Heat vs. Fatigue: Hyperthermia as a Possible Treatment Option for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)
Abstract
1. Introduction
2. Results
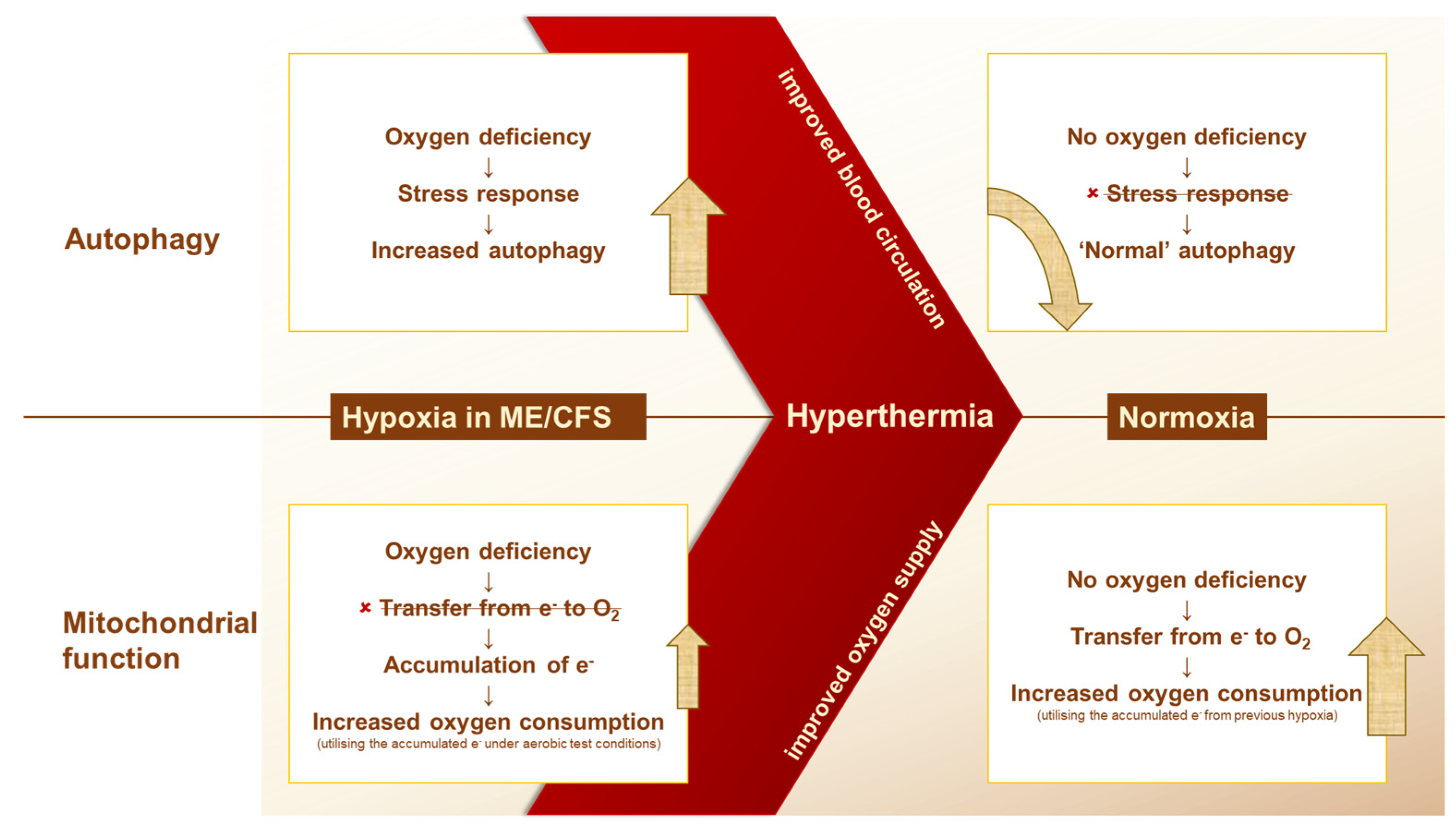
2.1. Autophagy
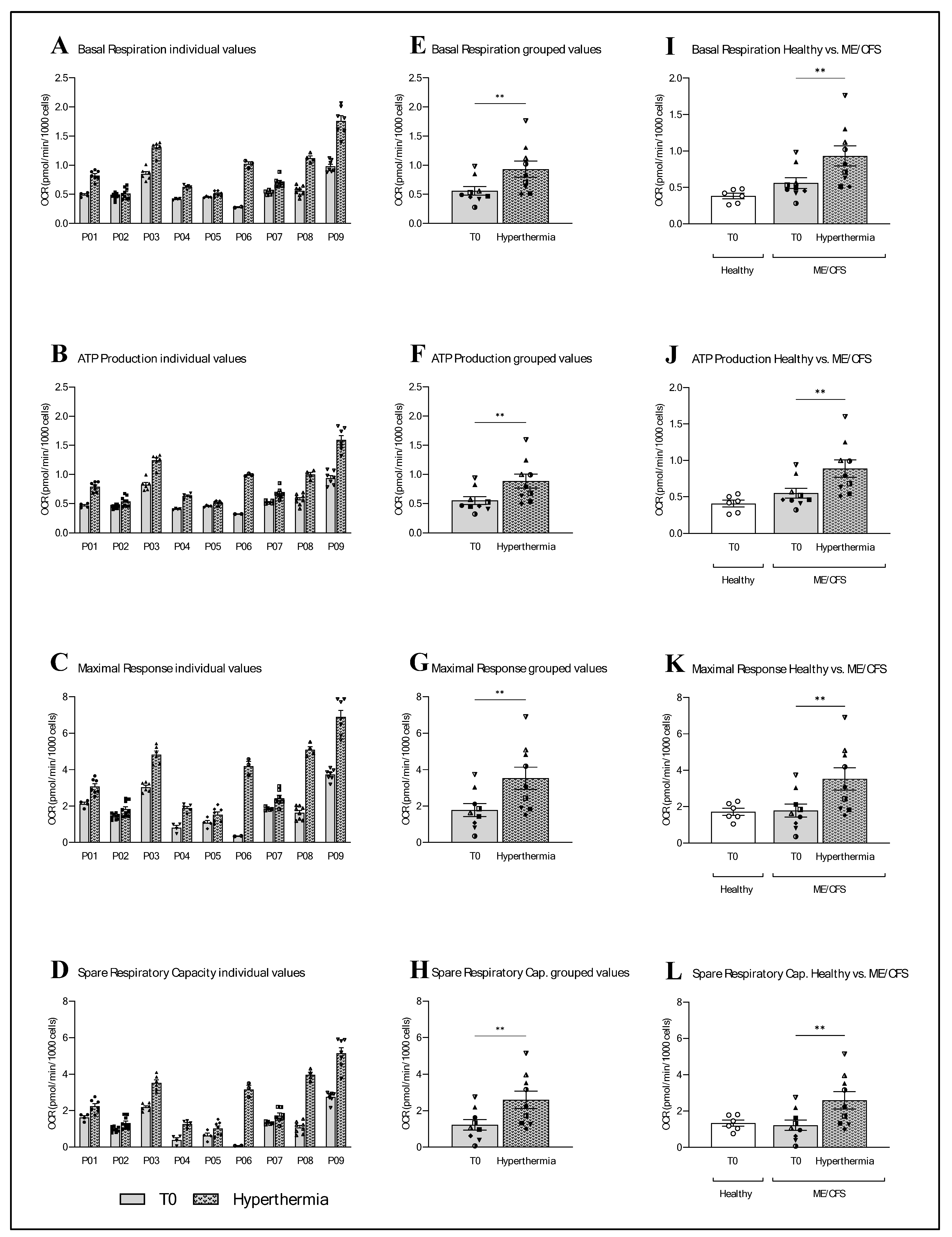
2.2. Mitochondrial Function
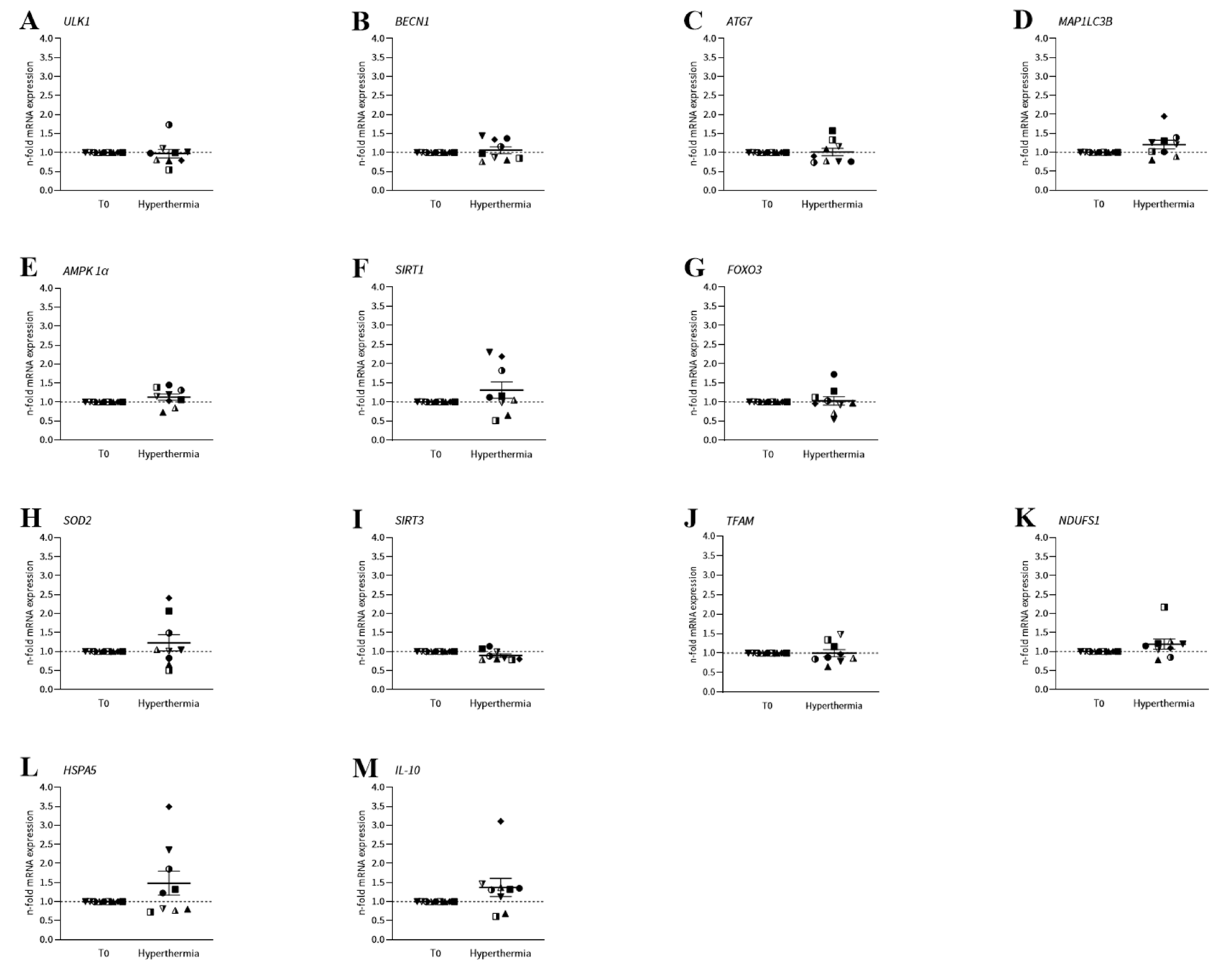
2.3. mRNA Expression
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Human Participants and Blood Collection
4.3. Whole-Body Hyperthermia Treatment
4.4. Isolation of Human PBMCs
4.5. Antibody-Based Quantification of LC3-II
4.6. Analysis of Mitochondrial Function—Seahorse XF Cell Mito Stress Test
4.7. Determination of mRNA Expression in Human PBMCs
4.8. Data Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK1α | protein kinase AMP-activated catalytic subunit alpha 1 |
| APT | Adaptive Pacing Therapy |
| ATG | autophagy-related |
| ATP | adenosine triphosphate |
| BECN1 | beclin 1 |
| FOXO3 | forkhead box O3 |
| HIF | hypoxia-inducible factor |
| HSPA5 | heat-shock protein family A (Hsp70) member 5 |
| IL-10 | interleukin-10 |
| IR | infrared |
| MAP1LC3B | microtubule-associated protein 1 light-chain 3 beta |
| ME/CFS | myalgic encephalomyelitis/chronic fatigue syndrome |
| mRNA | messenger ribonucleic acid |
| NDUFS1 | NADH-ubiquinone oxidoreductase 75 kDa subunit S1 |
| OCR | oxygen consumption rate |
| PBMCs | peripheral blood mononuclear cells |
| PBS | phosphate-buffered saline |
| PEM | post-exertional malaise |
| ROS | reactive oxygen species |
| RPLP0 | ribosomal protein lateral stalk subunit P0 |
| SIRT1 | sirtuin 1 |
| SIRT3 | sirtuin 3 |
| SOD2 | superoxide dismutase 2 |
| Tc | core body temperature |
| TFAM | mitochondrial transcription factor A |
