, K.C.; Onzi, G.R.; da Fonseca, F.N.; Guterres, S.S.; Paese, K. Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma. Drug Deliv. Transl. Res. 2023, 13, 642–657. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Franz-Montan, M.; Baltazar, F.; Chorilli, M. Innovative Mucoadhesive Precursor of Liquid Crystalline System Loading Anti-Gellatinolytic Peptide for Topical Treatment of Oral Cancer. J. Biomed. Nanotechnol. 2021, 17, 253–262. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the Study of Irritation and Toxicity of Substances Applied Topically to the Skin and Mucous Membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar] [CrossRef]
- Díaz-Tomé, V.; Bendicho-Lavilla, C.; García-Otero, X.; Varela-Fernández, R.; Martín-Pastor, M.; Llovo-Taboada, J.; Alonso-Alonso, P.; Aguiar, P.; González-Barcia, M.; Fernández-Ferreiro, A.; et al. Antifungal Combination Eye Drops for Fungal Keratitis Treatment. Pharmaceutics 2022, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Balážová, Ľ.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Tkáčiková, Ľ.; Takáčová, M.; Jacková, A. Silver Nanoparticles Produced In Vitro by Berberis vulgaris Fruit and Their Antioxidant, Antimicrobial and Ex Ovo Irritation Potential Study. BioNanoScience 2024, 14, 867–879. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Moller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Alstrup Jensen, K.; et al. Nanomaterials Versus Ambient Ultrafine Particles: An Opportunity to Exchange Toxicology Knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Barbara, D.; Phil, S.; Klaus Günter, S.; Alke, P.-F.; Barbara, R.-R. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Marchese Robinson, R.L.; Cronin, M.T.; Richarz, A.N.; Rallo, R. An ISA-TAB-Nano based data collection framework to support data-driven modelling of nanotoxicology. Beilstein J. Nanotechnol. 2015, 6, 1978–1999. [Google Scholar] [CrossRef]
- Benam, K.H.; Dauth, S.; Hassell, B.; Herland, A.; Jain, A.; Jang, K.J.; Karalis, K.; Kim, H.J.; MacQueen, L.; Mahmoodian, R.; et al. Engineered in vitro disease models. Annu. Rev. Pathol. 2015, 10, 195–262. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L552–L565. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Riess, O.; Sturm, M.; Menden, B.; Liebmann, A.; Demidov, G.; Witt, D.; Casadei, N.; Admard, J.; Schutz, L.; Ossowski, S.; et al. Genomes in clinical care. npj Genom. Med. 2024, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wu, H.; Wang, M.D.; Wang, G. Introduction of medical genomics and clinical informatics integration for p-Health care. Prog. Mol. Biol. Transl. Sci. 2022, 190, 1–37. [Google Scholar] [CrossRef]
- Gokulan, K.; Bekele, A.Z.; Drake, K.L.; Khare, S. Responses of intestinal virome to silver nanoparticles: Safety assessment by classical virology, whole-genome sequencing and bioinformatics approaches. Int. J. Nanomed. 2018, 13, 2857–2867. [Google Scholar] [CrossRef]
- Wu, K.; Li, H.; Wang, Y.; Liu, D.; Li, H.; Zhang, Y.; Lynch, M.; Long, H. Silver nanoparticles elevate mutagenesis of eukaryotic genomes. G3 2023, 13, jkad008. [Google Scholar] [CrossRef]
- Pan, B.; Kaldhone, P.R.; Alund, A.W.; Du, H.; Guo, X.; Yan, J.; Chen, Y.; Zhou, T.; Robison, T.W.; Chen, T. Mutagenicity of silver nanoparticles evaluated using whole-genome sequencing in mouse lymphoma cells. Nanotoxicology 2021, 15, 418–432. [Google Scholar] [CrossRef]
- Qing, T.; Mahmood, M.; Zheng, Y.; Biris, A.S.; Shi, L.; Casciano, D.A. A genomic characterization of the influence of silver nanoparticles on bone differentiation in MC3T3-E1 cells. J. Appl. Toxicol. 2018, 38, 172–179. [Google Scholar] [CrossRef]
- Grzesiakowska, A.; Kasprowicz, M.J.; Kuchta-Gladysz, M.; Rymuza, K.; Szeleszczuk, O. Genotoxicity of physical silver nanoparticles, produced by the HVAD method, for Chinchilla lanigera genome. Sci. Rep. 2021, 11, 18473. [Google Scholar] [CrossRef]
