Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts
Abstract
1. Introduction
2. Results
2.1. Pre-Treatment with Quercetin, Kaempferol or Capsaicin Did Not Affect αSMA Intensity in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
2.2. Pre-Treatment with Quercetin, Kaempferol or Capsaicin Did Not Affect MyoD Intensity in Two-Days Differentiated C2C12 Cells Exposed to TGF-β1
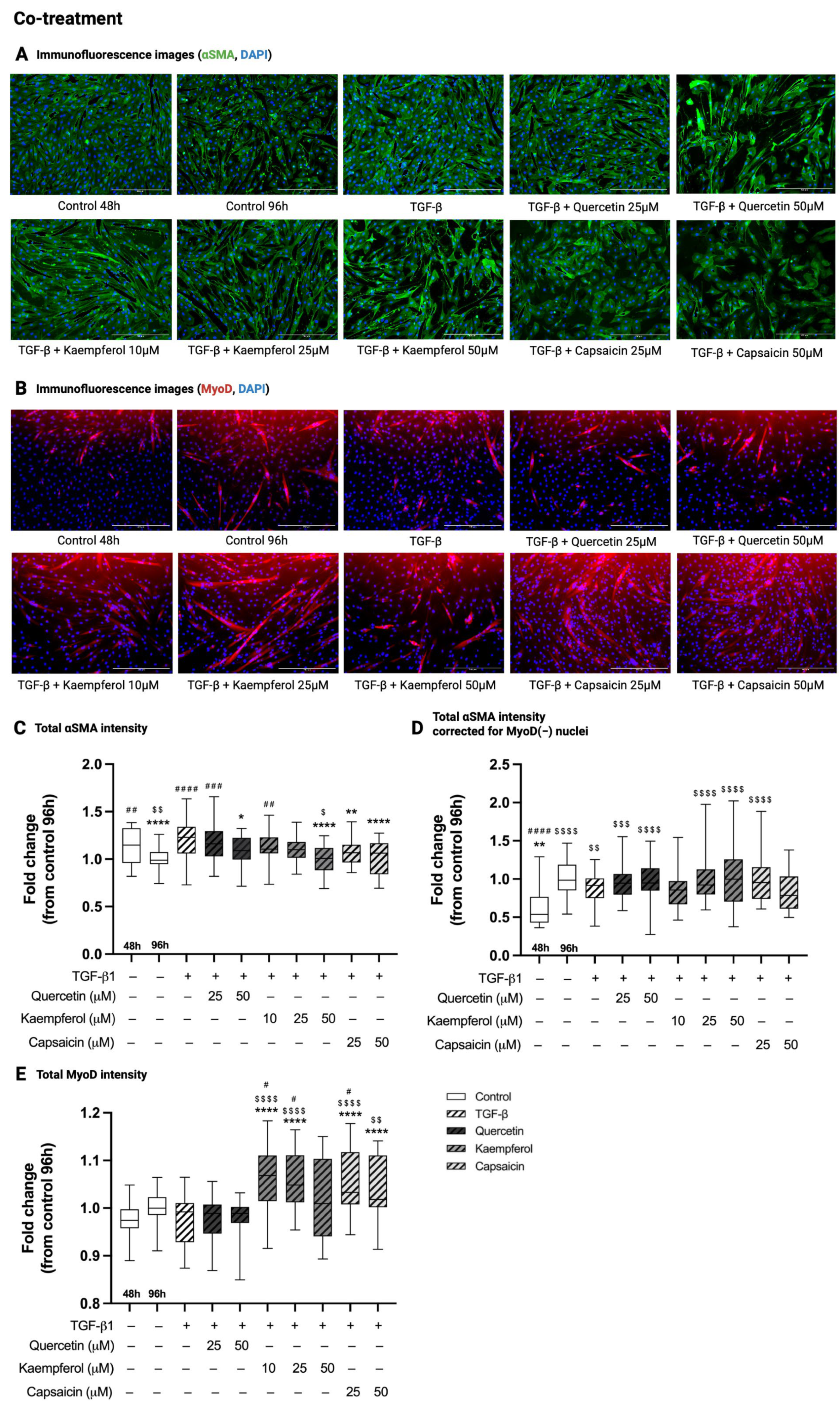
2.3. Co-Treatment with Quercetin, Kaempferol or Capsaicin Reduced αSMA Intensity in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
2.4. Co-Treatment with Kaempferol or Capsaicin Increased MyoD Intensity in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
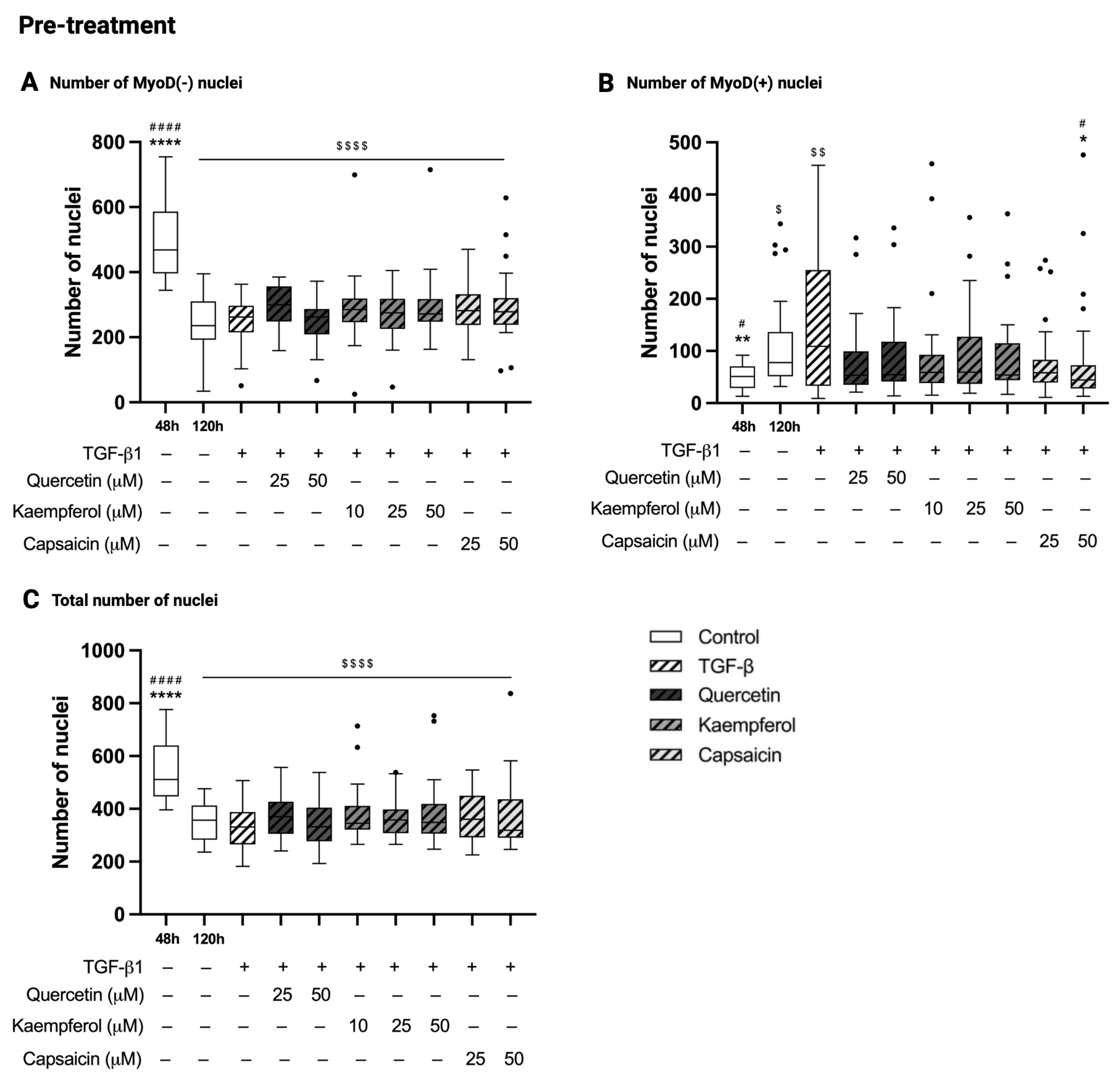
2.5. Pre-Treatment with Quercetin, Kaempferol or Capsaicin Did Not Affect the Number of Nuclei in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
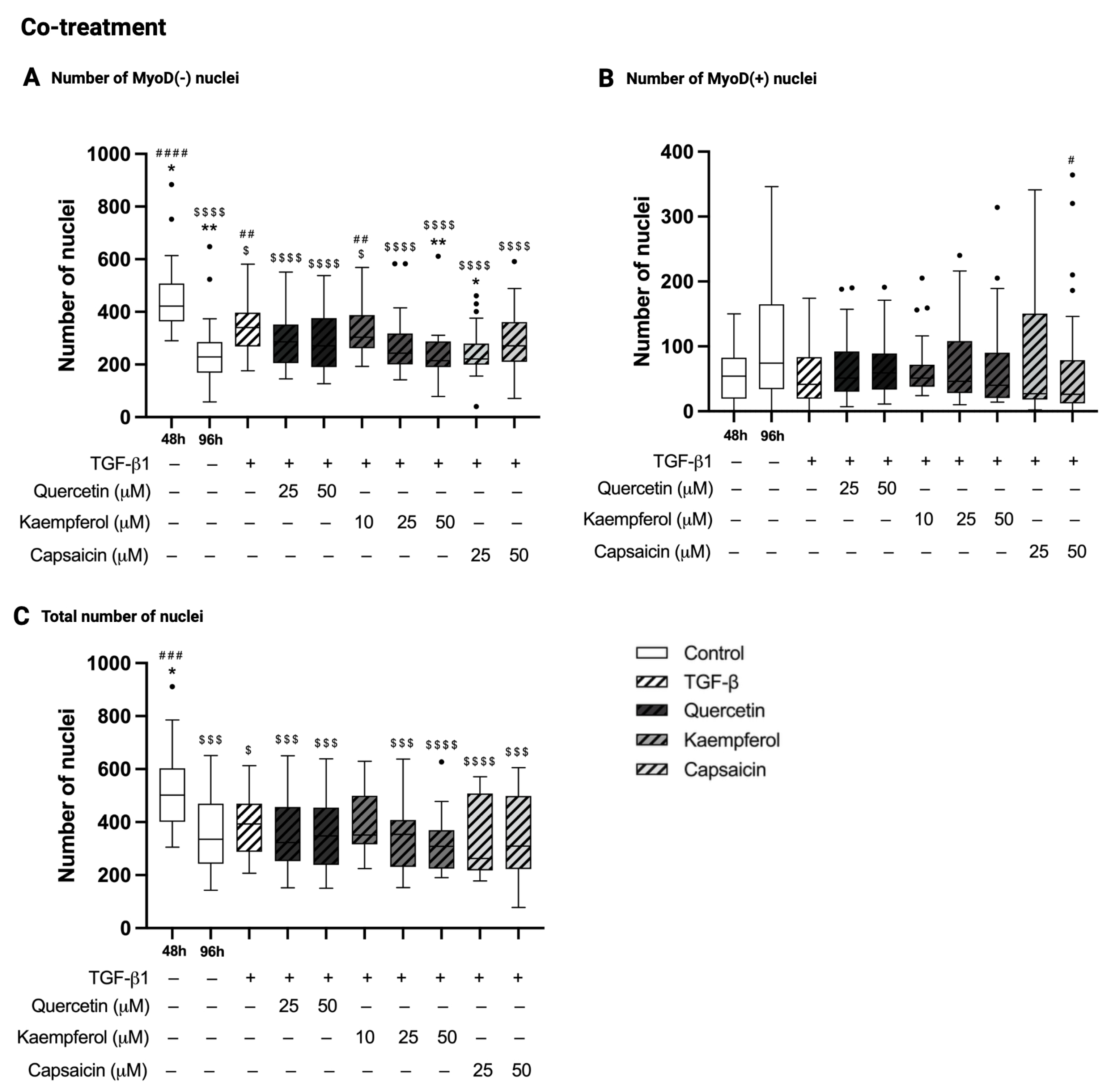
2.6. Co-Treatment with Kaempferol or Capsaicin Reduced the Number of MyoD-Negative Nuclei in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
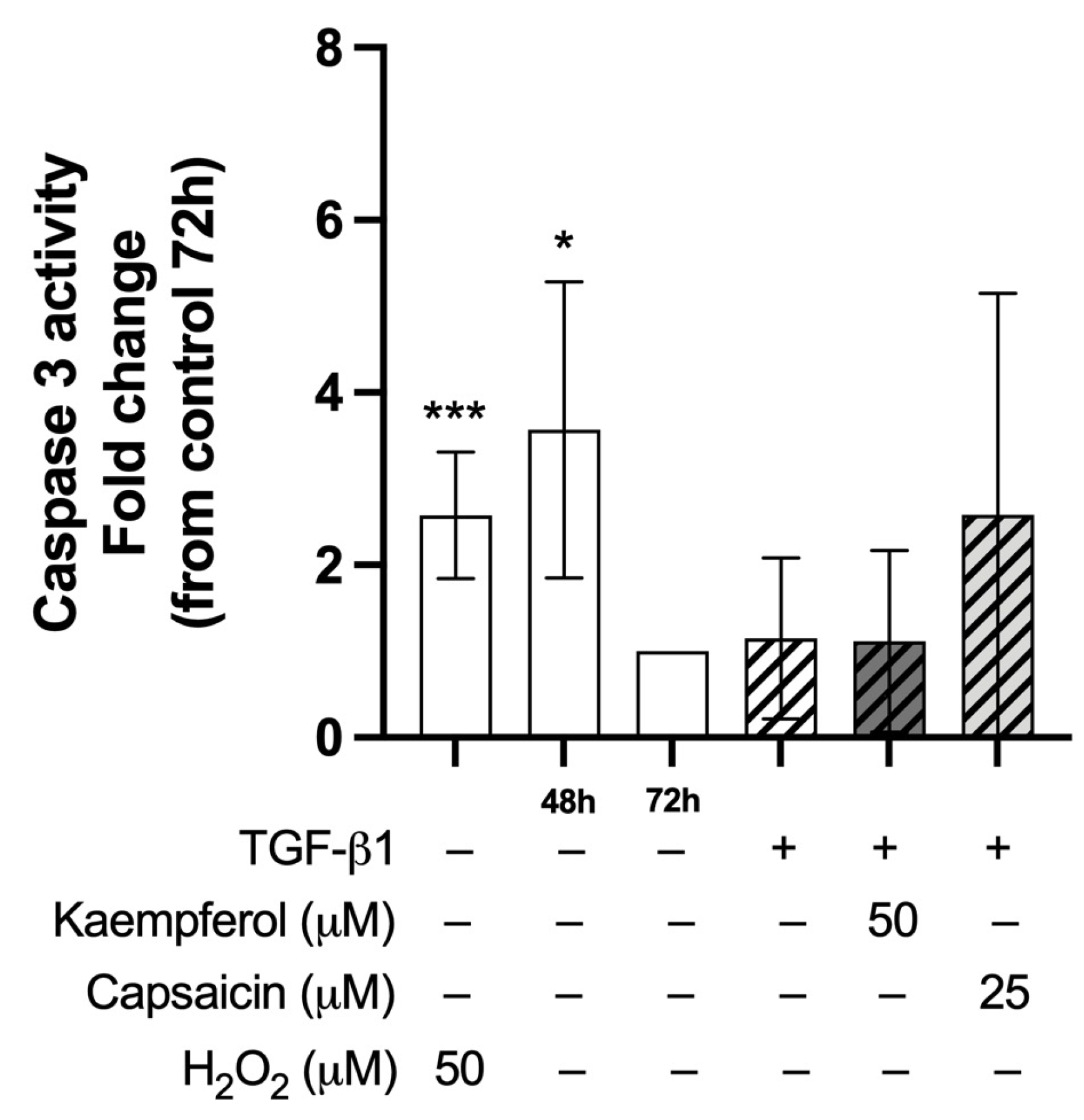
2.7. Co-Treatment with Kaempferol or Capsaicin Did Not Affect Caspase-3 Activity in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
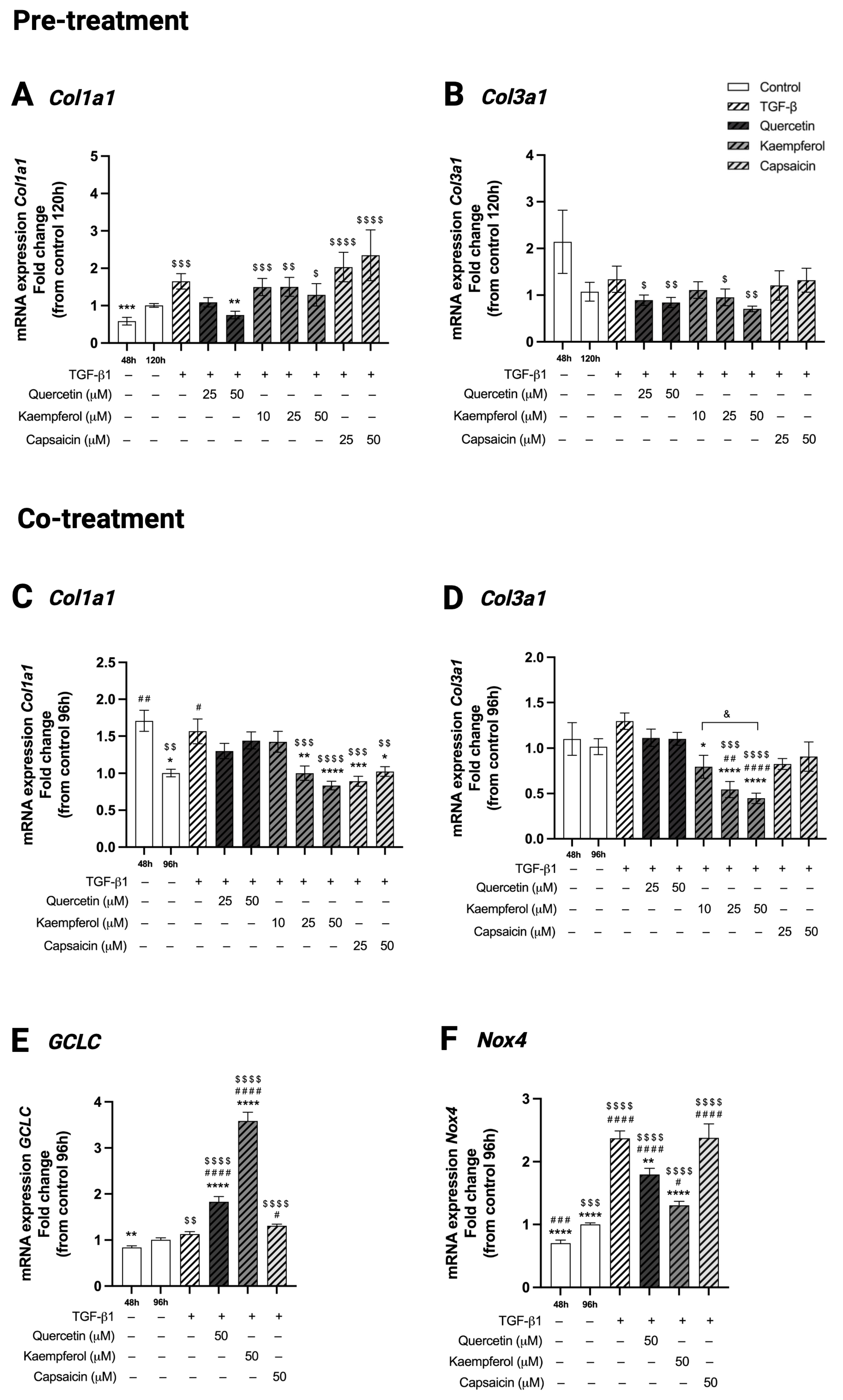
2.8. Pre-Treatment with Quercetin Reduced Collagen Type 1 (Col1a1) Gene Expression in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
2.9. Co-Treatment with Kaempferol or Capsaicin Reduced Collagen Type I (Col1a1) and Kaempferol Reduced Collagen Type 3 (Col3a1) Gene Expression in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
2.10. Co-Treatment with Quercetin or Kaempferol Increased GCLC Gene Expression in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
2.11. Co-Treatment with Quercetin or Kaempferol Decreased Nox4 Gene Expression in Two-Day-Differentiated C2C12 Cells Exposed to TGF-β1
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Myogenic Differentiation
4.3. Myofibroblast Differentiation and Phytochemical Treatment
4.4. Immunofluorescence Stainings of Myogenic and Fibrotic Markers
4.5. Image Analyses
4.6. RNA Extraction and qPCR
4.7. Caspase-3 Activity Assay
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ac-DEVD-pNA | N-Acetyl-Asp-Glu-Val-Asp p-nitroanilide |
| αSMA | alpha smooth muscle actin |
| BAX | BCL-2-like protein |
| β-catenin | catenin beta-1 |
| BCL-2 | B-cell lymphoma 2 |
| BSA | bovine serum albumin |
| cDNA | complementary DNA |
| Col1a1 | collagen type 1 |
