Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Patients Included in This Study
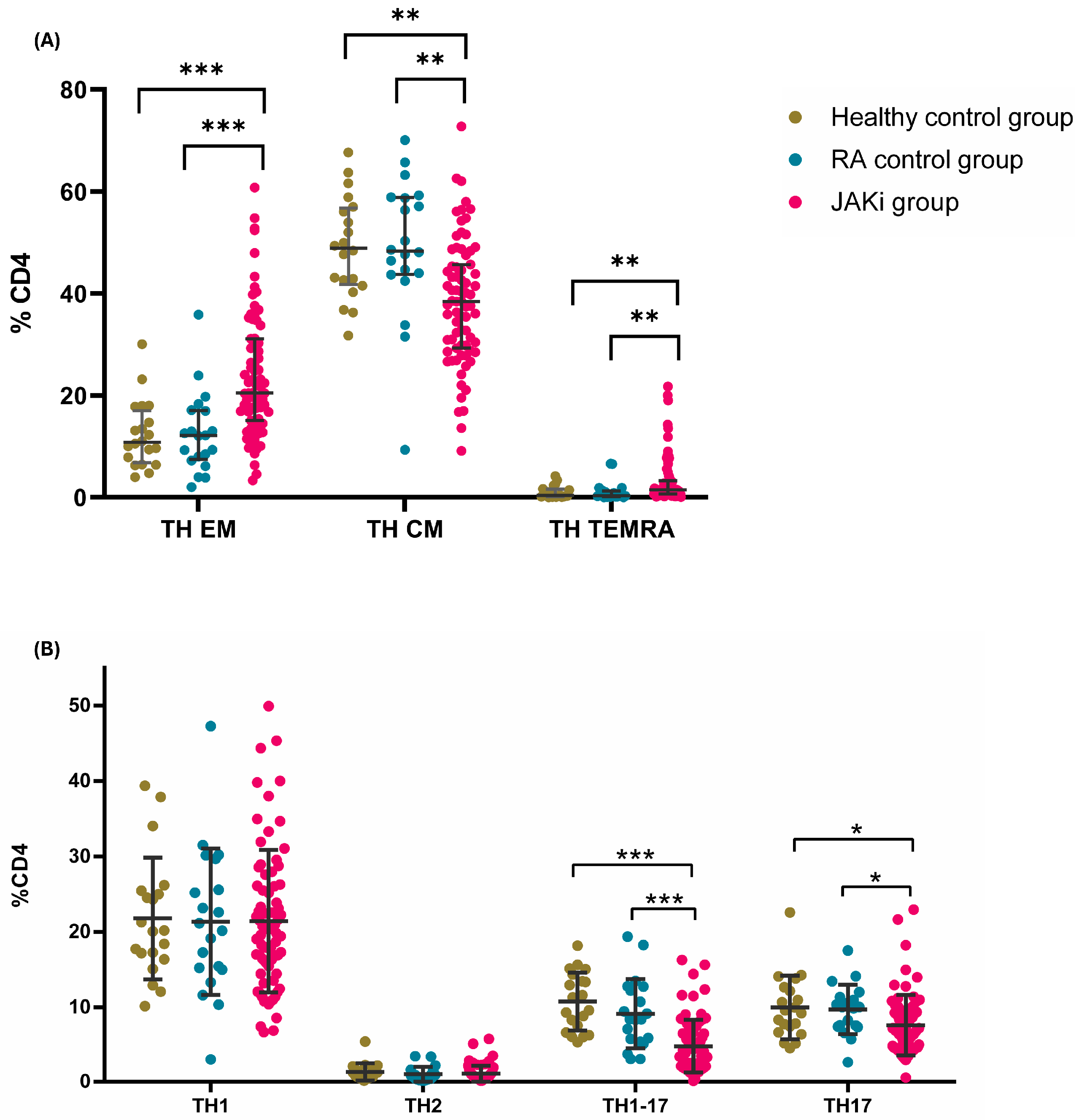
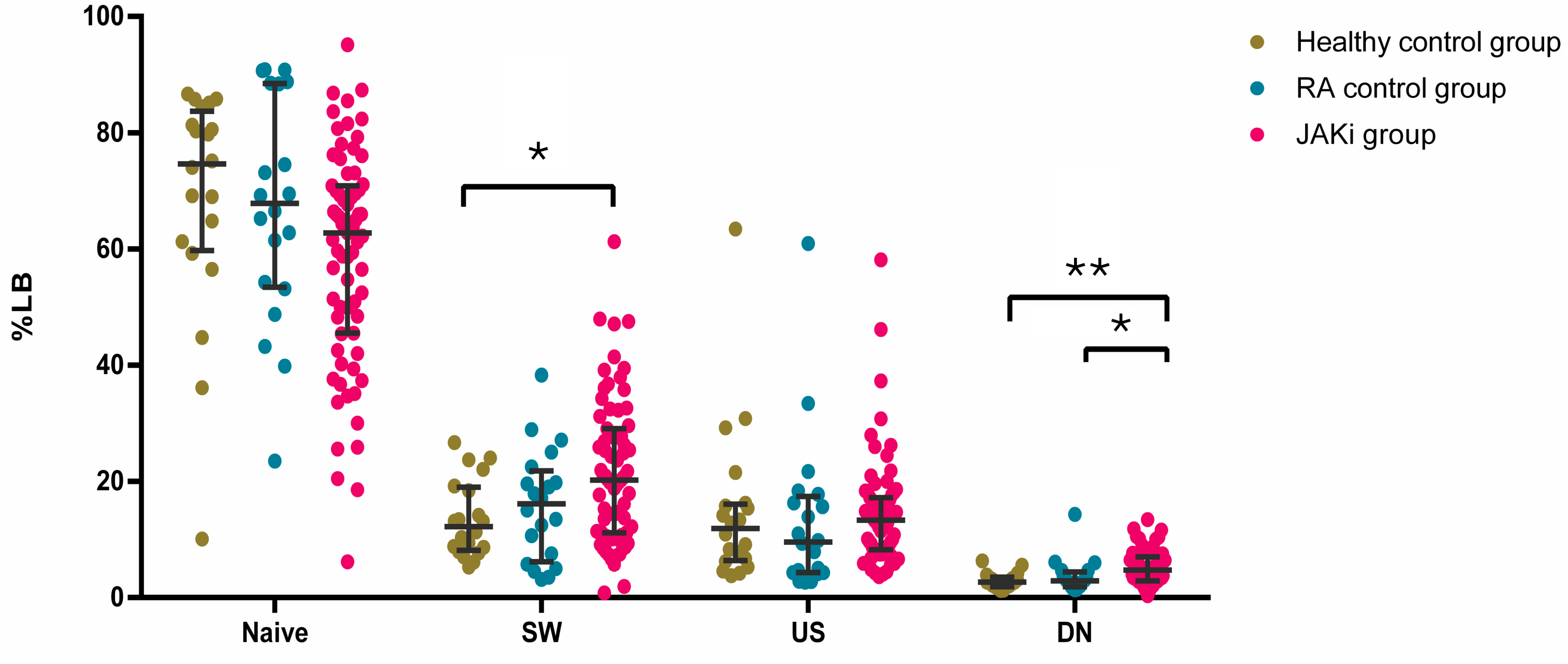
2.2. Effect of JAKi on the Frequencies of Circulating Adaptive Immune Cells
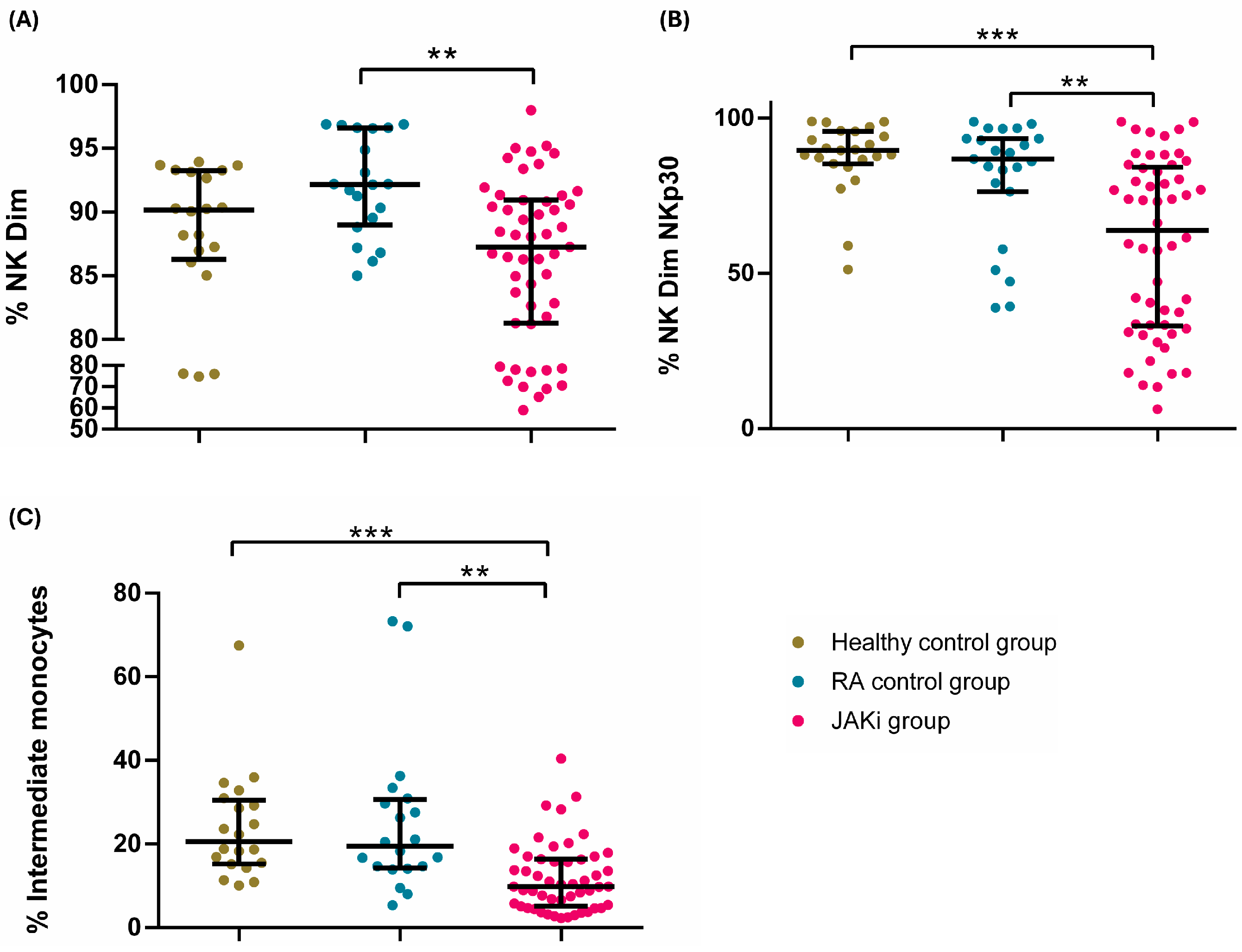
2.3. Effect of JAKi on Peripheral Blood Innate Immune Cells
2.4. Differential Impact of JAKi
2.5. Differential Effect of Selective and Non-Selective JAKi
2.6. Adverse Events
3. Discussion
4. Materials and Methods
4.1. Analysis by Flow Cytometry
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aCarp | anti-carbamylated protein antibodies |
| ACPA | anti-citrullinated protein antibodies |
| AMPA | anti-modified protein antibodies |
| aPAD | anti-peptidylarginine deiminases |
| Bari | baricitinib |
| bDMARD | biological disease-modifying antirheumatic drug |
| CD | cluster differentiation |
| CDAI | clinical disease activity index |
| cDMARD | conventional disease modifying antirheumatic drug |
| CEIM | Cantabria Ethical Committee |
| CM | central memory |
| CRP | C-reactive protein |
| CVRF | cardiovascular risk factor |
| DAS28 | disease activity score |
| DMARD | Disease-modifying antirheumatic drug |
| EM | effector memory |
| ESR | erythrocyte sedimentation rate |
| Filgo | filgotinib |
| IFN-γ | interferon γ |
| Ig | immunoglobulin |
| IQR | interquartile range |
| JAKi | Jak–Stat inhibitors |
| Jak–Stat | Janus kinase–signal transducer and activator of transcription |
| MTX | methotrexate |
| NK | natural killer |
| NSAIDs | non-steroid anti-inflammatory drugs |
| PBMCs | peripheral blood mononuclear cells |
| RA | rheumatoid arthritis |
| RAPID3 | routine assessment of patient index data 3 |
| RF | rheumatoid factor |
| SE | shared epitopes |
| TC | T cytotoxic |
| TEMRA | terminally differentiated CD45RA |
