Abstract
Probiotics play a critical role in promoting the health of both humans and animals, with growing interest in the potential of animal-derived strains. Safety and efficacy assessments are crucial, with rigorous testing required to ensure the absence of harmful effects. The health benefits of animal-derived probiotic strains include improved digestion, balanced microbiota, behavioral impact, reduced inflammation, and minimized risk of infections. Probiotics of animal origin show promise as complementary or alternative options to antibiotics, with potential applications in both veterinary and human medicine. While promising, the usage of animal-derived probiotics requires careful evaluation of safety and regulatory aspects. This research underscores their potential for promoting health across species and contributing to future therapeutic approaches.
1. Introduction
Probiotics are living microorganisms that, when administered in adequate amounts, may confer a health benefit on the host [1]. Probiotic bacteria have been demonstrated to exert only a marginal effect on microbial diversity and typically fail to establish persistent colonization in the gut due to competitive exclusion by the resident microbiota [2]. Probiotic-derived components, such as bacteriocins, amines, and hydrogen peroxide, interact with specific targets within these pathways, thereby regulating apoptosis, cell proliferation, inflammation, and differentiation [3]. A prebiotic is a non-digestible compound that, when metabolized by gut microorganisms, influences the composition and activity of the gut microbiota, thereby providing a beneficial physiological effect on the host [4]. Prebiotics, by serving as substrates for beneficial microbes, promote a favorable gut environment, leading to the production of short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate [5]. Synbiotics are formulations that combine probiotics and prebiotics in a synergistic manner. The prebiotic component supports the survival and activity of the probiotic strains, enhancing their efficacy in modulating the gut microbiota and conferring health benefits [6]. The term “postbiotic” denotes the formulation of inactivated bacteria and/or their components in a way that supports host health [7]. Postbiotics comprise bioactive compounds produced during the fermentation of probiotic bacteria [8].
Probiotic bacterial strains are specific microorganisms identified and selected for their beneficial effects on host health. Historically, these strains were isolated from traditional fermented foods and the intestines of healthy individuals. The utilization of fermented foods for health benefits has a long history, spanning thousands of years. Fermented dairy products, particularly yogurt, were widely consumed by nomadic societies due to their portability, extended shelf-life, and the health benefits attributed to their probiotic content [9]. This historical precedent underscores the enduring connection between probiotics and human health, with contemporary research now substantiating and refining these early observations. As a result, probiotics have transitioned from traditional remedies to scientifically validated agents for promoting gastrointestinal health and overall well-being [10]. Advancements in microbiological techniques have significantly enhanced the ability to isolate probiotic strains from a diverse range of sources, including human breast milk and the gastrointestinal tracts of animals. These modern techniques, such as genomic sequencing, metagenomics, and high-throughput screening, allow for the precise identification, characterization, and validation of microbial strains, ensuring their potential efficacy and safety for therapeutic applications [11]. The growing body of research surrounding animal-derived probiotics highlights their potential as valuable therapeutic agents, not only for human health but also in the context of veterinary medicine, animal husbandry, and the One Health concept [12] (Figure 1). Probiotic strains derived from animals have garnered increasing interest due to their potential health benefits. The gastrointestinal tracts of various animal species, including pigs, poultry, and even bees, have been identified as sources of beneficial microorganisms with probiotic potential. Animal-derived probiotics, defined as microbial strains isolated from the gastrointestinal tracts or mucosal surfaces of animals, have garnered increasing attention due to their potential to confer host-specific health benefits. Certain animal-derived Bifidobacterium species have demonstrated beneficial effects on gut microbiota composition, gut barrier integrity, and immune system modulation [13]. In addition, studies have shown that probiotics derived from the gastrointestinal microbiota of bees exhibit antimicrobial properties and can enhance the health and immune responses of the host [14]. As scientific understanding of these probiotics continues to expand, the therapeutic applications of probiotics derived from animal sources may play a more significant role in promoting gut health, immune function, and overall well-being. Despite these promising attributes, research on animal-derived probiotics remains relatively limited. In particular, more studies are needed to elucidate their strain-specific mechanisms of action, host interactions, and long-term effects, especially in the context of cross-species application. Additionally, the lack of standardized evaluation criteria and insufficient genomic characterization of many strains contribute to significant knowledge gaps in this field. Addressing these issues is crucial for advancing their safe and effective use in both veterinary and human medicine.
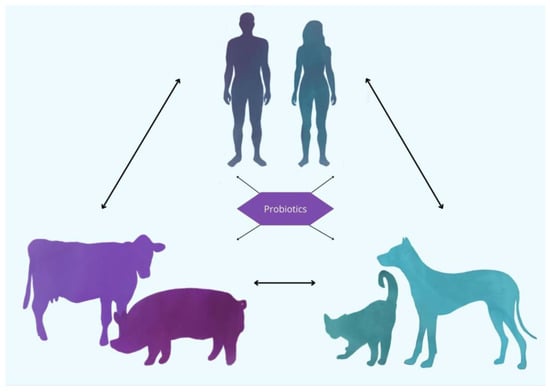
Figure 1.
Probiotics as a unifying factor in One Health: connecting humans, pets, and livestock. The use of probiotics can serve as a bridge, promoting a shared microbial ecosystem across different domains. By modulating the gut microbiota in humans, pets, and livestock, probiotics contribute to enhanced health outcomes and disease prevention. This integrated perspective underscores the importance of considering all species in the context of health interventions, where probiotics can play a vital role in maintaining a balanced microbiome, reducing the transmission of infectious diseases, and improving overall well-being across species.
2. Probiotics’ Role in Promoting Health in Humans and Animals
Probiotics offer a wide range of health benefits for both humans and animals, from improving digestion to supporting immune function and mental health. While antibiotics are used sub-therapeutically in animal feed to promote growth, excessive or improper use can lead to the development of antibiotic-resistant bacteria [15]. This overuse of antibiotics has become a major public health concern due to its potential to cause both human and animal diseases. In addition to the risks posed by antibiotic overuse, foodborne pathogenic bacteria are responsible for major zoonotic diseases such as salmonellosis, campylobacteriosis, and infections caused by pathogenic Escherichia coli in humans [16]. To address these challenges in livestock and aquaculture production, probiotics have emerged as an effective alternative, promoting animal growth and health without the negative side effects associated with antibiotics. Today, probiotic feed supplements are commonly provided to poultry, ruminants, and fish. These probiotics are mostly Gram-positive bacteria, although Gram-negative bacteria, yeast, and fungi are also used [17]. Common probiotics include Lactobacillus, Bifidobacterium, Lactococcus, Bacillus, Streptococcus, and yeasts such as Candida and Saccharomyces [18]. Probiotic strains derived from animals, such as those isolated from the gastrointestinal tract of mammals, are gaining significant attention due to their potential to modulate both local and systemic immune responses. These animal-derived probiotic strains contribute to immune health by influencing the gut-associated lymphoid tissue (GALT), improving intestinal barrier integrity, and enhancing the production of specific immune factors like immunoglobulins and cytokines [19]. In a recent study [20], a novel Limosilactobacillus reuteri (previously known as Lactobacillus reuteri) strain, RGW1, isolated from the feces of healthy calves, was characterized for its probiotic properties, including its immunomodulatory effects. Administration of RGW1 led to a marked increase in the levels of anti-inflammatory cytokines, including TGF-β and IL-10. Studies on BALB/c mice have shown that animal-derived probiotic strains have a significant impact on the immune system. Lactobacillus gasseri SBT2055 (LG2055), administered for 5 weeks, increased IgA production and the number of IgA+ patches in Peyer’s patches and the lamina propria [21]. Similarly, a mixture of the species L. acidophilus, L. casei, L. reuteri, B. bifidum, and Streptococcus thermophilus, given for 20 days, led to an increase in regulatory T cells (CD4+ Foxp3+) while reducing the population of Th1, Th2, and Th17 cells, suggesting a potential immunomodulatory effect [22]. The immunomodulatory function of probiotics is illustrated in Figure 2.
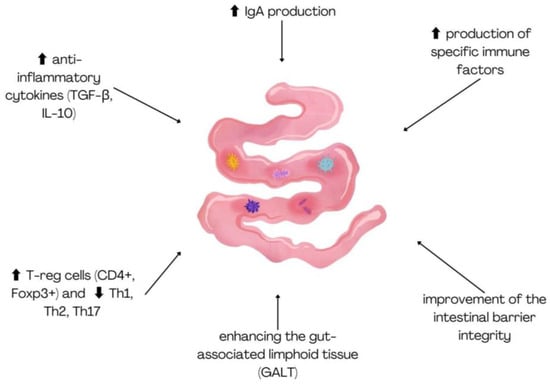
Figure 2.
Immunomodulatory effects of animal-derived probiotic strains on gut immunity.
Pet ownership has increasingly evolved, with many owners now treating their pets as integral members of the family, colleagues, and even friends [23]. The gastrointestinal system plays a vital role in the overall health of animals, hosting a complex and diverse microbial community. A balanced and healthy gut microbiome is crucial for influencing overall host physiology, well-being, nutrient absorption, metabolism, and the host’s immune functions. Probiotics, defined as living microorganisms that provide health benefits to the host when consumed in adequate amounts, are gaining popularity in pet care [24].
As more and more studies concerning microbiome and host intricate relationships emerge, both in animals and humans, probiotic science gains significant recognition. The scientists need to meet the clinicians and answer some of the most frequently asked questions: What product is the most suitable for the patient? In general, the key factors a clinical specialist should consider when selecting a probiotic product for their patient include the documented efficacy of a specific probiotic strain for a particular species, the therapeutic dose, exclusion of contraindications, and consideration of potential interactions between the probiotic preparation and the host organism.
Among the diverse intestinal microorganisms, those that are selected as probiotics are typically those that can positively influence the host by promoting a balanced intestinal microbiota. These include species from genera such as Bifidobacterium, Enterococcus, and the family Lactobacillaceae [25].
2.1. Probiotics Implications on Human Health with Clinical Application in Certain Diseases
2.1.1. Probiotic Therapy Alongside Antibiotics Administration
Research carried out on human medicine proves that probiotics show potential in decolonizing multidrug-resistant (MDR) pathogens from the gut (e.g., Escherichia coli, MDR Gram-negative Bacillus, Staphylococcus aureus, Clostridium difficile, and Helicobacter pylori), making them a viable alternative to antibiotics [26], which are known to disrupt gut microbiota and promote antibiotic resistance [27]. Probiotics and prebiotics, either alone or in combination with antibiotics, may help restore microbial diversity and improve long-term health outcomes. Findings from a meta-analysis [28] suggest that probiotics can effectively decolonize pathogens in the gut, regardless of bacterial type, making them a promising alternative to conventional antibiotic treatments. Subgroup analysis revealed variations in the effectiveness of probiotics in pathogen decolonization. Saccharomyces boulardii demonstrated the highest efficacy, particularly in clearing Clostridioides difficile (CDI) during or after antibiotic treatment, followed by other probiotics like E. coli Nissle 1917 (EcN), Enterococcus faecium, and non-toxigenic C. difficile [29]. The success rate of probiotics varied depending on the specific pathogen, with notable effects on CDI, multidrug-resistant Enterobacteriaceae, and vancomycin-resistant Enterococci (VRE). These differences suggest that the ability of probiotics to eliminate pathogens is influenced by factors such as competition for adhesion sites, production of antimicrobial compounds, immune modulation, and gut barrier reinforcement [30]. However, probiotic dosage, ranging from 109 to 1010 colony-forming units (CFU)/day, and study location did not significantly impact the results. While a minimum dose of 109 CFU/day appears necessary for beneficial effects, further clinical studies are required to standardize probiotic formulations, assess strain combinations, and optimize their decolonization potential [31].
2.1.2. Probiotics in Helicobacter pylori (H. pylori) Infection
Probiotics have emerged as a potential adjunct therapy for various diseases, including Helicobacter pylori (H. pylori) infection, autoimmune disorders, hypertension, inflammatory bowel disease (IBD), oral candidiasis, autism spectrum disorder (ASD), migraine, and diabetes [32]. H. pylori, a Gram-negative flagellated bacterium colonizing the stomach epithelium, has shown increasing resistance to standard triple therapy (STT) due to its impact on natural flora and adverse side effects [33]. Probiotics, particularly from the family Lactobacillaceae, exhibit immunomodulatory and antimicrobial properties that may inhibit H. pylori infection and enhance eradication rates while reducing gastrointestinal inflammation. Recent studies have explored genetically modified probiotics, such as Lactococcus lactis NZ9000 producing H. pylori lipoprotein Lpp20 and Bacillus subtilis spores expressing H. pylori urease B protein, demonstrating promising immune responses [34].
Various probiotic microorganisms, such as those from species Enterococcus faecium, Lactobacillus helveticus, and Lacticaseibacillus rhamnosus (previously known as Lactobacillus rhamnosus), have demonstrated reductions in total cholesterol (TC), Low-Density Lipoprotein Cholesterol, and Non-High-Density Lipoprotein Cholesterol, particularly in individuals with hypercholesterolemia [35]. The mechanisms behind these effects include cholesterol assimilation, bile salt deconjugation, production of SCFAs, and modulation of gut microbiota [36].
2.1.3. Probiotics in Metabolic Health
Additionally, probiotics have shown potential in improving metabolic health in patients with type 2 diabetes mellitus (T2DM) and dyslipidemia [37]. Studies suggest that multistrain probiotic formulations can reduce endotoxin levels, improve glycemic parameters, and enhance lipid profiles. Probiotic strains from species Lactiplantibacillus plantarum (previously known as Lactobacillus plantarum) have also been linked to beneficial changes in gut microbiota composition and SCFA production, which may contribute to obesity prevention and metabolic regulation [38]. Another study shows that probiotics offer benefits in both type 1 and type 2 diabetes (T2DM). In type 1 diabetes, specific strains like Bifidobacterium longum subsp. longum JCM 1217T, B. longum subsp. infantis 157F (BF)13, B. longum subsp. infantis JCM 1222T, L. brevis KLDS 1.0727, L. brevis KLDS 1.0373, Lactiplantibacillus plantarum TN627, and L. fermentum MTCC, as well as species like L.acidophilus, Lacticaseibacillus casei, and L. delbrueckii subsp. bulgaricus, modulate immune responses, reduce pancreatic inflammation, and delay beta-cell destruction [39]. In T2DM, probiotic species such as Lactiplantibacillus plantarum, Bifidobacterium lactis, Lacticaseibacillus rhamnosus, and L. gasseri influence glucose metabolism, lipid homeostasis, and gut microbiota composition, contributing to improved insulin sensitivity, reduced oxidative stress, and lower systemic inflammation [40]. Despite promising findings, some studies report inconsistent results, highlighting the need for further long-term clinical trials to confirm the efficacy and durability of probiotics in managing cholesterol and metabolic disorders [41].
2.1.4. Probiotics in IBD
Probiotics may play a significant role in IBD by inhibiting pathogenic bacteria, thereby protecting the intestinal cells [42]. They enhance the intestinal barrier by stimulating mucus production and antimicrobial peptide release. Additionally, probiotics modify the mucosal immune system, regulating inflammatory responses. By inducing T cell apoptosis, they increase the production of anti-inflammatory cytokines, such as IL-10 and TGF-B, while reducing pro-inflammatory cytokines like TNF-a, IFN-y, and IL-8, helping to manage IBD effectively [43]. In IBD, probiotic strains such as Lacticaseibacillus paracasei L74 and the species Streptococcus salivarius suppress NF-κB activation and inflammatory cytokine production, and Lactiplantibacillus plantarum Lp91 reduces tumor necrosis factor-alpha (TNF-α) and cyclooxygenase-2 (COX-2) expression, promoting intestinal integrity [44].
2.1.5. Probiotics in Gastrointestinal Integrity
Lactobacillaceae contribute to gut health by producing lactic acid, which triggers hypoxia-inducible factor (HIF)-2α signaling, reinforcing intestinal barrier integrity. This process was linked to a significant reduction in Vibrio cholerae in neonatal mice, suggesting the protective role of lactic acid-producing probiotics [45]. In premature infants, supplementation with Bifidobacterium bifidum NCDO 2203 and Lactobacillus acidophilus NCDO 1748 resulted in increased fecal acetate and lactate, effectively lowering intestinal pH and restricting the growth of opportunistic pathogens such as Klebsiella, Escherichia, and Enterobacter [46]. Furthermore, EcN has been reported to inhibit biofilm formation of Pseudomonas aeruginosa and disrupt mature biofilms, thereby reducing colonization by enterohemorrhagic Escherichia coli (EHEC) [47].
2.1.6. Potential Anticarcinogenic Properties of Probiotics
Probiotics exhibit potential anticarcinogenic properties through various mechanisms, including modification of the intestinal microbiota, production of beneficial metabolites, like SCFAs and conjugated linoleic acids (CLAs), and the inhibition of cancer cell growth. They induce apoptosis in cancer cells, e.g., gastric, colonic, and myeloid leukemia cells, modulate mutagenic factors, and enhance immune responses. Many researchers indicate a significant antiproliferative role and/or induction of apoptosis mus musculus colon carcinoma (HGC-27) and human colonic cancer cells (Caco-2, DLD-1, HT-29), and also lowering the level of IL–8 via the strain Lacticaseibacillus rhamnosus GG [48,49,50,51,52].
In addition, scientists’ reports indicate the effectiveness of probiotic microorganisms (e.g., Bacillus: polyfermenticus, subtilis; Bifidobacterium: lactis, adolescentis; Clostridium butyricum; Enterococcus faecium; Lactobacillaceae: L. acidophilus, L. casei, L. fermentum, L. delbrueckii, L. helveticus, L. paracasei, L. pentosus, L. plantarum, L. salivarius; Lactococcus lactis; Pediococcus pentosaceus, Propionibacterium acidopropionici, and Streptococcus thermophilus) in reducing proliferation and/or induction of apoptosis human colonic cancer cells such as Caco-2, HT-29, SW1116, HCT116, SW480, DLD-1, LoVo [53].
