Ano5 Deficiency Leads to Abnormal Bone Formation via miR-34c-5p/KLF4/β-Catenin in Gnathodiaphyseal Dysplasia
Abstract
1. Introduction
2. Results
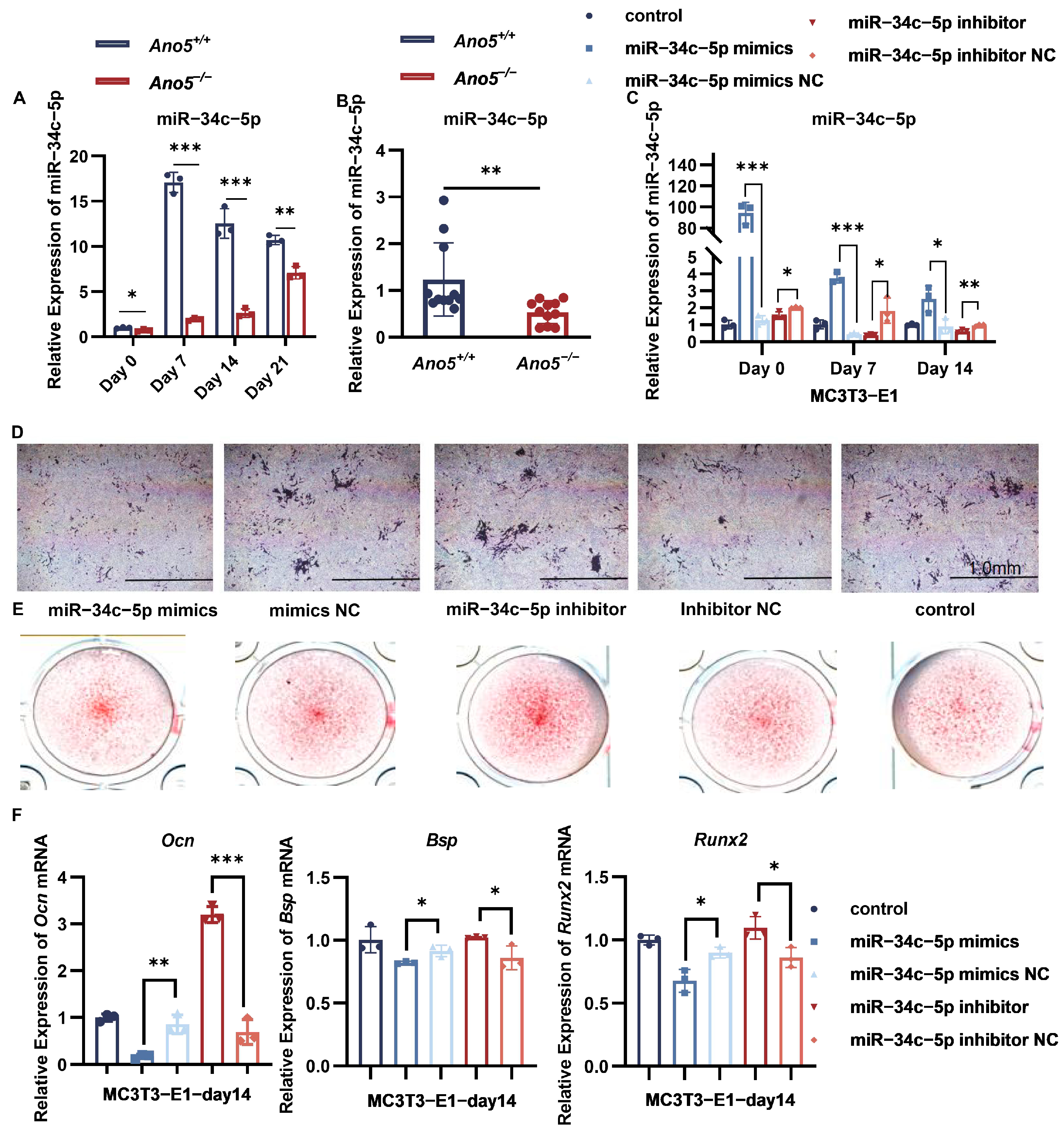
2.1. miR-34c-5p Was Down-Regulated in the Ano5−/− mCOBs
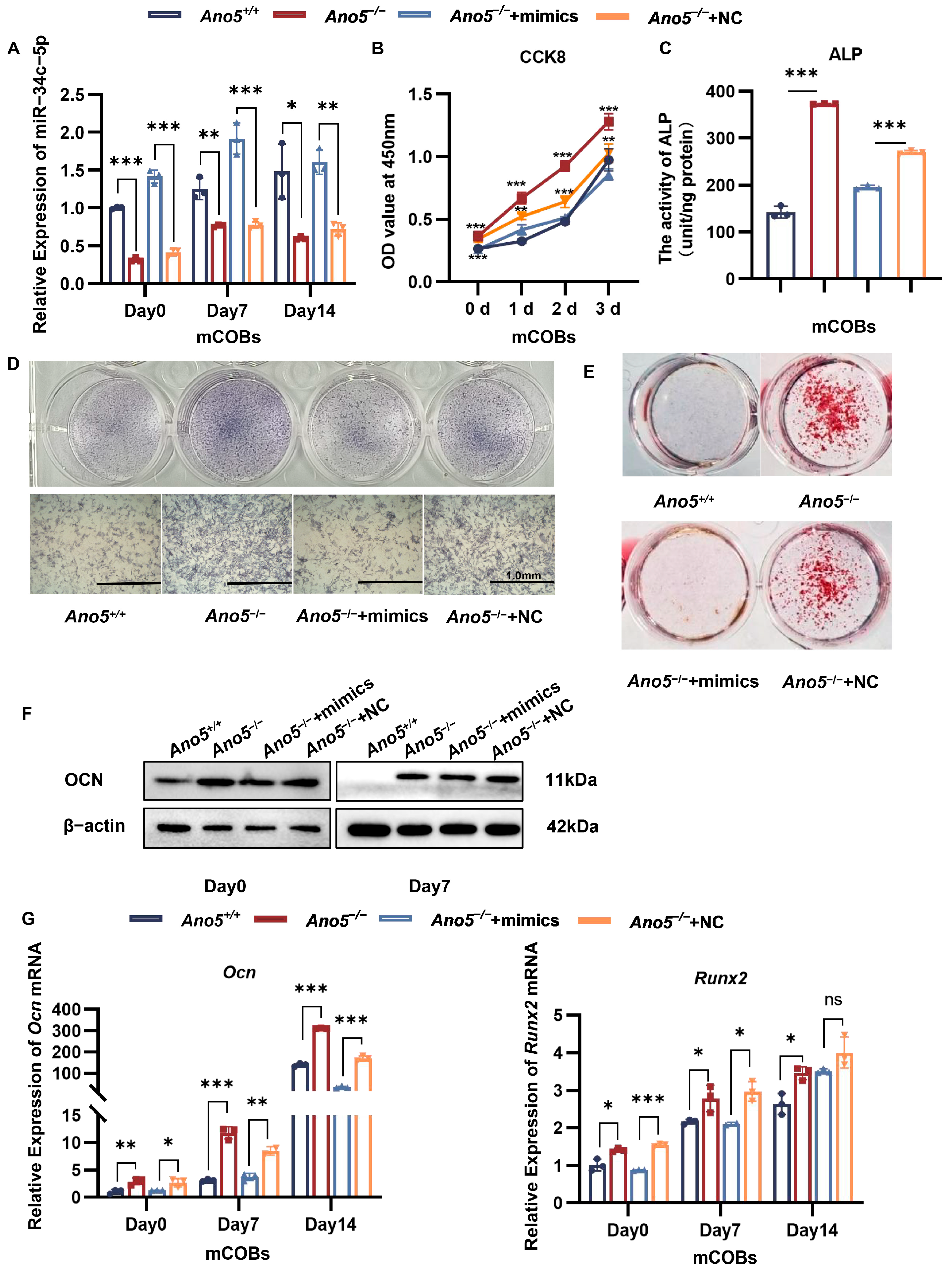
2.2. miR-34c-5p Rescued the Abnormal Osteogenic Ability of Ano5−/− mCOBs
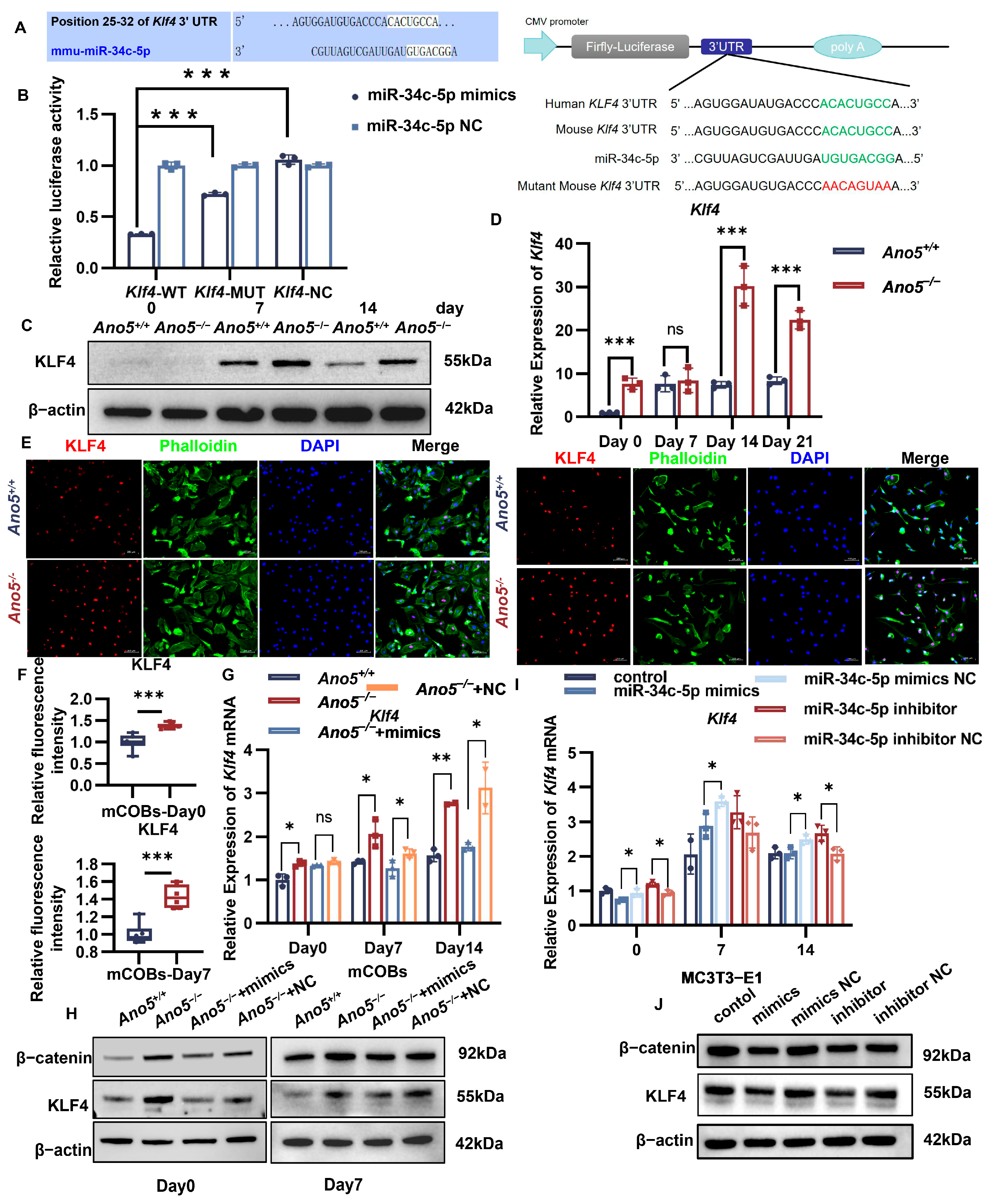
2.3. miR-34c-5p Directly Targeted KLF4
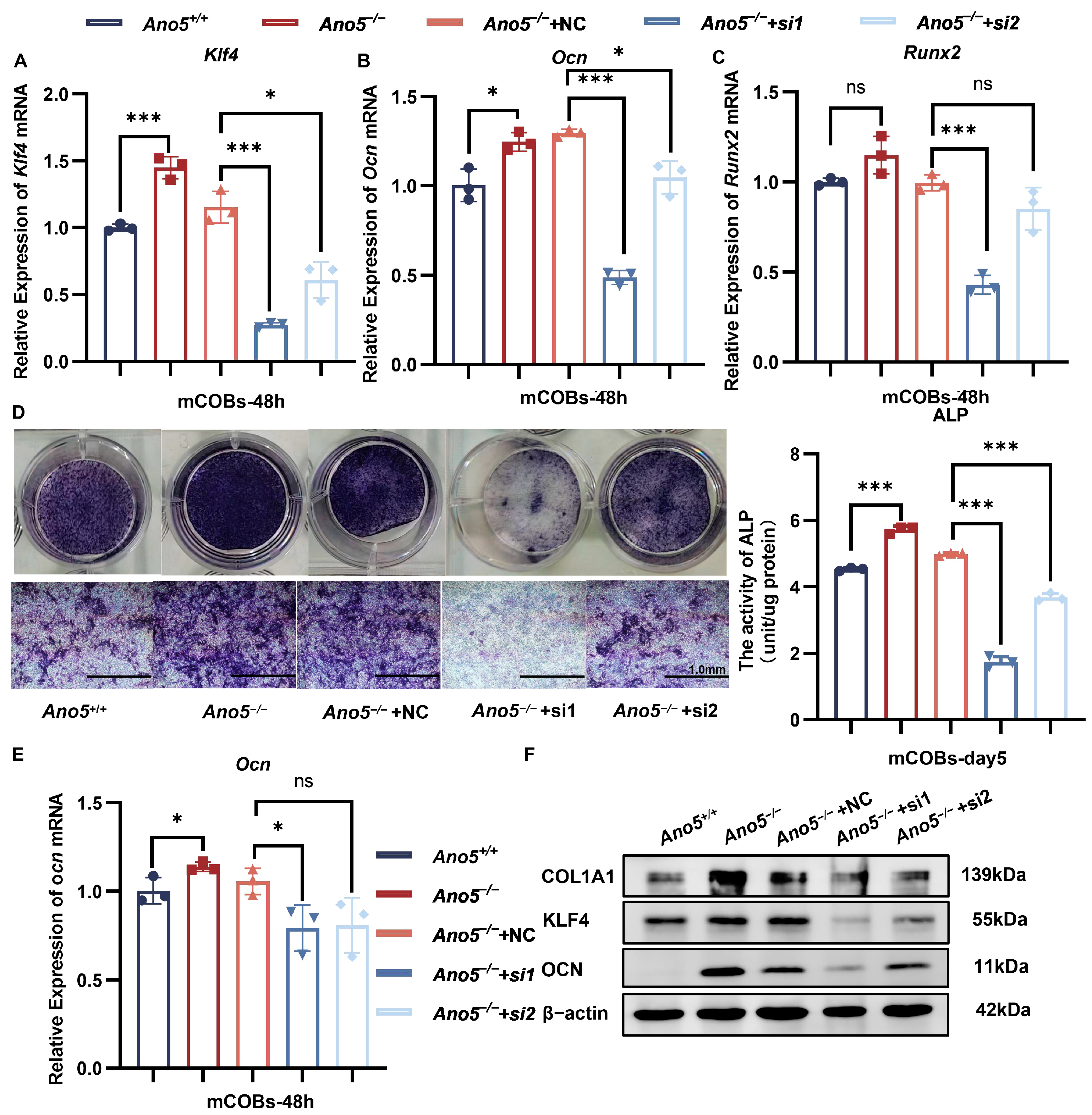
2.4. Inhibiting KLF4 Attenuated Abnormally Enhanced Osteogenesis of Ano5−/− mCOBs
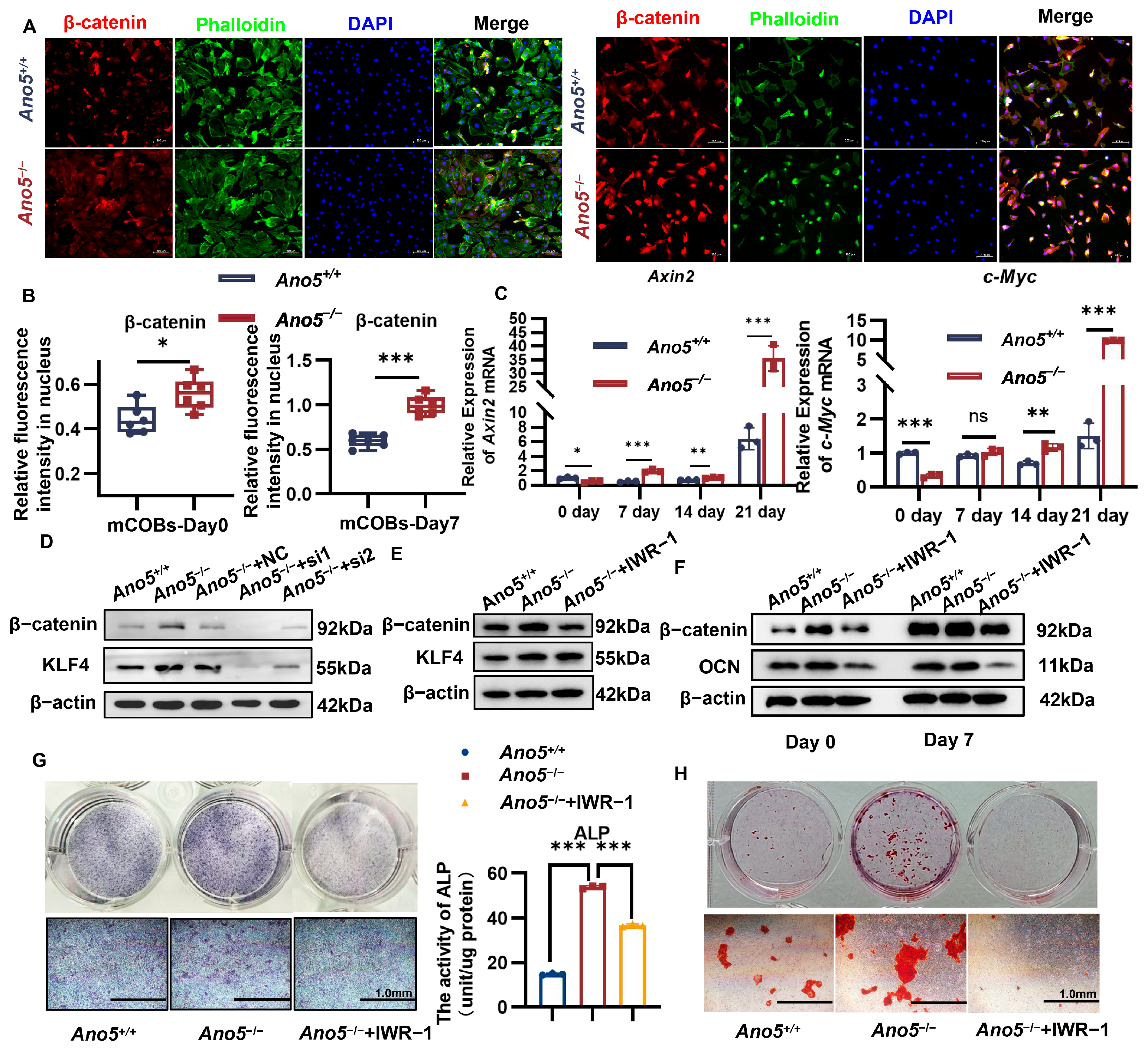
2.5. Ano5 Deficiency Upregulated β-Catenin Signaling Pathway
2.6. miR-34c-5p/KLF4 Regulated the β-Catenin Pathway and Influenced Osteogenic Differentiation
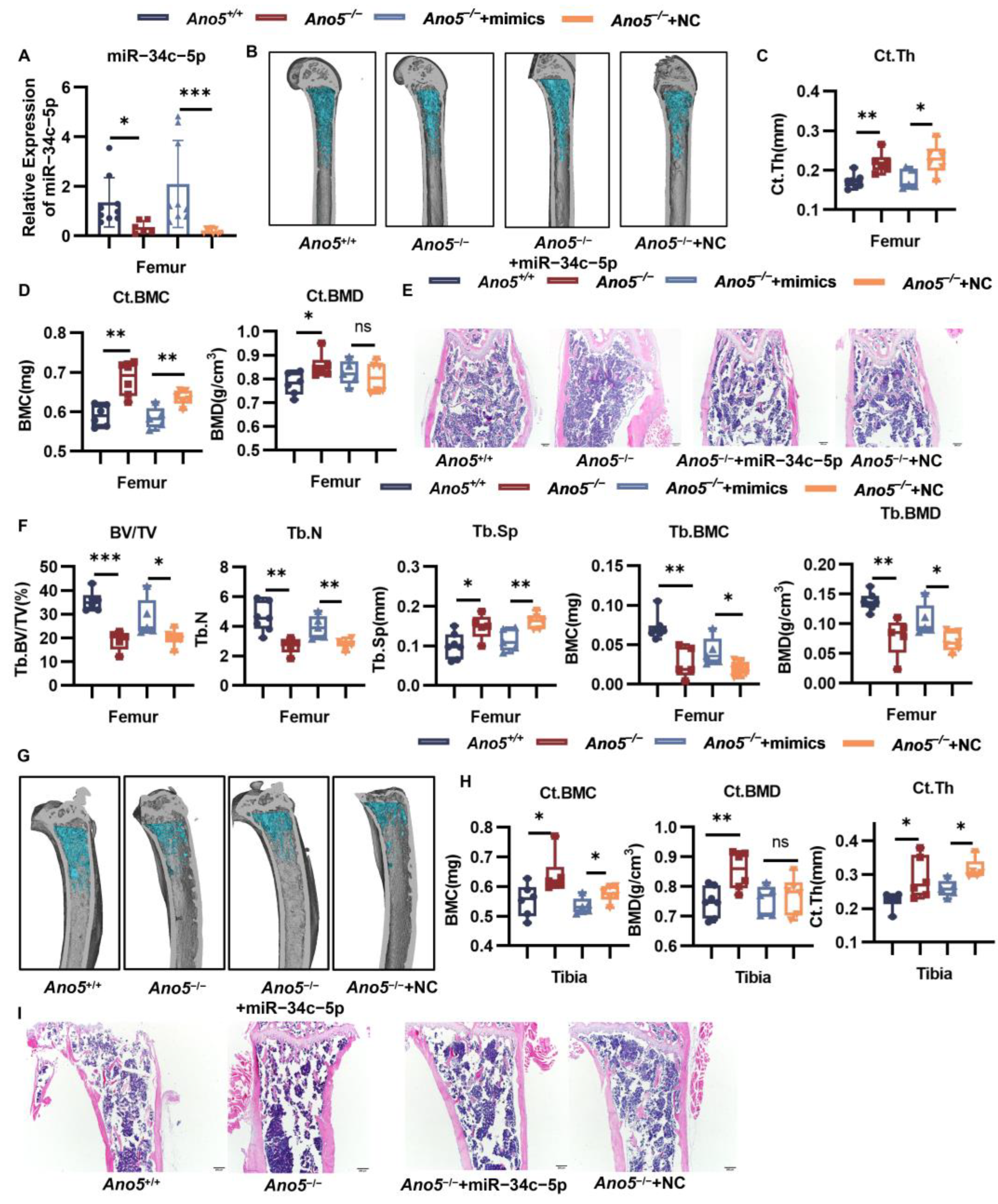
2.7. AAV9-miR-34c-5p Rescued Part of the Bone Phenotype in Ano5−/− Mice
3. Discussion
4. Materials and Methods
4.1. Subsection Animal
4.2. Cell Culture
4.3. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
4.4. Western Blot Analysis
4.5. Transfections
4.6. Cell Proliferation Assay
4.7. Alkaline Phosphatase (ALP) Staining and Activity Assay
4.8. Alizarin Red Staining
4.9. Dual-Luciferase Reporter Assay
4.10. Immunofluorescence
4.11. In Vivo AAV-miR-34c-5p-Treatment
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Skeletal Analysis
4.14. Frozen Section Preparation of Bone Tissue
4.15. H&E Staining of Bone Tissue
4.16. Three-Point Bending Test
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GDD | Gnathodiaphyseal dysplasia |
| Ano5 | Anoctamin 5 |
| ALP | alkaline phosphatase |
| AAV | adeno-associated virus |
| P1NP | N-terminal peptide of type I collagen |
| mCOBs | calvaria-derived osteoblasts |
| OCN | osteocalcin |
| RUNX2 | runt-related transcription factor 2 |
