Myofibroblast-like Cells and Junctional Complex Development Play a Role in Mouse Pubic Symphysis Remodeling During Pregnancy and Postpartum
Abstract
1. Introduction
2. Results
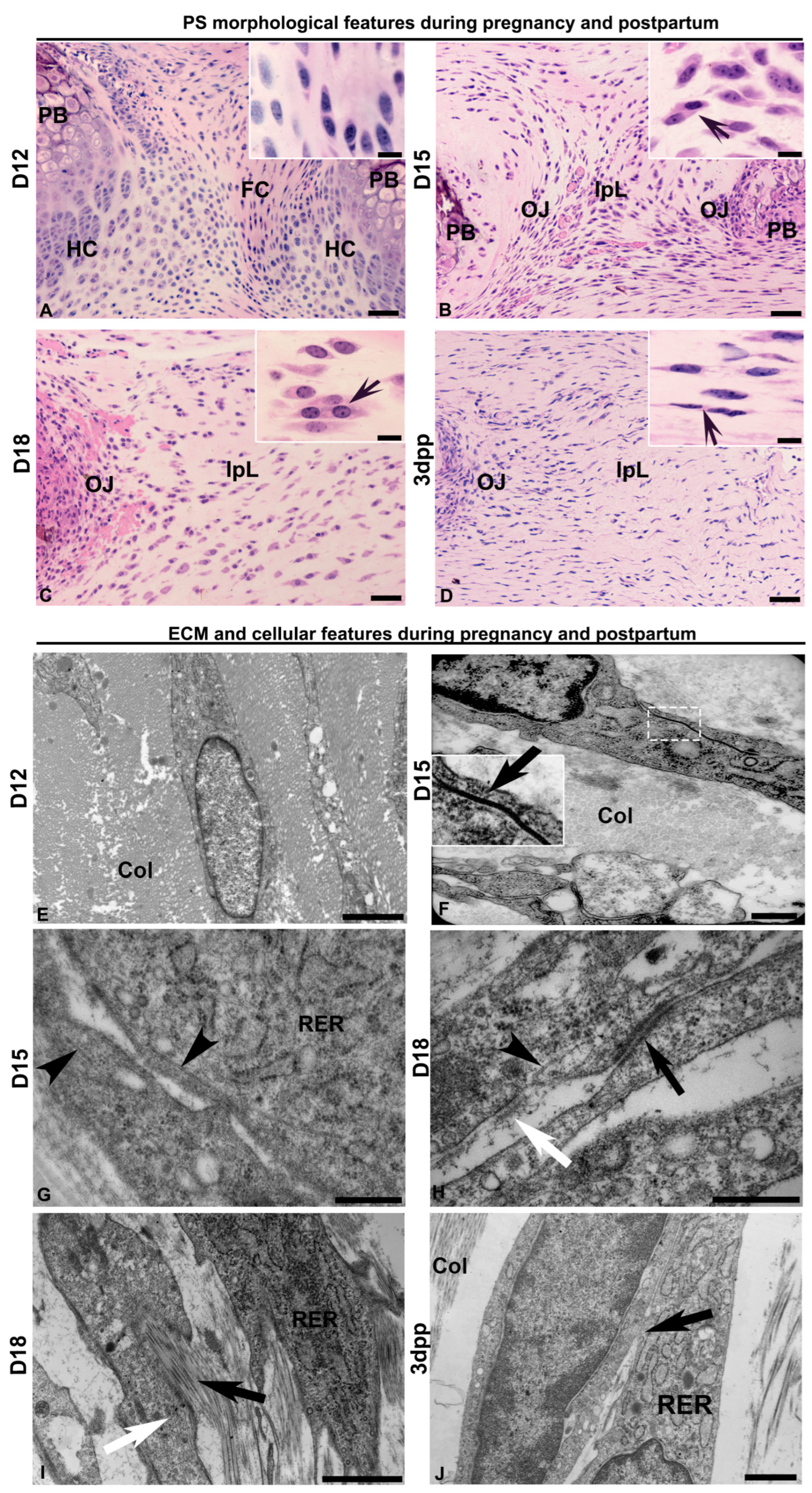
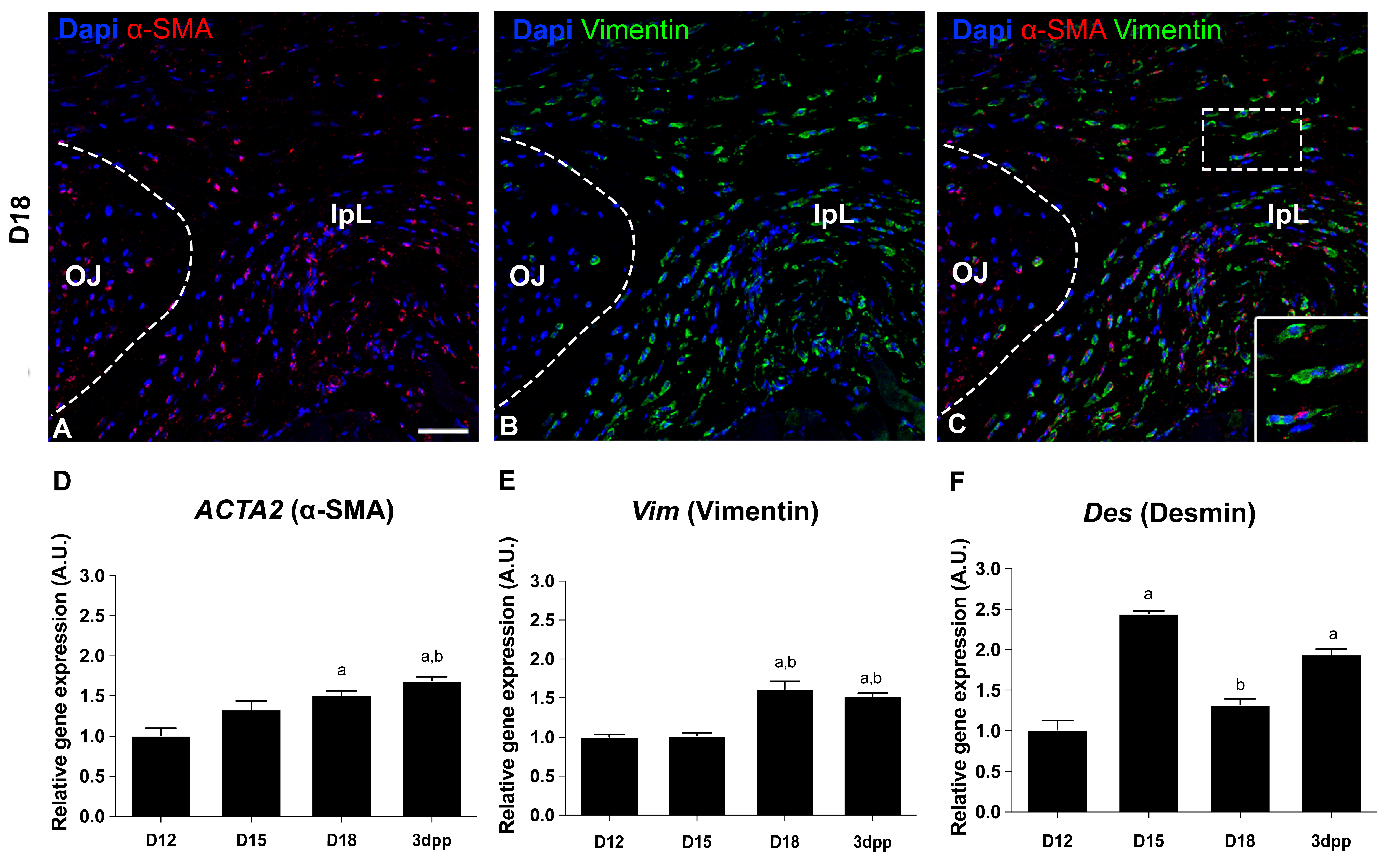
2.1. Dynamic Changes on Cellular Phenotypes and Cell–Cell and Cell–ECM Interactions in the Interpubic Tissues During Pregnancy and Postpartum
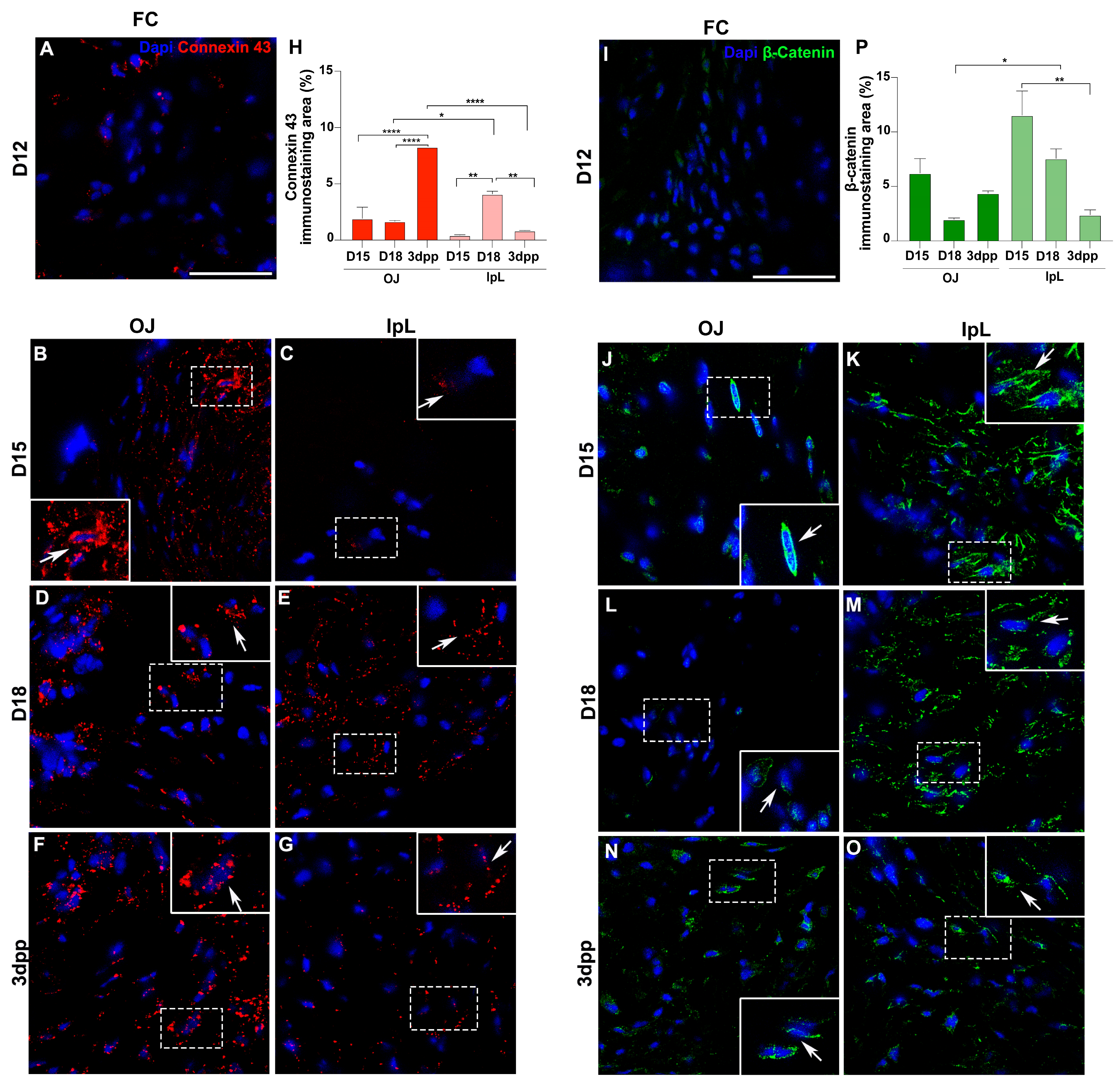
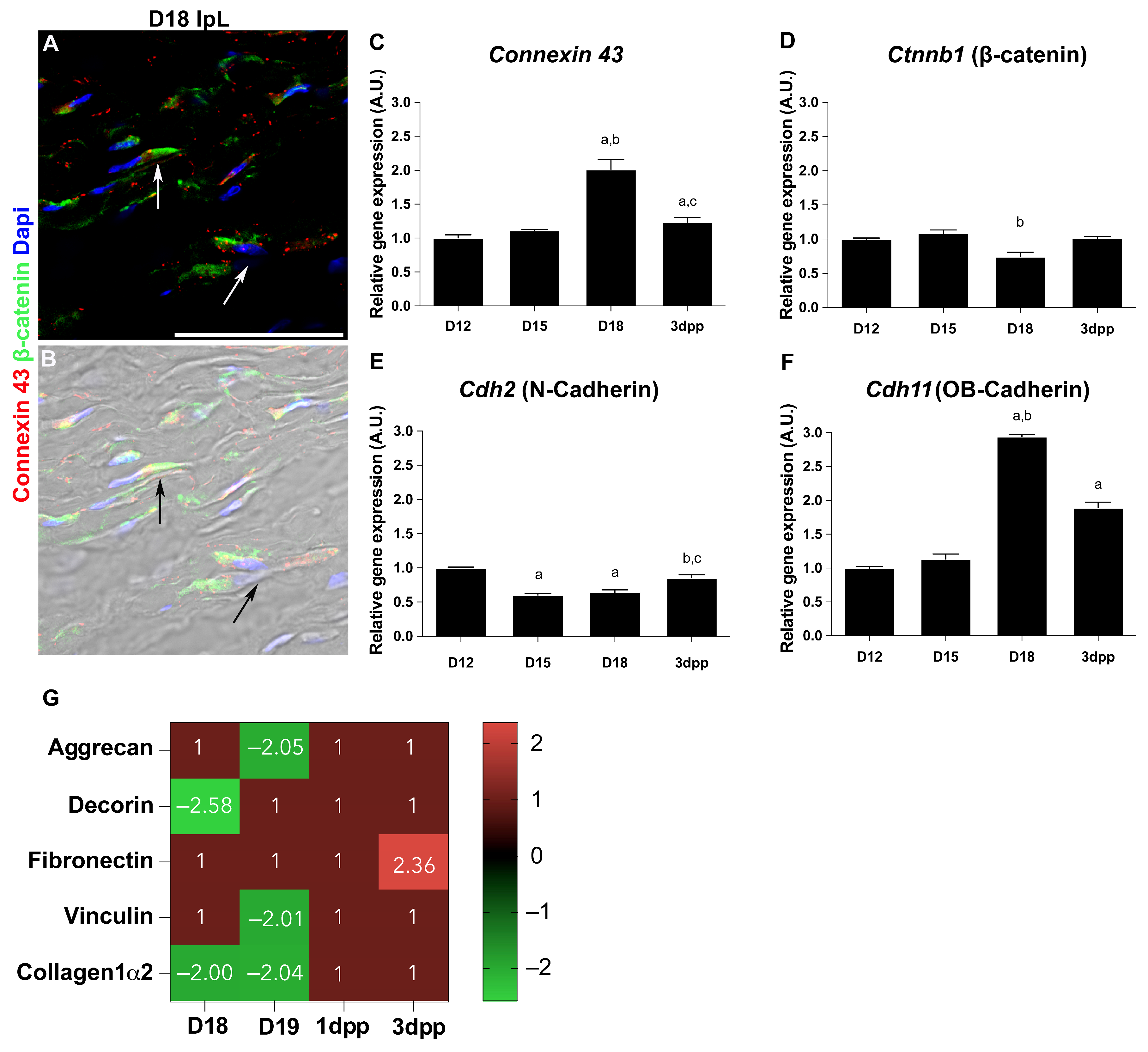
2.2. Cell–Cell and Cell–ECM Junctions Play a Role in the PS Remodeling During Pregnancy and Postpartum
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histology
4.3. Transmission Electron Microscopy
4.4. Immunofluorescence
4.5. Quantification of Immunofluorescence Images
4.6. Real-Time PCR
4.7. Proteomic Shotgun Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamora, C.; Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell Biol. 2002, 4, E101–E108. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hayami, T.; Kapila, S. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthr. Cartil. 2009, 17, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.H.; Munoz-de-Toro, M.M.; Luque, E.H.; Montes, G.S. Morphological characteristics of the interpubic joint (Symphysis pubica) of rats, guinea pigs and mice in different physiological situations. A comparative study. Cells Tissues Organs 2003, 173, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Joazeiro, P.P.; Consonni, S.R.; Rosa, R.G.; Toledo, O.M.S. Peri-partum changes to mouse pubic symphysis. In the Guide to Investigation of Mouse Pregnancy, 1st ed.; Croy, A., Yamada, A.T., DeMayo, F.J., Adamson, S.L., Eds.; Elsevier/Academic Press: London, UK, 2014; pp. 403–417. [Google Scholar] [CrossRef]
- Wang, N.; Tong, X.; Li, Y.K. The mouse pubic symphysis: A narrative review. Front. Physiol. 2025, 16, 1497250. [Google Scholar] [CrossRef]
- Crelin, E.S.; Newton, E.V. The pelvis of the free-tailed bat: Sexual dimorphism and pregnancy changes. Anat. Rec. 1969, 164, 349–357. [Google Scholar] [CrossRef]
- Talmage, R.V. Changes produced in the symphysis pubis of the guinea pig by the sex steroids and relaxin. Anat. Rec. 1947, 99, 91–113. [Google Scholar] [CrossRef]
- Becker, I.; Woodley, S.J.; Stringer, M.D. The adult human pubic symphysis: A systematic review. J. Anat. 2010, 217, 475–487. [Google Scholar] [CrossRef]