- Lu, C.; Lv, Y.; Kou, G.; Liu, Y.; Liu, Y.; Chen, Y.; Wu, X.; Yang, F.; Luo, J.; Yang, X. Silver nanoparticles induce developmental toxicity via oxidative stress and mitochondrial dysfunction in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 243, 113993. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Choi, Y.-J.; Jung, E.-J.; Yin, H.; Kwon, J.-T.; Kim, J.-E.; Im, H.-T.; Cho, M.-H.; Kim, J.H.; Kim, H.-Y.; et al. Genomics-based screening of differentially expressed genes in the brains of mice exposed to silver nanoparticles via inhalation. J. Nanopart. Res. 2010, 12, 1567–1578. [Google Scholar] [CrossRef]
- Chen, Y.; Sheng, F.; Wang, X.; Zhang, Z.; Qi, S.; Chen, L. Early Epigenetic Responses in the Genomic DNA Methylation Fingerprints in Cells in Response to Sublethal Exposure of Silver Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 927036. [Google Scholar] [CrossRef] [PubMed]
- Piersanti, A.; Juganson, K.; Mozzicafreddo, M.; Wei, W.; Zhang, J.; Zhao, K.; Ballarini, P.; Mortimer, M.; Pucciarelli, S.; Miao, W.; et al. Transcriptomic responses to silver nanoparticles in the freshwater unicellular eukaryote Tetrahymena thermophila. Environ. Pollut. 2021, 269, 115965. [Google Scholar] [CrossRef]
- Horstmann, C.; Davenport, V.; Zhang, M.; Peters, A.; Kim, K. Transcriptome Profile Alterations with Carbon Nanotubes, Quantum Dots, and Silver Nanoparticles: A Review. Genes 2021, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.K.; Kwon, S.J.; Choi, J.S.; Nguyen, N.T.; Song, J.; Lee, Y.; Kim, Y.E.; Shin, I.; Nam, J.W.; Yoon, T.H. Mass Cytometry and Single-Cell RNA-seq Profiling of the Heterogeneity in Human Peripheral Blood Mononuclear Cells Interacting with Silver Nanoparticles. Small 2020, 16, e1907674. [Google Scholar] [CrossRef]
- Gliga, A.R.; Di Bucchianico, S.; Lindvall, J.; Fadeel, B.; Karlsson, H.L. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci. Rep. 2018, 8, 6668. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Sanchez, J.C.; Gooley, A.A.; Appel, R.D.; Humphery-Smith, I.; Hochstrasser, D.F.; Williams, K.L. Progress with proteome projects: Why all proteins expressed by a genome should be identified and how to do it. Biotechnol. Genet. Eng. Rev. 1996, 13, 19–50. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef]
- Altelaar, A.F.; Munoz, J.; Heck, A.J. Next-generation proteomics: Towards an integrative view of proteome dynamics. Nat. Rev. Genet. 2013, 14, 35–48. [Google Scholar] [CrossRef]
- Xu, M.; Yang, Q.; Xu, L.; Rao, Z.; Cao, D.; Gao, M.; Liu, S. Protein target identification and toxicological mechanism investigation of silver nanoparticles-induced hepatotoxicity by integrating proteomic and metallomic strategies. Part. Fibre Toxicol. 2019, 16, 46. [Google Scholar] [CrossRef]
- Wong, T.Y.; Yan, N.; Kwan, K.K.L.; Pan, Y.; Liu, J.; Xiao, Y.; Wu, L.; Lam, H. Comparative proteomic analysis reveals the different hepatotoxic mechanisms of human hepatocytes exposed to silver nanoparticles. J. Hazard. Mater. 2023, 445, 130599. [Google Scholar] [CrossRef] [PubMed]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.Q.; Gao, Y.; Li, Q.Q.; Ling, J.; Chen, L.Q. Proteomic profiling reveals the differential toxic responses of gills of common carp exposed to nanosilver and silver nitrate. J. Hazard. Mater. 2020, 394, 122562. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.Q.; Kang, Y.H.; Lian, L.H.; Chen, Z.Y.; Wang, P.; Hu, J.M.; Chen, L.Q. Proteomic profiling reveals mitochondrial toxicity of nanosilver and silver nitrate in the gill of common carp. Aquat. Toxicol. 2022, 252, 106318. [Google Scholar] [CrossRef]
- Chen, L.; Meng, X.; Gu, J.; Fan, W.; Abdlli, N.; Peprah, F.A.; Wang, N.; Zhu, F.; Lu, P.; Ma, S.; et al. Silver nanoparticle toxicity in silkworms: Omics technologies for a mechanistic understanding. Ecotoxicol. Environ. Saf. 2019, 172, 388–395. [Google Scholar] [CrossRef]
