De Novo Transcriptome Assembly and Annotation Elucidate the Response to Extreme Temperature Stress in the Intermediate Host Bulinus globosus of Schistosoma haematobium
Abstract
1. Introduction
2. Results
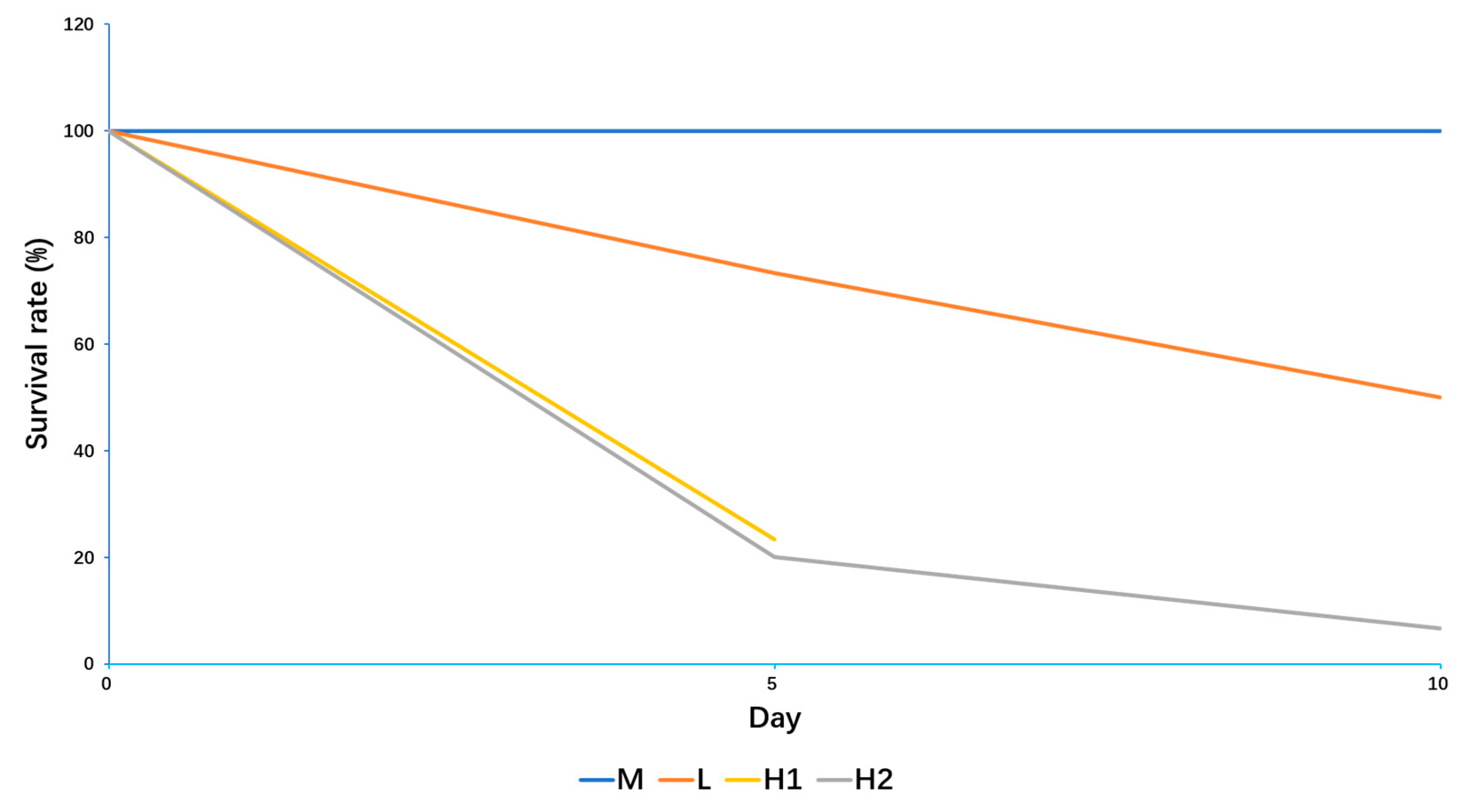
2.1. Responses of B. globosus to Extreme Temperature Stress
2.2. RNA Sequencing and De Novo Assembly
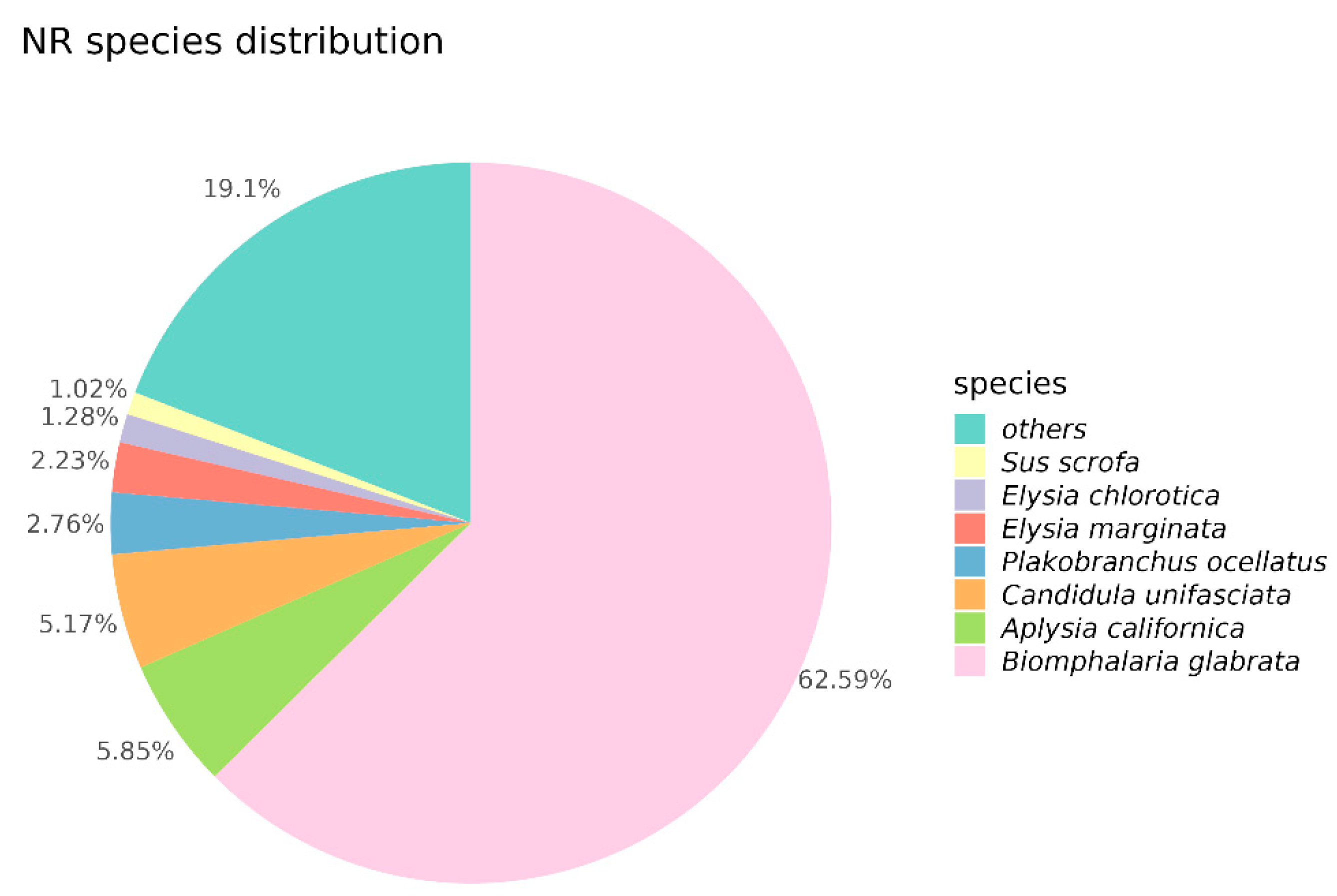
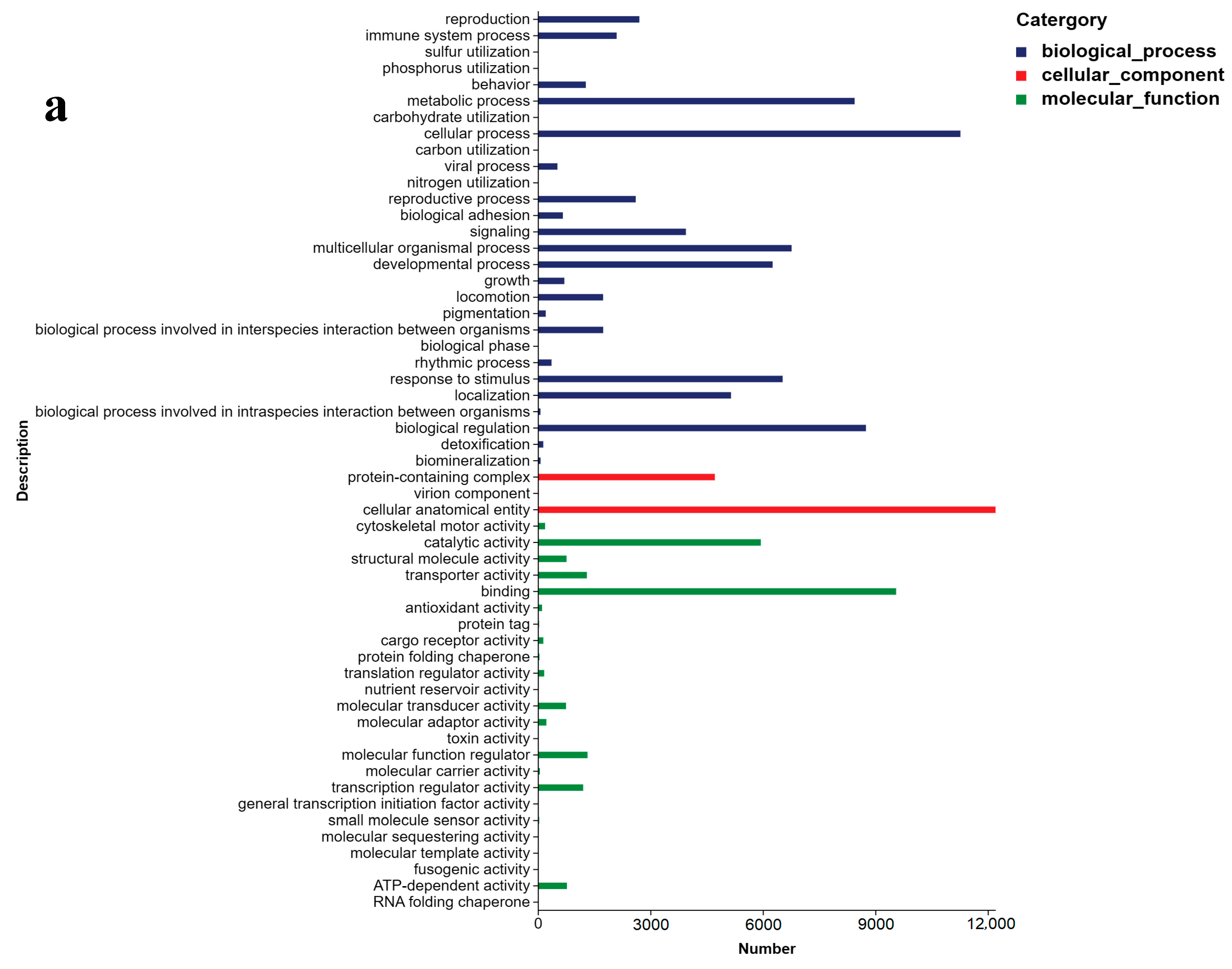
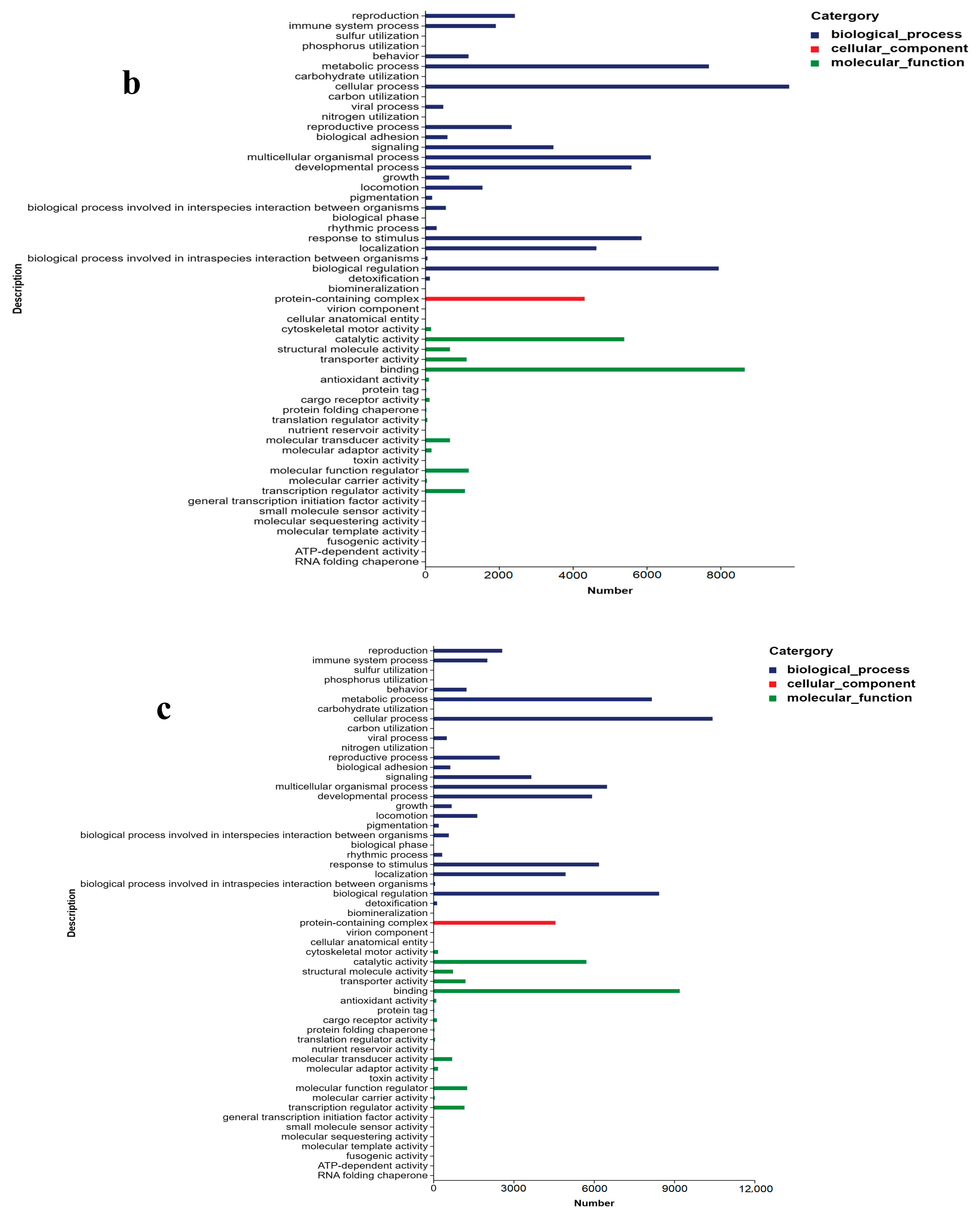
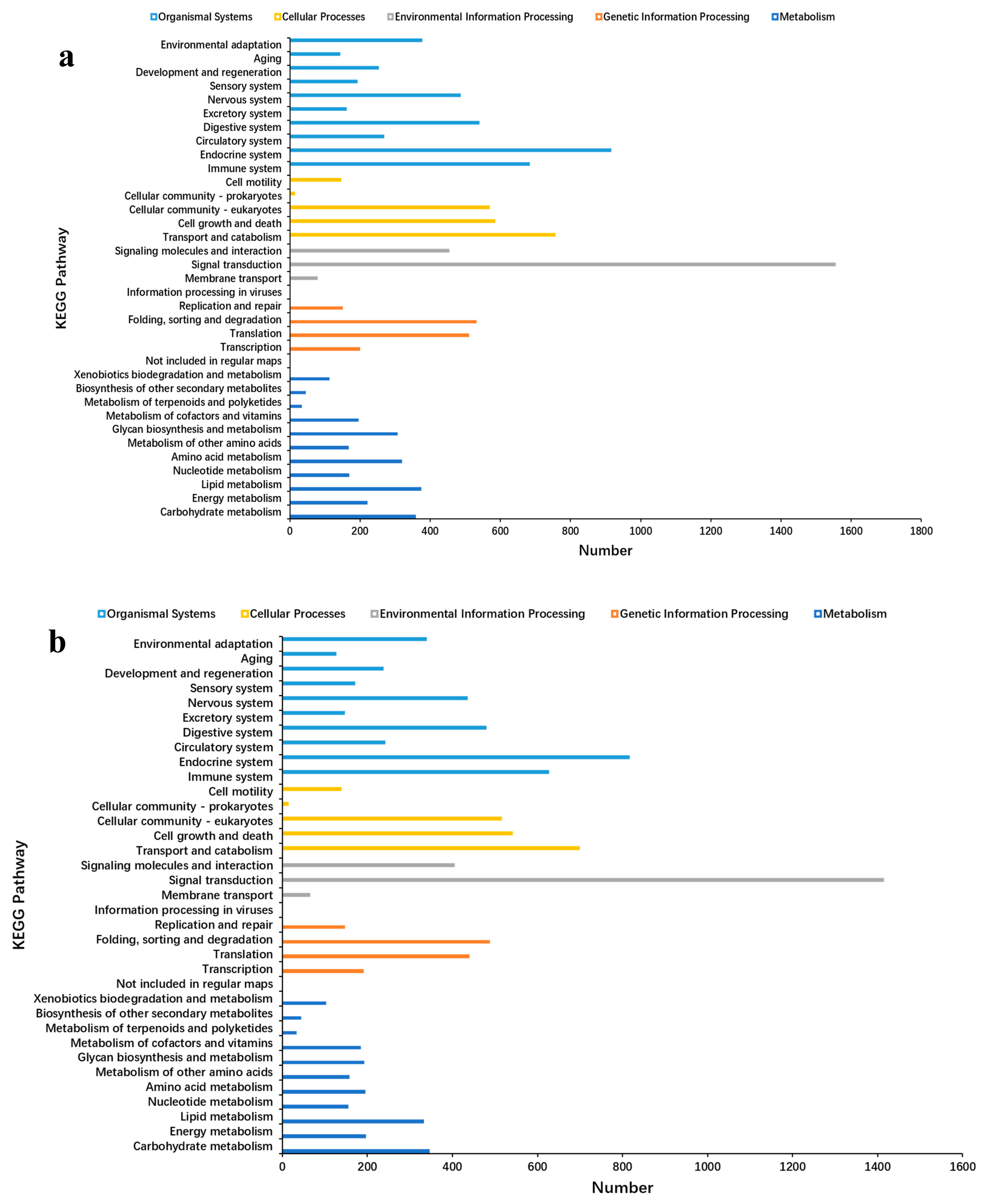
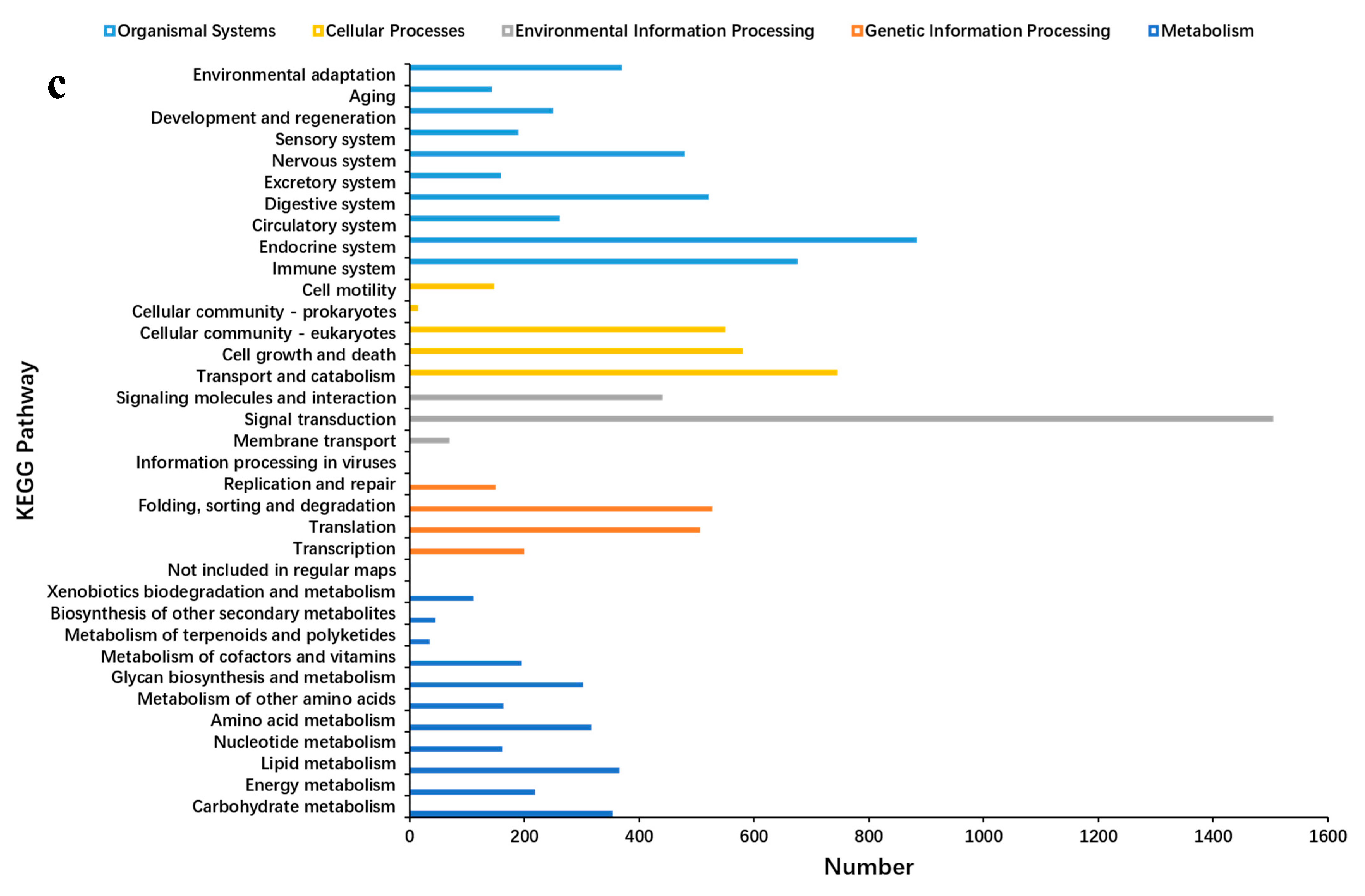
2.3. Unigene Annotation and Functional Classification
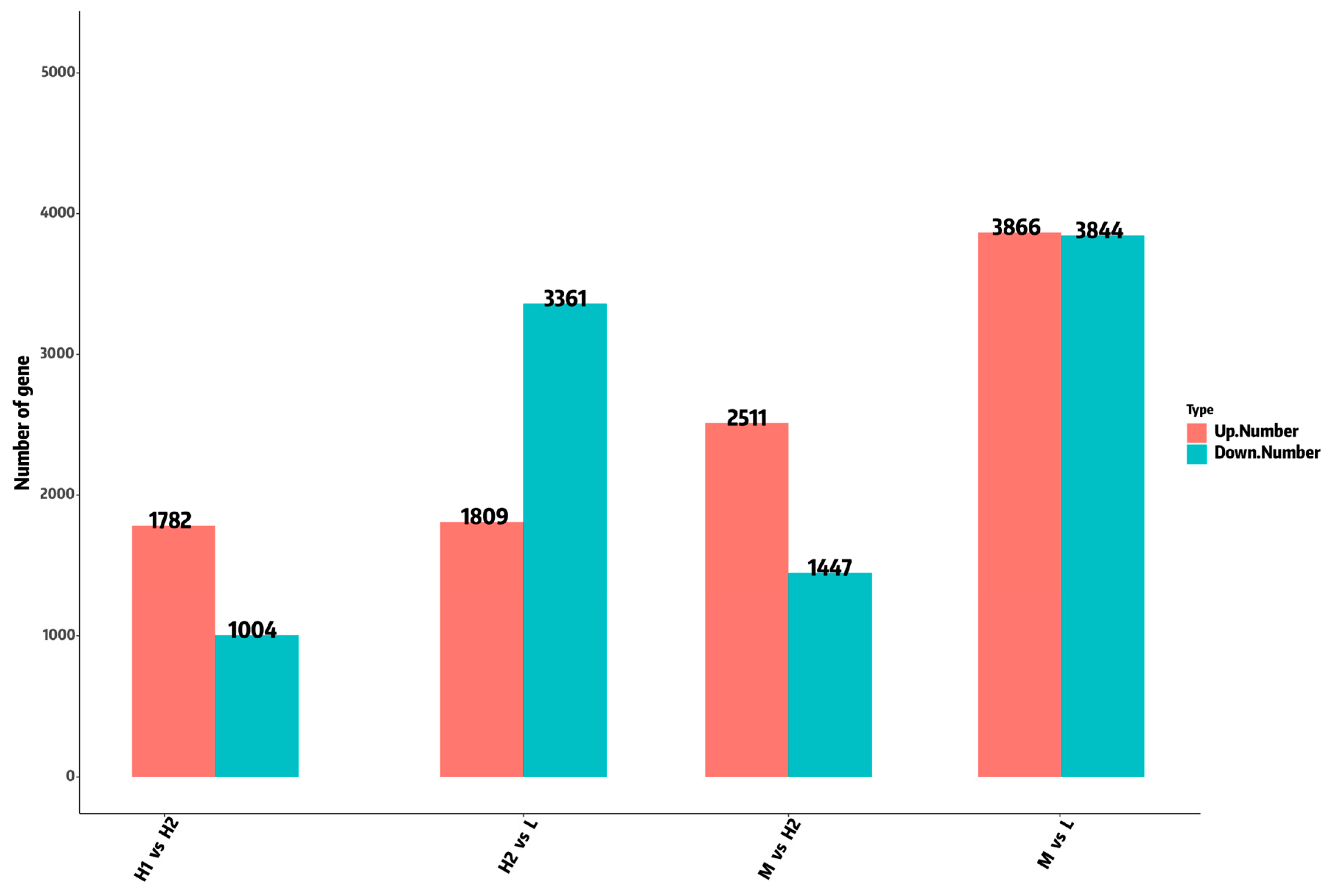
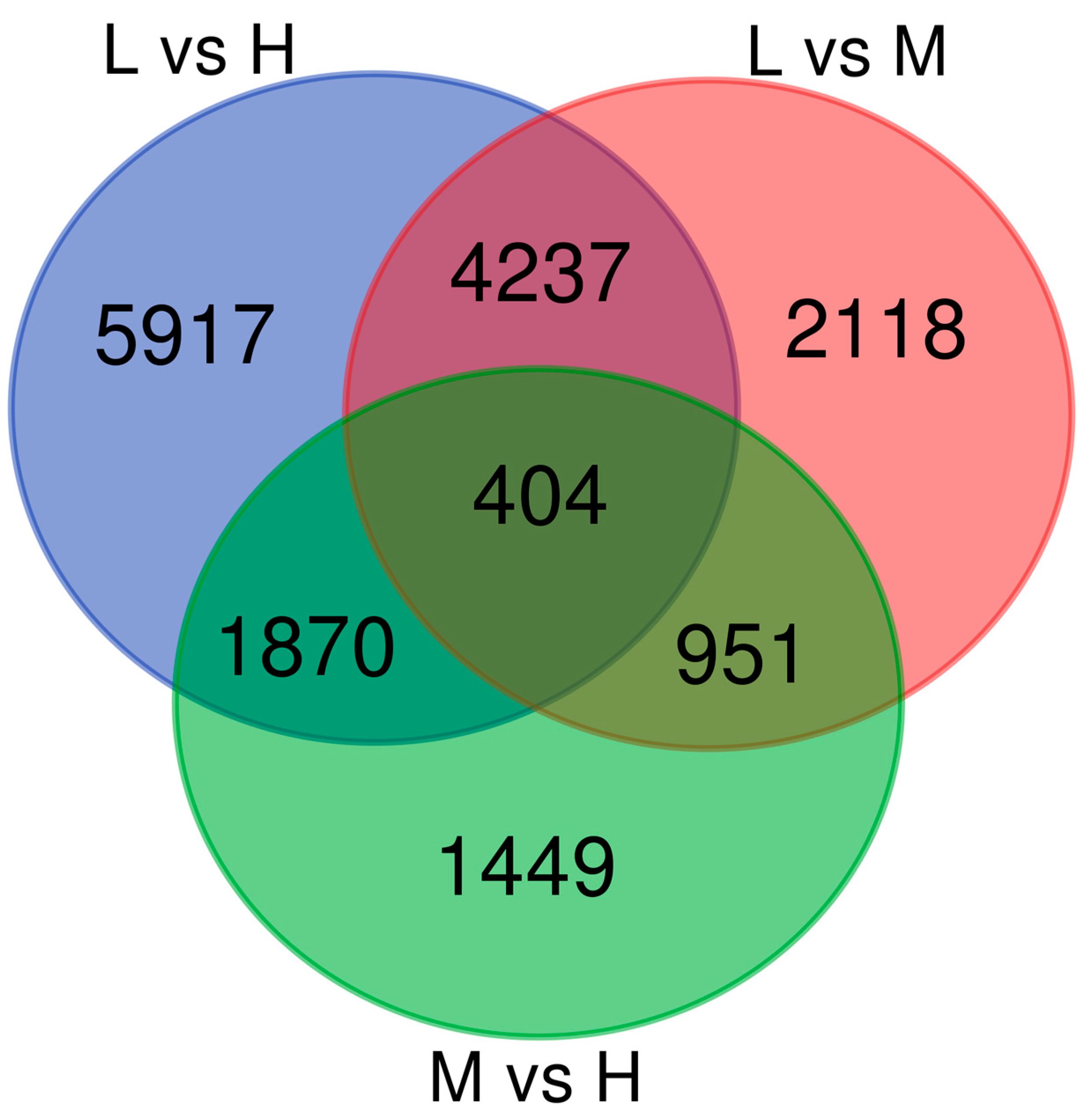
2.4. Analysis of DEGs Under Temperature Stress
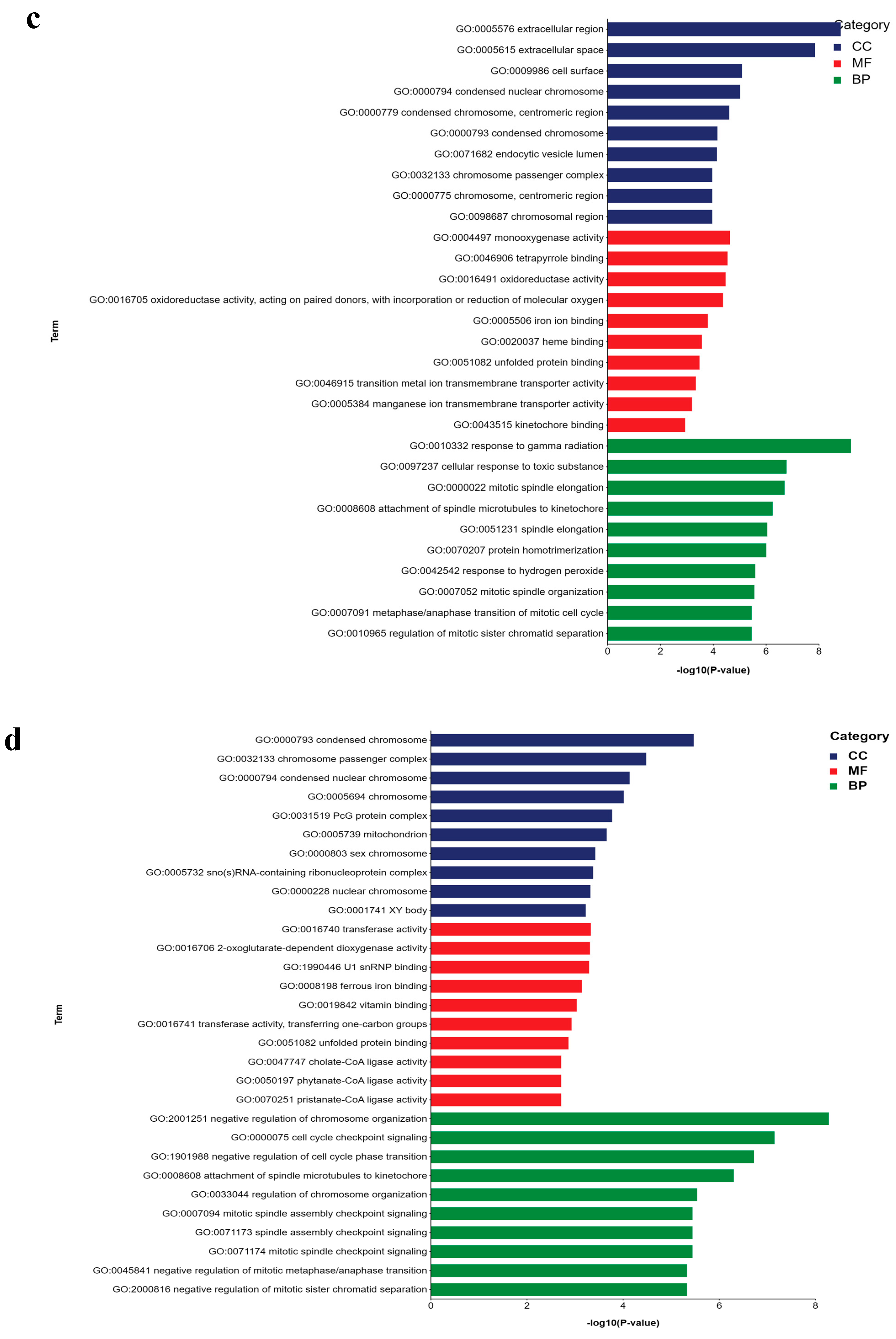
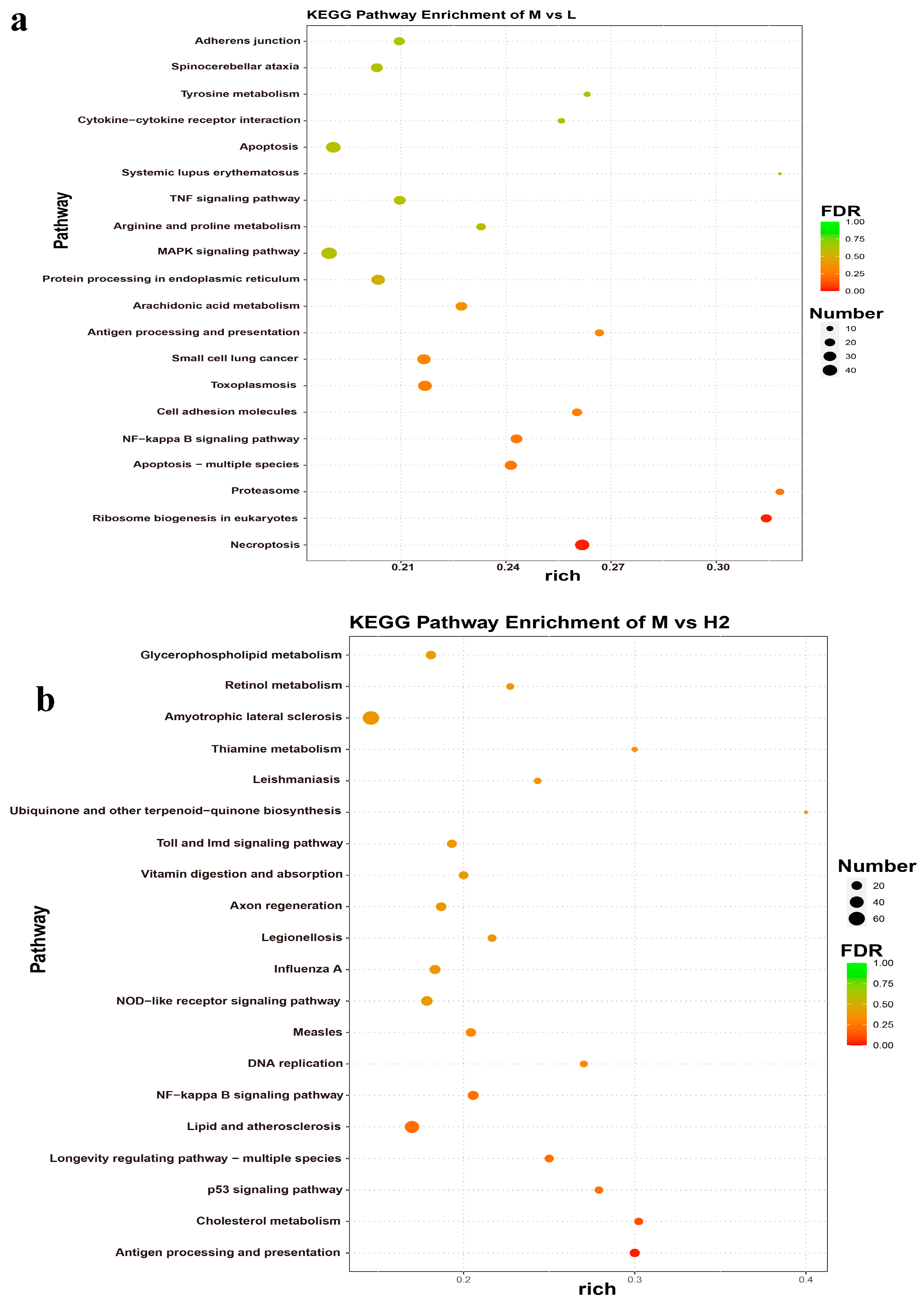
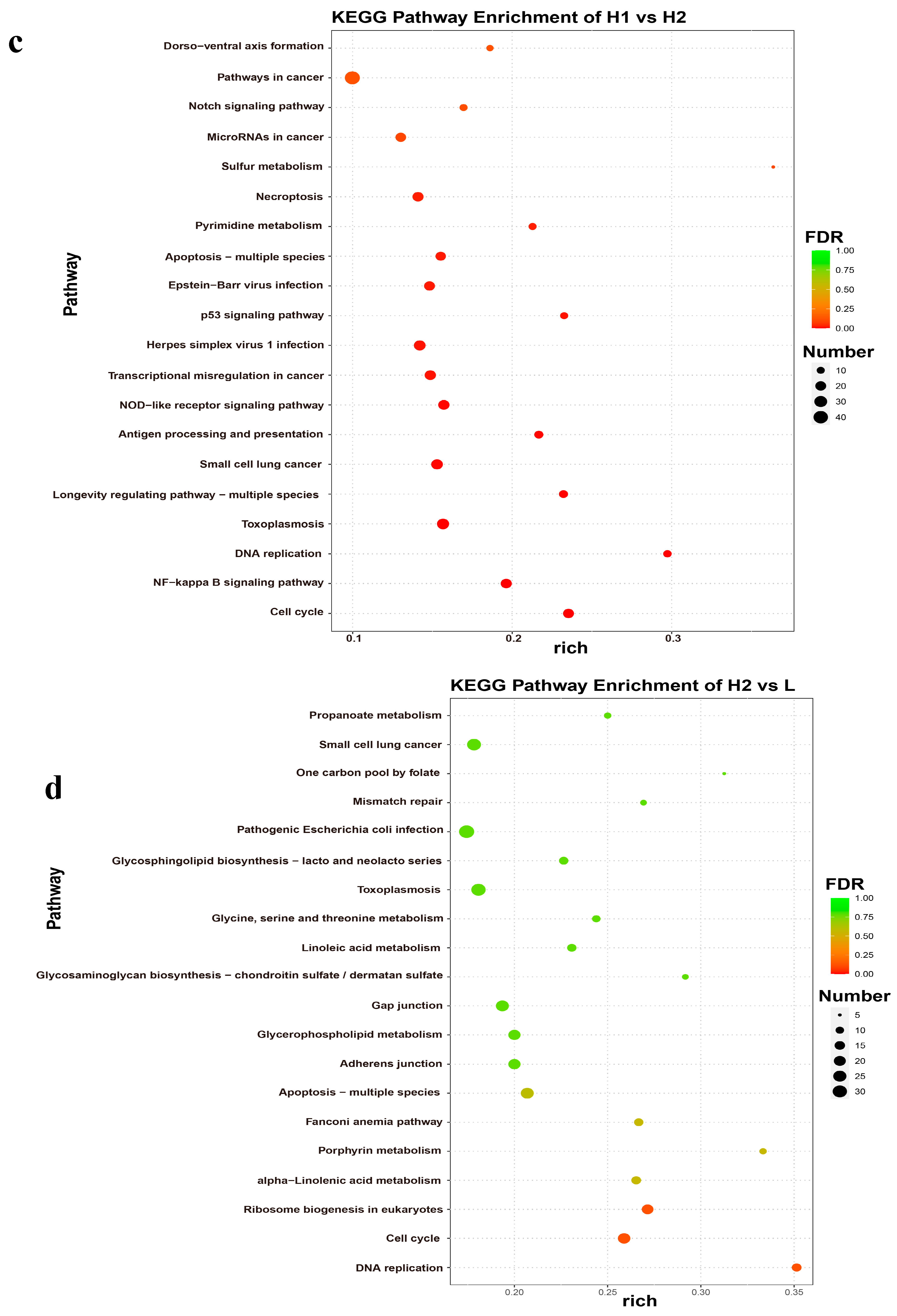
2.5. GO and KEGG Analyses of DEGs
2.6. Validation of RNA-Seq Results Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. B. globosus Preparation
4.2. RNA Sequencing
4.3. De Novo Transcriptome Data Processing and Differentially Expressed Gene Analysis
4.4. Annotation of the GO Function and Analysis of the KEGG Pathway
4.5. qRT-PCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, D.; Zhu, Y.; Liu, L.; Zhang, J.; Huang, P.; Bai, S.; Wang, X.; Yang, K. Schistosomiasis Burden and Trend Analysis in Africa: Insights from the Global Burden of Disease Study 2021. Trop. Med. Infect. Dis. 2025, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.W.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Primers 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Adenowo, A.F.; Oyinloye, B.E.; Ogunyinka, B.I.; Kappo, A.P. Impact of human schistosomiasis in sub-Saharan Africa. Braz. J. Infect. Dis. 2015, 19, 196–205. [Google Scholar] [CrossRef]
- Dejon-Agobé, J.C.; Adegnika, A.A.; Grobusch, M.P. Haematological changes in Schistosoma haematobium infections in school children in Gabon. Infection 2021, 49, 645–651. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Khurana, S.; Dubey, M.L.; Malla, N. ASSOCIATION OF PARASITIC INFECTIONS AND CANCERS. Indian J. Med. Microbiol. 2005, 23, 74–79. [Google Scholar] [CrossRef]
