Unraveling Botulinum Neurotoxin A Light-Chain-Induced Signaling Pathways: A Phosphoproteomic Analysis in a Controlled Cellular Model
Abstract
1. Introduction
2. Results
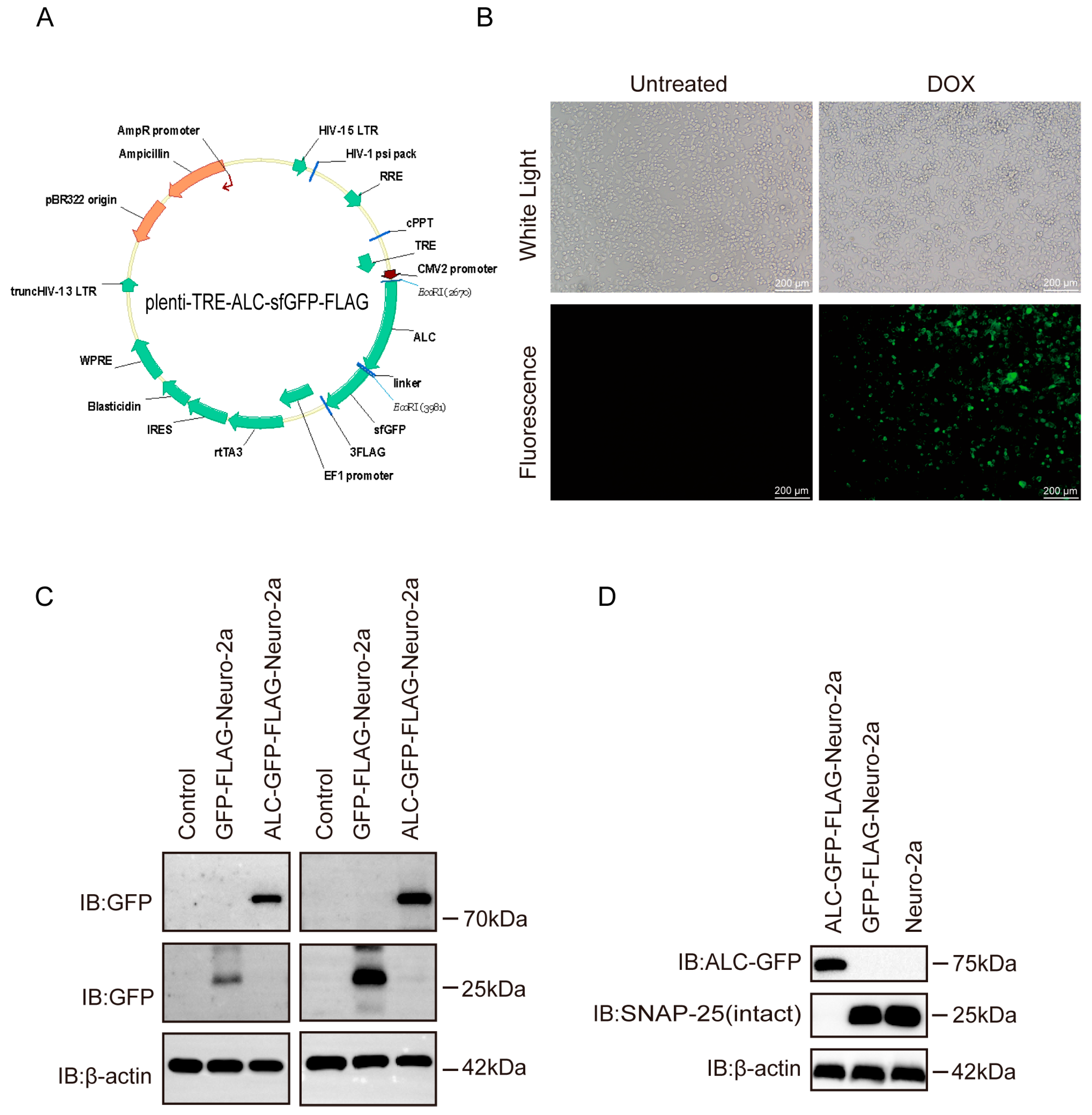
2.1. The Cell Line Model Successfully Mimics the Activity of the Botulinum Neurotoxin Type A Light Chain
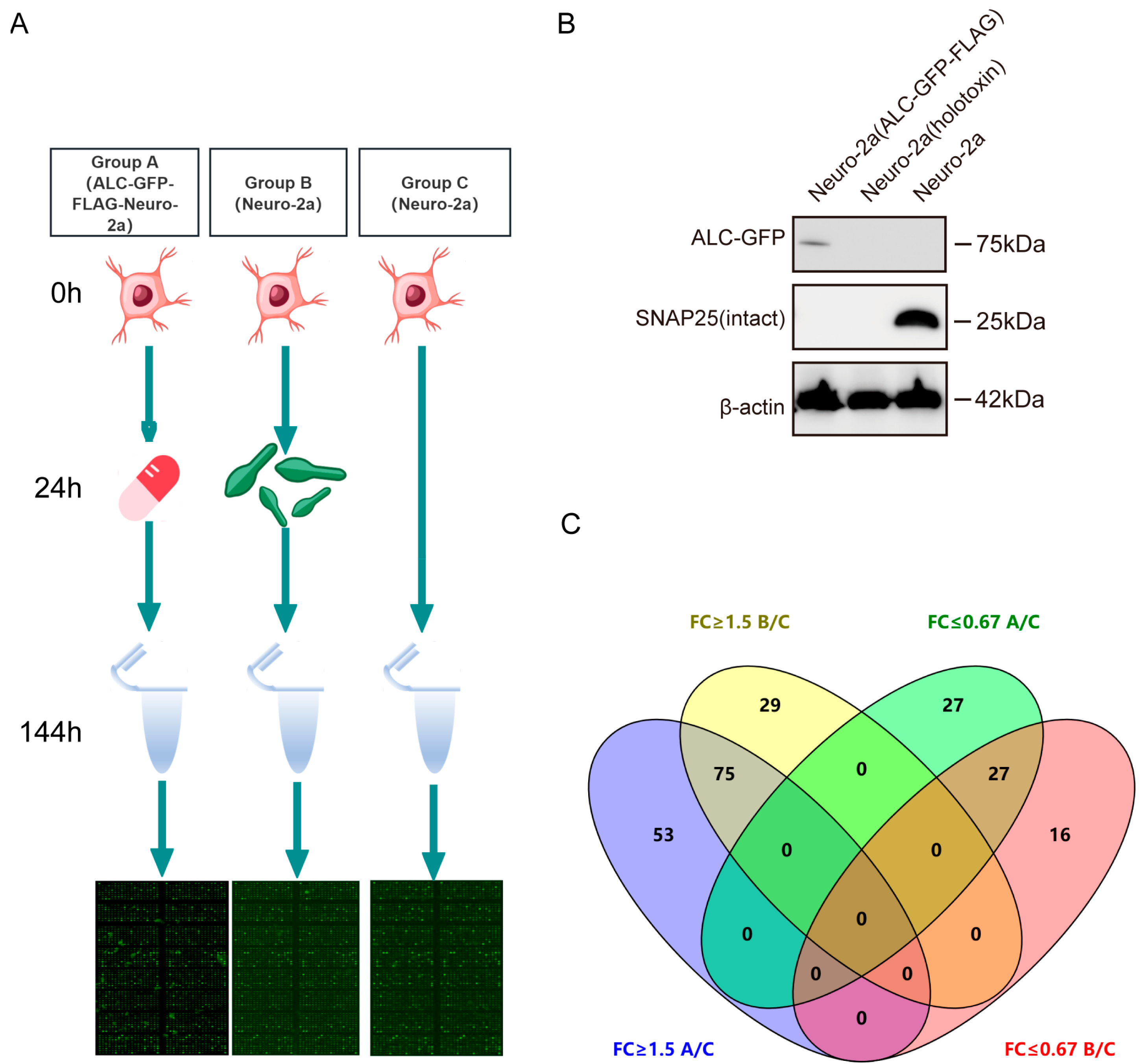
