Clinical Significance of NUDT1 (MTH1) Across Cancer Types
Abstract
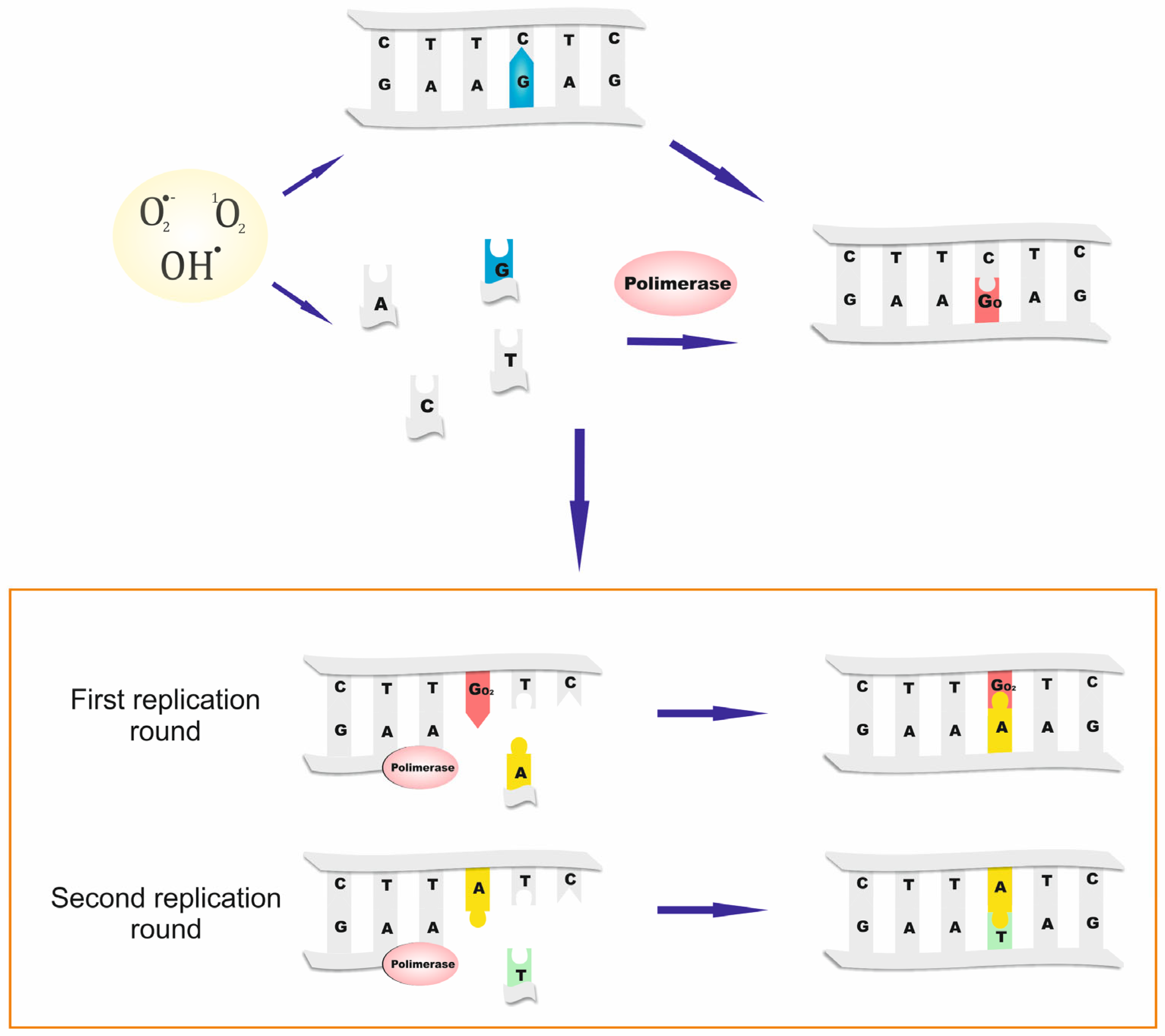
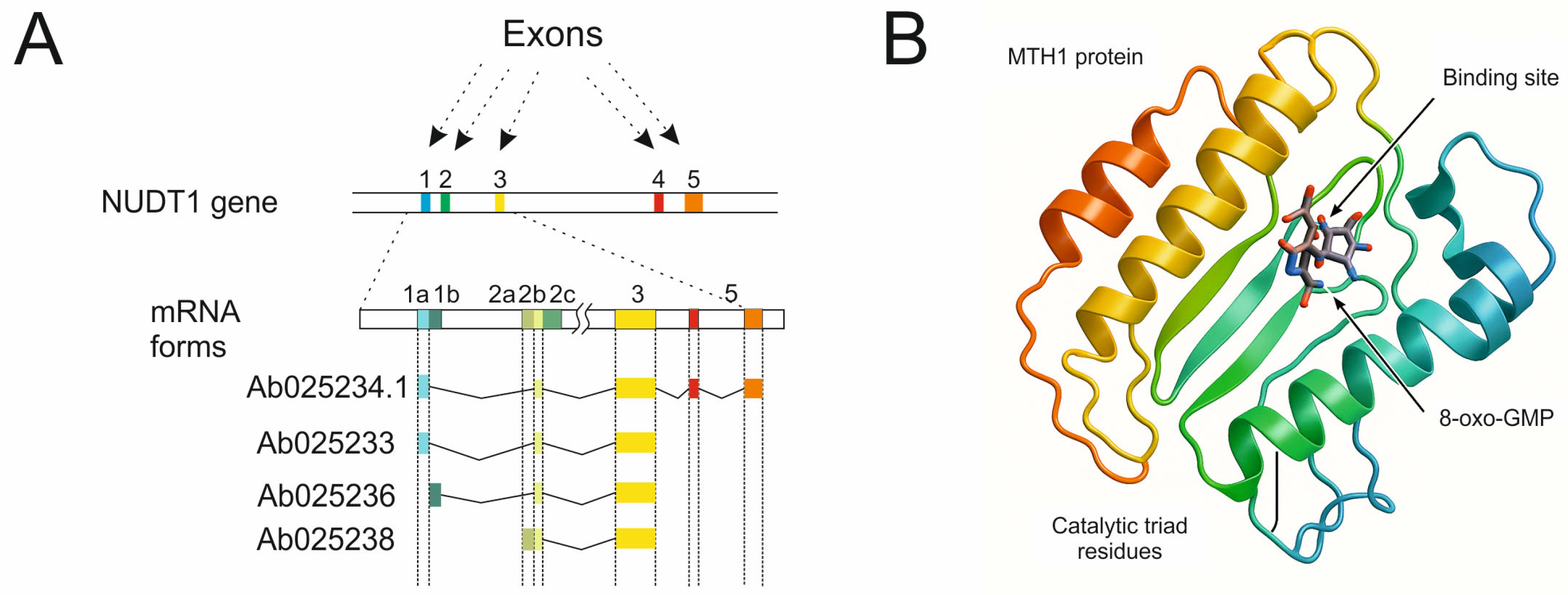
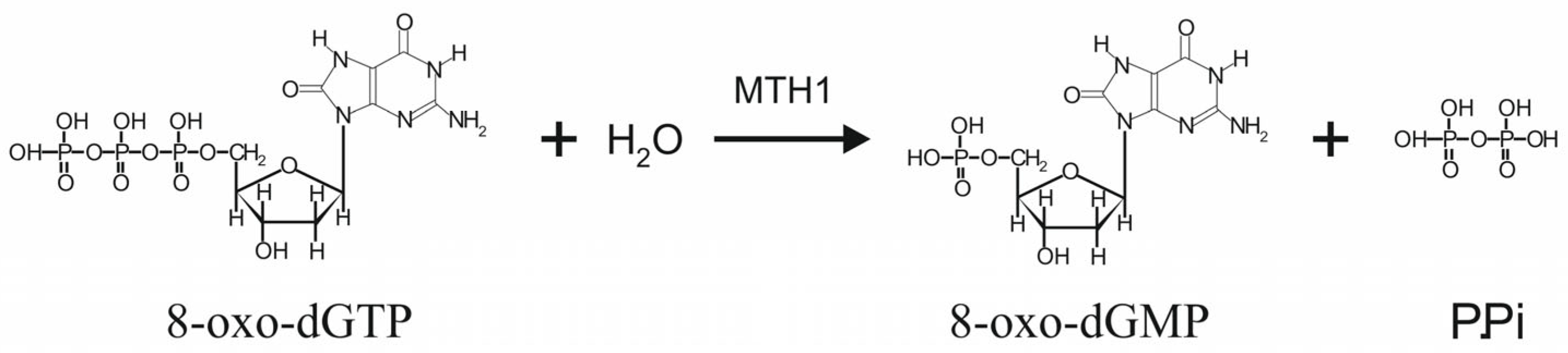
1. Introduction
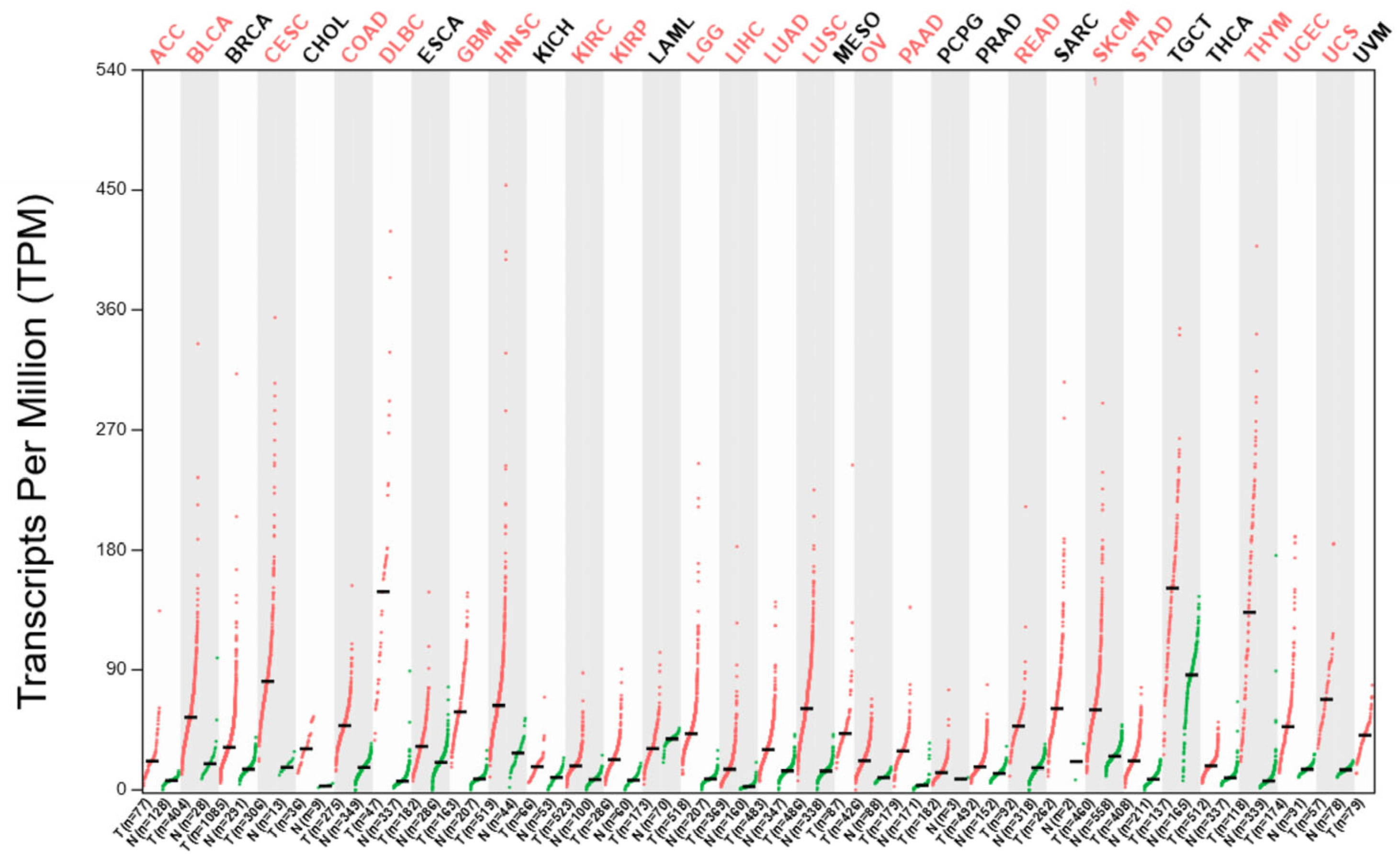
2. Overexpression of the NUDT1 Gene as a Quantitative Biomarker and a Prognostic Factor in Cancer
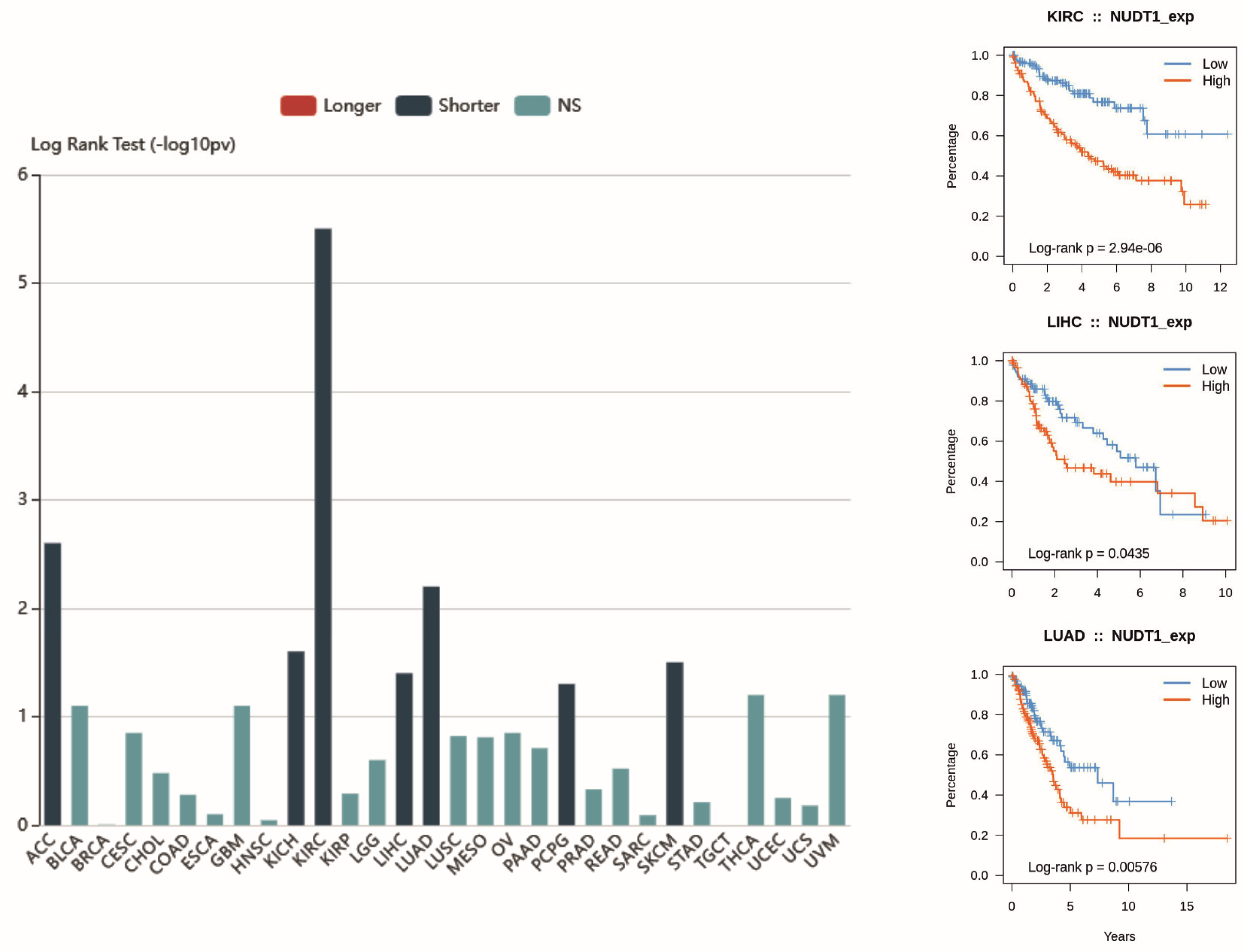
2.1. Renal-Cell Carcinoma
2.2. Hepatocellular Carcinoma
2.3. Colorectal Cancer
2.4. Non-Small-Cell Lung Cancer
2.5. Breast Cancer
2.6. Thyroid Cancer
2.7. Pancreatic Cancer
2.8. Esophageal Squamous Cell Carcinoma
2.9. B-Cell Lymphoma
2.10. Gastric Cancer
2.11. Multiple Myeloma
2.12. Osteosarcoma
2.13. Oral Squamous Cell Carcinoma
2.14. Brain Tumors
2.15. Malignant Melanoma
2.16. Mesothelioma
3. Small Molecular Inhibitors of MTH1 as a Therapeutic Approach
| Inhibitors | Authors | Cancers | IC50 |
|---|---|---|---|
| TH287 | Abbas et al. [59] | NSCLC | N |
| Gad et al. [56] | Colorectal cancer | 0.8 nmol/L | |
| Gad et al. [56] | Breast cancer | ||
| Gad et al. [56] | Melanoma | ||
| Huang et al. [57] | Osteosarcoma | 0.9 nmol/L (with DOX) | |
| TH588 | Zhou W et al. [21] | Gastric cancer | 10.23 nM |
| Moukengue et al. [46] | Osteosarcoma | 4.48–17.37 μmol/L | |
| Gad et al. [56] | Colorectal cancer | 5.0 nmol/L | |
| Pompsch et al. [65] | 5 mM | ||
| Van der Waals et al. [66] | N | ||
| Gad et al. [56] | Breast cancer | 5.0 nmol/L | |
| Zhang et al. [37] | 9.78, 6.96 and 8.97 μM | ||
| Gad et al. [56] | Melanoma | 5.0 nmol/L | |
| Berglund et al. [61] | N | ||
| Pudelko et al. [62] | Glioma | 12.1 µM | |
| Ikerjiri et al. [58] | Lymphoma | 5 nM | |
| Ikerjiri et al. [58] | Leukemia | 5 nM | |
| TH1579 | Hua et al. [22] | Hepatocellular carcinoma | N |
| Oksvold et al. [43] | Lymphomas (DLBCL, Burkitt’s) | 0.1–0.3 μM | |
| Moukengue et al. [46] | Osteosarcoma | 0.31–16.26 μmol/L | |
| Pudelko et al. [62] | Glioma | 1.1 μM | |
| Berglund et al. [61] | Melanoma | N | |
| Berglund et al. [61] | Colorectal cancer | N | |
| Magkouta et al. [55] | Mesothelioma | N | |
| Sanjiv et al. [63] | Leukemia | 417.4 nmol/L | |
| (S)-Crizotinib | Ji et al. [67] | Gastric cancer | 21.33 and 24.81 μM |
| Echinacoside | Zou et al. [68] | Hepatocarcinoma | 7.01 μmol/L |
| Osteosarcoma | |||
| Breast cancer | |||
| Colorectal cancer | |||
| Mi-743 | Zhou et al. [21] | Gastric cancer | 91.44 ± 1.45 nM |
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 8-oxo-Gua | 8-oxo-7,8-dihydroguanine |
| 8-oxo-dGTP | 8-oxo-7,8-dihydro-2′-deoxyguanosine 5′-triphosphate |
| ACC | Adenoid Cystic Carcinoma |
| BLCA | Bladder Urothelial Carcinoma |
| BRCA | Breast invasive carcinoma |
| CESC | Cervical squamous cell carcinoma and endocervical adenocarcinoma |
| CHOL | Cholangiocarcinoma |
| CMM | Cutaneous Malignant Melanoma |