| Col3a1 | collagen type 3 |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMD | Duchenne muscular dystrophy |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | dimethylsulfoxide |
| DPBS | Dulbecco’s phosphate-buffered saline |
| DTT | dithiothreitol |
| ECM | extracellular matrix |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GCLC | glutamate cysteine ligase catalytic subunit |
| GSH | glutathione |
| HEPES | N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid |
| hiFBS | heat-inactivated fetal bovine serum |
| H2O2 | hydrogen peroxide |
| IGF1R/AKT/mTOR | insulin-like growth factor 1/protein kinase B/mammalian target of rapamycin |
| ITG1B/FAK/paxillin | integrin-β1/focal adhesion kinase/paxillin |
| LDH | lactate dehydrogenase |
| MAPK | mitogen-activated protein kinase |
| mTOR | mammalian target of rapamycin |
| MyoD | myoblast determination protein 1 |
| MyoD(-) | myoblast determination protein 1-negative |
| MyoD(+) | myoblast determination protein 1-positive |
| NADPH oxidase/Nox | nicotinamide adenine dinucleotide phosphate oxidase |
| Nrf-2 | nuclear factor erythroid 2-related factor 2 |
| qPCR | quantitative polymerase chain reaction |
| ROS | reactive oxygen species |
| SEM | standard error of the mean |
| TGF-β1 | transforming growth factor beta 1 |
| YWHAZ | 14-3-3 protein zeta/delta |
Appendix A
| Antigen | Host Species | Species Reactivity | Dilution Factor | Supplier | Category Number |
|---|---|---|---|---|---|
| α-Smooth muscle actin | Mouse | Mouse, human, rat, chicken, frog, canine, rabbit, guinea pig, goat, bovine, sheep, snake | 1:500 | Sigma Aldrich (Saint Louis, MO, USA) | A5228 |
| MyoD | Rabbit | Mouse, human | 1:500 | Sigma Aldrich | ZRB1452 |
| Host species | Species reactivity | Dilution factor | Supplier | Category number | |
| Alexa Fluor™ Plus 488 | Goat | Mouse | 1:500 | ThermoFisher Scientific (Waltham, MA, USA) | A48286 |
| Texas Red | Goat | Rabbit | 1:500 | Sigma Aldrich | SAB3700888 |
References
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Talbot, L.A.; Schrager, M.; Conwit, R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, B359–B365. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Reginster, J.Y. The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef]
- Brack, A.S.; Bildsoe, H.; Hughes, S.M. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J. Cell Sci. 2005, 118, 4813–4821. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Gutarra, S.; García-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardí, M.; Ballestar, E.; González, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Neves, J.; Sousa-Victor, P. Understanding muscle regenerative decline with aging: New approaches to bring back youthfulness to aged stem cells. FEBS J. 2020, 287, 406–416. