Modeling Human Airway Epithelial Barrier Penetration Using Birch Bet v 1 and Alder Aln g 1 Pollen Allergens During Sensitization Process
Abstract
1. Introduction
2. Results
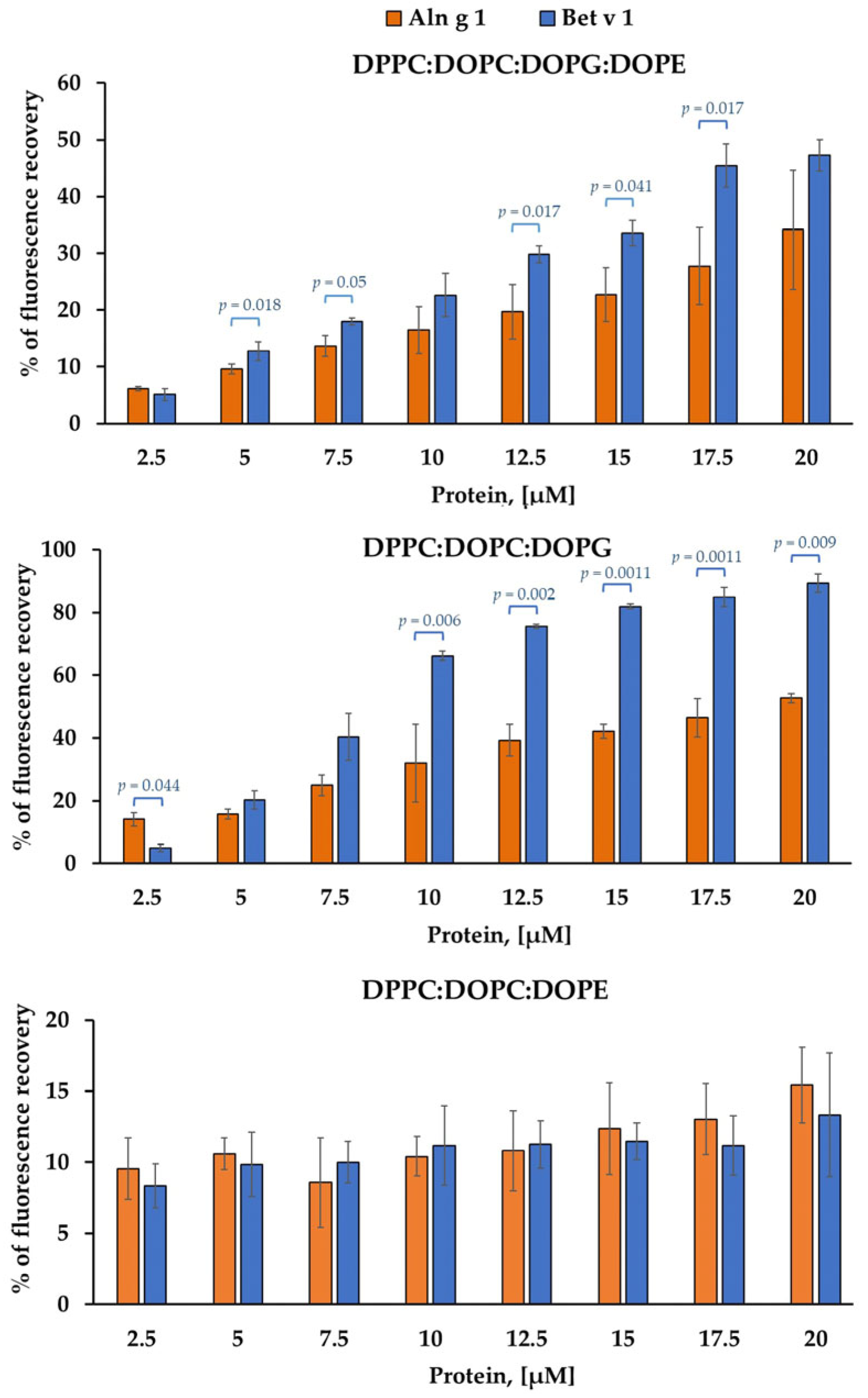
2.1. Calcein-Leakage Induced by Allergens
2.2. Transport of Aln g 1 and Bet v 1 Across the Calu-3 Epithelial Barrier
2.3. Influence on the Expression of Alarmin Genes in Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Cell Lines and Cultures
4.3. Stimulation of Calu-3 Cells with Aln g 1 and Bet v 1
4.4. RNA Extraction
4.5. Real-Time PCR
4.6. Preparation of Calcein-Loaded Liposomes and Leakage Experiments
4.7. Dynamic Light Scattering
4.8. Labeling of Aln g 1 with FITC
4.9. Transport of FITC-Aln g 1 Across the Calu-3 Epithelial Barrier
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPG | 1,2-dioleoyl-sn-glycero-3-phosphoglycerol |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| LTPs | Lipid transfer proteins |
| OVA | Ovalbumin |
| SUVs | Small unilamellar vesicles |
| TEER | Transepithelial electrical resistance |
References
- Pawankar, R. Allergic Diseases and Asthma: A Global Public Health Concern and a Call to Action. World Allergy Organ. J. 2014, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.W. Pollen Overload: Seasonal Allergies in a Changing Climate. Environ. Health Perspect. 2016, 125, A70–A75. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; van Cauwenberge, P. Allergenic Pollen and Pollen Allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant Pathogenesis-Related Proteins PR-10 and PR-14 as Components of Innate Immunity System and Ubiquitous Allergens. Curr. Med. Chem. 2017, 24, 1772–1787. [Google Scholar] [CrossRef]
- Biedermann, T.; Winther, L.; Till, S.; Panzner, P.; Knulst, A.; Valovirta, E. Birch Pollen Allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Polak, D.; Vollmann, U.; Grilo, J.; Bogdanov, I.V.; Aglas, L.; Ovchinnikova, T.V.; Ferreira, F.; Bohle, B. Bet v 1-Independent Sensitization to Major Allergens in Fagales Pollen: Evidence at the T-Cell Level. Allergy 2022, 78, 743–751. [Google Scholar] [CrossRef]
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial Cells: Liaisons of Immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef]
- Joenväärä, S.; Mattila, P.; Renkonen, J.; Mäkitie, A.; Toppila-Salmi, S.; Lehtonen, M.; Salmi, P.; Lehti, S.; Mäkinen, J.; Sormunen, R.; et al. Caveolar Transport through Nasal Epithelium of Birch Pollen Allergen Bet v 1 in Allergic Patients. J. Allergy Clin. Immunol. 2009, 124, 135–142.e21. [Google Scholar] [CrossRef]