- Crelin, E.S. The development of the bony pelvis and its changes during pregnancy and parturition. Trans. N. Y. Acad. Sci. 1969, 31, 1049–1058. [Google Scholar] [CrossRef]
- Castelucci, B.G.; Consonni, S.R.; Rosa, V.S.; Sensiate, L.A.; Delatti, P.C.R.; Alvares, L.E.; Joazeiro, P.P. Time-dependent regulation of morphological changes and cartilage differentiation markers in the mouse pubic symphysis during pregnancy and postpartum recovery. PLoS ONE 2018, 13, e0195304. [Google Scholar] [CrossRef]
- Storey, E. Relaxation in the pubic symphysis of the mouse during pregnancy and after relaxin administration, with special reference to the behaviour of collagen. J. Pathol. 1957, 74, 147–162. [Google Scholar] [CrossRef]
- Hall, K. The effects of pregnancy and relaxin on the histology of the pubic symphysis in the mouse. J. Endocrinol. 1947, 5, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Consonni, S.R.; Werneck, C.C.; Sobreira, D.R.; Kuhne, F.; Moraes, S.G.; Alvares, L.E.; Joazeiro, P.P. Elastic fiber assembly in the adult mouse pubic symphysis during pregnancy and postpartum. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Castelucci, B.G.; Consonni, S.R.; Rosa, V.S.; Joazeiro, P.P. Recruitment of monocytes and mature macrophages in mouse pubic symphysis relaxation during pregnancy and postpartum recoverydagger. Biol. Reprod. 2019, 101, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Crelin, E.S. Interpubic ligament: Elasticity in pregnant free-tailed bat. Science 1969, 164, 81–82. [Google Scholar] [CrossRef]
- de Sousa, L.M.; Castelucci, B.G.; Suarez, P.A.S.; Damas, I.I.; Mariano, F.V.; Joazeiro, P.P.; Consonni, S.R. Multiparity and Aging Impact Chondrogenic and Osteogenic Potential at Symphyseal Enthesis: New Insights into Interpubic Joint Remodeling. Int. J. Mol. Sci. 2023, 24, 4573. [Google Scholar] [CrossRef]
- Pinheiro, M.C.; Moraes, S.G.; Battlehner, C.N.; Caldini, E.G.; Toledo, O.M.; Joazeiro, P.P. Histochemical and ultrastructural study of collagen fibers in mouse pubic symphysis during late pregnancy. Micron 2004, 35, 685–693. [Google Scholar] [CrossRef]
- Moraes, S.G.; Campos Pinheiro, M.; Toledo, O.M.; Joazeiro, P.P. Phenotypic modulation of fibroblastic cells in mice pubic symphysis during pregnancy, partum and postpartum. Cell Tissue Res. 2004, 315, 223–231. [Google Scholar] [CrossRef]