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Nincheri, P.; Donati, C.; Bruni, P. Transforming growth factor-beta1 induces transdifferentiation of myoblasts into myofibroblasts via up-regulation of sphingosine kinase-1/S1P3 axis. Mol. Biol. Cell 2010, 21, 1111–1124. [Google Scholar] [CrossRef]
- Tai, Y.; Woods, E.L.; Dally, J.; Kong, D.; Steadman, R.; Moseley, R.; Midgley, A.C. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules 2021, 11, 1095. [Google Scholar] [CrossRef]
- Hu, B.; Wu, Z.; Phan, S.H. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am. J. Respir. Cell Mol. Biol. 2003, 29, 397–404. [Google Scholar] [CrossRef]
- Evans, R.A.; Tian, Y.C.; Steadman, R.; Phillips, A.O. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp. Cell Res. 2003, 282, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdóttir, S.; Deries, M.; Cachaço, A.S.; Bajanca, F. The extracellular matrix dimension of skeletal muscle development. Dev. Biol. 2011, 354, 191–207. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Phan, S.H. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am. J. Respir. Cell Mol. Biol. 1999, 21, 658–665. [Google Scholar] [CrossRef]
- Casalena, G.; Daehn, I.; Bottinger, E. Transforming growth factor-β, bioenergetics, and mitochondria in renal disease. Semin. Nephrol. 2012, 32, 295–303. [Google Scholar] [CrossRef]
- Herranz-Itúrbide, M.; Peñuelas-Haro, I.; Espinosa-Sotelo, R.; Bertran, E.; Fabregat, I. The TGF-β/NADPH Oxidases Axis in the Regulation of Liver Cell Biology in Health and Disease. Cells 2021, 10, 2312. [Google Scholar] [CrossRef]
- Liu, R.M.; Gaston Pravia, K.A. Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic. Biol. Med. 2010, 48, 1–15. [Google Scholar] [CrossRef]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef]
- Alnaqeeb, M.A.; Al Zaid, N.S.; Goldspink, G. Connective tissue changes and physical properties of developing and ageing skeletal muscle. J. Anat. 1984, 139, 677–689. [Google Scholar]
- Wood, L.K.; Kayupov, E.; Gumucio, J.P.; Mendias, C.L.; Claflin, D.R.; Brooks, S.V. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J. Appl. Physiol. 2014, 117, 363–369. [Google Scholar] [CrossRef]
