Eyes Are the Windows to the Soul: Reviewing the Possible Use of the Retina to Indicate Traumatic Brain Injury
Abstract
1. Introduction
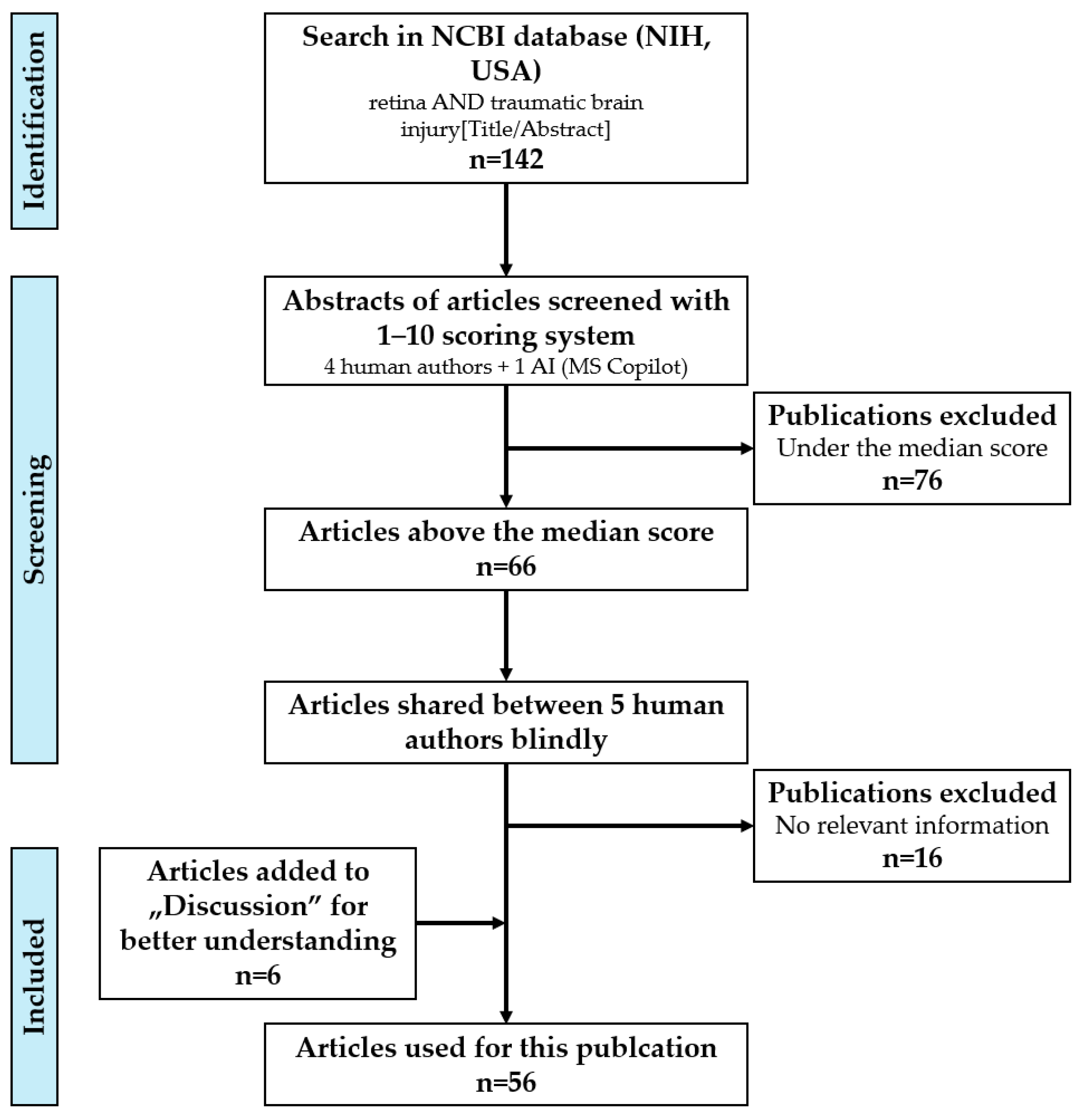
2. Methods
2.1. Searching, Scoring, and Processing of Articles
2.2. Figures
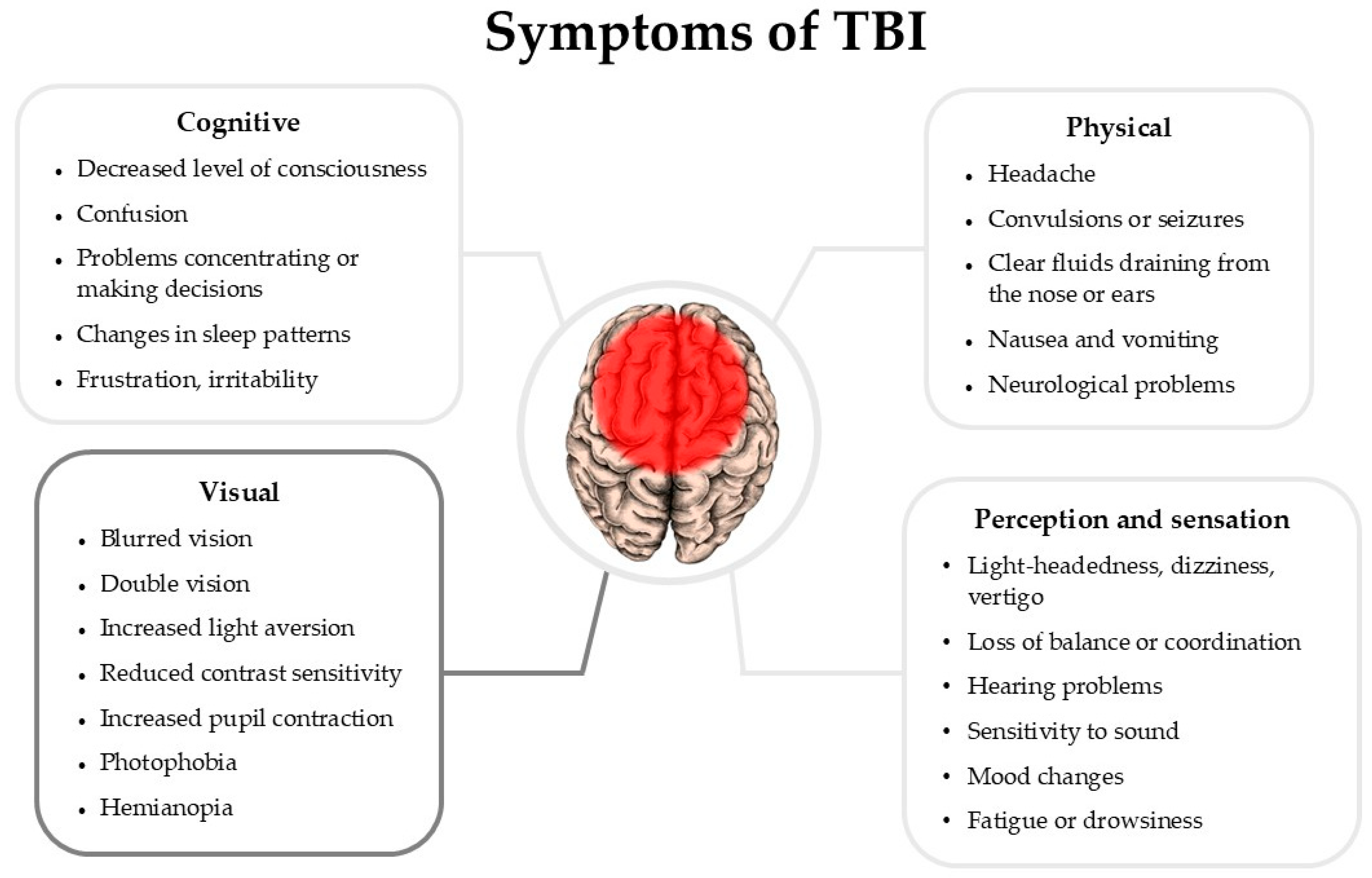
3. Manifestations of TBI in Patients
4. Effects of TBI on the Visual System
5. Cellular and Molecular Markers of TBI in the Retina
| Marker | Function | Study |
|---|---|---|
| GFAP (glial fibrillary acidic protein) | Indicates Müller glia activation in the retina post-TBI or acoustic blast overpressure | [17,31,32] |
| IBA1 (ionized calcium-binding adapter molecule 1) | Reflects microglial activation and inflammation post-TBI | [2,17,31] |
| CD68 (Cluster of Differentiation 68) | Marker of pro-inflammatory microglial activation and indicates phagocytic response in traumatic axonopathy | [17,37] |
