The Intrabody Against Murine Double Minute 2 via a p53-Dependent Pathway Induces Apoptosis of Cancer Cell
Abstract
1. Introduction
2. Results
2.1. Construction of a Single-Domain Antibody Against the RING Finger Domain of MDM2
2.2. The Intracellular Antibody Demonstrated an Apoptosis-Induced Activity
2.3. VH-HT3 Revealed a Specific Combination and Inhibitory Effect on MDM2
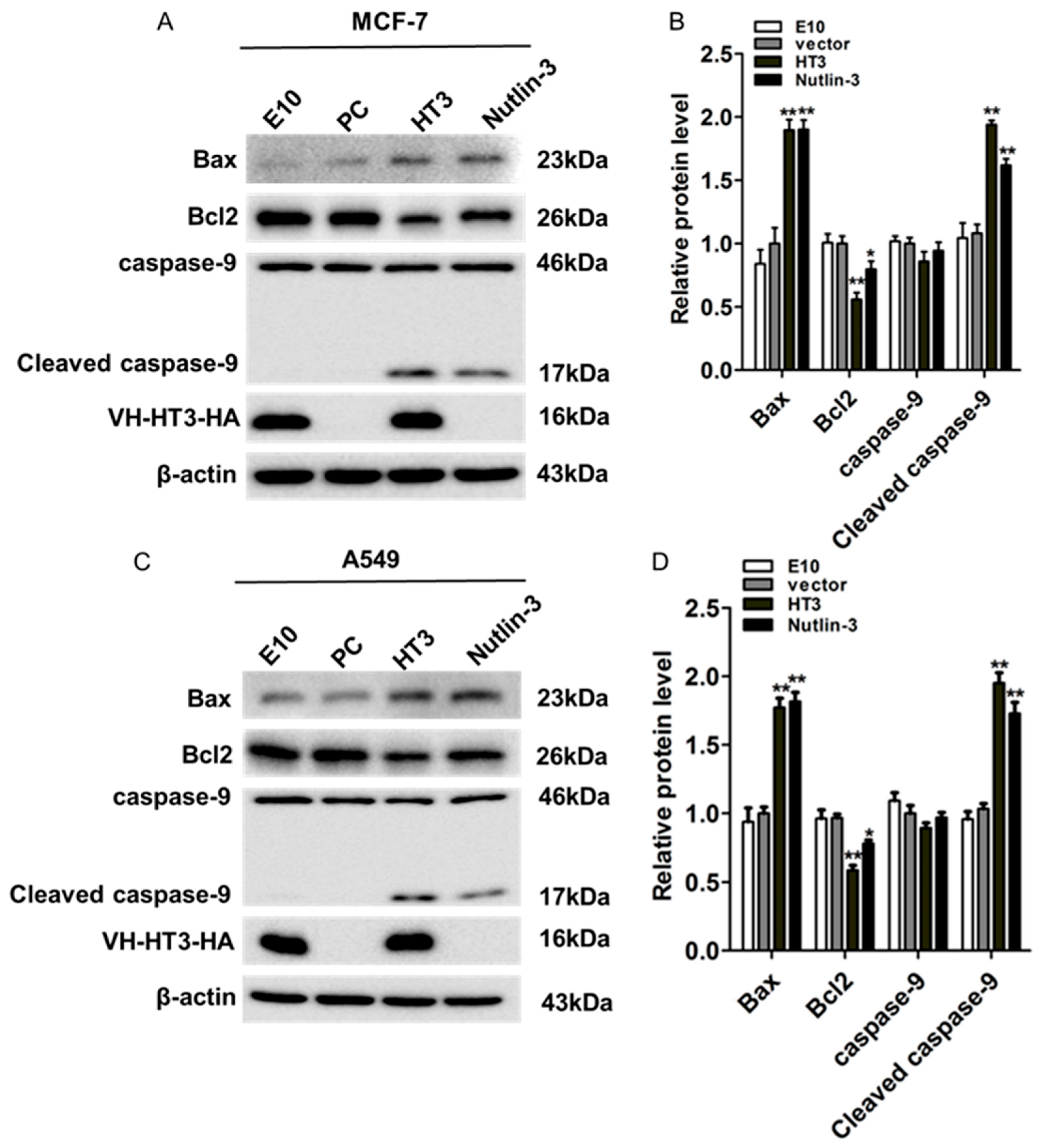
2.4. VH-HT3 Induced Apoptosis Is Affected by the Mitochondrial Pathway
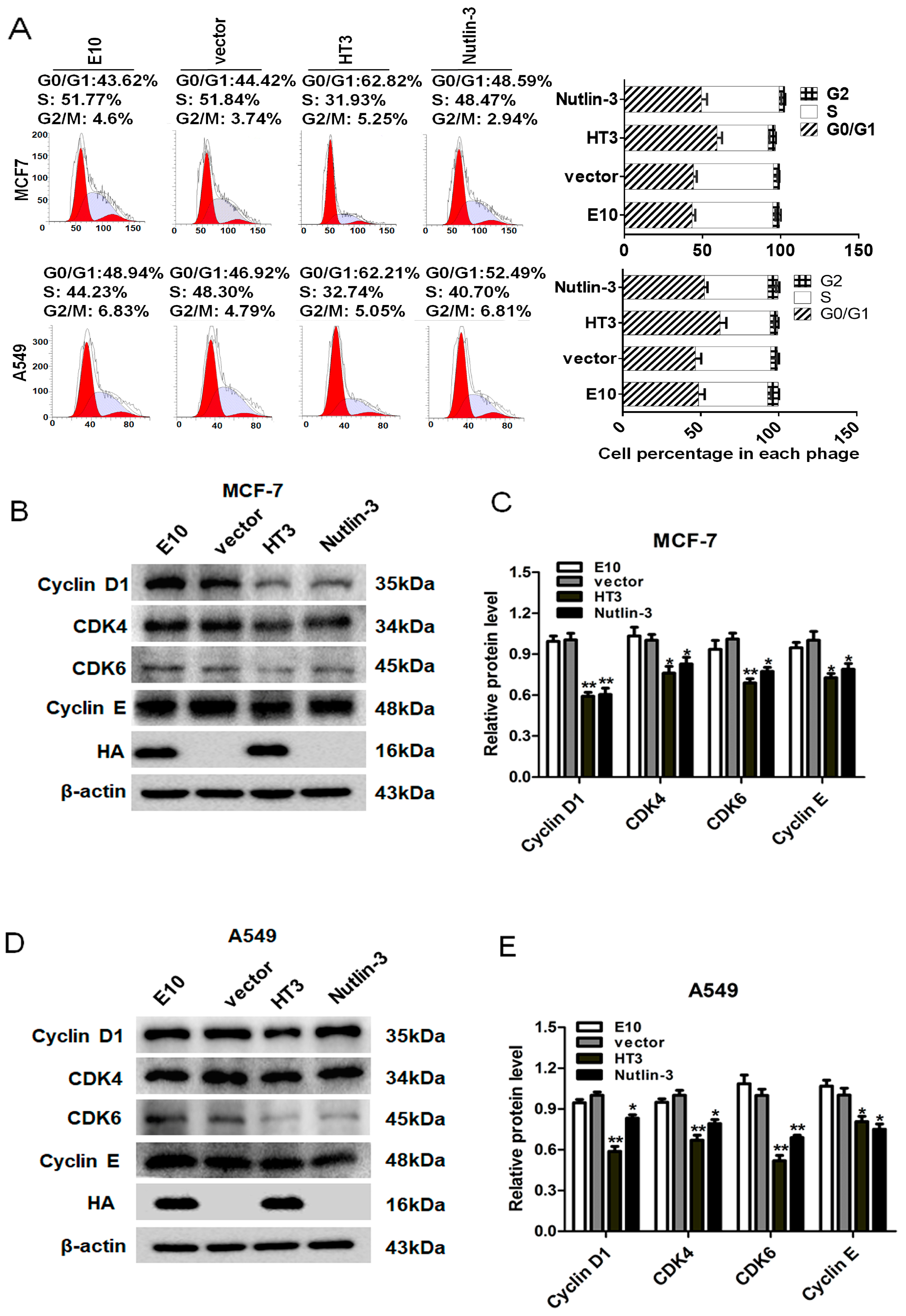
2.5. VH-HT3 Induced Cell Cycle Arrest
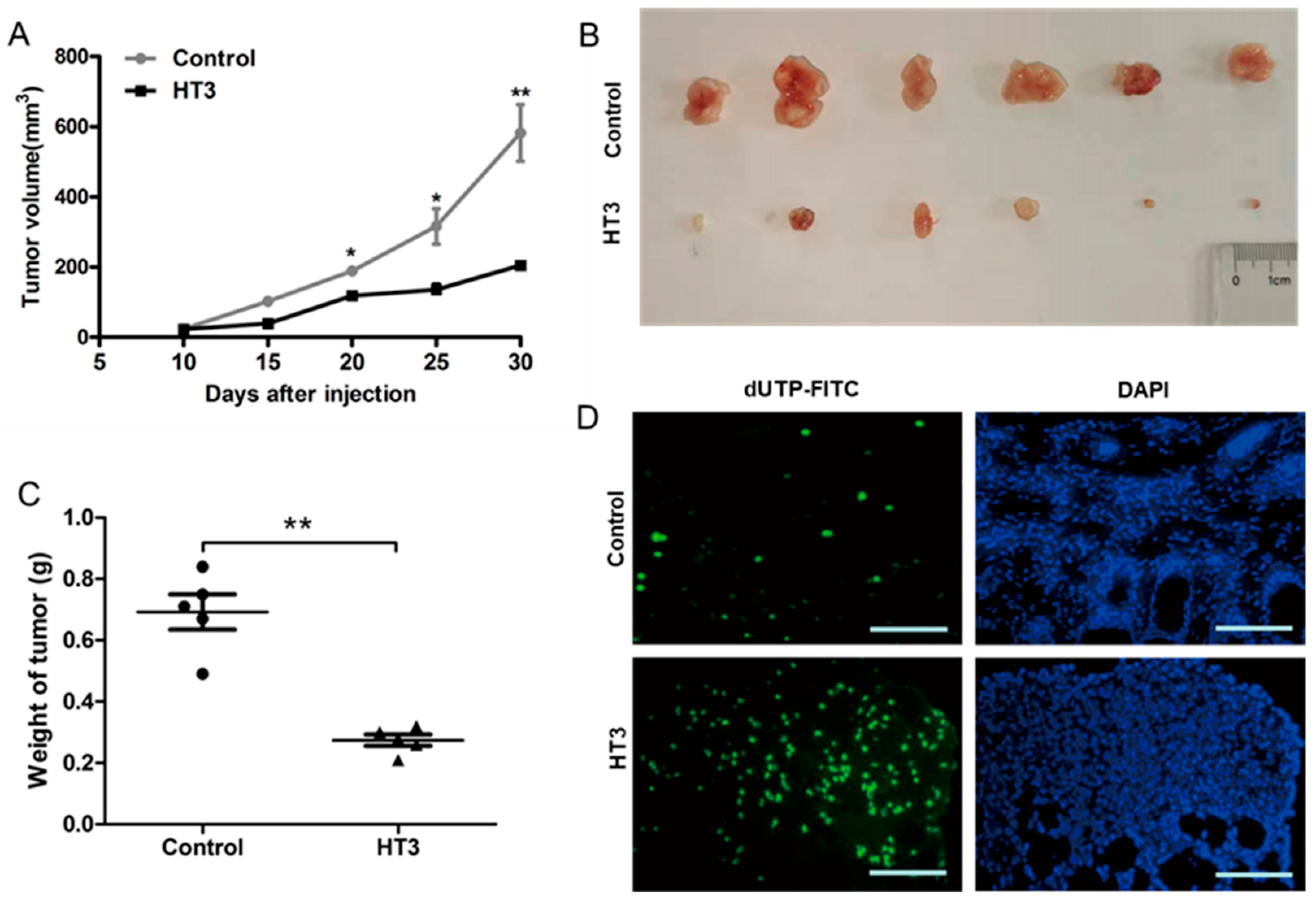
2.6. The Inhibitory Effects of Intrabody VH-HT3 In Vivo
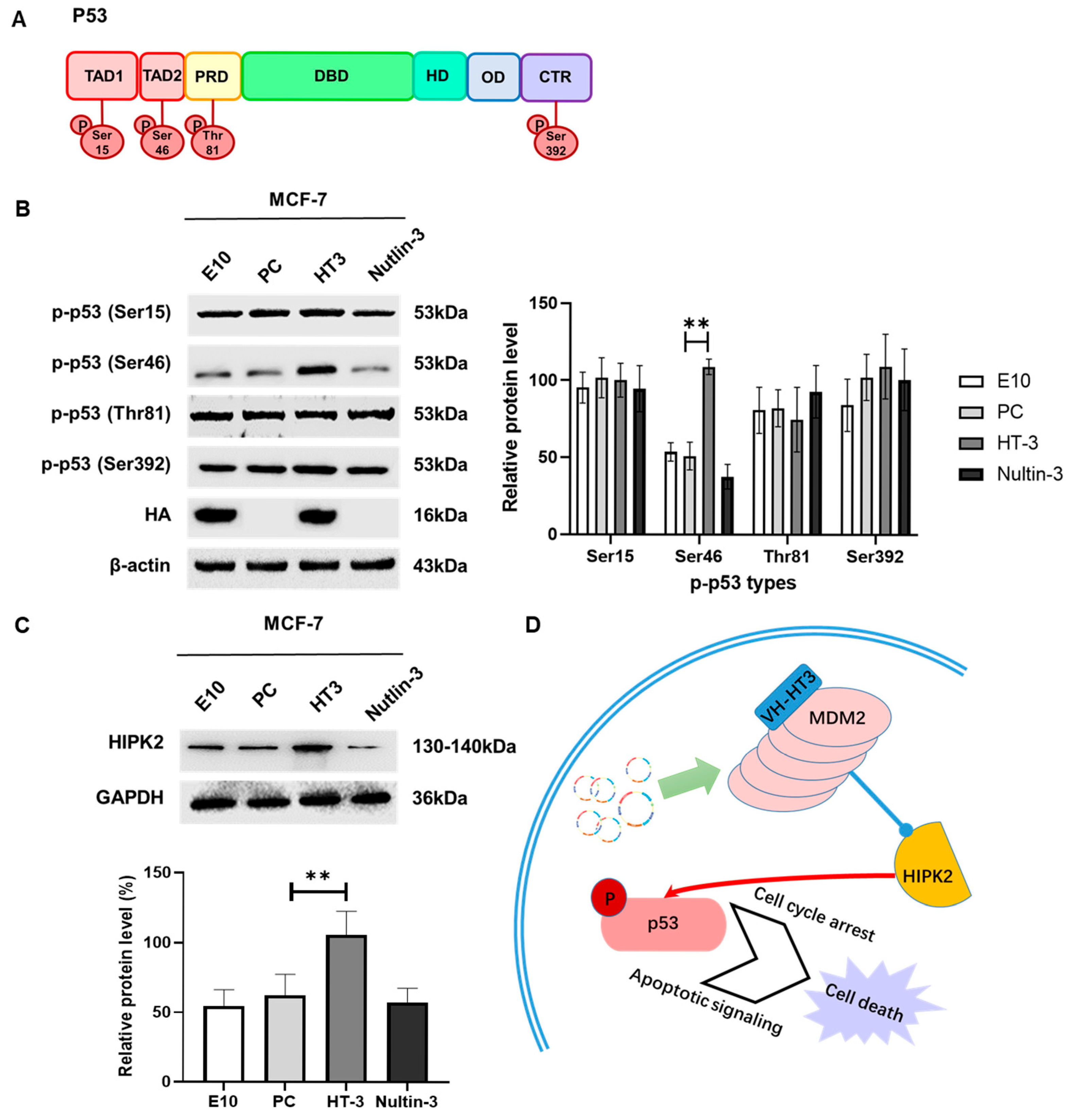
2.7. VH-HT3 Regulates p53 Phosphorylation via HIPK2
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Cell Culture
4.2. Construction of the Anti-MDM2 Human VH Single-Domain Antibody
4.3. Plasmid Transfection
4.4. Cell Viability Assay
4.5. Cell Cycle and Apoptosis Analysis
4.6. Anti-Tumor Assay In Vivo
4.7. TUNEL Staining
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. Immunoprecipitation
4.10. Western Blot Analysis
4.11. Confocal Laser Scanning Microscopy
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, D.; Gu, W. Dual roles of MDM2 in the regulation of p53: Ubiquitination dependent and ubiquitination independent mechanisms of MDM2 repression of p53 activity. Genes. Cancer 2012, 3, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Leenders, G.B.; Tuszynski, J.A. Stochastic and deterministic models of cellular p53 regulation. Front. Oncol. 2013, 3, 64. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Korotchkina, L.G.; Gudkov, A.V.; Blagosklonny, M.V. Paradoxical suppression of cellular senescence by p53. Proc. Natl. Acad. Sci. USA 2010, 107, 9660–9664. [Google Scholar] [CrossRef] [PubMed]
