Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers
Abstract
1. Introduction
2. Biomarker: A Concise Definition
3. Comparative Oncology: The Novel Approach to Biomarker Research
| Cancer Type | Key Biomarkers | Clinical Advances in Humans | Clinical Advances in Dogs | Translational Impact | References |
|---|---|---|---|---|---|
| Mammary (Breast) | HER2, ER, PR, Ki-67, p53 | HER2 and ER/PR are routinely used for prognostic assessment and to predict response to therapies | Similar expression of HER2 and ER/PR in canine mammary tumors (CMTs); under study for diagnostic and prognostic methods | Supports drug repurposing and predictive diagnostics across species | [18,23] |
| Lymphoma | CD20, CCL17 (TARC), PTPRK, Ki-67 | Anti-CD20 antibody rituximab is added to standard therapy protocols to extend survival rates for B-cell lymphoma patients; GS-9219 not clinically tested yet | CD20-targeted therapies are under research in canine B-cell lymphoma; the prodrug GS-9219 is under research trials that demonstrated safety and efficacy for treatment of canine lymphoma | Shared immune markers enable parallel development of antibody therapies; use of spontaneous tumors in canines as preclinical models for non-Hodgkin lymphoma | [24,25] |
| Melanoma | BRAF, NRAS, PTPRK, PD-L1 | BRAF mutations treated with vemurafenib; PD-L1 testing enables checkpoint inhibitor therapy | PD-L1 expressed in canine melanoma; c-KIT and TK inhibitors and other immunotherapies under clinical trials | Promotes checkpoint inhibitor research and immune profiling in veterinary oncology | [26,27] |
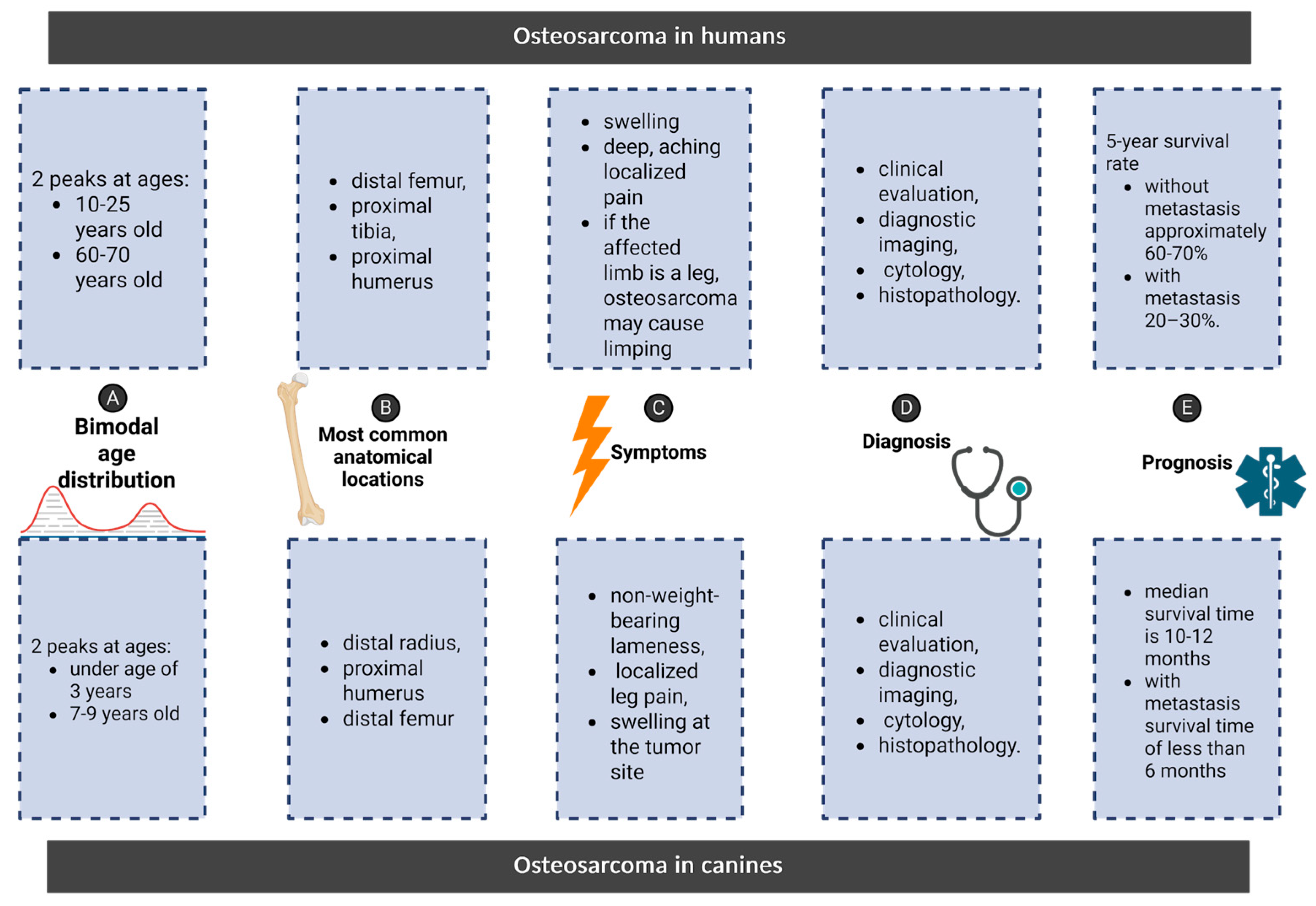
4. Osteosarcoma: Shared Features Between Humans and Canines
5. Parallels in Osteosarcoma: Comparing Clinical Features, Diagnosis, and Prognosis in Humans and Canines
5.1. Symptoms
5.2. Diagnosis
5.3. Prognosis
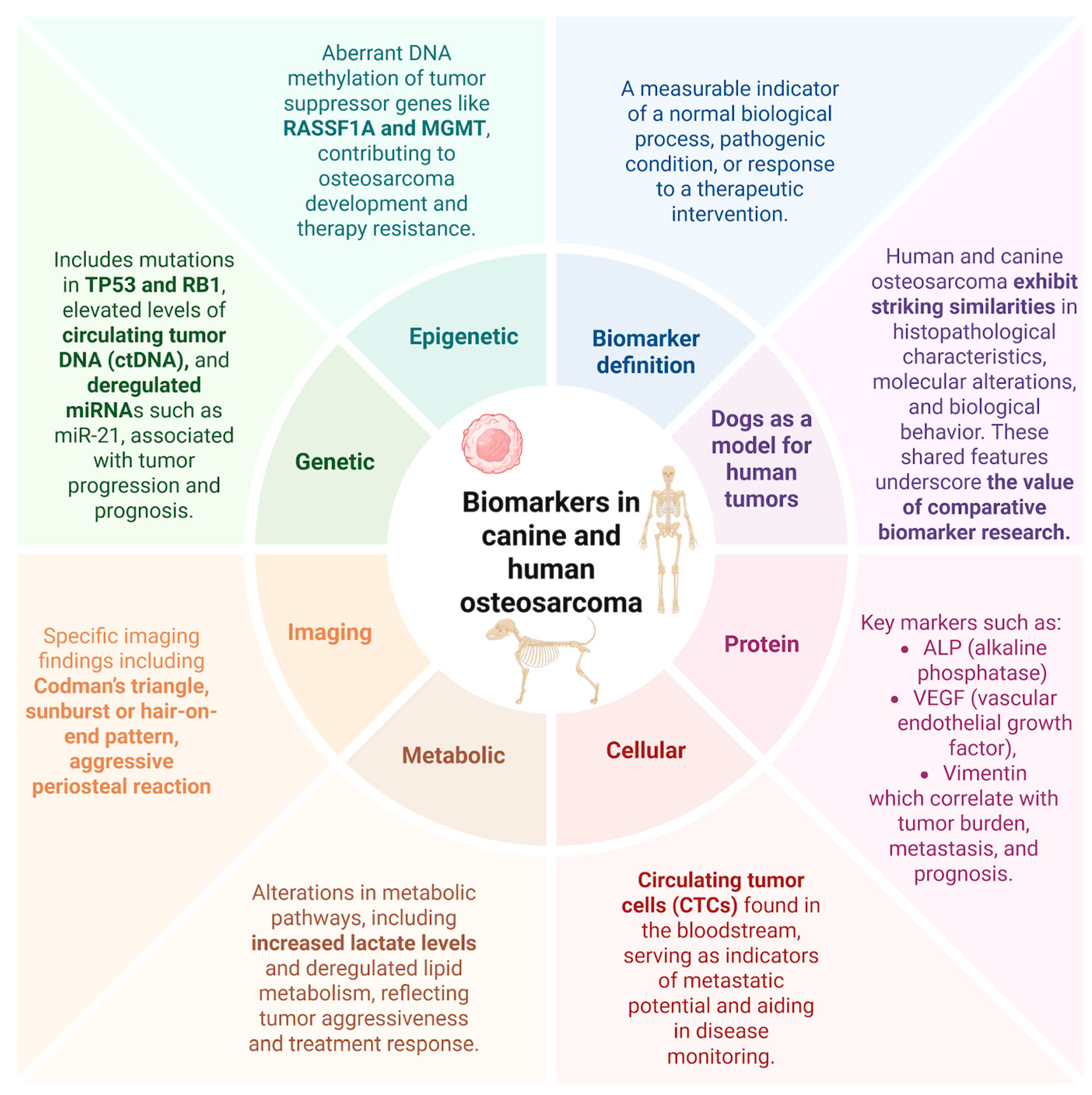
5.4. Biomarkers in Osteosarcoma: Translational Indicators of Disease and Therapeutic Response
6. Genetic and Epigenetic Biomarkers
7. Small Non-Coding RNAs (microRNA/miRNAs)
8. DNA Methylation Alterations
9. Protein Biomarkers
10. Cell-Based Biomarkers
11. Radiological Findings in Translational Osteosarcoma Research
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CTCs | Circulating Tumor Cells |
| ctDNA | Circulating Tumor DNA |
| DST | Dystonin |
| EMT | Epithelial Mesenchymal Transition |
| LDH | Dehydrogenase Lactate |
| miRNA | MicroRNA |
| ncRNA | Non-coding RNA |
| OSA | Osteosarcoma |
| VEGF | Vascular Endothelial Growth Factor |
| VIM | Vimentin |
References
- Makielski, K.M.; Mills, L.J.; Sarver, A.L.; Henson, M.S.; Spector, L.G.; Naik, S.; Modiano, J.F. Risk Factors for Development of Canine and Human Osteosarcoma: A Comparative Review. Vet. Sci. 2019, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Martano, M.; Buracco, P. Biology, diagnosis and treatment of canine appendicular osteosarcoma: Similarities and differences with human osteosarcoma. Vet. J. 2011, 189, 268–277. [Google Scholar] [CrossRef]
- Botter, S.M.; Neri, D.; Fuchs, B. Recent advances in osteosarcoma. Curr. Opin. Pharmacol. 2014, 16, 15–23. [Google Scholar] [CrossRef]
- Romanucci, M.; De Maria, R.; Morello, E.M.; Della Salda, L. Editorial: Canine osteosarcoma as a model in comparative oncology: Advances and perspective. Front. Vet. Sci. 2023, 10, 1141666. [Google Scholar] [CrossRef]
- Zamborsky, R.; Kokavec, M.; Harsanyi, S.; Danisovic, L. Identification of Prognostic and Predictive Osteosarcoma Biomarkers. Med. Sci. 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and other Tools (BEST) resource in neuro-oncology. Neuro-Oncology 2018, 20, 1162–1172. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dey, M.K.; Devireddy, R.; Gartia, M.R. Biomarkers in Cancer Detection, Diagnosis, and Prognosis. Sensors 2023, 24, 37. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, J.-Y. Comparative oncology: Overcoming human cancer through companion animal studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Simpson, S.; Dunning, M.D.; de Brot, S.; Grau-Roma, L.; Mongan, N.P.; Rutland, C.S. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet. Scand. 2017, 59, 71. [Google Scholar] [CrossRef] [PubMed]