- Cao, Y.; Chen, H.; Sun, Y.; Fan, Z.; Cheng, H. Quercetin inhibits fibroblasts proliferation and reduces surgery-induced epidural fibrosis via the autophagy-mediated PI3K/Akt/mTOR pathway. Bioengineered 2022, 13, 9973–9986. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jin, S.W.; Choi, C.Y.; Kim, H.G.; Lee, G.H.; Kim, Y.A.; Chung, Y.C.; Jeong, H.G. Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 2017, 65, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.H.; Cho, I.H.; Shin, D.; Han, S.Y.; Park, S.H.; Kang, Y.H. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab. Investig. 2014, 94, 297–308. [Google Scholar] [CrossRef]
- Guan, Y.; Quan, D.; Chen, K.; Kang, L.; Yang, D.; Wu, H.; Yan, M.; Wu, S.; Lv, L.; Zhang, G. Kaempferol inhibits renal fibrosis by suppression of the sonic hedgehog signaling pathway. Phytomedicine 2023, 108, 154246. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Guo, S.; Liu, Y.; Zhao, X.; Li, R.; Yan, X.; Li, Y.; Wang, S.; Niu, X.; et al. Kaempferol Alleviates Angiotensin II-Induced Cardiac Dysfunction and Interstitial Fibrosis in Mice. Cell. Physiol. Biochem. 2017, 43, 2253–2263. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Li, X.; Tang, S.; Meng, D.; Xia, W.; Wang, H.; Wu, Y.; Zhou, X.; Zhang, J. Capsaicin ameliorates renal fibrosis by inhibiting TGF-β1-Smad2/3 signaling. Phytomedicine 2022, 100, 154067. [Google Scholar] [CrossRef]
- Ren, J.; Li, J.; Liu, X.; Feng, Y.; Gui, Y.; Yang, J.; He, W.; Dai, C. Quercetin Inhibits Fibroblast Activation and Kidney Fibrosis Involving the Suppression of Mammalian Target of Rapamycin and β-catenin Signaling. Sci. Rep. 2016, 6, 23968. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Motegi, S.I.; Fujiwara, C.; Yamazaki, S.; Inoue, Y.; Uchiyama, A.; Akai, R.; Iwawaki, T.; Ishikawa, O. Inhibitory effect of kaempferol on skin fibrosis in systemic sclerosis by the suppression of oxidative stress. J. Dermatol. Sci. 2019, 96, 8–17. [Google Scholar] [CrossRef]
- Xu, T.; Huang, S.; Huang, Q.; Ming, Z.; Wang, M.; Li, R.; Zhao, Y. Kaempferol attenuates liver fibrosis by inhibiting activin receptor-like kinase 5. J. Cell. Mol. Med. 2019, 23, 6403–6410. [Google Scholar] [CrossRef]
- Abdulaal, W.H.; Asfour, H.Z.; Helmi, N.; Al Sadoun, H.; Eldakhakhny, B.; Alhakamy, N.A.; Alqarni, H.M.; Alzahrani, S.A.M.; El-Moselhy, M.A.; Sharkawi, S.S.; et al. Capsaicin ameliorate pulmonary fibrosis via antioxidant Nrf-2/PPAR-γ pathway activation and inflammatory TGF-β1/NF-κB/COX II pathway inhibition. Front. Pharmacol. 2024, 15, 1333715. [Google Scholar] [CrossRef]