- Eischen, C.M.; Lozano, G. p53 and MDM2: Antagonists or partners in crime? Cancer Cell 2009, 15, 161–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Maerken, T.; Rihani, A.; Van Goethem, A.; De Paepe, A.; Speleman, F.; Vandesompele, J. Pharmacologic activation of wild-type p53 by nutlin therapy in childhood cancer. Cancer Lett. 2014, 344, 157–165. [Google Scholar] [CrossRef]
- Tan, B.X.; Liew, H.P.; Chua, J.S.; Ghadessy, F.J.; Tan, Y.S.; Lane, D.P.; Coffill, C.R. Anatomy of Mdm2 and Mdm4 in evolution. J. Mol. Cell Biol. 2017, 9, 3–15. [Google Scholar] [CrossRef]
- Roxburgh, P.; Hock, A.K.; Dickens, M.P.; Mezna, M.; Fischer, P.M.; Vousden, K.H. Small molecules that bind the Mdm2 RING stabilize and activate p53. Carcinogenesis 2012, 33, 791–798. [Google Scholar] [CrossRef]
- Kawai, H.; Wiederschain, D.; Yuan, Z.-M. Critical contribution of the MDM2 acidic domain to p53 ubiquitination. Mol. Cell Biol. 2003, 23, 4939–4947. [Google Scholar] [CrossRef]
- Meulmeester, E.; Frenk, R.; Stad, R.; De Graaf, P.; Marine, J.C.; Vousden, K.H.; Jochemsen, A.G. Critical role for a central part of Mdm2 in the ubiquitylation of p53. Mol. Cell Biol. 2003, 23, 4929–4938. [Google Scholar] [CrossRef]
- Manfredi, J.J. The Mdm2-p53 relationship evolves: Mdm2 swings both ways as an oncogene and a tumor suppressor. Genes. Dev. 2010, 24, 1580–1589. [Google Scholar] [CrossRef]
- Poyurovsky, M.V.; Priest, C.; Kentsis, A.; Borden, K.L.; Pan, Z.Q.; Pavletich, N.; Prives, C. The Mdm2 RING domain C-terminus is required for supramolecular assembly and ubiquitin ligase activity. EMBO J. 2007, 26, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Uldrijan, S.; Pannekoek, W.