- Tarone, L.; Barutello, G.; Iussich, S.; Giacobino, D.; Quaglino, E.; Buracco, P.; Cavallo, F.; Riccardo, F. Naturally occurring cancers in pet dogs as pre-clinical models for cancer immunotherapy. Cancer Immunol. Immunother. CII 2019, 68, 1839–1853. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.; Ren, L.; Huang, S.; Berger, E.; Bardales, K.; Mannheimer, J.; Mazcko, C.; LeBlanc, A. Canine and murine models of osteosarcoma. Vet. Pathol. 2022, 59, 399–414. [Google Scholar] [CrossRef] [PubMed]
- McGee, L.E.; Pereira, J.S.; McEachron, T.A.; Mazcko, C.; LeBlanc, A.K.; Beck, J.A. The tumor microenvironment of metastatic osteosarcoma in the human and canine lung. Commun. Biol. 2025, 8, 756. [Google Scholar] [CrossRef]
- Riccardo, F.; Aurisicchio, L.; Impellizeri, J.A.; Cavallo, F. The importance of comparative oncology in translational medicine. Cancer Immunol. Immunother. CII 2015, 64, 137–148. [Google Scholar] [CrossRef]
- Paoloni, M.C.; Khanna, C. Comparative oncology today. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1023–1032. [Google Scholar] [CrossRef]
- Nance, R.L.; Sajib, A.M.; Smith, B.F. Canine models of human cancer: Bridging the gap to improve precision medicine. Prog. Mol. Biol. Transl. Sci. 2022, 189, 67–99. [Google Scholar] [CrossRef]
- Peña, L.; Gama, A.; Goldschmidt, M.H.; Abadie, J.; Benazzi, C.; Castagnaro, M.; Díez, L.; Gärtner, F.; Hellmén, E.; Kiupel, M.; et al. Canine mammary tumors: A review and consensus of standard guidelines on epithelial and myoepithelial phenotype markers, HER2, and hormone receptor assessment using immunohistochemistry. Vet. Pathol. 2014, 51, 127–145. [Google Scholar] [CrossRef]
- Ito, D.; Frantz, A.M.; Modiano, J.F. Canine lymphoma as a comparative model for human non-Hodgkin lymphoma: Recent progress and applications. Vet. Immunol. Immunopathol. 2014, 159, 192–201. [Google Scholar] [CrossRef]
- Vail, D.M.; Thamm, D.H.; Reiser, H.; Ray, A.S.; Wolfgang, G.H.I.; Watkins, W.J.; Babusis, D.; Henne, I.N.; Hawkins, M.J.; Kurzman, I.D.; et al. Assessment of GS-9219 in a pet dog model of non-Hodgkin’s lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 3503–3510. [Google Scholar] [CrossRef]
- Nishiya, A.T.; Massoco, C.O.; Felizzola, C.R.; Perlmann, E.; Batschinski, K.; Tedardi, M.V.; Garcia, J.S.; Mendonça, P.P.; Teixeira, T.F.; Zaidan Dagli, M.L. Comparative Aspects of Canine Melanoma. Vet. Sci. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Paoloni, M.; Mazcko, C.; Khanna, C. The Comparative Oncology Trials Consortium: Using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009, 6, e1000161. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.J.L.; Bowen, R.L.; Jones, J.L.; Wells, C.A. Predictive markers in breast cancer—The present. Histopathology 2008, 52, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Gelain, M.E.; Comazzi, S. The dog as a possible animal model for human non-Hodgkin lymphoma: A review. Hematol. Oncol. 2013, 31, 1–9. [Google Scholar] [CrossRef]
- Dias, J.N.R.; Almeida, A.; André, A.S.; Aguiar, S.I.; Bule, P.; Nogueira, S.; Oliveira, S.S.; Carrapiço, B.; Gil, S.; Tavares, L.; et al. Characterization of the canine CD20 as a therapeutic target for comparative passive immunotherapy. Sci. Rep. 2022, 12, 2678. [Google Scholar] [CrossRef]
- He, X.; Gao, Y.; Deng, Y.; He, J.; Nolte, I.; Murua Escobar, H.; Yu, F. The Comparative Oncology of Canine Malignant Melanoma in Targeted Therapy: A Systematic Review of In Vitro Experiments and Animal Model Reports. Int. J. Mol. Sci. 2024, 25, 10387. [Google Scholar] [CrossRef]
- Fisher, R.; Larkin, J. Vemurafenib: A new treatment for BRAF-V600 mutated advanced melanoma. Cancer Manag. Res. 2012, 4, 243–252. [Google Scholar] [CrossRef]
- Wittenburg, L.A.; Gustafson, D.L.; Thamm, D.H. Phase I pharmacokinetic and pharmacodynamic evaluation of combined valproic acid/doxorubicin treatment in dogs with spontaneous cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 4832–4842. [Google Scholar] [CrossRef]
- Zapata, I.; Moraes, L.E.; Fiala, E.M.; Zaldivar-Lopez, S.; Couto, C.G.; Rowell, J.L.; Alvarez, C.E. Risk-modeling of dog osteosarcoma genome scans shows individuals with Mendelian-level polygenic risk are common. BMC Genom. 2019, 20, 226. [Google Scholar] [CrossRef]
