Betaine Alleviates Bisphosphonate-Related Osteonecrosis of the Jaw by Rescuing BMSCs Function in an m6A-METTL3-Dependent Manner
Abstract
1. Introduction
2. Results
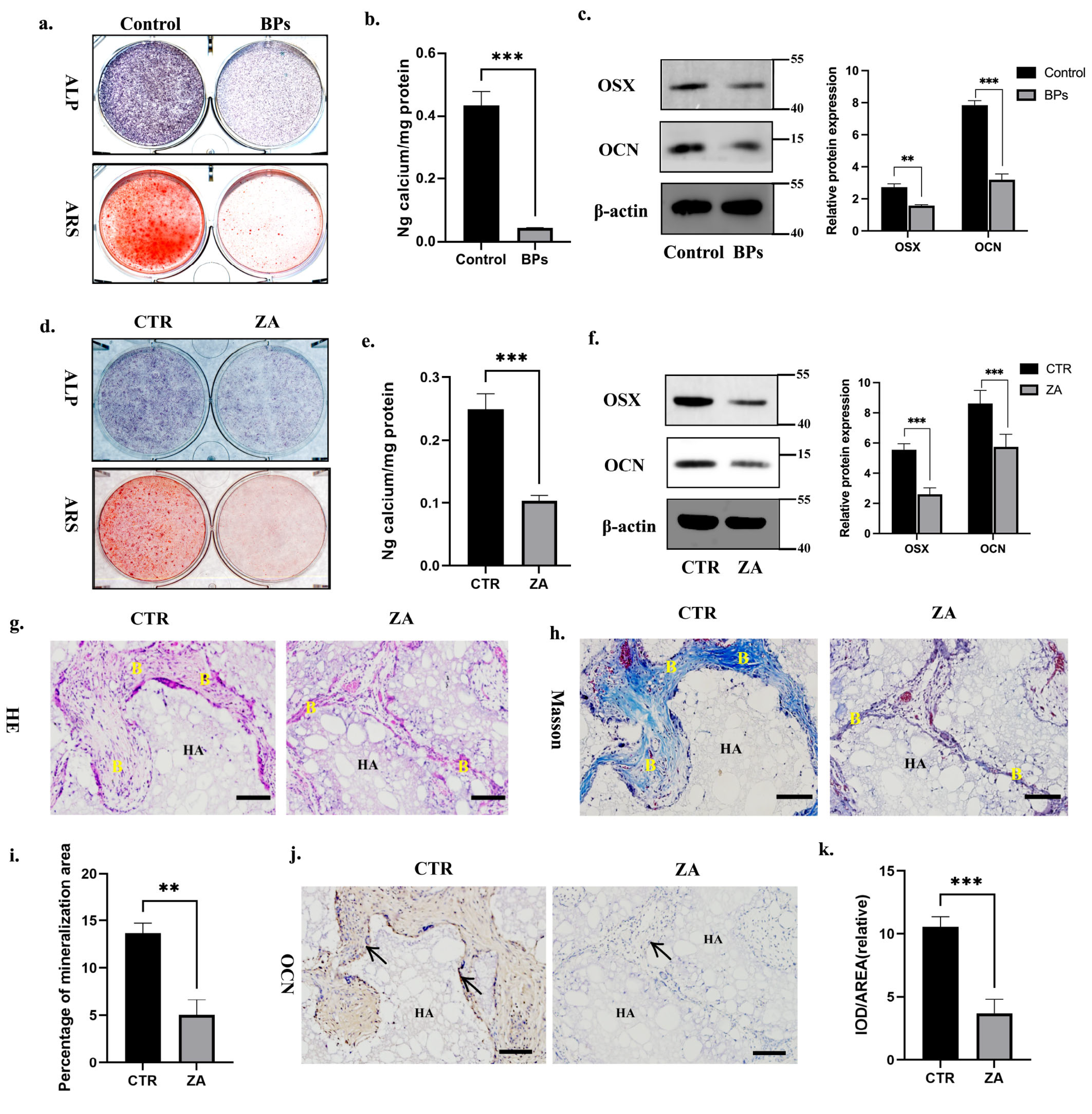
2.1. BPs Impaired BMSCs Function and Decreased Global m6A Methylation Level of BMSCs
2.2. Betaine Rescued BPs-Induced BMSCs Dysfunction In Vitro
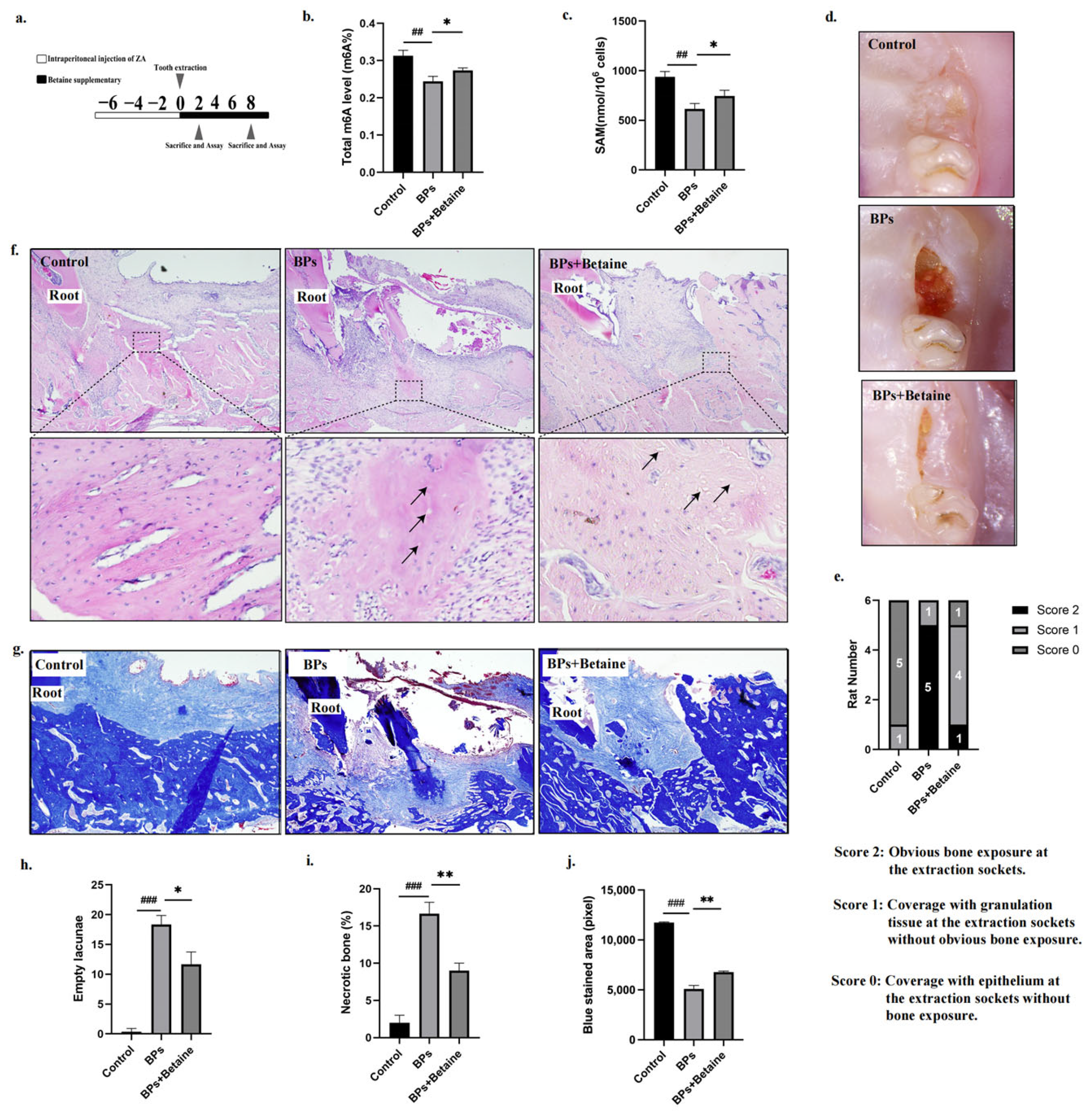
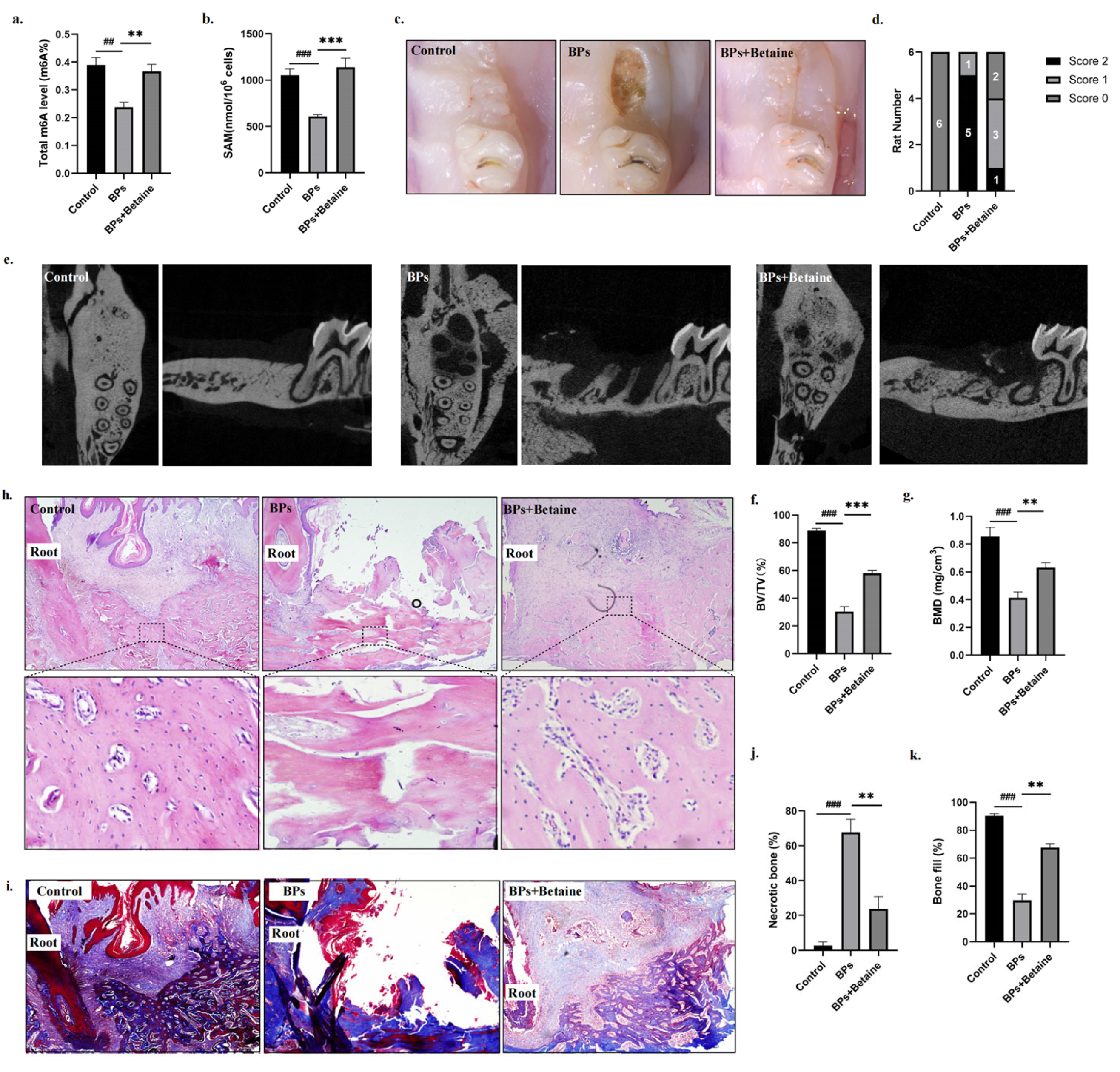
2.3. Betaine Alleviated Osteonecrosis of the Jaw in BRONJ Rat Model
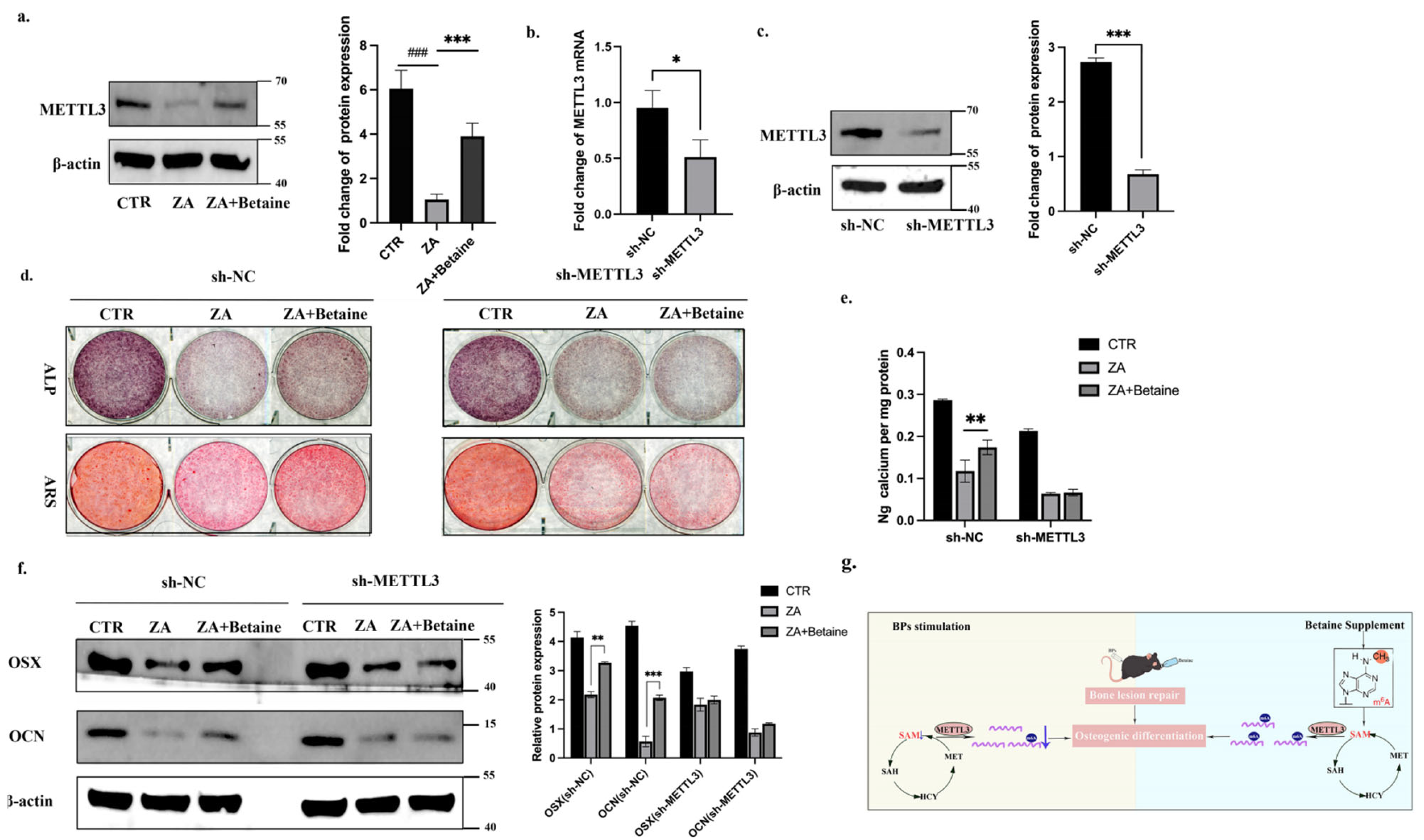
2.4. Betaine Regulated BMSCs Function in an m6A-METTL3-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Drug Administration
4.2. Animals and Treatments
4.3. Transplantation in Nude Mice
4.4. Alkaline Phosphatase and Alizarin Red Staining Detection
4.5. RNA Extraction and Real-Time PCR
4.6. Western Blot
4.7. m6A-ELISA
4.8. Immunofluorescence
4.9. SAM-ELISA
4.10. Construction of Plasmids and Transfection of Virus
4.11. Statistics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPs | Bisphosphonates |
| BRONJ | Bisphosphonate-related osteonecrosis of the jaw |
| BMSCs | bone marrow-derived mesenchymal stem cells |
| ZA | Zoledronate acid |
References
- Wu, S.; Li, F.; Tan, J.; Ye, X.; Le, Y.; Liu, N.; Everts, V.; Wan, Q. Porphyromonas gingivalis Induces Bisphosphonate-Related Osteonecrosis of the Femur in Mice. Front. Cell. Infect. Microbiol. 2022, 12, 886411. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Funayama, N.; Yagyuu, T.; Imada, M.; Ueyama, Y.; Nakagawa, Y.; Kirita, T. Impact of beta-tricalcium phosphate on preventing tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaw in rats. Sci. Rep. 2023, 13, 16032. [Google Scholar] [CrossRef]