| KLF4 | Krüppel-Like Factor 4 |
| BMC | bone mineral content |
| BMD | bone mineral density |
References
- Jin, L.; Liu, Y.; Sun, F.; Collins, M.T.; Blackwell, K.; Woo, A.S.; Reichenberger, E.J.; Hu, Y. Three novel ANO5 missense mutations in Caucasian and Chinese families and sporadic cases with gnathodiaphyseal dysplasia. Sci. Rep. 2017, 7, 40935. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, Y.; Nakajima, T.; Koyama, K.; Furuya, K.; Mitsuka, Y. Familial cases of a new systemic bone disease, hereditary gnatho-diaphyseal sclerosis. Nihon Seikeigeka Gakkai Zasshi 1969, 43, 381–394. [Google Scholar] [PubMed]
- Tsutsumi, S.; Kamata, N.; Maruoka, Y.; Ando, M.; Tezuka, O.; Enomoto, S.; Omura, K.; Nagayama, M.; Kudo, E.; Moritani, M.; et al. Autosomal dominant gnathodiaphyseal dysplasia maps to chromosome 11p14.3–15.1. J. Bone. Min. Res. 2003, 18, 413–418. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Kamata, N.; Vokes, T.J.; Maruoka, Y.; Nakakuki, K.; Enomoto, S.; Omura, K.; Amagasa, T.; Nagayama, M.; Saito-Ohara, F.; et al. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD). Am. J. Hum. Genet. 2004, 74, 1255–1261. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Chen, E.; Liu, X.; Ma, X.; Miao, C.; Tian, Z.; Dong, R.; Hu, Y. Introduction of a Cys360Tyr Mutation in ANO5 Creates a Mouse Model for Gnathodiaphyseal Dysplasia. J. Bone Miner. Res. 2022, 37, 515–530. [Google Scholar] [CrossRef]
- Li, H.; Liu, S.; Miao, C.; Lv, Y.; Hu, Y. Integration of metabolomics and transcriptomics provides insights into enhanced osteogenesis in Ano5(Cys360Tyr) knock-in mouse model. Front. Endocrinol. 2023, 14, 1117111. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Dong, R.; Liang, C.; Reichenberger, E.J.; Hu, Y. Genetic Disruption of Anoctamin 5 in Mice Replicates Human Gnathodiaphyseal Dysplasia (GDD). Calcif. Tissue Int. 2019, 104, 679–689. [Google Scholar] [CrossRef]