- Ravichandran, N.; Uvarajan, D.; Ravikumar, M.; Mahendhran, K.; Krishnamoorthy, K.; Vellingiri, B.; Govindasamy, C.; Narayanasamy, A. Gracilaria edulis-mediated silver nanoparticles as a targeted strategy for cervical cancer with integrated toxicity evaluation in zebrafish. Bioorg Chem. 2025, 159, 108361. [Google Scholar] [CrossRef]
- Gao, X.; Li, R.; Yourick, J.J.; Sprando, R.L. Transcriptomic and proteomic responses of silver nanoparticles in hepatocyte-like cells derived from human induced pluripotent stem cells. Toxicol. In Vitro 2022, 79, 105274. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef]
- Xiang, Q.Q.; Yan, H.; Luo, X.W.; Kang, Y.H.; Hu, J.M.; Chen, L.Q. Integration of transcriptomics and metabolomics reveals damage and recovery mechanisms of fish gills in response to nanosilver exposure. Aquat. Toxicol. 2021, 237, 105895. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Xiang, Q.Q.; Lian, L.H.; Chen, Z.Y.; Luo, X.; Ding, C.Z.; Chen, L.Q. Metabolic profiling of nanosilver toxicity in the gills of common carp. Ecotoxicol. Environ. Saf. 2021, 222, 112548. [Google Scholar] [CrossRef] [PubMed]
- Anh, N.H.; Min, Y.J.; Thi My Nhung, T.; Long, N.P.; Han, S.; Kim, S.J.; Jung, C.W.; Yoon, Y.C.; Kang, Y.P.; Park, S.K.; et al. Unveiling potentially convergent key events related to adverse outcome pathways induced by silver nanoparticles via cross-species omics-scale analysis. J. Hazard. Mater. 2023, 459, 132208. [Google Scholar] [CrossRef] [PubMed]
- Joana, C.; Verónica, B.; José Miguel, P.; Helena, O.; Conceição, S.; Ana, M.G.; Iola, F.D. Insights into the impact of silver nanoparticles on human keratinocytes metabolism through NMR metabolomics. Arch. Biochem. Biophys. 2016, 589, 53–61. [Google Scholar] [CrossRef]
- Carrola, J.; Bastos, V.; Jarak, I.; Oliveira-Silva, R.; Malheiro, E.; Daniel-da-Silva, A.L.; Oliveira, H.; Santos, C.; Gil, A.M.; Duarte, I.F. Metabolomics of silver nanoparticles toxicity in HaCaT cells: Structure-activity relationships and role of ionic silver and oxidative stress. Nanotoxicology 2016, 10, 1105–1117. [Google Scholar] [CrossRef]
- Nirmala, J.G.; Meher, K.; Lopus, M. Proteomic and metabolomic profiling combined with in vitro studies reveal the antiproliferative mechanism of silver nanoparticles in MDA-MB-231 breast carcinoma cells. J. Mater. Chem. B 2022, 10, 2148–2159. [Google Scholar] [CrossRef]
- Jarak, I.; Carrola, J.; Barros, A.S.; Gil, A.M.; Pereira, M.L.; Corvo, M.L.; Duarte, I.F. From the Cover: Metabolism Modulation in Different Organs by Silver Nanoparticles: An NMR Metabolomics Study of a Mouse Model. Toxicol. Sci. 2017, 159, 422–435. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, R.; Xu, M. Metabolomics Analysis for Unveiling the Toxicological Mechanism of Silver Nanoparticles Using an In Vitro Gastrointestinal Digestion Model. ACS Nanosci. Au 2024, 4, 327–337. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Koyilot, M.C.; Natarajan, P.; Hunt, C.R.; Sivarajkumar, S.; Roy, R.; Joglekar, S.; Pandita, S.; Tong, C.W.; Marakkar, S.; Subramanian, L.; et al. Breakthroughs and Applications of Organ-on-a-Chip Technology. Cells 2022, 11, 1828. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.O.; Stucki, J.D.; Hall, S.R.; Felder, M.; Mermoud, Y.; Schmid, R.A.; Geiser, T.; Guenat, O.T. A lung-on-a-chip array with an integrated bio-inspired respiration mechanism. Lab Chip 2015, 15, 1302–1310. [Google Scholar] [CrossRef]
- Fu, J.; Qiu, H.; Tan, C.S. Microfluidic Liver-on-a-Chip for Preclinical Drug Discovery. Pharmaceutics 2023, 15, 1300. [Google Scholar] [CrossRef]
