Dizocilpine Does Not Alter NOS1AP Gene Expression in Rats and in Cell Cultures
Abstract
1. Introduction
2. Materials and Methods
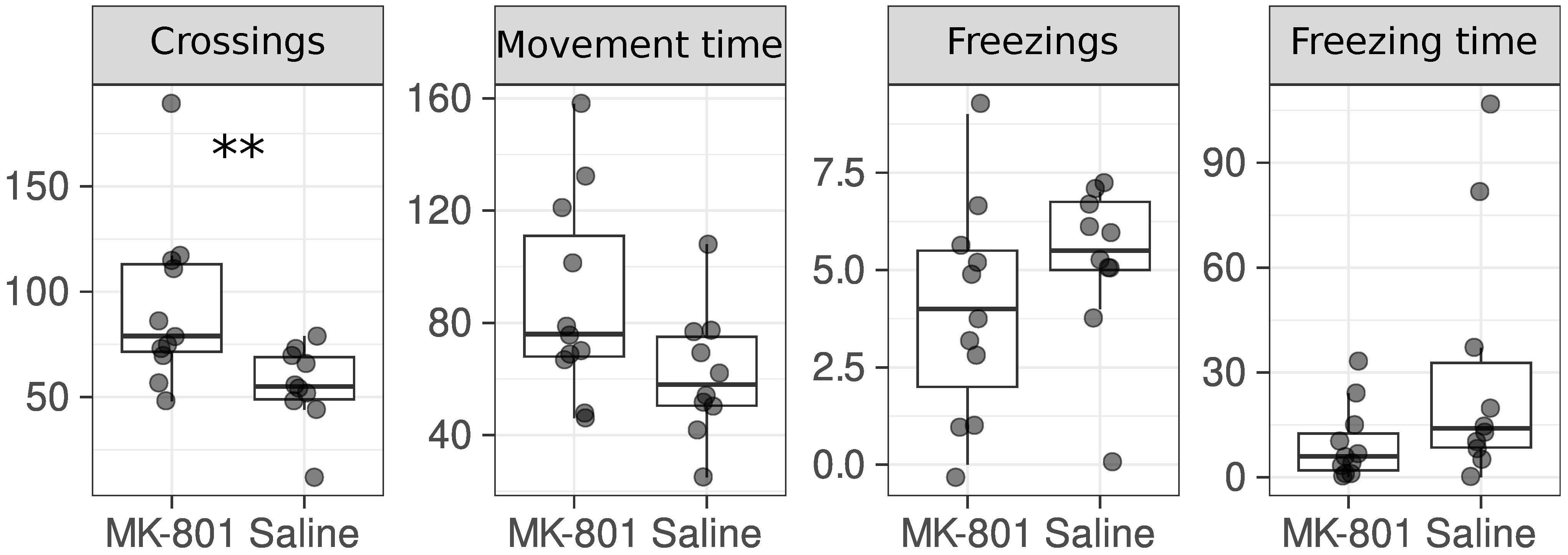
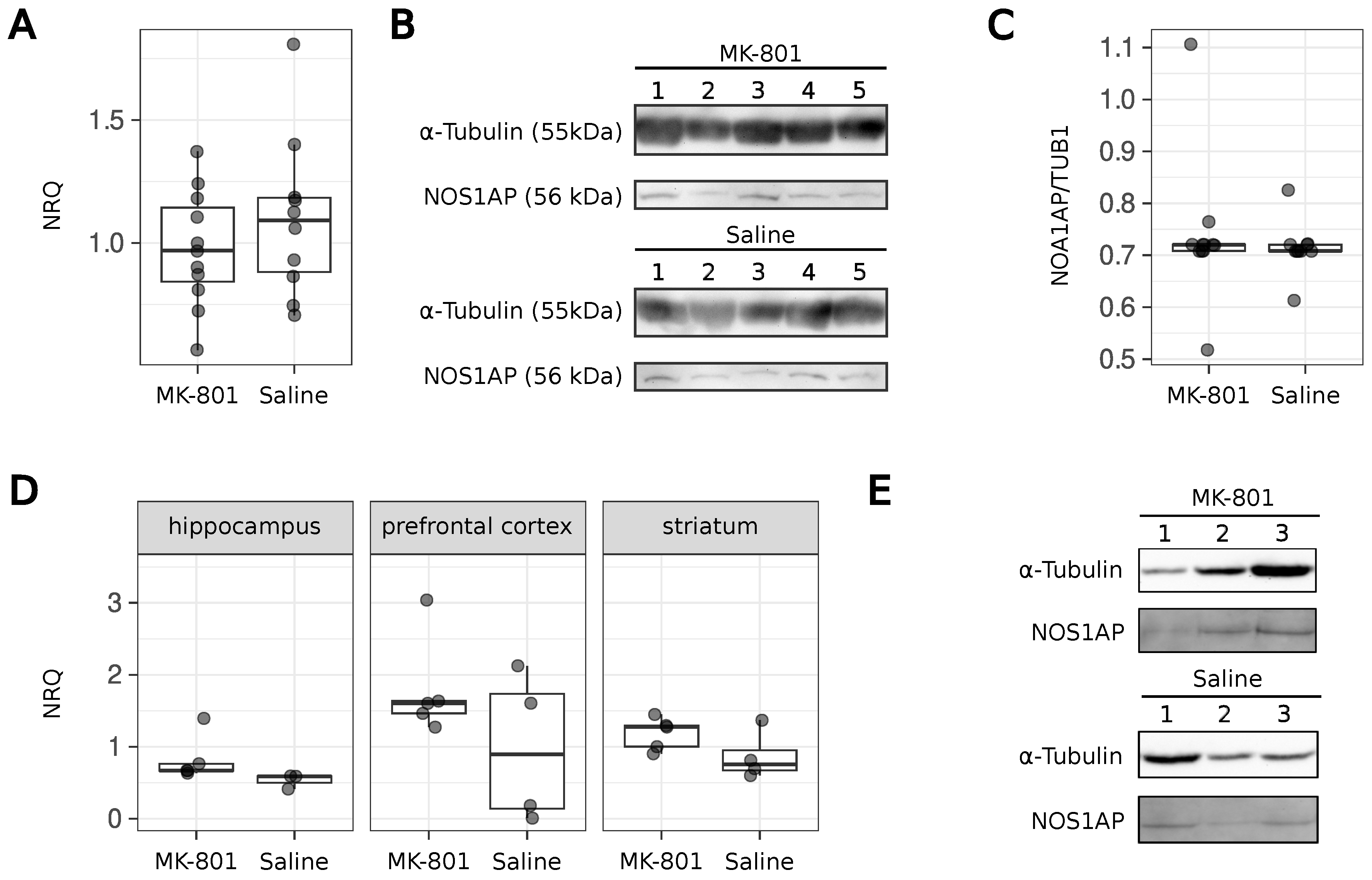
2.1. Animals with Symptoms of Schizophrenia
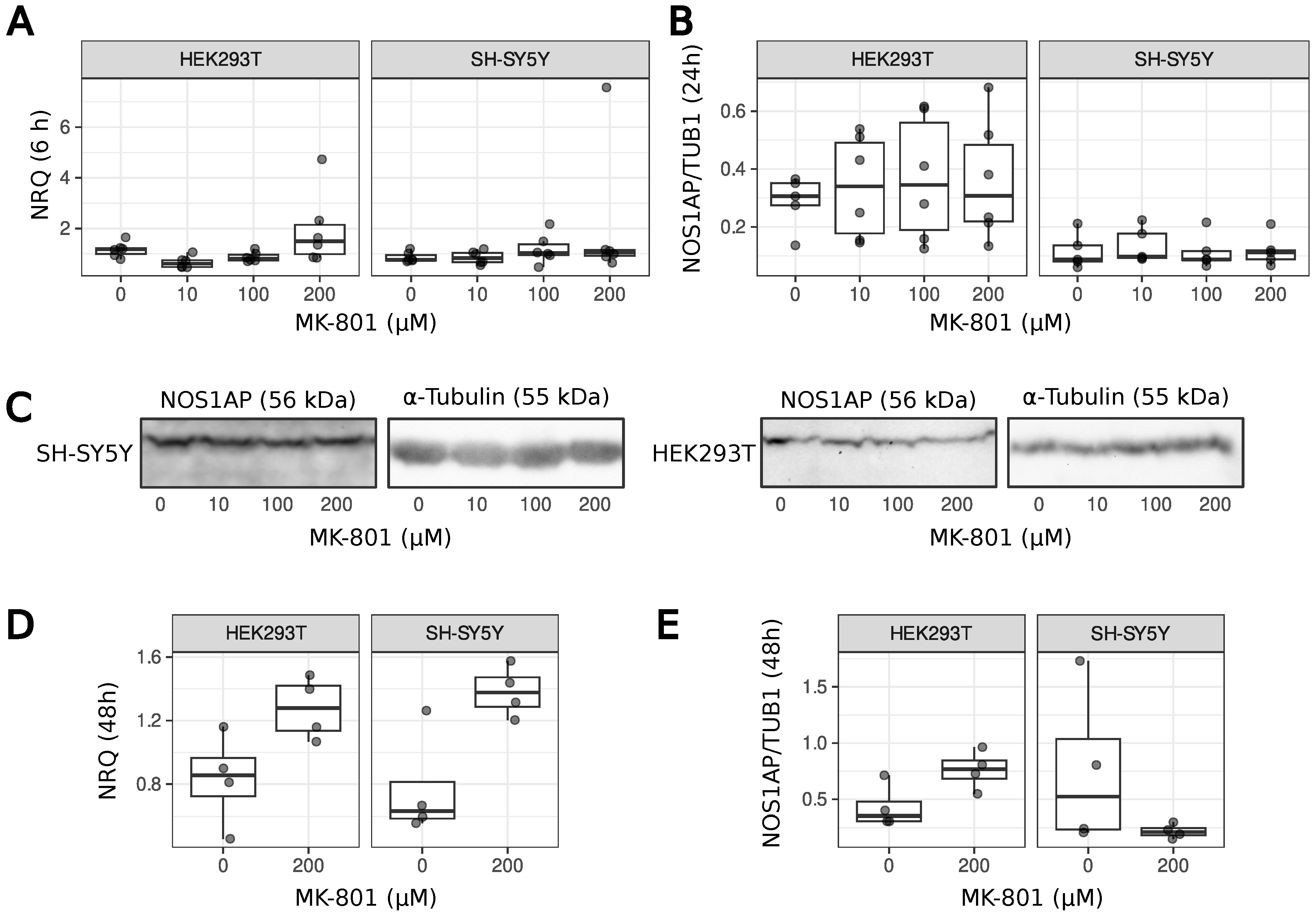
2.2. Treatment of Cells with MK-801
2.3. RNA Isolation
2.4. Protein Extraction
2.5. qRT-PCR
2.6. SDS-PAGE and Western Blotting
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FBS | Fetal bovine serum |
| MK-801 | Dizocilpine |
| NMDA | N-methyl-D-aspartate |
| NMDAR | NMDA receptor |
| NRQ | Normalized relative quantity |
| NOS1 | Nitric Oxide Synthase 1 |
| NOS1AP | Nitric Oxide Synthase 1 Adapter Protein |
| PDZ-BD | PDZ-binding domain |
| PTB | Phosphotyrosine-binding domain |
| RPLP0 | Ribosomal Protein Lateral Stalk Subunit P0 |
| SNVs | Single-nucleotide variations |
References
- Jaffrey, S.R.; Snowman, A.M.; Eliasson, M.J.; Cohen, N.A.; Snyder, S.H. CAPON: A protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron 1998, 20, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Buerger, F.; Forbes, T.A.; Klämbt, V.; Schneider, R.; Deutsch, K.; Kitzler, T.M.; Howden, S.E.; Scurr, M.; Tan, K.S.; et al. Recessive NOS1AP variants impair actin remodeling and cause glomerulopathy in humans and mice. Sci. Adv. 2021, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Clattenburg, L.; Wigerius, M.; Qi, J.; Rainey, J.K.; Rourke, J.L.; Muruganandan, S.; Sinal, C.J.; Fawcett, J.P. NOS1AP Functionally Associates with YAP To Regulate Hippo Signaling. Mol. Cell. Biol. 2015, 35, 2265–2277. [Google Scholar] [CrossRef]
- Hernandez, K.; Swiatkowski, P.; Patel, M.V.; Liang, C.; Dudzinski, N.R.; Brzustowicz, L.M.; Firestein, B.L. Overexpression of isoforms of nitric oxide synthase 1 adaptor protein, encoded by a risk gene for schizophrenia, alters actin dynamics and synaptic function. Front. Cell. Neurosci. 2016, 10, 6. [Google Scholar] [CrossRef]