- Cuvelier, V.; Trost, D.; Stichelbout, M.; Michot, C.; Cormier-Daire, V.; Boutry, N.; Machet, E.; Vincent-Delorme, C. Gnathodiaphyseal dysplasia: Diagnostic clues from two fetal cases and literature review. Prenat. Diagn. 2024, 44, 1098–1104. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Ma, X.; Li, H.; Miao, C.; Tian, Z.; Hu, Y. Genetic disruption of Ano5 leads to impaired osteoclastogenesis for gnathodiaphyseal dysplasia. Oral. Dis. 2024, 30, 1403–1415. [Google Scholar] [CrossRef]
- Yang, Y.; Yujiao, W.; Fang, W.; Linhui, Y.; Ziqi, G.; Zhichen, W.; Zirui, W.; Shengwang, W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol. Res. 2020, 53, 40. [Google Scholar] [CrossRef]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Ferragut Cardoso, A.P.; Banerjee, M.; Nail, A.N.; Lykoudi, A.; States, J.C. miRNA dysregulation is an emerging modulator of genomic instability. Semin. Cancer Biol. 2021, 76, 120–131. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Li, M.; Yuan, J.; Yu, Y.; Bi, X.; Hong, H.; Ye, J.; Liu, P. MicroRNA-34c-5p provokes isoprenaline-induced cardiac hypertrophy by modulating autophagy via targeting ATG4B. Acta Pharm. Sin. B 2022, 12, 2374–2390. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Sun, Y.; Zhang, X.; Zhao, Y.; Zhang, H.; Mei, Q.; Meng, J.; Zhang, F.; Zhang, T. ING5 overexpression upregulates miR-34c-5p/Snail1 to inhibit EMT and invasion of lung cancer cells. Acta Biochim. Et Biophys. Sin. 2023, 55, 809–817. [Google Scholar] [CrossRef]
- Cong, F.; Wu, N.; Tian, X.; Fan, J.; Liu, J.; Song, T.; Fu, H. MicroRNA-34c promotes osteoclast differentiation through targeting LGR4. Gene 2017, 610, 1–8. [Google Scholar] [CrossRef]