2.2. Phosphorylation Antibody Array Workflow Validation and Venn Diagram Analysis
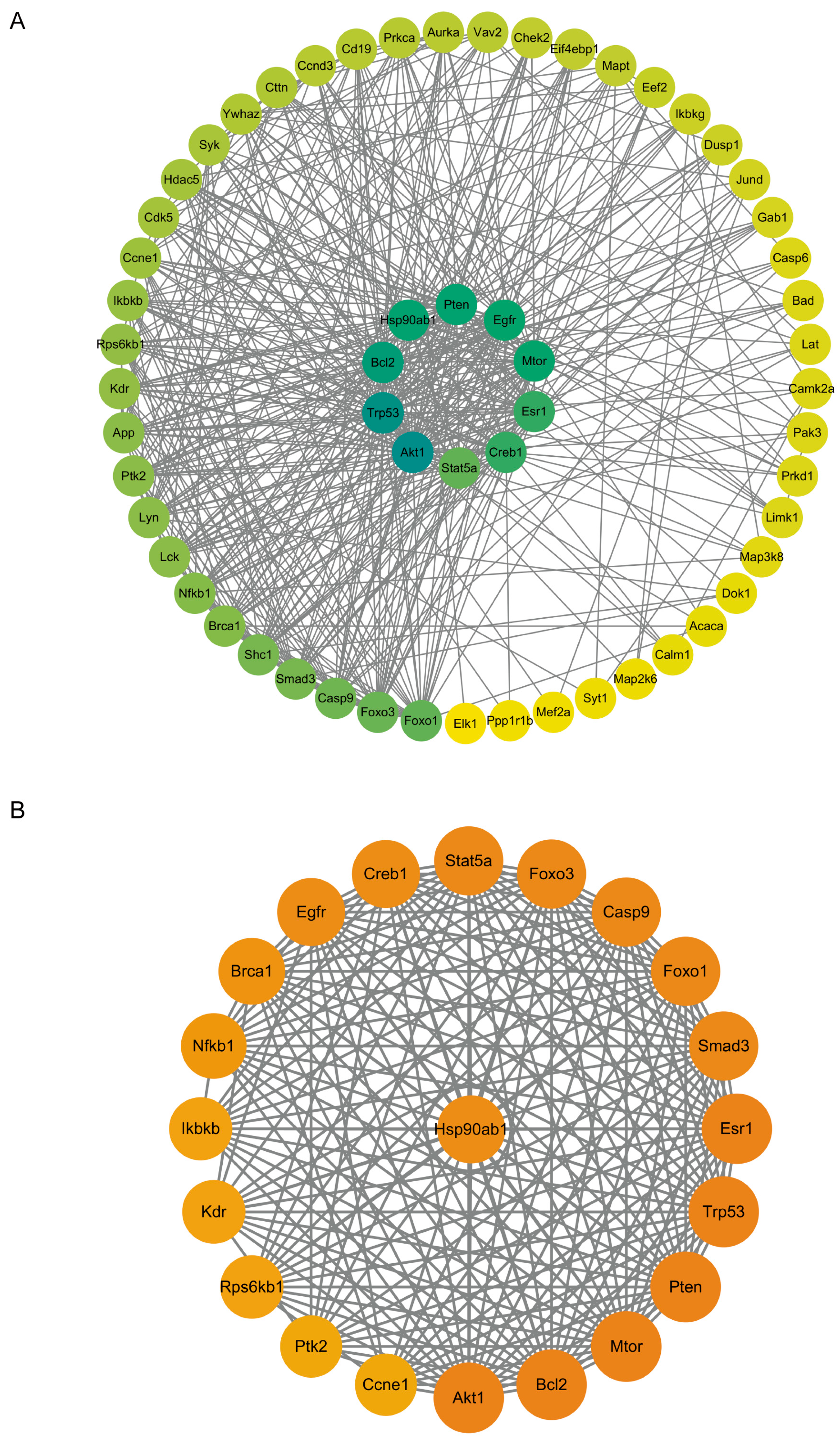
- To investigate the host responses and associated signaling pathways triggered by the intracellular entry of ALC, we conducted a phosphoproteomics analysis using a commercial antibody microarray (Figure 2A). This array is widely applied to identify key signaling proteins that undergo phosphorylation changes in biological processes [17,18,19,20]. The array covers 30 distinct signaling pathways, enabling the detection of 584 phosphorylation sites across 332 key signaling proteins. Our experimental design comprises three groups: Group A, Group B, and Group C.