Moreover, Lactobacillus acidophilus CL1285 and Lacticaseibacillus casei LBC80R (in the presence of 5-FU) induced apoptosis in human colorectal cells (LS513), while Lactobacillus acidophilus SNUL, Lacticaseibacillus casei YIT9029, and Bifidobacterium longum HY8001 suppressed proliferation of human colorectal (SNUC2A) and gastric carcinoma cells (SNU1) [54]. Probiotics improve intestinal barrier function, degrade carcinogenic compounds, and combat dysbiosis, which is linked to colorectal cancer development. Pathogenic bacteria such as Bacteroides fragilis and Clostridium spp. contribute to inflammation and tumor progression, while probiotics restore balance by outcompeting harmful bacteria and promoting protective biofilms [55]. Microbial metabolism, particularly the activity of enzymes like azoreductase, β-glucuronidase, and nitrate reductase, can convert dietary components and bile salts into carcinogenic compounds. By modulating these metabolic pathways, probiotics can inhibit harmful enzyme activity, thereby reducing cancer risk. Certain species, like Lactobacillus acidophilus, have been shown to lower the activity of these enzymes [56]. Lactic acid bacteria (LAB) contribute to intestinal health by producing organic acids such as lactic and acetic acid, which lower intestinal pH and disrupt pathogenic bacteria. Probiotics also produce bacteriocins with bactericidal properties [57]. SCFAs, particularly butyrate, play a significant role in cancer prevention by regulating inflammation, apoptosis, and cell cycle progression. Butyrate inhibits inflammatory cytokine production, suppresses COX-2 activity, and induces epigenetic changes that favor apoptosis in cancer cells [58]. Conjugated linoleic acids produced by probiotics, such as Streptococcus thermophilus: Strain BT01, Bifidobacterium breve: Strain BB02, Bifidobacterium longum: Strain BL03, Bifidobacterium infantis: Strain BI04, Lactobacillus acidophilus: Strain BA05, Lactiplantibacillus plantarum: Strain BP06, Lacticaseibacillus paracasei: Strain BP07, and Lactobacillus delbrueckii subsp. bulgaricus: Strain BD08, also have anticancer effects, conferred by regulating apoptosis-related genes and suppressing eicosanoid production, which is linked to colon cancer progression [59].
2.1.7. Influence of Probiotics on the Central Nervous System
The microbiome significantly influences the central nervous system (CNS) through a bidirectional communication pathway known as the gut–brain axis (GBA). This interaction occurs via microbial metabolites, which can cross the blood–brain barrier (BBB) and the vagus nerve, modulating the hypothalamic-pituitary-adrenal (HPA) axis and immune responses [60]. Conversely, the brain also impacts gut function by regulating secretion, motility, and permeability, thereby affecting the microbiota. Serotonin, a key signaling molecule, plays an essential role in both the CNS and the enteric nervous system (ENS) within the GBA [61]. Probiotic bacteria influence the CNS through three mechanisms: the production of neuroactive substances, such as neurotransmitters and their precursors, that affect emotions and behavior; the interaction of SCFAs and secondary bile acids with host cells to regulate signaling molecule production; and the activation of signaling molecules through bacterial enzymatic deconjugation. These mechanisms underscore the complex relationship between the microbiome and brain function [62]. The gut microbiota plays a crucial role in maintaining brain health, with imbalances linked to neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD) [63]. In AD, an altered microbiota, characterized by the overgrowth of pro-inflammatory bacteria like Proteobacteria and Escherichia/Shigella, contributes to BBB disruption and disease progression [64]. This imbalance enhances inflammation, a central feature of AD. Certain bacterial infections, such as Helicobacter pylori, Borrelia burgdorferi, and Chlamydia pneumoniae, have also been linked to amyloid-β accumulation and tau phosphorylation, key markers of AD [65]. In PD, microbial disruption, including small intestinal bacterial overgrowth and Helicobacter pylori infection, increases gut permeability and motor dysfunction, with a reduction in beneficial bacteria such as Roseburia [66]. In HD, a genetic disorder caused by the overexpression of the huntingtin gene, dysbiosis contributes to excessive hydrogen sulfide production and cytokine dysregulation, worsening neurodegenerative symptoms [67]. Research has been carried out on the effects of various probiotic strains on inflammatory cytokines and reactive oxygen species (ROS) production in Parkinson’s disease patients. The results indicated that strains such as Ligilactobacillus salivarius LS01 and the species Lactobacillus acidophilus significantly reduced pro-inflammatory cytokines and increased anti-inflammatory cytokines, suggesting potential therapeutic benefits [68,69].
2.1.8. Probiotic Preparation Application in Skin Diseases and Wound Healing
Several probiotic strains have been recognized for their positive impact on skin health, particularly in managing various skin conditions. Notable probiotic species include Lactiplantibacillus plantarum, Lactobacillus acidophilus, Bifidobacterium longum, and Streptococcus thermophilus. These microbes are known to inhibit the release of pro-inflammatory cytokines, block inflammatory mediators, and support the restoration of skin barrier function [70,71]. These probiotics have been utilized both topically and orally in the treatment of common dermatological conditions, such as acne, atopic dermatitis, and rosacea. Their use helps restore the balance of the skin microbiota, reduces skin inflammation, and improves overall skin health. Studies have investigated the effects of both topical and oral probiotics on skin health. Topical probiotics have demonstrated significant benefits in improving skin hydration and barrier function [72]. Moreover, topical probiotics have shown positive effects in managing various inflammatory skin conditions, including acne, rosacea, and psoriasis, and they also appear to support wound healing [73]. On the other hand, oral probiotics primarily work by modulating the gut microbiota, which indirectly benefits skin health. These probiotics can enhance gut barrier function and reduce systemic inflammation, ultimately improving skin conditions [74]. While topical probiotics tend to produce faster, localized improvements, oral probiotics have more systemic effects, suggesting that both methods may have complementary roles in skin health management. Further studies are needed to directly compare the efficacy of these two forms of probiotic administration using the same strains and to explore any potential synergistic effects between them. One of the main challenges for topical probiotics is their survival under the harsh conditions of the skin, such as low moisture, acidic pH, and immune defenses [75]. Certain strains, such as Lactiplantibacillus plantarum ATCC 10241, have shown good survival rates, especially in moist environments like wounds, making them ideal candidates for targeted therapeutic applications [76].
Topical probiotics have garnered scientific interest for their potential to enhance wound healing and prevent inflammation. A study demonstrated that treating burn wounds with Saccharomyces cerevisiae MYA-796 resulted in accelerated healing, including increased expression of collagen type 1 and growth factor beta 1 (TGF-β1), as well as improved biomechanical characteristics of the healing skin [77]. Probiotics such as Lactobacillus acidophilus CL1285 and Lacticaseibacillus casei LBC80R have also shown promise in inhibiting Methicillin-resistant Staphylococcus aureus (MRSA), a common wound pathogen. In one study, these probiotics eliminated MRSA growth by 99% after 24 h of incubation. Moreover, the species Lactobacillus reuteri and strain Lacticaseibacillus rhamnosus GG were found to protect epidermal keratinocytes from S. aureus-induced cell death by preventing pathogen adhesion, with the species L. reuteri showing superior protection compared to L. rhamnosus’s strains. These effects are believed to result from the exclusion of S. aureus from integrin binding sites on keratinocytes [78].
2.1.9. Probiotics and the Immune System
Allergic contact dermatitis (ACD), commonly referred to as eczema, occurs when the skin comes into contact with an allergenic substance, triggering an allergic response. Symptoms include skin inflammation, itching, dryness, and blisters, with the immune response primarily regulated by CD4+ T cells. Both pro- and prebiotics have demonstrated preventive effects on ACD and have been shown to help mediate its symptoms [79]. Lacticaseibacillus casei. has been found to reduce skin inflammation through multiple mechanisms, including the inhibition of INF-γ, a cytokine involved in the production of CD8+ effector T cells. Additionally, L. casei may promote regulatory CD4+ T cells, further enhancing its anti-inflammatory effects. The species has also been shown to stimulate the production of IL-10 by activating CD4+ CD25+ T regulatory cells (Tregs), which support its role in controlling skin inflammation [80]. EcN is another probiotic that has demonstrated the ability to prevent ACD by increasing the number of Foxp3+ cells, which play a role in suppressing lymphocyte antigen priming [81].
2.1.10. Probiotics in Urinary Tract Infections
Urinary tract infections (UTIs) are often caused by uropathogenic E. coli (UPEC). Vaginal probiotics, particularly those containing lactobacilli, could potentially reduce these UPEC reservoirs, thereby decreasing the recurrence of UTIs (rUTIs). Lactobacillus crispatus is known for its ability to maintain a healthy vaginal microbiome by reducing pathogen abundance, with L. crispatus CTV-05 being an active strain in Lactin-V, a product currently in clinical trials for bacterial vaginosis treatment [82,83]. Recent advancements also include the potential of intravesical delivery of lactobacilli for treating neurogenic lower urinary tract dysfunction. Preliminary studies have shown that intravesical L. rhamnosus GG is safe and well tolerated, though its effectiveness in reducing UTI occurrence remains inconclusive [84]. For UTIs, various probiotics have shown positive results, particularly Lacticaseibacillus rhamnosus GR-1 and Lactobacillus reuteri B-54 when administered vaginally [85,86]. Oerlemans et al. [87] conducted a study on 20 women with vulvovaginal candidiasis (VVC), investigating the effects of a probiotic formulation containing Lactobacillus pentosus KCA1, Lactiplantibacillus plantarum WCFS1, and Lacticaseibacillus rhamnosus GG. The probiotic was administered in doses ranging from 2.5 × 109 to 2.5 × 1010 CFU/day for 1.5 weeks. The results indicated that 45% of the women experienced restoration of the vaginal microbiota with the probiotic treatment. However, the remaining 55% required rescue medication, specifically fluconazole.
2.1.11. Probiotics Usage in Hypertension Management
In hypertension management, probiotics contribute to gut microbiota homeostasis, improve intestinal barrier integrity, and lower systemic inflammation. Specific probiotic species, including Lactobacillus helveticus and Saccharomyces cerevisiae, produce bioactive peptides with angiotensin-converting enzyme (ACE) inhibitory properties, mimicking the effects of ACE inhibitors. The consumption of fermented milk products containing L. casei strain Shirota (LcS) has been linked to reduced hypertension risk, attributed to its polysaccharide-glycopeptide complex promoting prostaglandin I2 synthesis and reducing vascular resistance [88].
2.1.12. Probiotics in Maintaining Oral Health
Some bacterial species also contribute to oral health, particularly in reducing Candida colonization in denture wearers. Lacticaseibacillus rhamnosus and Limosilactobacillus reuteri have demonstrated antifungal activity, with probiotic formulations containing Bifidobacterium longum, Lactobacillus bulgaricus, and Streptococcus thermophilus showing superior efficacy when combined with antifungal agents like nystatin. This suggests that probiotic-based interventions may help prevent oral candidiasis in immunocompromised individuals and elderly populations [89].
2.2. Health Benefits of Probiotic Usage in Companion Animals and Viable Strains Used in Specific Conditions
In recent years, the application of probiotics in pets, particularly dogs and cats, has been explored with promising outcomes. Research indicates that probiotics can modulate the immune system, enhance gut health, and protect against pathogenic bacteria in companion animals [90]. The consumption of probiotics offers several health advantages, including the prevention of diarrhea, the maintenance of a stable and healthy gastrointestinal microbiome, and support in managing mild enteropathies as well as small intestinal bacterial overgrowth [91].
Shelter animals, prone to diarrhea due to stress and dietary changes, have shown species-specific responses to probiotics; E. faecium SF68 reduced diarrhea in shelter cats but not in dogs [92]. Furthermore, probiotics have been investigated for dietary allergies and Helicobacter infections in dogs, especially specific strains from the species Lactobacillus acidophilus, Lactobacillus reuteri, and Lactobacillus johnsonii, improving gut health and clinical symptoms [93]. Given the effectiveness of Lactobacillus casei DN-114 001 in eradicating Helicobacter infections in pediatric patients, similar therapeutic applications in veterinary medicine may be possible [94]. In feline studies, Lactobacillus acidophilus DSM13241 supplementation over 4.5 weeks resulted in increased lactobacilli levels, reduced Enterococcus faecalis and Clostridium difficile, lowered fecal pH, and decreased endotoxin levels in the blood, suggesting a strengthened immune response [95]. Probiotics have been increasingly utilized in veterinary medicine, particularly in the management and prevention of gastrointestinal disorders in both dogs and cats. Several studies have investigated the efficacy of specific probiotic strains, each demonstrating a variety of health benefits in animals, as presented in Table 1.

Table 1.
Effects of application of some probiotic strains used in cats and dogs, along with their origin.
Probiotic supplementation in cats has demonstrated various health benefits, particularly in improving gut health and managing gastrointestinal and respiratory conditions. For instance, a combination of Saccharomyces boulardii (1 × 1010 CFU/kg) and Pediococcus acidilactici (1.25 × 1010 CFU/kg) was shown to modulate gut microbiota, enhance SCFA production, reduce inflammation, and promote the settlement of beneficial bacteria such as Lactobacillaceae and Bacillus species in 12 healthy cats [109]. Similarly, Enterococcus faecium strain SF68 (5 × 108 CFU/day) reduced the prevalence of diseases associated with chronic feline herpesvirus type 1 (FHV-1) infections in 12 cats [110]. Probiotics have also been beneficial for managing chronic gastrointestinal conditions. For example, a mixture of Streptococcus thermophilus DSM32245, Lactobacillus acidophilus DSM32241, Lactiplantibacillus plantarum DSM32244, Lacticaseibacillus casei DSM32243, Lactobacillus helveticus DSM32242, Lactobacillus brevis DSM27961, Bifidobacterium lactis DSM32246, and Bifidobacterium lactis DSM32247 (2 × 1011 lyophilized bacteria per 5 kg body weight) significantly improved clinical symptoms of constipation and idiopathic megacolon in seven cats [111]. In cats suffering from diarrhea, Enterococcus faecium SF68 (2.1 × 109 CFU/day) led to a reduction in diarrhea rates across a study group of 217 cats [112]. Additionally, Bacillus subtilis SC06 and Bacillus coagulans B10 (3 × 109 CFU/kg) improved digestion, antioxidant capacity, and weight gain in 20 healthy cats, while Bacillus licheniformis (1.1 mg/kg) alleviated chronic diarrhea in 8 cats [113]. Furthermore, Lactobacillus strains such as L. acidophilus CECT 4529 (5 × 109 CFU/kg) and L. reuteri NBF 2 DSM 32264 (5 × 109 CFU/kg) improved fecal quality and increased beneficial Lactobacillaceae populations in 10 and 12 healthy cats, respectively [114].
The benefits of using probiotics in the treatment of kidney diseases in dogs have also been described. Administration of VSL#3, a probiotic formulation containing Lacticaseibacillus casei, L. plantarum, L. acidophilus, L. delbrueckii subsp. bulgaricus, Bifidobacterium longum, B. breve, B. infantis, and Streptococcus salivarius subsp. thermophilus (112 to 225 × 109 CFU/10 kg body weight daily for 60 days), significantly enhanced the glomerular filtration rate (GFR) in dogs diagnosed with chronic kidney disease. The probiotic-treated group exhibited improved renal function compared to both baseline levels and the control group [115]. Chronic kidney disease (CKD) is prevalent among felines, leading to the accumulation of nitrogenous waste products, such as urea, which can be detrimental to health [116]. Recently, a synbiotic supplement was introduced to mitigate uremic toxins in cats with CKD. It combines specific bacterial strains from the species Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum, aiming to metabolize urea and other toxins within the gut, thereby reducing their systemic absorption (Vetoquinol, Fort Worth, TX, USA). The proposed mechanism, termed “enteric dialysis”, involves these probiotics utilizing urea in the intestines, potentially lowering blood urea nitrogen levels. However, clinical evaluations of the product’s efficacy have yielded mixed outcomes [117]. A study by Rishniw and Wynn [118] reported that the method of delivering certain synbiotic products to cats with CKD (sprinkling onto food) significantly reduces the potential of altering azotemia. Another study evaluating a commercial probiotic supplement, Enterococcus faecium SF68, in cats with CKD over an 8-week period found no significant changes in the gut microbiome or serum concentrations of indoxyl sulfate (IS) and p-cresyl sulfate (pCS), two uremic toxins [119]. Lactobacillaceae supplementation may influence gut microbiota composition and metabolic pathways, leading to potential benefits in CKD management. A feline study examining the effects of Lactiplantibacillus plantarum subsp. plantarum MFM 30 − 3 and Lacticaseibacillus paracasei subsp. paracasei MFM 18 intervention in cats with stage 2–3 CKD demonstrated significant changes in microbial composition and serum metabolites, highlighting mechanisms by which probiotics may modulate CKD progression [120].
In a study involving 35 dogs without a history of recurrent urinary tract infections (UTIs) [121], the results suggested that oral probiotics could potentially influence the vaginal microbiota in dogs, although further investigation is needed to understand the full effects and the optimal probiotic strains for canine health. The oral probiotic used contained Lactobacillaceae, Bifidobacterium, and Bacillus species. A case report from India suggests a positive impact of probiotic combination treatment in managing Feline Idiopathic Cystitis (FIC) [122]. The probiotic combination administered included the species Lacticaseibacillus casei (4 × 108 CFU), Lacticaseibacillus rhamnosus (3 × 108 CFU), Lactobacillus acidophilus (5 × 107 CFU), Lactobacillus bulgaricus (1 × 107 CFU), Bifidobacterium infantis (4 × 107 CFU), Bifidobacterium breve (5 × 107 CFU), and Streptococcus thermophilus (1 × 108 CFU). The exact probiotic strains have not been stated. After the initial treatment phase, medication was continued orally for an additional 5 days, including pipemidic acid, diazepam, neurotropic vitamins, and doxycycline. Lactobacillus species demonstrated antimicrobial properties by producing H2O2 [123], which inhibits the growth of uropathogenic E. coli (UPEC), Salmonella sp., and Helicobacter pylori. Furthermore, probiotics contributed to immune modulation, significantly reducing IL-6, IL-8, and lactic acid dehydrogenase levels, which in turn exerted anti-inflammatory effects [124].