| ULK1 | unc-51-like autophagy activating kinase 1 |
| wIRA | water-filtered infrared-A |
References
- Bested, A.C.; Marshall, L.M. Review of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health 2015, 30, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef]
- Almenar-Pérez, E.; Sánchez-Fito, T.; Ovejero, T.; Nathanson, L.; Oltra, E. Impact of Polypharmacy on Candidate Biomarker miRNomes for the Diagnosis of Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Striking Back on Treatments. Pharmaceutics 2019, 11, 126. [Google Scholar] [CrossRef]
- Goudsmit, E.M.; Nijs, J.; Jason, L.A.; Wallman, K.E. Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: A consensus document. Disabil. Rehabil. 2012, 34, 1140–1147. [Google Scholar] [CrossRef]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; González González, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef]
- Huilgol, N.G.; Gupta, S.; Sridhar, C.R. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: A report of randomized trial. J. Cancer Res. Ther. 2010, 6, 492–496. [Google Scholar] [CrossRef]
- Colombo, R.; Salonia, A.; Leib, Z.; Pavone-Macaluso, M.; Engelstein, D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC). BJU Int. 2011, 107, 912–918. [Google Scholar] [CrossRef]
- Harima, Y.; Nagata, K.; Harima, K.; Ostapenko, V.V.; Tanaka, Y.; Sawada, S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. nt. J. Hyperth. 2009, 25, 338–343. [Google Scholar] [CrossRef]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone, C.B. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Munemoto, T.; Soejima, Y.; Masuda, A.; Nakabeppu, Y.; Tei, C. Increase in the Regional Cerebral Blood Flow following Waon Therapy in Patients with Chronic Fatigue Syndrome: A Pilot Study. Intern. Med. 2017, 56, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Soejima, Y.; Munemoto, T.; Masuda, A.; Uwatoko, Y.; Miyata, M.; Tei, C. Effects of Waon therapy on chronic fatigue syndrome: A pilot study. Intern. Med. 2015, 54, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Tannock, C.; Brostoff, J. Brainstem perfusion is impaired in chronic fatigue syndrome. QJM Int. J. Med. 1995, 88, 767–773. [Google Scholar]
- van Campen, C.L.M.C.; Verheugt, F.W.A.; Rowe, P.C.; Visser, F.C. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: A quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020, 5, 50–58. [Google Scholar] [CrossRef]
- Newton, D.J.; Kennedy, G.; Chan, K.K.F.; Lang, C.C.; Belch, J.J.F.; Khan, F. Large and small artery endothelial dysfunction in chronic fatigue syndrome. Int. J. Cardiol. 2012, 154, 335–336. [Google Scholar] [CrossRef]
- Hochecker, B.; Molinski, N.; Matt, K.; Meßmer, A.; Scherer, M.; von Ardenne, A.; Bergemann, J. Heat treatment in health and disease: How water-filtered infrared-A (wIRA) irradiation affects key cellular mechanisms in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) patients compared to healthy donors. J. Therm. Biol. 2024, 120, 103813. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; White, E. Autophagy and metabolism. Science 2010, 330, 1344–1348. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Lawson, N.; Hsieh, C.-H.; March, D.; Wang, X. Elevated Energy Production in Chronic Fatigue Syndrome Patients. J. Nat. Sci. 2016, 2, e221. [Google Scholar]