- Group A consisted of ALC-GFP-FLAG-Neuro-2a cells where ALC was stably expressed as a GFP-FLAG fusion protein under doxycycline-inducible control. Doxycycline (10 µg/mL) was administered at the 24 h time point to induce ALC expression, allowing us to study the effects of ALC in a tightly regulated and inducible manner. This setup provided a controlled system to examine the intracellular effects of ALC expression independent of the toxin delivery complex. Group B utilized wild-type Neuro-2a cells treated with BoNT/A at a concentration of 1 ng/mL. This group simulated the natural process of ALC entering host cells through BoNT/A-mediated delivery, reflecting the physiological route of ALC intracellular activity. Group C served as the control group, utilizing untreated wild-type Neuro-2a cells to establish a baseline for phosphorylation levels in the absence of ALC.
- The key focus of this experimental design was to compare the phosphorylation profiles between Group B (natural ALC delivery via BoNT/A) and Group A (controlled ALC expression), with Group C as a reference. By identifying the common phosphorylation changes in both experimental groups, we aimed to elucidate the conserved host signaling networks that are specifically activated by ALC’s intracellular activity, regardless of the delivery method. Such proteins are postulated to influence the prolonged intracellular stability of the Botulinum neurotoxin type A light chain [21]. Western blot analysis confirmed the successful expression of ALC in the stable cell line and the intracellular entry of botulinum neurotoxin type A, as evidenced by substrate cleavage (Figure 2B).
- The level of protein phosphorylation in each group was expressed as the ratio of phosphorylated to non-phosphorylated proteins. A significant difference in the phosphorylation ratio between groups was considered to exist when the ratio in the experimental groups was ≥1.5 or ≤0.67 that of the control group. In Group A, 128 proteins showed elevated phosphorylation levels, while 54 proteins exhibited reduced phosphorylation levels, with the maximum ratio reaching 7.17. In Group B, 104 proteins demonstrated increased phosphorylation levels, and 43 proteins showed decreased levels, with the maximum ratio being 9.08 (Supplementary Table S1).
- To effectively visualize and analyze the overlap in phosphorylation changes between Group A and Group B, we employed a Venn diagram [22]. This graphical representation allowed us to clearly depict the intersection of upregulated and downregulated phosphorylation events between the two experimental groups. Venn diagram analysis revealed that 53 proteins showed elevated phosphorylation levels only in Group A, and 29 proteins showed elevated levels only in Group B. Additionally, 27 proteins exhibited reduced phosphorylation levels only in Group A, and 16 proteins showed reduced levels only in Group B. Furthermore, 75 proteins exhibited commonly upregulated phosphorylation levels, while 27 proteins showed commonly downregulated phosphorylation levels (Figure 2C). These core proteins with consistent phosphorylation changes in both groups are likely to represent key nodes in the signaling pathways directly modulated by ALC.
2.3. Enrichment Analysis of Proteins with Upregulated Phosphorylation Levels
2.4. The Phosphorylation Levels of Several Key Molecules in the PI3K-AKT Signaling Pathway Were Significantly Upregulated
2.5. Enrichment Analysis of Proteins with Downregulated Phosphorylation Levels
3. Discussion
4. Materials and Methods
4.1. Generation of Stable Cell Lines
4.2. Detection of ALC-GFP-FLAG Protein Expression and Activity
4.3. Phosphoproteomic Array
4.4. Data Analysis of Phosphoproteomic Array