-J.; Vousden, K.H. An essential function of the extreme C-terminus of MDM2 can be provided by MDMX. EMBO J. 2007, 26, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.A.; Neriah, D.B.; Senecal, A.; Benard, L.; Thiruthuvanathan, V.; Yatsenko, T.; Narayanagari, S.R.; Wheat, J.C.; Todorova, T.I.; Mitchell, K.; et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci. Transl. Med. 2018, 10, eaao3003. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, F.-E. Small-molecule MDM2 inhibitors in clinical trials for cancer therapy. Eur. J. Med. Chem. 2022, 236, 114334. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Oliveira, S.; van Bergen En Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287–1292. [Google Scholar] [CrossRef]
- Lim, C.C.; Chan, S.K.; Lim, Y.Y.; Ishikawa, Y.; Choong, Y.S.; Nagaoka, Y.; Lim, T.S. Development and structural characterisation of human scFv targeting MDM2 spliced variant MDM2(15kDa). Mol. Immunol. 2021, 135, 191–203. [Google Scholar] [CrossRef]
- Yokota, T.; Milenic, D.E.; Whitlow, M.; Schlom, J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992, 52, 3402–3408. [Google Scholar]
- Milenic, D.E.; Yokota, T.; Filpula, D.R.; Finkelman, M.A.; Dodd, S.W.; Wood, J.F.; Whitlow, M.; Snoy, P.; Schlom, J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991, 51, 6363–6371. [Google Scholar]
- Dong, J.; Finn, J.A.; Larsen, P.A.; Smith, T.P.L.; Crowe, J.E., Jr. Structural Diversity of Ultralong CDRH3s in Seven Bovine Antibody Heavy Chains. Front. Immunol. 2019, 10, 558. [Google Scholar] [CrossRef]