| TH | T helper |
| TNF | tumour necrosis factor |
| Tofa | tofacitinib |
| Treg | T regulatory |
| tsDMARD | targeted synthetic disease-modifying antirheumatic drug |
| Upa | upadacitinib |
References
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Jin, S.; Wang, Y.; Jiang, N.; Wu, C.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Remission Rate and Predictors of Remission in Patients with Rheumatoid Arthritis under Treat-to-Target Strategy in Real-World Studies: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2019, 38, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Ternant, D.; Bejan-Angoulvant, T.; Passot, C.; Mulleman, D.; Paintaud, G. Clinical Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies Approved to Treat Rheumatoid Arthritis. Clin. Pharmacokinet. 2015, 54, 1107–1123. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Eberhard, A.; Di Giuseppe, D.; Askling, J.; Bergman, S.; Bower, H.; Chatzidionysiou, K.; Forsblad-d’Elia, H.; Kastbom, A.; Olofsson, T.; Frisell, T.; et al. Effectiveness of JAK Inhibitors Compared with Biologic Disease-Modifying Antirheumatic Drugs on Pain Reduction in Rheumatoid Arthritis: Results from a Nationwide Swedish Cohort Study. Arthritis Rheumatol. 2025, 77, 253–262. [Google Scholar] [CrossRef]
- Balanescu, A.-R.; Citera, G.; Pascual-Ramos, V.; Bhatt, D.L.; Connell, C.A.; Gold, D.; Chen, A.-S.; Sawyerr, G.; Shapiro, A.B.; Pope, J.E.; et al. Infections in Patients with Rheumatoid Arthritis Receiving Tofacitinib versus Tumour Necrosis Factor Inhibitors: Results from the Open-Label, Randomised Controlled ORAL Surveillance Trial. Ann. Rheum. Dis. 2022, 81, 1491–1503. [Google Scholar] [CrossRef]
