Development of D-Limonene Nanoemulsions for Oral Cancer Inhibition: Investigating the Role of Ostwald Ripening Inhibitors and Cell Death Mechanisms
Abstract
1. Introduction
2. Results
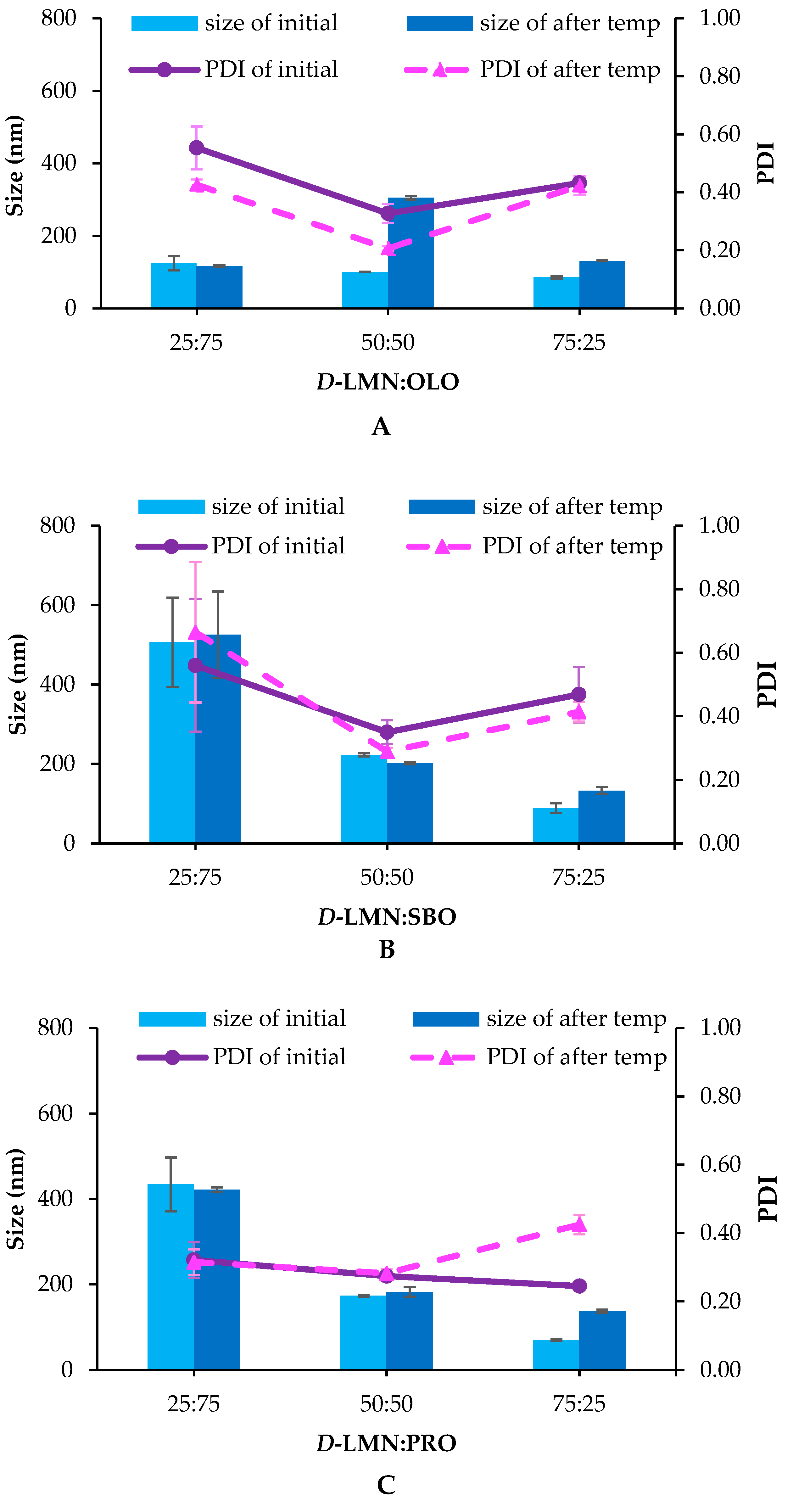
2.1. Droplet Size, Size Distribution (Polydispersity Index; PDI) and Zeta Potential
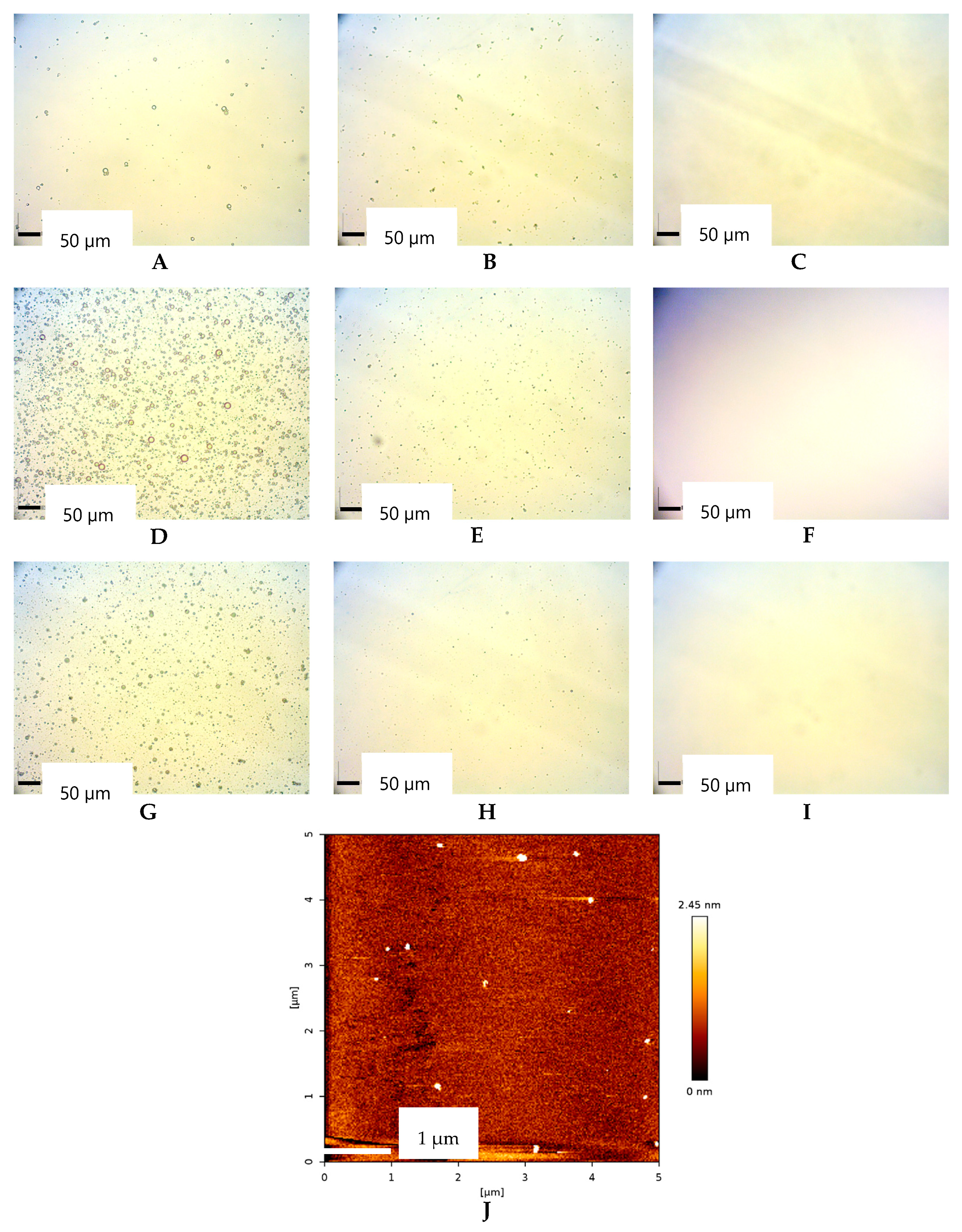
2.2. Morphological of Oil Droplet
2.3. Anticancer Activity
2.4. Antimetastatic and Anti-Invasive Properties
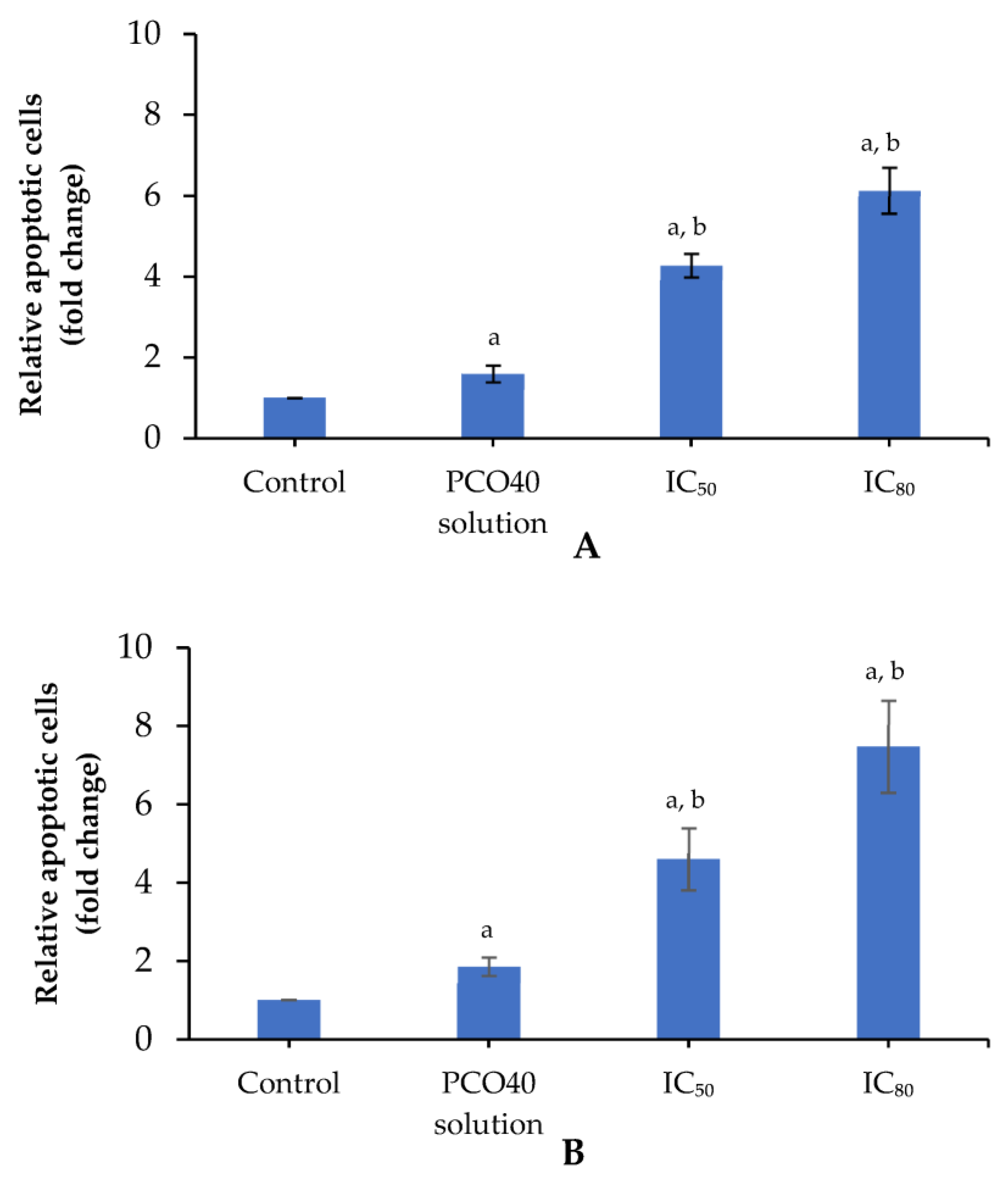
2.5. Apoptosis Induction
2.6. Cell Cycle Arrest
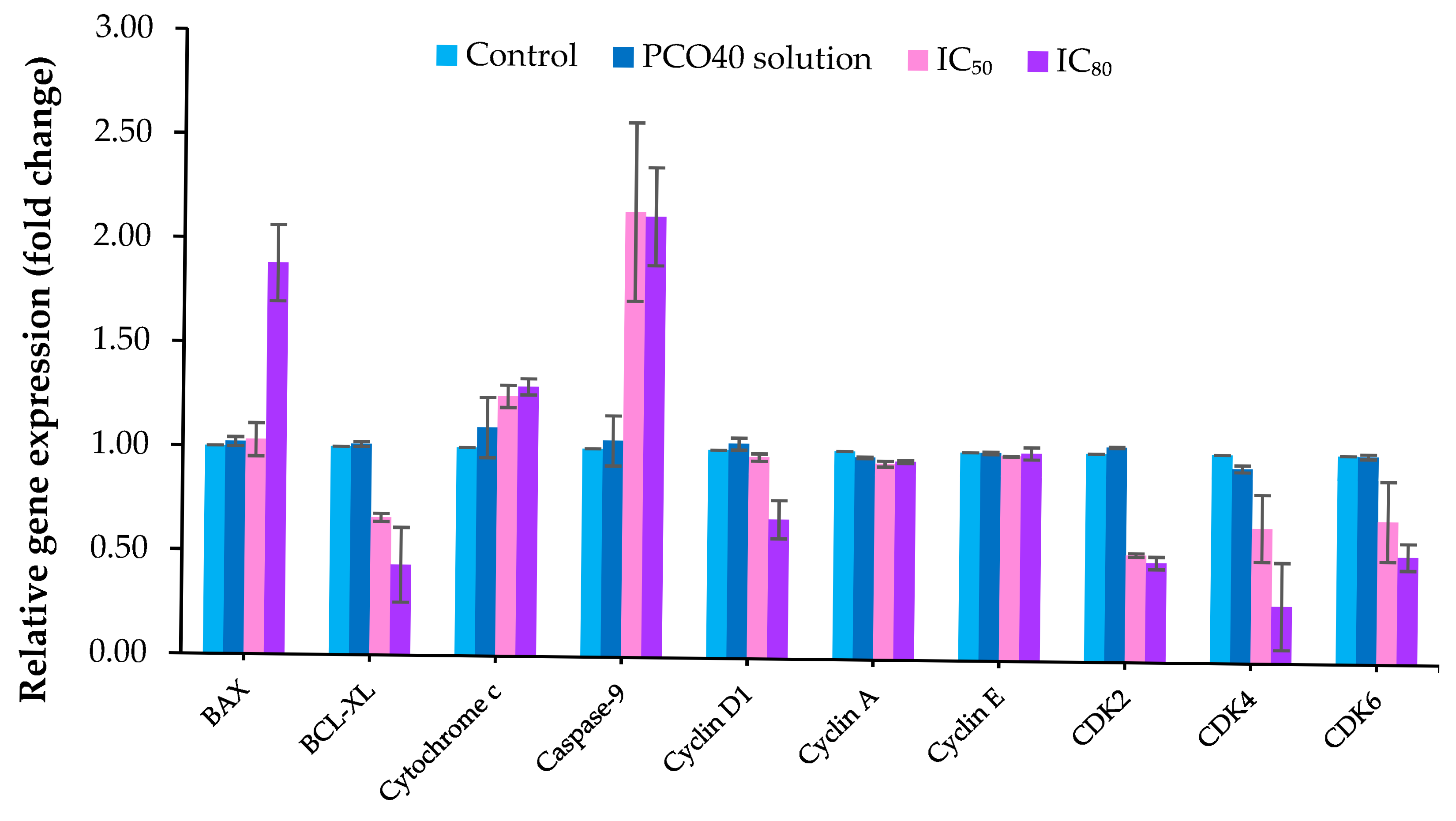
2.7. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Nanoemulsion Preparation
4.3. Measurement of Droplet Size, Size Distribution and Zeta Potential
4.4. Stability Assessment
4.5. Morphological Analysis of Oil Droplets
4.6. Anticancer Activity Study
4.7. Transwell Migration Assay
4.8. Transwell Invasion Assay
4.9. Nucleus Analysis by Hoechst 33258 Staining
4.10. Determination of Cell Death Using Annexin V-FITC/PI Staining with Flow Cytometry
4.11. Cell Cycle Arrest Analysis
4.12. Gene Expression Analysis by RT-PCR
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lekshmi Priya, K.S.; Maheswary, D.; Ravi, S.S.S.; Leela, K.V.; Lathakumari, R.H.; Malavika, G. The impact of probiotics on oral cancer: Mechanistic insights and therapeutic strategies. Oral Oncol. 2025, 13, 100715. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Lu, B.; Zhang, D.; Yin, Y.; Liu, S.; Chen, L.; Zhang, Z. Current applications, future perspectives and challenges of organoid technology in oral cancer research. Eur. J. Pharmacol. 2025, 993, 177368. [Google Scholar] [CrossRef]
