Elexacaftor/Tezacaftor/Ivacaftor Supports Treatment for CF with ΔI1023-V1024-CFTR
Abstract
1. Introduction
2. Case Presentation
2.1. Clinical Features Before ETI
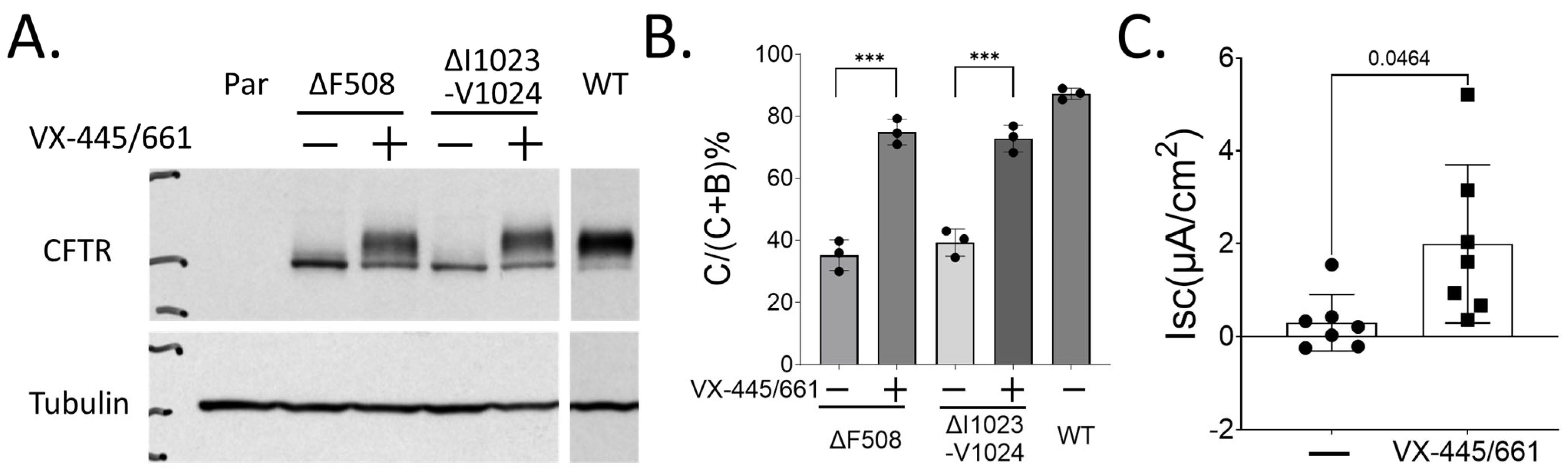
2.2. In Vitro Therapying Studies
2.3. Improvement in Clinical Outcomes with ETI Treatment
2.4. Materials and Methods
2.4.1. Ethics Statement
2.4.2. CFTR Modulators and Chemicals
2.4.3. Generation of Relevant Plasmids
2.4.4. Cell Lysate Preparation and Immunoblotting
2.4.5. Nasal Cells Culture
2.4.6. Ussing Chambers
2.4.7. Statistics
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Clancy, J.P.; Cotton, C.U.; Donaldson, S.H.; Solomon, G.M.; VanDevanter, D.R.; Boyle, M.P.; Gentzsch, M.; Nick, J.A.; Illek, B.; Wallenburg, J.C.; et al. CFTR modulator theratyping: Current status, gaps and future directions. J. Cyst. Fibros. 2019, 18, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Paul, G.; Lee, J.; Yarlagadda, S.; McCoy, K.; Naren, A.P. Elexacaftor/Tezacaftor/Ivacaftor Improved Clinical Outcomes in a Patient with N1303K-CFTR Based on In Vitro Experimental Evidence. Am. J. Respir. Crit. Care Med. 2021, 204, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Dreano, E.; Burgel, P.R.; Hatton, A.; Bouazza, N.; Chevalier, B.; Macey, J.; Leroy, S.; Durieu, I.; Weiss, L.; Grenet, D.; et al. Theratyping cystic fibrosis patients to guide elexacaftor/tezacaftor/ivacaftor out-of-label prescription. Eur. Respir. J. 2023, 62, 2300110. [Google Scholar] [CrossRef] [PubMed]
- Kleinfelder, K.; Lotti, V.; Eramo, A.; Amato, F.; Lo Cicero, S.; Castelli, G.; Spadaro, F.; Farinazzo, A.; Dell’Orco, D.; Preato, S.; et al. In silico analysis and theratyping of an ultra-rare CFTR genotype (W57G/A234D) in primary human rectal and nasal epithelial cells. iScience 2023, 26, 108180. [Google Scholar] [CrossRef]
- Bihler, H.; Sivachenko, A.; Millen, L.; Bhatt, P.; Patel, A.T.; Chin, J.; Bailey, V.; Musisi, I.; LaPan, A.; Allaire, N.E.; et al. In vitro modulator responsiveness of 655 CFTR variants found in people with cystic fibrosis. J. Cyst. Fibros. 2024, 23, 664–675. [Google Scholar] [CrossRef]