- Winthrop, K.L.; Park, S.-H.; Gul, A.; Cardiel, M.H.; Gomez-Reino, J.J.; Tanaka, Y.; Kwok, K.; Lukic, T.; Mortensen, E.; Ponce de Leon, D.; et al. Tuberculosis and Other Opportunistic Infections in Tofacitinib-Treated Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2016, 75, 1133–1138. [Google Scholar] [CrossRef]
- Benucci, M.; Damiani, A.; Infantino, M.; Manfredi, M.; Lari, B.; Grossi, V.; Gobbi, F.L.; Sarzi-Puttini, P. Cardiovascular Safety, Cancer and Jak-Inhibitors: Differences to Be Highlighted. Pharmacol. Res. 2022, 183, 106359. [Google Scholar] [CrossRef]
- Bonelli, M.; Kerschbaumer, A.; Kastrati, K.; Ghoreschi, K.; Gadina, M.; Heinz, L.X.; Smolen, J.S.; Aletaha, D.; O’Shea, J.; Laurence, A. Selectivity, Efficacy and Safety of JAKinibs: New Evidence for a Still Evolving Story. Ann. Rheum. Dis. 2023, 83, 139–160. [Google Scholar] [CrossRef]
- Kraev, K.; Geneva-Popova, M.G.; Hristov, B.K.; Uchikov, P.A.; Belova-Popova, S.D.; Kraeva, M.I.; Basheva-Kraeva, Y.M.; Stoyanova, N.S.; Mitkova-Hristova, V.T.; Koleva-Ivanova, M.S.; et al. Examining the Safety Profile of Janus Kinase (JAK) Inhibitors in the Management of Immune-Mediated Diseases: A Comprehensive Review. Life 2023, 13, 2244. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Reddig, A.; Voss, L.; Guttek, K.; Roggenbuck, D.; Feist, E.; Reinhold, D. Impact of Different JAK Inhibitors and Methotrexate on Lymphocyte Proliferation and DNA Damage. J. Clin. Med. 2021, 10, 1431. [Google Scholar] [CrossRef]
- Piscianz, E.; Valencic, E.; Cuzzoni, E.; De Iudicibus, S.; De Lorenzo, E.; Decorti, G.; Tommasini, A. Fate of Lymphocytes after Withdrawal of Tofacitinib Treatment. PLoS ONE 2014, 9, e85463. [Google Scholar] [CrossRef]
- Weinhold, K.J.; Bukowski, J.F.; Brennan, T.V.; Noveck, R.J.; Staats, J.S.; Lin, L.; Stempora, L.; Hammond, C.; Wouters, A.; Mojcik, C.F.; et al. Reversibility of Peripheral Blood Leukocyte Phenotypic and Functional Changes after Exposure to and Withdrawal from Tofacitinib, a Janus Kinase Inhibitor, in Healthy Volunteers. Clin. Immunol. 2018, 191, 10–20. [Google Scholar] [CrossRef]