- Lu, L.; Qi, H.; Zhu, J.; Sun, W.X.; Zhang, B.; Tang, C.Y.; Cheng, Q. Vascular-homing peptides for cancer therapy. Biomed. Pharmacother. 2017, 92, 187–195. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Huang, X.; Hou, H.; Zhou, S. Peptide-Based Nanocarriers for Cancer Therapy. Small Methods 2018, 2, 1700358. [Google Scholar] [CrossRef]
- Magnussen, H.M.; Ahmed, S.F.; Sibbet, G.J.; Hristova, V.A.; Nomura, K.; Hock, A.K.; Archibald, L.J.; Jamieson, A.G.; Fushman, D.; Vousden, K.H.; et al. Structural basis for DNA damage-induced phosphoregulation of MDM2 RING domain. Nat. Commun. 2020, 11, 2094. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, A.; Biocca, S. Gene-based antibody strategies for prion diseases. Int. J. Cell Biol. 2013, 2013, 710406. [Google Scholar] [CrossRef] [PubMed]
- Lobato, M.N.; Rabbitts, T.H. Intracellular antibodies and challenges facing their use as therapeutic agents. Trends Mol. Med. 2003, 9, 390–396. [Google Scholar] [CrossRef]
- Soetens, E.; Ballegeer, M.; Saelens, X. An Inside Job: Applications of Intracellular Single Domain Antibodies. Biomolecules 2020, 10, 1663. [Google Scholar] [CrossRef]
- Xi, X.; Guo, S.; Gu, Y.; Wang, X.; Wang, Q. Challenges and opportunities in single-domain antibody-based tumor immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189284. [Google Scholar] [CrossRef]
- Leroy, B.; Girard, L.; Hollestelle, A.; Minna, J.D.; Gazdar, A.F.; Soussi, T. Analysis of TP53 mutation status in human cancer cell lines: A reassessment. Hum. Mutat. 2014, 35, 756–765. [Google Scholar] [CrossRef]
- Leach, F.S.; Tokino, T.; Meltzer, P.; Burrell, M.; Oliner, J.D.; Smith, S.; Hill, D.E.; Sidransky, D.; Kinzler, K.W.; Vogelstein, B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993, 53, 2231–2234. [Google Scholar]
- Juven-Gershon, T.; Oren, M. Mdm2: The ups and downs. Mol. Med. Rep. 1999, 5, 71–83. [Google Scholar] [CrossRef]