- Roth, A. Human microphysiological systems for drug development. Science 2021, 373, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Huh, D.; Hamilton, G.; Ingber, D.E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab Chip 2012, 12, 2165–2174. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.J.; Chapron, A.; Chapron, B.D.; Voellinger, J.L.; Lidberg, K.A.; Yeung, C.K.; Wang, Z.; Yamaura, Y.; Hailey, D.W.; Neumann, T.; et al. Development of a microphysiological model of human kidney proximal tubule function. Kidney Int. 2016, 90, 627–637. [Google Scholar] [CrossRef]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef]
- Danku, A.E.; Dulf, E.-H.; Braicu, C.; Jurj, A.; Berindan-Neagoe, I. Organ-On-A-Chip: A Survey of Technical Results and Problems. Front. Bioeng. Biotechnol. 2022, 10, 840674. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Foo, G.W.; Aggarwal, N.; Chang, M.W. Organ-on-chip technology: Opportunities and challenges. Biotechnol. Notes 2024, 5, 8–12. [Google Scholar] [CrossRef]
- Morais, A.S.; Mendes, M.; Cordeiro, M.A.; Sousa, J.J.; Pais, A.C.; Mihaila, S.M.; Vitorino, C. Organ-on-a-Chip: Ubi sumus? Fundamentals and Design Aspects. Pharmaceutics 2024, 16, 615. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Chrastina, A.; Schnitzer, J.E. Iodine-125 radiolabeling of silver nanoparticles for in vivo SPECT imaging. Int. J. Nanomed. 2010, 5, 653–659. [Google Scholar] [CrossRef]
- Pijeira, M.S.O.; Viltres, H.; Kozempel, J.; Sakmar, M.; Vlk, M.; Ilem-Ozdemir, D.; Ekinci, M.; Srinivasan, S.; Rajabzadeh, A.R.; Ricci-Junior, E.; et al. Radiolabeled nanomaterials for biomedical applications: Radiopharmacy in the era of nanotechnology. EJNMMI Radiopharm. Chem. 2022, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhu, J.; Gu, H.; Deng, S. Biodistribution and Acute Toxicity of Intravenous Multifunctional 125I-Radiolabeled Fe3O4-Ag Heterodimer Nanoparticles in Mice. J. Nanomater. 2018, 2018, 3150351. [Google Scholar] [CrossRef]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue distribution and acute toxicity of silver after single intravenous administration in mice: Nano-specific and size-dependent effects. Part. Fibre Toxicol. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Ferreira de Oliveira, J.M.; Brown, D.; Jonhston, H.; Malheiro, E.; Daniel-da-Silva, A.L.; Duarte, I.F.; Santos, C.; Oliveira, H. The influence of Citrate or PEG coating on silver nanoparticle toxicity to a human keratinocyte cell line. Toxicol. Lett. 2016, 249, 29–41. [Google Scholar] [CrossRef]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moaca, E.A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-coated silver nanoparticles—A comprehensive toxicological profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef]
- Ema, M.; Okuda, H.; Gamo, M.; Honda, K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod. Toxicol. 2017, 67, 149–164. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Varani, M.; Lauri, C.; Ranieri, D.; Signore, A. Methods for Radiolabelling Nanoparticles: PET Use (Part 2). Biomolecules 2022, 12, 1517. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Nayak, P.; Varani, M.; Lauri, C.; Signore, A. Methods for Radiolabeling Nanoparticles (Part 3): Therapeutic Use. Biomolecules 2023, 13, 1241. [Google Scholar] [CrossRef]
- Wenk, M.R. The emerging field of lipidomics. Nat. Rev. Drug Discov. 2005, 4, 594–610. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.; Cusick, M.E.; Barabási, A.-L. Interactome Networks and Human Disease. Cell 2011, 144, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Kobos, L.M.; Xia, L.; Ferreira, C.; Franco, J.; Du, X.; Shannahan, J.H. Exacerbation of Nanoparticle-Induced Acute Pulmonary Inflammation in a Mouse Model of Metabolic Syndrome. Front. Immunol. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Subbotina, J.; Lobaskin, V. Multiscale Modeling of Bio-Nano Interactions of Zero-Valent Silver Nanoparticles. J. Phys. Chem. B 2022, 126, 1301–1314. [Google Scholar] [CrossRef]
- Martinez-Esquivias, F.; Gutierrez-Angulo, M.; Becerra-Ruiz, J.S.; Martinez-Perez, L.A.; de la Cruz-Ahumada, C.J.; Guzman-Flores, J.M. Bioinformatic Analysis of the Effect of Silver Nanoparticles on Colorectal Cancer Cell Line. BioMed Res. Int. 2022, 2022, 6828837. [Google Scholar] [CrossRef]