- Galbiatti, A.L.S.; Padovani-Junior, J.A.; Maníglia, J.V.; Rodrigues, C.D.S.; Pavarino, É.C.; Goloni-Bertollo, E.M. Head and neck cancer: Causes, prevention and treatment. Braz. J. Otorhinolaryngol. 2013, 79, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, S.; Wang, S.; Chen, S.; Dong, S.; Zhu, Y. Effect of D-limonene on volatile fatty acids production from anaerobic fermentation of waste activated sludge under pH regulation: Performance and mechanisms. J. Environ. Manag. 2024, 370, 122828. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Patel, P.; Verma, S.K.; Sahu, B.; Parija, T. Proximal discrepancy in intrinsic atomic interaction arrests G2/M phase by inhibiting Cyclin B1/CDK1 to infer molecular and cellular biocompatibility of D-limonene. Sci. Rep. 2022, 12, 18184. [Google Scholar] [CrossRef]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Yuan, Q.; Liang, H.; Vriesekoop, F. Process optimization and stability of D-limonene nanoemulsions prepared by catastrophic phase inversion method. J. Food Eng. 2013, 119, 419–424. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, D.; Tian, Y.; Wang, M.; Liu, R.; Xia, Z.; Huang, Y. Nanoemulsion for improving the oral bioavailability of hesperetin: Formulation optimization and absorption mecha-nism. J. Pharm. Sci. 2021, 110, 2555–2561. [Google Scholar] [CrossRef]
- Kotwiski, F.O.; São Paulo, Í.; Carneiro, P.I.S.; Barbosa, R.d.M.; Viseras, C.; Rangel, A.L.; Villarreal, C.F.; Cabral-Albuquerque, E.C.d.M.; Lucchese, A.M. Development of a nanoemulsion containing Lippia origanoides essential oil with anti-fungal activity by low energy method: From extraction to formulation. J. Drug Deliv. Sci. Technol. 2024, 102, 106392. [Google Scholar] [CrossRef]