- Fang, M.; Jaffrey, S.R.; Sawa, A.; Ye, K.; Luo, X.; Snyder, S.H. Dexras1: A G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron 2000, 28, 183–193. [Google Scholar] [CrossRef]
- Allen, N.C.; Bagade, S.; McQueen, M.B.; Ioannidis, J.P.; Kavvoura, F.K.; Khoury, M.J.; Tanzi, R.E.; Bertram, L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat. Genet. 2008, 40, 827–834. [Google Scholar] [CrossRef]
- Brzustowicz, L.M.; Hodgkinson, K.A.; Chow, E.W.C.; Honer, W.G.; Bassett, A.S. Location of a Major Susceptibility Locus for Familial Schizophrenia on Chromosome 1q21-q22. Science 2000, 288, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.H.; Kelly, M.; Smith, A.B.; Shields, G.; Hopkins, P.J.; Loftus, J.; Laval, S.H.; Vita, A.; De Hert, M.; Cardon, L.R.; et al. A genome-wide search for schizophrenia susceptibility genes. Am. J. Med Genet.-Neuropsychiatr. Genet. 1998, 81, 364–376. [Google Scholar] [CrossRef]
- Brzustowicz, L.M.; Hayter, J.E.; Hodgkinson, K.A.; Chow, E.W.; Bassett, A.S. Fine mapping of the schizophrenia susceptibility locus on chromosome 1q22. Hum. Hered. 2002, 54, 199–209. [Google Scholar] [CrossRef]
- Gurling, H.M.; Kalsi, G.; Brynjolfson, J.; Sigmundsson, T.; Sherrington, R.; Mankoo, B.S.; Read, T.; Murphy, P.; Blaveri, E.; McQuillin, A.; et al. Genomewide genetic linkage analysis confirms the presence of susceptibility loci for schizophrenia, on chromosomes 1q32.2, 5q33.2, and 8p21-22 and provides support for linkage to schizophrenia, on chromosomes 11q23.3-24 and 20q12.1-11.23. Am. J. Hum. Genet. 2001, 68, 661–673. [Google Scholar] [CrossRef]
- Hwu, H.G.; Liu, C.M.; Fann, C.S.; Ou-Yang, W.C.; Lee, S.F. Linkage of schizophrenia with chromosome 1q loci in Taiwanese families. Mol. Psychiatry 2003, 8, 445–452. [Google Scholar] [CrossRef]