| COAD | Colon adenocarcinoma |
| CRC | Colorectal cancer |
| DEGs | Differentially expressed genes |
| dGTP | 2′-deoxyguanosine 5′-triphosphate |
| DLBC | Lymphoid Neoplasm Diffuse Large B-cell Lymphoma |
| dNTPs, NTPs | 2′-deoxyribonucleoside 5′-triphosphates, ribonucleoside 5′-triphosphates |
| ESCA | Esophageal carcinoma |
| ESCC | Esophageal squamous cell carcinoma |
| GBM | Glioblastoma multiforme |
| HCC | Hepatocellular carcinoma |
| HGG | High grade gliomas |
| HIF1a | Hypoxia-inducible factor 1-alpha |
| HNSC | Head and Neck squamous cell carcinoma |
| KICH | Kidney Chromophobe |
| KIRC | Kidney renal clear cell carcinoma |
| KIRP | Kidney renal papillary cell carcinoma |
| LAML | Acute Myeloid Leukemia |
| LGG | Low grade gliomas |
| LIHC | Liver Hepatocellular Carcinoma |
| LSCs | Leukemic Stem Cells |
| LUAD | Lung Adenocarcinoma |
| LUSC | Lung Squamous Cell Carcinoma |
| MESO | Mesothelioma |
| MTH1 | MutT Homolog 1 |
| NUDT1 | Nudix Hydrolase 1 |
| OS | Overall survival |
| OSCC | Oral squamous cell carcinoma |
| OV | Ovarian serous cystadenocarcinoma |
| PAAD | Pancreatic Adenocarcinoma |
| PCPG | Pheochromocytoma and Paraganglioma |
| PDAC | Pancreatic ductal adenocarcinoma |
| PMNCs | Peripheral mononuclear cells |
| PRAD | Prostate Adenocarcinoma |
| RCC | Renal-cell carcinoma |
| READ | Rectum Adenocarcinoma |
| ROS | Reactive oxygen species |
| SARC | Sarcoma |
| SKCM | Skin Cutaneous Melanoma |
| STAD | Stomach Adenocarcinoma |
| TGCT | Testicular Germ Cell Tumors |
| THCA | Thyroid Carcinoma |
| THYM | Thymoma |
| UCEC | Uterine Corpus Endometrial Carcinoma |
| UCS | Uterine Carcinosarcoma |
| UVM | Uveal Melanoma |
| VEGF | Factors responsible for angiogenesis |
References
- Cheng, K.C.; Cahill, D.S.; Kasai, H.; Nishimura, S.; Loeb, L.A. 8-Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and A→C substitutions. J. Biol. Chem. 1992, 267, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Treffers, H.P.; Spinelli, V.; Belser, N.O. A factor (or mutator gene) influencing mutation rates in Escherichia coli. Proc. Natl. Acad. Sci. USA 1954, 40, 1064–1071. [Google Scholar] [CrossRef]
- Cox, E.C.; Yanofsky, C. Mutator gene studies in Escherichia coli. J. Bacteriol. 1969, 100, 390–397. [Google Scholar] [CrossRef]
- Bhatnagar, S.K.; Bessman, M.J. Studies on the mutator gene, mutT of Escherichia coli. Molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J. Biol. Chem. 1988, 263, 8953–8957. [Google Scholar] [CrossRef] [PubMed]
- Maki, H.; Sekiguchi, M. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 1992, 355, 273–275. [Google Scholar] [CrossRef]
- Sakumi, K.; Furuichi, M.; Tsuzuki, T.; Kakuma, T.; Kawabata, S.; Maki, H.; Sekiguchi, M. Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem. 1993, 268, 23524–23530. [Google Scholar] [CrossRef]