- Guryanova, S.V.; Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Bohle, B.; Ovchinnikova, T.V. How Do Pollen Allergens Sensitize? Front. Mol. Biosci. 2022, 9, 900533. [Google Scholar] [CrossRef]
- Camelo, A.; Rosignoli, G.; Ohne, Y.; Stewart, R.A.; Overed-Sayer, C.; Sleeman, M.A.; May, R.D. IL-33, IL-25, and TSLP Induce a Distinct Phenotypic and Activation Profile in Human Type 2 Innate Lymphoid Cells. Blood Adv. 2017, 1, 577–589. [Google Scholar] [CrossRef]
- Melnikova, D.N.; Finkina, E.I.; Potapov, A.E.; Danilova, Y.D.; Toropygin, I.Y.; Matveevskaya, N.S.; Ovchinnikova, T.V.; Bogdanov, I.V. Structural and Immunological Features of PR-10 Allergens: Focusing on the Major Alder Pollen Allergen Aln g 1. Int. J. Mol. Sci. 2024, 25, 4965. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-R.; Wei, S.-Z.; Song, X.-Y.; Wang, Y.; Zhang, W.-B.; Ren, C.; Mou, Y.-K.; Song, X.-C. IL-1β and Allergy: Focusing on Its Role in Allergic Rhinitis. Mediat. Inflamm. 2023, 2023, 1265449. [Google Scholar] [CrossRef] [PubMed]
- Pelst, M.; Höbart, C.; de Rooster, H.; Devriendt, B.; Cox, E. Immortalised Canine Buccal Epithelial Cells’ CXCL8 Secretion Is Affected by Allergen Extracts, Toll-like Receptor Ligands, IL-17A and Calcitriol. Vet. Res. 2022, 53, 72. [Google Scholar] [CrossRef] [PubMed]
- Tomee, J.F.; van Weissenbruch, R.; de Monchy, J.G.; Kauffman, H.F. Interactions between Inhalant Allergen Extracts and Airway Epithelial Cells: Effect on Cytokine Production and Cell Detachment. J. Allergy Clin. Immunol. 1998, 102, 75–85. [Google Scholar] [CrossRef]
- Shelley, S.A.; Balis, J.U.; Paciga, J.E.; Espinoza, C.G.; Richman, A.V. Biochemical Composition of Adult Human Lung Surfactant. Lung 1982, 160, 195–206. [Google Scholar] [CrossRef]
- Naiim, M.; Boualem, A.; Ferre, C.; Jabloun, M.; Jalocha, A.; Ravier, P. Multiangle dynamic light scattering for the improvement of multimodal particle size distribution measurements. Soft Matter 2015, 11, 28–32. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Caidi, H.; Anderson, L.J.; Haynes, L.M. Evaluation of the Calu-3 Cell Line as a Model of in Vitro Respiratory Syncytial Virus Infection. J. Virol. Methods 2011, 174, 144–149. [Google Scholar] [CrossRef]
- Meindl, C.; Stranzinger, S.; Dzidic, N.; Salar-Behzadi, S.; Mohr, S.; Zimmer, A.; Fröhlich, E. Permeation of Therapeutic Drugs in Different Formulations across the Airway Epithelium In Vitro. PLoS ONE 2015, 10, e0135690. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Hevir-Kene, N.; Rižner, T.L.; Peternel, L.; Kristan, K. The Characterization of the Human Cell Line Calu-3 under Different Culture Conditions and Its Use as an Optimized in Vitro Model to Investigate Bronchial Epithelial Function. Eur. J. Pharm. Sci. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Mathia, N.R.; Timoszyk, J.; Stetsko, P.I.; Megill, J.R.; Smith, R.L.; Wall, D.A. Permeability Characteristics of Calu-3 Human Bronchial Epithelial Cells: In Vitro-in Vivo Correlation to Predict Lung Absorption in Rats. J. Drug Target. 2002, 10, 31–40. [Google Scholar] [CrossRef]
- Untergasser, A.; Ruijter, J.M.; Benes, V.; van den Hoff, M.J.B. Web-Based LinRegPCR: Application for the Visualization and Analysis of (RT)-qPCR Amplification and Melting Data. BMC Bioinform. 2021, 22, 398. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Pfaffl, M.W.; Zhao, S.; Spiess, A.N.; Boggy, G.; Blom, J.; Rutledge, R.G.; Sisti, D.; Lievens, A.; De Preter, K.; et al. Evaluation of qPCR Curve Analysis Methods for Reliable Biomarker Discovery: Bias, Resolution, Precision, and Implications. Methods 2013, 59, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, X.; Wang, M.; Deng, Z.; Niu, B.; Chen, Q. Effect of Roasted Peanut Allergen Ara h 3 Protein on the Sensitization of Caco-2 Cells. J. Sci. Food Agric. 2021, 101, 5325–5336. [Google Scholar] [CrossRef]
- Tordesillas, L.; Gómez-Casado, C.; Garrido-Arandia, M.; Murua-García, A.; Palacín, A.; Varela, J.; Konieczna, P.; Cuesta-Herranz, J.; Akdis, C.A.; O’Mahony, L.; et al. Transport of Pru p 3 across Gastrointestinal Epithelium—An Essential Step towards the Induction of Food Allergy? Clin. Exp. Allergy 2013, 43, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Nešić, A.; Čavić, M.; Popović, M.; Gavrović-Jankulović, M. A New Approach for Activation of the Kiwifruit Cysteine Protease for Usage in In-Vitro Testing. Mol. Biol. Rep. 2021, 48, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Röschmann, K.; Farhat, K.; König, P.; Suck, R.; Ulmer, A.J.; Petersen, A. Timothy Grass Pollen Major Allergen Phl p 1 Activates Respiratory Epithelial Cells by a Non-protease Mechanism. Clin. Exp. Allergy 2009, 39, 1358–1369. [Google Scholar] [CrossRef]
- Mattila, P.; Renkonen, J.; Toppila-Salmi, S.; Parviainen, V.; Joenväärä, S.; Alff-Tuomala, S.; Nicorici, D.; Renkonen, R. Time-series Nasal Epithelial Transcriptomics during Natural Pollen Exposure in Healthy Subjects and Allergic Patients. Allergy 2010, 65, 175–183. [Google Scholar] [CrossRef]
- Bleck, B.; Kazeros, A.; Bakal, K.; Garcia-Medina, L.; Adams, A.; Liu, M.; Lee, R.A.; Tse, D.B.; Chiu, A.; Grunig, G.; et al. Coexpression of Type 2 Immune Targets in Sputum-Derived Epithelial and Dendritic Cells from Asthmatic Subjects. J. Allergy Clin. Immunol. 2015, 136, 619–627. [Google Scholar] [CrossRef]