Additionally, probiotics have been associated with enhanced immune responses, providing a protective effect against various diseases [125]. Probiotics exert immunomodulatory effects by promoting T-cell differentiation, regulating the balance of pro- and anti-inflammatory cytokines, and enhancing secretory IgA (sIgA) production [126]. The impact of Enterococcus faecium SF68 supplementation on immune responses following administration of a multivalent vaccine was assessed in kittens. E. faecium SF68 was detected in the feces of seven out of nine cats. Notably, the percentage of CD4+ lymphocytes was significantly increased in the treatment group, although no significant differences were observed in other immune parameters between the groups [99].
Probiotic therapy seems to engage in modulating the behavior and mental health of companion animals, particularly dogs and cats. The GBA, as described before, is a bidirectional communication network between the gastrointestinal tract and the central nervous system, playing a pivotal role in this interaction. A study conducted in dogs found that supplementation with Saccharomyces boulardii (1 × 109 CFU per kg of feed) led to a reduction in fecal calprotectin, immunoglobulin A (IgA), and cortisol levels, suggesting that S. boulardii may contribute to alleviating intestinal inflammation and decreasing stress hormone production [127]. Additionally, a 14-day supplementation with Lactiplantibacillus plantarum PS128 seemed to stabilize behaviors associated with aggression and separation anxiety. Plasma 5-HT turnover ratio was found to decrease after supplementation, particularly in dogs with separation anxiety. This suggests that 5-HT may play a role in the GBA, as the slower breakdown of 5-HT into its metabolites leads to higher availability of this neurotransmitter in the system [128]. Probiotic interventions have gained attention for their potential in alleviating anxiety-related behaviors in dogs. One study performed by Purina® researchers investigated the effects of Bifidobacterium longum (BL999) on anxious Labrador Retrievers. The results showed significant improvements in both behavioral and physiological markers, including heart rate and salivary cortisol, suggesting the potential of BL999 to reduce anxiety. In a separate double-blind, placebo-controlled trial [129], Relaxigen Pet dog®, a nutraceutical supplement containing prebiotics, probiotics (Lacticaseibacillus reuteri), postbiotics, and neuroprotective compounds, was evaluated in anxious dogs. The treated dogs exhibited a reduction in microbials like Bacteroides and Lactobacillus, along with a decrease in anxiety-like behaviors. However, a recent study showed that there were no major changes in the gut microbiota of dogs with phobic behavioral disorder, except for an increase in Lactobacillus, which is known for its probiotic properties [130]. Chronic treatment with Lacticaseibacillus rhamnosus JB—1 has been shown to impact anxiety and depression-related behaviors by modulating GABA receptor mRNA expression in specific brain regions [131]. Although the factors behind the increased abundance of Lactobacillus in phobic dogs are unclear, the authors speculated that the presence of this psychobiotic could contribute to the development of phobic behavioral traits.
A study conducted by Barthe et al. (2023) assessed the effects of topical probiotic bacteria on canine progenitor epidermal keratinocytes (CPEK) [132]. Non-formulated probiotics were applied at concentrations of 75, 750, and 7500 CFU/mL for 24 h. At 7500 CFU/mL, only 2% of the probiotic bacteria were dead, while at lower concentrations (750 and 75 CFU/mL), 81% and 84% of those bacterial agents were dead, respectively. This indicated that higher concentrations maintained more viable probiotic products. In a scratch wound assay, non-formulated probiotics enhanced CPEK cell migration in a dose-dependent manner, with 16% improvement at 750,000 CFU/mL. The vehicle used in the formulation also increased migration by up to 14%. However, formulated probiotics provided the most significant wound healing enhancement, increasing migration by 20% at the highest concentration.
Human and canine microbiomes seem to be similar, with canines posing as models for studying the human microbiome [133]. Thus, some probiotic products designed for humans may be utilized in canine medicine, provided that the probiotic strain is adequate and examined for canines and the amount of the product (CFU/day) corresponds to those tested for the species.
It is important to note that while the current evidence is promising, research on the use of probiotics in companion animals is still limited.
2.3. Probiotic Therapy in the Animal Production Sector
In recent years, there has been increasing scientific and commercial interest in the incorporation of probiotics into animal feed as a strategy for preventing or managing various animal diseases. This approach is gaining traction as a viable alternative to the use of growth-promoting antibiotics, which can lead to undesirable side effects and adverse reactions in animals [134]. The use of probiotics in animal production has become a promising strategy to improve growth, feed efficiency, health, and meat quality across various livestock species, including poultry, swine, and cattle [135].
Probiotic supplementation has been shown to enhance microbial diversity, improve gut health, and positively affect nutrient digestion and immunity [136]. Species like Lactiplantibacillus plantarum and Bacillus subtilis are among the most widely used probiotics in livestock, promoting beneficial metabolic processes such as short-chain fatty acid production and improving immune responses. Studies have demonstrated the efficacy of specific probiotic strains, such as L. plantarum PFM 105, in increasing short-chain fatty acid production compared to antibiotics, while B. subtilis strains have been found to reduce the abundance of harmful bacteria in the gastrointestinal tract of swine and poultry, contributing to enhanced health outcomes [137]. However, the effectiveness of probiotics can vary depending on factors like dosage, dietary formulation, and the microbiota composition of the host [138]. Research also suggests that probiotic treatment may have unintended consequences, such as disrupting the native gut microbiota, which could potentially increase the risk of future diseases [139]. Long-term colonization of probiotic strains is often desirable, but competition between introduced and native microbiota needs to be considered to ensure successful engraftment. Recent findings emphasize the potential of multistrain probiotics, as they may provide a broader ecological range, minimizing competition with resident microorganisms [140]. Available data also suggest a connection between the gut microbiome and neurological changes, influencing feeding behaviors in farm animals, although the underlying mechanisms remain unclear [141]. However, the efficacy of probiotics in comparison to traditional antibiotic growth promoters remains a challenge. Studies indicate that certain probiotic strains may not be as effective as antibiotics or implants in promoting faster growth [142]. The variation in efficacy is influenced by factors such as microbial strain composition, dosage, delivery methods, environmental stress, and the health condition of the animal.
2.3.1. Swine
Studies highlight the positive impact of probiotics such as Bacillus coagulans GBI-30, 6086 and the species Clostridium butyricum on growth performance and nutrient digestibility in pigs, with Bacillus strains enhancing protein consumption and nutritional absorption [143]. Among the various probiotic strains utilized in swine nutrition, those belonging to the genera Lactobacillus and Bacillus are particularly prominent. A meta-analysis focusing on Lactobacillus-based probiotics, including species such as Lactobacillus delbrueckii, Lactobacillus reuteri, Lactiplantibacillus plantarum, and Lactobacillus acidophilus, demonstrated improvements in the growth performance and intestinal morphology of piglets [144]. The supplementation of these probiotics was associated with enhanced villus height and a reduced crypt depth in the intestines, indicating better nutrient absorption and gut health [145]. Yet, probiotic efficacy needs to be carefully considered.
A study evaluating the impact of a probiotic containing Bacillus subtilis DSM25841 and Bacillus amyloliquefaciens DSM25840 on sows and their piglets found that dietary supplementation improved reproductive performance, increased the birth and weaning weights of piglets, and enhanced fecal microbiota composition. Specifically, sows receiving the probiotic exhibited higher average daily feed intake during lactation and reduced body weight loss, while their piglets had higher birth weights and improved growth rates [146].
In swine production, multistrain probiotics play a vital role in enhancing growth performance, feed efficiency, and overall metabolic utilization of nutrients. In piglets, combinations of probiotics, such as Ligilactobacillus salivarius ZJ614, Lactobacillus reuteri ZJ625, and Streptococcus salivarius NBRC13956, have shown positive impacts on blood profiles (hemoglobin and hematocrit, neutrophils, monocytes, lymphocytes, eosinophils, basophils, platelets, total serum protein, albumin, globulin, cholesterol, and glucose) and overall health. Supplementation with such probiotics also significantly increased IgG serum levels, which is crucial for preventing postweaning diarrhea, and reduced the population of enteric bacteria while increasing the population of lactic acid bacteria [147]. Probiotics containing strains from such species as Bacillus lichenformis, Bacillus coagulans, and Bacillus subtilis have been linked to increased weight gain, improved feed efficiency, and reductions in harmful gas emissions like hydrogen sulfide and mercaptans, which are of environmental concern [148]. Additionally, high doses of these probiotics have been shown to increase the digestibility of dry matter, nitrogen, and energy, as well as modulate the microbial populations in the feces, particularly by reducing E. coli counts. In reproductive swine, probiotics such as Enterococcus faecalis DSM 7134 (species Clostridium butyricum and Bacillus mesentericus) administered before farrowing have been shown to improve reproductive performance by enhancing the return of sows to estrus and optimizing farrowing outcomes [149]. However, some studies report no effect on the reproductive performance of lactating sows, suggesting that the benefits of probiotics in swine may be strain-specific [150].
There is emerging evidence on the use of probiotics in wound healing in pigs. In one study investigating the effects of topical treatments on three full-thickness skin wounds created on the dorsum of each animal [151], the pigs remained comfortable, with consistent feed intake and no changes in activity or social interaction. By day 15, all wounds had healed with normal progression, contracting to a minimal surface area. No significant difference in wound appearance was observed between the treatment and control groups. The study suggests that topical treatment with S. boulardii (no indication of specific strain used in the study) did not significantly alter the bacterial profile or wound healing in terms of surface area reduction, but histological analysis showed typical wound healing features.
The effects of the application of selected probiotic strains in swine are listed in the Table 2.

Table 2.
Effects of the application of selected probiotic strains used in pigs, along with their origin.
2.3.2. Poultry
Probiotics have been increasingly utilized in poultry production to enhance growth performance, bolster immune responses, and improve gut health. Specific strains from the species Lactobacillus acidophilus have been shown to stimulate cytokine production, thereby enhancing the immune response in broiler chickens. Additionally, strains from the genus Bacillus, known for their resilience to high temperatures and acidic pH, are commonly employed in poultry diets. These probiotics have demonstrated efficacy in improving nutrient utilization and maintaining gut health [156]. Furthermore, host-specific probiotics, derived from bacterial strains that have coevolved with poultry, have shown greater potential in providing health benefits compared to non-host-specific strains. This host-specific approach may enhance the colonization and efficacy of probiotics in the avian gut. Research into probiotic multistrain usage in poultry has demonstrated that mixtures containing microorganisms with probiotic potential, such as Saccharomyces cerevisiae, Lactobacillus fermentum, Pediococcus acidilactici, Lactiplantibacillus plantarum, and Enterococcus faecium, can enhance feed efficiency, growth, and intestinal health in broiler chickens, especially when challenged with Pasteurella multocida [157]. A combination of such species: Enterococcus faecium, Lactobacillus acidophilus, Lactiplantibacillus plantarum, and Bifidobacterium bifidum improved overall performance in chickens, including enhanced gut structure, reduced lipid peroxidation, and a reduction in Clostridium spp. populations [158]. Despite these promising results, the benefits of probiotics in poultry are not always uniform. For example, certain probiotic formulations have shown no effect on broiler breeder performance or cholesterol levels [159].
The effects of the application of selected probiotic strains in poultry are listed in Table 3.

Table 3.
Effects of the application of selected probiotic strains used in poultry, along with their origin.
2.3.3. Cattle
Furthermore, probiotics can enhance milk production in dairy cows. For example, supplementation with Bacillus subtilis and Bacillus licheniformis has increased milk protein and fat content, while Lactobacillus strains have been linked to higher milk output [163]. Additionally, probiotics can help reduce the incidence of mastitis in dairy cows [164]. In dairy cows, supplementation with yeast strains, particularly Saccharomyces cerevisiae, has been shown to improve ruminal fermentation, leading to increased fiber digestion and milk yield. These yeast probiotics enhance cellulolytic activity and microbial protein synthesis in the rumen, contributing to better nutrient utilization [165]. In beef cattle, the use of lactic acid bacteria such as Lactobacillus acidophilus has demonstrated benefits in growth performance and health. For instance, administering Lactobacillus acidophilus NP51 at a concentration of 109 CFU per day to steers over a 126-day period resulted in a 37% reduction in Escherichia coli O157:H7 shedding, thereby enhancing food safety [166]. In young calves, early-life probiotic supplementation has been linked to enhanced weight gain and disease resistance. Probiotics can modulate the gut microbiota, leading to improved nutrient absorption and immune function, which are critical during the early stages of development [167]. For instance, a multispecies probiotic consisting of Bifidobacterium bifidum, Pediococcus acidilactici, Lactobacillus acidophilus, Lacticaseibacillus casei, and Enterococcus faecium has been found to reduce the duration of diarrhea in dairy calves while also improving daily weight gain [168]. In buffaloes, a multistrain probiotic mixture containing Streptococcus faecium, Lacticaseibacillus casei, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus reuteri, and Lactobacillus lactis, along with Aspergillus oryzae and Saccharomyces cerevisiae, led to an increased milk yield and improved feed conversion ratio, despite no significant effects on body condition or dry matter intake [169].
Recent research has explored alternative therapies to antibiotics for preventing and treating uterine diseases in cows, with probiotics emerging as a promising option. One of the most commonly studied groups of probiotics is LAB, particularly Lactobacillus spp., due to their ability to produce lactic acid, which helps maintain optimal vaginal pH and inhibits the growth of pathogenic bacteria [170]. Various strains of Lactobacillus spp., such as L. rhamnosus, L. sakei, and Pediococcus acidilactici, have demonstrated potential for inhibiting pathogens like E. coli and T. pyogenes in vitro. For instance, P. acidilactici was found to produce bacteriocins that could inhibit pathogenic bacteria, while combinations of LAB strains showed enhanced effectiveness in reducing E. coli infection and related inflammation [171]. In vivo studies have focused on the application of intravaginal probiotics, demonstrating positive outcomes in reducing the incidence of uterine infections, improving uterine health, and even enhancing reproductive performance [172]. For example, a mixture of L. sakei, P. acidilactici, and L. reuteri administered intravaginally reduced the occurrence of purulent vaginal discharge and improved milk yield in dairy cows [173]. Additionally, intravaginal treatments have been shown to reduce metritis prevalence and improve uterine involution, leading to increased fertility rates [174].
Overall, while probiotics offer numerous benefits in animal husbandry, their effectiveness depends on various factors (age, sex, nutrition, and genetics), requiring careful consideration to maximize their potential.
The effects of the application of selected probiotic strains in cattle are listed in Table 4.

Table 4.
Effects of the application of selected probiotic strains used in cattle, along with their origin.
2.4. Influence of Probiotics on Bioavailability of Drugs
Probiotics can significantly influence the pharmacokinetics of various drugs by altering their absorption, metabolism, and bioavailability through several mechanisms, including changes in gut microbiota composition, microbial enzyme activity, and intestinal transport processes. For example, Lactobacillus acidophilus, Bifidobacterium lactis, and Streptococcus salivarius increased the activity of azoreductase and enhanced the metabolism of sulfasalazine in Wistar rats [180]. In humans, L. acidophilus decreased nitroreductase and azoreductase activity, reducing the toxicity of nitrazepam [181]. Similarly, B. lactis increased dopamine levels in human studies, and L. brevis enhanced tyrosine decarboxylase activity in vitro. Probiotics can also modulate the bioavailability of antidiabetic drugs such as gliclazide, with L. acidophilus, L. rhamnosus, and B. lactis increasing bioavailability in diabetic rats but decreasing it in healthy rats [182]. In contrast, Lacticaseibacillus casei delayed the peak plasma concentration of amiodarone in rats, whereas E. coli Nissile 1917 serotype O6:K5:H1 increased amiodarone bioavailability [183]. Additionally, B. lactis, B. longum, B. bifidum, L. acidophilus, L. rhamnosus, and S. thermophilus were shown to reduce toxicity in amlodipine-treated rabbits [184] and enhance bioavailability. Probiotics also play a role in reducing the toxicity of chemotherapy drugs like irinotecan, with L. plantarum, L. casei, L. acidophilus, and B. longum reducing β-glucuronidase activity and irinotecan toxicity [185]. Moreover, studies on pain medications, such as indomethacin and paracetamol, suggest that probiotics like L. casei CRL 431, L. paracasei CNCM I-1518, and L. reuteri K8 can mitigate drug toxicity and modulate biotransformation pathways [186].
2.5. The Role of Probiotics in Digestion and Nutrient Absorption
Probiotics may play a significant role in enhancing digestive processes and nutrient absorption. Recent studies have elucidated various mechanisms through which probiotics influence these physiological functions [187,188].
Probiotics contribute to digestion by modulating the gut microbiota, leading to improved breakdown of dietary components [189]. Certain Bifidobacterium bifidum strains, such as B. bifidum PRL2010, B. bifidum TMC3115, B. bifidum UCC2003, and B. bifidum JCM1217, produce enzymes that aid in the hydrolysis of complex carbohydrates and proteins, facilitating their assimilation [190]. Additionally, probiotics can stimulate the host’s digestive enzyme activity, further enhancing nutrient breakdown [191]. In animal models, probiotic supplementation has been associated with increased villus height and crypt depth in the small intestine, morphological changes that are indicative of enhanced nutrient absorption capacity. Moreover, probiotics have been shown to improve the expression of nutrient transporters, such as Glucose transporter 2 (GLUT2), thereby facilitating the uptake of glucose and other monosaccharides [192].