- Tomas, C.; Brown, A.E.; Newton, J.L.; Elson, J.L. Mitochondrial complex activity in permeabilised cells of chronic fatigue syndrome patients using two cell types. PeerJ 2019, 7, e6500. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, G.; Peterson, D.; Knox, K.; Maynard, M.; Whelan, R.J.; Roy, A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol. Cell. Neurosci. 2022, 120, 103731. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.M.; Kruger, A.; Proal, A.; Kell, D.B.; Pretorius, E. The Occurrence of Hyperactivated Platelets and Fibrinaloid Microclots in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Pharmaceuticals 2022, 15, 931. [Google Scholar] [CrossRef]
- Green, D.E.; Tzagoloff, A. The mitochondrial electron transfer chain. Arch. Biochem. Biophys. 1966, 116, 293–304. [Google Scholar] [CrossRef]
- Scherbakov, N.; Szklarski, M.; Hartwig, J.; Sotzny, F.; Lorenz, S.; Meyer, A.; Grabowski, P.; Doehner, W.; Scheibenbogen, C. Peripheral endothelial dysfunction in myalgic encephalomyelitis/chronic fatigue syndrome. ESC Heart Fail. 2020, 7, 1064–1071. [Google Scholar] [CrossRef]
- Hoffmann, G. Principles and working mechanisms of water-filtered infrared-A (wIRA) in relation to wound healing. GMS Krankenhaushygiene Interdiszip. 2007, 2, Doc54. [Google Scholar]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol. Cell. Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.J.; King, K.E.; Côté, M.D.; McManus, M.K.; Topshee, S.M.; Hsu, H.; Fujii, N.; Kenny, G.P. Regulation of autophagy following ex vivo heating in peripheral blood mononuclear cells from young adults. J. Therm. Biol. 2020, 91, 102643. [Google Scholar] [CrossRef]
- Amirkavei, M.; Plastino, F.; Kvanta, A.; Kaarniranta, K.; André, H.; Koskelainen, A. Hormetic Heat Shock Enhances Autophagy through HSF1 in Retinal Pigment Epithelium Cells. Cells 2022, 11, 1778. [Google Scholar] [CrossRef]