- Rizzi, M.; Lorenzetti, R.; Fischer, K.; Staniek, J.; Janowska, I.; Troilo, A.; Strohmeier, V.; Erlacher, M.; Kunze, M.; Bannert, B.; et al. Impact of Tofacitinib Treatment on Human B-Cells in Vitro and in Vivo. J. Autoimmun. 2017, 77, 55–66. [Google Scholar] [CrossRef]
- Meudec, L.; Richebé, P.; Pascaud, J.; Mariette, X.; Nocturne, G. Janus Kinase Inhibitors Alter NK Cell Phenotypes and Inhibit Their Antitumour Capacity. Rheumatology 2023, 62, 2855–2863. [Google Scholar] [CrossRef]
- Perniola, S.; Chimenti, M.; Spinelli, F.; Frediani, B.; Foti, R.; Ferrigno, S.; Garufi, C.; Cassone, G.; Venerito, V.; Atzeni, F.; et al. Rheumatoid Arthritis from Easy to Complex Disease: From the “2022 GISEA International Symposium”. J. Clin. Med. 2023, 12, 2781. [Google Scholar] [CrossRef]
- Vencovský, J.; Macháček, S.; Šedová, L.; Kafková, J.; Gatterová, J.; Pešáková, V.; Růžičková, Š. Autoantibodies Can Be Prognostic Markers of an Erosive Disease in Early Rheumatoid Arthritis. Ann. Rheum. Dis. 2003, 62, 427–430. [Google Scholar] [CrossRef]
- Long, S.A.; Muir, V.S.; Jones, B.E.; Wall, V.Z.; Ylescupidez, A.; Hocking, A.M.; Pribitzer, S.; Thorpe, J.; Fuchs, B.; Wiedeman, A.E.; et al. Abatacept Increases T Cell Exhaustion in Early RA Individuals Who Carry HLA Risk Alleles. Front. Immunol. 2024, 15, 1383110. [Google Scholar] [CrossRef]
- Long, S.A.; Thorpe, J.; DeBerg, H.A.; Gersuk, V.; Eddy, J.A.; Harris, K.M.; Ehlers, M.; Herold, K.C.; Nepom, G.T.; Linsley, P.S. Partial Exhaustion of CD8 T Cells and Clinical Response to Teplizumab in New-Onset Type 1 Diabetes. Sci. Immunol. 2016, 1, eaai7793. [Google Scholar] [CrossRef] [PubMed]