Probiotics also affect the bioavailability of various micronutrients through several mechanisms. Certain probiotic species, such as Lactiplantibacillus plantarum, Limosilactobacillus fermentum, Bifidobacterium bifidum, and Bifidobacterium longum, can synthesize B vitamins, including folate, vitamin B12, and riboflavin, directly within the gut lumen, contributing to the host’s vitamin pool [192]. Probiotic activity can lower intestinal pH through the production of short-chain fatty acids, enhancing the solubility and absorption of minerals like calcium, iron, and zinc [193]. Some probiotic species, such as Lactiplantibacillus plantarum, Limosilactobacillus fermentum, Lactobacillus acidophilus, and Bifidobacterium breve, can degrade phytates and oxalates, compounds that otherwise inhibit mineral absorption, thereby improving the bioavailability of these nutrients [194]. Clinical trials have demonstrated that supplementation with specific probiotic species such as Lactobacillus helveticus and Lacticaseibacillus paracasei (no particular strains were mentioned in the study) can lead to measurable increases in serum levels of these micronutrients, underscoring their potential role in addressing nutrient deficiencies [191]. Probiotics may also influence gastrointestinal motility, which is crucial for optimal digestion and nutrient absorption. For instance, Lacticaseibacillus rhamnosus GG has been shown to enhance gastric emptying and intestinal transit times, thereby facilitating more efficient nutrient assimilation. These effects are thought to be mediated through the modulation of gut hormones and neurotransmitters involved in motility regulation [195].
The integration of probiotics into the diet presents a promising avenue for enhancing digestive health and nutrient absorption. Through various mechanisms—including enzyme production, modulation of gut morphology, vitamin synthesis, and improvement of mineral bioavailability—probiotics can play a pivotal role in optimizing nutritional status. However, the efficacy of probiotic interventions is strain-specific and influenced by factors such as dosage, duration of administration, and individual host characteristics. Further research is warranted to delineate these variables and to establish standardized guidelines for probiotic use in nutritional therapy.
2.6. Probiotics as the Connecting Link in the One Health Concept
In the One Health approach, great emphasis is put on the interconnection between humans, animals, and the environment. These factors cannot function properly if one of them is missing or harmed. In this framework, probiotics play a great role as a link connecting all parts, potentially benefiting all three sectors simultaneously. The growing use in veterinary medicine, agriculture, and environmental management positions probiotics as one of the strategies of the One Health concept [196].
The use of probiotics in both human and animal medicine lowers the use of antibiotics, reducing the reliance of treatment on these drugs, therefore addressing one of the most critical One Health issues: antimicrobial resistance [197].
Probiotics used in agriculture enhance biodiversity, reduce the ecological footprint of food production, and, as mentioned above, limit the spread of resistant bacteria and genes into the environment [198].
Probiotics serve as a bridge between human, animal, and environmental health. Their responsible use aligns with One Health goals. As global health challenges become more and more connected in these three sectors, as proven, for example, by the SARS-CoV-2 pandemic, probiotics serve as a possibility of managing all of them by using one solution [199].
3. Key Characteristics of New Probiotic Strains Derived from Animals
3.1. Tolerance to Environmental Conditions
Newly isolated probiotic strains from animals ought to exhibit key characteristics that determine their survival and efficacy under various environmental conditions. A fundamental aspect of these probiotics is their resilience to harsh conditions, particularly the challenges posed by the gastrointestinal tract (GIT). To function effectively in humans, companion animals, and livestock, probiotic strains must demonstrate tolerance to factors such as low pH, bile salts, enzymatic degradation, and temperature fluctuations [200]. Their ability to thrive in such environments is critical for ensuring their viability and effectiveness when administered.
The successful application of probiotic strains isolated from animals requires optimization of manufacturing processes, ensuring viability during storage and transportation, and resilience through gastrointestinal transit. Each stage presents significant challenges that must be addressed to maintain probiotic efficacy [201]. Scaling up probiotic production introduces variations in cultivation conditions, including pH, medium composition, and gas atmosphere, affecting cell survival and metabolic stability [202]. Large-scale fermentation requires stringent control of homogeneity and holding times to maintain quality [203]. Post-fermentation processing, particularly freeze-drying and spray-drying, imposes osmotic, oxidative, and thermal stresses, potentially leading to membrane damage and viability loss. Freeze-drying risks intracellular ice formation, while spray-drying primarily induces heat stress, affecting membrane integrity [204].
Studies have shown that probiotics derived from livestock, including ruminants, pigs, and poultry, must be rigorously tested for survival under conditions mimicking the GIT environment. These conditions include acidic gastric environments (pH 2–3), bile salt concentrations, and the presence of digestive enzymes, such as pepsin, which challenge microbial survival and activity. Upon ingestion, probiotics encounter salivary enzymes, such as lysozyme, though their impact on viability is minimal due to the transient exposure time [205]. However, the gastric environment presents a major barrier, with acidic pH (0.9–3.0), digestive enzymes, and hydrochloric acid posing significant threats to bacterial survival [206]. Acid stress can lead to intracellular acidification, disruption of membrane integrity, and depletion of ATP due to the reversal of the F1F0-ATPase function [207]. Strains such as Lacticaseibacillus rhamnosus GG and those from the species Saccharomyces boulardii have been evaluated for their ability to withstand these stressors and demonstrate efficacy in animal models as well as in humans [208]. Probiotic yeasts isolated from animal feces, such as Kodamaea ohmeri, Trichosporon asahii, Trichosporon spp., Pichia kudriavzevii, and Wickerhamomyces anomalus, have shown promising resistance to low pH and bile salt concentrations. Among these, W. anomalus exhibited the highest capacity for agglutination and adherence, demonstrating its ability to grow under acidic and stressful conditions, which enhances its potential as a probiotic in harsh gastrointestinal environments [209]. Furthermore, yeasts isolated from ruminal liquid, including Magnusiomyces capitatus, Candida ethanolica, Candida paraugosa, Candida rugosa, and P. kudriavzevii, have been shown to effectively reduce pH, accumulate acids, and improve the digestibility of neutral detergent fiber. These findings indicate the survival and functionality of these microorganisms within the ruminal environment, offering potential benefits for ruminant nutrition [210]. Additionally, Debaryomyces hansenii has been highlighted for its strong immunomodulatory effects in in vitro studies, likely attributed to its production of polyamines and the presence of β-D-glucan in its cell wall, which contribute to its beneficial immune-stimulating properties [211]. These studies underscore the promising applications of yeasts as probiotics for both gastrointestinal health and animal nutrition.
Upon entering the small intestine, probiotics must withstand bile salts, pancreatic enzymes, and a sudden shift to a more neutral pH (~6.0), which can destabilize membranes and proteins [212]. Bile acids, particularly their conjugated forms, act as biological detergents, disrupting membrane integrity and dissipating the proton motive force, leading to ion leakage, oxidative stress, and potential cell death. However, probiotic strains with bile salt hydrolase (BSH) activity demonstrate enhanced survival by deconjugating bile acids, reducing their toxicity [213]. Species like Lactobacillus spp. isolated from ruminants and swine have demonstrated enhanced resistance to digestive enzymes such as pepsin and pancreatin, ensuring their survival and retention of probiotic functionality after GIT transit [214]. Furthermore, biofilm formation—a key adaptive mechanism—enhances the resilience of probiotics by promoting adherence to intestinal mucosal surfaces and increasing resistance to environmental stressors. Lactobacillus spp. and Bifidobacterium spp., derived from animals, exhibit strong biofilm-forming capabilities, allowing them to establish stable populations in the host’s intestine [215].
To counteract these stressors, newly animal-derived probiotics should employ both innate and adaptive mechanisms. Intrinsic resistance includes cell envelope modifications and metabolic adjustments, while adaptive responses involve changes in membrane composition, upregulation of chaperones, stress-response proteins, and DNA repair enzymes [216]. These strategies collectively enhance survival and functional stability, highlighting the importance of strain-specific selection and formulation techniques in probiotic development.
3.2. Production of Antimicrobial Properties and Bioactive Compounds
3.2.1. Bacteriocins
Another essential characteristic of new animal-derived probiotics is their ability to produce antimicrobial compounds that inhibit pathogenic microorganisms within the gut. Bacteriocins are cationic peptides with antimicrobial properties that exert their action primarily through the formation of pores in the target cell membranes, leading to the dissipation of cytosolic contents and subsequent cell death. In addition to their direct antimicrobial activity, bacteriocins are also involved in modulating the host’s native microbiota. This modulation can positively influence host immune responses, contributing to enhanced health outcomes. The ability of bacteriocins to alter microbial community structures and promote beneficial host–microbe interactions highlights their potential as therapeutic agents in preventing or treating infections. Furthermore, the immune-regulatory effects of bacteriocins may enhance the host’s resilience to pathogens, thus supporting overall health-promoting functions [217].
Strains isolated from pigs and poultry, such as those from Lactobacillus spp., have been shown to secrete lactic acid and bacteriocins, contributing to microbiota balance and pathogen suppression. In vitro studies have shown that Lacticaseibacillus rhamnosus GG effectively inhibits the growth and adherence of several pathogenic bacteria, including Salmonella, Shigella, Escherichia coli, and Streptococcus species, highlighting its potential as a broad-spectrum probiotic with antimicrobial properties [218]. Some probiotic strains, such as Ligilactobacillus salivarius NRRL B-30514, isolated from chicken ceca, have demonstrated potential for producing bacteriocins that inhibit pathogenic bacteria. Specifically, studies have shown that this strain significantly reduces the presence of Campylobacter jejuni in the intestinal environment [219]. Although poorly documented, there is growing evidence suggesting that LAB, particularly those capable of producing bacteriocins or adhering to host cells, may have the potential to modify the microbiota of the teat apex and reduce the proliferation of pathogenic bacteria. Isolates from the genera Lactobacillus and Lactococcus have been evaluated for their ability to colonize the epithelium of the teat apex, where they demonstrated inhibitory activities against pathogenic bacteria such as Staphylococcus aureus, Streptococcus uberis, and Escherichia coli [220]. Bacteriocin-mediated effects of probiotic strains on pathogen inhibition have been demonstrated, as seen in Figure 3. Moreover, examples of animal derived bacterial strains and bacteriocins they produce are listed in Table 5.
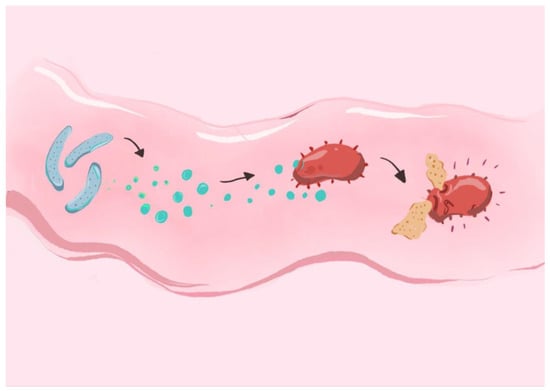
Figure 3.
Bacteriocin-mediated effects of probiotic strains on pathogen inhibition: probiotic bacteria, such as those from Lactobacillus spp., secrete bacteriocins that act on the pathogenic bacterial cells. By creating pores in the target cell membranes, bacteria-derived antimicrobial compounds lead to the dissipation of cytosolic contents and subsequent cell death.

Table 5.
Examples of animal-derived bacterial strains and the bacteriocins they produce.
3.2.2. Organic Acids, Vitamins, and Exopolysaccharides
Animal-derived probiotic bacteria can produce organic acids such as lactic acid, acetic acid, and butyric acid, which acidify the gut environment and inhibit the growth of pathogenic bacteria. The production of amino acids by gut bacteria plays a critical role in the synthesis of SCFAs and the regulation of host metabolism. Bacteria in the gut produce several amino acids de novo, which act as precursors for SCFAs, substances that assist in the fermentation of undigested carbohydrates and influence the host’s physiology. These amino acids and their derived metabolites, including SCFAs, are known to regulate the metabolism of carbohydrates and lipids, ultimately contributing to the overall health of the host [225]. Additionally, LAB are involved in the proteolysis of casein molecules, producing small peptides and amino acids, which further contribute to various metabolic processes [226]. These acids also serve as a source of energy for the host and can modulate the immune response. The Bifidobacterium species and Lactobacillus species (Lacticaseibacillus rhamnosus GG, Lacticaseibacillus rhamnosus HA-114, among other animal-derived strains) produce lactic acid, which lowers the pH of the gut, creating an inhospitable environment for many pathogens [227].
Numerous different bacteria produce essential vitamins that are incredibly beneficial for the host’s organism. A summary of the bacterial strains and the vitamins they produce is to be found in Table 6.

Table 6.
A summary of the bacterial strains and the vitamins they produce.
Exopolysaccharides (EPSs) produced by probiotic bacteria, particularly LAB, have garnered attention due to their various health benefits, including immunostimulation, antitumor effects, antioxidant activity, and cholesterol-lowering properties. EPSs are synthesized through the action of enzymes such as glycosyltransferases and glycantransferases, which convert sugar nucleotide precursors into polysaccharides [237]. Among LAB, Lactobacillus helveticus has shown antitumor effects against cancer cell lines, including HepG-2, BGC-823, and HT-29 [238].
4. Factors Influencing the Safety and Effectiveness of the New Strains
Before implementing new strains of probiotics into both human and veterinary medicine, it is of great importance to thoroughly test them and understand the possible risks associated with their use. Unfortunately, the fact that products based on probiotic bacterial strains have been tested does not rule out the occurrence of adverse reactions following their administration. Such a situation sometimes occurs after the use of preparations containing Clostridium spp. strains. In Japan, a case was reported of an elderly man dying of bacteremia, which developed after consuming a commonly used (and one of the 10 prescribed) probiotics containing strains from the species Clostridium butyricum [239]. New, untested strains could pose a similar threat to the health of future consumers. In order to prevent this, all new strains must go through a rigorous series of testing. There were also particular cases where the administration of Lactobacillus probiotics has been associated with developing infections in immunocompromised patients [240]. In addition to what is written above, probiotic therapy may pose a threat when administered to patients with CKD. Tryptophan metabolites produced by gut microbiota, such as indoxyl sulfate, have been implicated in the progression of chronic kidney disease due to their pro-inflammatory and oxidative properties [241]. The role of probiotic therapy in modulating these metabolites remains contentious. Some studies caution against probiotic use, suggesting it may elevate levels of tryptophan-derived uremic toxins, potentially accelerating CKD progression [242]. Conversely, other research indicates that specific probiotic formulations can reduce these toxins, thereby exerting a protective effect on renal function [243].
A study from D’Agostin (2021) [244] identified 49 cases of probiotic-associated invasive infection in children, with sepsis being the most frequently reported outcome. Importantly, the majority of these cases occurred in infants under 2 years of age who had underlying risk factors, such as prematurity or the presence of indwelling intravenous catheters. Fortunately, 94% of affected children responded successfully to antimicrobial therapy, suggesting that early detection and treatment are effective.
The essential components required to establish the safety of a probiotic are the correct identification of the bacteria from the samples obtained using modern techniques, followed by the determination of the ability of these bacteria to colonize the relevant niche of the organism, the characterization of their resistance and sensitivity to various agents, and their stability and possible pathogenicity.
4.1. Stability and Safety Testing of New Probiotic Strains
The stability of probiotic strains is an area in which not much research has been conducted. The high stability of the genome is a factor that ensures that passage through multiple hosts and long-term colonization keep the strains in their original, unchanged form. Sequencing the whole genome is required to obtain all needed information, and such studies have not been conducted in multiple strains, leaving this area open for further research [245].
In order to register new strains of probiotics, they must go through a series of tests to prove that they are safe for human and animal consumption. Tests can be divided into three groups: in vitro safety assessments, in vivo animal studies, and human clinical trials.
To check for carrying antibiotic resistance genes, the most common method is Minimum Inhibitory Concentration (MIC) testing. Hemolysis tests, gelatinase and protease activity, biofilm formation assay, adhesion and invasion assays, toxin gene screening, and DNAse tests enable potential virulence factor identification. Metabolic activity screening ensures no production of harmful compounds like D-lactate. In vitro test results may differ from those received after an in vivo method of testing [246].
If the in vitro tests are promising, animal models (usually rodents) are used to assess safety. These studies provide insight into the effects of probiotics on a living organism and its microbiota, offering data that can not be obtained during in vitro testing. Rodents such as mice and rats are mainly chosen for this purpose due to their well-known anatomy and physiology. Gnotobiotic animals are used for testing the effect of probiotics on chosen microbes by intentionally inserting them into the animal organisms that were previously free of germs. To test the impact of probiotics on vulnerable populations, immunocompromised models are used [247].
To identify any toxic response, both single and repeated doses should be given to the model. This will result in checking the risk of both methods of supplementation, which can inhibit acute, sub-chronic, and chronic reactions to the given strain, identifying any kind of toxic response. Bacterial translocation has to be checked in order to observe the probiotics’ possible route into the body. Potential pathogenicity can be indicated by the translocation of the strain to sterile tissues like the liver, spleen, or the bloodstream. Infectivity in vulnerable populations is checked using immunocompromised models [248]. Both ante-mortem and postmortem examination of the samples is crucial. Histopathological and microbiological examinations of the sample are conducted to detect any adverse effects or bacterial translocation [249].
Conducting human clinical trials is a pivotal step in validating the safety of new probiotic strains. Randomized Controlled Trials (RCTs) are the gold standard for clinical research. The length of the study should enable the observation of effects on the human body. Immunocompromised individuals require additional testing. The results have to be thoroughly analyzed, and a report of the study should be written to finish the trial. Any adverse effects should be reported and analyzed closely [250].
To ensure the quality, safety, and efficiency of new probiotic strains, test method guidelines need to be met. Several organizations have developed rules for the tests. The Food and Agriculture Organization (FAO) and the World Health Organization (WHO) prepared the FAO/WHO Guidelines for the Evaluation of Probiotics in Food, which provides information on the testing methods for probiotics intended for food use. The International Probiotics Association (IPA) wrote guidelines focusing on the production and marketing of probiotic-containing products. In Europe, the guidelines of the European Federation of Associations of Health Product Manufacturers (EHPM) must be met [251].
Conducting in vivo studies on both animal models and humans requires adherence to strict ethical standards. Animal welfare, as well as scientific validity, are key factors in obtaining data.