- Bae, Y.; Yang, T.; Zeng, H.C.; Campeau, P.M.; Chen, Y.; Bertin, T.; Dawson, B.C.; Munivez, E.; Tao, J.; Lee, B.H. miRNA-34c regulates Notch signaling during bone development. Hum. Mol. Genet. 2012, 21, 2991–3000. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Elkarim, E.A.; Bialkowska, A.B.; Yang, V.W. KLF4 Suppresses Tumor Formation in Genetic and Pharmacological Mouse Models of Colonic Tumorigenesis. Mol. Cancer Res. 2016, 14, 385–396. [Google Scholar] [CrossRef]
- Wen, Y.; Lu, X.; Ren, J.; Privratsky, J.R.; Yang, B.; Rudemiller, N.P.; Zhang, J.; Griffiths, R.; Jain, M.K.; Nedospasov, S.A.; et al. KLF4 in Macrophages Attenuates TNFalpha-Mediated Kidney Injury and Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 1925–1938. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Amin, R.S.; Bustani, G.S.; Romero-Parra, R.M.; Zabibah, R.S.; Oz, T.; Jalil, A.T.; Soltani, A.; Kujawska, M. Therapeutic Targeting of Kruppel-Like Factor 4 and Its Pharmacological Potential in Parkinson’s Disease: A Comprehensive Review. Mol. Neurobiol. 2024, 61, 3596–3606. [Google Scholar] [CrossRef] [PubMed]
- Frazzi, R. KLF4 is an epigenetically modulated, context-dependent tumor suppressor. Front. Cell Dev. Biol. 2024, 12, 1392391. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Chen, Y.; Sun, Y.; Wang, X.; Wang, Y.; Guo, S.; Shang, Z.; Shao, Z. EphA2 promotes the transcription of KLF4 to facilitate stemness in oral squamous cell carcinoma. Cell. Mol. Life Sci. 2024, 81, 278. [Google Scholar] [CrossRef] [PubMed]
- Martins-Neves, S.R.; Corver, W.E.; Paiva-Oliveira, D.I.; van den Akker, B.E.; Briaire-de-Bruijn, I.H.; Bovee, J.V.; Gomes, C.M.; Cleton-Jansen, A.M. Osteosarcoma Stem Cells Have Active Wnt/beta-catenin and Overexpress SOX2 and KLF4. J. Cell. Physiol. 2016, 231, 876–886. [Google Scholar] [CrossRef]