- Patel, P.; Rose, C.E.; Kjetland, E.F.; Downs, J.A.; Mbabazi, P.S.; Sabin, K.; Chege, W.; Watts, D.H.; Secor, W.E. Association of schistosomiasis and HIV infections: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 102, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Zirimenya, L.; Mahmud-Ajeigbe, F.; McQuillan, R.; Li, Y. A systematic review and meta-analysis to assess the association between urogenital schistosomiasis and HIV/AIDS infection. PLoS Neglected Trop. Dis. 2020, 14, e0008383. [Google Scholar] [CrossRef] [PubMed]
- Chibwana, F.D.; Tumwebaze, I.; Mahulu, A.; Sands, A.F.; Albrecht, C. Assessing the diversity and distribution of potential intermediate hosts snails for urogenital schistosomiasis: Bulinus spp. (Gastropoda: Planorbidae) of Lake Victoria. Parasites Vectors 2020, 13, 418. [Google Scholar] [CrossRef]
- Zhang, S.-M.; Bu, L.; Lu, L.; Babbitt, C.; Adema, C.M.; Loker, E.S. Comparative mitogenomics of freshwater snails of the genus Bulinus, obligatory vectors of Schistosoma haematobium, causative agent of human urogenital schistosomiasis. Sci. Rep. 2022, 12, 5357. [Google Scholar] [CrossRef]
- Muhsin, M.A.; Wang, X.; Kabole, F.M.; Zilabumba, J.; Yang, K. The Indispensability of Snail Control for Accelerating Schistosomiasis Elimination: Evidence from Zanzibar. Trop. Med. Infect. Dis. 2022, 7, 347. [Google Scholar] [CrossRef] [PubMed]
- Pennance, T.; Ame, S.M.; Amour, A.K.; Suleiman, K.R.; Muhsin, M.A.; Kabole, F.; Ali, S.M.; Archer, J.; Allan, F.; Emery, A.; et al. Transmission and diversity of Schistosoma haematobium and S. bovis and their freshwater intermediate snail hosts Bulinus globosus and B. nasutus in the Zanzibar Archipelago, United Republic of Tanzania. PLoS Neglected Trop. Dis. 2022, 16, e0010585. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Juma, S.; Li, W.; Suleman, M.; Muhsin, M.A.; He, J.; He, M.; Xu, D.; Zhang, J.; Bergquist, R.; et al. Potential risk of colonization of Bulinus globosus in the mainland of China under climate change. Infect. Dis. Poverty 2022, 11, 52. [Google Scholar] [CrossRef]