- Bessman, M.J.; Frick, D.N.; O’Handley, S.F. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 1996, 271, 25059–25062. [Google Scholar] [CrossRef] [PubMed]
- McLennan, A.G. The Nudix hydrolase superfamily. Cell. Mol. Life Sci. 2006, 63, 123–143. [Google Scholar] [CrossRef]
- Oda, H.; Nakabeppu, Y.; Furuichi, M.; Sekiguchi, M. Regulation of expression of the human MTH1 gene encoding 8-oxo-dGTPase. Alternative splicing of transcription products. J. Biol. Chem. 1997, 272, 17843–17850. [Google Scholar] [CrossRef]
- Oda, H.; Taketomi, A.; Maruyama, R.; Itoh, R.; Nishioka, K.; Yakushiji, H.; Suzuki, T.; Sekiguchi, M.; Nakabeppu, Y. Multi-forms of human MTH1 polypeptides produced by alternative translation initiation and single nucleotide polymorphism. Nucleic Acids Res. 1999, 27, 4335–4343. [Google Scholar] [CrossRef]
- Samaranayake, G.J.; Huynh, M.; Rai, P. MTH1 as a Chemotherapeutic Target: The Elephant in the Room. Cancers 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, K.; Oliński, R. Metabolism of phosphorylated derivatives of 8-oxo-2′-deoxyguanosine. Postepy Biochem. 1997, 43, 199–208. [Google Scholar] [PubMed]
- Qiu, Y.; Zheng, H.; Sun, L.-H.; Peng, K.; Xiao, W.-D.; Yang, H. Hypoxia-inducible factor-1 modulates upregulation of mutT homolog-1 in colorectal cancer. World J. Gastroenterol. 2015, 21, 13447–13456. [Google Scholar] [CrossRef]
- Okamoto, K.; Toyokuni, S.; Kim, W.-J.; Ogawa, O.; Kakehi, Y.; Arao, S.; Hiai, H.; Yoshida, O. Overexpression of humanmutT homologue gene messenger RNA in renal-cell carcinoma: Evidence of persistent oxidative stress in cancer. Int. J. Cancer 1996, 65, 437–441. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, F.; Chang, K.; Lu, X.; Dai, B.; Ye, D. NUDT expression is predictive of prognosis in patients with clear cell renal cell carcinoma. Oncol. Lett. 2017, 14, 6121–6128. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, F.; Jin, Y.; Zhong, Q.; Tan, W.; Liu, J.; Wu, Z. NUDT1 Could Be a Prognostic Biomarker and Correlated with Immune Infiltration in Clear Cell Renal Cell Carcinoma. Appl. Bionics Biomech. 2022, 2022, e3669296. [Google Scholar] [CrossRef]
- Xie, J.; Cui, L.; Pan, S.; Liu, D.; Liu, F.; Liu, Z. Metabolic Understanding of the Genetic Dysregulation in the Tumor Microenvironment of Kidney Renal Clear Cell Carcinoma. Dis. Markers 2022, 2022, 6085072. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, Z.; Wang, K.; Yuan, C.; Huang, Y.; Xiao, W.; Meng, X.; Chen, Z.; Lv, Q.; Miao, D.; et al. HIF2α promotes tumour growth in clear cell renal cell carcinoma by increasing the expression of NUDT1 to reduce oxidative stress. Clin. Transl. Med. 2021, 11, e592. [Google Scholar] [CrossRef]