- Rosa, R.G.; Akgul, Y.; Joazeiro, P.P.; Mahendroo, M. Changes of large molecular weight hyaluronan and versican in the mouse pubic symphysis through pregnancy. Biol. Reprod. 2012, 86, 44. [Google Scholar] [CrossRef]
- Castelucci, B.G.; Pereira, A.H.M.; Fioramonte, M.; Carazzolle, M.F.; de Oliveira, P.S.L.; Franchini, K.G.; Kobarg, J.; Martins-de-Souza, D.; Joazeiro, P.P.; Consonni, S.R. Evidence of macrophage modulation in the mouse pubic symphysis remodeling during the end of first pregnancy and postpartum. Sci. Rep. 2020, 10, 12403. [Google Scholar] [CrossRef]
- Mant, J.; Painter, R.; Vessey, M. Epidemiology of genital prolapse: Observations from the Oxford Family Planning Association Study. Br. J. Obstet. Gynaecol. 1997, 104, 579–585. [Google Scholar] [CrossRef]
- Patel, D.A.; Xu, X.; Thomason, A.D.; Ransom, S.B.; Ivy, J.S.; DeLancey, J.O. Childbirth and pelvic floor dysfunction: An epidemiologic approach to the assessment of prevention opportunities at delivery. Am. J. Obstet. Gynecol. 2006, 195, 23–28. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O. The anatomy of the pelvic floor. Curr. Opin. Obstet. Gynecol. 1994, 6, 313–316. [Google Scholar] [CrossRef]
- Norton, P.A. Pelvic floor disorders: The role of fascia and ligaments. Clin. Obstet. Gynecol. 1993, 36, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Serini, G.; Gabbiani, G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp. Cell Res. 1999, 250, 273–283. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef]
- Follonier, L.; Schaub, S.; Meister, J.J.; Hinz, B. Myofibroblast communication is controlled by intercellular mechanical coupling. J. Cell Sci. 2008, 121, 3305–3316. [Google Scholar] [CrossRef]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003, 14, 538–546. [Google Scholar] [CrossRef]
- Younesi, F.S.; Miller, A.E.; Barker, T.H.; Rossi, F.M.V.; Hinz, B. Fibroblast and myofibroblast activation in normal tissue repair and fibrosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 617–638. [Google Scholar] [CrossRef]
- Younesi, F.S.; Son, D.O.; Firmino, J.; Hinz, B. Myofibroblast Markers and Microscopy Detection Methods in Cell Culture and Histology. Methods Mol. Biol. 2021, 2299, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.E.; Guan, J.L.; Trevithick, J.E.; Hynes, R.O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990, 1, 597–604. [Google Scholar] [CrossRef]
