Cancer Pain: Radiotherapy as a Double-Edged Sword
Abstract
1. Introduction
2. Radiotherapy as a Mainstream Procedure for the Treatment of Cancer Pain
- Teleradiotherapy (or external beam radiation therapy, EBRT) is the most commonly used irradiation technique in oncological treatment. In EBRT, the radiation source is placed at a certain distance from the tumor, and the patient, lying in a predetermined position, is exposed to a beam of ionizing radiation (either photon or electron) generated by a therapeutic device—a medical linear accelerator. The irradiation area includes not only the tumor itself, but also an appropriate margin of surrounding tissue, which has been visualized in imaging studies (computed tomography and magnetic resonance imaging), in order to account for the possibility that cancerous cells may have also occupied areas in the vicinity of the tumor. A patient who is to undergo EBRT is prepared for the procedure by means of selecting the appropriate form of immobilization and conducting a computed tomography-guided localization scan to define the exact irradiation targets. Additionally, important points are marked on the patient’s skin, which are essential for reproducing the planned irradiation field [5]. The basic EBRT technique is 3D conformal radiation therapy (3D-CRT), which uses fixed angles to deliver radiation in discrete steps from multiple directions, with the beam shapes and angles adjusted to match the tumor’s three-dimensional structure. Here, 3D-CRT does not modulate the intensity of the radiation during treatment. Volumetric modulated arc therapy (VMAT) is a more sophisticated technique that delivers radiation in a continuous, arc-based rotation around the patient from the treatment source, often a linear accelerator (LINAC), which rotates 360 degrees around the patient while adjusting the intensity of the radiation beam as it moves. VMAT is a conformal radiotherapy technique, which means that the radiation beam and dose are more closely adapted to the shape and size of the tumor than in 3D-CRT. Traditional RT techniques, primarily based on photon (X-ray) therapy, often affect healthy tissues adjacent to the tumor, leading to unintended side effects. More recently, image-guided RT (IGRT) and stereotactic radiosurgery (SRS) have made strides in real-time tumor tracking and high-precision delivery, enabling the treatment of tumors in challenging locations with sub-millimeter accuracy.
- Brachytherapy (BRT) is a form of localized RT used to treat various types of cancer, which involves placing a radioactive source directly inside or very close to the tumor. This allows for high doses of radiation to be delivered directly to the cancerous tissue, simultaneously minimizing radiation exposure to surrounding healthy tissues. The localized radiation can help shrink tumors, alleviate obstruction, and reduce inflammation, which may lead to significant pain relief [6]. This technique is particularly effective in cases where cancer causes localized pain due to soft tissue masses; for instance, in head and neck cancers, prostate cancers, and gynecological malignancies [7]. Sometimes BRT is used to treat painful bone metastases, particularly in areas like the spine, pelvis, or other bones where radiation can be delivered precisely [8]. In cancers of the gastrointestinal tract, urinary tract, or reproductive organs, BRT can be used to treat obstruction-related pain, especially when tumors exert pressure on other organs or nerves. Various radioactive isotopes are used in BRT, such as iridium-192 (192Ir), cesium-137 (137Cs), cobalt-60 (60Co), radium-226 (226Ra), or yttrium-90 (90Y), which are placed in close proximity to the tumor for a strictly defined period of time. The various types of BRT are described in Table 1.
- 3.
- Proton and heavy ion therapy are some of the most significant developments in RT. These novel therapies use charged particles—protons (positively charged particles—hydrogen nuclei) and heavier ions (such as carbon ions)—to deliver radiation to tumors. Compared to traditional photon therapy, proton and heavy ion therapy offer remarkable benefits, especially in terms of reducing radiation exposure to surrounding healthy tissues and minimizing side effects. Protons have mass and energy, and when they enter tissue, they deposit most of their energy at a specific depth known as the Bragg peak. This allows the proton beam to release a significant portion of its radiation directly at the tumor site, with little to no radiation affecting the tissues beyond the tumor. As a result, healthy organs and structures surrounding the tumor are spared from unnecessary radiation exposure. This precise dose distribution is particularly valuable when treating tumors located near critical structures such as the brain, spinal cord, eyes, and especially in pediatric cancers, where minimizing side effects is of utmost importance [16,17].Heavy ion therapy, often involving carbon ions, takes the precision of proton therapy a step further. These ions are heavier than protons and possess greater energy, allowing them to deliver a higher biological effectiveness (i.e., having higher cell-death potential) at the same physical dose. Carbon ions can penetrate deeper into tissue while maintaining their precise dose distribution, making them ideal for treating tumors in challenging locations, including those that are resistant to conventional radiation [18]. One of the distinct advantages of heavy ion therapy over proton therapy is its ability to overcome the resistance of hypoxic tumor cells—cells that are deprived of oxygen, which are often more resistant to conventional radiation [19]. These techniques, due to their high cost and limited availability, should be selected for use in patients with oligometastatic/oligorecurrent disease with a low metastatic burden [20,21]. To date, proton therapy has been used with encouraging results for the treatment of bone or liver metastases, as an option in which the reduced radiation exposure to normal tissues leads to a clinically significant reduction in treatment-related toxicities [22]. It is hoped that the increase in the number of proton therapy treatment centers available worldwide will potentially lead to an expansion of its commissioning to include indications that are currently not routinely funded [23].The future of RT appears promising, with continued advancements in technology that aim to further improve precision and reduce toxicity. The development of FLASH RT, which involves delivering very high doses of radiation at ultra-fast speeds (ultra-high dose rates ≥ 40 Gy/s), is one such breakthrough that is currently being explored. The preliminary data suggest that the lower levels of toxic oxygen reactive species in normal tissues may explain why fewer side effects may be produced by FLASH than by conventional RT [24]. The first-in-human FAST-01 clinical trial demonstrated the clinical feasibility of proton FLASH in the treatment of extremity bone metastases [25]. The FAST-02 trial is currently assessing the toxicities of treatment (eight Gy in a single fraction) and pain relief in patients with painful thoracic bone metastases [26]. FLASH therapy has shown potential for sparing normal tissues from radiation toxicity while still effectively targeting tumors [27].Furthermore, as proton and heavy ion therapy centers become more accessible and cost-effective, it is expected that more patients will benefit from these advanced treatments. In addition, personalized medicine—where treatment is tailored to the individual characteristics of both the patient and the tumor—will continue to play an essential role in RT.
- 4.
- The most common indication for the use of radioactive strontium isotopes is multiple, painful bone metastases that cannot be irradiated with external sources (teletherapy) due to their extensive distribution. The effects of isotopic therapy last from 3 to 12 months, and the onset of action may not become apparent until several weeks after the treatment. Occasionally, before the therapeutic effect occurs, there may be a transient increase in pain symptoms, in which case the patient may require a temporary increase in doses of analgesics [34,35]. Isotope treatment can be used in combination with RT, provided there is an appropriate time interval after prior monitoring of blood morphological parameters.