- Aghbash, P.S.; Hemmat, N.; Nahand, J.S.; Shamekh, A.; Memar, M.Y.; Babaei, A.; Baghi, H.B. The Role of Th17 Cells in Viral Infections. Int. Immunopharmacol. 2021, 91, 107331. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
| Treatment or Group | |||||||
|---|---|---|---|---|---|---|---|
| BARICITINIB n = 36 | FILGOTINIB n = 19 | TOFACITINIB n = 14 | UPADACITINIB n = 9 | ABATACEPT n = 9 | TOCILIZUMAB n = 11 | Healthy Controls n = 20 | |
| Age, years, mean ± SD | 64.4 ± 10.4 | 61 ± 11.4 | 55.5 ± 8.6 | 63.1 ± 7.8 | 66.2 ± 9 | 60.3 ± 10.2 | 60 ± 4.3 |
| Women, n (%) | 28 (77.8) | 18 (94.7) | 14 (100) | 9 (100) | 5 (55.6) | 8 (72.7) | 17 (85) |
| RA duration, months, mean ± SD | 178.3 ± 135.4 | 256 ± 307.1 | 166.9 ± 65.4 | 65.1 ± 53.9 | 236.1 ± 135.9 | 238.1 ± 96.1 | NA |
| CVRF, n (%) | 31 (86.1) | 14 (73.7) | 9 (64.3) | 5 (55.6) | 9 (100) | 9 (81.8) | NA |
| RF/ACPA, n (%) | 25 (69.4) | 17 (89.5) | 10 (71.4) | 6 (66.7) | 8 (88.9) | 8 (72.7) | 1 (5) |
| Extra-articular manifestations, n (%) | 6 (16.6) | 0 | 2 (14.3) | 0 | 2 (22.2) | 0 | NA |
| Erosive, n (%) | 12 (33.3) | 7 (36.8) | 6 (42.9) | 6 (66.7) | 7 (77.8) | 6 (54.5) | NA |
| Comorbidities, n (%) | 34 (94.4) | 18 (94.7) | 14 (100) | 9 (100) | 9 (100) | 10 (90.9) | NA |
| Current treatment | |||||||
| Prednisone dose, mg/day, median [IQR] | 2.5 [0–5] | 2.5 [0–5] | 2.5 [0–7.5] | 5 [0–10] | 5 [5–7.5] | 0 [0–2] | 0 [0–0] |
| NSAIDs, n (%) | 12 (33.3) | 6 (31.6) | 9 (64.3) | 8 (88.9) | 6 (66.7) | 8 (72.7) | NA |
| MTX, n (%) | 7 (19.4) | 4 (21.1) | 4 (28.6) | 1 (11.1) | 1 (11.1) | 2 (18.2) | 0 |
| JAKi or bDMARD monotherapy #, n (%) | 19 (52.8) | 9 (47.4) | 8 (57.1) | 5 (55.5) | 3 (33.3) | 6 (54.5) | 0 |
| Previous treatment | |||||||
| MTX, n (%) | 36 (100) | 19 (100) | 14 (100) | 9 (100) | 8 (88.9) | 10 (90.9) | NA |