- Dugina, V.; Fontao, L.; Chaponnier, C.; Vasiliev, J.; Gabbiani, G. Focal adhesion features during myofibroblastic differentiation are controlled by intracellular and extracellular factors. J. Cell Sci. 2001, 114, 3285–3296. [Google Scholar] [CrossRef]
- Zamir, E.; Geiger, B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 2001, 114, 3583–3590. [Google Scholar] [CrossRef]
- Hinz, B.; Mastrangelo, D.; Iselin, C.E.; Chaponnier, C.; Gabbiani, G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am. J. Pathol. 2001, 159, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Gabbiani, G. Cell-matrix and cell-cell contacts of myofibroblasts: Role in connective tissue remodeling. Thromb. Haemost. 2003, 90, 993–1002. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Pittet, P.; Lee, K.; Kulik, A.J.; Meister, J.J.; Hinz, B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J. Cell Sci. 2008, 121, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Pittet, P.; Smith-Clerc, J.; Chaponnier, C.; Meister, J.J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol. Biol. Cell 2004, 15, 4310–4320. [Google Scholar] [CrossRef]
- Black, M.; Milewski, D.; Le, T.; Ren, X.; Xu, Y.; Kalinichenko, V.V.; Kalin, T.V. FOXF1 Inhibits Pulmonary Fibrosis by Preventing CDH2-CDH11 Cadherin Switch in Myofibroblasts. Cell Rep. 2018, 23, 442–458. [Google Scholar] [CrossRef]
- Gabbiani, G.; Chaponnier, C.; Huttner, I. Cytoplasmic filaments and gap junctions in epithelial cells and myofibroblasts during wound healing. J. Cell Biol. 1978, 76, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef]
- Spanakis, S.G.; Petridou, S.; Masur, S.K. Functional gap junctions in corneal fibroblasts and myofibroblasts. Invest. Ophthalmol. Vis. Sci. 1998, 39, 1320–1328. [Google Scholar] [PubMed]
- Jamieson, S.; Going, J.J.; D’Arcy, R.; George, W.D. Expression of gap junction proteins connexin 26 and connexin 43 in normal human breast and in breast tumours. J. Pathol. 1998, 184, 37–43. [Google Scholar] [CrossRef]
- Olk, S.; Zoidl, G.; Dermietzel, R. Connexins, cell motility, and the cytoskeleton. Cell Motil. Cytoskelet. 2009, 66, 1000–1016. [Google Scholar] [CrossRef]