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT/A | Botulinum neurotoxin type A |
| DOX | Doxycycline |
| ALC | BoNT/A light chain |
| LC | Light chain |
| HC | Heavy chain |
| SNAP-25 | Synaptosome-associated protein 25 |
| PPI | Protein–protein interaction |
| TBST | Tris-buffered saline with Tween 20 |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| IB | Immunoblotting |
References
- Lin, L.; Olson, M.E.; Eubanks, L.M.; Janda, K.D. Strategies to Counteract Botulinum Neurotoxin A: Nature’s Deadliest Biomolecule. Acc. Chem. Res. 2019, 52, 2322–2331. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum Toxin as a Biological Weapon: Medical and Public Health Management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef]
- Humeau, Y.; Doussau, F.; Grant, N.J.; Poulain, B. How Botulinum and Tetanus Neurotoxins Block Neurotransmitter Release. Biochimie 2000, 82, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Dolly, J.O.; Black, J.; Williams, R.S.; Melling, J. Acceptors for Botulinum Neurotoxin Reside on Motor Nerve Terminals and Mediate Its Internalization. Nature 1984, 307, 457–460. [Google Scholar] [CrossRef]
- Dong, M.; Masuyer, G.; Stenmark, P. Botulinum and Tetanus Neurotoxins. Annu. Rev. Biochem. 2019, 88, 811–837. [Google Scholar] [CrossRef]
- Serrera-Figallo, M.-A.; Ruiz-de-León-Hernández, G.; Torres-Lagares, D.; Castro-Araya, A.; Torres-Ferrerosa, O.; Hernández-Pacheco, E.; Gutierrez-Perez, J.-L. Use of Botulinum Toxin in Orofacial Clinical Practice. Toxins 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Adib Saberi, F.; Rosales, R.L. Botulinum Toxin Therapy of Dystonia. J. Neural Transm. 2021, 128, 531–537. [Google Scholar] [CrossRef]
- Small, R. Botulinum Toxin Injection for Facial Wrinkles. Am. Fam. Physician 2014, 90, 168–175. [Google Scholar]
- Ben David, A.; Barnea, A.; Torgeman, A.; Diamant, E.; Dor, E.; Schwartz, A.; Rosen, O.; Caspi, N.; Saraf, M.; Lerer, E.; et al. Immunologic and Protective Properties of Subunit- vs. Whole Toxoid-Derived Anti-Botulinum Equine Antitoxin. Vaccines 2022, 10, 1522. [Google Scholar] [CrossRef]
- Kiris, E.; Burnett, J.C.; Nuss, J.E.; Wanner, L.M.; Peyser, B.D.; Du, H.T.; Gomba, G.Y.; Kota, K.P.; Panchal, R.G.; Gussio, R.; et al. Src Family Kinase Inhibitors Antagonize the Toxicity of Multiple Serotypes of Botulinum Neurotoxin in Human Embryonic Stem Cell-Derived Motor Neurons. Neurotox. Res. 2015, 27, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Luo, D.; Zhuang, C.; Ning, N.; Zhang, Y.; He, Z.; Gao, J.; Hong, Z.; Xv, X.; et al. Discovery of a Potent Botulinum Neurotoxin A Inhibitor ZM299 with Effective Protections in Botulism Mice. Chin. J. Chem. 2022, 40, 357–364. [Google Scholar] [CrossRef]
- Wang, J.; Zurawski, T.H.; Bodeker, M.O.; Meng, J.; Boddul, S.; Aoki, K.R.; Dolly, J.O. Longer-Acting and Highly Potent Chimaeric Inhibitors of Excessive Exocytosis Created with Domains from Botulinum Neurotoxin A and B. Biochem. J. 2012, 444, 59–67. [Google Scholar] [CrossRef]
- Lietz, M.; Ullrich, A.; Schulte-Eversum, C.; Oberhoffner, S.; Fricke, C.; Müller, H.W.; Schlosshauer, B. Physical and Biological Performance of a Novel Block Copolymer Nerve Guide. Biotechnol. Bioeng. 2006, 93, 99–109. [Google Scholar] [CrossRef]
- Pantano, S.; Montecucco, C. The Blockade of the Neurotransmitter Release Apparatus by Botulinum Neurotoxins. Cell Mol. Life Sci. 2014, 71, 793–811. [Google Scholar] [CrossRef]
- Gossen, M.; Bujard, H. Tight Control of Gene Expression in Mammalian Cells by Tetracycline-Responsive Promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef]