- Anettová, L.; Šipková, A.; Izquierdo-Rodriguez, E.; Velič, V.; Modrý, D. Rat lungworm survives winter: Experimental overwintering of Angiostrongylus cantonensis larvae in European slugs. Parasitology 2023, 150, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Polechová, J.; Barton, N.H. Limits to adaptation along environmental gradients. Proc. Natl. Acad. Sci. USA 2015, 112, 6401–6406. [Google Scholar] [CrossRef]
- Caldeira, R.L.; Jannotti-Passos, L.K.; Carvalho, O.S. Molecular epidemiology of Brazilian Biomphalaria: A review of the identification of species and the detection of infected snails. Acta Trop. 2009, 111, 1–6. [Google Scholar] [CrossRef]
- Pedersen, U.B.; Stendel, M.; Midzi, N.; Mduluza, T.; Soko, W.; Stensgaard, A.-S.; Vennervald, B.J.; Mukaratirwa, S.; Kristensen, T.K. Modelling climate change impact on the spatial distribution of fresh water snails hosting trematodes in Zimbabwe. Parasites Vectors 2014, 7, 536. [Google Scholar] [CrossRef]
- Ahn, A.-C.; Jongepier, E.; Schuurmans, J.M.; Rijpstra, W.I.C.; Sinninghe Damsté Jaap, S.; Galinski Erwin, A.; Roman, P.; Sorokin, D.; Muyzer, G. Molecular and Physiological Adaptations to Low Temperature in Thioalkalivibrio Strains Isolated from Soda Lakes with Different Temperature Regimes. mSystems 2021, 6, e01202-20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. The Influence of Temperature Stress on Energy Substances and Some Respiratory Enzymes in the Body of Oncomelania hupensis in Hubei Province. Master’s Thesis, Wannan Medical College, Wuhu, China, 2021. [Google Scholar]
- Bu, L.; Habib, M.R.; Lu, L.; Mutuku, M.W.; Loker, E.S.; Zhang, S.-M. Transcriptional profiling of Bulinus globosus provides insights into immune gene families in snails supporting the transmission of urogenital schistosomiasis. Dev. Comp. Immunol. 2024, 154, 105150. [Google Scholar] [CrossRef]
- Brown, D.S. Freshwater Snails of Africa and Their Medical Importance; CRC Press: London, UK, 2014. [Google Scholar]
- Rivera-Vicéns, R.E.; Garcia-Escudero, C.A.; Conci, N.; Eitel, M.; Wörheide, G. TransPi—A comprehensive TRanscriptome ANalysis PIpeline for de novo transcriptome assembly. Mol. Ecol. Resour. 2022, 22, 2070–2086. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Q.; Li, Y.-L.; Guo, S.-Y.; Li, S.-Z.; Zhou, X.-N.; Guo, J.-G.; Bergquist, R.; Juma, S.; Zhang, J.