- McCoy, B.M.; Brassington, L.; Jin, K.; Dolby, G.A.; Shrager, S.; Collins, D.; Dunbar, M.; Dog Aging Project Consortium; Ruple, A.; Snyder-Mackler, N. Social determinants of health and disease in companion dogs: A cohort study from the Dog Aging Project. Evol. Med. Public Health 2023, 11, 187–201. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Division on Earth and Life Studies; Institute for Laboratory Animal Research; Committee on Assessment of the Use and Care of Dogs in Biomedical Research Funded by or Conducted at the U.S. Department of Veterans Affairs. Necessity, Use, and Care of Laboratory Dogs at the U.S. Department of Veterans Affairs; National Academies Press: Washington DC, USA, 2020; ISBN 978-0-309-67641-0. [Google Scholar]
- Morrow, J.J.; Khanna, C. Osteosarcoma Genetics and Epigenetics: Emerging Biology and Candidate Therapies. Crit. Rev. Oncog. 2015, 20, 173–197. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.M.; Khanna, C. Comparative Aspects of Osteosarcoma Pathogenesis in Humans and Dogs. Vet. Sci. 2015, 2, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.S.; Gorlick, R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin. Adv. Hematol. Oncol. 2010, 8, 705–718. [Google Scholar]
- Sakthikumar, S.; Elvers, I.; Kim, J.; Arendt, M.L.; Thomas, R.; Turner-Maier, J.; Swofford, R.; Johnson, J.; Schumacher, S.E.; Alföldi, J.; et al. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 2018, 78, 3421–3431. [Google Scholar] [CrossRef]
- Varshney, J.; Scott, M.C.; Largaespada, D.A.; Subramanian, S. Understanding the Osteosarcoma Pathobiology: A Comparative Oncology Approach. Vet. Sci. 2016, 3, 3. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, M.; Malhotra, K.; Patel, S. Primary Osteosarcoma in the Elderly Revisited: Current Concepts in Diagnosis and Treatment. Curr. Oncol. Rep. 2018, 20, 13. [Google Scholar] [CrossRef]
- Meyers, P.A.; Gorlick, R. Osteosarcoma. Pediatr. Clin. N. Am. 1997, 44, 973–989. [Google Scholar] [CrossRef]
- Fenger, J.M.; London, C.A.; Kisseberth, W.C. Canine Osteosarcoma: A Naturally Occurring Disease to Inform Pediatric Oncology. ILAR J. 2014, 55, 69–85. [Google Scholar] [CrossRef]
- Crombé, A.; Simonetti, M.; Longhi, A.; Hauger, O.; Fadli, D.; Spinnato, P. Imaging of Osteosarcoma: Presenting Findings, Metastatic Patterns, and Features Related to Prognosis. J. Clin. Med. 2024, 13, 5710. [Google Scholar] [CrossRef]
- Choi, L.E.; Healey, J.H.; Kuk, D.; Brennan, M.F. Analysis of Outcomes in Extraskeletal Osteosarcoma: A Review of Fifty-three Cases. J. Bone Jt. Surg. 2014, 96, e2. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, A.; Anderson, M.; Dambach, D.; Sorenmo, K.; Shofer, F. Extraskeletal osteosarcomas in dogs: A retrospective study of 169 cases (1986–1996). J. Am. Anim. Hosp. Assoc. 1998, 34, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Orrapin, S.; Moonmuang, S.; Udomruk, S.; Yongpitakwattana, P.; Pruksakorn, D.; Chaiyawat, P. Unlocking the tumor-immune microenvironment in osteosarcoma: Insights into the immune landscape and mechanisms. Front. Immunol. 2024, 15, 1394284. [Google Scholar] [CrossRef] [PubMed]
- Silver, K.I.; Patkar, S.; Mazcko, C.; Berger, E.P.; Beck, J.A.; LeBlanc, A.K. Patterns of metastatic progression and association with clinical outcomes in canine osteosarcoma: A necropsy study of 83 dogs. Vet. Comp. Oncol. 2023, 21, 646–655. [Google Scholar] [CrossRef]
- Niswander, L.M.; Kim, S.Y. Stratifying osteosarcoma: Minimizing and maximizing therapy. Curr. Oncol. Rep. 2010, 12, 266–270. [Google Scholar] [CrossRef]
- Cam, M.; Gardner, H.L.; Roberts, R.D.; Fenger, J.M.; Guttridge, D.C.; London, C.A.; Cam, H. ΔNp63 mediates cellular survival and metastasis in canine osteosarcoma. Oncotarget 2016, 7, 48533–48546. [Google Scholar] [CrossRef]
- Doppelt-Flikshtain, O.; Younis, A.; Tamari, T.; Ginesin, O.; Shentzer-Kutiel, T.; Nikomarov, D.; Bar-Sela, G.; Coyac, B.R.; Assaraf, Y.G.; Zigdon-Giladi, H. Endothelial Progenitor Cells Promote Osteosarcoma Progression and Invasiveness via AKT/PI3K Signaling. Cancers 2023, 15, 1818. [Google Scholar] [CrossRef]
- Widhe, B.; Widhe, T. Initial symptoms and clinical features in osteosarcoma and Ewing sarcoma. J. Bone Jt. Surg. Am. 2000, 82, 667–674. [Google Scholar] [CrossRef]
- Wallack, S.T.; Wisner, E.R.; Werner, J.A.; Walsh, P.J.; Kent, M.S.; Fairley, R.A.; Hornof, W.J. Accuracy of Magnetic Resonance Imaging for Estimating Intramedullary Osteosarcoma Extent in Pre-Operative Planning of Canine Limb-Salvage Procedures. Vet. Radiol. Ultrasound 2002, 43, 432–441. [Google Scholar] [CrossRef]