- Faheem, M.S.; Ghanem, N.; Gad, A.; Procházka, R.; Dessouki, S.M. Adaptive and Biological Responses of Buffalo Granulosa Cells Exposed to Heat Stress under In Vitro Condition. Animals 2021, 11, 794. [Google Scholar] [CrossRef]
- Zauner, D.; Quehenberger, F.; Hermann, J.; Dejaco, C.; Stradner, M.H.; Stojakovic, T.; Angerer, H.; Rinner, B.; Graninger, W.B. Whole body hyperthermia treatment increases interleukin 10 and toll-like receptor 4 expression in patients with ankylosing spondylitis: A pilot study. Int. J. Hyperth. 2014, 30, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kozłowski, H.M.; Sobocińska, J.; Jędrzejewski, T.; Maciejewski, B.; Dzialuk, A.; Wrotek, S. Fever-range whole body hyperthermia leads to changes in immune-related genes and miRNA machinery in Wistar rats. Int. J. Hyperth. 2023, 40, 2216899. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Jason. The Development of a Revised Canadian Myalgic Encephalomyelitis Chronic Fatigue Syndrome Case Definition. Am. J. Biochem. Biotechnol. 2010, 6, 120–135. [Google Scholar] [CrossRef]
- Fukuda, K.; Straus, S.E.; Hickie, I.; Sharpe, M.C.; Dobbins, J.G.; Komaroff, A. The chronic fatigue syndrome: A comprehensive approach to its definition and study. Ann. Intern. Med. 1994, 121, 953–959. [Google Scholar] [CrossRef]
- Bell, D.S. The Doctor’s Guide to Chronic Fatigue Syndrome: Understanding, Treating, and Living with CFIDS; Da Capo Press: Boston, MA, USA, 1995; ISBN 978-0201407976. [Google Scholar]
- Hochecker, B.; Scherer, M. LC3-II Fluorescence Intensity in PBMCs from Healthy Donors and ME/CFS Patients (CTRL + Whole-Body Hyperthermia). 2024. Available online: https://data.mendeley.com/datasets/vy5z29r5pg/1 (accessed on 27 May 2025).