- Obersteiner, A.; Gilles, S.; Frank, U.; Beck, I.; Häring, F.; Ernst, D.; Rothballer, M.; Hartmann, A.; Traidl-Hoffmann, C.; Schmid, M. Pollen-Associated Microbiome Correlates with Pollution Parameters and the Allergenicity of Pollen. PLoS ONE 2016, 11, e0149545. [Google Scholar] [CrossRef]
- González Roldán, N.; Engel, R.; Düpow, S.; Jakob, K.; Koops, F.; Orinska, Z.; Vigor, C.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Lipid Mediators From Timothy Grass Pollen Contribute to the Effector Phase of Allergy and Prime Dendritic Cells for Glycolipid Presentation. Front. Immunol. 2019, 10, 974. [Google Scholar] [CrossRef]
- Toppila-Salmi, S.; van Drunen, C.M.; Fokkens, W.J.; Golebski, K.; Mattila, P.; Joenvaara, S.; Renkonen, J.; Renkonen, R. Molecular Mechanisms of Nasal Epithelium in Rhinitis and Rhinosinusitis. Curr. Allergy Asthma Rep. 2015, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Echaide, M.; Autilio, C.; Arroyo, R.; Perez-Gil, J. Restoring Pulmonary Surfactant Membranes and Films at the Respiratory Surface. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, J.E.; Ferreras, M.; Wimmer, R.; Petersen, S.V.; Enghild, J.J.; Otzen, D.E. The Major Allergen from Birch Tree Pollen, Bet v 1, Binds and Permeabilizes Membranes. Biochemistry 2007, 46, 3356–3365. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodriguez, E.; Pérez-Gil, J. Structure-Function Relationships in Pulmonary Surfactant Membranes: From Biophysics to Therapy. Biochim. Biophys. Acta 2014, 1838, 1568–1585. [Google Scholar] [CrossRef]
- Pérez-Gil, J. Structure of Pulmonary Surfactant Membranes and Films: The Role of Proteins and Lipid-Protein Interactions. Biochim. Biophys. Acta 2008, 1778, 1676–1695. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Weaver, T.E. Pulmonary Surfactant Pathophysiology: Current Models and Open Questions. Physiology 2010, 25, 132–141. [Google Scholar] [CrossRef]
- Vazquez-de-Lara, L.G.; Tlatelpa-Romero, B.; Romero, Y.; Fernández-Tamayo, N.; Vazquez-de-Lara, F.; Justo-Janeiro, J.M.; Garcia-Carrasco, M.; de-la-Rosa Paredes, R.; Cisneros-Lira, J.G.; Mendoza-Milla, C.; et al. Phosphatidylethanolamine Induces an Antifibrotic Phenotype in Normal Human Lung Fibroblasts and Ameliorates Bleomycin-Induced Lung Fibrosis in Mice. Int. J. Mol. Sci. 2018, 19, 2758. [Google Scholar] [CrossRef]
- Renkonen, J.; Mattila, P.; Lehti, S.; Mäkinen, J.; Sormunen, R.; Tervo, T.; Paavonen, T.; Renkonen, R. Birch Pollen Allergen Bet v 1 Binds to and Is Transported through Conjunctival Epithelium in Allergic Patients. Allergy 2009, 64, 868–875. [Google Scholar] [CrossRef]