- Laselva, O.; Ardelean, M.C.; Bear, C.E. Phenotyping Rare CFTR Mutations Reveal Functional Expression Defects Restored by TRIKAFTA(TM). J. Pers. Med. 2021, 11, 301. [Google Scholar] [CrossRef]
- Terlizzi, V.; Castaldo, G.; Salvatore, D.; Lucarelli, M.; Raia, V.; Angioni, A.; Carnovale, V.; Cirilli, N.; Casciaro, R.; Colombo, C.; et al. Genotype-phenotype correlation and functional studies in patients with cystic fibrosis bearing CFTR complex alleles. J. Med. Genet. 2017, 54, 224–235. [Google Scholar] [CrossRef]
- Lee, M.; Roos, P.; Sharma, N.; Atalar, M.; Evans, T.A.; Pellicore, M.J.; Davis, E.; Lam, A.N.; Stanley, S.E.; Khalil, S.E.; et al. Systematic Computational Identification of Variants That Activate Exonic and Intronic Cryptic Splice Sites. Am. J. Hum. Genet. 2017, 100, 751–765. [Google Scholar] [CrossRef]
- Raraigh, K.S.; Aksit, M.A.; Hetrick, K.; Pace, R.G.; Ling, H.; O’Neal, W.; Blue, E.; Zhou, Y.H.; Bamshad, M.J.; Blackman, S.M.; et al. Complete CFTR gene sequencing in 5,058 individuals with cystic fibrosis informs variant-specific treatment. J. Cyst. Fibros. 2022, 21, 463–470. [Google Scholar] [CrossRef]
- Schrijver, I.; Pique, L.; Graham, S.; Pearl, M.; Cherry, A.; Kharrazi, M. The Spectrum of CFTR Variants in Nonwhite Cystic Fibrosis Patients: Implications for Molecular Diagnostic Testing. J. Mol. Diagn. 2016, 18, 39–50. [Google Scholar] [CrossRef]
- Claustres, M.; Altieri, J.P.; Guittard, C.; Templin, C.; Chevalier-Porst, F.; Des Georges, M. Are p.I148T, p.R74W and p.D1270N cystic fibrosis causing mutations? BMC Med. Genet. 2004, 5, 19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Y.; Arora, K.; Mun, K.S.; Yang, F.; Moon, C.; Yarlagadda, S.; Jegga, A.; Weaver, T.; Naren, A.P. Targeting DNAJB9, a novel ER luminal co-chaperone, to rescue DeltaF508-CFTR. Sci. Rep. 2019, 9, 9808. [Google Scholar] [CrossRef]
- Fulcher, M.L.; Randell, S.H. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol. Biol. 2013, 945, 109–121. [Google Scholar] [CrossRef]