- Watanabe, T.; Ichikawa, A.; Saito, H.; Hotta, T. Overexpression of the MDM2 oncogene in leukemia and lymphoma. Leuk. Lymphoma 1996, 21, 391–397. [Google Scholar] [CrossRef]
- Moll, U.M.; Petrenko, O. The MDM2-p53 interaction. Mol. Cancer Res. 2003, 1, 1001–1008. [Google Scholar] [PubMed]
- Vassilev, L.T.; Vu, B.T.; Graves, B.; Carvajal, D.; Podlaski, F.; Filipovic, Z.; Kong, N.; Kammlott, U.; Lukacs, C.; Klein, C.; et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science 2004, 303, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Liu, F.; Wang, W. Interplay between Mdm2 and HIPK2 in the DNA damage response. J. R. Soc. Interface 2014, 11, 20140319. [Google Scholar] [CrossRef] [PubMed]
- Elimam, H.; Hussein, J.; Abdel-Latif, Y.; Abdel-Aziz, A.K.; El-Say, K.M. Preclinical activity of fluvastatin-loaded self-nanoemulsifying delivery system against breast cancer models: Emphasis on apoptosis. J. Cell Biochem. 2022, 123, 947–963. [Google Scholar] [CrossRef]
- Burns, T.F.; EI-Deiry, W.S. The p53 pathway and apoptosis. J. Cell. Physiol. 1999, 22, 264–269. [Google Scholar] [CrossRef]
- Kim, D.; Kwon, W.; Park, S.; Kim, W.; Park, J.K.; Han, J.E.; Cho, G.J.; Yun, S.; Han, S.H.; Kim, M.O.; et al. Anticancer effects of veratramine via the phosphatidylinositol-3-kinase/serine-threonine kinase/mechanistic target of rapamycin and its downstream signaling pathways in human glioblastoma cell lines. Life Sci. 2022, 288, 120170. [Google Scholar] [CrossRef]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Horton, R.M.; Hunt, H.D.; Ho, S.N.; Pullen, J.K.; Pease, L.R. Engineering hybrid genes without the use of restriction enzymes: Gene splicing by overlap extension. Gene 1989, 77, 61–68. [Google Scholar] [CrossRef]