- Bielack, S.S.; Hecker-Nolting, S.; Blattmann, C.; Kager, L. Advances in the management of osteosarcoma. F1000Research 2016, 5, 2767. [Google Scholar] [CrossRef]
- Mueller, F.; Fuchs, B.; Kaser-Hotz, B. Comparative Biology of Human and Canine Osteosarcoma. Anticancer Res. 2007, 27, 155–164. [Google Scholar] [PubMed]
- Wittig, J.C.; Bickels, J.; Priebat, D.; Jelinek, J.; Kellar-Graney, K.; Shmookler, B.; Malawer, M.M. Osteosarcoma: A Multidisciplinary Approach to Diagnosis and Treatment. Am. Fam. Physician 2002, 65, 1123–1133. [Google Scholar] [PubMed]
- de Lorimier, L.-P.; Fan, T.M. Delayed diagnosis of fungal osteomyelitis with early scintigraphic lesions in a dog. Can. Vet. J. Rev. Vet. Can. 2010, 51, 1394–1396. [Google Scholar]
- Mannheimer, J.D.; Tawa, G.; Gerhold, D.; Braisted, J.; Sayers, C.M.; McEachron, T.A.; Meltzer, P.; Mazcko, C.; Beck, J.A.; LeBlanc, A.K. Transcriptional profiling of canine osteosarcoma identifies prognostic gene expression signatures with translational value for humans. Commun. Biol. 2023, 6, 856. [Google Scholar] [CrossRef]
- Das, S.; Idate, R.; Fowles, J.S.; Lana, S.E.; Regan, D.P.; Gustafson, D.L.; Duval, D.L. Molecular Landscape of Canine Osteosarcoma. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Selvarajah, G.T.; Kirpensteijn, J. Prognostic and predictive biomarkers of canine osteosarcoma. Vet. J. 2010, 185, 28–35. [Google Scholar] [CrossRef]
- Fu, H.-L.; Shao, L.; Wang, Q.; Jia, T.; Li, M.; Yang, D.-P. A systematic review of p53 as a biomarker of survival in patients with osteosarcoma. Tumor Biol. 2013, 34, 3817–3821. [Google Scholar] [CrossRef]
- Levine, R.A.; Fleischli, M.A. Inactivation of p53 and Retinoblastoma Family Pathways in Canine Osteosarcoma Cell Lines. Vet. Pathol. 2000, 37, 54–61. [Google Scholar] [CrossRef]
- Vimalraj, S.; Sekaran, S. RUNX Family as a Promising Biomarker and a Therapeutic Target in Bone Cancers: A Review on Its Molecular Mechanism(s) behind Tumorigenesis. Cancers 2023, 15, 3247. [Google Scholar] [CrossRef]
- Barger, A.; Graca, R.; Bailey, K.; Messick, J.; de Lorimier, L.-P.; Fan, T.; Hoffmann, W. Use of Alkaline Phosphatase Staining to Differentiate Canine Osteosarcoma from Other Vimentin-positive Tumors. Vet. Pathol. 2005, 42, 161–165. [Google Scholar] [CrossRef]
- Leonardi, L.; Manuali, E.; Bufalari, A.; Porcellato, I. Canine soft tissue sarcomas: The expression of RUNX2 and karyopherin alpha-2 in extraskeletal (soft tissues) and skeletal osteosarcomas. Front. Vet. Sci. 2024, 11, 1292852. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ito, T.; Alex, D.; Vanderbilt, C.M.; Chang, J.C.; Islamdoust, N.; Zhang, Y.; Nafa, K.; Healey, J.; Ladanyi, M.; et al. RUNX2 (6p21.1) Amplification in Osteosarcoma. Hum. Pathol. 2019, 94, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Alegre, F.; Ormonde, A.R.; Godinez, D.R.; Illendula, A.; Bushweller, J.H.; Wittenburg, L.A. The interaction between RUNX2 and core binding factor beta as a potential therapeutic target in canine osteosarcoma. Vet. Comp. Oncol. 2020, 18, 52–63. [Google Scholar] [CrossRef]
- Patrașcu, A.-V.; Țarcă, E.; Lozneanu, L.; Ungureanu, C.; Moroșan, E.; Parteni, D.-E.; Jehac, A.; Bernic, J.; Cojocaru, E. The Role of Epithelial-Mesenchymal Transition in Osteosarcoma Progression: From Biology to Therapy. Diagnostics 2025, 15, 644. [Google Scholar] [CrossRef]
- Tufail, M. PTEN-mediated resistance in cancer: From foundation to future therapies. Toxicol. Rep. 2025, 14, 101987. [Google Scholar] [CrossRef]
- Georgescu, M.-M. PTEN Tumor Suppressor Network in PI3K-Akt Pathway Control. Genes Cancer 2010, 1, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Su, X.; Xia, Q.; Wang, J.; Kan, S. Expression of NF-κB and PTEN in osteosarcoma and its clinical significance. Oncol. Lett. 2017, 14, 6744–6748. [Google Scholar] [CrossRef]
- Chen, M.-W.; Wu, X.-J. SLC25A22 Promotes Proliferation and Metastasis of Osteosarcoma Cells via the PTEN Signaling Pathway. Technol. Cancer Res. Treat. 2018, 17, 1533033818811143. [Google Scholar] [CrossRef]
- Hinton, K.; Kirk, A.; Paul, P.; Persad, S. Regulation of the Epithelial to Mesenchymal Transition in Osteosarcoma. Biomolecules 2023, 13, 398. [Google Scholar] [CrossRef]