- Tao, H.; Lin, H.; Sun, Z.; Pei, F.; Zhang, J.; Chen, S.; Liu, H.; Chen, Z. Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-beta Signaling Pathway and Interaction with Histone Acetylation. J. Bone Min. Res. 2019, 34, 1502–1516. [Google Scholar] [CrossRef]
- Fei, Z.; Sun, C.; Peng, X.; Han, M.; Zhou, Q. Kruppel-like factor 4 promotes the proliferation and osteogenic differentiation of BMSCs through SOX2/IGF2 pathway. Acta Biochim. Pol. 2022, 69, 349–355. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Kito, A.; Itoh, S.; Naruse, H.; Fujikawa, J.; Sadek, K.M.; Akiyama, S.; Yamashiro, T.; Wakisaka, S.; Abe, M. Kruppel-Like Factor 4 represses osteoblast differentiation via ciliary Hedgehog signaling. Exp. Cell Res. 2018, 371, 417–425. [Google Scholar] [CrossRef]
- Lin, C.; Yang, Y.; Wang, Y.; Jing, H.; Bai, X.; Hong, Z.; Zhang, C.; Gao, H.; Zhang, L. Periodontal ligament fibroblasts-derived exosomes induced by PGE (2) inhibit human periodontal ligament stem cells osteogenic differentiation via activating miR-34c-5p/SATB2/ERK. Exp. Cell Res. 2022, 419, 113318. [Google Scholar] [CrossRef]
- Kikuchi, A. Modulation of Wnt signaling by Axin and Axil. Cytokine Growth Factor Rev. 1999, 10, 255–265. [Google Scholar] [CrossRef]
- Martins-Neves, S.R.; Paiva-Oliveira, D.I.; Fontes-Ribeiro, C.; Bovee, J.; Cleton-Jansen, A.M.; Gomes, C.M.F. IWR-1, a tankyrase inhibitor, attenuates Wnt/beta-catenin signaling in cancer stem-like cells and inhibits in vivo the growth of a subcutaneous human osteosarcoma xenograft. Cancer Lett. 2018, 414, 1–15. [Google Scholar] [CrossRef]