- Jüngst, C.; Cheng, B.; Gehrke, R.; Schmitz, V.; Nischalke, H.D.; Ramakers, J.; Schramel, P.; Schirmacher, P.; Sauerbruch, T.; Caselmann, W.H. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology 2004, 39, 1663–1672. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, B.; Lin, J. Expression of DNA repair enzyme hMTH1 mRNA and protein in hepatocellular carcinoma. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2005, 25, 389–392. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, L.; Yang, J.; Qiao, H.; Li, L.; Guo, Q.; Ma, J.; Zhao, L.; Wang, J.; Jiang, G.; et al. Potent and specific MTH1 inhibitors targeting gastric cancer. Cell Death Dis. 2019, 10, 434. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Sanjiv, K.; Gad, H.; Pham, T.; Gokturk, C.; Rasti, A.; Zhao, Z.; He, K.; Feng, M.; Zang, Y.; et al. Karonudib is a promising anticancer therapy in hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2019, 11, 1758835919866960. [Google Scholar] [CrossRef]
- Ou, Q.; Ma, N.; Yu, Z.; Wang, R.; Hou, Y.; Wang, Z.; Chen, F.; Li, W.; Bi, J.; Ma, J.; et al. Nudix hydrolase 1 is a prognostic biomarker in hepatocellular carcinoma. Aging 2020, 12, 7363–7379. [Google Scholar] [CrossRef]
- Notterman, D.A.; Alon, U.; Sierk, A.J.; Levine, A.J. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001, 61, 3124–3130. [Google Scholar]
- Kumagae, Y.; Hirahashi, M.; Takizawa, K.; Yamamoto, H.; Gushima, M.; Esaki, M.; Matsumoto, T.; Nakamura, M.; Kitazono, T.; Oda, Y. Overexpression of MTH1 and OGG1 proteins in ulcerative colitis-associated carcinogenesis. Oncol. Lett. 2018, 16, 1765–1776. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.-C.; Tian, X.-Y.; Li, Y.-X.; Cui, J.; Chen, Z.; Deng, Z.-L.; Chen, F.-J.; Hayakawa, H.; Sekiguchi, M.; et al. MutT-related proteins are novel progression and prognostic markers for colorectal cancer. Oncotarget 2017, 8, 105714–105726. [Google Scholar] [CrossRef]
- Ji, D.; Beharry, A.A.; Ford, J.M.; Kool, E.T. A Chimeric ATP-Linked Nucleotide Enables Luminescence Signaling of Damage Surveillance by MTH1, a Cancer Target. J. Am. Chem. Soc. 2016, 138, 9005–9008. [Google Scholar] [CrossRef] [PubMed]
- McPherson, L.A.; Troccoli, C.I.; Ji, D.; Bowles, A.E.; Gardiner, M.L.; Mohsen, M.G.; Nagathihalli, N.S.; Nguyen, D.M.; Robbins, D.J.; Merchant, N.B.; et al. Increased MTH1-specific 8-oxodGTPase activity is a hallmark of cancer in colon, lung and pancreatic tissue. DNA Repair 2019, 83, 102644. [Google Scholar] [CrossRef] [PubMed]
- Bialkowski, K.; Szpila, A. Specific 8-oxo-dGTPase activity of MTH1 (NUDT1) protein as a quantitative marker and prognostic factor in human colorectal cancer. Free. Radic. Biol. Med. 2021, 176, 257–264. [Google Scholar] [CrossRef]
- Chong, I.-W.; Chang, M.-Y.; Chang, H.-C.; Yu, Y.-P.; Sheu, C.-C.; Tsai, J.-R.; Hung, J.-Y.; Chou, S.-H.; Tsai, M.-S.; Hwang, J.-J.; et al. Great potential of a panel of multiple hMTH1, SPD, ITGA11 and COL11A1 markers for diagnosis of patients with non-small cell lung cancer. Oncol. Rep. 2006, 16, 981–988. [Google Scholar] [CrossRef]