- Macarron, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef]
- Inglese, J.; Johnson, R.L.; Simeonov, A.; Xia, M.; Zheng, W.; Austin, C.P.; Auld, D.S. High-throughput screening assays for the identification of chemical probes. Nat. Chem. Biol. 2007, 3, 466–479. [Google Scholar] [CrossRef]
- Mayr, L.M.; Bojanic, D. Novel trends in high-throughput screening. Curr. Opin. Pharmacol. 2009, 9, 580–588. [Google Scholar] [CrossRef]
- Krewski, D.; Andersen, M.E.; Mantus, E.; Zeise, L. Toxicity testing in the 21st century: Implications for human health risk assessment. Risk Anal. 2009, 29, 474–479. [Google Scholar] [CrossRef]
- Tong, Z.; Rajeev, G.; Guo, K.; Ivask, A.; McCormick, S.; Lombi, E.; Priest, C.; Voelcker, N.H. Microfluidic Cell Microarray Platform for High Throughput Analysis of Particle-Cell Interactions. Anal. Chem. 2018, 90, 4338–4347. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, G.; Fenech, M.; Pompa, P.P.; Voelcker, N.H. Lab-on-a-chip-based high-throughput screening of the genotoxicity of engineered nanomaterials. Small 2014, 10, 2721–2734. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Lacconi, V.; Filippi, J.; Martinelli, E. Twenty years of in vitro nanotoxicology: How AI could make the difference. Front. Toxicol. 2024, 6, 1470439. [Google Scholar] [CrossRef] [PubMed]
- Puzyn, T.; Rasulev, B.; Gajewicz, A.; Hu, X.; Dasari, T.P.; Michalkova, A.; Hwang, H.-M.; Toropov, A.; Leszczynska, D.; Leszczynski, J. Using nano-QSAR to predict the cytotoxicity of metal oxide nanoparticles. Nat. Nanotechnol. 2011, 6, 175–178. [Google Scholar] [CrossRef]
- Toschi, N.; Ciulli, S.; Diciotti, S.; Duggento, A.; Guerrisi, M.; Magrini, A.; Campagnolo, L.; Pietroiusti, A. Forecasting nanoparticle toxicity using nonlinear predictive regressor learning systems. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 137–140. [Google Scholar] [CrossRef]
- Desai, A.S.; Ashok, A.; Edis, Z.; Bloukh, S.H.; Gaikwad, M.; Patil, R.; Pandey, B.; Bhagat, N. Meta-Analysis of Cytotoxicity Studies Using Machine Learning Models on Physical Properties of Plant Extract-Derived Silver Nanoparticles. Int. J. Mol. Sci. 2023, 24, 4220. [Google Scholar] [CrossRef]
- Bilgi, E.; Karakus, C.O. Machine learning-assisted prediction of the toxicity of silver nanoparticles: A meta-analysis. J. Nanopart. Res. 2023, 25, 157. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorpeh, F. Toxicity prediction of nanoparticles using machine learning approaches. Toxicology 2024, 501, 153697. [Google Scholar] [CrossRef]
- Dusinska, M.; Boland, S.; Saunders, M.; Juillerat-Jeanneret, L.; Tran, L.; Pojana, G.; Marcomini, A.; Volkovova, K.; Tulinska, J.; Knudsen, L.E.; et al. Towards an alternative testing strategy for nanomaterials used in nanomedicine: Lessons from NanoTEST. Nanotoxicology 2015, 9 (Suppl. 1), 118–132. [Google Scholar] [CrossRef]
- Park, E.J.; Yi, J.; Kim, Y.; Choi, K.; Park, K. Silver nanoparticles induce cytotoxicity by a Trojan-horse type mechanism. Toxicol. In Vitro 2010, 24, 872–878. [Google Scholar] [CrossRef]
- Lankveld, D.P.; Oomen, A.G.; Krystek, P.; Neigh, A.; Troost-de Jong, A.; Noorlander, C.W.; Van Eijkeren, J.C.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Summer, M.; Ashraf, R.; Ali, S.; Bach, H.; Noor, S.; Noor, Q.; Riaz, S.; Khan, R.R.M. Inflammatory response of nanoparticles: Mechanisms, consequences, and strategies for mitigation. Chemosphere 2024, 363, 142826. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Rufino, A.T.; Fernandes, R.; Malheiro, A.; Carvalho, F.; Fernandes, E.; Freitas, M. Silver nanoparticles exert toxic effects in human monocytes and macrophages associated with the disruption of Δψm and release of pro-inflammatory cytokines. Arch. Toxicol. 2023, 97, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Salim, E.I.; Abdel-Halim, K.Y.; El-Mahalawy, M.E.; Badr, H.A.; Ahmed, H. Tissue Distribution, Pharmacokinetics, and Effect of Hematological and Biochemical Parameters of Acute Intravenous Administration of Silver Nanoparticles in Rats. Nanomaterials 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Bachler, G.; von Goetz, N.; Hungerbühler, K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int. J. Nanomed. 2013, 8, 3365–3382. [Google Scholar] [CrossRef]