- Krege, J.H.; Lane, N.E.; Harris, J.M.; Miller, P.D. PINP as a biological response marker during teriparatide treatment for osteoporosis. Osteoporos. Int. 2014, 25, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Durdan, M.M.; Azaria, R.D.; Weivoda, M.M. Novel insights into the coupling of osteoclasts and resorption to bone formation. Semin. Cell Dev. Biol. 2022, 123, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bartold, M.; Gronthos, S.; Haynes, D.; Ivanovski, S. Mesenchymal stem cells and biologic factors leading to bone formation. J. Clin. Periodontol. 2019, 46 (Suppl. S21), 12–32. [Google Scholar] [CrossRef]
- Liu, B.; Gan, W.; Jin, Z.; Wang, M.; Cui, G.; Zhang, H.; Wang, H. The Role of miR-34c-5p in Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Int. J. Stem Cells 2021, 14, 286–297. [Google Scholar] [CrossRef]
- Yang, S.; Dong, F.; Li, D.; Sun, H.; Wu, B.; Sun, T.; Wang, Y.; Shen, P.; Ji, F.; Zhou, D. Persistent distention of colon damages interstitial cells of Cajal through Ca (2+) -ERK-AP-1-miR-34c-SCF deregulation. J. Cell. Mol. Med. 2017, 21, 1881–1892. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, F.; Pei, H.X.; Zhu, X.; Lin, X.; Song, C.Y.; Liang, Q.H.; Liao, E.Y.; Yuan, L.Q. Vaspin regulates the osteogenic differentiation of MC3T3-E1 through the PI3K-Akt/miR-34c loop. Sci. Rep. 2016, 6, 25578. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Wang, H.; Qin, A.; Qin, X. Ano5 modulates calcium signaling during bone homeostasis in gnathodiaphyseal dysplasia. NPJ Genom. Med. 2022, 7, 48. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Zhang, S.; Dong, R.; Miao, C.; Tian, Z.; Hu, Y. Ano5(Cys360Tyr) mutation leads to bone dysfunction of gnathodiaphyseal dysplasia via disturbing Akt signaling. Bone Rep. 2025, 24, 101825. [Google Scholar] [CrossRef]