| Phosphorylated tau | Indicates neurodegeneration; associated with neurofibrillary tangles | [17] |
| LPA (lysophosphatidic acid) | Induces inflammatory processes, astrocyte proliferation, and tau phosphorylation | [33,41,42] |
| IL-1B, IL-1a, IL-6, TNF (interleukin; tumor necrosis factor) | Cytokines involved in inflammation and oxidative stress post-TBI | [31] |
| KLF4 (Kruppel-like factor 4) | Inhibits pro-survival STAT3 and triggers pro-apoptotic p53 in RGCs | [34] |
| CCL20 (chemokine (C-C motif) ligand 20) | Involved in neurodegeneration and inflammation post-TBI | [35] |
| Caspase-3 | Marker of apoptosis, expressed in astrocytes in retinal damage | [36] |
| Complement C3 | Deposits in retinogeniculate synapses post-TBI; inhibition is neuroprotective | [27] |
| β-amyloid and 4HNE (4-hydroxy-trans-2-nonenal) | Oxidative stress markers post-TBI | [38] |
| PTEN (phosphatase and tensin homolog) | Downregulation promotes regeneration of α RGCs | [40] |
| Osteopontin and IGF-1 (insulin-like growth factor 1) | Enhances RGC regeneration | [40] |
6. Possible Translational Implementation of Retinal Markers in TBI
6.1. The Importance of Early TBI Detection
6.2. Methods for Early TBI Detection
6.3. Possible Interventions for Early TBI Treatment
7. Discussion
8. Conclusions
Funding
Conflicts of Interest
References
- National Institue of Neurological Disorders and Stroke. Traumatic Brain Injury (TBI); National Institue of Neurological Disorders and Stroke: Bethesda, MD, USA, 2024. Available online: https://www.ninds.nih.gov/health-information/disorders/traumatic-brain-injury-tbi# (accessed on 13 March 2025).