- Sampaio, C.I.; Bourbon, A.I.; Gonçalves, C.; Pastrana, L.M.; Dias, A.M.; Cerqueira, M.A. Low energy nanoemulsions as carriers of thyme and lemon balm essential oils. LWT 2022, 154, 112748. [Google Scholar] [CrossRef]
- Jawaid, T.; Alaseem, A.M.; Khan, M.M.; Mukhtar, B.; Kamal, M.; Anwer, R.; Ahmed, S.; Alam, A. Prepa-ration and evaluation of nanoemulsion of citronella essential oil with improved antimi-crobial and anti-cancer properties. Antibiotics 2023, 12, 478. [Google Scholar] [CrossRef] [PubMed]
- Manaa, A.O.; Baghdadi, H.H.; El-Nikhely, N.A.; Heikal, L.A.; El-Hosseiny, L.S. Oregano oil-nanoemulsions: Formulation and evaluation of antibacterial and anticancer potentials. J. Drug Deliv. Sci. Technol. 2022, 78, 103978. [Google Scholar] [CrossRef]
- Alharbi, D.S.; Albalawi, S.F.; Alghrid, S.T.; Alhwity, B.S.; Qushawy, M.; Mortagi, Y.; El-Sherbiny, M.; Prabahar, K.; Elsherbiny, N. Ginger oil nanoemulsion formulation augments its antiproliferative effect in ehrlich solid tumor model. Foods 2023, 12, 4139. [Google Scholar] [CrossRef] [PubMed]
- Tubtimsri, S.; Limmatvapirat, C.; Limsirichaikul, S.; Akkaramongkolporn, P.; Piri-yaprasarth, S.; Patomchaiviwat, V.; Limmatvapirat, S. Incorporation of fixed oils into spearmint oil-loaded nanoemulsions and their influence on characteristic and cytotoxic properties against human oral cancer cells. J. Drug Deliv. Sci. Technol. 