4.2. Effectiveness
The implementation of new probiotic strains requires a complicated series of tests, including those examining the effectiveness of potential new strains. In order to determine the effects of the strain and register them for human or animal consumption, multiple factors need to be checked, including clinical evidence of probiotic therapy, the mechanism of action, and interactions [252]. Without proving the effectiveness of new strains, implementing them for consumption is redundant [253].
The mechanism of action (MoA) is crucial in probiotic research and implementation. Without determining the MoA, probiotics may be inconsistently effective, unsafe, or not effective at all. Different strains have different MoAs, which influence their effects. Subtle differences between species may cause crucial differences in their functioning. Because of this, seemingly similar strains can act differently. Identifying the MoA ensures the selection of strains based on their specific medical outcomes, making targeted therapy possible. Establishing the MoA is one of the steps required by national federations responsible for probiotic analysis, which has to be conducted before registering them. In a study by Mingkang et al., the mechanisms of action of probiotics are divided into three main groups: the production of bioactive compounds, the regulation of the gastrointestinal microbiome, and the modulation of the immune and nervous systems [254].
The effectiveness of probiotics largely depends on strain specification, dose, and the formula of application. Proper dosing and formulation ensure survival, colonization, and therapeutic benefits, reducing the risk of adverse effects or failing to deliver desired results [255]. The recommended daily dose generally ranges from 106 to 1012 CFU. However, it can vary widely based on the specific strain of probiotics, the health problem being treated, and the patient’s health status and general condition. Probiotics with lower CFUs (1–5 billion CFUs per day) are recommended for maintaining the general proper condition of gut microbial flora or as the starting point for probiotic therapy. On the contrary, for conditions like irritable bowel syndrome (IBS), IBD, or antibiotic-associated diarrhea, higher doses (10–50 billion CFUs per day) are proven to be more effective [256]. For example, research on the probiotic Lacticaseibacillus rhamnosus Lcr35 revealed that its effect on human dendritic cells is dose-dependent. At higher doses, the probiotic-induced semi-maturation of dendritic cells and a strong pro-inflammatory response are characterized by increased production of pro-Th1/Th17 cytokines. This finding underscores the necessity of precise dosing to achieve the desired immunomodulatory effects [257].
5. Conclusions
Probiotics derived from animal origin present a promising tool in both human and veterinary medicine, offering a wide array of health benefits across species. From mitigating gastrointestinal disorders, enhancing immune responses, and improving wound healing to enhancing livestock productivity, their applications are extensive and continue to evolve. However, despite their therapeutic potential, probiotics remain underappreciated, not only for their benefits but also for the risks they may pose if not thoroughly investigated and carefully applied.
While numerous strains are already in clinical or commercial use, ongoing research continues to unveil new candidates with unique properties, including enhanced resilience to environmental conditions with greater precision and efficacy. These discoveries pave the way for tailored probiotic therapies that could target specific health conditions with greater precision and efficacy. Yet, such advancements need rigorous safety evaluations, including in vivo and in vitro studies, to assess factors like pathogenicity, drug resistance, and stability.
Looking ahead, the future of probiotic research lies in striking a careful balance between innovation and safety. Probiotics should not be viewed as universally benign; individual patient history, potential interactions with medications, and strain-specific characteristics must all be considered. The benefit/risk ratio must guide clinical decisions, particularly as many probiotic products remain available over the counter without prescription. This widespread accessibility, while advantageous, also calls for increased public awareness and potentially stricter regulatory guidelines to ensure that probiotic use aligns with scientific evidence and patient safety.
To become more beneficial for the future, the ongoing research on probiotics should focus on the development of standardized protocols for probiotic strain identification, characterization, and clinical testing to ensure reproducibility and comparability across studies. Investigating host-specific responses, including differences in gut microbiota composition, immune modulation, and pharmacokinetics, can help tailor probiotic therapies to individual patients or animal species. Expanding longitudinal studies and real-world trials will further clarify long-term safety, efficacy, and optimal usage parameters, guiding both regulatory policy and clinical practice.
Ultimately, novel animal-origin probiotics represent a frontier of opportunity within the One Health framework, linking human and animal well-being. Their future development holds immense promise, but their responsible integration into healthcare requires continued research, thoughtful risk assessment, and a commitment to prioritizing patient safety alongside therapeutic innovation.
Author Contributions
Conceptualization, J.B. and M.M.; literature review, Z.G., A.M., J.B., and M.M.; writing-original draft preparation, Z.G. and A.M.; writing-review and editing, Z.G., A.M., J.B., and M.M.; figures conceptualization, Z.G.; figures preparation, D.B.; supervision, J.B. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Wrocław University of Environmental and Life Sciences.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ACE | Angiotensin-converting enzyme |
| ACD | Allergic contact dermatitis |
| AD | Alzheimer’s disease |
| AFLP | Amplified Fragment Length Polymorphism |
| ARGs | Antibiotic resistance genes |
| ASD | Autism spectrum disorder |
| ATP | Adenosine Triphosphate |
| BSH | Bile salt hydrolase |
| BL999 | Bifidobacterium longum (strain 999) |
| CLA | Conjugated linoleic acid |
| CDI | Clostridioides difficile Infection |
| CFU | Colony-forming unit |
| CKD | Chronic kidney disease |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase-2 |
| CPEK | Canine progenitor epidermal keratinocytes |
| DNA | Deoxyribonucleic Acid |
| EcN | Escherichia coli Nissle 1917 |
| EHEC | Enterohemorrhagic Escherichia coli |
| EHPM | European Federation of Associations of Health Product Manufacturers |
| ENS | Enteric nervous system |
| EPSs | Exopolysaccharides |
| EPEC | Enteropathogenic Escherichia coli |
| FAO | Food and Agriculture Organization |
| FHV-1 | Feline herpesvirus type 1 |
| FIC | Feline Idiopathic Cystitis |
| FISH | Fluorescent In Situ Hybridization |
| GALT | Gut-associated lymphoid tissue |
| GBA | Gut–brain axis |
| GIT | Gastrointestinal tract |
| GFR | Glomerular filtration rate |
| GLUT2 | Glucose transporter 2 |
| H. pylori | Helicobacter pylori |
| HPA | Hypothalamic-pituitary-adrenal |
| HIF | Hypoxia-inducible factor |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| Is | Indoxyl sulfate |
| IgA | Immunoglobulin A |
| LAB | Lactic acid bacteria |
| LG2055 | Lactobacillus gasseri SBT2055 |
| LcS | Lactobacillus casei strain Shirota |
| MIC | Minimum Inhibitory Concentration |
| MDR | Multidrug-resistant |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MOA | Mechanism of action |
| UTI | Urinary tract infection |
| pCS | p-Cresyl Sulfate |
| RCTs | Randomized Controlled Trials |
| ROS | Reactive oxygen species |
| rUTIs | Recurrence of Urinary Tract Infections |
| SCFA | Short-chain fatty acids |
| sIgA | Secretory Immunoglobulin A |
| STT | Standard triple therapy |
| TC | Total cholesterol |
| T2DM | Type 2 diabetes mellitus |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| Tregs | T regulatory cells |
| UPEC | Uropathogenic Escherichia coli |
| VRE | Vancomycin-resistant Enterococci |
| VBNC | Viable but Non-Culturable |
| VVC | Vulvovaginal Candidiasis |
| WHO | World Health Organization |
References
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.O.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef] [PubMed]
- Hand, D.; Wallis, C.; Colyer, A.; Penn, C.W. Pyrosequencing the canine fecal microbiota: Breadth and depth of biodiversity. PLoS ONE 2013, 8, e53115. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.A.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 380–388. [Google Scholar] [CrossRef]
- Pinna, C.; Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windish, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations, and apparent total tract digestibility in dogs. BMC Vet. Res. 2018, 14, 106. [Google Scholar] [CrossRef]
- Wang, G.; Ding, T.; Ai, L. Editorial: Effects and mechanisms of probiotics, prebiotics, synbiotics and postbiotics on intestinal health and disease. Front. Cell. Infect. Microbiol. 2024, 14, 1430312. [Google Scholar] [CrossRef]
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077. [Google Scholar] [CrossRef]
- Collado, M.C.; Vinderola, G.; Salminen, S. Postbiotics: Facts and Open Questions. A Position Paper on the Need for a Consensus Definition. Benef. Microbes 2019, 10, 711–720. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–166. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Bonvicini, F.; Gramenzi, A. Probiotics History. J. Clin. Gastroenterol. 2016, 50, S116–S119. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Abdou, A.M.; Hedia, R.H.; Omara, S.T.; Mahmoud, M.A.E.; Kandil, M.M.; Bakry, M.A. Interspecies comparison of probiotics isolated from different animals. Vet. World. 2018, 11, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Bunesova, V.; Vlkova, E.; Rada, V.; Killer, J.; Musilova, S. Bifidobacteria from the gastrointestinal tract of animals: Differences and similarities. Benef. Microbes 2014, 5, 377–388. [Google Scholar] [CrossRef]
- Elzeini, H.M.; Ali, A.R.A.A.; Nasr, N.F.; Hassan, M.; Hassan, A.A.M.; Elenany, Y.E. Probiotic capability of novel lactic acid bacteria isolated from worker honey bees gut microbiota. FEMS Microbiol. Lett. 2021, 368, fnab030. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.; Liu, S.; Wang, Q.; Guo, Z.; Chen, C.; Feng, J. What drives changes in the virulence and antibiotic resistance of Vibrio harveyi in the South China Sea? J. Fish Dis. 2020, 43, 1103–1112. [Google Scholar] [CrossRef]
- Anee, I.J.; Alam, S.; Begum, R.A.; Shahjahan, R.M.; Khandaker, A.M. The role of probiotics on animal health and nutrition. J. Basic Appl. Zool. 2021, 82, 52. [Google Scholar] [CrossRef]
- Park, Y.H.; Hamidon, F.; Rajangan, C.; Soh, K.P.; Gan, C.Y.; Lim, T.S.; Abdullah, W.N.W.; Liong, M.T. Application of Probiotics for the Production of Safe and High-Quality Poultry Meat. Korean J. Food Sci. Anim. Resour. 2016, 36, 567–576. [Google Scholar] [CrossRef]
- Goel, G.; Kumar, A. (Eds.) Advances in Probiotics for Sustainable Food and Medicine; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Kober, A.K.M.H.; Riaz Rajoka, M.S.; Mehwish, H.M.; Villena, J.; Kitazawa, H. Immunomodulation Potential of Probiotics: A Novel Strategy for Improving Livestock Health, Immunity, and Productivity. Microorganisms 2022, 10, 388. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Shi, W.; Yang, B.; Wang, J. The Probiotic and Immunomodulation Effects of Limosilactobacillus reuteri RGW1 Isolated from Calf Feces. Front. Cell. Infect. Microbiol. 2023, 12, 1086861. [Google Scholar] [CrossRef]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus gasseri SBT2055 Induces TGF-β Expression in Dendritic Cells and Activates TLR2 Signal to Produce IgA in the Small Intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef]
- Kwon, H.-K.; Lee, C.-G.; So, J.-S.; Chae, C.-S.; Hwang, J.-S.; Sahoo, A.; Nam, J.H.; Rhee, J.H.; Hwang, K.-C.; Im, S.-H. Generation of Regulatory Dendritic Cells and CD4+Foxp3+ T Cells by Probiotics Administration Suppresses Immune Disorders. Proc. Natl. Acad. Sci. USA 2010, 107, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Nugent, W.R.; Daugherty, L. A Measurement Equivalence Study of the Family Bondedness Scale: Measurement Equivalence Between Cat and Dog Owners. Front. Vet. Sci. 2021, 8, 812922. [Google Scholar] [CrossRef]
- Deng, P.; Swanson, K.S. Gut microbiota of humans, dogs, and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 2015, 113, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Probiotics in human medicine. Gut 1991, 32, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.N.; Barua, N.; Tin, M.C.F.; Dharmaratne, P.; Wong, S.H.; Ip, M. The use of probiotics and prebiotics in decolonizing pathogenic bacteria from the gut; A systematic review and meta-analysis of clinical outcomes. Gut Microbes 2024, 16, 2356279. [Google Scholar] [CrossRef]
- Shah, T.; Baloch, Z.; Shah, Z.; Cui, X.; Xia, X. The intestinal microbiota: Impacts of antibiotics therapy, colonisation resistance, and diseases. Int. J. Mol. Sci. 2021, 22, 6597. [Google Scholar] [CrossRef]
- Roson-Calero, N.; Ballesté-Delpierre, C.; Fernández, J.; Vila, J. Insights on current strategies to decolonize the gut from multidrug-resistant bacteria: Pros and cons. Antibiotics 2023, 12, 1074. [Google Scholar] [CrossRef]
- Wombwell, E.; Patterson, M.E.; Bransteitter, B.; Gillen, L. The effect of Saccharomyces Boulardii primary prevention on risk of hospital-onset Clostridioides difficile infection in hospitalized patients administered antibiotics frequently associated with C. difficile infection. Clin. Infect. Dis. 2021, 73, e2512–e2518. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef]
- Christensen, H.R.; Larsen, C.N.; Kæstel, P.; Rosholm, L.B.; Sternberg, C.; Michaelsen, K.F.; Frøkiær, H. Immunomodulating potential of supplementation with probiotics: A dose-response study in healthy young adults. FEMS Immunol. Med. Microbiol. 2006, 47, 380–390. [Google Scholar] [CrossRef]
- Tegegne, B.A.; Kebede, B. Probiotics, Their Prophylactic and Therapeutic Applications in Human Health Development: A Review of the Literature. Heliyon 2022, 8, e09725. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Cho, I.K.; Lee, C.H. Clinical Outcomes of Standard Triple Therapy Plus Probiotics or Concomitant Therapy for Helicobacter pylori Infection. Gut Liver 2018, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Qi, Z.; Luo, J.; Chen, T. Potential Applications of Engineered Bacteria in Disease Diagnosis and Treatment. Microbiome Res. Rep. 2025, 4, 10. [Google Scholar] [CrossRef]
- Wu, T.; Wang, G.; Xiong, Z.; Xia, Y.; Song, X.; Zhang, H.; Wu, Y.; Ai, L. Probiotics Interact with Lipids Metabolism and Affect Gut Health. Front. Nutr. 2022, 9, 917043. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Z.; Chen, X.; Huang, Y.; Yin, B.; Guo, F.; Zhao, H.; Zhao, T.; Qu, H.; Huang, J.; et al. The Effect of Lactobacillus rhamnosus Hsryfm 1301 on the Intestinal Microbiota of a Hyperlipidemic Rat Model. BMC Complement. Altern. Med. 2014, 14, 386. [Google Scholar]
- Ballan, R.; Saad, S.M.I. Characteristics of the Gut Microbiota and Potential Effects of Probiotic Supplements in Individuals with Type 2 Diabetes Mellitus. Foods 2021, 10, 2528. [Google Scholar] [CrossRef] [PubMed]
- Sohn, M.; Na, G.Y.; Chu, J.; Joung, H.; Kim, B.-K.; Lim, S. Efficacy and Safety of Lactobacillus plantarum K50 on Lipids in Koreans with Obesity: A Randomized, Double-Blind Controlled Clinical Trial. Front. Endocrinol. 2022, 12, 790046. [Google Scholar] [CrossRef]
- Mishra, S.; Wang, S.; Nagpal, R.; Miller, B.; Singh, R.; Taraphder, S.; Yadav, H. Probiotics and Prebiotics for the Amelioration of Type 1 Diabetes: Present and Future Perspectives. Microorganisms 2019, 7, 67. [Google Scholar] [CrossRef]
- Caesar, R. Pharmacologic and Non-Pharmacologic Therapies for Type 2 Diabetes on the Gut Microbiota. Can. J. Diabetes 2019, 43, 224–231. [Google Scholar] [CrossRef]