- Cobarg, C.C. Physikalische Grundlagen der wassergefilterten Infrarot-A-Strahlung. In Wärmetherapie Mit Wassergefilterter Infrarot-A-Strahlung. Grundlagen und Anwendungsmöglichkeiten; Vaupel, P., Krüger, W., Eds.; Stuttgart: Hippokrates, Germany, 1995; pp. 19–28. [Google Scholar]
- Hellige, G. Wärmetherapie Mit Wassergefilterter Infrarot-A-Strahlung. Grundlagen und Anwendungsmöglichkeiten; Vaupel, P., Krüger, W., Eds.; Stuttgart: Hippokrates, Germany, 1995. [Google Scholar]
- Schöller-Mann, A.; Matt, K.; Hochecker, B.; Bergemann, J. Ex vivo Assessment of Mitochondrial Function in Human Peripheral Blood Mononuclear Cells Using XF Analyzer. Bio-Protoc. 2021, 11, e3980. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| ME/CFS Patients | Healthy Donors | |||||||
|---|---|---|---|---|---|---|---|---|
| Autophagy—Mitochondrial—mRNA Expression | Autophagy | Mitochondrial | ||||||
| Participant | Sex | Age | Bell Scale | Symbol | Sex | Age | Sex | Age |
| 01 | f | 31 | 20 | ● | f | 61 | f | 30 |
| 02 | f * | 53 * | 40 * | ■ | m | 57 | f | 39 |
| 03 | m | 41 | 30 | ▲ | f | 61 | f | 29 |
| 04 | m | 50 | 50 | ▼ | f | 39 | m | 37 |
| 05 | f | 35 | 30–40 | ♦ | m | 42 | m | 44 |
| 06 | f | 32 | 30–40 | ◑ | m | 41 | f | 24 |
| 07 | f | 47 | 20 | ◨ | f | 34 | - | - |
| 08 | f | 32 | 40–60 | ◮ | m | 45 | - | - |
| 09 | f | 25 | 20–30 | ⧩ | f | 53 | - | - |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| RPLP0 | ccc gag aag acc tcc ttt tt | aga agg ggg aga tgt tga gc |
| β-Actin | gga ctt cga gca aga gat gg | agc act gtg ttg gcg tac ag |
| ULK1 | ggt cac acg cca cat aac ag | gcc cca caa ggt gag aat aa |
| ATG7 | acc cag aag aag ctg aac ga | ggt ggg agc act cat gtc aa |
| BECN1 | ggc tga gag act gga tca gg | ctg tcc act gtg cca gat gt |
| MAP1LC3B | ggt gag aag cag ctt cct gt | aga ttg gtg tgg aga cgc tg |
| AMPK1α | ttc aag tga ttc tcc cgc ct | gaa gct gag gtg gtg gat ca |
| FOXO3 | gca agc aca gag ttg gat ga | cag gtc gtc cat gag gtt tt |
| SIRT1 | gca gat tag tag gcg gct tg | tct ggc atg tcc cac tat ca |
| SIRT3 | cat gag ctg cag tga ctg gt | gag ctt gcc gtt caa cta gg |
| TFAM | ggg aag gtc tgg agc aga g | acg ctg ggc aat tct tct aa |
| NDUFS1 | agg cag ttc tgc act cca aa | tcc atc tgc tcc cag gag aa |
| SOD2 | cac cag cac tag cag cat gt | ggt gac gtt cag gtt gtt ca |
| HSPA5 | ggt gaa aga ccc ctg aca aa | gtc agg cga ttc tgg tca tt |
| IL-10 | gag aac agc tgc acc cac tt | gca tca cct cct cca ggt aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hochecker, B.; Matt, K.; Scherer, M.; Meßmer, A.; von Ardenne, A.; Bergemann, J. Heat vs. Fatigue: Hyperthermia as a Possible Treatment Option for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Mol. Sci. 2025, 26, 5339. https://doi.org/10.3390/ijms26115339
Hochecker B, Matt K, Scherer M, Meßmer A, von Ardenne A, Bergemann J. Heat vs. Fatigue: Hyperthermia as a Possible Treatment Option for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). International Journal of Molecular Sciences. 2025; 26(11):5339. https://doi.org/10.3390/ijms26115339
Chicago/Turabian StyleHochecker, Barbara, Katja Matt, Melanie Scherer, Alica Meßmer, Alexander von Ardenne, and Jörg Bergemann. 2025. "Heat vs. Fatigue: Hyperthermia as a Possible Treatment Option for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)" International Journal of Molecular Sciences 26, no. 11: 5339. https://doi.org/10.3390/ijms26115339
APA StyleHochecker, B., Matt, K., Scherer, M., Meßmer, A., von Ardenne, A., & Bergemann, J. (2025). Heat vs. Fatigue: Hyperthermia as a Possible Treatment Option for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). International Journal of Molecular Sciences, 26(11), 5339. https://doi.org/10.3390/ijms26115339