- Calder, A.A. Prostaglandins and biological control of cervical function. Aust. N. Z. J. Obs. Obstet. Gynaecol. 1994, 34, 347–351. [Google Scholar] [CrossRef]
- Mahendroo, M. Cervical remodeling in term and preterm birth: Insights from an animal model. Reproduction 2012, 143, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Runyan, C.E.; Schnaper, H.W.; Poncelet, A.C. The phosphatidylinositol 3-kinase/Akt pathway enhances Smad3-stimulated mesangial cell collagen I expression in response to transforming growth factor-beta1. J. Biol. Chem. 2004, 279, 2632–2639. [Google Scholar] [CrossRef]
- Hall, K.; Newton, W.H. The normal course of separation of the pubes in pregnant mice. J. Physiol. 1946, 104, 346–352. [Google Scholar] [CrossRef]
- Weiss, M.; Nagelschmidt, M.; Struck, H. Relaxin and collagen metabolism. Horm. Metab. Res. 1979, 11, 408–410. [Google Scholar] [CrossRef]
- Samuel, C.S.; Butkus, A.; Coghlan, J.P.; Bateman, J.F. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: The influence of estrogen and progesterone in regulating relaxin activity. Endocrinology 1996, 137, 3884–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Roche, P.J.; Gunnersen, J.M.; Hammond, V.E.; Tregear, G.W.; Wintour, E.M.; Beck, F. Mice without a functional relaxin gene are unable to deliver milk to their pups. Endocrinology 1999, 140, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Samuel, C.S.; Tregear, G.W.; Beck, F.; Wintour, E.M. Collagen studies in late pregnant relaxin null mice. Biol. Reprod. 2000, 63, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Darby, I.A.; Gabbiani, G. Normal and pathologic soft tissue remodeling: Role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab. Invest. 2003, 83, 1689–1707. [Google Scholar] [CrossRef]
- Kapila, S.; Wang, W.; Uston, K. Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints. Ann. N. Y. Acad. Sci. 2009, 1160, 322–328. [Google Scholar] [CrossRef]
- Leivonen, S.K.; Chantry, A.; Hakkinen, L.; Han, J.; Kahari, V.M. Smad3 mediates transforming growth factor-beta-induced collagenase-3 (matrix metalloproteinase-13) expression in human gingival fibroblasts. Evidence for cross-talk between Smad3 and p38 signaling pathways. J. Biol. Chem. 2002, 277, 46338–46346. [Google Scholar] [CrossRef]