| Other cDMARDS, n (%) | 29 (80.6) | 17 (89.5) | 8 (57.1) | 8 (88.9) | 3 (33.3) | 3 (27.3) | NA |
| AntiTNF, n (%) | 29 (80.6) | 16 (84.2) | 13 (92.9) | 6 (66.7) | 3 (33.3) | 5 (45.5) | NA |
| Anti-IL6, n (%) | 15 (41.7) | 14 (73.7) | 7 (50) | 6 (66.7) | 4 (44.4) | 0 | NA |
| Previous cDMARDS, number, median [IQR] | 2 [2–3] | 3 [2–3] | 1.5 [1–4] | 2.5 [1.3–3.8] | 1 [1–1] | 1 [1–1] | NA |
| Previous bDMARDS, number, median [IQR] | 2 [1–3] | 2 [1.3–3] | 2 [1–2] | 2 [0.5–3.75] | 1 [1–2] | 0 [0–2] | NA |
| RA status | |||||||
| DAS28, RCP, remission, n (%) | 14 (38.9) | 12 (63.2) | 7 (50) | 2 (25) | NA | NA | NA |
| DAS28, RCP, low disease activity, n (%) | 9 (25) | 0 | 3 (27.3) | 0 | NA | NA | NA |
| RAPID3, remission, n (%) | 17 (47.2) | 12/18 (66.7) | 3/5 (60) | 1/4 (25) | NA | NA | NA |
| CDAI, remission, n (%) | 12 (33.3) | 6 (31.6) | 0 | 2 (33.3) | NA | NA | NA |
| Ultrasonography remission, n (%) | 22 (61.1) | 6 (31.6) | 8 (57.1) | 3 (50) | NA | NA | NA |
| Inflammatory markers | |||||||
| CRP (mg/dL), median [IQR] | 0.4 [0.4–0.4] | 0.4 [0.4–0.7] | 0.4 [0.4–0.9] | 1 [0.4–1.4] | 0.4 [0.4–0.6] | 0.4 [0.4–0.4] | 0.4 [0.4–0.4] |
| ESR (mm/1st hour], mean ± DS | 15.2 ± 21.9 | 10.7 ± 22.8 | 28.4 ± 15.1 | 25.8 ± 27.1 | 25.2 ± 20.7 | 7.0 ± 6.7 | NA |
| Circulating calprotectin (μg/mL), median [IQR] * | 1.28 [0.98–1.8] | 0.87 [0.72–1.23] | - | 1.14 [0.91–1.25] | 1.8 [1.01–1.85] | 0.87 [0.73–1.2] | 1.39 [1.01–1.65] |
| FL1 | FL2 | FL3 | FL4 | FL5 | FL6 | FL7 | FL8 | FL9 | FL10 | FL11 | FL12 | FL13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T cells | CXCR3 AF488 | CD127 PE | CD62L ECD | CD4 PC5.5 | CD8 PC7 | CD3 APC | CD45RA AF700 | CD294 APCCy7 | CCR6 PB | CD25 BV785 | |||
| Monocytes and NK | CD57 FITC | CD14 PE | CD56 ECD | NKp30 PC7 | CD3 APC | CD16 APCCy7 | SLAN VB | HLA-DR BV650 | |||||