- Poxleitner, P.; Engelhardt, M.; Schmelzeisen, R.; Voss, P. The prevention of medication-related osteonecrosis of the jaw. Dtsch. Aerzteblatt Online 2017, 114, 63–69. [Google Scholar] [CrossRef]
- He, L.L.H.; Xiao, E.; An, J.G.; He, Y.; Chen, S.; Zhao, L.; Zhang, T.; Zhang, Y. Role of Bone Marrow Stromal Cells in Impaired Bone Repair from BRONJ Osseous Lesions. J. Dent. Res. 2017, 98, 539–546. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Mao, L.; Liu, Y.; Gao, R.; Zheng, Z.; Chen, W.; Le, A.; Shi, S.; Wang, S. Allogeneic Mesenchymal Stem Cell Therapy for Bisphosphonate-Related Jaw Osteonecrosis in Swine. Stem Cells Dev. 2013, 22, 2047–2056. [Google Scholar] [CrossRef]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw–like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef]
- Ide, C.; Nakai, Y.; Nakano, N.; Seo, T.-B.; Yamada, Y.; Endo, K.; Noda, T.; Saito, F.; Suzuki, Y.; Fukushima, M.; et al. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in the rat. Brain Res. 2010, 1332, 32–47. [Google Scholar] [CrossRef]
- Mummery, C.L.; Davis, R.P.; Krieger, J.E. Challenges in Using Stem Cells for Cardiac Repair. Perspective 2010, 2, 17–27. [Google Scholar] [CrossRef]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular Vesicles of Stem Cells to Prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef]