- Peinado, H.; Quintanilla, M.; Cano, A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: Mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 2003, 278, 21113–21123. [Google Scholar] [CrossRef] [PubMed]
- MA, T. Pelvic Organ Prolapse in Pregnancy. Obs. Obstet. Gynecol. Int. J. 2017, 8, 00284. [Google Scholar] [CrossRef][Green Version]
- Yiou, R.; Delmas, V.; Carmeliet, P.; Gherardi, R.K.; Barlovatz-Meimon, G.; Chopin, D.K.; Abbou, C.C.; Lefaucheur, J.P. The pathophysiology of pelvic floor disorders: Evidence from a histomorphologic study of the perineum and a mouse model of rectal prolapse. J. Anat. 2001, 199, 599–607. [Google Scholar] [CrossRef]
- Couri, B.M.; Lenis, A.T.; Borazjani, A.; Paraiso, M.F.; Damaser, M.S. Animal models of female pelvic organ prolapse: Lessons learned. Expert. Rev. Obstet. Gynecol. 2012, 7, 249–260. [Google Scholar] [CrossRef]
- Abramowitch, S.D.; Feola, A.; Jallah, Z.; Moalli, P.A. Tissue mechanics, animal models, and pelvic organ prolapse: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144 (Suppl. 1), S146–S158. [Google Scholar] [CrossRef] [PubMed]
- Drewes, P.G.; Yanagisawa, H.; Starcher, B.; Hornstra, I.; Csiszar, K.; Marinis, S.I.; Keller, P.; Word, R.A. Pelvic organ prolapse in fibulin-5 knockout mice: Pregnancy-induced changes in elastic fiber homeostasis in mouse vagina. Am. J. Pathol. 2007, 170, 578–589. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Rahn, D.D.; Acevedo, J.F.; Roshanravan, S.; Keller, P.W.; Davis, E.C.; Marmorstein, L.Y.; Word, R.A. Failure of pelvic organ support in mice deficient in fibulin-3. Am. J. Pathol. 2009, 174, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, U.J.; Gustilo-Ashby, A.M.; Daneshgari, F.; Kuang, M.; Vurbic, D.; Lin, D.L.; Flask, C.A.; Li, T.; Damaser, M.S. Lower urogenital tract anatomical and functional phenotype in lysyl oxidase like-1 knockout mice resembles female pelvic floor dysfunction in humans. Am. J. Physiol. Ren. Physiol. 2008, 295, F545–F555. [Google Scholar] [CrossRef]
- Han, X.J.; Cai, C.F.; Huang, J.Z.; Li, Q.F.; Huang, L.; Xuan, Q.S.; Yang, J.Y. The intervention effect of nicotine on cervical fibroblast-myofibroblast differentiation in lipopolysaccharide-induced preterm birth model through activating the TGF-β1/Smad3 pathway. Biomed. Pharmacother. 2021, 134, 111135. [Google Scholar] [CrossRef]