| B cells | IgD FITC | CD27 PC7 | CD20 APC | CD21 APC700 | CD38 APC750 | CD19 PB | |||||||
| Extended NK cell analysis | CD57 FITC | CD16 PE | CD56 ECD | LILRB (ILT2) PerCP Cy5.5 | NKG2C APC | CD14 APC700 | CD69 APCCy7 | NKG2A BV421 | CD3 KrO | NKp30BBV605 | HLA-DR BV650 | NKp46 BV785 |
| Cell Subset | Surface Expression Marker |
|---|---|
| T cells | CD3+ |
| T helper cells | CD3+CD4+ |
| T cytotoxic cells | CD3+CD8+ |
| T helper 1 | CD3+CD4+CD45RA−CXCR3+CCR6− |
| T helper 17 | CD3+CD4+CD45RA−CXCR3−CCR6+ |
| T helper 1-17 | CD3+CD4+CD45RA−CXCR3+CCR6+ |
| T helper 2 | CD3+CD4+CD45RA−CXCR3−CCR6−CD294+ |
| T cytotoxic 1 | CD3+CD8+CD45RA−CXCR3+CCR6− |
| T cytotoxic 17 | CD3+CD8+CD45RA−CXCR3−CCR6+ |
| T cytotoxic 1-17 | CD3+CD8+CD45RA−CXCR3+CCR6+ |
| T cytotoxic 2 | CD3+CD8+CD45RA−CXCR3−CCR6−CD294+ |
| Effector memory T cells | CD3+CD62L−CD45RA− |
| Central memory T cells | CD3+CD62L+CD45RA− |
| Effector memory re-expressing RA | CD3+CD62L−CD45RA+ |
| Naïve T cells | CD3+CD62L+CD45RA+ |
| Regulatory T cells | CD3+CD4+CD127−CD25+ |
| Effector memory T helper cells | CD3+CD4+CD62L−CD45RA− |
| Central memory T helper cells | CD3+CD4+CD62L+CD45RA− |
| Effector memory re-expressing RA T helper cells | CD3+CD4+CD62L−CD45RA+ |
| Naïve T helper cells | CD3+CD4+CD62L+CD45RA+ |
| Effector memory T cytotoxic cells | CD3+CD8+CD62L−CD45RA− |
| Central memory T cytotoxic cells | CD3+CD8+CD62L+CD45RA− |
| Effector memory re-expressing RA T cytotoxic cells | CD3+CD8+CD62L−CD45RA+ |
| Naïve T cytotoxic cells | CD3+CD8+CD62L+CD45RA+ |
| B cells | CD19+ |
| Double negative B cells | CD19+CD27−IgD− |
| Naïve B cells | CD19+IgD+CD27− |
| Switched memory B cells | CD19+IgD−CD27+ |
| Unswitched memory B cells | CD19+IgD+CD27+ |
| CD21 low B cells | CD19+CD27−CD21− |
| Plasmablasts | CD19+CD20−CD19+CD38++CD27++ |