- Alderman, M.H.; Xiao, A.Z. N(6)-Methyladenine in eukaryotes. Cell. Mol. Life Sci. 2019, 76, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, X.; Xie, J.; Xu, Y.; Li, X.-J.; Lin, L. Emerging Roles for DNA 6mA and RNA m6A Methylation in Mammalian Genome. Int. J. Mol. Sci. 2023, 24, 13897. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xie, L.; Wang, M.; Xiong, Q.; Guo, Y.; Liang, Y.; Li, J.; Sheng, R.; Deng, P.; Wang, Y.; et al. Mettl3-mediated m6A RNA methylation regulates the fate of bone marrow mesenchymal stem cells and osteoporosis. Nat. Commun. 2018, 9, 4772. [Google Scholar] [CrossRef]
- Chen, X.; Gong, W.; Shao, X.; Shi, T.; Zhang, L.; Dong, J.; Shi, Y.; Shen, S.; Qin, J.; Jiang, Q.; et al. METTL3-mediated m6A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 2022, 81, 87–99. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, Z.; Yang, C.; Luo, Z.; Bao, X. ALKBH5 regulates STAT3 activity to affect the proliferation and tumorigenicity of osteosarcoma via an m6A-YTHDF2-dependent manner. EBioMedicine 2022, 80, 104019. [Google Scholar] [CrossRef]
- Xie, Z.; Luo, H.; Wang, T.; Wang, L.; Zhang, J.; Dong, W.; Liu, G.; Li, F.; Kang, Q.; Zhu, X.; et al. METTL3 inhibits BMSC apoptosis and facilitates osteonecrosis repair via an m6A-IGF2BP2-dependent mechanism. Heliyon 2024, 10, e30195. [Google Scholar] [CrossRef]