- Brzustowicz, L.M.; Simone, J.; Mohseni, P.; Hayter, J.E.; Hodgkinson, K.A.; Chow, E.W.; Bassett, A.S. Linkage Disequilibrium Mapping of Schizophrenia Susceptibility to the CAPON Region of Chromosome 1q22. Am. J. Hum. Genet. 2004, 74, 1057–1063. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, B.; Wratten, N.; Charych, E.I.; Buyske, S.; Firestein, B.L.; Brzustowicz, L.M. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2005, 2, 0999–1007. [Google Scholar] [CrossRef] [PubMed]
- Brzustowicz, L.M. NOS1AP in schizophrenia. Curr. Psychiatry Rep. 2008, 10, 158–163. [Google Scholar] [CrossRef]
- Kruse, A.O.; Bustillo, J.R. Glutamatergic dysfunction in Schizophrenia. Transl. Psychiatry 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Watson, D.; Fone, K. Animal models of schizophrenia. Br. J. Pharmacol. 2011, 164, 1162–1194. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, M.; Lv, Q.; Cheng, C.; Li, X.; Guo, Z.; Tao, T.; Shen, A. Inhibition of nitric oxide-induced nuclear localization of CAPON by NMDA receptor antagonist in cultured rat primary astrocytes. Neurochem. Int. 2010, 56, 561–568. [Google Scholar] [CrossRef]
- Neill, J.C.; Barnes, S.; Cook, S.; Grayson, B.; Idris, N.F.; McLean, S.L.; Snigdha, S.; Rajagopal, L.; Harte, M.K. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol. Ther. 2010, 128, 419–432. [Google Scholar] [CrossRef]
- Eyjolfsson, E.M.; Brenner, E.; Kondziella, D.; Sonnewald, U. Repeated injection of MK801: An animal model of schizophrenia? Neurochem. Int. 2006, 48, 541–546. [Google Scholar] [CrossRef]
- Sestakova, N.; Puzserova, A.; Kluknavsky, M.; Bernatova, I. Determination of motor activity and anxiety-related behaviour in rodents: Methodological aspects and role of nitric oxide. Interdiscip. Toxicol. 2013, 6, 126–135. [Google Scholar] [CrossRef]
- Freudenberg, F.; Alttoa, A.; Reif, A. Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes Brain Behav. 2015, 14, 46–63. [Google Scholar] [CrossRef]