| Radionuclide | Cancer | Indications | Pain Relief Effect | Pain-Free Period |
|---|---|---|---|---|
| Strontium-89 Chloride | Prostate [36,37] breast lung, head and neck, colorectal [38] | Bone pain | 63–88% | 6 weeks–6 months |
| Samarium-153-EDTMP | lung, prostate [38,39] breast, osteosarcoma [40] | Bone pain | 62–78% | 3–8 months |
| Radium-223-Dichloride | Prostate [41,42] | Castration resistant prostate bone pain | 41–72% | Up to 16 weeks |
| Rhenium-186-HEDP | Prostate [43], breast [44] | Bone pain | 38% and 82% | 5–12 months |
| Rhenium-188-HEDP | Prostate [45,46] | progressive hormone-resistant prostate carcinoma and bone pain | 64–76% | 6 weeks |
3. The Molecular Mechanism of Radiotherapy
4. Radiotherapy as an Analgesic
4.1. Radiotherapy as a Mainstage Procedure in Painful Metastatic Bone Cancer
- Bone pain resulting from the presence of metastatic lesions, e.g., in the spine;
- Osteolytic metastases with significant bone loss that threaten fractures;
- Conditions following pathological bone fractures;
| Study | RT Scheme | Complete Pain Response | Partial Pain Response |
|---|---|---|---|
| Steenland et al., 1999 [107] | 1 × 8 Gy | 72% | 37% |
| 6 × 4 Gy | 69% | 33% | |
| Koswing et al., 1999 [108] | 1 × 8 Gy | 79% | 31% |
| 10 × 3 Gy | 82% | 33% | |
| Roos et al., 2005 [94] | 1 × 8 Gy | 61% | 15% |
| 10 × 3 Gy | 53% | 18% | |
| Hartsell et al., 2005 [109] | 1 × 8 Gy | 65% | 15% |
| 10 × 3 Gy | 66% | 18% | |
| Foro Arnalot et al., 2008 [89] | 1 × 8 Gy | 75% | 15% |
| 10 × 3 Gy | 86% | 13% | |
| Nongkynrih et al., 2018 [109] | 1 × 8 Gy | 80% | 20% |
| 5 × 4 Gy | 75% | 20% | |
| 10 × 3 Gy | 85% | 20% | |
| Nguygen et al., 2019 [110] | 1 × 12 Gy–16 Gy | 55% | 52% |
| 10 × 3 Gy | 34% | 19% | |
| Nguygen et al., 2023 [111] | 2 × 12 Gy | 83–94% | - |
| Ryu et al., 2023 [112] | 1 × 16–18 Gy | 60% | - |
| 1 × 8 Gy | 41% |
4.2. Radiotherapy as an Effective Analgesic Procedure in Advanced Head and Neck Cancer Patients
4.3. Radiotherapy as an Effective Procedure in Inflammatory Joint Diseases
4.4. Factors Predicting RT Effectiveness
5. The Other Side of the Coin in the Effect of Radiotherapy
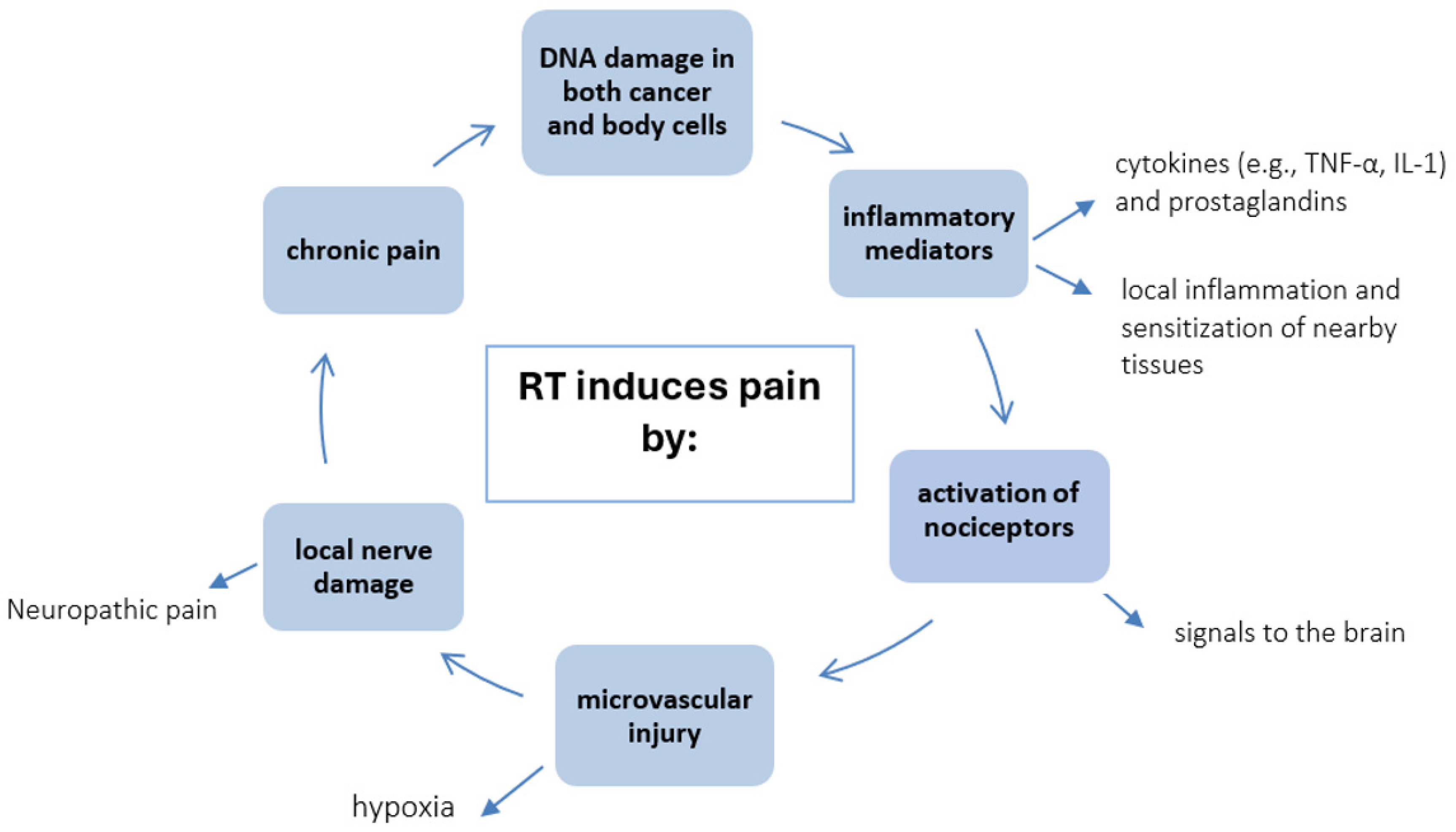
5.1. Pain as a Consequence of Radiotherapy
5.2. Painful Complications After RT
5.3. Pain Flares as a Temporary Side Effect
5.4. Promising Technological Progress in RT
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agarawal, J.P.; Swangsilpa, T.; van der Linden, Y.; Rades, D.; Jeremic, B.; Hoskin, P.J. The Role of External Beam Radiotherapy in the Management of Bone Metastases. Clin. Oncol. 2006, 8, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hsieh, R.K.; Chen, J.S.; Lee, K.D.; Rau, K.M.; Shao, Y.Y.; Sung, Y.C.; Yeh, S.P.; Chang, C.S.; Liu, T.C.; et al. Satisfaction with pain management and impact of pain on quality of life in cancer patients. Asia Pac. J. Clin. Oncol. 2020, 16, e91–e98. [Google Scholar] [CrossRef] [PubMed]
- Sierko, E.; Hempel, D.; Zuzda, K.; Wojtukiewicz, M.Z. Personalized Radiation Therapy in Cancer Pain Management. Cancers 2019, 11, 390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, E.; Zeng, L.; Salvo, N.; Dennis, K.; Tsao, M.; Lutz, S. Update on the Systematic Review of Palliative Radiotherapy Trials for Bone Metastases. Clin. Oncol. 2012, 24, 112–124. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jooya, A.; Talla, K.; Wei, R.; Huang, F.; Dennis, K.; Gaudet, M. Systematic review of brachytherapy for symptom palliation. Brachytherapy 2022, 6, 912–932. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Bolaños, Á.; Jiménez-Gil, M.; Federico, M.; Domínguez-Díaz, Y.; Valencia, L.; Rodríguez-Pérez, A. Pain in High-Dose-Rate Brachytherapy for Cervical Cancer: A Retrospective Cohort Study. J. Pers. Med. 2023, 13, 1187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, G.; Theodore, N. Spinal brachytherapy. Neuro Oncol. 2022, 24, S62–S68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bossi, A.; Foulon, S.; Maldonado, X.; Sargos, P.; MacDermott, R.; Kelly, P.; Fléchon, A.; Tombal, B.; Supiot, S.; Berthold, D.; et al. Efficacy and safety of prostate radiotherapy in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet 2024, 404, 2065–2076. [Google Scholar] [CrossRef] [PubMed]
- Krzysztofiak, T.; Kamińska-Winciorek, G.; Pilśniak, A.; Wojcieszek, P. High dose rate brachytherapy in nonmelanoma skin cancer—Systematic review. Dermatol. Ther. 2022, 9, e15675. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, H.; Meng, N.; Jiang, Y.; Jiang, P.; Gao, Y.; Tian, S.; Liu, C.; Yang, R.; Wang, J.; et al. CT-guidance interstitial 125Iodine seed brachytherapy as a salvage therapy for recurrent spinal primary tumors. Radiat. Oncol. 2014, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Bao, Z.; Zou, J.; Yang, H. Effect of pedicle fixation combined with 125I seed implantation for metastatic thoracolumbar tumors. J. Pain Res. 2016, 9, 271–278. [Google Scholar]
- Nag, S.; Hu, K.S. Intraoperative high-dose-rate brachytherapy. Surg. Oncol. Clin. N. Am. 2003, 4, 1079–1097. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Mai, Q.; Yang, F.; Zhuang, W.; Gou, Q.; Zhou, Z.; Xu, R.; Chen, X.; Mo, Z. Feasibility and Clinical Value of CT-Guided 125I Brachytherapy for Pain Palliation in Patients with Breast Cancer and Bone Metastases After External Beam Radiotherapy Failure. Front. Oncol. 2021, 11, 627158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zuckerman, S.L.; Lim, J.; Yamada, Y.; Bilsky, M.H.; Laufer, I. Brachytherapy in Spinal Tumors: A Systematic Review. World Neurosurg. 2018, 118, e235–e244. [Google Scholar] [CrossRef] [PubMed]
- Berlin, E.; Eisenberg, R.; Hill-Kayser, C.; Lustig, R.A.; Kurtz, G.; Cummings, E.; LaRiviere, M. Delivery of re-irradiation and complex palliative radiotherapy using proton therapy in pediatric cancer patients. Pediatr. Blood Cancer 2023, 70, e30708. [Google Scholar] [CrossRef] [PubMed]