- Lee, C.M.; Iorno, N.; Sierro, F.; Christ, D. Selection of human antibody fragments by phage display. Nat. Protoc. 2007, 2, 3001–3008. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Y.; Song, L.; Kang, T.; Du, T.; Wu, Y.; Xiong, M.; Luo, L.; Long, J.; Men, K.; et al. A Vesicular Stomatitis Virus-Inspired DNA Nanocomplex for Ovarian Cancer Therapy. Adv. Sci. 2018, 5, 1700263. [Google Scholar] [CrossRef]
- Xu, X.; Jin, L.; Jiang, T.; Lu, Y.; Aosai, F.; Piao, H.N.; Xu, G.H.; Jin, C.H.; Jin, X.J.; Ma, J.; et al. Ginsenoside Rh2 attenuates microglial activation against toxoplasmic encephalitis via TLR4/NF-κB signaling pathway. J. Ginseng Res. 2020, 44, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Xue, M.; Jiang, J.; Zhang, Y.; Gao, X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J. Exp. Clin. Cancer Res. 2020, 39, 149. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, W.; Gu, X.; Chang, C.; Wu, J. Repression of deoxynivalenol-triggered cytotoxicity and apoptosis by mannan/β-glucans from yeast cell wall: Involvement of autophagy and PI3K-AKT-mTOR signaling pathway. Int. J. Biol. Macromol. 2020, 164, 1413–1421. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J.; Xie, F.; Lu, Y.; Yin, C.; Shen, X. Co-Delivery of Docetaxel and Salinomycin to Target Both Breast Cancer Cells and Stem Cells by PLGA/TPGS Nanoparticles. Int. J. Nanomed. 2019, 14, 9199–9216. [Google Scholar] [CrossRef]
- Huang, B.; Luo, L.; Wang, J.; He, B.; Feng, R.; Xian, N.; Zhang, Q.; Chen, L.; Huang, G. B7-H3 specific T cells with chimeric antigen receptor and decoy PD-1 receptors eradicate established solid human tumors in mouse models. Oncoimmunology 2020, 9, 1684127. [Google Scholar] [CrossRef]