- Candemir, E.; Fattakhov, N.; Leary, A.O.; Slattery, D.A.; Courtney, M.J.; Reif, A.; Freudenberg, F. Disrupting the nNOS/NOS1AP interaction in the medial prefrontal cortex impairs social recognition and spatial working memory in mice. Eur. Neuropsychopharmacol. 2023, 67, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Gou, G.; Roca-Fernandez, A.; Kilinc, M.; Serrano, E.; Reig-Viader, R.; Araki, Y.; Huganir, R.L.; de Quintana-Schmidt, C.; Rumbaugh, G.; Bayés, À. SynGAP splice variants display heterogeneous spatio-temporal expression and subcellular distribution in the developing mammalian brain. J. Neurochem. 2020, 154, 618–634. [Google Scholar] [CrossRef]
- He, T.; Huang, Y.; Chak, J.C.; Klar, R.M. Recommendations for improving accuracy of gene expression data in bone and cartilage tissue engineering. Sci. Rep. 2018, 8, 14874. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2008, 8, R19. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbour, NY, USA, 1989. [Google Scholar]
- Kyhse-Andersen, J. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef]
- Matiiv, A.B.; Moskalenko, S.E.; Sergeeva, O.S.; Zhouravleva, G.A.; Bondarev, S.A. NOS1AP Interacts with α-Synuclein and Aggregates in Yeast and Mammalian Cells. Int. J. Mol. Sci. 2022, 23, 9102. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wilcoxon, F. Individual Comparison By Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Brenner, E.; Kondziella, D.; Håberg, A.; Sonnewald, U. Impaired glutamine metabolism in NMDA receptor hypofunction induced by MK801. J. Neurochem. 2005, 94, 1594–1603. [Google Scholar] [CrossRef] [PubMed]
- Koszła, O.; Targowska-Duda, K.M.; Kȩdzierska, E.; Kaczor, A.A. In vitro and in vivo models for the investigation of potential drugs against schizophrenia. Biomolecules 2020, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Zhang, J.; Wu, J.; Li, G.; Yao, W.; Hao, J.; Sun, J. Paliperidone Protects SH-SY5Y Cells Against MK-801-Induced Neuronal Damage Through Inhibition of Ca2+ Influx and Regulation of SIRT1/miR-134 Signal Pathway. Mol. Neurobiol. 2016, 53, 2498–2509. [Google Scholar] [CrossRef]