- Fujishita, T.; Okamoto, T.; Akamine, T.; Takamori, S.; Takada, K.; Katsura, M.; Toyokawa, G.; Shoji, F.; Shimokawa, M.; Oda, Y.; et al. Association of MTH1 expression with the tumor malignant potential and poor prognosis in patients with resected lung cancer. Lung Cancer 2017, 109, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-N.; Yang, C.-C.; Li, J.; Yang, Q.-G.O.; Zeng, L.-T.; Fan, G.-Q.; Liu, T.-H.; Tian, X.-Y.; Wang, J.-J.; Zhang, H.; et al. The high expression of MTH1 and NUDT5 promotes tumor metastasis and indicates a poor prognosis in patients with non-small-cell lung cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118895. [Google Scholar] [CrossRef] [PubMed]
- Speina, E.; Arczewska, K.D.; Gackowski, D.; Zielińska, M.; Siomek, A.; Kowalewski, J.; Oliński, R.; Tudek, B.; Kuśmierek, J.T. Contribution of hMTH1 to the maintenance of 8-oxoguanine levels in lung DNA of non-small-cell lung cancer patients. J. Natl. Cancer Inst. 2005, 97, 384–395. [Google Scholar] [CrossRef]
- Patel, A.; Burton, D.G.A.; Halvorsen, K.; Balkan, W.; Reiner, T.; Perez-Stable, C.; Cohen, A.; Munoz, A.; Giribaldi, M.G.; Singh, S.; et al. MutT Homolog 1 (MTH1) maintains multiple KRAS-driven pro-malignant pathways. Oncogene 2014, 34, 2586–2596. [Google Scholar] [CrossRef]
- Kennedy, C.H.; Pass, H.I.; Mitchell, J.B. Expression of human MutT homologue (hMTH1) protein in primary non-small-cell lung carcinomas and histologically normal surrounding tissue. Free Radic. Biol. Med. 2003, 34, 1447–1457. [Google Scholar] [CrossRef]
- Giribaldi, M.G.; Munoz, A.; Halvorsen, K.; Patel, A.; Rai, P. MTH1 expression is required for effective transformation by oncogenic HRAS. Oncotarget 2015, 6, 11519–11529. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.; Zhou, Y.; Mao, F.; Lin, Y.; Guan, J.; Sun, Q. Expression and function of MutT homolog 1 in distinct subtypes of breast cancer. Oncol. Lett. 2017, 13, 2161–2168. [Google Scholar] [CrossRef]
- Wani, G.; Milo, G.E.; D’Ambrosio, S.M. Enhanced expression of the 8-oxo-7,8-dihydrodeoxyguanosine triphosphatase gene in human breast tumor cells. Cancer Lett. 1998, 125, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.H.G.; Beato, M. Role of the NUDT Enzymes in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 2267. [Google Scholar] [CrossRef]
- Arczewska, K.D.; Krasuska, W.; Stachurska, A.; Karpińska, K.; Sikorska, J.; Kiedrowski, M.; Lange, D.; Stępień, T.; Czarnocka, B. hMTH1 and GPX1 expression in human thyroid tissue is interrelated to prevent oxidative DNA damage. DNA Repair 2020, 95, 102954. [Google Scholar] [CrossRef]