- Takeda, T.; Tsubaki, M.; Genno, S.; Tomita, K.; Nishida, S. RANK/RANKL axis promotes migration, invasion, and metastasis of osteosarcoma via activating NF-κB pathway. Exp. Cell Res. 2024, 436, 113978. [Google Scholar] [CrossRef]
- Ge, R.; Huang, G.M. Targeting transforming growth factor beta signaling in metastatic osteosarcoma. J. Bone Oncol. 2023, 43, 100513. [Google Scholar] [CrossRef] [PubMed]
- Issagholian, L.; Tabaie, E.; Reddy, A.J.; Ghauri, M.S.; Patel, R. Expression of E-cadherin and N-cadherin in Epithelial-to-Mesenchymal Transition of Osteosarcoma: A Systematic Review. Cureus 2023, 15, e49521. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Marycz, K.; Smieszek, A. Small and Long Non-coding RNAs as Functional Regulators of Bone Homeostasis, Acting Alone or Cooperatively. Mol. Ther. Nucleic Acids 2020, 21, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Skipar, P.; Bartnik, E.; Piątkowski, J.; Sulejczak, D.; Czarnecka, A.M. MicroRNA signatures in osteosarcoma: Diagnostic insights and therapeutic prospects. Mol. Cell. Biochem. 2024, 480, 2065–2075. [Google Scholar] [CrossRef]
- Kobayashi, E.; Hornicek, F.J.; Duan, Z. MicroRNA Involvement in Osteosarcoma. Sarcoma 2012, 2012, 359739. [Google Scholar] [CrossRef]
- Llobat, L.; Gourbault, O. Role of MicroRNAs in Human Osteosarcoma: Future Perspectives. Biomedicines 2021, 9, 463. [Google Scholar] [CrossRef]
- Gally, T.B.; Aleluia, M.M.; Borges, G.F.; Kaneto, C.M. Circulating MicroRNAs as Novel Potential Diagnostic Biomarkers for Osteosarcoma: A Systematic Review. Biomolecules 2021, 11, 1432. [Google Scholar] [CrossRef]
- Leonardi, L.; Scotlandi, K.; Pettinari, I.; Benassi, M.S.; Porcellato, I.; Pazzaglia, L. MiRNAs in Canine and Human Osteosarcoma: A Highlight Review on Comparative Biomolecular Aspects. Cells 2021, 10, 428. [Google Scholar] [CrossRef]
- Dailey, D.D.; Hess, A.M.; Bouma, G.J.; Duval, D.L. MicroRNA Expression Changes and Integrated Pathways Associated with Poor Outcome in Canine Osteosarcoma. Front. Vet. Sci. 2021, 8, 637622. [Google Scholar] [CrossRef]
- Zhu, X.-B.; Zhang, Z.-C.; Han, G.-S.; Han, J.-Z.; Qiu, D.-P. Overexpression of miR-214 promotes the progression of human osteosarcoma by regulating the Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2017, 15, 1884–1892. [Google Scholar] [CrossRef]
- Cai, H.; Miao, M.; Wang, Z. miR-214-3p promotes the proliferation, migration and invasion of osteosarcoma cells by targeting CADM1. Oncol. Lett. 2018, 16, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Rehei, A.-L.; Zhang, L.; Fu, Y.-X.; Mu, W.-B.; Yang, D.-S.; Liu, Y.; Zhou, S.-J.; Younusi, A. MicroRNA-214 functions as an oncogene in human osteosarcoma by targeting TRAF3. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5156–5164. [Google Scholar] [CrossRef]
- Ludwig, L.; Edson, M.; Treleaven, H.; Viloria-Petit, A.M.; Mutsaers, A.J.; Moorehead, R.; Foster, R.A.; Ali, A.; Wood, R.D.; Wood, G.A. Plasma microRNA signatures predict prognosis in canine osteosarcoma patients. PLoS ONE 2024, 19, e0311104. [Google Scholar] [CrossRef]
- Ren, X.; Shen, Y.; Zheng, S.; Liu, J.; Jiang, X. miR-21 predicts poor prognosis in patients with osteosarcoma. Br. J. Biomed. Sci. 2016, 73, 158–162. [Google Scholar] [CrossRef]
- Wang, S.; Ma, F.; Feng, Y.; Liu, T.; He, S. Role of exosomal miR-21 in the tumor microenvironment and osteosarcoma tumorigenesis and progression (Review). Int. J. Oncol. 2020, 56, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Z.; Wu, Z.-Z.; Lv, Z. The Clinical Significance of miR-21 in Guiding Chemotherapy for Patients with Osteosarcoma. Pharmacogenomics Pers. Med. 2021, 14, 1247–1261. [Google Scholar] [CrossRef]
- Zamboni, C.; Zamarian, V.; Stefanello, D.; Ferrari, R.; Auletta, L.; Milanesi, S.; Mauri, S.; Grieco, V.; Ceciliani, F.; Lecchi, C. Plasma small extracellular vesicles from dogs affected by cutaneous mast cell tumors deliver high levels of miR-21-5p. Front. Vet. Sci. 2022, 9, 1083174. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Liu, Y.; Liao, W.; Liu, R.; Shi, P.; Wang, L. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J. Bone Oncol. 2016, 5, 74–79. [Google Scholar] [CrossRef]