- Press, R.H.; Mehta, M.P. Proton Therapy: Current Status and Controversies. JCO Oncol. Pract. 2024, 20, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Rief, H.; Chaudhri, N.; Tonndorf-Martini, E.; Bruckner, T.; Rieken, S.; Bostel, T.; Förster, R.; Schlampp, I.; Debus, J.; Sterzing, F. Intensity-modulated radiotherapy versus proton radiotherapy versus carbon ion radiotherapy for spinal bone metastases: A treatment planning study. J. Appl. Clin. Med. Phys. 2015, 16, 186–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, X.; Mohammadi, H.; Moreno, K.C.; Beltran, C.J.; Holtzman, A.L. Heavy Ion Particle Therapy in Modern Day Radiation Oncology. Hematol. Oncol. Clin. N. Am. 2025, 39, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Worawongsakul, R.; Steinmeier, T.; Lin, Y.L.; Bauer, S.; Hardes, J.; Hecker-Nolting, S.; Dirksen, U.; Timmermann, B. Proton Therapy for Primary Bone Malignancy of the Pelvic and Lumbar Region—Data From the Prospective Registries ProReg and KiProReg. Front. Oncol. 2022, 12, 805051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gaito, S.; Marvaso, G.; Ortiz, R.; Crellin, A.; Aznar, M.C.; Indelicato, D.J.; Pan, S.; Whitfield, G.; Alongi, F.; Jereczek-Fossa, B.A.; et al. Proton Beam Therapy in the Oligometastatic/Oligorecurrent Setting: Is There a Role? A Literature Review. Cancers 2023, 26, 2489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grau, C.; Durante, M.; Georg, D.; Langendijk, J.A.; Weber, D.C. Particle therapy in Europe. Mol. Oncol. 2020, 14, 1492–1499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsang, D.S.; Timmerman, B. Improving Access to Proton Therapy in the United States and Around the World. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951, Erratum in Proc. Natl. Acad. Sci. USA 2020, 117, 25946–25947. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daugherty, E.C.; Mascia, A.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.; Levine, L.; et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR Res. Protoc. 2023, 12, e41812. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daugherty, E.C.; Zhang, Y.; Xiao, Z.; Mascia, A.E.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.J.; Sharma, R.A.; Khuntia, D.; et al. FLASH radiotherapy for the treatment of symptomatic bone metastases in the thorax (FAST-02): Protocol for a prospective study of a novel radiotherapy approach. Radiat. Oncol. 2024, 19, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93, Erratum in Sci. Transl. Med. 2019, 11, eaba4525. [Google Scholar] [CrossRef] [PubMed]
- Fettich, J.; Padhy, A.; Nair, N.; Morales, R.; Tanumihardja, M.; Riccabonna, G.; Nair, G. Comparative clinical efficacy and safety of Phosphorus-32 and Strontium-89 in the palliative treatment of metastatic bone pain: Results of an IAEA Coordinated Research Project. World J. Nucl. Med. 2003, 34, 226–231. [Google Scholar]
- Parker, C.; Heinrich, D.; O’Sullivan, J.M.; Fossa, S.; Chodacki, A.; Demkow, T.; Cross, A.; Bolstad, B.; Garcia-Vargas, J.; Sartor, O. Sartor Overall survival benefit of radium-223 chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in Castration-resistant Prostate Cancer (CRPC): A phase III randomized trial (ALSYMPCA). Eur. J. Cancer. 2011, 47, 3. [Google Scholar] [CrossRef]
- Choi, J.Y. Treatment of Bone Metastasis with Bone-Targeting Radiopharmaceuticals. Nucl. Med. Mol. Imaging 2018, 52, 200–207. [Google Scholar] [CrossRef]
- Ma, Y.-B.; Yan, W.-L.; Dai, J.-C.; Xu, F.; Yuan, Q.; Shi, H.-H. Strontium-89: A desirable therapeutic for bone metastases of prostate cancer. Zhonghua Nan Ke Xue 2008, 14, 819–822. [Google Scholar]
- Ogawa, K.; Washiyama, K. Bone Target Radiotracers for Palliative Therapy of Bone Metastases. Curr. Med. Chem. 2012, 19, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Finlay, I.G.; Mason, M.D.; Shelley, M. Radioisotopes for the palliation of metastatic bone cancer: A systematic review. Lancet Oncol. 2005, 6, 392–400. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Dolezal, J.; Vizda, J.; Odrazka, K. Prospective evaluation of samarium-153-EDTMP radionuclide treatment for bone metastases in patients with hormone-refractory prostate cancer. Urol. Int. 2007, 78, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Dafermou, A.; Colamussi, P.; Giganti, M.; Cittanti, C.; Bestagno, M.; Piffanelli, A. A multicentre observational study of radionuclide therapy in patients with painful bone metastases of prostate cancer. Eur. J. Nucl. Med. 2001, 28, 788–798. [Google Scholar] [CrossRef]
- Baczyk, M.; Milecki, P.; Baczyk, E.; Sowiński, J. The effectivness of strontium 89 in palliative therapy of painful prostate cancer bone metastases. Ortop. Traumatol. Rehabil. 2003, 5, 364–368. [Google Scholar] [PubMed]
- Zenda, S.; Nakagami, Y.; Toshima, M.; Arahira, S.; Kawashima, M.; Matsumoto, Y.; Kinoshita, H.; Satake, M.; Akimoto, T. Strontium-89 (Sr-89) chloride in the treatment of various cancer patients with multiple bone metastases. Int. J. Clin. Oncol. 2014, 19, 739–743. [Google Scholar] [CrossRef]
- Sciuto, R.; Festa, A.; Pasqualoni, R.; Semprebene, A.; Rea, S.; Bergomi, S.; Maini, C.L. Metastatic bone pain palliation with 89-Sr and 186-Re-HEDP in breast cancer patients. Breast Cancer Res. Treat. 2001, 66, 101–109. [Google Scholar] [CrossRef]
- Laing, A.H.; Ackery, D.M.; Bayly, R.J.; Buchanan, R.B.; Lewington, V.J.; McEwan, A.J.B.; Macleod, P.M.; Zivanovic, M.A. Strontium-89 chloride for pain palliation in prostatic skeletal malignancy. Br. J. Radiol. 1991, 64, 817–822. [Google Scholar] [CrossRef]
- Sartor, O.; Reid, R.H.; Hoskin, P.J.; Quick, D.P.; Ell, P.J.; Coleman, R.E.; Kotler, J.A.; Freeman, L.M.; Olivier, P. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004, 63, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Serafini, A.N.; Houston, S.J.; Resche, I.; Quick, D.P.; Grund, F.M.; Ell, P.J.; Bertrand, A.; Ahmann, F.R.; Orihuela, E.; Reid, R.H.; et al. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: A double-blind placebo-controlled clinical trial. J. Clin. Oncol. 1998, 16, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.; de Klerk, J.M.H.; Tan, S.; van het Schip, A.D.; Derksen, B.H.; van Dijk, A.; Kruitwagen, C.L.J.J.; Blijham, G.H.; van Rijk, P.P.; Zonnenberg, B.A. The PLACORHEN study: A double-blind, placebo-controlled, randomized radionuclide study with (186) Re-etidronate in hormone-resistant prostate cancer patients with painful bone metastases. Placebo Controlled Rhenium Study. J. Nucl. Med. 2002, 43, 1150–1156. [Google Scholar]
- Han, S.H.; Zonneberg, B.A.; de Klerk, J.M.; Quirijnen, J.M.; van het Schip, A.D.; van Dijk, A.; Blijham, G.H.; van Rijk, P.P. 186Re-etidronate in breast cancer patients with metastatic bone pain. J. Nucl. Med. 1999, 40, 639–642. [Google Scholar]
- Palmedo, H.; Guhlke, S.; Bender, H.; Sartor, J.; Schoeneich, G.; Risse, J.; Grünwald, F.; Knapp, F.F.; Biersack, H.J. Dose escalation study with rhenium-188 hydroxyethylidene diphosphonate in prostate cancer patients with osseous metastases. Eur. J. Nucl. Med. 2000, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Liepe, K.; Hliscs, R.; Kropp, J.; Gruning, T.; Runge, R.; Koch, R.; Knapp, F.F.J.; Franke, W.G. Rhenium-188-HEDP in the palliative treatment of bone metastases. Cancer Biother. Radiopharm. 2000, 15, 261–265. [Google Scholar] [CrossRef]
- Goblirsch, M.J.; Zwolak, P.P.; Clohisy, D.R. Biology of Bone Cancer Pain. Clin. Cancer Res. 2006, 12, 6231s–6235s. [Google Scholar] [CrossRef]
- Goblirsch, M.; Mathews, W.; Lynch, C.; Alaei, P.; Gerbi, B.J.; Mantyh, P.W.; Clohisy, D.R. Radiation treatment decreases bone cancer pain, osteolysis and tumor size. Radiat. Res. 2004, 161, 228–234. [Google Scholar] [CrossRef]