- Qin, X.; Xu, Y.; Zhou, X.; Gong, T.; Zhang, Z.R.; Fu, Y. An injectable micelle-hydrogel hybrid for localized and prolonged drug delivery in the management of renal fibrosis. Acta Pharm. Sin. B 2021, 11, 835–847. [Google Scholar] [CrossRef]
- Nakamori, S.; Dohi, K.; Ishida, M.; Goto, Y.; Imanaka-Yoshida, K.; Omori, T.; Goto, I.; Kumagai, N.; Fujimoto, N.; Ichikawa, Y.; et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 48–59. [Google Scholar] [CrossRef]
- Yagi, Y.; Aly, R.G.; Tabata, K.; Barlas, A.; Rekhtman, N.; Eguchi, T.; Montecalvo, J.; Hameed, M.; Manova-Todorova, K.; Adusumilli, P.S.; et al. Three-Dimensional Histologic, Immunohistochemical and Multiplex Immunofluorescence Analyses of Dynamic Vessel Co-Option of Spread Through Air Spaces in Lung Adenocarcinoma. J. Thorac. Oncol. 2020, 15, 589–600. [Google Scholar] [CrossRef]
- Gao, Q.G.; Zhou, L.P.; Lee, V.H.Y.; Chan, H.Y.; Man, C.W.Y.; Wong, M.S. Ginsenoside Rg1 activates ligand-independent estrogenic effects via rapid estrogen receptor signaling pathway. J. Ginseng Res. 2019, 43, 527–538. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Z.; Lan, R.; Cai, S.; Lin, Z.; Li, J.; Wang, J.; Li, Z.; Liu, P. The poly(ADP-ribosyl)ation of BRD4 mediated by PARP1 promoted pathological cardiac hypertrophy. Acta Pharm. Sin. B 2021, 11, 1286–1299. [Google Scholar] [CrossRef]
- Qin, J.J.; Li, X.; Wang, W.; Zi, X.; Zhang, R. Targeting the NFAT1-MDM2-MDMX Network Inhibits the Proliferation and Invasion of Prostate Cancer Cells, Independent of p53 and Androgen. Front. Pharmacol. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yan, L.; Zhao, X.; Wu, J.; Fang, Y.; Xin, Z.; Wang, H.; Yang, X. Aberrantly High FBXO31 Impairs Oocyte Quality in Premature Ovarian Insufficiency. Aging Dis. 2024, 15, 804–823. [Google Scholar] [PubMed]