2021, 63, 102443. [Google Scholar] [CrossRef]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions stabilized by non-ionic surfactants: Stability and degradation mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Weerapol, Y.; Manmuan, S.; Chaothanaphat, N.; Okonogi, S.; Limmatvapirat, C.; Limmatvapirat, S.; Tubtimsri, S. Impact of fixed oil on ostwald ripening of anti-oral cancer nanoemulsions loaded with Amomum kravanh essential oil. Pharmaceutics 2022, 14, 938. [Google Scholar] [CrossRef]
- McClements, D.J.; Henson, L.; Popplewell, L.M.; Decker, E.A.; Choi, S.J. Inhibition of Ostwald ripening in model beverage emulsions by addition of poorly water soluble triglyceride oils. J. Food Sci. 2012, 77, C33–C38. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Muranushi, N.; Takagi, N.; Muranishi, S.; Sezaki, H. Effect of fatty acids and mono-glycerides on permeability of lipid bilayer. Chem. Phys. Lipids 1981, 28, 269–279. [Google Scholar] [CrossRef]
- Han, W.; Wang, S.; Liang, R.; Wang, L.; Chen, M.; Li, H.; Wang, Y. Non-ionic surfactant vesicles simultaneously enhance antitumor activity and reduce the toxicity of cantharidin. Int. J. Nanomed. 2013, 8, 2187–2196. [Google Scholar]
- Rao, S.; Song, Y.; Peddie, F.; Evans, A.M. Particle size reduction to the nanometer range: A promising approach to improve buccal absorption of poorly water-soluble drugs. Int. J. Nanomed. 2011, 6, 1245–1251. [Google Scholar]
- Gokhale, J.P.; Mahajan, H.S.; Surana, S.J. Quercetin loaded nanoemulsion-based gel for rheumatoid arthritis: In vivo and in vitro studies. Biomed. Pharmacother. 2019, 112, 108622. [Google Scholar] [CrossRef]