- Jin, Y.; Han, X.; Wang, Y.; Fan, Z. METTL7A-mediated m6A modification of corin reverses bisphosphonates-impaired osteogenic differentiation of orofacial BMSCs. Int. J. Oral Sci. 2024, 16, 42. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Barak, A.J.; Beckenhauer, H.C.; Tuma, D.J. Betaine, ethanol, and the liver: A review. Alcohol 1996, 13, 395–398. [Google Scholar] [CrossRef]
- Huang, W.; Chen, T.-Q.; Fang, K.; Zeng, Z.-C.; Ye, H.; Chen, Y.-Q. N6-methyladenosine methyltransferases: Functions, regulation, and clinical potential. J. Hematol. Oncol. 2021, 14, 117. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, J.; Guo, F.; Yu, L.; Ren, X.; Yin, X. Betaine regulates adipogenic and osteogenic differentiation of hAD-MSCs. Mol. Biol. Rep. 2023, 50, 5081–5089. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, W.; Chen, Y.; Zhu, D.; Yin, J.; Zhang, C.; Gao, Y. Betaine alleviates alcohol-induced osteonecrosis of the femoral head via mTOR signaling pathway regulation. Biomed. Pharmacother. 2019, 120, 109486. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- Sendinc, E.; Yu, H.; Fu, Y.H.H.; Santos, J.; Johnson, Z.; Kirstein, J.R.; Niu, J.; Chabot, M.B.; Cantu, V.A.; Džakula, Ž.; et al. Mapping multiple RNA modifications simultaneously by proximity barcode sequencing. BioRxiv 2024. [Google Scholar] [CrossRef]
- Han, X.; Li, G.; Yang, H.; Zhang, C.; Cao, Y.; Wang, N.; Ge, L.; Fan, Z. METTL3 Promotes Osteo/Odontogenic Differentiation of Stem Cells by Inhibiting miR-196b-5p Maturation. Stem Cells Int. 2023, 2023, 8992284. [Google Scholar] [CrossRef]
- Sato, M.; Grasser, W. Effects of bisphosphonates on isolated rat osteoclasts as examined by reflected light microscopy. J. Bone Miner. Res. 1990, 5, 30–41. [Google Scholar] [CrossRef]