- Ji, Q.; Xu, X.; Song, Q.; Xu, Y.; Tai, Y.; Goodman, S.B.; Bi, W.; Xu, M.; Jiao, S.; Maloney, W.J.; et al. miR-223-3p Inhibits Human Osteosarcoma Metastasis and Progression by Directly Targeting CDH6. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 1299–1312. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, W.; Liu, Y.; Guo, A.; Yang, D. Let-7b acts as a tumor suppressor in osteosarcoma via targeting IGF1R. Oncol. Lett. 2019, 17, 1646–1654. [Google Scholar] [CrossRef]
- Wei, H.; Cui, R.; Bahr, J.; Zanesi, N.; Luo, Z.; Meng, W.; Liang, G.; Croce, C.M. miR-130a Deregulates PTEN and Stimulates Tumor Growth. Cancer Res. 2017, 77, 6168–6178. [Google Scholar] [CrossRef]
- Ludwig, L.; Vanderboon, E.N.; Treleaven, H.; Wood, R.D.; Schott, C.R.; Wood, G.A. Patient-matched tumours, plasma, and cell lines reveal tumour microenvironment- and cell culture-specific microRNAs. Biol. Open 2024, 13, bio060483. [Google Scholar] [CrossRef]
- Mills, L.J.; Scott, M.C.; Shah, P.; Cunanan, A.R.; Deshpande, A.; Auch, B.; Curtin, B.; Beckman, K.B.; Spector, L.G.; Sarver, A.L.; et al. Comparative analysis of genome-wide DNA methylation identifies patterns that associate with conserved transcriptional programs in osteosarcoma. Bone 2022, 158, 115716. [Google Scholar] [CrossRef]
- González-Rodríguez, P.; Engskog-Vlachos, P.; Zhang, H.; Murgoci, A.-N.; Zerdes, I.; Joseph, B. SETD2 mutation in renal clear cell carcinoma suppress autophagy via regulation of ATG12. Cell Death Dis. 2020, 11, 69. [Google Scholar] [CrossRef]
- Xie, Y.; Sahin, M.; Sinha, S.; Wang, Y.; Nargund, A.M.; Lyu, Y.; Han, S.; Dong, Y.; Hsieh, J.J.; Leslie, C.S.; et al. SETD2 loss perturbs the kidney cancer epigenetic landscape to promote metastasis and engenders actionable dependencies on histone chaperone complexes. Nat. Cancer 2022, 3, 188–202. [Google Scholar] [CrossRef]
- Suehara, Y.; Alex, D.; Bowman, A.; Middha, S.; Zehir, A.; Chakravarty, D.; Wang, L.; Jour, G.; Nafa, K.; Hayashi, T.; et al. Clinical Genomic Sequencing of Pediatric and Adult Osteosarcoma Reveals Distinct Molecular Subsets with Potentially Targetable Alterations. Clin. Cancer Res. 2019, 25, 6346–6356. [Google Scholar] [CrossRef]
- Espinoza Pereira, K.N.; Shan, J.; Licht, J.D.; Bennett, R.L. Histone mutations in cancer. Biochem. Soc. Trans. 2023, 51, 1749–1763. [Google Scholar] [CrossRef]
- Mancini, M.; Monaldi, C.; De Santis, S.; Papayannidis, C.; Rondoni, M.; Sartor, C.; Bruno, S.; Pagano, L.; Criscuolo, M.; Zanotti, R.; et al. SETD2 non genomic loss of function in advanced systemic mastocytosis is mediated by an Aurora kinase A/MDM2 axis and can be therapeutically targeted. Biomark. Res. 2023, 11, 29. [Google Scholar] [CrossRef]
- Gardner, H.L.; Sivaprakasam, K.; Briones, N.; Zismann, V.; Perdigones, N.; Drenner, K.; Facista, S.; Richholt, R.; Liang, W.; Aldrich, J.; et al. Canine osteosarcoma genome sequencing identifies recurrent mutations in DMD and the histone methyltransferase gene SETD2. Commun. Biol. 2019, 2, 266. [Google Scholar] [CrossRef]
- Beheshtizadeh, N.; Gharibshahian, M.; Bayati, M.; Maleki, R.; Strachan, H.; Doughty, S.; Tayebi, L. Vascular endothelial growth factor (VEGF) delivery approaches in regenerative medicine. Biomed. Pharmacother. 2023, 166, 115301. [Google Scholar] [CrossRef]
- Scheidegger, P.; Weiglhofer, W.; Suarez, S.; Kaser-Hotz, B.; Steiner, R.; Ballmer-Hofer, K.; Jaussi, R. Vascular Endothelial Growth Factor (VEGF) and Its Receptors in Tumor-Bearing Dogs. Biol. Chem. 1999, 380, 1449–1454. [Google Scholar] [CrossRef]
- Thamm, D.H.; O’Brien, M.G.; Vail, D.M. Serum vascular endothelial growth factor concentrations and postsurgical outcome in dogs with osteosarcoma. Vet. Comp. Oncol. 2008, 6, 126–132. [Google Scholar] [CrossRef]
- Tabone, M.-D.; Brugières, L.; Piperno-Neumann, S.; Selva, M.-A.; Marec-Bérard, P.; Pacquement, H.; Lervat, C.; Corradini, N.; Gentet, J.-C.; Couderc, R.; et al. Prognostic impact of blood and urinary angiogenic factor levels at diagnosis and during treatment in patients with osteosarcoma: A prospective study. BMC Cancer 2017, 17, 419. [Google Scholar] [CrossRef]
- Qu, Y.; Xu, J.; Jiang, T.; Zhao, H.; Gao, Y.; Zheng, C.; Shi, X. Difference in Pre- and Postchemotherapy Vascular Endothelial Growth Factor Levels as a Prognostic Indicator in Osteosarcoma. J. Int. Med. Res. 2011, 39, 1474–1482. [Google Scholar] [CrossRef]