- MacLeod, K.; Laird, B.J.A.; Carragher, N.O.; Hoskin, P.; Fallon, M.T.; Sande, T.A. Predicting Response to Radiotherapy in Cancer-Induced Bone Pain: Cytokines as a Potential Biomarker? Clin. Oncol. 2020, 32, e203–e208. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Chen, Y.; Yuan, Y.; Wang, R.; Shan, H. Study on the Correlation between Pain and Cytokine Expression in the Peripheral Blood of Patients with Bone Metastasis of Malignant Cancer Treated Using External Radiation Therapy. Pain Res. Manag. 2022, 2022, 1119014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cun-Jin, S.; Jian-Hao, X.; Xu, L.; Feng-Lun, Z.; Jie, P.; Ai-Ming, S.; Duan-Min, H.; Yun-Li, Y.; Tong, L.; Yu-Song, Z. X-ray induces mechanical and heat allodynia in mouse via TRPA1 and TRPV1 activation. Mol. Pain 2019, 15, 1744806919849201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habberstad, R.; Aass, N.; Mollnes, T.E.; Damås, J.K.; Brunelli, C.; Rossi, R.; Garcia-Alonso, E.; Kaasa, S.; Klepstad, P. Inflammatory Markers and Radiotherapy Response in Patients With Painful Bone Metastases. J. Pain Symptom Manag. 2022, 64, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bertho, A.; Iturri, L.; Prezado, Y. Radiation-induced immune response in novel radiotherapy approaches FLASH and spatially fractionated radiotherapies. Int. Rev. Cell Mol. Biol. 2023, 376, 37–68. [Google Scholar] [CrossRef] [PubMed]
- Dutt, S.; Ahmed, M.M.; Loo, B.W., Jr.; Strober, S. Novel Radiation Therapy Paradigms and Immunomodulation: Heresies and Hope. Semin. Radiat. Oncol. 2020, 30, 194–200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, X.J.; Wang, Z.B.; Gan, Q.; Walters, E.T. cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J. Neurophysiol. 2006, 95, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Walters, E.T.; Song, X.J. Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP. J. Neurophysiol. 2007, 97, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.P.; Song, W.B.; Song, X.J. EphrinB-EphB receptor signaling contributes to bone cancer pain via Toll-like receptor and proinflammatory cytokines in rat spinal cord. Pain 2013, 154, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Dong, Y.; He, X.; Zhao, P.; Yang, A.; Zhou, R.; Ma, J.; Xie, Z.; Song, X.J. Radiotherapy Suppresses Bone Cancer Pain through Inhibiting Activation of cAMP Signaling in Rat Dorsal Root Ganglion and Spinal Cord. Mediat. Inflamm. 2016, 5093095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koswig, S.; Budach, V. Remineralization and pain relief in bone metastases after after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study. Strahlenther. Onkol. 1999, 175, 500–508. [Google Scholar] [CrossRef]
- Sprave, T.; Verma, V.; Förster, R.; Schlampp, I.; Hees, K.; Bruckner, T.; Bostel, T.; El Shafie, R.A.; Welzel, T.; Nicolay, N.H.; et al. Bone density and pain response following intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for vertebral metastases—Secondary results of a randomized trial. Radiat. Oncol. 2018, 13, 212. [Google Scholar] [CrossRef]
- Wei, R.L.; Mattes, M.D.; Yu, J.; Thrasher, A.; Shu, H.-K.; Paganetti, H.; De Los Santos, J.; Koontz, B.; Abraham, C.; Balboni, T. Attitudes of radiation oncologists toward palliative and supportive care in the United States: Report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract. Radiat. Oncol. 2017, 7, 113–119. [Google Scholar] [CrossRef]
- Amini, A.; Shinde, A.; Wong, J. Palliative Radiation for Cancer Pain Management. Cancer Treat. Res. 2021, 182, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Culleton, S.; Kwok, S.; Chow, E. Radiotherapy for pain. Clin. Oncol. 2011, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Kitani, A.; Kubota, T.; Uezono, Y. Increased pain after palliative radiotherapy: Not only due to cancer progression. Ann. Palliat. Med. 2024, 13, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Brozović, G.; Lesar, N.; Janev, D.; Bošnjak, T.; Muhaxhiri, B. Cancer Pain And Therapy. Acta Clin. Croat. 2022, 61, 103–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, S.P.; Koay, E.J. Current and emerging radiotherapy strategies for pancreatic adenocarcinoma: Stereotactic, intensity modulated and particle radiotherapy. Ann. Pancreat. Cancer. 2018, 1, 22. [Google Scholar] [CrossRef]
- Ryan, J.F.; Rosati, L.M.; Groot, V.P.; Le, D.T.; Zheng, L.; Laheru, D.A.; Shin, E.J.; Jackson, J.; Moore, J.; Narang, A.K.; et al. Stereotactic body radiation therapy for palliative management of pancreatic adenocarcinoma in elderly and medically inoperable patients. Oncotarget 2018, 9, 16427–16436. [Google Scholar] [CrossRef]
- Arscott, W.T.; Emmett, J.; Ghiam, A.F.; Jones, J.A. Palliative Radiotherapy: Inpatients, Outpatients, and the Changing Role of Supportive Care in Radiation Oncology. Hematol. Oncol. Clin. N. Am. 2020, 34, 253–277. [Google Scholar] [CrossRef]
- Fairchild, A.; Harris, K.; Barnes, E.; Wong, R.; Lutz, S.; Bezjak, A.; Cheung, P.; Chow, E. Palliative thoracic radiotherapy for lung cancer: A systematic review. J. Clin. Oncol. 2008, 26, 4001–4011. [Google Scholar] [CrossRef]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, G.; Pinnarò, P.; Rambone, R.; Giannarelli, D.; Benassi, M. A phase III randomized study on the sequencing of radiotherapy and chemotherapy in the conservative management of early-stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 61–167. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Maffulli, N.; Trivellas, A.; Eschweiler, J.; Tingart, M.; Driessen, A. Bone metastases: A comprehensive review of the literature. Mol. Biol. Rep. 2020, 47, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
- Harstell, W.F.; Santosh, Y. Palliation of bone metastases. In Principles and Practice of Radiation Oncology; Halperin, E., Perez, C., Brady, L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 1778–1791. [Google Scholar]
- Cook, G.J.R.; Goh, V. Molecular Imaging of Bone Metastases and Their Response to Therapy. J. Nucl. Med. 2020, 61, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kakhki, V.R.; Anvari, K.; Sadeghi, R.; Mahmoudian, A.S.; Torabian-Kakhki, M. Pattern and distribution of bone metastases in common malignant tumors. Nucl. Med. Rev. Cent. East. Eur. 2013, 16, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef] [PubMed]
- Käkönen, S.M.; Mundy, G.R. Mechanisms of osteolytic bone metastases in breast carcinoma. Cancer 2003, 1, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A.; Mohammad, K.S.; Clines, G.; Stebbins, E.G.; Wong, D.H.; Higgins, L.S.; Vessella, R.; Corey, E.; Padalecki, S.; Suva, L.; et al. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin. Cancer Res. 2006, 12, 6213s–6216s. [Google Scholar] [CrossRef] [PubMed]