- Reyes-Avendaño, I.; Reyes-Jiménez, E.; González-García, K.; Pérez-Figueroa, D.C.; Baltiérrez-Hoyos, R.; Tapia-Pastrana, G.; Sánchez-Chino, X.M.; Villa-Treviño, S.; Arellanes-Robledo, J.; Vásquez-Garzón, V.R. Quercetin Regulates Key Components of the Cellular Microenvironment during Early Hepatocarcinogenesis. Antioxidants 2022, 11, 358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Zhou, S.; Xie, Z.; Wang, C.; Gao, Y.; Zhou, J.; Zhang, X.; Li, Q. Flavones hydroxylated at 5, 7, 3′ and 4′ ameliorate skin fibrosis via inhibiting activin receptor-like kinase 5 kinase activity. Cell Death Dis. 2019, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Morré, D.J.; Chueh, P.J.; Morré, D.M. Capsaicin inhibits preferentially the NADH oxidase and growth of transformed cells in culture. Proc. Natl. Acad. Sci. USA 1995, 92, 1831–1835. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Q.; Huang, Z.; Zhu, J.; Qiu, J.; Ma, W.; Yang, X.; Ding, F.; Sun, H. Isoquercitrin Delays Denervated Soleus Muscle Atrophy by Inhibiting Oxidative Stress and Inflammation. Front. Physiol. 2020, 11, 988. [Google Scholar] [CrossRef]
- Wang, W.; Ma, B.L.; Xu, C.G.; Zhou, X.J. Dihydroquercetin protects against renal fibrosis by activating the Nrf2 pathway. Phytomedicine 2020, 69, 153185. [Google Scholar] [CrossRef]
- Hao, W.; Li, M.; Cai, Q.; Wu, S.; Li, X.; He, Q.; Hu, Y. Roles of NRF2 in Fibrotic Diseases: From Mechanisms to Therapeutic Approaches. Front. Physiol. 2022, 13, 889792. [Google Scholar] [CrossRef]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: A key event in muscle fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Shi, A.; Hillege, M.M.G.; Wüst, R.C.I.; Wu, G.; Jaspers, R.T. Synergistic short-term and long-term effects of TGF-β1 and 3 on collagen production in differentiating myoblasts. Biochem. Biophys. Res. Commun. 2021, 547, 176–182. [Google Scholar] [CrossRef]
- Liu, D.; Black, B.L.; Derynck, R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001, 15, 2950–2966. [Google Scholar] [CrossRef]
- Massagué, J.; Cheifetz, S.; Endo, T.; Nadal-Ginard, B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc. Natl. Acad. Sci. USA 1986, 83, 8206–8210. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhou, L.; Zhang, T.; Qin, C.; Wei, P.; Luo, L.; Luo, L.; Huang, G.; Chen, A.; Liu, G. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/mTOR signaling pathway. Life Sci. 2020, 250, 117552. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, S.; Li, D.; Zhang, Y.; Tang, B.; Qiu, C.; Yang, Y.; Yang, D. Dietary capsaicin ameliorates pressure overload-induced cardiac hypertrophy and fibrosis through the transient receptor potential vanilloid type 1. Am. J. Hypertens. 