- Unal, G.; Dokumaci, A.H.; Ozkartal, C.S.; Yerer, M.B.; Aricioglu, F. Famotidine has a neuroprotective effect on MK-801 induced toxicity via the Akt/GSK-3β/β-catenin signaling pathway in the SH-SY5Y cell line. Chem.-Biol. Interact. 2019, 314, 108823. [Google Scholar] [CrossRef]
- Shaw, G.; Morse, S.; Ararat, M.; Graham, F.L. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 869–871. [Google Scholar] [CrossRef]
- Pan, B.; Wang, Y.; Shi, Y.; Yang, Q.; Han, B.; Zhu, X.; Liu, Y. Altered expression levels of miR-144-3p and ATP1B2 are associated with schizophrenia. World J. Biol. Psychiatry 2022, 23, 666–676. [Google Scholar] [CrossRef]
- Wallach, J.; Kang, H.; Colestock, T.; Morris, H.; Bortolotto, Z.A.; Collingridge, G.L.; Lodge, D.; Halberstadt, A.L.; Brandt, S.D.; Adejare, A. Pharmacological Investigations of the Dissociative ’Legal Highs’ Diphenidine, Methoxphenidine and Analogues. PLoS ONE 2016, 11, e0157021. [Google Scholar] [CrossRef]
- Nakazawa, K.; Sapkota, K. The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol. Ther. 2020, 205, 107426. [Google Scholar] [CrossRef]
- Paulson, L.; Martin, P.; Ljung, E.; Blennow, K.; Davidsson, P. Effects on rat thalamic proteome by acute and subchronic MK-801-treatment. Eur. J. Pharmacol. 2004, 505, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wang, C.; Zhang, X.; Wang, Z.; Liang, X.; Alachkar, A.; Civelli, O. A Natural Product with High Affinity to Sigma and 5-HT7 Receptors as Novel Therapeutic Drug for Negative and Cognitive Symptoms of Schizophrenia. Neurochem. Res. 2019, 44, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Fendt, M.; Pedersen, V.; Koch, M. Sensitization of prepulse inhibition deficits by repeated administration of dizocilpine. Psychopharmacology 2001, 156, 177–181. [Google Scholar] [CrossRef] [PubMed]