- Honig, M.G.; Del Mar, N.A.; Henderson, D.L.; Ragsdale, T.D.; Doty, J.B.; Driver, J.H.; Li, C.; Fortugno, A.P.; Mitchell, W.M.; Perry, A.M.; et al. Amelioration of Visual Deficits and Visual System Pathology after Mild TBI via the Cannabinoid Type-2 Receptor Inverse Agonism of Raloxifene. Exp. Neurol. 2019, 322, 113063. [Google Scholar] [CrossRef] [PubMed]
- Waddell, P.A.; Gronwall, D.M.A. Sensitivity to Light and Sound Following Minor Head Injury. Acta Neurol. Scand. 1984, 69, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, P.T.; Shorter, P.D.; McDaniel, C.E.; Earley, M.J.; Hartwick, A.T.E. Blue and Red Light-Evoked Pupil Responses in Photophobic Subjects with TBI. Optom. Vis. Sci. 2017, 94, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Decramer, T.; Van Keer, K.; Stalmans, P.; Dupont, P.; Sunaert, S.; Theys, T. Tracking Posttraumatic Hemianopia. J. Neurol. 2018, 265, 41–45. [Google Scholar] [CrossRef]
- Honig, M.G.; Del Mar, N.A.; Henderson, D.L.; O’Neal, D.; Yammanur, M.; Cox, R.; Li, C.; Perry, A.M.; Moore, B.M.; Reiner, A. Raloxifene, a Cannabinoid Type-2 Receptor Inverse Agonist, Mitigates Visual Deficits and Pathology and Modulates Microglia after Ocular Blast. Exp. Eye Res. 2022, 218, 108966. [Google Scholar] [CrossRef]
- Lemke, S.; Cockerham, G.C.; Glynn-Milley, C.; Lin, R.; Cockerham, K.P. Automated Perimetry and Visual Dysfunction in Blast-Related Traumatic Brain Injury. Ophthalmology 2016, 123, 415–424. [Google Scholar] [CrossRef]
- Lyons, H.S.; Sassani, M.; Hyder, Y.; Mitchell, J.L.; Thaller, M.; Mollan, S.P.; Sinclair, A.J.; mTBI Predict Consortium; Sinclair, A.; Finch, A.; et al. A Systematic Review of Optical Coherence Tomography Findings in Adults with Mild Traumatic Brain Injury. Eye 2024, 38, 1077–1083. [Google Scholar] [CrossRef]
- Mufti, O.; Mathew, S.; Harris, A.; Siesky, B.; Burgett, K.M.; Verticchio Vercellin, A.C. Ocular Changes in Traumatic Brain Injury: A Review. Eur. J. Ophthalmol. 2020, 30, 867–873. [Google Scholar] [CrossRef]
- Evans, L.P.; Roghair, A.M.; Gilkes, N.J.; Bassuk, A.G. Visual Outcomes in Experimental Rodent Models of Blast-Mediated Traumatic Brain Injury. Front. Mol. Neurosci. 2021, 14, 659576. [Google Scholar] [CrossRef]
- Das, M.; Tang, X.; Mohapatra, S.S.; Mohapatra, S. Vision Impairment after Traumatic Brain Injury: Present Knowledge and Future Directions. Rev. Neurosci. 2019, 30, 305–315. [Google Scholar] [CrossRef]
- Ryan, A.K.; Rich, W.; Reilly, M.A. Oxidative Stress in the Brain and Retina after Traumatic Injury. Front. Neurosci. 2023, 17, 1021152. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, C.N.; Watson, J.B.; Higgins, E.K.; Quan, N.; Bachstetter, A.D. Inflammatory Regulation of CNS Barriers After Traumatic Brain Injury: A Tale Directed by Interleukin-1. Front. Immunol. 2021, 12, 688254. [Google Scholar] [CrossRef] [PubMed]
- Rauchman, S.H.; Albert, J.; Pinkhasov, A.; Reiss, A.B. Mild-to-Moderate Traumatic Brain Injury: A Review with Focus on the Visual System. Neurol. Int. 2022, 14, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Qiu, T.; Xiao, Z. Photophobia in Headache Disorders: Characteristics and Potential Mechanisms. J. Neurol. 2022, 269, 4055–4067. [Google Scholar] [CrossRef]