2014, 27, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Choi, B.M.; Chen, X.Y.; Zhu, R.Z.; Kim, Y.; So, H.; Park, R.; Sung, M.; Kim, B.R. Kaempferol suppresses cisplatin-induced apoptosis via inductions of heme oxygenase-1 and glutamate-cysteine ligase catalytic subunit in HEI-OC1 cell. Pharm. Res. 2010, 27, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Weng, Q.; Gong, S.; Zhang, W.; Wang, J.; Huang, Y.; Li, Y.; Guo, J.; Lan, T. Kaempferol prevents acetaminophen-induced liver injury by suppressing hepatocyte ferroptosis via Nrf2 pathway activation. Food Funct. 2023, 14, 1884–1896. [Google Scholar] [CrossRef]
- Hinz, B.; Lagares, D. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef]
- Loomis, T.; Hu, L.Y.; Wohlgemuth, R.P.; Chellakudam, R.R.; Muralidharan, P.D.; Smith, L.R. Matrix stiffness and architecture drive fibro-adipogenic progenitors’ activation into myofibroblasts. Sci. Rep. 2022, 12, 13582. [Google Scholar] [CrossRef]
- Duan, D.; Derynck, R. Transforming growth factor-β (TGF-β)-induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J. Biol. Chem. 2019, 294, 8490–8504. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Birkova, A. Quercetin as an effective antioxidant against superoxide radical. Funct. Food Sci. 2023, 3, 15–25. [Google Scholar] [CrossRef]
- Fazilatun, N.; Nornisah, M.; Zhari, I. Superoxide radical scavengingproperties of extracts and flavonoids isolated from the leavesof Blumea balsamifera. Pharm. Biol. 2005, 43, 15–20. [Google Scholar] [CrossRef]
- Hour, T.C.; Lan Nhi, N.T.; Lai, I.J.; Chuu, C.P.; Lin, P.C.; Chang, H.W.; Su, Y.F.; Chen, C.H.; Chen, Y.K. Kaempferol-Enhanced Migration and Differentiation of C2C12 Myoblasts via ITG1B/FAK/Paxillin and IGF1R/AKT/mTOR Signaling Pathways. Mol. Nutr. Food Res. 2024, 68, e2300685. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.Y.; Jang, E.J.; Lee, E.H.; Bae, M.A.; Hong, J.H.; Hwang, E.S. Hesperedin promotes MyoD-induced myogenic differentiation in vitro and in vivo. Br. J. Pharmacol. 2011, 163, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Yang, J.M.; Wu, Y.; Li, H.; Zhong, Y.B.; Luo, Y.; Xie, R.L. Curcumin-activated Wnt5a pathway mediates Ca(2+) channel opening to affect myoblast differentiation and skeletal muscle regeneration. J. Cachexia Sarcopenia Muscle 2024, 15, 1834–1849. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Lee, S.J.; Kim, Y.K.; Seo, D.W.; Baek, N.I.; Ryu, J.H.; Kang, J.S.; Bae, G.U. Dehydrocorydaline promotes myogenic differentiation via p38 MAPK activation. Mol. Med. Rep. 2016, 14, 3029–3036. [Google Scholar] [CrossRef]
- Artaud-Macari, E.; Goven, D.; Brayer, S.; Hamimi, A.; Besnard, V.; Marchal-Somme, J.; Ali, Z.E.; Crestani, B.; Kerdine-Römer, S.; Boutten, A.; et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox Signal. 2013, 18, 66–79. [Google Scholar] [CrossRef]