| Primer | Sequence | Annealing T (°C) | Primer Efficiency (%) |
|---|---|---|---|
| NOS1AP_1&3i_F | GAAAGTGATTCTGAAGAAGAAG | 55.7 and 62.0 | 101 |
| NOS1AP_R | CGATTCTCATAGCTTGGC | 55.7 and 62.0 | 101 |
| b-actin_F | CATCCTCACCCTGAAGTACCC | 55.7 | 100 |
| b-actin_R | CTCAAACATGATCTGGGTCATCTT | 55.7 | 100 |
| RPLP0_F | CAACCCAGCTCTGGAGA | 62.0 | 114 |
| RPLP0_R | CAGCTGGCACCTTATTGG | 62.0 | 114 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matiiv, A.B.; Rogoza, T.M.; Razgovorova, I.A.; Zhdanova, M.I.; Trubitsina, N.P.; Bezgina, M.D.; Isaeva, I.G.; Markov, A.G.; Zhouravleva, G.A.; Bondarev, S.A. Dizocilpine Does Not Alter NOS1AP Gene Expression in Rats and in Cell Cultures. Int. J. Mol. Sci. 2025, 26, 5329. https://doi.org/10.3390/ijms26115329
Matiiv AB, Rogoza TM, Razgovorova IA, Zhdanova MI, Trubitsina NP, Bezgina MD, Isaeva IG, Markov AG, Zhouravleva GA, Bondarev SA. Dizocilpine Does Not Alter NOS1AP Gene Expression in Rats and in Cell Cultures. International Journal of Molecular Sciences. 2025; 26(11):5329. https://doi.org/10.3390/ijms26115329
Chicago/Turabian StyleMatiiv, Anton B., Tatyana M. Rogoza, Irina A. Razgovorova, Maria I. Zhdanova, Nina P. Trubitsina, Mariya D. Bezgina, Irina G. Isaeva, Alexander G. Markov, Galina A. Zhouravleva, and Stanislav A. Bondarev. 2025. "Dizocilpine Does Not Alter NOS1AP Gene Expression in Rats and in Cell Cultures" International Journal of Molecular Sciences 26, no. 11: 5329. https://doi.org/10.3390/ijms26115329
APA StyleMatiiv, A. B., Rogoza, T. M., Razgovorova, I. A., Zhdanova, M. I., Trubitsina, N. P., Bezgina, M. D., Isaeva, I. G., Markov, A. G., Zhouravleva, G. A., & Bondarev, S. A. (2025). Dizocilpine Does Not Alter NOS1AP Gene Expression in Rats and in Cell Cultures. International Journal of Molecular Sciences, 26(11), 5329. https://doi.org/10.3390/ijms26115329