-F.; et al. Prevalence and correlations of schistosomiasis mansoni and schistosomiasis haematobium among humans and intermediate snail hosts: A systematic review and meta-analysis. Infect. Dis. Poverty 2024, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Bergquist, R. Potential Impact of Climate Change on Schistosomiasis: A Global Assessment Attempt. Trop. Med. Infect. Dis. 2018, 3, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-N.; Yang, G.-J.; Yang, K.; Wang, X.-H.; Hong, Q.-B.; Sun, L.-P.; Malone, J.B.; Kristensen, T.K.; Bergquist, N.R.; Utzinger, J. Potential impact of climate change on schistosomiasis transmission in China. Am. J. Trop. Med. Hyg. 2008, 78, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.R.; Posavi, M.; Lekired, A.; Zhang, S.-M. Exploring the genome-wide transcriptomic responses of Bulinus truncatus to Schistosoma haematobium infection: An important host-parasite system involved in the transmission of human urogenital schistosomiasis. Mol. Immunol. 2024, 175, 74–88. [Google Scholar] [CrossRef]
- Xiao, Q.; Lin, Y.; Li, H.; Chen, Y.; Wei, W.; Li, P.; Chen, L. Transcriptome sequencing reveals the differentially expressed lncRNAs and mRNAs in response to cold acclimation and cold stress in Pomacea canaliculata. BMC Genom. 2022, 23, 382. [Google Scholar] [CrossRef]
- Enriquez, T.; Renault, D.; Charrier, M.; Colinet, H. Cold Acclimation Favors Metabolic Stability in Drosophila suzukii. Front. Physiol. 2018, 9, 1506. [Google Scholar] [CrossRef]
- Colinet, H.; Larvor, V.; Laparie, M.; Renault, D. Exploring the plastic response to cold acclimation through metabolomics. Funct. Ecol. 2012, 26, 711–722. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, R.S.; Zhang, J.; Deng, Z.X.; Zhang, C.X.; Guo, J. Survivorship of geographic Pomacea canaliculata populations in responses to cold acclimation. Ecol. Evol. 2020, 10, 3715–3726. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Z.; Wang, Z.; Peng, Y. A Comparative Transcriptomics Approach to Analyzing the Differences in Cold Resistance in Pomacea canaliculata between Guangdong and Hunan. J. Immunol. Res. 2020, 2020, 8025140. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-d.; Cartwright, S.R.; Ganmanee, M.; Chan, B.K.K.; Adzis, K.A.A.; Hutchinson, N.; Wang, J.; Hui, T.Y.; Williams, G.A.; Dong, Y.-w. High thermal stress responses of Echinolittorina snails at their range edge predict population vulnerability to future warming. Sci. Total Environ. 2019, 647, 763–771. [Google Scholar] [CrossRef]