- Batoon, L.; Koh, A.J.; Millard, S.M.; Grewal, J.; Choo, F.M.; Kannan, R.; Kinnaird, A.; Avey, M.; Teslya, T.; Pettit, A.R.; et al. Induction of osteoblast apoptosis stimulates macrophage efferocytosis and paradoxical bone formation. Bone Res. 2024, 12, 43. [Google Scholar] [CrossRef]
- Ott, S.M. Cortical or Trabecular Bone: What’s the Difference? Am. J. Nephrol. 2018, 47, 373–375. [Google Scholar] [CrossRef]
- Jaruga, A.; Ksiazkiewicz, J.; Kuzniarz, K.; Tylzanowski, P. Orofacial Cleft and Mandibular Prognathism-Human Genetics and Animal Models. Int. J. Mol. Sci. 2022, 23, 953. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Guo, J.; Sun, Z.; Lin, C.; Tao, H.; Zhang, Q.; Cui, Y.; Zuo, H.; Lin, Y.; Chen, S.; et al. BMP2-dependent gene regulatory network analysis reveals Klf4 as a novel transcription factor of osteoblast differentiation. Cell Death Dis. 2021, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Youn, B.U.; Lee, J.; Kim, I.; Shin, H.I.; Akiyama, H.; Choi, Y.; Kim, N. Kruppel-like factor 4 attenuates osteoblast formation, function, and cross talk with osteoclasts. J. Cell Biol. 2014, 204, 1063–1074. [Google Scholar] [CrossRef]
- Guo, J.; Yu, S.; Zhang, H.; Zhang, L.; Yuan, G.; Liu, H.; Chen, Z. Klf4 haploinsufficiency in Sp7+ lineage leads to underdeveloped mandibles and insufficient elongation of mandibular incisor. Biochim. Et Biophys. Acta Mol. Basis Dis. 2023, 1869, 166636. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef]
- Campagnolo, P.; Tsai, T.N.; Hong, X.; Kirton, J.P.; So, P.W.; Margariti, A.; Di Bernardini, E.; Wong, M.M.; Hu, Y.; Stevens, M.M.; et al. c-Kit+ progenitors generate vascular cells for tissue-engineered grafts through modulation of the Wnt/Klf4 pathway. Biomaterials 2015, 60, 53–61. [Google Scholar] [CrossRef]