- Mou, H.; Vinarsky, V.; Tata, P.R.; Brazauskas, K.; Choi, S.H.; Crooke, A.K.; Zhang, B.; Solomon, G.M.; Turner, B.; Bihler, H.; et al. Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 2016, 19, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Levardon, H.; Yonker, L.M.; Hurley, B.P.; Mou, H. Expansion of Airway Basal Cells and Generation of Polarized Epithelium. Bio Protoc. 2018, 8, e2877. [Google Scholar] [CrossRef]
- Shik Mun, K.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D.; et al. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat. Commun. 2019, 10, 3124. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Z.; Csanady, L.; Gadsby, D.C.; Chen, J. Molecular Structure of the Human CFTR Ion Channel. Cell 2017, 169, 85–95.e8. [Google Scholar] [CrossRef]
- Fiedorczuk, K.; Chen, J. Molecular structures reveal synergistic rescue of Delta508 CFTR by Trikafta modulators. Science 2022, 378, 284–290. [Google Scholar] [CrossRef]
- Billet, A.; Mornon, J.P.; Jollivet, M.; Lehn, P.; Callebaut, I.; Becq, F. CFTR: Effect of ICL2 and ICL4 amino acids in close spatial proximity on the current properties of the channel. J. Cyst. Fibros. 2013, 12, 737–745. [Google Scholar] [CrossRef]
- Brewington, J.J.; Filbrandt, E.T.; LaRosa, F.J., 3rd; Moncivaiz, J.D.; Ostmann, A.J.; Strecker, L.M.; Clancy, J.P. Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight 2018, 3, e99385. [Google Scholar] [CrossRef]
- Suprynowicz, F.A.; Upadhyay, G.; Krawczyk, E.; Kramer, S.C.; Hebert, J.D.; Liu, X.; Yuan, H.; Cheluvaraju, C.; Clapp, P.W.; Boucher, R.C., Jr.; et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, 20035–20040. [Google Scholar] [CrossRef] [PubMed]
- Sondo, E.; Cresta, F.; Pastorino, C.; Tomati, V.; Capurro, V.; Pesce, E.; Lena, M.; Iacomino, M.; Baffico, A.M.; Coviello, D.; et al. The L467F-F508del Complex Allele Hampers Pharmacological Rescue of Mutant CFTR by Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Patients: The Value of the Ex Vivo Nasal Epithelial Model to Address Non-Responders to CFTR-Modulating Drugs. Int. J. Mol. Sci. 2022, 23, 3175. [Google Scholar] [CrossRef] [PubMed]
- Calucho, M.; Gartner, S.; Barranco, P.; Fernandez-Alvarez, P.; Perez, R.G.; Tizzano, E.F. Validation of nasospheroids to assay CFTR functionality and modulator responses in cystic fibrosis. Sci. Rep. 2021, 11, 15511. [Google Scholar] [CrossRef] [PubMed]