- Troschinski, S.; Dieterich, A.; Krais, S.; Triebskorn, R.; Köhler, H.-R. Antioxidant defence and stress protein induction following heat stress in the Mediterranean snail Xeropicta derbentina. J. Exp. Biol. 2014, 217, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L.; Lee, R.E., Jr. Low Temperature Biology of Insects. Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Park, J.C.; Lee, M.-C.; Yoon, D.-S.; Han, J.; Park, H.G.; Hwang, U.-K.; Lee, J.-S. Genome-wide identification and expression of the entire 52 glutathione S-transferase (GST) subfamily genes in the Cu2+-exposed marine copepods Tigriopus japonicus and Paracyclopina nana. Aquat. Toxicol. 2019, 209, 56–69. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Manso, B.; Cossins, A.R. Molecular basis of chill resistance adaptations in poikilothermic animals. J. Exp. Biol. 2014, 217, 6–15. [Google Scholar] [CrossRef]
- Mock, E.D.; Mustafa, M.; Gunduz-Cinar, O.; Cinar, R.; Petrie, G.N.; Kantae, V.; Di, X.; Ogasawara, D.; Varga, Z.V.; Paloczi, J.; et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat. Chem. Biol. 2020, 16, 667–675. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Mehmood Khan, T.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Farhadi, A.; Lv, L.; Song, J.; Zhang, Y.; Ye, S.; Zhang, N.; Zheng, H.; Li, S.; Zhang, Y.; Ikhwanuddin, M.; et al. Whole-transcriptome RNA sequencing revealed the roles of chitin-related genes in the eyestalk abnormality of a novel mud crab hybrid (Scylla serrata ♀ × S. paramamosain ♂). Int. J. Biol. Macromol. 2022, 208, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhao, Z.; Feng, S.; Zhang, Y.; Wang, Z.; Li, Z. Multi-omics analysis reveal the fall armyworm Spodoptera frugiperda tolerate high temperature by mediating chitin-related genes. Insect Biochem. Mol. Biol. 2024, 174, 104192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, W.; Zhang, Z. miR-306-5p is involved in chitin metabolism in Aedes albopictus pupae via linc8338-miR-306-5p-XM_019678125.2 axis. Pestic. Biochem. Physiol. 2024, 200, 105811. [Google Scholar] [CrossRef]