- Zhang, M.; Chang, G.; Gao, S.; Wei, J.; Chen, M.; Song, L.; Lu, J.; Sheng, J.; Ma, X. Kaempferol protects against dexamethasone-induced muscle atrophy in mice by increasing PI3K/AKT/mTOR and NRF2/HO-1/KEAP1 signaling pathways: Network pharmacology, molecular docking, and experimental validation studies. Food Sci. Hum. Wellness 2024. Available online: https://www.sciopen.com/article/10.26599/FSHW.2024.9250362 (accessed on 22 May 2025). [CrossRef]
- Gabbiani, G. The biology of the myofibroblast. Kidney Int. 1992, 41, 530–532. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Gonzalez, K.N.; Kiosses, W.B.; Meech, R. Barx2 controls myoblast fusion and promotes MyoD-mediated activation of the smooth muscle alpha-actin gene. J. Biol. Chem. 2009, 284, 14866–14874. [Google Scholar] [CrossRef]
- Springer, M.L.; Ozawa, C.R.; Blau, H.M. Transient production of alpha-smooth muscle actin by skeletal myoblasts during differentiation in culture and following intramuscular implantation. Cell Motil. Cytoskelet. 2002, 51, 177–186. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Mora, M.; Krol, S.; Arrigoni, C.; Candrian, C.; Zanotti, S.; Moretti, M. Tackling muscle fibrosis: From molecular mechanisms to next generation engineered models to predict drug delivery. Adv. Drug Deliv. Rev. 2018, 129, 64–77. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Ugolini, G.S.; Sansoni, V.; Talò, G.; Perego, S.; Zanotti, S.; Ostano, P.; Mora, M.; Soncini, M.; et al. Engineering an Environment for the Study of Fibrosis: A 3D Human Muscle Model with Endothelium Specificity and Endomysium. Cell Rep. 2018, 25, 3858–3868.e3854. [Google Scholar] [CrossRef]
- Fede, C.; Fan, C.; Pirri, C.; Petrelli, L.; Biz, C.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. The Effects of Aging on the Intramuscular Connective Tissue. Int. J. Mol. Sci. 2022, 23, 11061. [Google Scholar] [CrossRef] [PubMed]
- Babraj, J.A.; Cuthbertson, D.J.; Smith, K.; Langberg, H.; Miller, B.; Krogsgaard, M.R.; Kjaer, M.; Rennie, M.J. Collagen synthesis in human musculoskeletal tissues and skin. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E864–E869. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Carrithers, J.A.; Trappe, S.W.; Trappe, T.A. Collagen, cross-linking, and advanced glycation end products in aging human skeletal muscle. J. Appl. Physiol. 2007, 103, 2068–2076. [Google Scholar] [CrossRef]