- Canty, E.G.; Lu, Y.; Meadows, R.S.; Shaw, M.K.; Holmes, D.F.; Kadler, K.E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004, 165, 553–563. [Google Scholar] [CrossRef]
- Canty, E.G.; Starborg, T.; Lu, Y.; Humphries, S.M.; Holmes, D.F.; Meadows, R.S.; Huffman, A.; O’Toole, E.T.; Kadler, K.E. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006, 281, 38592–38598. [Google Scholar] [CrossRef]
- Wehrle-Haller, B.; Imhof, B.A. Actin, microtubules and focal adhesion dynamics during cell migration. Int. J. Biochem. Cell Biol. 2003, 35, 39–50. [Google Scholar] [CrossRef]
- Bershadsky, A.D.; Ballestrem, C.; Carramusa, L.; Zilberman, Y.; Gilquin, B.; Khochbin, S.; Alexandrova, A.Y.; Verkhovsky, A.B.; Shemesh, T.; Kozlov, M.M. Assembly and mechanosensory function of focal adhesions: Experiments and models. Eur. J. Cell Biol. 2006, 85, 165–173. [Google Scholar] [CrossRef]
- Harris, T.J.; Tepass, U. Adherens junctions: From molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Montes, G.S.; Zugaib, M.; Joazeiro, P.P.; Varayoud, J.; Ramos, J.G.; Muñoz-De-Toro, M.; Luque, E.H. Phenotypic modulation of fibroblastic cells in the mucous layer of the human uterine cervix at term. Reproduction 2002, 124, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Schwartz, A.G.; Moore, E.R.; Sup, M.E.; Thomopoulos, S. Primary cilia as the nexus of biophysical and hedgehog signaling at the tendon enthesis. Sci. Adv. 2020, 6, eabc1799. [Google Scholar] [CrossRef]
- Doring, B.; Shynlova, O.; Tsui, P.; Eckardt, D.; Janssen-Bienhold, U.; Hofmann, F.; Feil, S.; Feil, R.; Lye, S.J.; Willecke, K. Ablation of connexin43 in uterine smooth muscle cells of the mouse causes delayed parturition. J. Cell Sci. 2006, 119, 1715–1722. [Google Scholar] [CrossRef]
- Lefort, C.T.; Wojciechowski, K.; Hocking, D.C. N-cadherin cell-cell adhesion complexes are regulated by fibronectin matrix assembly. J. Biol. Chem. 2011, 286, 3149–3160. [Google Scholar] [CrossRef]
- Chen, X.; Gumbiner, B.M. Crosstalk between different adhesion molecules. Curr. Opin. Cell Biol. 2006, 18, 572–578. [Google Scholar] [CrossRef]
- Gamble, J.G.; Simmons, S.C.; Freedman, M. The symphysis pubis. Anatomic and pathologic considerations. Clin. Orthop. Relat. Res. 1986, 203, 261–272. [Google Scholar] [CrossRef]