- Le, X.T.; Mai, T.V.T.; Ratkiewicz, A.; Huynh, L.K. Mechanism and Kinetics of Low-Temperature Oxidation of a Biodiesel Surrogate: Methyl Propanoate Radicals with Oxygen Molecule. J. Phys. Chem. A 2015, 119, 3689–3703. [Google Scholar] [CrossRef]
- Roginski, A.C.; Wajner, A.; Cecatto, C.; Wajner, S.M.; Castilho, R.F.; Wajner, M.; Amaral, A.U. Disturbance of bioenergetics and calcium homeostasis provoked by metabolites accumulating in propionic acidemia in heart mitochondria of developing rats. Biochim. et Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165682. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Chinta, S.; Singh, P.; Wang, Y.; Nunnery, J.K.; Butcher, R.A. Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.A.; Douglas, P.M.; Simic, M.S.; Grant, A.R.; Moresco, J.J.; Wolff, S.C.; Yates, J.R.; Manning, G.; Dillin, A. HSF-1–mediated cytoskeletal integrity determines thermotolerance and life span. Science 2014, 346, 360–363. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Sukhotin, A.A.; Lannig, G. Stress Effects on Metabolism and Energy Budgets in Mollusks. Oxidative Stress in Aquatic Ecosystems 2011, 261–280. [Google Scholar] [CrossRef]
- Song, H.-M.; Mu, X.-D.; Gu, D.-E.; Luo, D.; Yang, Y.-X.; Xu, M.; Luo, J.-R.; Zhang, J.-E.; Hu, Y.-C. Molecular characteristics of the HSP70 gene and its differential expression in female and male golden apple snails (Pomacea canaliculata) under temperature stimulation. Cell Stress Chaperones 2014, 19, 579–589. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, Z.; Zhang, R.; Guo, K.; Wang, S.; Xu, W.; Wang, C.a. The effects of temperature changes on the isozyme and Hsp70 levels of the Amur sturgeon, Acipenser schrenckii, at two acclimation temperatures. Aquaculture 2022, 551, 737743. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef]
- Yang, C.; Ran, X.; Zhou, Y.; Huang, Y.; Yue, G.; Zhang, M.; Gong, G.; Chang, X.; Qiu, X.; Chen, H. Study on the relationship of Hsp70 with the temperature sensitivity of pedunsaponin A poisoning Pomacea canaliculata. Pestic. Biochem. Physiol. 2022, 188, 105243. [Google Scholar] [CrossRef] [PubMed]