- Hensel, N.; Cieri, F.; Santonicola, P.; Tapken, I.; Schüning, T.; Taiana, M.; Pagliari, E.; Joseph, A.; Fischer, S.; Heidrich, N.; et al. Impairment of the Neurotrophic Signaling Hub B-Raf Contributes to Motoneuron Degeneration in Spinal Muscular Atrophy. Proc. Natl. Acad. Sci. USA 2021, 118, e2007785118. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Xiang, J.; Ruan, X.; Peng, P.; Ruan, Y.; Chen, Y.-G.; Wang, Y.; Yu, Q.; Zhang, H.; et al. Gut Stem Cell Aging Is Driven by mTORC1 via a P38 MAPK-P53 Pathway. Nat. Commun. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Vehlow, A.; Klapproth, E.; Jin, S.; Hannen, R.; Hauswald, M.; Bartsch, J.-W.; Nimsky, C.; Temme, A.; Leitinger, B.; Cordes, N. Interaction of Discoidin Domain Receptor 1 with a 14-3-3-Beclin-1-Akt1 Complex Modulates Glioblastoma Therapy Sensitivity. Cell Rep. 2019, 26, 3672–3683.e7. [Google Scholar] [CrossRef]
- Dai, B.; Shi, X.; Ma, N.; Ma, W.; Zhang, Y.; Yang, T.; Zhang, J.; He, L. HMQ-T-B10 Induces Human Liver Cell Apoptosis by Competitively Targeting EphrinB2 and Regulating Its Pathway. J. Cell Mol. Med. 2018, 22, 5231–5243. [Google Scholar] [CrossRef]
- Zanotti, S.; Kapetis, D.; Gibertini, S.; Salerno, F.; Ciusani, E.; Colombo, C.; Gronchi, A.; Morandi, L.; Mantegazza, R.; Molteni, F.; et al. Botulinum Toxin Type A Affects the Transcriptome of Cell Cultures Derived from Muscle Biopsies of Controls and Spastic Patients. Toxicol. In Vitro 2018, 50, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Xu, L.; Wang, Y. Venn Diagrams in Bioinformatics. Brief. Bioinform. 2021, 22, bbab108. [Google Scholar] [CrossRef] [PubMed]
- Piredda, M.L.; Gaur, G.; Catalano, G.; Divona, M.; Banella, C.; Travaglini, S.; Puzzangara, M.C.; Voso, M.T.; Lo-Coco, F.; Noguera, N.I. PML/RARA Inhibits Expression of HSP90 and Its Target AKT. Br. J. Haematol. 2019, 184, 937–948. [Google Scholar] [CrossRef]
- Azarnia Tehran, D.; Pirazzini, M.; Leka, O.; Mattarei, A.; Lista, F.; Binz, T.; Rossetto, O.; Montecucco, C. Hsp90 Is Involved in the Entry of Clostridial Neurotoxins into the Cytosol of Nerve Terminals. Cell Microbiol. 2017, 19, e12647. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Albert, V.; Hall, M.N. mTOR Signaling in Cellular and Organismal Energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Rounds, S. Focal Adhesion Kinase and Endothelial Cell Apoptosis. Microvasc. Res. 2012, 83, 56–63. [Google Scholar] [CrossRef]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-Regulated FAK-Src Signaling in Normal and Cancer Cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal Adhesion Kinase: In Command and Control of Cell Motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Krisenko, M.O.; Higgins, R.L.; Ghosh, S.; Zhou, Q.; Trybula, J.S.; Wang, W.-H.; Geahlen, R.L. Syk Is Recruited to Stress Granules and Promotes Their Clearance through Autophagy. J. Biol. Chem. 2015, 290, 27803–27815. [Google Scholar] [CrossRef]
- Inuzuka, H.; Shaik, S.; Onoyama, I.; Gao, D.; Tseng, A.; Maser, R.S.; Zhai, B.; Wan, L.; Gutierrez, A.; Lau, A.W.; et al. SCF(FBW7) Regulates Cellular Apoptosis by Targeting MCL1 for Ubiquitylation and Destruction. Nature 2011, 471, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chung, M.E. Botulinum Toxin for Central Neuropathic Pain. Toxins 2018, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.O. Cosmetic Treatment Using Botulinum Toxin in the Oral and Maxillofacial Area: A Narrative Review of Esthetic Techniques. Toxins 2023, 15, 82. [Google Scholar] [CrossRef]
- Hassell, T.J.W.; Charles, D. Treatment of Blepharospasm and Oromandibular Dystonia with Botulinum Toxins. Toxins 2020, 12, 269. [Google Scholar] [CrossRef]
- Cb, S.; Ga, O. Persistence of Botulinum Neurotoxin Inactivation of Nerve Function. Curr. Top. Microbiol. Immunol. 2013, 364, 179–196. [Google Scholar] [CrossRef]