- Mahboobi, S.; Iraj, B.; Maghsoudi, Z.; Feizi, A.; Ghiasvand, R.; Askari, G.; Maayeshi, N. The Effects of Probiotic Supplementation on Markers of Blood Lipids, and Blood Pressure in Patients with Prediabetes: A Randomized Clinical Trial. Int. J. Prev. Med. 2014, 5, 1239–1246. [Google Scholar]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Veerappan, G.R.; Betteridge, J.; Young, P.E. Probiotics for the treatment of inflammatory bowel disease. Curr. Gastroenterol. Rep. 2012, 14, 324–333. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.D.S.S.; Barbalho, S.M.; de Alvares Goulart, R.; Quesada, K.; Bechara, M.D. The Current and Future Role of Drugs and Probiotics in the Management of Inflammatory Bowel Disease. J. Biosci. Med. 2015, 3, 76–85. [Google Scholar] [CrossRef]
- Mao, N.; Cubillos-Ruiz, A.; Cameron, D.E.; Collins, J.J. Probiotic strains detect and suppress cholera in mice. Sci. Transl. Med. 2018, 10, eaao2586. [Google Scholar] [CrossRef]
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: An observational study. Cell Rep. Med. 2020, 1, 100077. [Google Scholar] [CrossRef]
- Aljohani, A.M.; El-Chami, C.; Alhubail, M.; Ledder, R.G.; O’Neill, C.A.; McBain, A.J. Escherichia coli Nissle 1917 inhibits biofilm formation and mitigates virulence in Pseudomonas aeruginosa. Front. Microbiol. 2023, 14, 1108273. [Google Scholar] [CrossRef] [PubMed]
- Altonsy, M.O.; Andrews, S.C.; Tuohy, K.M. Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: Mediation by the mitochondrial pathway. Int. J. Food Microbiol. 2010, 137, 190–203. [Google Scholar] [CrossRef]
- Borowicki, A.; Michelmann, A.; Stein, K.; Scharlau, D.; Scheu, K. Fermented wheat aleurone enriched with probiotic strains LGG and Bb12 modulates markers of tumor progression in human colon cells. Nutr. Cancer 2011, 63, 151–160. [Google Scholar] [CrossRef]
- Orlando, A.; Refolo, M.G.; Messa, C.; Amati, L.; Lavermicocca, P.; Guerra, V.; Russo, F. Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr. Cancer 2012, 64, 1103–1111. [Google Scholar] [CrossRef]
- Russo, F.; Orlando, A.; Linsalata, M.; Cavallini, A.; Messa, C. Effects of Lactobacillus rhamnosus GG on the cell growth and polyamine metabolism in HGC-27 human gastric cancer cells. Nutr. Cancer 2007, 59, 106–114. [Google Scholar] [CrossRef]
- Sadeghi-Aliabadi, H.; Mohammadi, F.; Fazeli, H.; Mirlohi, M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran. J. Basic Med. Sci. 2014, 17, 815–819. [Google Scholar] [PubMed]
- Lee, J.W.; Shin, J.G.; Kim, E.H.; Kang, H.E.; Yim, I.B.; Kim, J.Y.; Joo, H.G.; Woo, H.J. Immunomodulatory and antitumor effects in vivo by the cytoplasmic fraction of Lactobacillus casei and Bifidobacterium longum. J. Vet. Sci. 2004, 5, 41–48. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2021, 13, 20. [Google Scholar] [CrossRef]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Shim, Y.Y.; Cha, S.K.; Reaney, M.J.T.; Chee, K.M. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J. Med. Microbiol. 2012, 61, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Rountree, R. Proven therapeutic benefits of high-quality probiotics. Appl. Nutr. Sci. Rep. 2002, 4, 1–6. [Google Scholar]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Hautefort, I.; Thompson, A.; Hinton, J.C.; Van Immerseel, F. Butyrate specifically down-regulates salmonella pathogenicity island 1 gene expression. Appl. Environ. Microbiol. 2006, 72, 946–949. [Google Scholar] [CrossRef]
- Bassaganya-Riera, J.; Viladomiu, M.; Pedragosa, M.; Simone, C.; Hontecillas, R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS ONE 2012, 7, e0275512. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota–Gut–Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23, 11245. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-Gut-Microbe Communication in Health and Disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Wang, Z.; Xie, G.; Liu, M.; Yuan, B.; Chai, H.; Wang, W.; Cheng, P. Implications of gut microbiota in neurodegenerative diseases. Front. Immunol. 2022, 13, 785644. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.F.; Hardingham, G.E. The influence of synaptic activity on neuronal health. Curr. Opin. Neurobiol. 2011, 21, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Patil, N.; Jain, M.; Kole, C.; Kaushik, P. Probiotics for Neurodegenerative Diseases: A Systemic Review. Microorganisms 2023, 11, 1083. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The role of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Böttner, M.; Zunke, F.; Cossais, F.; Berg, D.; Arnold, P. Alpha synuclein connects the gut-brain axis in Parkinson’s disease patients—A view on clinical aspects, cellular pathology and analytical methodology. Front. Cell Dev. Biol. 2020, 8, 573696. [Google Scholar] [CrossRef]
- Magistrelli, L.; Amoruso, A.; Mogna, L.; Graziano, T.; Cantello, R.; Pane, M.; Comi, C. Probiotics May Have Beneficial Effects in Parkinson’s Disease: In vitro Evidence. Front. Immunol. 2021, 10, 969. [Google Scholar] [CrossRef]
- Nowak, A.; Paliwoda, A.; Blasiak, J. Anti-proliferative, pro-apoptotic and anti-oxidative activity of Lactobacillus and Bifidobacterium strains: A review of mechanisms and therapeutic perspectives. Crit. Rev. Food Sci. Nutr. 2018, 59, 3456–3467. [Google Scholar] [CrossRef]
- Mohammedsaeed, W.; Cruickshank, S.; McBain, A.J.; O’Neill, C.A. Lactobacillus rhamnosus GG lysate increases re-epithelialization of keratinocyte scratch assays by promoting migration. Sci. Rep. 2015, 5, 16147. [Google Scholar] [CrossRef]
- Puebla-Barragan, S.; Reid, G. Probiotics in Cosmetic and Personal Care Products: Trends and Challenges. Molecules 2021, 26, 1249. [Google Scholar] [CrossRef]
- Gueniche, A.; Delattre, C.; Winstall, E.; Bastien, P.; Bernard, D.; Castiel-Higounec, I.; Breton, L. An Original Topical Probiotic-Related Ingredient for Dry Skin: Efficacy Evaluated in a Clinical Trial with the Help of Bioinstrumental Measurements and Proteomic Tools. J. Investig. Dermatol. 2010, 130, S65. [Google Scholar]
- Habeebuddin, M.; Karnati, R.K.; Shiroorkar, P.N.; Nagaraja, S.; Asdaq, S.M.B.; Anwer, M.K.; Fattepur, S. Topical Probiotics: More Than a Skin Deep. Pharmaceutics 2022, 14, 557. [Google Scholar] [CrossRef]
- Karimi, F.; Montazeri-Najafabady, N.; Mohammadi, F.; Azadi, A.; Koohpeyma, F.; Gholami, A. A Potential Therapeutic Strategy of an Innovative Probiotic Formulation Toward Topical Treatment of Diabetic Ulcer: An In Vivo Study. Nutr. Diabetes 2024, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Alziadi, R.; Thaher, A.A.S.; Al-Issawi, N. Utilization of Isolated Therapeutic Bacteria in Topical Ointment for Infection Treatment. Iraqi J. Agric. Sci. 2020, 51, 1661–1669. [Google Scholar] [CrossRef]
- Aybar, J.N.A.; Mayor, S.O.; Olea, L.; Garcia, J.J.; Nisoria, S.; Kolling, Y.; Melian, C.; Rachid, M.; Dimani, R.T.; Werenitzky, C.; et al. Topical Administration of Lactiplantibacillus plantarum Accelerates the Healing of Chronic Diabetic Foot Ulcers through Modifications of Infection, Angiogenesis, Macrophage Phenotype, and Neutrophil Response. Microorganisms 2022, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Jalili, M.; Kamali, A.; Nikahval, B. The concurrent use of probiotic microorganism and collagen hydrogel/scaffold enhances burn wound healing: An in vivo evaluation. Burns 2018, 44, 1775–1786. [Google Scholar] [CrossRef]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef]
- Woodfolk, J.A. T-cell responses to allergens. J. Allergy Clin. Immunol. 2007, 119, 280–294. [Google Scholar] [CrossRef]
- Chapat, L.; Chemin, K.; Dubois, B.; Bourdet-Sicard, R.; Kaiserlian, D. Lactobacillus casei reduces CD8+T cell-mediated skin inflammation. Eur. J. Immunol. 2004, 3, 2520–2528. [Google Scholar] [CrossRef]
- Weise, C.; Zhu, Y.; Ernst, D.; Ku, A.A.; Worm, M. Oral administration of Escherichia coli Nissle 1917 prevents allergen-induced dermatitis in mice. Exp. Dermatol. 2011, 20, 805–809. [Google Scholar] [CrossRef]
- Cohen, C.R.; Wierzbicki, M.R.; French, A.L.; Morris, S.; Newmann, S.; Reno, H.; Green, L.; Miller, S.; Powell, J.; Parks, T.; et al. Randomized Trial of Lactin-V to Prevent Recurrence of Bacterial Vaginosis. N. Engl. J. Med. 2020, 382, 1906–1915. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.E.; Au-yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, Placebo-Controlled Phase 2 Trial of a Lactobacillus crispatus Probiotic Given Intravaginally for Prevention of Recurrent Urinary Tract Infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Groah, S.L.; Rounds, A.K.; Ljungberg, I.H.; Sprague, B.M.; Frost, J.K.; Tractenberg, R.E. Intravesical Lactobacillus rhamnosus GG Is Safe and Well Tolerated in Adults and Children with Neurogenic Lower Urinary Tract Dysfunction: First-in-Human Trial. Neurourol. Urodyn. 2019, 38, 1441–1449. [Google Scholar] [CrossRef]
- Reid, G.; Bruce, A.W.; Taylor, M. Instillation of Lactobacillus and Stimulation of Indigenous Organisms to Prevent Recurrence of Urinary Tract Infections. Microecol. Ther. 1995, 23, 32–45. [Google Scholar]
- Gupta, V.; Mastromarino, P.; Garg, R. Effectiveness of Prophylactic Oral and/or Vaginal Probiotic Supplementation in the Prevention of Recurrent Urinary Tract Infections: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2024, 78, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, E.F.M.; Bellen, G.; Claes, I.; Henkens, T.; Allonsius, C.N.; Wittouck, S.; van den Broek, M.F.L.; Wuyts, S.; Kiekens, F.; Donders, G.G.G.; et al. Impact of a Lactobacilli-Containing Gel on Vulvovaginal Candidosis and the Vaginal Microbiome. Sci. Rep. 2020, 10, 7976. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Gut Microbiota and Hypertension: From Pathogenesis to New Therapeutic Strategies. Clin. Res. Hepatol. Gastroenterol. 2018, 42, 110–117. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, M.; Young, A.; Zhao, W.; Yan, Z. In Vivo Effectiveness and Safety of Probiotics on Prophylaxis and Treatment of Oral Candidiasis: A Systematic Review and Meta-Analysis. BMC Oral Health 2019, 19, 140. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, Z. Gut Probiotics and Health of Dogs and Cats: Benefits, Applications, and Underlying Mechanisms. Microorganisms 2023, 11, 2452. [Google Scholar] [CrossRef]
- Mondo, E.; Marliani, G.; Accorsi, P.A.; Cocchi, M.; Di Leone, A. Role of gut microbiota in dog and cat’s health and diseases. Open Vet. J. 2019, 9, 253–258. [Google Scholar] [CrossRef]
- Gookin, J.L.; Strong, S.J.; Bruno-Bárcena, J.M.; Stauffer, S.H.; Williams, S.; Wassack, E.; Azcarate-Peril, M.A.; Estrada, M.; Seguin, A.; Balzer, J. Randomized placebo-controlled trial of feline-origin Enterococcus hirae probiotic effects on preventative health and fecal microbiota composition of fostered shelter kittens. Front. Vet. Sci. 2022, 9, 923792. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef]
- Sýkora, J.; Valecková, K.; Amlerová, J.; Siala, K.; Dedek, P.; Watkins, S.; Varvarovská, J.; Stožický, F.; Pazdiora, P.; Schwarz, J. Effects of a specially designed fermented milk product containing probiotic Lactobacillus casei DN-114 001 and the eradication of H. pylori in children: A prospective randomized double-blind study. J. Clin. Gastroenterol. 2005, 39, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F. Evaluation of the Gastrointestinal Microbiota in Response to Dietary and Therapeutic Factors in Cats and Dogs Using Molecular Methods; Texas A&M University: College Station, TX, USA, 2011. [Google Scholar]
- Kelley, R.L.; Minikhiem, D.; Kiely, B.; O’Mahony, L.; O’Sullivan, D.; Boileau, T.; Park, J.S. Clinical Benefits of Probiotic Canine-Derived Bifidobacterium animalis Strain AHC7 in Dogs with Acute Idiopathic Diarrhea. Vet. Ther. 2009, 10, 121–130. [Google Scholar] [PubMed]
- Strompfová, V.; Simonová, M.P.; Gancarčíková, S.; Mudroňová, D.; Farbáková, J.; Mad’Ari, A.; Lauková, A. Effect of Bifidobacterium animalis B/12 Administration in Healthy Dogs. Anaerobe 2014, 28, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Strompfová, V.; Kubašová, I.; Lauková, A. Health benefits observed after probiotic Lactobacillus fermentum CCM 7421 application in dogs. Appl. Microbiol. Biotechnol. 2017, 101, 6309–6319. [Google Scholar] [CrossRef]
- Veir, J.K.; Knorr, R.; Cavadini, C.; Sherrill, S.J.; Benyacoub, J.; Satyaraj, E.; Lappin, M.R. Effect of Supplementation with Enterococcus faecium (SF68) on Immune Functions in Cats. Vet. Ther. 2007, 8, 229–238. [Google Scholar]
- Watson, V.E.; Jacob, M.E.; Bruno-Bárcena, J.M.; Amirsultan, S.; Stauffer, S.H.; Píqueras, V.O.; Frias, R.; Gookin, J.L. Influence of the intestinal microbiota on disease susceptibility in kittens with experimentally-induced carriage of atypical enteropathogenic Escherichia coli. Vet. Microbiol. 2019, 231, 197–206. [Google Scholar] [CrossRef]
- Fusi, E.; Rizzi, R.; Polli, M.; Cannas, S.; Giardini, A.; Bruni, N.; Marelli, S.P. Effects of Lactobacillus acidophilus D2/CSL (CECT 4529) supplementation on healthy cat performance. Vet. Rec. Open 2019, 6, e000368. [Google Scholar] [CrossRef]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.-Y.; Laga, W.; Wang, Y.; Ma, H.; Sun, Z.; Zhang, H. Oral administration of compound probiotics improved canine feed intake, weight gain, immunity, and intestinal microbiota. Front. Immunol. 2019, 10, 666. [Google Scholar] [CrossRef]
- Strompfová, V.; Marciňáková, M.; Simonová, M.; Bogovič-Matijašić, B.; Lauková, A. Application of potential probiotic Lactobacillus fermentum AD1 strain in healthy dogs. Anaerobe 2006, 12, 75–79. [Google Scholar] [CrossRef]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Gupta, R.; Jadhav, S.E.; Dutta, N. Comparative assessment of canine-origin Lactobacillus johnsonii CPN23 and dairy-origin Lactobacillus acidophilus NCDC 15 for nutrient digestibility, fecal fermentative metabolites and selected gut health indices in dogs. J. Nutr. Sci. 2017, 6, e38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.; Pattanaik, A.K.; Sharma, S.; Jadhav, S.E. Species-specific probiotic Lactobacillus johnsonii CPN23 supplementation modulates blood biochemical profile and erythrocytic antioxidant indices in Labrador dogs. Indian J. Anim. Sci. 2016, 86, 918–924. [Google Scholar] [CrossRef]
- Delucchi, L.; Fraga, M.; Zunino, P. Effect of the probiotic Lactobacillus murinus LbP2 on clinical parameters of dogs with distemper-associated diarrhea. Can. J. Vet. Res. 2017, 81, 118–121. [Google Scholar] [PubMed]
- Segovia, B.M.; Torras, M.D.L.Á.C. Communication of the results of the treatment with probiotics in two cats with chronic gingivostomatitis. Open J. Vet. Med. 2018, 8, 9–14. [Google Scholar] [CrossRef][Green Version]
- Garcia-Mazcorro, J.F.; Lanerie, D.J.; Dowd, S.E.; Paddock, C.G.; Grützner, N.; Steiner, J.M.; Ivanek, R.; Suchodolski, J.S. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol. Ecol. 2011, 78, 542–554. [Google Scholar] [CrossRef]
- Li, Y.; Ali, I.; Lei, Z.; Li, Y.; Yang, M.; Yang, C.; Li, L. Effect of a Multistrain Probiotic on Feline Gut Health through the Fecal Microbiota and Its Metabolite SCFAs. Metabolites 2023, 13, 228. [Google Scholar] [CrossRef]
- Lappin, M.R.; Veir, J.K.; Satyaraj, E.; Czarnecki-Maulden, G. Pilot study to evaluate the effect of oral supplementation of Enterococcus faecium SF68 on cats with latent feline herpesvirus 1. J. Feline Med. Surg. 2009, 11, 650–654. [Google Scholar] [CrossRef]
- Rossi, G.; Jergens, A.; Cerquetella, M.; Berardi, S.; Di Cicco, E.; Bassotti, G.; Pengo, G.; Suchodolski, J.S. Effects of a probiotic (SLAB51TM) on clinical and histologic variables and microbiota of cats with chronic constipation/megacolon: A pilot study. Benef. Microbes 2018, 9, 101–110. [Google Scholar] [CrossRef]
- Bybee, S.N.; Scorza, A.V.; Lappin, M.R. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. Vet. Intern. Med. 2011, 25, 856–860. [Google Scholar] [CrossRef]
- Wang, C.; He, R.; Dong, G. Comparative experiment on quality evaluation and deodorization antibacterial effect of probiotic bentonite cat litter. China Anim. Health 2022, 24, 110–112. [Google Scholar]