- Russell, R.G. Bisphosphonates: The first 40 years. Bone 2011, 49, 2–19. [Google Scholar] [CrossRef]
- Yang, J.-G.; Sun, B.; Wang, Z.; Li, X.; Gao, J.-H.; Qian, J.-J.; Li, J.; Wei, W.-J.; Zhang, P.; Wang, W. Exosome-targeted delivery of METTL14 regulates NFATc1 m6A methylation levels to correct osteoclast-induced bone resorption. Cell Death Dis. 2023, 14, 738. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Zhong, K.-Y.; Huang, W.-G.; Huang, Z.-H.; He, T.-T.; Yang, M.-T.; Wusiman, M.; Zhou, D.-D.; Chen, S.; et al. Betaine alleviates cognitive impairment induced by homocysteine through attenuating NLRP3-mediated microglial pyroptosis in an m6A-YTHDF2-dependent manner. Redox Biol. 2024, 69, 103026. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Hao, L.; Zhao, Y.; Zhang, L.; Ding, W.; Qi, Y.; Xu, Q. One-carbon metabolism supports S-adenosylmethionine and m6A methylation to control the osteogenesis of bone marrow stem cells and bone formation. J. Bone Miner. Res. 2024, 39, 1356–1370. [Google Scholar] [CrossRef]
- Yajun, W.; Jin, C.; Zhengrong, G.; Chao, F.; Yan, H.; Weizong, W.; Xiaoqun, L.; Qirong, Z.; Huiwen, C.; Hao, Z.; et al. Betaine Attenuates Osteoarthritis by Inhibiting Osteoclastogenesis and Angiogenesis in Subchondral Bone. Front. Pharmacol. 2021, 12, 723988. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liang, Y.; Cao, Y.; Cao, Y.; Fan, Z. Homeobox C8 inhibited the osteo-/dentinogenic differentiation and migration ability of stem cells of the apical papilla via activating KDM1A. J. Cell. Physiol. 