- Motshwene, P.; Karreman, R.; Kgari, G.; Brandt, W.; Lindsey, G. LEA (late embryonic abundant)-like protein Hsp 12 (heat-shock protein 12) is present in the cell wall and enhances the barotolerance of the yeast Saccharomyces cerevisiae. Biochem. J. 2004, 377, 769–774. [Google Scholar] [CrossRef]
- Cardoza, E.; Singh, H. Temporal changes in cold-inducible and uncharacterized Csps under heat and oxidative stress signify a role in bacterial stress response and adaptation. Arch. Microbiol. 2025, 207, 133. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative Stress, Redox Signaling, and Autophagy: Cell Death Versus Survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Rahman, I.A.; Islam, T.; Ghosh, A. Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: Gaining an insight to their physiological and stress-specific roles. PLoS ONE 2017, 12, e0187504. [Google Scholar] [CrossRef]
- Sieber, J.; Wieder, N.; Ostrosky-Frid, M.; Dvela-Levitt, M.; Aygün, O.; Udeshi, N.D.; Carr, S.A.; Greka, A. Lysine trimethylation regulates 78-kDa glucose-regulated protein proteostasis during endoplasmic reticulum stress. J. Biol. Chem. 2017, 292, 18878–18885. [Google Scholar] [CrossRef]
- Kölsch, H.; Linnebank, M.; Lütjohann, D.; Jessen, F.; Wüllner, U.; Harbrecht, U.; Thelen, K.M.; Kreis, M.; Hentschel, F.; Schulz, A.; et al. Polymorphisms in glutathione S-transferase omega-1 and AD, vascular dementia, and stroke. Neurology 2004, 63, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Pila, E.A.; Tarrabain, M.; Kabore, A.L.; Hanington, P.C. A Novel Toll-Like Receptor (TLR) Influences Compatibility between the Gastropod Biomphalaria glabrata, and the Digenean Trematode Schistosoma mansoni. PLoS Pathog. 2016, 12, e1005513. [Google Scholar] [CrossRef]
- Sandoval, C.; Mella, L.; Godoy, K.; Adeli, K.; Farías, J. β-Carotene Increases Activity of Cytochrome P450 2E1 during Ethanol Consumption. Antioxidants 2022, 11, 1033. [Google Scholar] [CrossRef]

| Gene ID | Forward Primer | Reverse Primer |
|---|---|---|