- Weerapol, Y.; Jarerattanachat, V.; Limmatvapirat, S.; Limmatvapirat, C.; Manmuan, S.; Tubtimsri, S. Unveiling the molecular dynamics, anticancer activity, and stability of spearmint oil nanoemulsions with triglycerides. Mol. Pharm. 2024, 21, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hong, C.R.; Choi, S.J. Prevention of Ostwald ripening in orange oil emul-sions: Impact of surfactant type and Ostwald ripening inhibitor type. LWT 2020, 134, 110180. [Google Scholar] [CrossRef]
- Li, P.-H.; Chiang, B.-H. Process optimization and stability of D-limonene-in-water nanoemulsions prepared by ultrasonic emulsification using response surface method-ology. Ultrason. Sonochem. 2012, 19, 192–197. [Google Scholar] [CrossRef]
- Wooster, T.J.; Golding, M.; Sanguansri, P. Impact of oil type on nanoemulsion formation and Ostwald ripening stability. Langmuir 2008, 24, 12758–12765. [Google Scholar] [CrossRef]
- Li, Y.; Le Maux, S.; Xiao, H.; McClements, D.J. Emulsion-based delivery systems for tributyrin, a potential colon cancer preventative agent. J. Agric. Food Chem. 2009, 57, 9243–9249. [Google Scholar] [CrossRef]
- Partearroyo, M.A.; Ostolaza, H.; Goñi, F.M.; Barberá-Guillem, E. Surfactant-induced cell toxicity and cell lysis: A study using B16 melanoma cells. Biochem. Pharmacol. 1990, 40, 1323–1328. [Google Scholar] [CrossRef]
- Shi, X.; Wang, X.; Yao, W.; Shi, D.; Shao, X.; Lu, Z.; Chai, Y.; Song, J.; Tang, W.; Wang, X. Mechanism insights and therapeutic intervention of tumor metastasis: Latest developments and perspectives. Signal Transduct. 2024, 9, 192. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the hallmarks of metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Lu, X.G.; Zhan, L.B.; Feng, B.A.; Qu, M.Y.; Yu, L.H.; Xie, J.H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by D-limonene. World J. Gastroenterol. 