- Challapalli, A.; Aziz, S.; Khoo, V.; Kumar, A.; Olson, R.; Ashford, R.U.; Gabbar, O.A.; Rai, B.; Bahl, A. Spine and Non-spine Bone Metastases—Current Controversies and Future Direction. Clin. Oncol. R Coll. Radiol. 2020, 32, 728–744. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Luporsi, E.; Krakowski, I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain 2017, 158, 1118–1125. [Google Scholar] [CrossRef]
- Zinboonyahgoon, N.; Luansritisakul, C. Neuropathic pain feature in cancer-induced bone pain: Does it matter? A prospective observational study. Korean J. Pain 2023, 36, 253–267. [Google Scholar] [CrossRef]
- Roos, D.E. Radiotherapy for neuropathic pain due to bone metastases. Ann. Palliat. Med. 2015, 4, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.G.; Chan, D.C.W.; Hoskin, P.J.; Marta, G.N.; Trippa, F.; Maranzano, E.; Chow, E.; Silva, M.F. Advances in radiotherapy in bone metastases in the context of new target therapies and ablative alternatives: A critical review. Radiother. Oncol. 2021, 163, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takei, D.; Tagami, K. Management of cancer pain due to bone metastasis. J. Bone Miner. Metab. 2023, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lutz, S.; Berk, L.; Chang, E.; Chow, E.; Hahn, C.; Hoskin, P.; Howell, D.; Konski, A.; Kachnic, L.; Lo, S.; et al. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 965–976. [Google Scholar] [CrossRef]
- Tseng, Y.D. Radiation Therapy for Painful Bone Metastases: Fractionation, Recalcification, and Symptom Control. Semin. Radiat. Oncol. 2023, 2, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.C.; Tseng, Y. Defining the radiation oncologist’s role in palliative care and radiotherapy. Ann. Palliat. Med. 2018, 7, 1002. [Google Scholar] [CrossRef]
- Foro Arnalot, P.; Fontanals, A.V.; Galcerán, J.C.; Lynd, F.; Latiesas, X.S.; de Dios, N.R.; Castillejo, A.R.; Bassols, M.L.; Galán, J.L.; Conejo, I.M.; et al. Randomized clinical trial with two palliative radiotherapy regimens in painful bone metastases: 30 Gy in 10 fractions compared with 8 Gy in single fraction. Radiother. Oncol. 2008, 89, 150–155. [Google Scholar] [CrossRef]
- Nongkynrih, A.; Dhull, A.K.; Kaushal, V.; Atri, R.; Dhankhar, R.; Kamboj, K. Comparison of Single Versus Multifraction Radiotherapy in Palliation of Painful Bone Metastases. World J. Oncol. 2018, 9, 91–95. [Google Scholar] [CrossRef]
- Bianchi, S.P.; Faccenda, V.; Pacifico, P.; Parma, G.; Saufi, S.; Ferrario, F.; Belmonte, M.; Sala, L.; De Ponti, E.; Panizza, D.; et al. Short-term pain control after palliative radiotherapy for uncomplicated bone metastases: A prospective cohort study. Med. Oncol. 2023, 41, 13. [Google Scholar] [CrossRef] [PubMed]
- Kachnic, L.; Berk, L. Palliative Single-Fraction Radiation Therapy: How Much More Evidence Is Needed? JNCI 2005, 97, 786–788. [Google Scholar] [CrossRef]
- Chow, E.; van der Linden, Y.M.; Roos, D.; Hartsell, W.F.; Hoskin, P.; Wu, J.S.Y.; Brundage, M.D.; Nabid, A.; Tissing-Tan, C.J.A.; Oei, B.; et al. Single versus multiple fractions of repeat radiation for painful bone metastases: A randomised, controlled, non-inferiority trial. Lancet Oncol. 2014, 15, 164–171. [Google Scholar] [CrossRef]
- Roos, D.E.; Turner, S.L.; O’Brien, P.C.; Smith, J.G.; Spry, N.A.; Burmeister, B.H.; Hoskin, P.J.; Ball, D.L. Randomized trial of 8 Gy in 1 versus 20 Gy in 5 fractions of radiotherapy for neuropathic pain due to bone metastases (Trans-Tasman Radiation Oncology Group, TROG 96.05). Radiother. Oncol. 2005, 75, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Tomitaka, E.; Toya, R.; Matsuyama, T.; Ninomura, S.; Watakabe, T.; Oya, N. A neuropathic pain component as a predictor of improvement in pain interference after radiotherapy for painful tumors: A secondary analysis of a prospective observational study. Clin. Transl. Radiat. Oncol. 2018, 12, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.I.; Hirai, T.; Inoue, T.; Hojo, N.; Kawai, S.; Kato, Y.; Ito, K.; Kato, M.; Ozawa, Y.; Shinjo, H.; et al. Time to Pain Relapse After Palliative Radiotherapy for Bone Metastasis: A Prospective Multi-institutional Study. Anticancer Res. 2023, 43, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wei, J.; Sun, R.; Jiang, W.; Chen, Y.; Shao, Y.; Gu, W. Stereotactic Body Radiation Therapy Versus Conventional Radiation Therapy in Pain Relief for Bone Metastases: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Taguchi, K.; Nakajima, Y.; Ogawa, H.; Murofushi, K.N. Palliative Efficacy of High-Dose Stereotactic Body Radiotherapy Versus Conventional Radiotherapy for Painful Non-Spine Bone Metastases: A Propensity Score-Matched Analysis. Cancers 2022, 14, 4014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, C.C.; Soon, Y.Y.; Cheo, T.; Vellayappan, B.; Tey, J. Stereotactic body radiation therapy versus conventional external beam radiation therapy for painful bone metastases: A systematic review and meta-analysis of randomized trials. Crit. Rev. Oncol. Hematol. 2022, 178, 103775. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.L.; van der Velden, J.M.; Wong, E.; Seravalli, E.; Sahgal, A.; Chow, E.; Verlaan, J.J.; Verkooijen, H.M.; van der Linden, Y.M. Systematic Review of the Role of Stereotactic Radiotherapy for Bone Metastases. J. Natl. Cancer Inst. 2019, 111, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grosinger, A.J.; Alcorn, S.R. An Update on the Management of Bone Metastases. Curr. Oncol. Rep. 2024, 26, 400–408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, H.C.Y.; Lee, S.F.; Chan, A.W.; Caini, S.; Hoskin, P.; Simone, C.B., 2nd; Johnstone, P.; van der Linden, Y.; van der Velden, J.M.; Martin, E.; et al. Stereotactic body radiation therapy versus conventional external beam radiotherapy for spinal metastases: A systematic review and meta-analysis of randomized controlled trials. Radiother. Oncol. 2023, 189, 109914. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shi, D.D.; Shin, K.Y.; Buckley, E.; Gunasti, L.; Hall, E.; Mann, E.; Spicer, B.; Chen, Y.H.; Hammoudeh, L.; et al. A Prospective Study Assessing the Efficacy and Toxicity of Stereotactic Body Radiation Therapy for Oligometastatic Bone Metastases. Adv. Radiat. Oncol. 2023, 9, 101411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lutz, S.; Balboni, T.; Jones, J.; Lo, S.; Petit, J.; Rich, S.E.; Wong, R.; Hahn, C. Palliative radiation therapy for bone metastases: Update of an ASTRO Evidence-Based Guideline. Pract. Radiat. Oncol. 2017, 7, 4–12. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Willmann, J.; Spałek, M.; Oldenburger, E.; Brown, S.; Kazmierska, J.; Andratschke, N.; Menten, J.; van der Linden, Y.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with uncomplicated bone metastases. Radiother. Oncol. 2022, 173, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Oldenburger, E.; Brown, S.; Willmann, J.; van der Velden, J.M.; Spałek, M.; van der Linden, Y.M.; Kazmierska, J.; Menten, J.; Andratschke, N.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with complicated bone metastases. Radiother. Oncol. 2022, 173, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Steenland, E.; Leer, J.W.; van Houwelingen, H.; Post, W.J.; van den Hout, W.B.; Kievit, J.; de Haes, H.; Martijn, H.; Oei, B.; Vonk, E.; et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother. Oncol. 1999, 2, 101–109, Erratum in Radiother. Oncol. 1999, 53, 167. [Google Scholar] [CrossRef] [PubMed]