- Kim, S.H.; Shin, K.-H.; Moon, S.-H.; Jang, J.; Kim, H.S.; Suh, J.-S.; Yang, W.-I. Reassessment of alkaline phosphatase as serum tumor marker with high specificity in osteosarcoma. Cancer Med. 2017, 6, 1311–1322. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Sun, L.-L.; Li, H.-Y.; Ye, Z.-M. Prognostic Significance of Serum Alkaline Phosphatase Level in Osteosarcoma: A Meta-Analysis of Published Data. BioMed Res. Int. 2015, 2015, 160835. [Google Scholar] [CrossRef]
- Hao, H.; Chen, L.; Huang, D.; Ge, J.; Qiu, Y.; Hao, L. Meta-analysis of alkaline phosphatase and prognosis for osteosarcoma. Eur. J. Cancer Care 2017, 26, e12536. [Google Scholar] [CrossRef]
- Shu, J.; Tan, A.; Li, Y.; Huang, H.; Yang, J. The correlation between serum total alkaline phosphatase and bone mineral density in young adults. BMC Musculoskelet. Disord. 2022, 23, 467. [Google Scholar] [CrossRef]
- Gu, R.; Sun, Y. Does serum alkaline phosphatase level really indicate the prognosis in patients with osteosarcoma? A meta-analysis. J. Cancer Res. Ther. 2018, 14, S468. [Google Scholar] [CrossRef]
- McKenna, R.J.; Schwinn, C.P.; Soong, K.Y.; Higinbotham, N.L. Osteogenic sarcoma arising in Paget’s disease. Cancer 1964, 17, 42–66. [Google Scholar] [CrossRef]
- Ehrhart, N.; Dernell, W.S.; Hoffmann, W.E.; Weigel, R.M.; Powers, B.E.; Withrow, S.J. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990–1996). J. Am. Vet. Med. Assoc. 1998, 213, 1002–1006. [Google Scholar] [CrossRef]
- Garzotto, C.K.; Berg, J.; Hoffmann, W.E.; Rand, W.M. Prognostic Significance of Serum Alkaline Phosphatase Activity in Canine Appendicular Osteosarcoma. J. Vet. Intern. Med. 2000, 14, 587–592. [Google Scholar] [CrossRef]
- Walenta, S.; Mueller-Klieser, W.F. Lactate: Mirror and motor of tumor malignancy. Semin. Radiat. Oncol. 2004, 14, 267–274. [Google Scholar] [CrossRef]
- Chen, J.; Sun, M.; Hua, Y.; Cai, Z. Prognostic significance of serum lactate dehydrogenase level in osteosarcoma: A meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1205–1210. [Google Scholar] [CrossRef]
- Bao, J.; Zeng, J.; Song, C.; Yu, H.; Shi, Q.; Mai, W.; Qu, G. A Retrospective Clinicopathological Study of Osteosarcoma Patients with Metachronous Metastatic Relapse. J. Cancer 2019, 10, 2982–2990. [Google Scholar] [CrossRef]
- Ogenyi, S.I.; Madukwe, J.; Onyemelukwe, A.O.; Ngokere, A.A. Vimentin and Cytokeratin Immunostaining: The Role in Basic Diagnosis and Prognosis of Sarcomas. J. Drug Deliv. Ther. 2020, 10, 175–178. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Sittiju, P.; Chaiyawat, P.; Pruksakorn, D.; Klangjorhor, J.; Wongrin, W.; Phinyo, P.; Kamolphiwong, R.; Phanphaisarn, A.; Teeyakasem, P.; Kongtawelert, P.; et al. Osteosarcoma-Specific Genes as a Diagnostic Tool and Clinical Predictor of Tumor Progression. Biology 2022, 11, 698. [Google Scholar] [CrossRef]
- Wilk, S.S.; Zabielska-Koczywąs, K.A. Molecular Mechanisms of Canine Osteosarcoma Metastasis. Int. J. Mol. Sci. 2021, 22, 3639. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Wycislo, K.L.; Pondenis, H.; Fan, T.M.; Das, A. Comparative proteomic investigation of metastatic and non-metastatic osteosarcoma cells of human and canine origin. PLoS ONE 2017, 12, e0183930. [Google Scholar] [CrossRef] [PubMed]
- Al-Khan, A.A.; Gunn, H.J.; Day, M.J.; Tayebi, M.; Ryan, S.D.; Kuntz, C.A.; Saad, E.S.; Richardson, S.J.; Danks, J.A. Immunohistochemical Validation of Spontaneously Arising Canine Osteosarcoma as a Model for Human Osteosarcoma. J. Comp. Pathol. 2017, 157, 256–265. [Google Scholar] [CrossRef]
- Batth, I.S.; Li, S. Discovery of Cell-Surface Vimentin (CSV) as a Sarcoma Target and Development of CSV-Targeted IL12 Immune Therapy. Adv. Exp. Med. Biol. 2020, 1257, 169–178. [Google Scholar] [CrossRef]
- Nieminen, M.; Henttinen, T.; Merinen, M.; Marttila-Ichihara, F.; Eriksson, J.E.; Jalkanen, S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat. Cell Biol. 2006, 8, 156–162. [Google Scholar] [CrossRef]
- Arrindell, J.; Desnues, B. Vimentin: From a cytoskeletal protein to a critical modulator of immune response and a target for infection. Front. Immunol. 2023, 14, 1224352. [Google Scholar] [CrossRef]
- Le, M.-C.N.; Smith, K.A.; Dopico, P.J.; Greer, B.; Alipanah, M.; Zhang, Y.; Siemann, D.W.; Lagmay, J.P.; Fan, Z.H. Investigating surface proteins and antibody combinations for detecting circulating tumor cells of various sarcomas. Sci. Rep. 2024, 14, 12374. [Google Scholar] [CrossRef]