- Akiyama, S.; Saeki, H.; Nakashima, Y.; Iimori, M.; Kitao, H.; Oki, E.; Oda, Y.; Nakabeppu, Y.; Kakeji, Y.; Maehara, Y. Prognostic impact of MutT homolog-1 expression on esophageal squamous cell carcinoma. Cancer Med. 2017, 6, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Liu, T.-H.; Li, J.; Li, D.-N.; Tian, X.-Y.; Ouyang, Q.-G.; Cai, J.-P. The high expression of MTH1 and NUDT5 predict a poor survival and are associated with malignancy of esophageal squamous cell carcinoma. PeerJ 2020, 8, e9195. [Google Scholar] [CrossRef] [PubMed]
- Oksvold, M.P.; Berglund, U.W.; Gad, H.; Bai, B.; Stokke, T.; Rein, I.D.; Pham, T.; Sanjiv, K.; Øy, G.F.; Norum, J.H.; et al. Karonudib has potent anti-tumor effects in preclinical models of B-cell lymphoma. Sci. Rep. 2021, 11, 6317. [Google Scholar] [CrossRef]
- Borrego, S.; Vazquez, A.; Dasí, F.; Cerdá, C.; Iradi, A.; Tormos, C.; Sánchez, J.M.; Bagán, L.; Boix, J.; Zaragoza, C.; et al. Oxidative Stress and DNA Damage in Human Gastric Carcinoma: 8-Oxo-7’8-dihydro-2’-deoxyguanosine (8-oxo-dG) as a Possible Tumor Marker. Int. J. Mol. Sci. 2013, 14, 3467–3486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jian, Y.; Leng, Y.; Liu, N.; Tian, Y.; Wang, G.; Gao, W.; Yang, G.; Chen, W. Human MutT homologue 1 mRNA overexpression correlates to poor response of multiple myeloma. Int. J. Hematol. 2017, 105, 318–325. [Google Scholar] [CrossRef]
- Moukengue, B.; Brown, H.K.; Charrier, C.; Battaglia, S.; Baud’Huin, M.; Quillard, T.; Pham, T.M.; Pateras, I.S.; Gorgoulis, V.G.; Helleday, T.; et al. TH1579, MTH1 inhibitor, delays tumour growth and inhibits metastases development in osteosarcoma model. EBioMedicine 2020, 53, 102704. [Google Scholar] [CrossRef]
- Qing, X.; Shao, Z.; Lv, X.; Pu, F.; Gao, F.; Liu, L.; Shi, D. Anticancer effect of (S)-crizotinib on osteosarcoma cells by targeting MTH1 and activating reactive oxygen species. Anti-Cancer Drugs 2018, 29, 341–352. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, L.; Piao, S.; Li, L.; Li, J.; Xia, Y.; Li, J.; Saiyin, W. NUDT1: A potential independent predictor for the prognosis of patients with oral squamous cell carcinoma. J. Oral Pathol. Med. 2020, 49, 210–218. [Google Scholar] [CrossRef]
- Iida, T.; Furuta, A.; Kawashima, M.; Nishida, J.-I.; Nakabeppu, Y.; Iwaki, T. Accumulation of 8-oxo-2’-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro-oncology 2001, 3, 73–81. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, Z.; Wang, X.; Yang, H.; Zhang, P.; Johnson, M.; Liu, N.; Liu, H.; Jin, W.; Zhang, Y.; et al. Birth of MTH1 as a therapeutic target for glioblastoma: MTH1 is indispensable for gliomatumorigenesis. Am. J. Transl. Res. 2016, 8, 2803–2811. [Google Scholar]
- Bhavya, B.; Easwer, H.; Vilanilam, G.; Anand, C.; Sreelakshmi, K.; Urulangodi, M.; Rajalakshmi, P.; Neena, I.; Padmakrishnan, C.; Menon, G.R.; et al. MutT Homolog1 has multifaceted role in glioma and is under the apparent orchestration by Hypoxia Inducible factor1 alpha. Life Sci. 2021, 264, 118673. [Google Scholar] [CrossRef]