- Koswig, S.; Buchali, A.; Böhmer, D.; Schlenger, L.; Budach, V. Palliative Strahlentherapie von Knochenmetastasen. Eine retrospective Analyse von 176 Patienten [Palliative radiotherapy of bone metastases. A retrospective analysis of 176 patients]. Strahlenther. Onkol. 1999, 175, 509–514. (In German) [Google Scholar] [CrossRef] [PubMed]
- Hartsell, W.F.; Scott, C.B.; Bruner, D.W.; Scarantino, C.W.; Ivker, R.A.; Roach, M., 3rd; Suh, J.H.; Demas, W.F.; Movsas, B.; Petersen, I.A.; et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J. Natl. Cancer Inst. 2005, 97, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.N.; Chun, S.G.; Chow, E.; Komaki, R.; Liao, Z.; Zacharia, R.; Szeto, B.K.; Welsh, J.W.; Hahn, S.M.; Fuller, C.D.; et al. Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol. 2019, 5, 872–878, Erratum in JAMA Oncol. 2021, 7, 1581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, E.K.; Ruschin, M.; Zhang, B.; Soliman, H.; Myrehaug, S.; Detsky, J.; Chen, H.; Sahgal, A.; Tseng, C.L. Stereotactic body radiotherapy for spine metastases: A review of 24 Gy in 2 daily fractions. J. Neurooncol. 2023, 163, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Deshmukh, S.; Timmerman, R.D.; Movsas, B.; Gerszten, P.; Yin, F.F.; Dicker, A.; Abraham, C.D.; Zhong, J.; Shiao, S.L.; et al. Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine: Phase 3 Results of NRG Oncology/RTOG 0631 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 800–807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bilski, M.; Konat-Bąska, K.; Mastroleo, F.; Hoskin, P.; Alicja Jereczek-Fossa, B.; Marvaso, G.; Korga, M.; Klas, J.; Zych, K.; Bijak, P.; et al. Half body irradiation (HBI) for bone metastases in the modern radiotherapy technique era—A systematic review. Clin. Transl. Radiat. Oncol. 2024, 49, 100845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delinikolas, P.; Patatoukas, G.; Kouloulias, V.; Dilvoi, M.; Plousi, A.; Efstathopoulos, E.; Platoni, K. A novel Hemi-Body Irradiation technique using electron beams (HBIe−). Phys. Medica 2018, 46, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Dutta, S.; Adhikary, S.; Bhattacharya, B.; Ghosh, B.; Patra, N. Hemi body irradiation: An economical way of palliation of pain in bone metastasis in advanced cancer. South Asian J. Cancer. 2014, 3, 28. [Google Scholar] [CrossRef]
- Palma, D.A.; Salama, J.K.; Lo, S.S.; Senan, S.; Treasure, T.; Govindan, R.; Weichselbaum, R. The oligometastatic state-separating truth from wishful thinking. Nat. Rev. Clin. Oncol. 2014, 11, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Osborn, V.W.; Lee, A.; Yamada, Y. Stereotactic Body Radiation Therapy for Spinal Malignancies. Technol. Cancer Res. Treat. 2018, 17, 1533033818802304. [Google Scholar] [CrossRef]
- Bindels, B.J.J.; Mercier, C.; Gal, R.; Verlaan, J.J.; Verhoeff, J.J.C.; Dirix, P.; Ost, P.; Kasperts, N.; van der Linden, Y.M.; Verkooijen, H.M.; et al. Stereotactic Body and Conventional Radiotherapy for Painful Bone Metastases: A Systematic Review and Meta-Analysis. JAMA Netw. Open. 2024, 7, e2355409. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahid Iqbal, M.; Kelly, C.; Kovarik, J.; Goranov, B.; Shaikh, G.; Morgan, D.; Dobrowsky, W.; Paleri, V. Palliative radiotherapy for locally advanced non-metastatic head and neck cancer: A systematic review. Radiother. Oncol. 2018, 126, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Zingeta, G.T.; Worku, Y.T.; Awol, M.; Woldetsadik, E.S.; Assefa, M.; Chama, T.Z.; Feyisa, J.D.; Bedada, H.F.; Adem, M.I.; Mengesha, T.; et al. Outcome of Hypofractionated Palliative Radiotherapy Regimens for Patients with Advanced Head and Neck Cancer in Tikur Anbessa Hospital, Ethiopia: A Prospective Cohort Study. JCO Glob. Oncol. 2024, 10, e2300253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaller, A.K.C.S.; Peterson, A.; Bäckryd, E. Pain management in patients undergoing radiation therapy for head and neck cancer—A descriptive study. Scand. J. Pain 2020, 21, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Trott, K.R.; Kamprad, F. Radiobiological mechanisms of anti-inflammatory radiotherapy. Radiother. Oncol. 1999, 51, 197–203. [Google Scholar] [CrossRef] [PubMed]
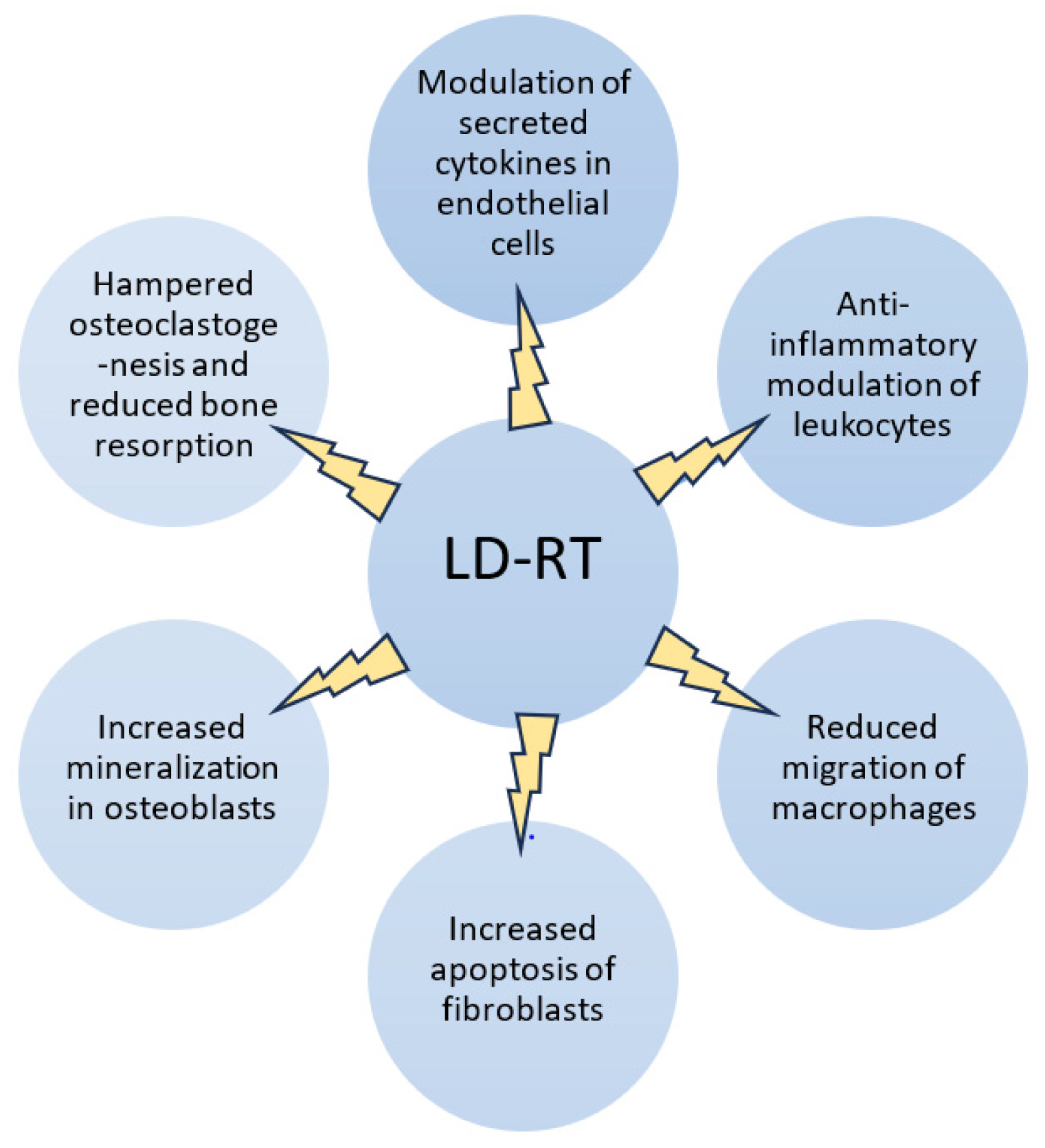
- Arenas, M.; Sabater, S.; Hernández, V.; Rovirosa, A.; Lara, P.C.; Biete, A.; Panés, J. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther. Onkol. 2012, 188, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Rühle, A.; Tkotsch, E.; Mravlag, R.; Haehl, E.; Spohn, S.K.B.; Zamboglou, C.; Huber, P.E.; Debus, J.; Grosu, A.L.; Sprave, T.; et al. Low-dose radiotherapy for painful osteoarthritis of the elderly: A multicenter analysis of 970 patients with 1185 treated sites. Strahlenther. Onkol. 2021, 197, 895–902. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdelmaqsoud, A.; Vorotniak, N.; Strauß, D.; Hentschel, B. The analgesic effect of low-dose radiotherapy in treating benign musculoskeletal painful disorders using different energies: A retrospective cohort study. JRP 2023, 22, e78. [Google Scholar] [CrossRef]
- Weissmann, T.; Rückert, M.; Zhou, J.G.; Seeling, M.; Lettmaier, S.; Donaubauer, A.J.; Nimmerjahn, F.; Ott, O.J.; Hecht, M.; Putz, F.; et al. Low-Dose Radiotherapy Leads to a Systemic Anti-Inflammatory Shift in the Pre-Clinical K/BxN Serum Transfer Model and Reduces Osteoarthritic Pain in Patients. Front. Immunol. 2022, 12, 777792. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Javadinia, S.A.; Nazeminezhad, N.; Ghahramani-Asl, R.; Soroosh, D.; Fazilat-Panah, D.; PeyroShabany, B.; Saberhosseini, S.N.; Mehrabian, A.; Taghizadeh-Hesary, F.; Nematshahi, M.; et al. Low-dose radiation therapy for osteoarthritis and enthesopathies: A review of current data. Int. J. Radiat. Biol. 2021, 97, 1352–1367. [Google Scholar] [CrossRef]