- Xu, L.; Nguyen, J.V.; Lehar, M.; Menon, A.; Rha, E.; Arena, J.; Ryu, J.; Marsh-Armstrong, N.; Marmarou, C.R.; Koliatsos, V.E. Repetitive Mild Traumatic Brain Injury with Impact Acceleration in the Mouse: Multifocal Axonopathy, Neuroinflammation, and Neurodegeneration in the Visual System. Exp. Neurol. 2016, 275, 436–449. [Google Scholar] [CrossRef]
- Mammadova, N.; Ghaisas, S.; Zenitsky, G.; Sakaguchi, D.S.; Kanthasamy, A.G.; Greenlee, J.J.; West Greenlee, M.H. Lasting Retinal Injury in a Mouse Model of Blast-Induced Trauma. Am. J. Pathol. 2017, 187, 1459–1472. [Google Scholar] [CrossRef]
- Wang, J.; Fox, M.A.; Povlishock, J.T. Diffuse Traumatic Axonal Injury in the Optic Nerve Does Not Elicit Retinal Ganglion Cell Loss. J. Neuropathol. Exp. Neurol. 2013, 72, 768–781. [Google Scholar] [CrossRef]
- Dutca, L.M.; Stasheff, S.F.; Hedberg-Buenz, A.; Rudd, D.S.; Batra, N.; Blodi, F.R.; Yorek, M.S.; Yin, T.; Shankar, M.; Herlein, J.A.; et al. Early Detection of Subclinical Visual Damage After Blast-Mediated TBI Enables Prevention of Chronic Visual Deficit by Treatment with P7C3-S243. Investig. Ophthalmol. Vis. Sci. 2014, 55, 8330–8341. [Google Scholar] [CrossRef]
- Harper, M.M.; Gramlich, O.W.; Elwood, B.W.; Boehme, N.A.; Dutca, L.M.; Kuehn, M.H. Immune Responses in Mice after Blast-Mediated Traumatic Brain Injury TBI Autonomously Contribute to Retinal Ganglion Cell Dysfunction and Death. Exp. Eye Res. 2022, 225, 109272. [Google Scholar] [CrossRef]
- Stern-Green, E.A.; Klimo, K.R.; Day, E.; Shelton, E.R.; Robich, M.L.; Jordan, L.A.; Racine, J.; VanNasdale, D.A.; McDaniel, C.E.; Yuhas, P.T. Henle Fiber Layer Thickening and Deficits in Objective Retinal Function in Participants with a History of Multiple Traumatic Brain Injuries. Front. Neurol. 2024, 15, 1330440. [Google Scholar] [CrossRef]
- Chan, J.W.; Hills, N.K.; Bakall, B.; Fernandez, B. Indirect Traumatic Optic Neuropathy in Mild Chronic Traumatic Brain Injury. Investig. Opthalmol. Vis. Sci. 2019, 60, 2005. [Google Scholar] [CrossRef] [PubMed]
- Honig, M.G.; Del Mar, N.A.; Henderson, D.L.; O’Neal, D.; Doty, J.B.; Cox, R.; Li, C.; Perry, A.M.; Moore, B.M.; Reiner, A. Raloxifene Modulates Microglia and Rescues Visual Deficits and Pathology After Impact Traumatic Brain Injury. Front. Neurosci. 2021, 15, 701317. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Palacios, K.; Vásquez-García, S.; Fariyike, O.A.; Robba, C.; Rubiano, A.M.; The Noninvasive ICP Monitoring International Consensus Group; Taccone, F.S.; Rasulo, F.; Badenes, R.R.; Menon, D.; et al. Using Optic Nerve Sheath Diameter for Intracranial Pressure (ICP) Monitoring in Traumatic Brain Injury: A Scoping Review. Neurocrit. Care 2024, 40, 1193–1212. [Google Scholar] [CrossRef]
- Tzekov, R.; Quezada, A.; Gautier, M.; Biggins, D.; Frances, C.; Mouzon, B.; Jamison, J.; Mullan, M.; Crawford, F. Repetitive Mild Traumatic Brain Injury Causes Optic Nerve and Retinal Damage in a Mouse Model. J. Neuropathol. Exp. Neurol. 2014, 73, 345–361. [Google Scholar] [CrossRef]