- Ozbek, O.; Genc, D.E.; Ulgen, K.O. Advances in Physiologically Based Pharmacokinetic (PBPK) Modeling of Nanomaterials. ACS Pharmacol. Transl. Sci. 2024, 7, 2251–2279. [Google Scholar] [CrossRef]
- Joost, W.; van de Steeg, E.; Dimitri, G.; Evelijn, E.Z.; Cyrille, A.M.K.; Miriam, V.; Heleen, M.W. A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices. Eur. J. Pharm. Sci. 2014, 63, 167–177. [Google Scholar] [CrossRef]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically Based Pharmacokinetic Modeling of Nanoparticles. J. Pharm. Sci. 2019, 108, 58–72. [Google Scholar] [CrossRef]
- Klaessig, F.C. PBPK Modeling of Slightly Soluble Silver Nanomaterials and Regulatory Acceptance. Small 2020, 16, e1907667. [Google Scholar] [CrossRef]
- Antsiferova, A.A.; Loginova, M.A.; Kashkarov, P.K. Physiologically Based Pharmacokinetic Modelling of Accumulation Kinetics of Silver Nanoparticles in a Mammalian Organism. Nanobiotechnol. Rep. 2023, 18, 927–935. [Google Scholar] [CrossRef]
- Liu, R.; Rallo, R.; Weissleder, R.; Tassa, C.; Shaw, S.; Cohen, Y. Nano-SAR Development for Bioactivity of Nanoparticles with Considerations of Decision Boundaries. Small 2013, 9, 1842–1852. [Google Scholar] [CrossRef]
- Buglak, A.A.; Zherdev, A.V.; Dzantiev, B.B. Nano-(Q)SAR for Cytotoxicity Prediction of Engineered Nanomaterials. Molecules 2019, 24, 4537. [Google Scholar] [CrossRef]
- Jing, L.; Chuanxi, W.; Le, Y.; Feiran, C.; Xuesong, C.; Zhenyu, W. Nano-QSAR modeling for predicting the cytotoxicity of metallic and metal oxide nanoparticles: A review. Ecotoxicol. Environ. Saf. 2022, 243, 113955. [Google Scholar] [CrossRef]
- Gajewicz, A.; Puzyn, T.; Odziomek, K.; Urbaszek, P.; Haase, A.; Riebeling, C.; Luch, A.; Irfan, M.A.; Landsiedel, R.; van der Zande, M.; et al. Decision tree models to classify nanomaterials according to the DF4nanoGrouping scheme. Nanotoxicology 2018, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mulenos, M.R.; Lujan, H.; Pitts, L.R.; Sayes, C.M. Silver Nanoparticles Agglomerate Intracellularly Depending on the Stabilizing Agent: Implications for Nanomedicine Efficacy. Nanomaterials 2020, 10, 1953. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Chouaib, R. Nanoparticle Drug Delivery Systems for Cancer Treatment, 1st ed.; Jenny Stanford Publishing: Singapore, 2020; p. 342. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Tiago, M.; Caksa, S.; Capparelli, C.; Purwin, T.J.; Kumar, G.; Glasheen, M.; Pomante, D.; Kotas, D.; Chervoneva, I.; et al. SOX10 requirement for melanoma tumor growth is due, in part, to immune-mediated effects. Cell Rep. 2021, 37, 110085. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef]
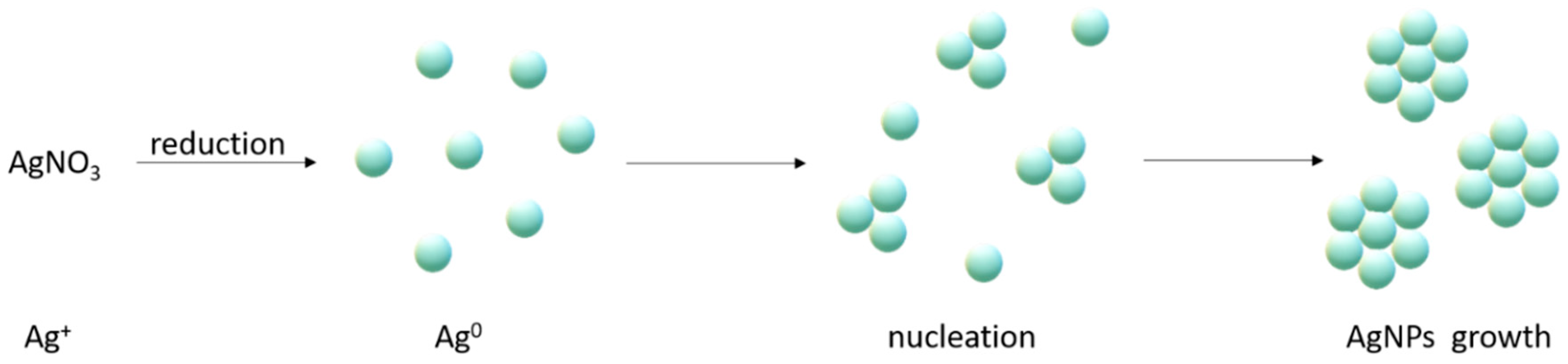
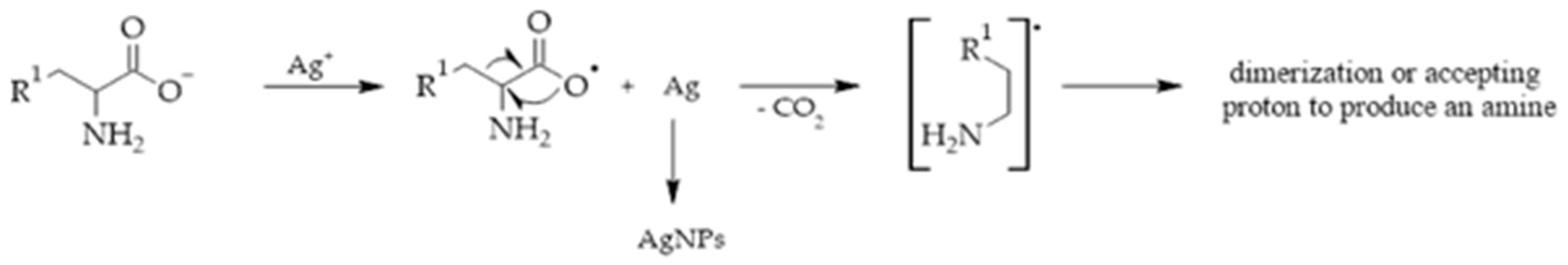

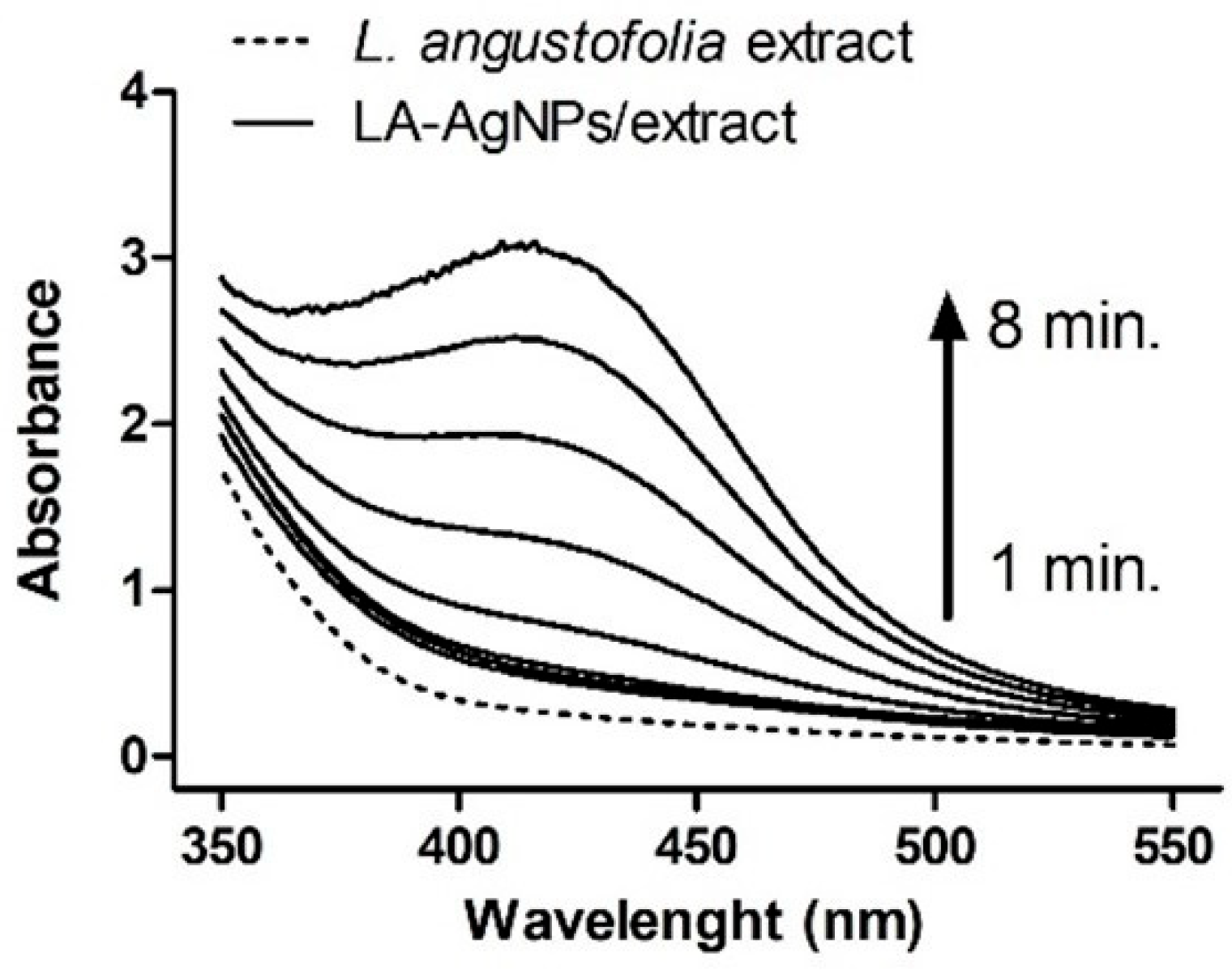
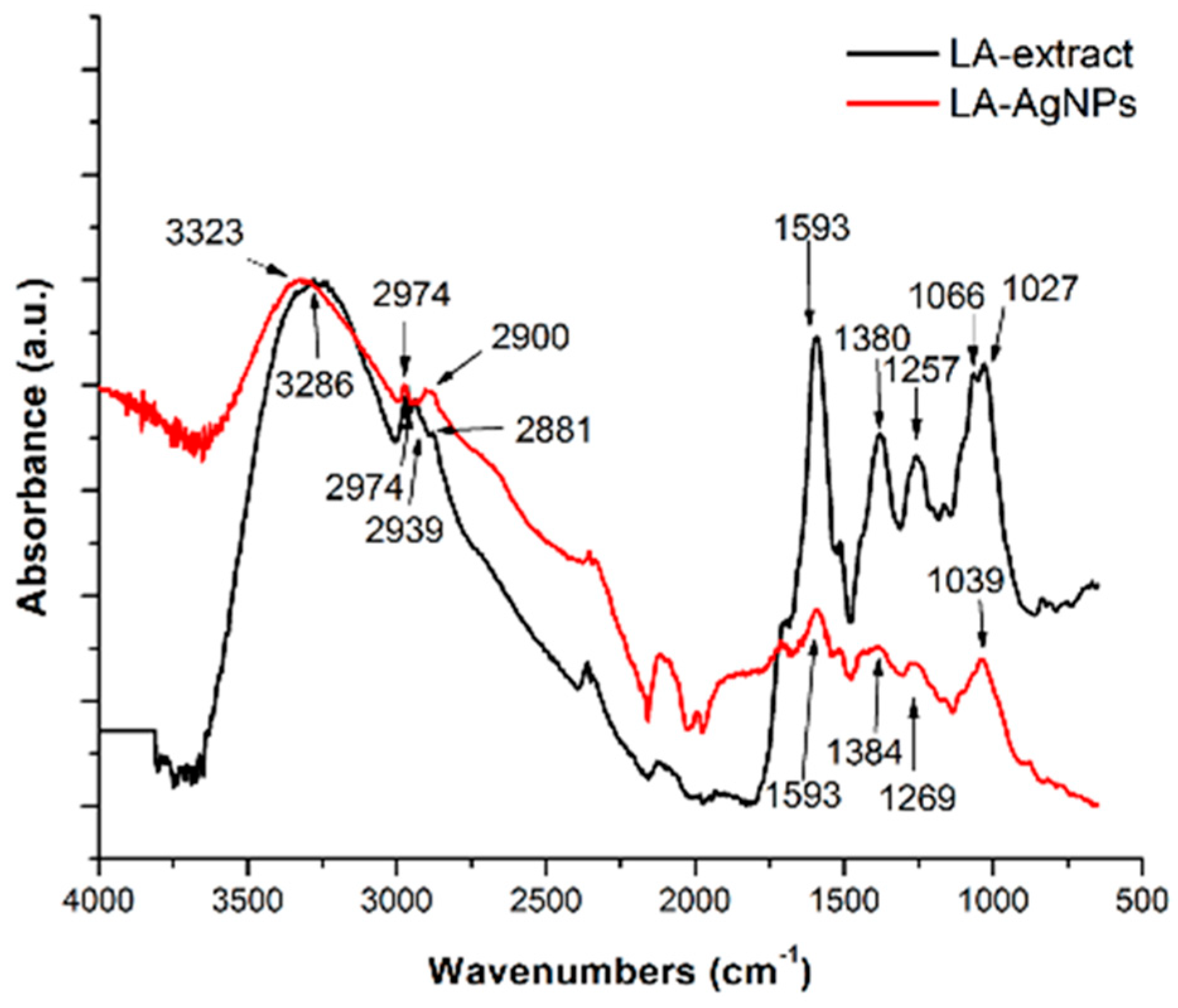
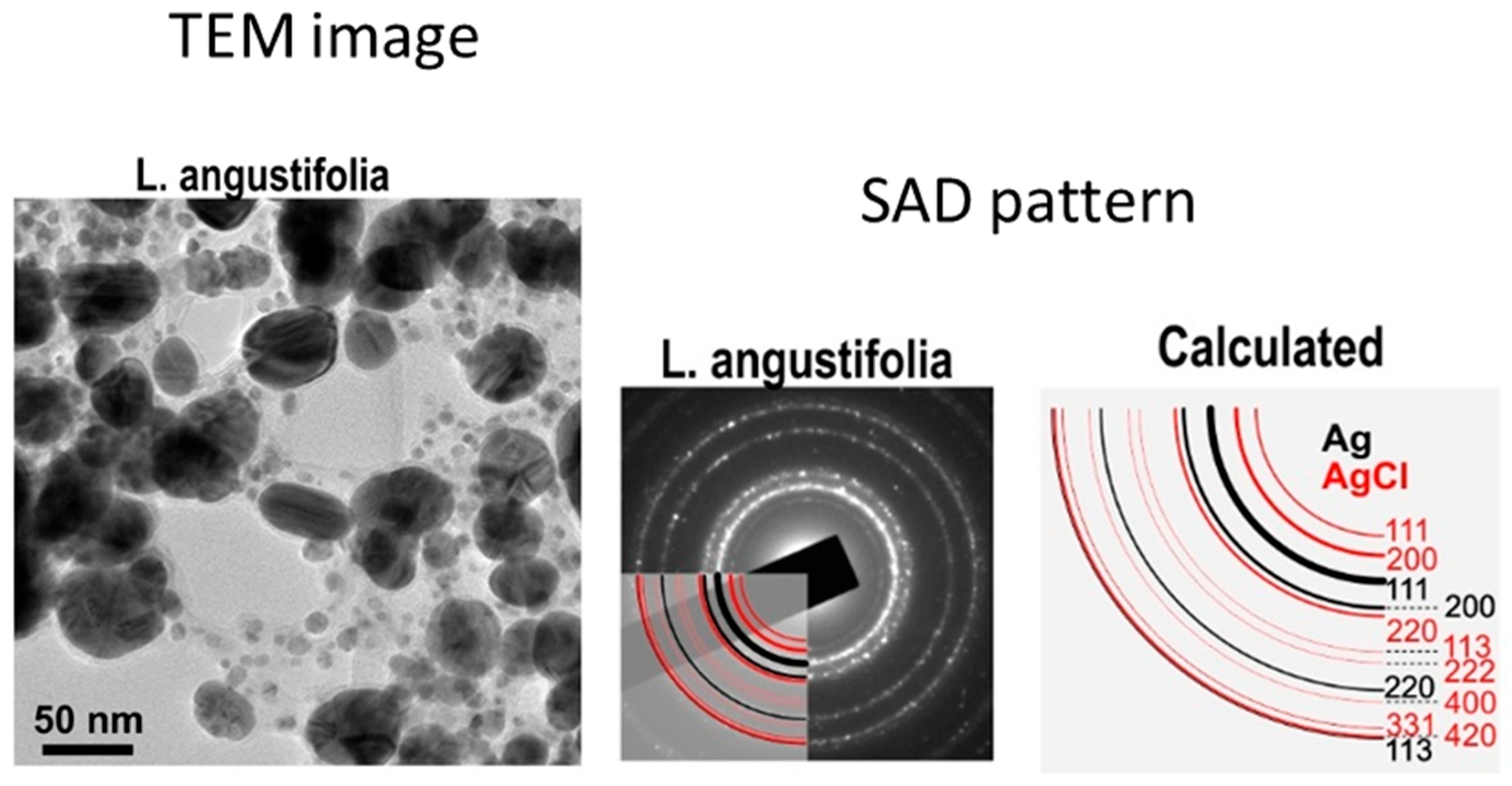


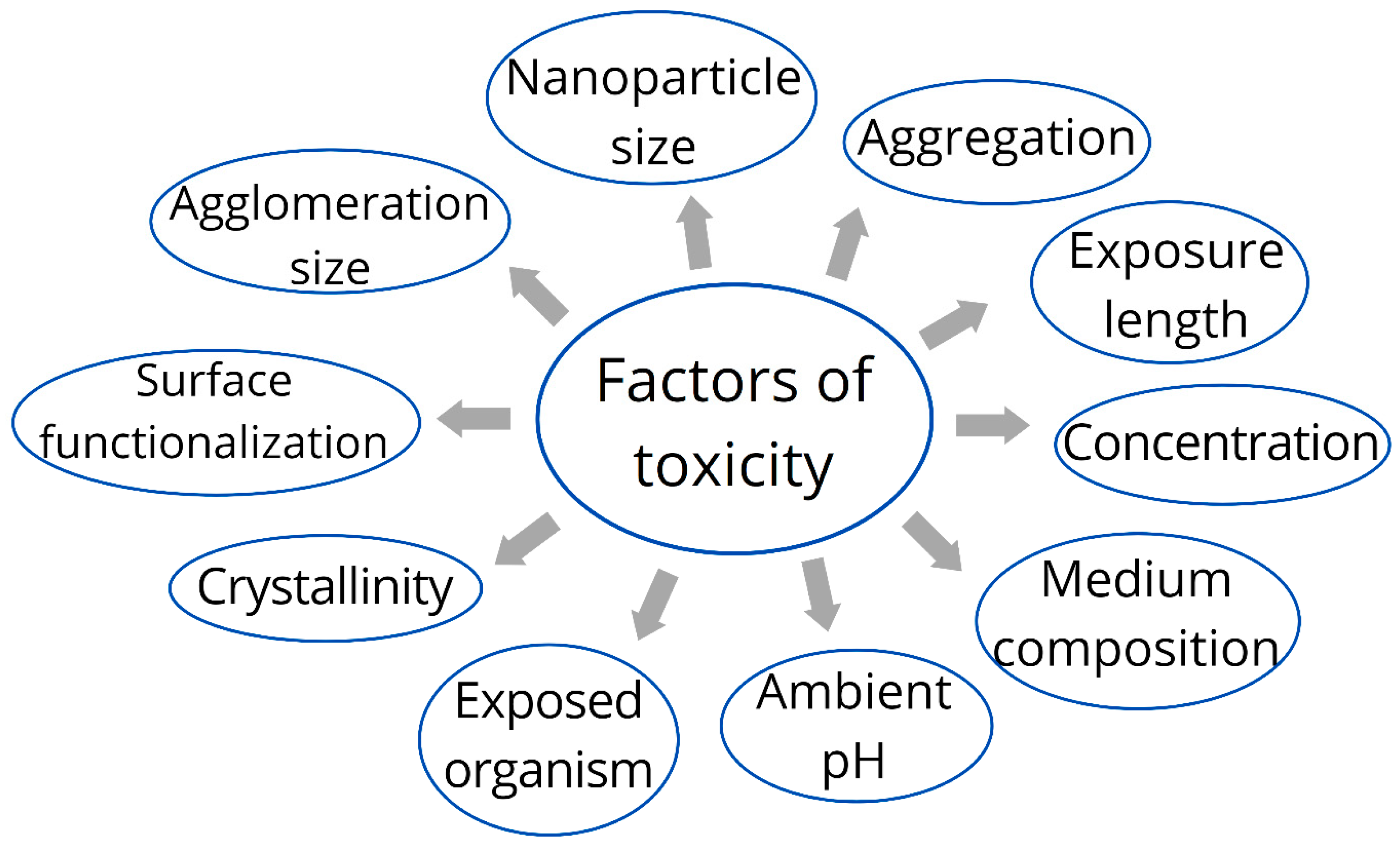
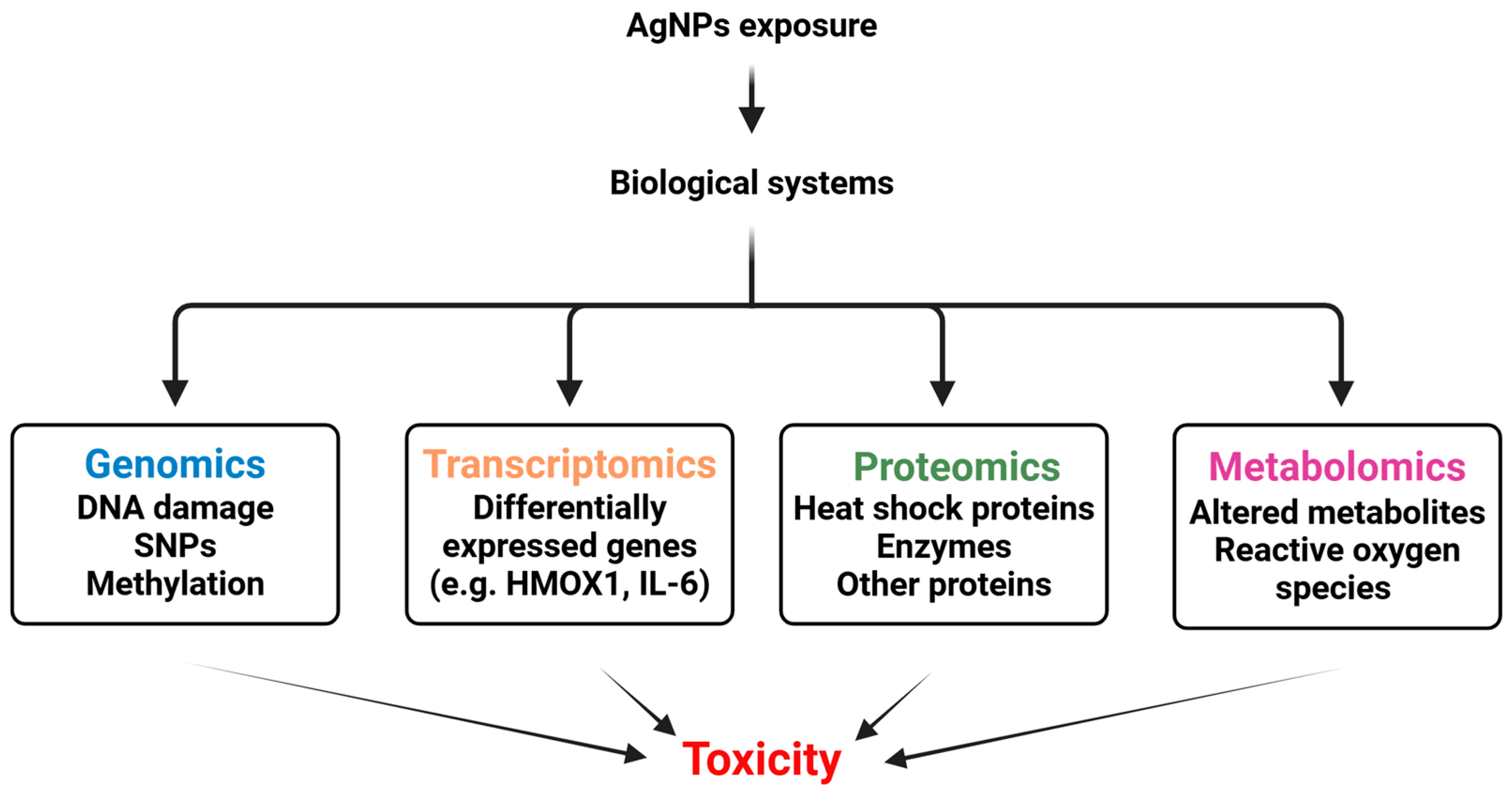
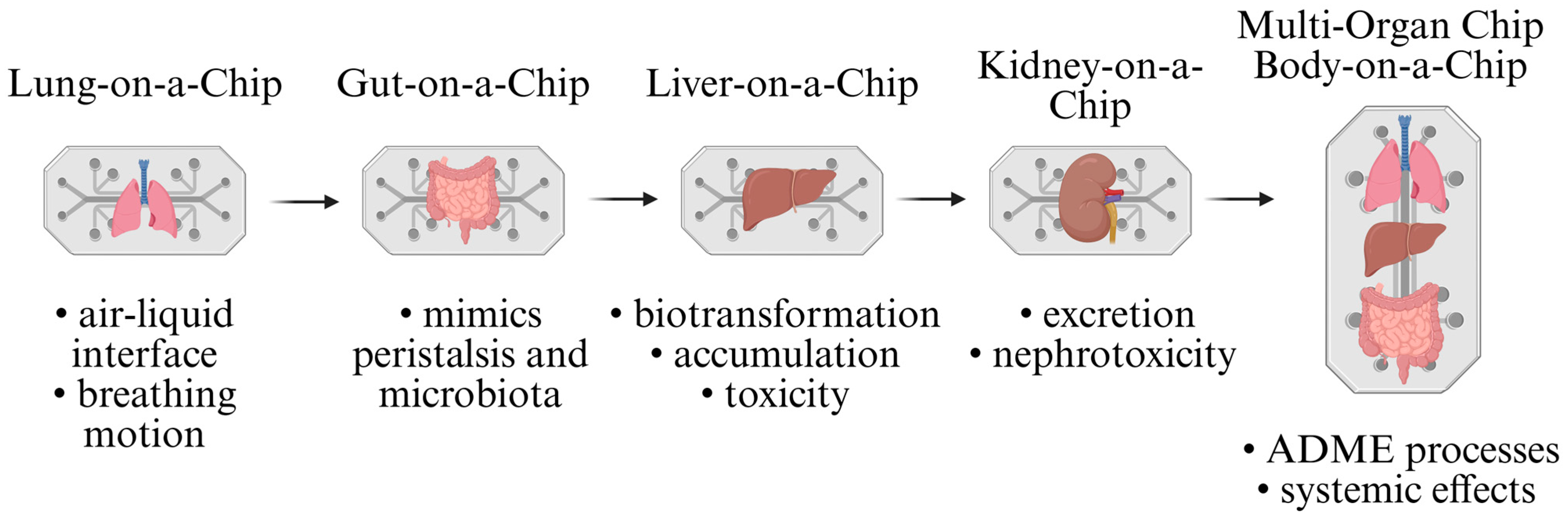
| Bottom-Up Methods | Top-Down Methods | ||
|---|---|---|---|
| Chemical | Biological | Physical | |
| Reduction | In vivo | In vitro | Milling |
| Sonochemical | By algae | By biomolecules | Thermal decomposition |
| Photochemical | By plants | By essential oils | Laser techniques |
| Microwave assisted | By microorganisms | By cell biomass filtrate | Spray pyrolysis |