| Natural killers | CD3−CD56+/CD16+ |
| NK bright | CD3−CD56++CD16− |
| NK Dim | CD3−CD56+CD16+ |
| NK CD16 | CD3−CD56−CD16+ |
| NKT | CD3+CD56+ |
| Monocytes | |
| Classical monocytes | CD14+CD16− |
| Intermediate monocytes | CD14+CD16+ |
| Non-classical monocytes | CD14−CD16+ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Cabero, J.J.; Lasa-Teja, C.; San Segundo, D.; Comins-Boo, A.; Irure-Ventura, J.; Walias Rivera, D.; Martín-Varillas, J.L.; Mata, C.; Santos, M.; Aurrecoechea, E.; et al. Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects. Int. J. Mol. Sci. 2025, 26, 5160. https://doi.org/10.3390/ijms26115160
Fernández-Cabero JJ, Lasa-Teja C, San Segundo D, Comins-Boo A, Irure-Ventura J, Walias Rivera D, Martín-Varillas JL, Mata C, Santos M, Aurrecoechea E, et al. Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects. International Journal of Molecular Sciences. 2025; 26(11):5160. https://doi.org/10.3390/ijms26115160
Chicago/Turabian StyleFernández-Cabero, Juan José, Carmen Lasa-Teja, David San Segundo, Alejandra Comins-Boo, Juan Irure-Ventura, David Walias Rivera, Jose Luis Martín-Varillas, Cristina Mata, Montserrat Santos, Elena Aurrecoechea, and et al. 2025. "Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects" International Journal of Molecular Sciences 26, no. 11: 5160. https://doi.org/10.3390/ijms26115160
APA StyleFernández-Cabero, J. J., Lasa-Teja, C., San Segundo, D., Comins-Boo, A., Irure-Ventura, J., Walias Rivera, D., Martín-Varillas, J. L., Mata, C., Santos, M., Aurrecoechea, E., Blanco, R., & López-Hoyos, M. (2025). Changes in NK Cells and Exhausted Th Cell Phenotype in RA Patients Treated with Janus Kinase Inhibitors: Implications for Adverse Effects. International Journal of Molecular Sciences, 26(11), 5160. https://doi.org/10.3390/ijms26115160