- Järvinen, T.A.; Józsa, L.; Kannus, P.; Järvinen, T.L.; Järvinen, M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. An immunohistochemical, polarization and scanning electron microscopic study. J. Muscle Res. Cell Motil. 2002, 23, 245–254. [Google Scholar] [CrossRef]
- Malis, V.; Sinha, U.; Smitaman, E.; Obra, J.K.L.; Langer, H.T.; Mossakowski, A.A.; Baar, K.; Sinha, S. Time-dependent diffusion tensor imaging and diffusion modeling of age-related differences in the medial gastrocnemius and feasibility study of correlations to histopathology. NMR Biomed. 2023, 36, e4996. [Google Scholar] [CrossRef]
- Cuijpers, I.; Dohmen, C.G.M.; Bouwman, F.G.; Troost, F.J.; Sthijns, M. Hesperetin but not ellagic acid increases myosin heavy chain expression and cell fusion in C2C12 myoblasts in the presence of oxidative stress. Front. Nutr. 2024, 11, 1377071. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequence Forward (5′ to 3′) | Primer Sequence Reverse (5′ to 3′) |
|---|---|---|
| Collagen type 1 (Col1a1) | CCTGGACGCCATCAAGGTCT | TTTTCCTTGGGGTTCGGGCT |
| Collagen type 3 (Col3a1) | GACCAAAAGGTGATGCTGGACAG | CAAGACCTCGTGCTCCAGTTAG |
| Glutamate cysteine ligase catalytic subunit (GCLC) | ATGTGGACACCCGATGCAGTATT | TGTCTTGCTTGTAGTCAGGATGGTTT |
| NADPH-oxidase 4 (Nox4) | GAACCCAAGTTCCAAGCTCATT | GGCACAAAGGTCCAGAAATCC |
| 14-3-3 protein zeta/delta (YWHAZ) | TGCTGGTGATGACAAGAAAGGAA | AACACAGAGAAGTTGAGGGCCA |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | CAACTCACTCAAGATTGTCAGCAA | TGGCAGTGATGGCATGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuijpers, I.; Sthijns, M.M.J.P.E.; van den Bogart, V.A.R.; Katsburg, J.; Leenders, C.F.M.; Troost, F.J. Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts. Int. J. Mol. Sci. 2025, 26, 5151. https://doi.org/10.3390/ijms26115151
Cuijpers I, Sthijns MMJPE, van den Bogart VAR, Katsburg J, Leenders CFM, Troost FJ. Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts. International Journal of Molecular Sciences. 2025; 26(11):5151. https://doi.org/10.3390/ijms26115151
Chicago/Turabian StyleCuijpers, Iris, Mireille M. J. P. E. Sthijns, Veerle A. R. van den Bogart, Joey Katsburg, Cliff F. M. Leenders, and Freddy J. Troost. 2025. "Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts" International Journal of Molecular Sciences 26, no. 11: 5151. https://doi.org/10.3390/ijms26115151
APA StyleCuijpers, I., Sthijns, M. M. J. P. E., van den Bogart, V. A. R., Katsburg, J., Leenders, C. F. M., & Troost, F. J. (2025). Quercetin, Kaempferol and Capsaicin Counteract the TGF-β1-Induced Upregulation of αSMA and Collagen in Myoblasts. International Journal of Molecular Sciences, 26(11), 5151. https://doi.org/10.3390/ijms26115151