| Electrochemical | By yeasts | Cell-free culture medium | Nanolitography |
| Nanoparticle Formulation | Drug Delivered | Key Features | Citation |
|---|---|---|---|
| Chitosan-coated AgNPs | Tamoxifen | Enhanced cytotoxicity, G2/M cell cycle arrest, apoptosis induction | [129] |
| Green-synthesized AgNPs | Cisplatin | Synergistic cytotoxicity, reduced IC50 value, increased apoptosis | [130] |
| Amine-functionalized MSNs-AgNPs | Doxorubicin | High drug loading, uniform shape, small size, biocompatibility | [131] |
| Carboplatin-loaded AgNPs | Carboplatin | High anticancer activity, low toxicity, pro-apoptotic effects | [132] |
| Paclitaxel-loaded AgNPs | Paclitaxel | Enhanced anticancer activity, high selectivity, apoptosis induction | [133] |
| Alginate hydrogel-AgNPs-CisPt | Cisplatin | Synergistic cytotoxic effects, enhanced ROS levels, apoptosis induction | [134] |
| Hyaluronic acid-QtN-conjugated AgNPs | Quercetin | Targeted delivery, enhanced anticancer efficacy, biocompatibility | [135] |
| Biosynthesized SeAgNPs | Doxorubicin | High drug encapsulation, biocompatibility, eco-friendly synthesis | [136] |
| NR1/AgNP-decorated PTX nanocrystals | Paclitaxel | Enhanced cellular uptake, anti-migratory effect, apoptosis induction | [137] |
| CendR peptide-targeted AgNPs | Monomethyl auristatin E | Selective cytotoxicity, apoptosis induction, targeted delivery | [138] |
| Silver nanotriangles | Doxorubicin | Synergistic antibreast cancer effect, ROS/ERK1/2 signalling pathway | [139] |
| PA-AgNPs | Doxorubicin | Targeted delivery, high drug release efficiency, biocompatibility | [140] |
| CS-AgNPs-DOX-FA | Doxorubicin | Effective drug delivery, apoptosis induction, targeted delivery | [141] |
| Principal Mechanism | A Specific Target/Process Affected by AgNPs Within the Principal Mechanism | References |
|---|---|---|
| Oxidative stress |
| [188,189,190] |
| Endoplasmic reticulum stress |
| [191,192] |
| Mitochondrial dysfunction via non-ROS pathways |
| [193,194] |
| Autophagy |
| [185] |
| Inflammatory response |
| [195] |
| Organ | Animal Model | Dose | Exposure Route | End-Point | Toxic Effect | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| Liver | Healthy adult male mice | 2 mg·kg−1 | intraperitoneal injection | 35 days | Alterations in the ultrastructure of the liver; focal hepatocytes necrosis and apoptosis | Free radical production and oxidative stress induction | [196] |
| Lungs | Balb/c mice | 0.1 mg/kg body weight | intranasal instillation | 1 or 24 h | Impaired lung function | Alterations in lung tissue O2 consumption due to increased mitochondrial active respiration and NOX activity leading to oxidative damage. | [197] |
| Heart | Mice | 10−9–10−6 g/mL ≥4 mg/kg | intravenous injection | 60–90 min | Loss of excitability in mice cardiac papillary muscle cells in vitro associated with sinus bradycardia, complete atrio-ventricular conduction block, and cardiac asystole | Inhibition of the activity of rectifying the inward potassium current (IK1) and inward sodium current (INa) channels of cardiomyocytes, leading to rapid collapse of cardiac cell transmembrane potential (TMP) | [198] |
| Vaginal mucosa, urethra, and rectum | Healthy female New Zealand rabbits | 0.1 g·kg−1 | intravaginal application | 24 and 72 h | Ultrastructural pathological changes to the vaginal mucosa, urethra, and rectum, and the promoted cytotoxic reactions | - | [199] |
| Fat body and wing imaginal disc | Drosophila melanogaster | 50 mg·L−1 | by ingestion of food | 10, 20, and 30 days | Behavioral abnormalities and altered metabolic activity at early larval stage | Impaired essential metabolic Components and increased reactive oxygen species | [200] |
| Cell Line | Toxic Effect(s) and Mechanism(s) | Ref. |
|---|---|---|
| human neural stem cells (NSCs) | by increasing mitochondrial production of reactive oxygen species led to apoptosis and necrosis of NSCs | [202] |