- Rao, A.K.; Sobel, J.; Chatham-Stephens, K.; Luquez, C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recomm. Rep. 2021, 70, 1–30. [Google Scholar] [CrossRef]
- Das, A.T.; Tenenbaum, L.; Berkhout, B. Tet-On Systems For Doxycycline-Inducible Gene Expression. Curr. Gene Ther. 2016, 16, 156–167. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Maditz, R.; Kuo, C.-L.; Fishman, P.S.; Shoemaker, C.B.; Oyler, G.A.; Weissman, A.M. Targeting Botulinum Neurotoxin Persistence by the Ubiquitin-Proteasome System. Proc. Natl. Acad. Sci. USA 2010, 107, 16554–16559. [Google Scholar] [CrossRef]
- Sakuma, T.; Barry, M.A.; Ikeda, Y. Lentiviral Vectors: Basic to Translational. Biochem. J. 2012, 443, 603–618. [Google Scholar] [CrossRef]
- Winner, B.M.; Bodt, S.M.L.; McNutt, P.M. Special Delivery: Potential Mechanisms of Botulinum Neurotoxin Uptake and Trafficking within Motor Nerve Terminals. Int. J. Mol. Sci. 2020, 21, 8715. [Google Scholar] [CrossRef]
- Bilbrough, T.; Piemontese, E.; Seitz, O. Dissecting the Role of Protein Phosphorylation: A Chemical Biology Toolbox. Chem. Soc. Rev. 2022, 51, 5691–5730. [Google Scholar] [CrossRef]
- Cesaro, L.; Pinna, L.A. Prevalence and Significance of the Commonest Phosphorylated Motifs in the Human Proteome: A Global Analysis. Cell Mol. Life Sci. 2020, 77, 5281–5298. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Lei, Z.; Yu, Y.; Lu, Z.; Qiang, L.; Chai, Q.; Zhang, Y.; Zhao, D.; Li, B.; Pang, Y.; et al. Tuberculosis PknG Manipulates Host Autophagy Flux to Promote Pathogen Intracellular Survival. Autophagy 2022, 18, 576–594. [Google Scholar] [CrossRef]
- Day, A.M.; Quinn, J. Stress-Activated Protein Kinases in Human Fungal Pathogens. Front. Cell Infect. Microbiol. 2019, 9, 261. [Google Scholar] [CrossRef]
- Chai, Q.; Yu, S.; Zhong, Y.; Lu, Z.; Qiu, C.; Yu, Y.; Zhang, X.; Zhang, Y.; Lei, Z.; Qiang, L.; et al. A Bacterial Phospholipid Phosphatase Inhibits Host Pyroptosis by Hijacking Ubiquitin. Science 2022, 378, eabq0132. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, C.; Blanes-Mira, C.; Fernández-Ballester, G.; Planells-Cases, R.; Ferrer-Montiel, A. Modulation of Botulinum Neurotoxin A Catalytic Domain Stability by Tyrosine Phosphorylation. FEBS Lett. 2004, 578, 121–127. [Google Scholar] [CrossRef]
- Encinar, J.A.; Fernández, A.; Ferragut, J.A.; González-Ros, J.M.; DasGupta, B.R.; Montal, M.; Ferrer-Montiel, A. Structural Stabilization of Botulinum Neurotoxins by Tyrosine Phosphorylation. FEBS Lett. 1998, 429, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Toth, S.; Brueggmann, E.E.; Oyler, G.A.; Smith, L.A.; Hines, H.B.; Ahmed, S.A. Tyrosine Phosphorylation of Botulinum Neurotoxin Protease Domains. Front. Pharmacol. 2012, 3, 102. [Google Scholar] [CrossRef]
- Ferrer-Montiel, A.V.; Canaves, J.M.; DasGupta, B.R.; Wilson, M.C.; Montal, M. Tyrosine Phosphorylation Modulates the Activity of Clostridial Neurotoxins. J. Biol. Chem. 1996, 271, 18322–18325. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, Z.; Gao, H.; Liu, Z.; Xv, Q.; Jiang, X.; Yu, W. LYC Inhibits the AKT Signaling Pathway to Activate Autophagy and Ameliorate TGFB-Induced Renal Fibrosis. Autophagy 2024, 20, 1114–1133. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Wang, X.; Zhang, D.; Sun, G.; Wang, M.; Wang, M.; Han, Y.; Chai, R.; Wang, H. PRDX1 Activates Autophagy via the PTEN-AKT Signaling Pathway to Protect against Cisplatin-Induced Spiral Ganglion Neuron Damage. Autophagy 2021, 17, 4159–4181. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, J.; Jiang, N.; Xian, Y.; Ju, H.; Wei, Y.; Zhang, X. Corin Protects H2O2-Induced Apoptosis through PI3K/AKT and NF-κB Pathway in Cardiomyocytes. Biomed. Pharmacother. 