- Das, I.; Gad, H.; Bräutigam, L.; Pudelko, L.; Tuominen, R.; Höiom, V.; Almlöf, I.; Rajagopal, V.; Hansson, J.; Helleday, T.; et al. AXL and CAV-1 play a role for MTH1 inhibitor TH1579 sensitivity in cutaneous malignant melanoma. Cell Death Differ. 2020, 27, 2081–2098. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Tuominen, R.; Helleday, T.; Hansson, J.; Berglund, U.W.; Brage, S.E. Coexpression of MTH1 and PMS2 is associated with advanced disease and disease progression after therapy in melanoma. J. Investig. Dermatol. 2022, 142, 736–740.e6. [Google Scholar] [CrossRef]
- Magkouta, S.F.; Pappas, A.G.; Vaitsi, P.C.; Agioutantis, P.C.; Pateras, I.S.; Moschos, C.A.; Iliopoulou, M.P.; Kosti, C.N.; Loutrari, H.V.; Gorgoulis, V.G.; et al. MTH1 favors mesothelioma progression and mediates paracrine rescue of bystander endothelium from oxidative damage. J. Clin. Investig. 2020, 5, e134885. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.F.; Vaitsi, P.C.; Iliopoulou, M.P.; Pappas, A.G.; Kosti, C.N.; Psarra, K.; Kalomenidis, I.T. MTH1 Inhibition Alleviates Immune Suppression and Enhances the Efficacy of Anti-PD-L1 Immunotherapy in Experimental Mesothelioma. Cancers 2023, 15, 4962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gad, H.; Koolmeister, T.; Jemth, A.-S.; Eshtad, S.; Jacques, S.A. MTH1 inhibition eradicates cancer by preventing sanitation of the dNTP pool. Nature 2014, 508, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, J.; Wu, W.; Yang, W.; Zhong, B.; Qing, X.; Shao, Z. Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma. Acta Biomater. 2020, 109, 229–243. [Google Scholar] [CrossRef]
- Ikejiri, F.; Honma, Y.; Kasukabe, T.; Urano, T.; Suzumiya, J. TH588, an MTH1 inhibitor, enhances phenethylisothiocyanate-induced growth inhibition in pancreatic cancer cells. Oncol. Lett. 2018, 15, 3240–3244. [Google Scholar] [CrossRef]
- Abbas, H.H.K.; Alhamoudi, K.M.H.; Evans, M.D.; Jones, G.D.D.; Foster, S.S. MTH1 deficiency selectively increases non-cytotoxic oxidative DNA damage in lung cancer cells: More bad news than good? BMC Cancer 2018, 18, 423. [Google Scholar] [CrossRef]
- Gul, N.; Karlsson, J.; Tängemo, C.; Linsefors, S.; Tuyizere, S.; Perkins, R.; Ala, C.; Zou, Z.; Larsson, E.; Bergö, M.O.; et al. The MTH1 inhibitor TH588 is a microtubule-modulating agent that eliminates cancer cells by activating the mitotic surveillance pathway. Sci. Rep. 2019, 9, 14667. [Google Scholar] [CrossRef]