| TRINITY_DN14794_c0_g1 | CGGTAGTAGTAGTTGAAGTAGCA | GTTATGGAGAATCTGAGGTAGGAA |
| TRINITY_DN6699_c1_g1 | ACACCACCAGATTCATAA | TAACAGCCACATTACTTG |
| TRINITY_DN1701_c0_g2 | GTACGATTGGCTGTTGTC | GGCTATGTGTGTTCTTTCTG |
| TRINITY_DN1300_c6_g1 | TGTTCTGTTGTCCTCCTT | TCTGTTGACCACTCTGTT |
| TRINITY_DN14794_c0_g2 | TGTATCCAGTGTCATAAGC | ATTGTTCGTCAACCAGTT |
| TRINITY_DN13712_c0_g1 | CTTCCTCGCAATCACCTT | TTACTACAACTACAGCCAAGAA |
| TRINITY_DN4554_c0_g1 | AGAGACTACTGTGATACTAC | ATGGCGTAATATCTGGAT |
| TRINITY_DN66651_c0_g1 | TAACCTTGAATGACCAGAT | TGATACTAGCCACTATGC |
| TRINITY_DN4880_c0_g1 | TACGAGTGGTGCTATATTAC | CAATCACGCAATGTATTCA |
| Actin | AATGAGCGATTCAGATGT | GATGGAGTTGTAGGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, J.; Yang, Y.; Guo, S.; Li, Y.; Qin, Z.; Juma, H.; Juma, S.; Yang, K.; Li, S.; et al. De Novo Transcriptome Assembly and Annotation Elucidate the Response to Extreme Temperature Stress in the Intermediate Host Bulinus globosus of Schistosoma haematobium. Int. J. Mol. Sci. 2025, 26, 5326. https://doi.org/10.3390/ijms26115326
Wang X, Zhang J, Yang Y, Guo S, Li Y, Qin Z, Juma H, Juma S, Yang K, Li S, et al. De Novo Transcriptome Assembly and Annotation Elucidate the Response to Extreme Temperature Stress in the Intermediate Host Bulinus globosus of Schistosoma haematobium. International Journal of Molecular Sciences. 2025; 26(11):5326. https://doi.org/10.3390/ijms26115326
Chicago/Turabian StyleWang, Xinyao, Jianfeng Zhang, Ying Yang, Suying Guo, Yinlong Li, Zhiqiang Qin, Hamza Juma, Saleh Juma, Kun Yang, Shizhu Li, and et al. 2025. "De Novo Transcriptome Assembly and Annotation Elucidate the Response to Extreme Temperature Stress in the Intermediate Host Bulinus globosus of Schistosoma haematobium" International Journal of Molecular Sciences 26, no. 11: 5326. https://doi.org/10.3390/ijms26115326
APA StyleWang, X., Zhang, J., Yang, Y., Guo, S., Li, Y., Qin, Z., Juma, H., Juma, S., Yang, K., Li, S., & Xu, J. (2025). De Novo Transcriptome Assembly and Annotation Elucidate the Response to Extreme Temperature Stress in the Intermediate Host Bulinus globosus of Schistosoma haematobium. International Journal of Molecular Sciences, 26(11), 5326. https://doi.org/10.3390/ijms26115326