2020, 235, 8432–8445. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Han, X.; Yang, H.; Xia, D.; Fan, Z. miR-6807-5p Inhibited the Odontogenic Differentiation of Human Dental Pulp Stem Cells Through Directly Targeting METTL7A. Front. Cell Dev. Biol. 2021, 9, 759192. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Song, J.; Diao, Z.; Han, X.; Fan, Z. Betaine Alleviates Bisphosphonate-Related Osteonecrosis of the Jaw by Rescuing BMSCs Function in an m6A-METTL3-Dependent Manner. Int. J. Mol. Sci. 2025, 26, 5233. https://doi.org/10.3390/ijms26115233
Jin Y, Song J, Diao Z, Han X, Fan Z. Betaine Alleviates Bisphosphonate-Related Osteonecrosis of the Jaw by Rescuing BMSCs Function in an m6A-METTL3-Dependent Manner. International Journal of Molecular Sciences. 2025; 26(11):5233. https://doi.org/10.3390/ijms26115233
Chicago/Turabian StyleJin, Yizhou, Jiaxin Song, Zhanqiu Diao, Xiao Han, and Zhipeng Fan. 2025. "Betaine Alleviates Bisphosphonate-Related Osteonecrosis of the Jaw by Rescuing BMSCs Function in an m6A-METTL3-Dependent Manner" International Journal of Molecular Sciences 26, no. 11: 5233. https://doi.org/10.3390/ijms26115233
APA StyleJin, Y., Song, J., Diao, Z., Han, X., & Fan, Z. (2025). Betaine Alleviates Bisphosphonate-Related Osteonecrosis of the Jaw by Rescuing BMSCs Function in an m6A-METTL3-Dependent Manner. International Journal of Molecular Sciences, 26(11), 5233. https://doi.org/10.3390/ijms26115233