- Awatade, N.T.; Wong, S.L.; Capraro, A.; Pandzic, E.; Slapetova, I.; Zhong, L.; Turgutoglu, N.; Fawcett, L.K.; Whan, R.M.; Jaffe, A.; et al. Significant functional differences in differentiated Conditionally Reprogrammed (CRC)- and Feeder-free Dual SMAD inhibited-expanded human nasal epithelial cells. J. Cyst. Fibros. 2021, 20, 364–371. [Google Scholar] [CrossRef]
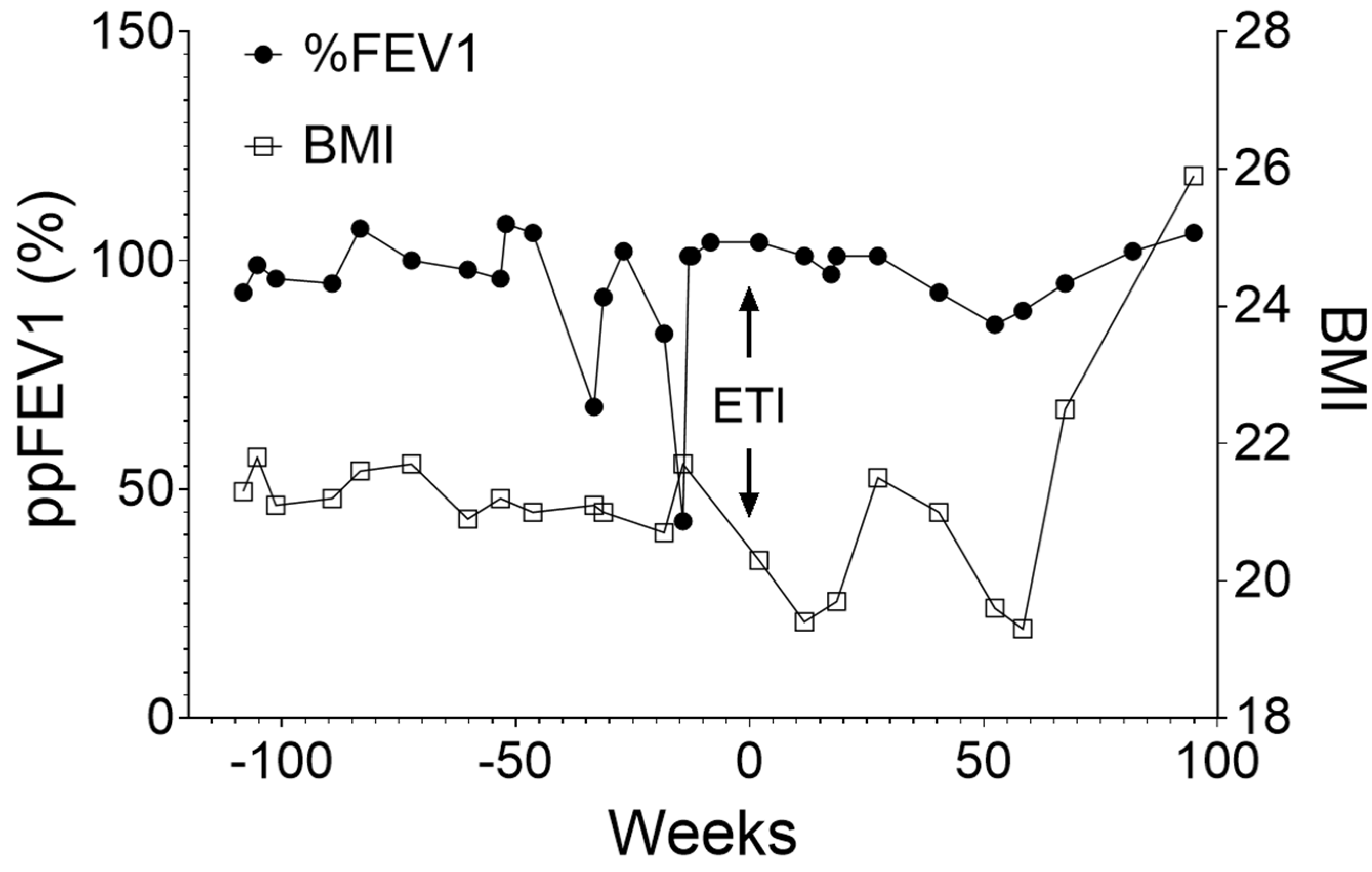
| Mean | y2 Pre-ETI | y1 Pre-ETI | y1 Post-ETI | y2 Post-ETI |
|---|---|---|---|---|
| ppFEV1 (%) | 99.1 | 89.0 | 97.6 | 98.0 |
| Coefficient of variation (ppFEV1) | 16.79% | 6.49% | ||
| BMI (kg/m2) | 21.3 | 21.2 | 20.4 | 21.8 |
| Cultures | PA, MSSA | |||
| PEx req IV (n) | 1 | 1 | 1 | 0 |
| Sweat Chloride * (mmol/L) | 116 | 108 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Gonzales Cordova, J.M.; Penrod, S.; Bendy, L.L.; Cheng, P.C.; Sanders, D.B.; Davis, M.D.; Gaston, B.; Chmiel, J.F. Elexacaftor/Tezacaftor/Ivacaftor Supports Treatment for CF with ΔI1023-V1024-CFTR. Int. J. Mol. Sci. 2025, 26, 5306. https://doi.org/10.3390/ijms26115306
Huang Y, Gonzales Cordova JM, Penrod S, Bendy LL, Cheng PC, Sanders DB, Davis MD, Gaston B, Chmiel JF. Elexacaftor/Tezacaftor/Ivacaftor Supports Treatment for CF with ΔI1023-V1024-CFTR. International Journal of Molecular Sciences. 2025; 26(11):5306. https://doi.org/10.3390/ijms26115306
Chicago/Turabian StyleHuang, Yunjie, Jorge Moises Gonzales Cordova, Sarah Penrod, Lisa Lynn Bendy, Pi Chun Cheng, Don B. Sanders, Michael Denning Davis, Benjamin Gaston, and James Francis Chmiel. 2025. "Elexacaftor/Tezacaftor/Ivacaftor Supports Treatment for CF with ΔI1023-V1024-CFTR" International Journal of Molecular Sciences 26, no. 11: 5306. https://doi.org/10.3390/ijms26115306
APA StyleHuang, Y., Gonzales Cordova, J. M., Penrod, S., Bendy, L. L., Cheng, P. C., Sanders, D. B., Davis, M. D., Gaston, B., & Chmiel, J. F. (2025). Elexacaftor/Tezacaftor/Ivacaftor Supports Treatment for CF with ΔI1023-V1024-CFTR. International Journal of Molecular Sciences, 26(11), 5306. https://doi.org/10.3390/ijms26115306