- Belà, B.; Di Simone, D.; Pignataro, G.; Fusaro, I.; Gramenzi, A. Effects of L. reuteri NBF 2 DSM 32264 consumption on the body weight, body condition score, fecal parameters, and intestinal microbiota of healthy persian cats. Vet. Sci. 2024, 11, 61. [Google Scholar] [CrossRef]
- Lippi, I.; Perondi, F.; Ceccherini, G.; Marchetti, V.; Guidi, G. Effects of probiotic VSL#3 on glomerular filtration rate in dogs affected by chronic kidney disease: A pilot study. Can. Vet. J. 2017, 58, 1301–1305. [Google Scholar] [PubMed]
- Kongtasai, T.; Paepe, D.; Meyer, E.; Mortier, F.; Marynissen, S.; Stammeleer, L.; Defauw, P.; Daminet, S. Renal Biomarkers in Cats: A Review of the Current Status in Chronic Kidney Disease. J. Vet. Intern. Med. 2022, 36, 379–396. [Google Scholar] [CrossRef]
- Prajapati, A.S.; Panchasara, H.H.; Sutaria, P.T.; Chauhan, P.M.; Suthar, A.N. Diagnosis of Chronic Renal Failure in Canine Using Enteric Dialysis. In Advances in Renal and Bladder Sciences; BP International: Kolkata, India, 2021; Chapter 9. [Google Scholar] [CrossRef]
- Rishniw, M.; Wynn, S.G. Azodyl, a Synbiotic, Fails to Alter Azotemia in Cats with Chronic Kidney Disease When Sprinkled onto Food. J. Feline Med. Surg. 2011, 13, 425–431. [Google Scholar] [CrossRef]
- Summers, S. Assessment of Novel Causes and Investigation into the Gut Microbiome in Cats with Chronic Kidney Disease. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2020. [Google Scholar]
- Huang, H.-W.; Kuo, T.-C.; Lee, Y.-J.; Chen, M.J. Multi-Omics Reveal That Two Probiotic Strains Associated with the Gut Microbiome and Host Metabolome Contribute to the Efficacy of Lactobacillus Intervention in Alleviating Feline Chronic Kidney Disease. Preprints 2023, 2023120403. [Google Scholar] [CrossRef]
- Hutchins, R.G.; Bailey, C.S.; Jacob, M.E.; Harris, T.L.; Wood, M.W.; Saker, K.E.; Vaden, S.L. The Effect of an Oral Probiotic Containing Lactobacillus, Bifidobacterium, and Bacillus Species on the Vaginal Microbiota of Spayed Female Dogs. Animals 2013, 27, 1368–1371. [Google Scholar] [CrossRef]
- Sofyan, M.S.; Rosman, N.; Krisnu, B.; Kamaludeen, J.B.; Dadi, T.B.; Pertiwi, H. Management of Feline Idiopathic Cystitis (FIC) Using Probiotic Combination Treatment. Indian Vet. J. 2019, 96, 20–22. [Google Scholar]
- Grin, P.M.; Kowalewska, P.M.; Alhazzani, W.; Fox-Robichaud, A.E. Lactobacillus for preventing recurrent urinary tract infections in women: Meta-analysis. Can. J. Urol. 2013, 20, 6607–6614. [Google Scholar]
- Liu, Y.H.; Ho, C.Y.; Huang, C.C.; Tsai, C.C. Inhibitory effect of lactic acid bacteria on uropathogenic Escherichia coli-induced urinary tract infections. J. Prob. Health 2016, 4, 144. [Google Scholar] [CrossRef]
- Shah, H.; Trivedi, M.; Gurjar, T.; Sahoo, D.K.; Jergens, A.E.; Yadav, V.K.; Patel, A.; Pandya, P. Decoding the Gut Microbiome in Companion Animals: Impacts and Innovations. Microorganisms 2024, 12, 1831. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Calinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Meineri, G.; Martello, E.; Atuahene, D.; Miretti, S.; Stefanon, B.; Sandri, M.; Biasato, I.; Corvaglia, M.R.; Ferrocino, I.; Cocolin, L.S. Effects of Saccharomyces boulardii Supplementation on Nutritional Status, Fecal Parameters, Microbiota, and Mycobiota in Breeding Adult Dogs. Vet. Sci. 2022, 9, 389. [Google Scholar] [CrossRef]
- Yeh, Y.-M.; Lye, X.-Y.; Lin, H.-Y.; Wong, J.-Y.; Wu, C.-C.; Huang, C.-L.; Tsai, Y.-C.; Wang, L.-C. Effects of Lactiplantibacillus plantarum PS128 on alleviating canine aggression and separation anxiety. Appl. Anim. Behav. Sci. 2022, 247, 105569. [Google Scholar] [CrossRef]
- Cannas, S.; Tonini, B.; Bela, B.; Di Prinzio, R.; Pignataro, G.; Di Simone, D.; and Gramenzi, A. Effect of a novel nutraceutical supplement (Relaxigen Pet dog) on the fecal microbiome and stress-related behaviors in dogs: A pilot study. J. Vet. Behav. 2021, 42, 37–47. [Google Scholar] [CrossRef]
- Mondo, E.; Barone, M.; Soverini, M.; Mariani, G.; Candelà, M.; Accorsi, P.A. Gut Microbiome Structure and Adrenocortical Activity in Dogs with Aggressive and Phobic Behavioral Disorders. Heliyon 2020, 6, e03311. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Barthe, M.; Gillot, L.; Perdigon, L.; Jacobs, A.; Schoonbroodt, G.; Mauhin, P.; Bouhajja, E.; Osman-Ponchet, H. Topical Probiotic Formulation Promotes Rapid Healing in Dog Keratinocyte Cells: A Promising Approach for Wound Management. Int. J. Mol. Sci. 2023, 24, 12360. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the Dog and Human Gut Microbiomes in Gene Content and Response to Diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Wu, D.; Cao, M.; Zhou, J.; Yan, S.; Peng, J.; Yu, Z.; Zhang, A.; Wu, J.; Yan, X.; Zhao, J. Lactobacillus casei T1 from Kurut against Helicobacter pylori-induced inflammation and the gut microbial disorder. J. Funct. Foods 2021, 85, 104611. [Google Scholar] [CrossRef]
- Mia, N.; Alam, A.M.M.N.; Rahman, M.M.; Ali, M.S.; Hashem, M.A. Probiotics to enhance animal production performance and meat quality: A review. Meat Res. 2024, 4, 1–7. [Google Scholar] [CrossRef]
- Ngunjiri, J.M.; Taylor, K.J.M.; Abundo, M.C.; Jang, H.; Elaish, M.; Kc, M.; Ghorbani, A.; Wijeratne, S.; Weber, B.P.; Johnson, T.J.; et al. Farm Stage, Bird Age, and Body Site Dominantly Affect the Quantity, Taxonomic Composition, and Dynamics of Respiratory and Gut Microbiota of Commercial Layer Chickens. Appl. Environ. Microbiol. 2019, 85, e03137-18. [Google Scholar] [CrossRef]
- Ngunjiri, J.M.; Taylor, K.J.M.; Abundo, M.C.; Jang, H.; Elaish, M.; Kc, M.; Ghorbani, A.; Wijeratne, S.; Weber, B.P.; Johnson, T.J.; et al. Longitudinal Investigation of the Swine Gut Microbiome from Birth to Market Reveals Stage and Growth Performance Associated Bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef]
- Krueger, L.A.; Spangler, D.A.; Sims, M.D. Titration of Supplemental Bacillus subtilis Subsp. subtilis American Type Culture Collection PTA-125135 to Broiler Chickens Fed Diets of 2 Different Metabolizable Energy Concentrations. Poult. Sci. 2020, 99, 3987–3996. [Google Scholar] [CrossRef]
- Arshad, M.A.; Rehman, M.S.; Huws, S.A.; Cheng, Y.; Din, A.U. Gut Microbiome Colonization and Development in Neonatal Ruminants: Strategies, Prospects, and Opportunities. Anim. Nutr. 2021, 7, 883–895. [Google Scholar] [CrossRef]
- Lambo, M.T.; Chang, X.; Liu, D. The Recent Trend in the Use of Multistrain Probiotics in Livestock Production: An Overview. Animals 2021, 11, 2805. [Google Scholar] [CrossRef] [PubMed]
- Kraimi, N.; Dawkins, M.; Gebhardt-Henrich, S.G.; Velge, P.; Rychlik, I.; Volf, J.; Creach, P.; Smith, A.; Colles, F.; Leterrier, C. Influence of the microbiota-gut-brain axis on behavior and welfare in farm animals: A review. Physiol. Behav. 2019, 210, 112658. [Google Scholar] [CrossRef]
- Ran, T.; Gomaa, W.; Shen, Y.; Saleem, A.; Yang, W.; McAllister, T. Use of naturally sourced feed additives (Lactobacillus fermentation products and enzymes) in growing and finishing steers: Effects on performance, carcass characteristics and blood metabolites, Anim. Feed Sci. Technol. 2019, 254, 114190. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Farmer, S.; Cash, H.A.; Keller, D. Probiotic Bacillus coagulans GBI-30, 6086 improves protein absorption and utilization, Probiotics Antimicrob. Proteins 2018, 10, 611–615. [Google Scholar]
- Zhu, C.; Yao, J.; Zhu, M.; Zhu, C.; Yuan, L.; Li, Z.; Cai, D.; Chen, S.; Hu, P.; Liu, H.-Y. A Meta-Analysis of Lactobacillus-Based Probiotics for Growth Performance and Intestinal Morphology in Piglets. Front. Vet. Sci. 2022, 9, 1045965. [Google Scholar] [CrossRef]
- Tsukahara, T.; Inoue, R.; Nakatani, M.; Fukuta, K.; Kishino, E.; Ito, T.; Ushida, K. Influence of Weaning Age on the Villous Height and Disaccharidase Activities in the Porcine Small Intestine. Anim. Sci. J. 2016, 87, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Kuśnirek, M.; Lipiński, K.; Jørgensen, J.N.; Hansen, L.H.B.; Antoszkiewicz, Z.; Zabielski, R.; Konieczka, P. The Effect of a Bacillus-Based Probiotic on Sow and Piglet Performance in Two Production Cycles. Animals 2023, 13, 3163. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.C.; Langa, R.L.S.; Aiyegoro, O.A.; Okoh, A.I. Effects of probiotics on growth performance, blood parameters, and antibody stimulation in piglets. S. Afr. J. Anim. Sci. 2017, 47, 766–775. [Google Scholar] [CrossRef]
- Aalaei, M.; Khatibjoo, A.; Zaghari, M.; Taherpou, K.; Akbari-Gharaei, M.; Soltani, M. Effect of single- and multistrain probiotics on broiler breeder performance, immunity and intestinal toll-like receptors expression. J. Appl. Anim. Res. 2019, 47, 236–242. [Google Scholar] [CrossRef]
- Bohmer, B.M.; Kramer, W.; Roth-Maier, D.A. Dietary probiotic supplementation and resulting effects on performance, health status, and microbial characteristics of primiparous sows. J. Anim. Physiol. Anim. Nutr. 2006, 90, 309–315. [Google Scholar] [CrossRef]
- Arsène, M.M.; Davares, A.K.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319–328. [Google Scholar] [CrossRef]
- Partlow, J.; Blikslager, A.; Matthews, C.; Mac Law, M.; Daniels, J.; Baker, R.; Labens, R. Effect of Topically Applied Saccharomyces boulardii on the Healing of Acute Porcine Wounds: A Preliminary Study. BMC Res. Notes 2016, 9, 210. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium animalis Promotes the Growth of Weaning Piglets by Improving Intestinal Development, Enhancing Antioxidant Capacity, and Modulating Gut Microbiota. Appl. Environ. Microbiol. 2022, 88, e0129622. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Cao, M.; Li, Y.; Zhuo, Y.; Fang, Z.; Che, L.; Xu, S.; Feng, B.; Lin, Y.; et al. Dietary Supplementation of Bacillus subtilis PB6 Improves Sow Reproductive Performance and Reduces Piglet Birth Intervals. Animal Nutr. 2020, 6, 278–287. [Google Scholar] [CrossRef]
- Joysowal, M.; Saikia, B.N.; Dowarah, R.; Tamuly, S.; Kalita, D.; Dev Choudhury, K.B. Effect of Probiotic Pediococcus acidilactici FT28 on Growth Performance, Nutrient Digestibility, Health Status, Meat Quality, and Intestinal Morphology in Growing Pigs. Vet. World 2018, 11, 1669–1675. [Google Scholar] [CrossRef]
- Laskowska, E.; Jarosz, Ł.; Grądzki, Z. Effect of Multi-Microbial Probiotic Formulation Bokashi on Pro- and Anti-Inflammatory Cytokines Profile in the Serum, Colostrum and Milk of Sows, and in a Culture of Polymorphonuclear Cells Isolated from Colostrum. Home Probiotics Antimicrob. Probiotics Antimicrob. Proteins 2019, 11, 220–232. [Google Scholar] [CrossRef]
- Luise, D.; Bosi, P.; Raff, L.; Amatucci, L.; Virdis, S.; Trevisi, P. Bacillus spp. Probiotic Strains as a Potential Tool for Limiting the Use of Antibiotics, and Improving the Growth and Health of Pigs and Chickens. Front. Microbiol. 2022, 13, 801827. [Google Scholar] [CrossRef] [PubMed]
- Monteverde, V.; Congiu, F.; Vazzana, I.; Dara, S.; Di Pietro, S.; Piccione, G. Serum Lipid Profile Modification Related to Polyunsaturated Fatty Acid Supplementation in Thoroughbred Horses. J. Appl. Anim. Res. 2017, 45, 615–618. [Google Scholar] [CrossRef]
- Kembabazi, B.; Ondiek, J.O.; Migwi, P.K. Effect of single or mixed strain probiotics on milk yield of dairy cows. Livest. Res. Rural Dev. 2021, 33. Available online: http://www.lrrd.org/lrrd33/1/brend3307.html (accessed on 18 February 2025).
- Chapman, C.M.C.; Gibson, G.R.; Todd, S.; Rowland, I. Comparative in vitro inhibition of urinary tract pathogens by single- and multistrain probiotics. Eur. J. Nutr. 2013, 52, 1669–1677. [Google Scholar] [CrossRef]
- Reuben, R.C.; Sarkar, S.L.; Ibnat, H.; Setu, M.A.A.; Roy, P.C.; Jahid, I.K. Novel Multi-Strain Probiotics Reduces Pasteurella multocida-Induced Fowl Cholera Mortality in Broilers. Sci. Rep. 2021, 11, 8885. [Google Scholar] [CrossRef] [PubMed]
- Revajová, V.; Benková, T.; Karaffová, V.; Levkut, M.; Selecká, E. Dvorožňáková, E.; Ševčíková, Z.; Herich, R.; Levkut, M. Influence of Immune Parameters after Enterococcus faecium AL41 Administration and Salmonella Infection in Chickens. Life 2022, 12, 201. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Dong, Y.; Lei, J.; Ito, K.; Zhang, B. Enterococcus faecium PNC01 Isolated from the Intestinal Mucosa of Chicken as an Alternative for Antibiotics to Reduce Feed Conversion Rate in Broiler Chickens. Microb. Cell Fact. 2021, 20, 122. [Google Scholar] [CrossRef]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef]
- Alhussien, M.N.; Dang, A.K. Milk somatic cells, factors influencing their release, future prospects, and practical utility in dairy animals: An overview. Vet. World 2018, 11, 562–577. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Tan, Z.L.; Abu Hafsa, S.H.; Adegbeye, M.J.; Greiner, R.; Ugbogu, E.A.; Cedillo, M.N.; Salem, A.Z.M. Saccharomyces cerevisiae as a Probiotic Feed Additive to Non and Pseudo-Ruminant Feeding: A Review. J. Appl. Microbiol. 2020, 128, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawi, S.G.; Dang, D.S.; Yousif, A.Y.; Al-Younis, Z.K.; Najm, T.A.; Matarneh, S.K. The Potential Use of Probiotics to Improve Animal Health, Efficiency, and Meat Quality: A Review. Agriculture 2020, 10, 452. [Google Scholar] [CrossRef]
- Nalla, K.; Manda, N.K.; Dhillon, H.S.; Kanade, S.R.; Rokana, N.; Hess, M.; Puniya, A.K. Impact of Probiotics on Dairy Production Efficiency. Front Microbiol. 2022, 13, 805963. [Google Scholar] [CrossRef]
- Renaud, D.L.; Kelton, D.F.; Weese, J.S.; Noble, C.; Duffield, T.F. Evaluation of a multispecies probiotic as a supportive treatment for diarrhea in dairy calves: A randomized clinical trial. J. Dairy Sci. 2019, 102, 4498–4505. [Google Scholar] [CrossRef]
- Rao, Y.Y.N.K.A.; Kumar, C.V.S.D.S.; Lekha, M.S. Effect of Feeding Multi-Strain Probiotic on Feed Intake and Milk Production Performance in Murrah Buffaloes. Int. J. Curr. Microbiol. Appl. Sci. 2021, 10, 409–417. [Google Scholar]
- Genís, S.; Sánchez-Chardi, A.; Bach, A.; Fàbregas, F.; Arís, A. A combination of lactic acid bacteria regulates Escherichia coli infection and inflammation of the bovine endometrium. J. Dairy Sci. 2017, 100, 479–492. [Google Scholar] [CrossRef]
- Liu, M.; Wu, Q.; Wang, M.; Fu, Y.; Wang, J. Lactobacillus rhamnosus GR-1 Limits Escherichia coli-Induced Inflammatory Responses via Attenuating MyD88-Dependent and MyD88-Independent Pathway Activation in Bovine Endometrial Epithelial Cells. Inflammation 2016, 39, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Gänzle, M.G.; Ametaj, B.N. Intravaginally administered lactic acid bacteria expedited uterine involution and modulated hormonal profiles of transition dairy cows. J. Dairy Sci. 2015, 98, 6018–6028. [Google Scholar] [CrossRef]
- Ametaj, B.N.; Iqbal, S.; Selami, F.; Odhiambo, J.F.; Wang, Y.; Gänzle, M.G.; Dunn, S.M.; Zebeli, Q. Intravaginal administration of lactic acid bacteria modulated the incidence of purulent vaginal discharges, plasma haptoglobin concentrations, and milk production in dairy cows. Res. Vet. Sci. 2014, 96, 365–370. [Google Scholar] [CrossRef]
- El-Garhi, M.S.; El-Bordeny, N.E. Impact of intravaginal probiotics inoculation on reproductive performance of Holstein dairy cattle during transition period. Assiut Vet. Med. J. 2019, 65, 63–70. [Google Scholar]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.Y.; Sun, Z.; Ma, H.; Zhao, F.; Lee, Y.K.; Zhang, H. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Jatkauskas, J.; Vrotniakiene, V. Effects of Probiotic Dietary Supplementation on Diarrhoea Patterns, Faecal Microbiota and Performance of Early Weaned Calves. Vet. Med. 2010, 55, 494–503. [Google Scholar] [CrossRef]
- Azzaz, H.H.; Kholif, A.E.; Murad, H.A.; Vargas-Bello-Pérez, E. A Newly Developed Strain of Enterococcus faecium Isolated from Fresh Dairy Products to Be Used as a Probiotic in Lactating Holstein Cows. Front. Vet. Sci. 2022, 9, 989606. [Google Scholar] [CrossRef]
- Yasmin, F.; Alam, M.J.; Kabir, M.E.; Maruf, A.A.; Islam, M.A.; Hossain, M.M. Influence of Probiotics Supplementation on Growth and Haemato-Biochemical Parameters in Growing Cattle. Int. J. Livest. Res. 2021, 11, 36–42. [Google Scholar] [CrossRef]