| human gingival fibroblast cells | oxidative stress, inflammation, and apoptosis | [203] |
| normal human lung fibroblast cells (IMR-90), | ROS production or decreased ATP production, resulting in aberrations of the chromosomes and altering energy-dependent DNA repair mechanisms | [204] |
| HEK-293 cells (human embryonic kidney) cells | direct cytotoxic and viability-lowering effects | [205,206] |
| human immune cells |
| [207,208] |
| Classical Method | Main Purpose | Main Methods | Principle | Ref. |
|---|---|---|---|---|
| Size and Surface Charge Evaluation | to understand and predict their biological interactions, toxicity, and environmental impact |
|
| [83,209] |
| Cellular Interaction Assays | to understand nanoparticle’s ability to transport across and interact with cellular barriers | Flow cytometry, confocal microscopy, inductively coupled plasma mass spectroscopy (ICP-MS),TEM, transmission X-ray microscopy (TXM) | [210,211,212] | |
| Viability assays | to assess the toxicity of nanoparticles towards cells | MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide), 96AQueous One (96AQ), alamarBlue, LDH, live/dead, neutral red etc. | Enzymatic conversion of dye precursor into a detectable dye within living cells allows evaluating of cell viability and metabolic activity | [213] |
| Genotoxic assays | to identify potential genotoxic carcinogens and germ cell mutagens |
|
| [214,215,216,217,218] |
| Toxicity Type | Guideline | Study Durations/Endpoints | Result of Existing AgNP Study: | Ref. |
|---|---|---|---|---|
| Acute (oral) | e.g., OECD TG 420, 423 | Single-dose studies over 14 days. | No deaths or abnormal finding were observed at the maximum concentration for 14 days. LD50 of cAgNPs was considered to be higher than 2000 mg/kg bw in male rats | [220] |
| Subacute and subchronic | e.g., TG 407, 408 | 28- or 90-day repeated-dose studies. | Bioaccumulation in liver/spleen at 10 mg/kg | [221] |
| Carcinogenity and genotoxicity | e.g., TG 451, 471, 474, 487 | Long-term studies evaluating tumour risk and genetic damage. | AgNPs (≤ 20 mg/kg) do not cause carcinogenesis in CB6F1 Tg mice via a single-dose intravenous injection | [222] |
| Reproductive and developmental Toxicity | e.g., TG 414, 421, 422 | Assess effects on fertility and offspring development. | The harmful effect of AgNPs on reproductive tissues in female Wistar rats (in a dose of 4 mg/kg of AgNPs) | [223] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takáč, P., Jr.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Laca Megyesi, Š.; Mačeková, Z.; Takáčová, G.; Moreno-Borrallo, A.; Ruiz-Hernandez, E.; et al. Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches. Int. J. Mol. Sci. 2025, 26, 5344. https://doi.org/10.3390/ijms26115344
Takáč P Jr., Michalková R, Čižmáriková M, Bedlovičová Z, Balážová Ľ, Laca Megyesi Š, Mačeková Z, Takáčová G, Moreno-Borrallo A, Ruiz-Hernandez E, et al. Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches. International Journal of Molecular Sciences. 2025; 26(11):5344. https://doi.org/10.3390/ijms26115344
Chicago/Turabian StyleTakáč, Peter, Jr., Radka Michalková, Martina Čižmáriková, Zdenka Bedlovičová, Ľudmila Balážová, Štefánia Laca Megyesi, Zuzana Mačeková, Gabriela Takáčová, Almudena Moreno-Borrallo, Eduardo Ruiz-Hernandez, and et al. 2025. "Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches" International Journal of Molecular Sciences 26, no. 11: 5344. https://doi.org/10.3390/ijms26115344
APA StyleTakáč, P., Jr., Michalková, R., Čižmáriková, M., Bedlovičová, Z., Balážová, Ľ., Laca Megyesi, Š., Mačeková, Z., Takáčová, G., Moreno-Borrallo, A., Ruiz-Hernandez, E., Isakov, L., & Takáč, P., Sr. (2025). Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches. International Journal of Molecular Sciences, 26(11), 5344. https://doi.org/10.3390/ijms26115344