2004, 10, 2140–2144. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Jayaprakasha, G.K.; Patil, B.S. D-limonene rich volatile oil from blood oranges inhibits angiogenesis, metastasis and cell death in human colon cancer cells. Life Sci. 2012, 91, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Chaudhary, S.; Siddiqui, M.; Athar, M.; Alam, M.S. D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 2012, 31, 798–811. [Google Scholar] [CrossRef]
- Vidula, N.; Rugo, H.S. Cyclin-Dependent Kinase 4/6 Inhibitors for the Treatment of Breast Cancer: A Review of Preclinical and Clinical Data. Clin. Breast Cancer 2016, 16, 8–17. [Google Scholar] [CrossRef]
- Tubtimsri, S.; Chuenbarn, T.; Manmuan, S. Quercetin triggers cell apoptosis-associated ROS-mediated cell death and induces S and G2/M-phase cell cycle arrest in KON oral cancer cells. BMC Complement. Med. Ther. 2025, 25, 34. [Google Scholar] [CrossRef]
- Fan, S.; Ge, Y.; Liu, J.; Liu, H.; Yan, R.; Gao, T.; Fan, X.; Xiao, Z.; An, G. Combination of anlotinib and gemcitabine promotes the G0/G1 cell cycle arrest and apoptosis of intrahepatic cholangiocarcinoma in vitro. J. Clin. Lab. Anal. 2021, 35, e23986. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Wang, L.; Liu, D.-W.; Tang, G.-Y.; Zhang, H.-Y. Synergistic inhibitory effect of berberine and d-limonene on human gastric carcinoma cell line MGC803. J. Med. Food 2014, 17, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Venkatesan, J.; Prabhu, A. D-limonene-loaded liposomes target malignant glioma cells via the downregulation of angiogenic growth factors. J. Drug Deliv. Sci. Technol. 2023, 82, 104358. [Google Scholar] [CrossRef]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef]