- Eng, J. Sample size estimation: How many individuals should be studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Consonni, S.R.; Rosa, R.G.; Nascimento, M.A.; Vinagre, C.M.; Toledo, O.M.; Joazeiro, P.P. Recovery of the pubic symphysis on primiparous young and multiparous senescent mice at postpartum. Histol. Histopathol. 2012, 27, 885–896. [Google Scholar] [CrossRef]
- Bennett, H.S.; Wyrick, A.D.; Lee, S.W.; McNeil, J.H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain. Technol. 1976, 51, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Laborda, J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991, 19, 3998. [Google Scholar] [CrossRef]
- Silva, J.C.; Denny, R.; Dorschel, C.A.; Gorenstein, M.; Kass, I.J.; Li, G.Z.; McKenna, T.; Nold, M.J.; Richardson, K.; Young, P.; et al. Quantitative proteomic analysis by accurate mass retention time pairs. Anal. Chem. 2005, 77, 2187–2200. [Google Scholar] [CrossRef]
- Conover, W.J.; Iman, R.L. Rank Transformations as a Bridge between Parametric and Nonparametric Statistics. Am. Stat. 1981, 35, 124–129. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, V.S.; Castelucci, B.G.; Moreira, M.; Joazeiro, P.P.; Consonni, S.R. Myofibroblast-like Cells and Junctional Complex Development Play a Role in Mouse Pubic Symphysis Remodeling During Pregnancy and Postpartum. Int. J. Mol. Sci. 2025, 26, 5307. https://doi.org/10.3390/ijms26115307
Rosa VS, Castelucci BG, Moreira M, Joazeiro PP, Consonni SR. Myofibroblast-like Cells and Junctional Complex Development Play a Role in Mouse Pubic Symphysis Remodeling During Pregnancy and Postpartum. International Journal of Molecular Sciences. 2025; 26(11):5307. https://doi.org/10.3390/ijms26115307
Chicago/Turabian StyleRosa, Viviane Souza, Bianca Gazieri Castelucci, Monica Moreira, Paulo Pinto Joazeiro, and Sílvio Roberto Consonni. 2025. "Myofibroblast-like Cells and Junctional Complex Development Play a Role in Mouse Pubic Symphysis Remodeling During Pregnancy and Postpartum" International Journal of Molecular Sciences 26, no. 11: 5307. https://doi.org/10.3390/ijms26115307
APA StyleRosa, V. S., Castelucci, B. G., Moreira, M., Joazeiro, P. P., & Consonni, S. R. (2025). Myofibroblast-like Cells and Junctional Complex Development Play a Role in Mouse Pubic Symphysis Remodeling During Pregnancy and Postpartum. International Journal of Molecular Sciences, 26(11), 5307. https://doi.org/10.3390/ijms26115307