- Evans, L.P.; Newell, E.A.; Mahajan, M.; Tsang, S.H.; Ferguson, P.J.; Mahoney, J.; Hue, C.D.; Vogel, E.W.; Morrison, B.; Arancio, O.; et al. Acute Vitreoretinal Trauma and Inflammation after Traumatic Brain Injury in Mice. Ann. Clin. Transl. Neurol. 2018, 5, 240–251. [Google Scholar] [CrossRef]
- Borucki, D.M.; Rohrer, B.; Tomlinson, S. Complement Propagates Visual System Pathology Following Traumatic Brain Injury. J. Neuroinflamm. 2024, 21, 98. [Google Scholar] [CrossRef]
- Morriss, N.J.; Conley, G.M.; Hodgson, N.; Boucher, M.; Ospina-Mora, S.; Fagiolini, M.; Puder, M.; Mejia, L.; Qiu, J.; Meehan, W.; et al. Visual Dysfunction after Repetitive Mild Traumatic Brain Injury in a Mouse Model and Ramifications on Behavioral Metrics. J. Neurotrauma 2021, 38, 2881–2895. [Google Scholar] [CrossRef]
- Elenberger, J.; Kim, B.; De Castro-Abeger, A.; Rex, T.S. Connections between Intrinsically Photosensitive Retinal Ganglion Cells and TBI Symptoms. Neurology 2020, 95, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C.W.; Likova, L.T. Brain Trauma Impacts Retinal Processing: Photoreceptor Pathway Interactions in Traumatic Light Sensitivity. Doc. Ophthalmol. 2022, 144, 179–190. [Google Scholar] [CrossRef]
- Evans, L.P.; Woll, A.W.; Wu, S.; Todd, B.P.; Hehr, N.; Hedberg-Buenz, A.; Anderson, M.G.; Newell, E.A.; Ferguson, P.J.; Mahajan, V.B.; et al. Modulation of Post-Traumatic Immune Response Using the IL-1 Receptor Antagonist Anakinra for Improved Visual Outcomes. J. Neurotrauma 2020, 37, 1463–1480. [Google Scholar] [CrossRef]
- Skelton, L.A.; Ramachandra Rao, S.; Allen, R.S.; Motz, C.T.; Pardue, M.T.; Fliesler, S.J. Retinal Gliosis and Phenotypic Diversity of Intermediate Filament Induction and Remodeling upon Acoustic Blast Overpressure (ABO) Exposure to the Rat Eye. Exp. Eye Res. 2023, 234, 109585. [Google Scholar] [CrossRef] [PubMed]
- Arun, P.; Rossetti, F.; DeMar, J.C.; Wang, Y.; Batuure, A.B.; Wilder, D.M.; Gist, I.D.; Morris, A.J.; Sabbadini, R.A.; Long, J.B. Antibodies Against Lysophosphatidic Acid Protect Against Blast-Induced Ocular Injuries. Front. Neurol. 2020, 11, 611816. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Zeng, T.; Ren, J.; Wang, K.; Jin, Y.; Zhou, L.; Gao, L. KLF 4 Knockdown Attenuates TBI-Induced Neuronal Damage through P53 and JAK—STAT 3 Signaling. CNS Neurosci. Ther. 2017, 23, 106–118. [Google Scholar] [CrossRef]
- Das, M.; Tang, X.; Han, J.Y.; Mayilsamy, K.; Foran, E.; Biswal, M.R.; Tzekov, R.; Mohapatra, S.S.; Mohapatra, S. CCL20-CCR6 Axis Modulated Traumatic Brain Injury-Induced Visual Pathologies. J. Neuroinflamm. 2019, 16, 115. [Google Scholar] [CrossRef]
- Kovács-Öller, T.; Zempléni, R.; Balogh, B.; Szarka, G.; Fazekas, B.; Tengölics, Á.J.; Amrein, K.; Czeiter, E.; Hernádi, I.; Büki, A.; et al. Traumatic Brain Injury Induces Microglial and Caspase3 Activation in the Retina. Int. J. Mol. Sci. 2023, 24, 4451. [Google Scholar] [CrossRef]