- van der Velden, J.M.; Peters, M.; Verlaan, J.J.; Versteeg, A.L.; Zhang, L.; Tsao, M.; Danjoux, C.; Barnes, E.; van Vulpen, M.; Chow, E.; et al. Development and Internal Validation of a Clinical Risk Score to Predict Pain Response After Palliative Radiation Therapy in Patients With Bone Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 859–866, Erratum in Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1186. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, C.; Almendros, S.; Navarro-Martin, A.; Gonzalez, J. Pain Flare-Effect Prophylaxis With Corticosteroids on Bone Radiotherapy Treatment: A Systematic Review. Pain Pract. 2020, 20, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Habberstad, R.; Frøseth, T.C.S.; Aass, N.; Bjerkeset, E.; Abramova, T.; Garcia-Alonso, E.; Caputo, M.; Rossi, R.; Boland, J.W.; Brunelli, C.; et al. Clinical Predictors for Analgesic Response to Radiotherapy in Patients with Painful Bone Metastases. J. Pain Symptom Manag. 2021, 62, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.M.; Maggiore, C.M.; Maltoni, M.; Rossi, R.; Nardi, E.; Zamagni, A.; Siepe, G.; Mammini, F.; Cellini, F.; Di Rito, A.; et al. Adequacy of Pain Management in Patients Referred for Radiation Therapy: A Subanalysis of the Multicenter ARISE-1 Study. Cancers 2023, 16, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delanian, S.; Lefaix, J.L.; Pradat, P.F. Radiation-induced neuropathy in cancer survivors. Radiother. Oncol. 2012, 105, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Fathers, E.; Thrush, D.; Huson, S.M.; Norman, A. Radiation-induced brachial plexopathy in women treated for carcinoma of the breast. Clin. Rehabil. 2002, 16, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, H.J.; Wotherspoon, A.; Denham, J.W.; Hauer-Jensen, M. Defining pelvic-radiation disease for the survivorship era. Lancet Oncol. 2010, 11, 310–312. [Google Scholar] [CrossRef]
- Elahi, F.; Callahan, D.; Greenlee, J.; Dann, T.L. Pudendal entrapment neuropathy: A rare complication of pelvic radiation therapy. Pain Physician 2013, 16, E793–E797. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for the Pharmacological and Radiotherapeutic Management of Cancer Pain in Adults and Adolescents; WHO: Geneva, Switzerland, 2018. [PubMed]
- Doo, A.R.; Shin, Y.S.; Yoo, S.; Park, J.K. Radiation-induced neuropathic pain successfully treated with systemic lidocaine administration. J. Pain Res. 2018, 11, 545–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moisset, X. Neuropathic pain: Evidence based recommendations. Presse Med. 2024, 53, 104232. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, I.T.; Su, P.F.; Huang, Y.T.; Chiu, G.L.; Chen, Y.Y.; Lai, W.S.; Lin, P.C. Lidocaine transdermal patches reduced pain intensity in neuropathic cancer patients already receiving opioid treatment. BMC Palliat. Care. 2023, 22, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guangmei, D.; Weishan, H.; Wenya, L.; Fasheng, W.; Jibing, C. Evolution of radiation-induced dermatitis treatment. Clin. Transl. Oncol. 2024, 26, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Dilalla, V.; Chaput, G.; Williams, T.; Sultanem, K. Radiotherapy side effects: Integrating a survivorship clinical lens to better serve patients. Curr. Oncol. 2020, 2, 107–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stubblefield, M.D. Cancer Rehabilitation: Principles and Practice, 2nd ed.; Demos Medical: New York, NY, USA, 2018. [Google Scholar]
- Pradat, P.F.; Bouche, P.; Delanian, S. Sciatic nerve moneuropathy: An unusual late effect of radiotherapy. Muscle Nerve 2009, 40, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tsai, W.; Sokolof, J. Sciatic Neuropathy After Radiation Treatment. Am. J. Phys. Med. Rehabil. 2021, 100, e198–e199. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.M.; Benson, J.C.; DeLone, D.R.; Diehn, F.E.; Kim, D.K.; Ma, D.; Nagelschneider, A.A.; Madhavan, A.A.; Johnson, D.R. Manifestations of radiation toxicity in the head, neck, and spine: An image-based review. Neuroradiol. J. 2022, 4, 427–436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chopra, S.; Kamdar, D.; Tulunay Ugur, O.E.; Chen, G.; Peshek, B.; Marunick, M.; Kim, H.; Lin, H.S.; Jacobs, J. Factors predictive of severity of osteoradionecrosis of the mandible. Head Neck 2011, 33, 1600–1605. [Google Scholar] [CrossRef]
- Alhilali, L.; Reynolds, A.R.; Fakhran, S. Osteoradionecrosis after radiation therapy for head and neck cancer: Differentiation from recurrent disease with CT and PET/CT imaging. Am. J. Neuroradiol. 2014, 35, 1405–1411. [Google Scholar] [CrossRef]
- Ito, K.; Nakajima, Y.; Ogawa, H.; Taguchi, K. Fracture risk following stereotactic body radiotherapy for long bone metastases. Jpn. J. Clin. Oncol. 2022, 52, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Demura, S.; Kato, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Oku, N.; Murakami, H.; Tsuchiya, H. Effects of Radiation on the Bone Strength of Spinal Vertebrae in Rats. Spine Phila Pa 1976 2022, 47, E514–E520. [Google Scholar] [CrossRef] [PubMed]
- Berk, L. The effects of high-dose radiation therapy on bone: A scoping review. Radiat. Oncol. J. 2024, 42, 95–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yaprak, G.; Gemici, C.; Temizkan, S.; Ozdemir, S.; Dogan, B.C.; Seseogullari, O.O. Osteoporosis development and vertebral fractures after abdominal irradiation in patients with gastric cancer. BMC Cancer 2018, 11, 972. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.J. Cancer treatment-induced bone loss. Korean J. Intern. Med. 2024, 39, 731–745. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adlakha, P.; Maheshwari, G.; Dhanawat, A.; Sinwer, R.; Singhal, M.; Jakhar, S.L.; Sharma, N.; Kumar, H.S. Comparison of two schedules of hypo-fractionated radiotherapy in locally advanced head-and-neck cancers. J. Cancer Res. Ther. 2022, 18, S151–S156. [Google Scholar] [CrossRef] [PubMed]
- Grewal, A.S.; Jones, J.; Lin, A. Palliative Radiation Therapy for Head and Neck Cancers. Int. J. Radiat. Oncol. Biol. Phys. 2019, 1, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Walker, J.; Heckler, C.E.; Morrow, G.R.; Ryan, J.L. Characterization of skin reactions and pain reported by patients receiving radiation therapy for cancer at different sites. J. Support. Oncol. 