- Morey, P.; Pfannkuch, L.; Pang, E.; Boccellato, F.; Sigal, M.; Imai-Matsushima, A.; Dyer, V.; Koch, M.; Mollenkopf, H.J.; Schlaermann, P.; et al. Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response. Gastroenterology 2018, 154, 1391–1404.e9. [Google Scholar] [CrossRef] [PubMed]
- Yekula, A.; Minciacchi, V.R.; Morello, M.; Shao, H.; Park, Y.; Zhang, X.; Muralidharan, K.; Freeman, M.R.; Weissleder, R.; Lee, H.; et al. Large and small extracellular vesicles released by glioma cells in vitro and in vivo. J. Extracell. Vesicles 2020, 9, 1689784. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, W.; Guo, H.; Lan, T.; Wang, T.; Wang, B. The Intrabody Against Murine Double Minute 2 via a p53-Dependent Pathway Induces Apoptosis of Cancer Cell. Int. J. Mol. Sci. 2025, 26, 5286. https://doi.org/10.3390/ijms26115286
Wang C, Liu W, Guo H, Lan T, Wang T, Wang B. The Intrabody Against Murine Double Minute 2 via a p53-Dependent Pathway Induces Apoptosis of Cancer Cell. International Journal of Molecular Sciences. 2025; 26(11):5286. https://doi.org/10.3390/ijms26115286
Chicago/Turabian StyleWang, Changli, Wanting Liu, Haotian Guo, Tian Lan, Tianyi Wang, and Bing Wang. 2025. "The Intrabody Against Murine Double Minute 2 via a p53-Dependent Pathway Induces Apoptosis of Cancer Cell" International Journal of Molecular Sciences 26, no. 11: 5286. https://doi.org/10.3390/ijms26115286
APA StyleWang, C., Liu, W., Guo, H., Lan, T., Wang, T., & Wang, B. (2025). The Intrabody Against Murine Double Minute 2 via a p53-Dependent Pathway Induces Apoptosis of Cancer Cell. International Journal of Molecular Sciences, 26(11), 5286. https://doi.org/10.3390/ijms26115286