- Alexandris, A.S.; Lee, Y.; Lehar, M.; Alam, Z.; McKenney, J.; Perdomo, D.; Ryu, J.; Welsbie, D.; Zack, D.J.; Koliatsos, V.E. Traumatic Axonal Injury in the Optic Nerve: The Selective Role of SARM1 in the Evolution of Distal Axonopathy. J. Neurotrauma 2023, 40, 1743–1761. [Google Scholar] [CrossRef]
- Mohan, K.; Kecova, H.; Hernandez-Merino, E.; Kardon, R.H.; Harper, M.M. Retinal Ganglion Cell Damage in an Experimental Rodent Model of Blast-Mediated Traumatic Brain Injury. Investig. Opthalmol. Vis. Sci. 2013, 54, 3440. [Google Scholar] [CrossRef]
- Xu, L.; Ryu, J.; Nguyen, J.V.; Arena, J.; Rha, E.; Vranis, P.; Hitt, D.; Marsh-Armstrong, N.; Koliatsos, V.E. Evidence for Accelerated Tauopathy in the Retina of Transgenic P301S Tau Mice Exposed to Repetitive Mild Traumatic Brain Injury. Exp. Neurol. 2015, 273, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Crair, M.C.; Mason, C.A. Reconnecting Eye to Brain. J. Neurosci. 2016, 36, 10707–10722. [Google Scholar] [CrossRef]
- Shano, S.; Moriyama, R.; Chun, J.; Fukushima, N. Lysophosphatidic Acid Stimulates Astrocyte Proliferation through LPA1. Neurochem. Int. 2008, 52, 216–220. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ariaens, A.; Ponsioen, B.; Moolenaar, W.H. GSK-3 Is Activated by the Tyrosine Kinase Pyk2 during LPA1-Mediated Neurite Retraction. Mol. Biol. Cell 2006, 17, 1834–1844. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, C.K.; Stagner, A.M. The Eyes Have It: How Critical Are Ophthalmic Findings to the Diagnosis of Pediatric Abusive Head Trauma? Semin. Ophthalmol. 2023, 38, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; Hedberg-Buenz, A.; Herlein, J.; Abrahamson, E.E.; Anderson, M.G.; Kuehn, M.H.; Kardon, R.H.; Poolman, P.; Ikonomovic, M.D. Blast-Mediated Traumatic Brain Injury Exacerbates Retinal Damage and Amyloidosis in the APPswePSENd19e Mouse Model of Alzheimer’s Disease. Investig. Opthalmol. Vis. Sci. 2019, 60, 2716. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Thamaraikani, T.; Vellapandian, C. A Review of the Retinal Impact of Traumatic Brain Injury and Alzheimer’s Disease: Exploring Inflammasome Complexes and Nerve Fiber Layer Alterations. Cureus 2024, 16, e67093. [Google Scholar] [CrossRef]
- Kumar Das, N.; Das, M. Structural Changes in Retina (Retinal Nerve Fiber Layer) Following Mild Traumatic Brain Injury and Its Association with Development of Visual Field Defects. Clin. Neurol. Neurosurg. 2022, 212, 107080. [Google Scholar] [CrossRef]
- Banbury, C.; Styles, I.; Eisenstein, N.; Zanier, E.R.; Vegliante, G.; Belli, A.; Logan, A.; Goldberg Oppenheimer, P. Spectroscopic Detection of Traumatic Brain Injury Severity and Biochemistry from the Retina. Biomed. Opt. Express 2020, 11, 6249. [Google Scholar] [CrossRef]
- Sabeti, F.; Carle, C.F.; Jaros, R.K.; Rohan, E.M.F.; Waddington, G.; Lueck, C.J.; Hughes, D.; Maddess, T. Objective Perimetry in Sporting-Related Mild Traumatic Brain Injury. Ophthalmology 2019, 126, 1053–1055. [Google Scholar] [CrossRef]