2018, 97, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.J.; Klein, J.B. Regulation of Neutrophil Apoptosis by Modulation of PKB/Akt Activation. Front. Biosci. 2009, 14, 2400–2412. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Nesterova, A.; Sable, C.; Heidenreich, K.A.; Boxer, L.M.; Heasley, L.E.; Reusch, J.E. Akt/Protein Kinase B up-Regulates Bcl-2 Expression through cAMP-Response Element-Binding Protein. J. Biol. Chem. 2000, 275, 10761–10766. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Rossetto, O.; Montecucco, C. Hsp90 and Thioredoxin-Thioredoxin Reductase Enable the Catalytic Activity of Clostridial Neurotoxins inside Nerve Terminals. Toxicon 2018, 147, 32–37. [Google Scholar] [CrossRef]
- Baldwin, M.R.; Bradshaw, M.; Johnson, E.A.; Barbieri, J.T. The C-Terminus of Botulinum Neurotoxin Type A Light Chain Contributes to Solubility, Catalysis, and Stability. Protein Expr. Purif. 2004, 37, 187–195. [Google Scholar] [CrossRef]
- Gräwe, A.; Stein, V. Linker Engineering in the Context of Synthetic Protein Switches and Sensors. Trends Biotechnol. 2021, 39, 731–744. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.-C. Fusion Protein Linkers: Property, Design and Functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Luo, S.; Li, T.; Wang, Q.; Tian, R.; Liu, H.; Fang, H.; Chen, F.; Wang, H. Development of a Fusion Protein SNVP as Substrate for Assaying Multi-Serotype Botulinum Neurotoxins. Anal. Biochem. 2014, 463, 75–81. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Cai, K.; Tian, M.; Wang, Q.; Shi, J.; Gao, X.; Wang, H. Hypersensitive Detection and Quantitation of BoNT/A by IgY Antibody against Substrate Linear-Peptide. PLoS ONE 2013, 8, e58908. [Google Scholar] [CrossRef]
- He, Q.; Liu, C.; Wang, X.; Rong, K.; Zhu, M.; Duan, L.; Zheng, P.; Mi, Y. Exploring the Mechanism of Curcumin in the Treatment of Colon Cancer Based on Network Pharmacology and Molecular Docking. Front. Pharmacol. 2023, 14, 1102581. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, J.; Gao, W.; Sun, J.; Liu, G.; Zhou, J. PPP2R1B Abolishes Colorectal Cancer Liver Metastasis and Sensitizes Oxaliplatin by Inhibiting MAPK/ERK Signaling Pathway. Cancer Cell Int. 2024, 24, 90. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Osterman, T.J.; Terry, M.; Miller, R.S. Improving Cancer Data Interoperability: The Promise of the Minimal Common Oncology Data Elements (mCODE) Initiative. JCO Clin. Cancer Inform. 2020, 4, 993–1001. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Zhang, L.; Yu, W.; Tu, Y.; Yang, X.; Li, D.; Wang, H.; Li, T. Unraveling Botulinum Neurotoxin A Light-Chain-Induced Signaling Pathways: A Phosphoproteomic Analysis in a Controlled Cellular Model. Int. J. Mol. Sci. 2025, 26, 5168. https://doi.org/10.3390/ijms26115168
Zhu C, Zhang L, Yu W, Tu Y, Yang X, Li D, Wang H, Li T. Unraveling Botulinum Neurotoxin A Light-Chain-Induced Signaling Pathways: A Phosphoproteomic Analysis in a Controlled Cellular Model. International Journal of Molecular Sciences. 2025; 26(11):5168. https://doi.org/10.3390/ijms26115168
Chicago/Turabian StyleZhu, Chensi, Liangyan Zhang, Wenjing Yu, Yeqing Tu, Xiaolan Yang, Deyu Li, Hui Wang, and Tao Li. 2025. "Unraveling Botulinum Neurotoxin A Light-Chain-Induced Signaling Pathways: A Phosphoproteomic Analysis in a Controlled Cellular Model" International Journal of Molecular Sciences 26, no. 11: 5168. https://doi.org/10.3390/ijms26115168
APA StyleZhu, C., Zhang, L., Yu, W., Tu, Y., Yang, X., Li, D., Wang, H., & Li, T. (2025). Unraveling Botulinum Neurotoxin A Light-Chain-Induced Signaling Pathways: A Phosphoproteomic Analysis in a Controlled Cellular Model. International Journal of Molecular Sciences, 26(11), 5168. https://doi.org/10.3390/ijms26115168