- Gladden, A.B.; Diehl, J.A. Cell cycle progression without cyclin E/CDK2: Breaking down the walls of dogma. Cancer Cell 2003, 4, 160–162. [Google Scholar] [CrossRef]
- Chebet, J.J.; Ehiri, J.E.; McClelland, D.J.; Taren, D.; Hakim, I.A. Effect of D-limonene and its derivatives on breast cancer in human trials: A scoping review and narrative synthesis. BMC Cancer 2021, 21, 902. [Google Scholar] [CrossRef]




| Ingredient | Concentration (%w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 25:75 | 50:50 | 75:25 | |||||||
| D-LMN | 2.5 | 2.5 | 2.5 | 5.0 | 5.0 | 5.0 | 7.5 | 7.5 | 7.5 |
| OLO | 7.5 | - | - | 5.0 | - | - | 2.5 | - | - |
| SBO | - | 7.5 | - | - | 5.0 | - | - | 2.5 | - |
| PRO | - | - | 7.5 | - | 5.0 | - | - | 2.5 | |
| PCO40 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Water | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| Gene | Forward Primers | Reverse Primers |
|---|---|---|
| GAPDH | 5′-AGGGCTGCTTTTAACTCT GGT-3′ | 5′-CCCCACTTGATTTTGGAGGGA-3′ |
| BAX | 5′-CCCTTTTGCTTCAGG GTTTC-3′ | 5′-TGTTACTGT CCA GTT CGT CC-3′ |
| BCL-XL | 5′-GATCCCCATGGCAGCAGTAAA GCAAG-3′ | 5′-CCCCATCCCGGAAGAGTTCATTCACT-3′ |
| Cytochrome c | 5′-CCCAGAAGTACATCCCTGGAAC-3′ | 5′-GGCAGTGGCCAATTATTACTCA-3′ |
| Caspase-9 | 5′-GCTGTGTCAAGTTTGCCTACCC-3′ | 5′-CCAGAATGCCATCCAAGGTCTC -3′ |
| Cyclin D1 | 5′-AACTACCTGGACCGCTTCCT-3′ | 5′- CCACTTGAGCTTGTTCACCA-3′ |
| Cyclin E | 5′-CGGCCTATATATTGGGTTGGC-3′ | 5′-GGCTGCTGCTTAGCTTGTAAAC-3′ |
| Cyclin A | 5′-GCCATTAGTTTACCTGGACCCAGA-3′ | 5′-CACTGACATGGAAGACAGGAACCT-3′ |