- Wang, Q.; Fan, W.; Cai, Y.; Wu, Q.; Mo, L.; Huang, Z.; Huang, H. Protective Effects of Taurine in Traumatic Brain Injury via Mitochondria and Cerebral Blood Flow. Amino Acids 2016, 48, 2169–2177. [Google Scholar] [CrossRef]
- Merezhinskaya, N.; Mallia, R.K.; Park, D.; Bryden, D.W.; Mathur, K.; Barker, F.M. Visual Deficits and Dysfunctions Associated with Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Optom. Vis. Sci. 2019, 96, 542–555. [Google Scholar] [CrossRef]
- Viano, D.C.; Parenteau, C.S.; Xu, L.; Faul, M. Head Injuries (TBI) to Adults and Children in Motor Vehicle Crashes. Traffic Inj. Prev. 2017, 18, 616–622. [Google Scholar] [CrossRef]
- Baker, C.E.; Martin, P.; Wilson, M.H.; Ghajari, M.; Sharp, D.J. The Relationship between Road Traffic Collision Dynamics and Traumatic Brain Injury Pathology. Brain Commun. 2022, 4, fcac033. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Zhang, M.; Lee, D.M.W.; Snyder, V.C.; Raghuraman, R.; Gofas-Salas, E.; Mecê, P.; Yadav, S.; Tiruveedhula, P.; Grieve, K.; et al. Label-Free Imaging of Inflammation at the Level of Single Cells in the Living Human Eye. Ophthalmol. Sci. 2024, 4, 100475. [Google Scholar] [CrossRef] [PubMed]
- Hammer, D.X.; Kovalick, K.; Liu, Z.; Chen, C.; Saeedi, O.J.; Harrison, D.M. Cellular-Level Visualization of Retinal Pathology in Multiple Sclerosis With Adaptive Optics. Investig. Opthalmol. Vis. Sci. 2023, 64, 21. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.Y.; Eldahshan, W.; Narayanan, S.P.; Caldwell, R.W.; Caldwell, R.B. Arginase Pathway in Acute Retina and Brain Injury: Therapeutic Opportunities and Unexplored Avenues. Front. Pharmacol. 2020, 11, 277. [Google Scholar] [CrossRef]
- Saliman, N.H.; Belli, A.; Blanch, R.J. Afferent Visual Manifestations of Traumatic Brain Injury. J. Neurotrauma 2021, 38, 2778–2789. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péntek, L.; Szarka, G.; Ross, L.; Balogh, B.; Telkes, I.; Völgyi, B.; Kovács-Öller, T. Eyes Are the Windows to the Soul: Reviewing the Possible Use of the Retina to Indicate Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 5171. https://doi.org/10.3390/ijms26115171
Péntek L, Szarka G, Ross L, Balogh B, Telkes I, Völgyi B, Kovács-Öller T. Eyes Are the Windows to the Soul: Reviewing the Possible Use of the Retina to Indicate Traumatic Brain Injury. International Journal of Molecular Sciences. 2025; 26(11):5171. https://doi.org/10.3390/ijms26115171
Chicago/Turabian StylePéntek, Loretta, Gergely Szarka, Liliana Ross, Boglárka Balogh, Ildikó Telkes, Béla Völgyi, and Tamás Kovács-Öller. 2025. "Eyes Are the Windows to the Soul: Reviewing the Possible Use of the Retina to Indicate Traumatic Brain Injury" International Journal of Molecular Sciences 26, no. 11: 5171. https://doi.org/10.3390/ijms26115171
APA StylePéntek, L., Szarka, G., Ross, L., Balogh, B., Telkes, I., Völgyi, B., & Kovács-Öller, T. (2025). Eyes Are the Windows to the Soul: Reviewing the Possible Use of the Retina to Indicate Traumatic Brain Injury. International Journal of Molecular Sciences, 26(11), 5171. https://doi.org/10.3390/ijms26115171