- Hens, G.; Bobic, S.; Reekmans, K.; Ceuppens, J.L.; Hellings, P.W. Rapid Systemic Uptake of Allergens through the Respiratory Mucosa. J. Allergy Clin. Immunol. 2007, 120, 472–474. [Google Scholar] [CrossRef]
- Kamekura, R.; Yamashita, K.; Jitsukawa, S.; Nagaya, T.; Ito, F.; Ichimiya, S.; Himi, T. Role of Crosstalk between Epithelial and Immune Cells, the Epimmunome, in Allergic Rhinitis Pathogenesis. Adv. Otorhinolaryngol. 2016, 77, 75–82. [Google Scholar] [CrossRef]
- Sudharson, S.; Kalic, T.; Eckl-Dorna, J.; Lengger, N.; Breiteneder, H.; Hafner, C. Modulation of Bronchial Epithelial Barrier Integrity by Low Molecular Weight Components from Birch Pollen. Int. J. Mol. Sci. 2024, 25, 7374. [Google Scholar] [CrossRef] [PubMed]
- Röschmann, K.I.L.; Luiten, S.; Jonker, M.J.; Breit, T.M.; Fokkens, W.J.; Petersen, A.; Drunen, C.M. van. Timothy Grass Pollen Extract-induced Gene Expression and Signalling Pathways in Airway Epithelial Cells. Clin. Exp. Allergy 2011, 41, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Watson, B.G.; Mattoo, S.; Rochet, J.-C. Calcein Release Assay to Measure Membrane Permeabilization by Recombinant Alpha-Synuclein. Bio Protoc. 2020, 10, e3690. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, D.N.; Potapov, A.E.; Ovchinnikova, T.V.; Bogdanov, I.V. Modeling Human Airway Epithelial Barrier Penetration Using Birch Bet v 1 and Alder Aln g 1 Pollen Allergens During Sensitization Process. Int. J. Mol. Sci. 2025, 26, 5169. https://doi.org/10.3390/ijms26115169
Melnikova DN, Potapov AE, Ovchinnikova TV, Bogdanov IV. Modeling Human Airway Epithelial Barrier Penetration Using Birch Bet v 1 and Alder Aln g 1 Pollen Allergens During Sensitization Process. International Journal of Molecular Sciences. 2025; 26(11):5169. https://doi.org/10.3390/ijms26115169
Chicago/Turabian StyleMelnikova, Daria N., Andrey E. Potapov, Tatiana V. Ovchinnikova, and Ivan V. Bogdanov. 2025. "Modeling Human Airway Epithelial Barrier Penetration Using Birch Bet v 1 and Alder Aln g 1 Pollen Allergens During Sensitization Process" International Journal of Molecular Sciences 26, no. 11: 5169. https://doi.org/10.3390/ijms26115169
APA StyleMelnikova, D. N., Potapov, A. E., Ovchinnikova, T. V., & Bogdanov, I. V. (2025). Modeling Human Airway Epithelial Barrier Penetration Using Birch Bet v 1 and Alder Aln g 1 Pollen Allergens During Sensitization Process. International Journal of Molecular Sciences, 26(11), 5169. https://doi.org/10.3390/ijms26115169