- Mu, H.; Zuo, D.; Chen, J.; Liu, Z.; Wang, Z.; Yang, L.; Shi, Q.; Hua, Y. Detection and surveillance of circulating tumor cells in osteosarcoma for predicting therapy response and prognosis. Cancer Biol. Med. 2022, 19, 1397–1409. [Google Scholar] [CrossRef]
- Dai, S.; Shao, X.; Wei, Q.; Du, S.; Hou, C.; Li, H.; Jin, D. Association of circulating tumor cells and IMP3 expression with metastasis of osteosarcoma. Front. Oncol. 2023, 13, 819357. [Google Scholar] [CrossRef]
- Wright, T.; Brisson, B.A.; Wood, G.A.; Oblak, M.; Mutsaers, A.J.; Sabine, V.; Skowronski, K.; Belanger, C.; Tiessen, A.; Bienzle, D. Flow Cytometric Detection of Circulating Osteosarcoma Cells in Dogs. Cytom. Part A 2019, 95, 997–1007. [Google Scholar] [CrossRef]
- Wright, T.F.; Brisson, B.A.; Belanger, C.R.; Tiessen, A.; Sabine, V.; Skowronski, K.; Wood, G.A.; Oblak, M.L.; Mutsaers, A.J.; Sears, W.; et al. Quantification of circulating tumour cells over time in dogs with appendicular osteosarcoma. Vet. Comp. Oncol. 2023, 21, 541–550. [Google Scholar] [CrossRef]
- Peihong, T.; Lingling, R.; Haifeng, H.; Chang, L.; Guifeng, L. Application and progress of X-ray, computed tomography, and magnetic resonance imaging radiomics in osteosarcoma. iRADIOLOGY 2023, 1, 262–268. [Google Scholar] [CrossRef]
- Hameed, M.; Dorfman, H. Primary malignant bone tumors—Recent developments. Semin. Diagn. Pathol. 2011, 28, 86–101. [Google Scholar] [CrossRef]
- Kundu, Z.S. Classification, imaging, biopsy and staging of osteosarcoma. Indian J. Orthop. 2014, 48, 238–246. [Google Scholar] [CrossRef]
- Musaddaq, T.; Musaddaq, B. Recent Advances in Image-Guided Tissue Sampling. Cureus 2024, 16, e71613. [Google Scholar] [CrossRef]
- Wang, T.; Ni, Y.; Liu, L. Innovative Imaging Techniques for Advancing Cancer Diagnosis and Treatment. Cancers 2024, 16, 2607. [Google Scholar] [CrossRef]
- Cè, M.; Cellina, M.; Ueanukul, T.; Carrafiello, G.; Manatrakul, R.; Tangkittithaworn, P.; Jaovisidha, S.; Fuangfa, P.; Resnick, D. Multimodal Imaging of Osteosarcoma: From First Diagnosis to Radiomics. Cancers 2025, 17, 599. [Google Scholar] [CrossRef]
- Kalus, S.; Vidoni, A.; Oliveira, I.; Saifuddin, A. Image-guided core needle biopsy for Ewing sarcoma of bone: A 10-year single-institution review. Eur. Radiol. 2020, 30, 5308–5314. [Google Scholar] [CrossRef]
- Krimins, R.A.; Fritz, J.; Gainsburg, L.A.; Gavin, P.R.; Ihms, E.A.; Huso, D.L.; Kraitchman, D.L. Use of magnetic resonance imaging-guided biopsy of a vertebral body mass to diagnose osteosarcoma in a Rottweiler. J. Am. Vet. Med. Assoc. 2017, 250, 779–784. [Google Scholar] [CrossRef]
- Zhong, J.; Hu, Y.; Zhang, G.; Xing, Y.; Ding, D.; Ge, X.; Pan, Z.; Yang, Q.; Yin, Q.; Zhang, H.; et al. An updated systematic review of radiomics in osteosarcoma: Utilizing CLAIM to adapt the increasing trend of deep learning application in radiomics. Insights Imaging 2022, 13, 138. [Google Scholar] [CrossRef]
- Gao, Z.; Dai, Z.; Ouyang, Z.; Li, D.; Tang, S.; Li, P.; Liu, X.; Jiang, Y.; Song, D. Radiomics analysis in differentiating osteosarcoma and chondrosarcoma based on T2-weighted imaging and contrast-enhanced T1-weighted imaging. Sci. Rep. 2024, 14, 26594. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolnicka, A.; Fosse, V.; Raciborska, A.; Śmieszek, A. Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers. Int. J. Mol. Sci. 2025, 26, 5152. https://doi.org/10.3390/ijms26115152
Dolnicka A, Fosse V, Raciborska A, Śmieszek A. Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers. International Journal of Molecular Sciences. 2025; 26(11):5152. https://doi.org/10.3390/ijms26115152
Chicago/Turabian StyleDolnicka, Agnieszka, Vibeke Fosse, Anna Raciborska, and Agnieszka Śmieszek. 2025. "Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers" International Journal of Molecular Sciences 26, no. 11: 5152. https://doi.org/10.3390/ijms26115152
APA StyleDolnicka, A., Fosse, V., Raciborska, A., & Śmieszek, A. (2025). Building a Therapeutic Bridge Between Dogs and Humans: A Review of Potential Cross-Species Osteosarcoma Biomarkers. International Journal of Molecular Sciences, 26(11), 5152. https://doi.org/10.3390/ijms26115152