2013, 11, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.; Lee, J.E. Comparison between 1-week and 2-week palliative radiotherapy courses for superior vena cava syndrome. Radiat. Oncol. J. 2023, 41, 178–185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, J.; Yang, G.; An, W.; Wang, W.; Li, F.; Meng, Y.; Wang, X. Correlations between the severity of radiation-induced oral mucositis and salivary epidermal growth factor as well as inflammatory cytokines in patients with head and neck cancer. Head Neck 2023, 45, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Young, J.; Rattan, D.; Cheung, A.; Lazarakis, S.; McGilvray, S. Pain management for persistent pain post radiotherapy in head and neck cancers: Systematic review. Scand. J. Pain 2023, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Wong, G.; Zhang, L.; Drost, L.; Karam, I.; Yee, C.; McCurdy-Franks, E.; Razvi, Y.; Ariello, K.; Wan, B.A.; et al. Self-reported pain in breast cancer patients receiving adjuvant radiotherapy. Support. Care Cancer 2021, 29, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.A.; Pidduck, W.; Zhang, L.; Nolen, A.; Yee, C.; Wang, K.; Chow, S.; Chan, S.; Drost, L.; Soliman, H.; et al. Patient-Reported Pain in Patients with Breast Cancer Who Receive Radiotherapy. Pain Manag. Nurs. 2021, 22, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Buwenge, M.; Macchia, G.; Arcelli, A.; Frakulli, R.; Fuccio, L.; Guerri, S.; Grassi, E.; Cammelli, S.; Cellini, F.; Morganti, A.G. Stereotactic radiotherapy of pancreatic cancer: A systematic review on pain relief. J. Pain Res. 2018, 4, 2169–2178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedes, C.; Butala, A.A. Palliative radiotherapy for pancreatic cancer. Ann. Palliat. Med. 2024, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Biran, A.; Bolnykh, I.; Rimmer, B.; Cunliffe, A.; Durrant, L.; Hancock, J.; Ludlow, H.; Pedley, I.; Rees, C.; Sharp, L. A Systematic Review of Population-Based Studies of Chronic Bowel Symptoms in Cancer Survivors following Pelvic Radiotherapy. Cancers 2023, 15, 4037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisson, E.; Piton, L.; Durand, B.; Sarrade, T.; Huguet, F. Palliative pelvic radiotherapy for symptomatic frail or metastatic patients with rectal adenocarcinoma: A systematic review. Dig. Liver Dis. 2025, 57, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Dhanachai, M.; Theerapancharoen, V.; Laothamatas, J.; Pongpech, S.; Kraiphibul, P.; Chanwitayanuchit, T.; Pochanugool, L.; Dangprasert, S.; Sarnvivad, P.; Sinpornchai, V.; et al. Early neurological complications after stereotactic radiosurgery/radiotherapy. J. Med. Assoc. Thai. 2001, 84, 1729–1737. [Google Scholar] [PubMed]
- Zaki, P.; Barbour, A.; Zaki, M.M.; Tseng, Y.D.; Amin, A.G.; Venur, V.; McGranahan, T.; Vellayappan, B.; Palmer, J.D.; Chao, S.T.; et al. Emergent radiotherapy for spinal cord compression/impingement—A narrative review. Ann. Palliat. Med. 2023, 12, 1447–1462. [Google Scholar] [CrossRef] [PubMed]
- Gojsevic, M.; Shariati, S.; Chan, A.W.; Bonomo, P.; Zhang, E.; Kennedy, S.K.F.; Rajeswaran, T.; Rades, D.; Vassiliou, V.; Soliman, H.; et al. Quality of life in patients with malignant spinal cord compression: A systematic review. Support. Care Cancer 2023, 31, 736. [Google Scholar] [CrossRef] [PubMed]
- Vistad, I.; Cvancarova, M.; Kristensen, G.B.; Fosså, S.D. A study of chronic pelvic pain after radiotherapy in survivors of locally advanced cervical cancer. J. Cancer Surviv. 2011, 5, 208–216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robijns, J.; Censabella, S.; Bollen, H.; Claes, S.; Van Bever, L.; Becker, J.; Pannekoeke, L.; Bulens, P.; Van de Werf, E. Vaginal mucositis in patients with gynaecological cancer undergoing (chemo-)radiotherapy: A retrospective analysis. J. Obstet. Gynaecol. 2022, 42, 2156–2163. [Google Scholar] [CrossRef] [PubMed]
- Loblaw, D.A.; Wu, J.S.; Kirkbride, P.; Panzarella, T.; Smith, K.; Aslanidis, J.; Warde, P. Pain flare in patients with bone metastases after palliative radiotherapy—A nested randomized control trial. Support. Care Cancer 2007, 15, 451–455. [Google Scholar] [CrossRef]
- Chow, E.; Ling, A.; Davis, L.; Panzarella, T.; Danjoux, C. Pain flare following external beam radiotherapy and meaningful change in pain scores in the treatment of bone metastases. Radiother. Oncol. 2005, 75, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hird, A.; Chow, E.; Zhang, L.; Wong, R.; Wu, J.; Sinclair, E.; Danjoux, C.; Tsao, M.; Barnes, E.; Loblaw, A. Determining the incidence of pain flare following palliative radiotherapy for symptomatic bone metastases: Results from three canadian cancer centers. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 193–197. [Google Scholar] [CrossRef]
- Chow, E.; Meyer, R.M.; Ding, K.; Nabid, A.; Chabot, P.; Wong, P.; Ahmed, S.; Kuk, J.; Dar, A.R.; Mahmud, A.; et al. Dexamethasone in the prophylaxis of radiation-induced pain flare after palliative radiotherapy for bone metastases: A double-blind, randomised placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 1463–1472. [Google Scholar] [CrossRef]
- Sheng, K. Artificial intelligence in radiotherapy: A technological review. Front. Med. 2020, 14, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Giraud, P.; Bibault, J.E. Artificial intelligence in radiotherapy: Current applications and future trends. Diagn. Interv. Imaging. 2024, 105, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ma, C.; Hu, M.; Qiu, R.L.J.; Salari, E.; Martini, R.; Yang, X. Machine learning in image-based outcome prediction after radiotherapy: A review. J. Appl. Clin. Med. Phys. 2025, 26, e14559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Type of Brachytherapy | Location of Applicator |
|---|---|
| interstitial | The applicator is placed inside the tumor, e.g., prostate cancer [9] |
| surface | A contact applicator is used in the treatment of skin cancers [10] |
| intracavitary | The radiation source is placed within body cavities, e.g., in the uterus, oral cavity cancers, or spinal canal cancers [11] |
| intraluminal | An applicator is inserted into the lumen of a cancer-infiltrated bronchus, e.g., irradiation of an intrabronchial lesion leads to its reduction and improved bronchial patency, thereby decreasing dyspnea and cancer pain [12] |
| intraoperative | An applicator is placed in the post-operative cavity, e.g., following removal of a breast tumor [13] |
| Radiotherapy as a Double-Edged Sword—Examples | |
|---|---|
| Analgesic in: | Pain Factor: |
|
|
| |
| |
|
|
|
|
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka-Filippow, M.; Politynska, B.; Wojtukiewicz, A.M.; Wojtukiewicz, M.Z. Cancer Pain: Radiotherapy as a Double-Edged Sword. Int. J. Mol. Sci. 2025, 26, 5223. https://doi.org/10.3390/ijms26115223
Konopka-Filippow M, Politynska B, Wojtukiewicz AM, Wojtukiewicz MZ. Cancer Pain: Radiotherapy as a Double-Edged Sword. International Journal of Molecular Sciences. 2025; 26(11):5223. https://doi.org/10.3390/ijms26115223
Chicago/Turabian StyleKonopka-Filippow, Monika, Barbara Politynska, Anna M. Wojtukiewicz, and Marek Z. Wojtukiewicz. 2025. "Cancer Pain: Radiotherapy as a Double-Edged Sword" International Journal of Molecular Sciences 26, no. 11: 5223. https://doi.org/10.3390/ijms26115223
APA StyleKonopka-Filippow, M., Politynska, B., Wojtukiewicz, A. M., & Wojtukiewicz, M. Z. (2025). Cancer Pain: Radiotherapy as a Double-Edged Sword. International Journal of Molecular Sciences, 26(11), 5223. https://doi.org/10.3390/ijms26115223