- Nusse, R.; Clevers, H. Wnt/beta-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Fan, J.; Su, Y.W.; Hassanshahi, M.; Fan, C.M.; Peymanfar, Y.; Piergentili, A.; Del Bello, F.; Quaglia, W.; Xian, C.J. beta-Catenin signaling is important for osteogenesis and hematopoiesis recovery following methotrexate chemotherapy in rats. J. Cell. Physiol. 2021, 236, 3740–3751. [Google Scholar] [CrossRef]
- Cao, Q.; Zhang, X.; Lu, L.; Yang, L.; Gao, J.; Gao, Y.; Ma, H.; Cao, Y. Klf4 is required for germ-layer differentiation and body axis patterning during Xenopus embryogenesis. Development 2012, 139, 3950–3961. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, S.; Xu, H.; Zhang, M.; Liu, X.; Liu, S.; Li, H.; Hu, Y. Ano5 Deficiency Leads to Abnormal Bone Formation via miR-34c-5p/KLF4/β-Catenin in Gnathodiaphyseal Dysplasia. Int. J. Mol. Sci. 2025, 26, 5267. https://doi.org/10.3390/ijms26115267
Wang S, Zhang S, Xu H, Zhang M, Liu X, Liu S, Li H, Hu Y. Ano5 Deficiency Leads to Abnormal Bone Formation via miR-34c-5p/KLF4/β-Catenin in Gnathodiaphyseal Dysplasia. International Journal of Molecular Sciences. 2025; 26(11):5267. https://doi.org/10.3390/ijms26115267
Chicago/Turabian StyleWang, Shengnan, Shuai Zhang, Huichong Xu, Mingyue Zhang, Xiu Liu, Sirui Liu, Hongyu Li, and Ying Hu. 2025. "Ano5 Deficiency Leads to Abnormal Bone Formation via miR-34c-5p/KLF4/β-Catenin in Gnathodiaphyseal Dysplasia" International Journal of Molecular Sciences 26, no. 11: 5267. https://doi.org/10.3390/ijms26115267
APA StyleWang, S., Zhang, S., Xu, H., Zhang, M., Liu, X., Liu, S., Li, H., & Hu, Y. (2025). Ano5 Deficiency Leads to Abnormal Bone Formation via miR-34c-5p/KLF4/β-Catenin in Gnathodiaphyseal Dysplasia. International Journal of Molecular Sciences, 26(11), 5267. https://doi.org/10.3390/ijms26115267