- Luan, S.; Duersteler, M.; Galbraith, E.A.; Cardoso, F.C. Effects of Direct-Fed Bacillus pumilus 8G-134 on Feed Intake, Milk Yield, Milk Composition, Feed Conversion, and Health Condition of Pre- and Postpartum Holstein Cows. J. Dairy Sci. 2015, 98, 6423–6432. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, F.; Liu, Y.; Ma, T.; Jin, H.; Quan, K.; Leng, B.; Zhao, J.; Yuan, X.; Li, Z.; et al. Probiotics synergized with conventional regimen in managing Parkinson’s disease. NPJ Park. Dis. 2022, 8, 62. [Google Scholar] [CrossRef]
- Goldin, B.R.; Gorbach, S.L. The Effect of Milk and Lactobacillus Feeding on Human Intestinal Bacterial Enzyme Activity. Am. J. Clin. Nutr. 1984, 39, 756–761. [Google Scholar] [CrossRef]
- Al-Salami, H.; Butt, G.; Fawcett, J.P.; Tucker, I.G.; Golocorbin-Kon, S.; Mikov, M. Probiotic Treatment Reduces Blood Glucose Levels and Increases Systemic Absorption of Gliclazide in Diabetic Rats. Eur. J. Drug Metab. Pharm. 2008, 33, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Matuskova, Z.; Anzenbacher, P.; Vecera, R.; Siller, M.; Tlaskalova-Hogenova, H.; Strojil, J.; Anzenbacherova, E. Effect of Lactobacillus casei on the Pharmacokinetics of Amiodarone in Male Wistar Rats. Eur. J. Drug Metab. Pharm. 2017, 42, 29–36. [Google Scholar] [CrossRef]
- Saputri, F.A.; Kang, D.; Kusuma, A.S.W.; Rusdiana, T.; Hasanah, A.N.; Mutakin; Surono, I.S.; Koyama, H.; Abdulah, R. Lactobacillus plantarum IS-10506 Probiotic Administration Increases Amlodipine Absorption in a Rabbit Model. J. Int. Med. Res. 2018, 46, 5004–5010. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, J.; Ji, R.; Zhou, Y.; Shao, L.; Chen, D.; Tan, J. Preventative Effects of Selenium-Enriched. Benef. Microbes 2019, 10, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, X.; Jiang, Z.; Wang, T.; Wu, Q.; Wang, J. LA14 Alleviates Liver Injury. mSystems 2021, 6, e0038421. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, H.; Zhang, A.; Chen, Z.; Chang, W.; Liu, G.; Deng, X.; Bryden, W.L.; Zheng, A. Enterococcus faecium Modulates the Gut Microbiota of Broilers and Enhances Phosphorus Absorption and Utilization. Animals 2020, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota medicine: Towards clinical revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Katoh, T.; Ojima, M.N.; Sakanaka, M.; Ashida, H.; Gotoh, A.; Katayama, T. Enzymatic Adaptation of Bifidobacterium bifidum to Host Glycans, Viewed from Glycoside Hydrolyases and Carbohydrate-Binding Modules. Microorganisms 2020, 8, 481. [Google Scholar] [CrossRef]
- Barkhidarian, B.; Roldos, L.; Iskandar, M.M.; Saedisomeolia, A.; Kubow, S. Probiotic Supplementation and Micronutrient Status in Healthy Subjects: A Systematic Review of Clinical Trials. Nutrients 2021, 13, 3001. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Zhang, R.; Jia, H.; Liu, X.; Zhu, Z. Effects of three probiotics and their interactions on the growth performance of and nutrient absorption in broilers. PeerJ 2022, 10, e13308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut Microbiome–Micronutrient Interaction: The Key to Controlling the Bioavailability of Minerals and Vitamins? BioFactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Enhancing Micronutrients Bioavailability through Fermentation of Plant-Based Foods: A Concise Review. Fermentation 2021, 7, 63. [Google Scholar] [CrossRef]
- Gong, P.; Tang, X. The impact of probiotic supplementation on gastric motility and nutrient absorption in elderly patients with Gastrointestinal disorders. BMC Gastroenterol. 2025, 25, 192. [Google Scholar] [CrossRef]
- Harutyunyan, N.; Kushugulova, A.; Hovhannisyan, N.; Pepoyan, A. One Health Probiotics as Biocontrol Agents: One Health Tomato Probiotics. Plants 2022, 11, 1334. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, A.C.; Forssten, S.; Hibberd, A.A.; Lyra, A.; Stahl, B. Probiotic approach to prevent antibiotic resistance. Ann Med. 2016, 48, 246–255. [Google Scholar] [CrossRef]
- Raman, J.; Kim, J.-S.; Choi, K.R.; Eun, H.; Yang, D.; Ko, Y.-J.; Kim, S.-J. Application of Lactic Acid Bacteria (LAB) in Sustainable Agriculture: Advantages and Limitations. Int. J. Mol. Sci. 2022, 23, 7784. [Google Scholar] [CrossRef]
- Ma, L.-C.; Zhao, H.-Q.; Wu, L.B.; Cheng, Z.-L.; Liu, C. Impact of the Microbiome on Human, Animal, and Environmental Health from a One Health Perspective. Sci. One Health 2023, 2, 100037. [Google Scholar] [CrossRef] [PubMed]
- Melara, E.G.; Avellaneda, M.C.; Valdivié, M.; García-Hernández, Y.; Aroche, R.; Martínez, Y. Probiotics: Symbiotic Relationship with the Animal Host. Animals 2022, 12, 719. [Google Scholar] [CrossRef]
- Wendel, U. Assessing Viability and Stress Tolerance of Probiotics—A Review. Front Microbiol. 2022, 12, 818468. [Google Scholar] [CrossRef] [PubMed]
- Kolaček, S.; Hojsak, I.; Berni Canani, R.; Guarino, A.; Indrio, F.; Orel, R.; Pot, B.; Shamir, R.; Szajewska, H.; Vandenplas, Y.; et al. Commercial probiotic products: A call for improved quality control. A position paper by the ESPGHAN working group for probiotics and prebiotics. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 117–124. [Google Scholar] [CrossRef]
- Fenster, K.; Freeburg, B.; Hollard, C.; Wong, C.; Laursen, R.R. Ouwehand AC. The production and delivery of probiotics: A review of a practical approach. Microorganisms 2019, 7, 83. [Google Scholar] [CrossRef]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef]
- Pérez, H.A.; Bustos, A.Y.; Taranto, M.P.; Frías, M.D.L.A.; Ledesma, A.E. Effects of lysozyme on the activity of ionic fluoroquinolone species. Molecules 2018, 23, 741. [Google Scholar] [CrossRef] [PubMed]
- Castro-López, C.; Romero-Luna, H.E.; García, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Key Stress Response Mechanisms of Probiotics During Their Journey Through the Digestive System: A Review. Probiotics Antimicrob. Proteins 2023, 15, 1250–1270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y. F1F0-ATPase functions under markedly acidic conditions in bacteria. In Regulation of Ca2+-ATPases, V-ATPases and F-ATPases; Chakraborti, S., Dhalla, N.S., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 459–468. [Google Scholar]
- Kadja, L.; Dib, A.L.; Lakhdara, N.; Bouaziz, A.; Espigares, E.; Gagaoua, M. Influence of Three Probiotics Strains, Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. Lactis BB-12 and Saccharomyces boulardii CNCM I-745 on the Biochemical and Haematological Profiles and Body Weight of Healthy Rabbits. Biology 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, Y.; Rodríguez, Z.; Brandão, L.R.; Rosa, C.A.; Nicoli, J.R.; Elías Iglesias, A.; Peréz-Sanchez, T.; Salabarría, R.B.; Halaihel, N. Identification and in vitro screening of avian yeasts for use as probiotic. Res. Vet. Sci. 2012, 93, 798–802. [Google Scholar] [CrossRef]
- Fernandes, T.; Carvalho, B.F.; Mantovani, H.C.; Schwan, R.F.; Ávila, C.L.S. Identification and characterization of yeasts from bovine rumen for potential use as probiotics. J. Appl. Microbiol. 2019, 127, 845–855. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Medina-Córdova, N.; Tovar-Ramírez, D.; Angulo, C. Probiotic and Nutritional Effects of Debaryomyces hansenii on Animals. Appl. Microbiol. Biotechnol. 2020, 104, 7689–7699. [Google Scholar] [CrossRef]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, A.K.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Yang, F.; Hou, C.; Zeng, X.; Qiao, S. The Use of Lactic Acid Bacteria as a Probiotic in Swine Diets. Pathogens 2015, 4, 34–45. [Google Scholar] [CrossRef]
- Kelly, S.M.; Lanigan, N.; O’Neill, I.J.; Bottacini, F.; Lugli, G.A.; Viappiani, A.; Turroni, F.; Ventura, M.; van Sinderen, D. Bifidobacterial Biofilm Formation Is a Multifactorial Adaptive Phenomenon in Response to Bile Exposure. Sci. Rep. 2020, 10, 11598. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Wang, M.; Du, G.; Chen, J. Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J. Ind. Microbiol. Biotechnol. 2012, 39, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Anjana, T.; Tiwari, S.K. Bacteriocin-Producing Probiotic Lactic Acid Bacteria in Controlling Dysbiosis of the Gut Microbiota. Front. Cell Infect. Microbiol. 2022, 12, 851140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Li, N.; Xie, J.; Wang, L.; Xu, Y.; Zhang, M.; Yang, X.; Zheng, Y. Antimicrobial Activity against Shigella sonnei and Probiotic Properties of Wild Lactobacilli from Fermented Food. Microbiol. Res. 2011, 167, 27–31. [Google Scholar] [CrossRef]
- Stern, N.; Eruslanov, B.; Pokhilenko, V.; Kovalev, Y.; Volodina, L.; Vladimir, P.; Mitsevich, E.; Mitsevich, I.; Borzenkov, V.; Levchuk, V.; et al. Bacteriocins reduce Campylobacter jejuni colonization while bacteria producing bacteriocins are ineffective. Microb. Ecol. Health. Dis. 2008, 20, 74–79. [Google Scholar]
- Rainard, P.; Foucras, G. A critical appraisal of probiotics for mastitis control. Front. Vet. Sci. 2018, 5, 251. [Google Scholar] [CrossRef]
- Sevillano, E.; Lafuente, I.; Peña, N.; Cintas, L.M.; Muñoz-Atienza, E.; Hernández, P.E.; Borrero, J. Isolation, Genomics-Based and Biochemical Characterization of Bacteriocinogenic Bacteria and Their Bacteriocins, Sourced from the Gastrointestinal Tract of Meat-Producing Pigs. Int. J. Mol. Sci. 2024, 25, 12210. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Han, S.K.; Ji, A.R.; Kim, K.S.; Lee, W.K. Isolation and characterization of bacteriocin-producing bacteria from the gastrointestinal tract of broiler chickens for probiotic use. J. Appl. Microbiol. 2008, 105, 2203–2212. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Mihailovskaya, V.S.; Remezovskaya, N.B.; Starčič Erjavec, M. Bacteriocin-Producing Escherichia coli Isolated from the Gastrointestinal Tract of Farm Animals: Prevalence, Molecular Characterization and Potential for Application. Microorganisms 2022, 10, 1558. [Google Scholar] [CrossRef]
- Öztürk, H.; Geniş, B.; Özden Tuncer, B.; Tuncer, Y. Bacteriocin Production and Technological Properties of Enterococcus mundtii and Enterococcus faecium Strains Isolated from Sheep and Goat Colostrum. Vet. Res. Commun. 2023, 47, 1321–1345. [Google Scholar] [CrossRef]
- Feng, W.; Ao, H.; Peng, C. Gut Microbiota, Short-Chain Fatty Acids, and Herbal Medicines. Front. Pharmacol. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Front. Nutr. 2017, 57, 1354. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, S.C.J.; Verhoeven, T.L.A.; Desair, J.; Marchal, K.; Vanderleyden, J.; Nagy, I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006, 259, 89–96. [Google Scholar] [CrossRef]
- Masuda, M.; Ide, M.; Utsumi, H.; Niiro, T.; Shimamura, Y.; Murata, M. Production potency of folate, vitamin B12, and thiamine by lactic acid bacteria isolated from Japanese pickles. Biosci. Biotechnol. Biochem. 2012, 76, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Dalto, D.; Matte, J.J. Pyridoxine (Vitamin B6) and the glutathione peroxidase system: A link between one-carbon metabolism and anti-oxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef]
- Patel, A.; Shah, N.; Prajapati, J.B. Biosynthesis of vitamins and enzymes in fermented foods by lactic acid bacteria and related genera: A promising approach. Croat. J. Food Sci. Technol. 2013, 5, 85–91. [Google Scholar]
- Mohammed, Y.; Lee, B.; Kang, Z.; Du, G. Capability of Lactobacillus reuteri to produce an active form of vitamin B12 under optimized fermentation conditions. J. Acad. Ind. Res. 2014, 2, 617–621. [Google Scholar]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef]
- Indira, M.; Venkateswarulu, T.C.; Peele, K.A.; Bobby, M.N.; Krupanidhi, S. Bioactive molecules of probiotic bacteria and their mechanism of action: A review. 3 Biotech 2019, 9, 306. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mitra Mazumder, P.; Roy Sarkar, S.; Saha, R.; Chatterjee, A.; Sarkar, B.; Banerjee, S. Neuroprotective effect of Vitamin K2 against gut dysbiosis associated cognitive decline. Physiol. Behav. 2023, 269, 114252. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Miller, J.K.; Harrison, M.T.; D’Andrea, A.; Endsley, A.N.; Yin, F.; Kodukula, K.; Watson, D.S. β-Carotene biosynthesis in probiotic bacteria. Probiotics Antimicrob. Proteins 2013, 5, 69–80. [Google Scholar] [CrossRef]
- Ates, O. Systems biology of microbial exopolysaccharides production. Front. Bioeng. Biotechnol. 2015, 3, 200. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, Q.; Dong, M. Structural characterization and anticancer activity of cell-bound exopolysaccharide from Lactobacillus helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef] [PubMed]
- Minoda Sada, R.; Matsuo, H.; Motooka, D.; Kutsuna, S.; Hamaguchi, S.; Yamamoto, G.; Ueda, A. Clostridium butyricum Bacteremia Associated with Probiotic Use, Japan. Emerg. Infect. Dis. 2024, 30, 655. [Google Scholar] [CrossRef]
- Pararajasingam, A.; Uwagwu, J. Lactobacillus: The Not So Friendly Bacteria. Case Rep. 2017, 2017, bcr-2016-218423. [Google Scholar] [CrossRef] [PubMed]
- Madella, A.M.; Van Bergenhenegouwen, J.; Garssen, J.; Masereeuw, R.; Overbeek, S.A. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins 2022, 14, 645. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Rudiansyah, M.; Jasim, S.A.; Renuka, J.S.; Kumar, A.; Yumashev, A.; Mohammed, J.S.; Sinha, A.; Alizadeh, F.; Tesfaye, A. Clinical Efficacy of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins and Renal Function in Patients with Kidney Disease: An Umbrella Meta-Analysis. Front. Med. 2024, 11, 1475145. [Google Scholar]
- Ribeiro, F.P.B.; Freire, M.O.L.; Coutinho, D.O.; Cirilo, M.A.S.; Alves, J.L.B. Gut Dysbiosis and Probiotic Therapy in Chronic Kidney Disease: A Comprehensive Review. Probiotics Antimicrob. Proteins 2024, in press. [Google Scholar] [CrossRef]
- D’Agostin, M.; Squillaci, D.; Lazzerini, M.; Barbi, E.; Wijers, L.; Da Lozzo, P. Invasive Infections Associated with the Use of Probiotics in Children: A Systematic Review. Children 2021, 8, 924. [Google Scholar] [CrossRef]
- Huys, G.; Vancanneyt, M.; D’Haene, K.; Vankerckhoven, V.; Goossens, H.; Swings, J. Accuracy of Species Identity of Commercial Bacterial Cultures Intended for Probiotic or Nutritional Use. Res. Microbiol. 2006, 157, 803–810. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Boyle, R.J.; Margolles, A.; Frias, R.; Gueimonde, M. Safety Assessment of Probiotics. In Prebiotics and Probiotics Science and Technology; Charalampopoulos, D., Rastall, R.A., Eds.; Springer: New York, NY, USA, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Thumu, S.C.R.; Halami, P.M. In Vivo Safety Assessment of Lactobacillus fermentum Strains, Evaluation of Their Cholesterol-Lowering Ability and Intestinal Microbial Modulation. J. Sci. Food Agric. 2020, 100, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Safety of Probiotics: Translocation and Infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Kailasapathy, K.; Chin, J. Survival and Therapeutic Potential of Probiotic Organisms with Reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Al-Madhagi, H.; Alramo, A. Clinical Trials of Probiotics: Current Outlook. Food Sci. Nutr. 2023, 12, 2192–2194. [Google Scholar] [CrossRef]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; EHPM: 2022; International Probiotics Association: Dollard-des-Ormeaux, QC, Canada, 2021. [Google Scholar]
- Al-Fakhrany, O.; Elekhnawy, E. Next-Generation Probiotics: The Upcoming Biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Ertem, H.; Çakmakçı, S. Shelf Life and Quality of Probiotic Yogurt Produced with Lactobacillus acidophilus and Gobdin. Int. J. Food Sci. Technol. 2018, 53, 776–783. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Dharmasiddhi, I.P.W.; Chen, S.; Liu, Y.; Liu, H. Review of the Potential of Probiotics in Disease Treatment: Mechanisms, Engineering, and Applications. Processes 2024, 12, 316. [Google Scholar] [CrossRef]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Pramanik, S.; Venkatraman, S.; Karthik, P.; Vaidyanathan, V.K. A Systematic Review on Selection, Characterization, and Implementation of Probiotics in Human Health. Food Sci. Biotechnol. 2023, 32, 423–440. [Google Scholar] [CrossRef]
- Evrard, B.; Coudeyras, S.; Dosgilbert, A.; Charbonnel, N.; Alamé, J.; Tridon, A.; Forestier, C. Dose-Dependent Immunomodulation of Human Dendritic Cells by the Probiotic Lactobacillus rhamnosus Lcr35. PLoS ONE 2011, 6, e18735. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).