| CDK2 | 5′-GCTTTCTGCCATTCTCATCG -3′ | 5′-GTCCCCAGAGTCCGAAAGAT-3′ |
| CDK4 | 5′-ACGGGTGTAAGTGCCATCTG-3′ | 5′-TGGTGTCGGTGCCTATGGGA-3′ |
| CDK6 | 5′-CGAATGCGTGGCGGAGATC -3′ | 5′-CCACTGAGGTTAGAGCCATC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manmuan, S.; Weerapol, Y.; Chuenbarn, T.; Limmatvapirat, S.; Limmatvapirat, C.; Tubtimsri, S. Development of D-Limonene Nanoemulsions for Oral Cancer Inhibition: Investigating the Role of Ostwald Ripening Inhibitors and Cell Death Mechanisms. Int. J. Mol. Sci. 2025, 26, 5279. https://doi.org/10.3390/ijms26115279
Manmuan S, Weerapol Y, Chuenbarn T, Limmatvapirat S, Limmatvapirat C, Tubtimsri S. Development of D-Limonene Nanoemulsions for Oral Cancer Inhibition: Investigating the Role of Ostwald Ripening Inhibitors and Cell Death Mechanisms. International Journal of Molecular Sciences. 2025; 26(11):5279. https://doi.org/10.3390/ijms26115279
Chicago/Turabian StyleManmuan, Suwisit, Yotsanan Weerapol, Tiraniti Chuenbarn, Sontaya Limmatvapirat, Chutima Limmatvapirat, and Sukannika Tubtimsri. 2025. "Development of D-Limonene Nanoemulsions for Oral Cancer Inhibition: Investigating the Role of Ostwald Ripening Inhibitors and Cell Death Mechanisms" International Journal of Molecular Sciences 26, no. 11: 5279. https://doi.org/10.3390/ijms26115279
APA StyleManmuan, S., Weerapol, Y., Chuenbarn, T., Limmatvapirat, S., Limmatvapirat, C., & Tubtimsri, S. (2025). Development of D-Limonene Nanoemulsions for Oral Cancer Inhibition: Investigating the Role of Ostwald Ripening Inhibitors and Cell Death Mechanisms. International Journal of Molecular Sciences, 26(11), 5279. https://doi.org/10.3390/ijms26115279