- Berglund, U.W.; Sanjiv, K.; Gad, H.; Kalderén, C.; Koolmeister, T.; Pham, T.; Gokturk, C.; Jafari, R.; Maddalo, G.; Seashore-Ludlow, B.; et al. Validation and development of MTH1 inhibitors for treatment of cancer. Ann. Oncol. 2016, 27, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Pudelko, L.; Rouhi, P.; Sanjiv, K.; Gad, H.; Kalderén, C.; Höglund, A.; Squatrito, M.; Schuhmacher, A.J.; Edwards, S.; Hägerstrand, D.; et al. Glioblastoma and glioblastoma stem cells are dependent on functional MTH1. Oncotarget 2017, 8, 84671–84684. [Google Scholar] [CrossRef] [PubMed]
- Sanjiv, K.; Calderón-Montaño, J.M.; Pham, T.M.; Erkers, T.; Tsuber, V.; Almlöf, I.; Höglund, A.; Heshmati, Y.; Seashore-Ludlow, B.; Danda, A.N.; et al. MTH1 Inhibitor TH1579 Induces Oxidative DNA Damage and Mitotic Arrest in Acute Myeloid Leukemia. Cancer Res. 2021, 81, 5733–5744. [Google Scholar] [CrossRef] [PubMed]
- Deneberg, S.; Uggla, B.; Smith, A.; Sandvall, T.; Klockare, M.; Breuer, O.; Berglund, U.W.; Helleday, T. A phase I/II study investigating Karonudib in patients with refractory haematologigal malignancies. Blood 2024, 144 (Suppl. 1), 6010. [Google Scholar] [CrossRef]
- Pompsch, M.; Vogel, J.; Classen, F.; Kranz, P.; Iliakis, G.; Riffkin, H.; Brockmeier, U.; Metzen, E. The presumed MTH1-inhibitor TH588 sensitizes colorectal carcinoma cells to ionizing radiation in hypoxia. BMC Cancer 2018, 18, 1190. [Google Scholar] [CrossRef]
- van der Waals, L.M.; Laoukili, J.; Jongen, J.M.J.; Raats, D.A.; Rinkes, I.H.M.B.; Kranenburg, O. Differential anti-tumour effects of MTH1 inhibitors in patient-derived 3D colorectal cancer cultures. Sci. Rep. 2019, 9, 819. [Google Scholar] [CrossRef]
- Ji, J.; Chen, W.; Lian, W.; Chen, R.; Yang, J.; Zhang, Q.; Weng, Q.; Khan, Z.; Hu, J.; Chen, X.; et al. (S)-crizotinib reduces gastric cancer growth through oxidative DNA damage and triggers pro-survival akt signal. Cell Death Dis. 2018, 9, 660. [Google Scholar] [CrossRef]
- Zou, Z.; Dong, L.; Wang, H.; Niu, J.; Zou, M.; Wu, N.; Yu, D.; Wang, Y. Echinacoside induces apoptotic cancer cell death by inhibiting the nucleotide pool sanitizing enzyme MTH1. OncoTargets Ther. 2015, 8, 3649–3664. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misiak, R.; Białkowski, K.; Dondajewska, E. Clinical Significance of NUDT1 (MTH1) Across Cancer Types. Int. J. Mol. Sci. 2025, 26, 5137. https://doi.org/10.3390/ijms26115137
Misiak R, Białkowski K, Dondajewska E. Clinical Significance of NUDT1 (MTH1) Across Cancer Types. International Journal of Molecular Sciences. 2025; 26(11):5137. https://doi.org/10.3390/ijms26115137
Chicago/Turabian StyleMisiak, Radosław, Karol Białkowski, and Ewelina Dondajewska. 2025. "Clinical Significance of NUDT1 (MTH1) Across Cancer Types" International Journal of Molecular Sciences 26, no. 11: 5137. https://doi.org/10.3390/ijms26115137
APA StyleMisiak, R., Białkowski, K., & Dondajewska, E. (2025). Clinical Significance of NUDT1 (MTH1) Across Cancer Types. International Journal of Molecular Sciences, 26(11), 5137. https://doi.org/10.3390/ijms26115137






