Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases
Abstract
1. Introduction
2. Mechanisms Underlying Far-Infrared Light Therapy
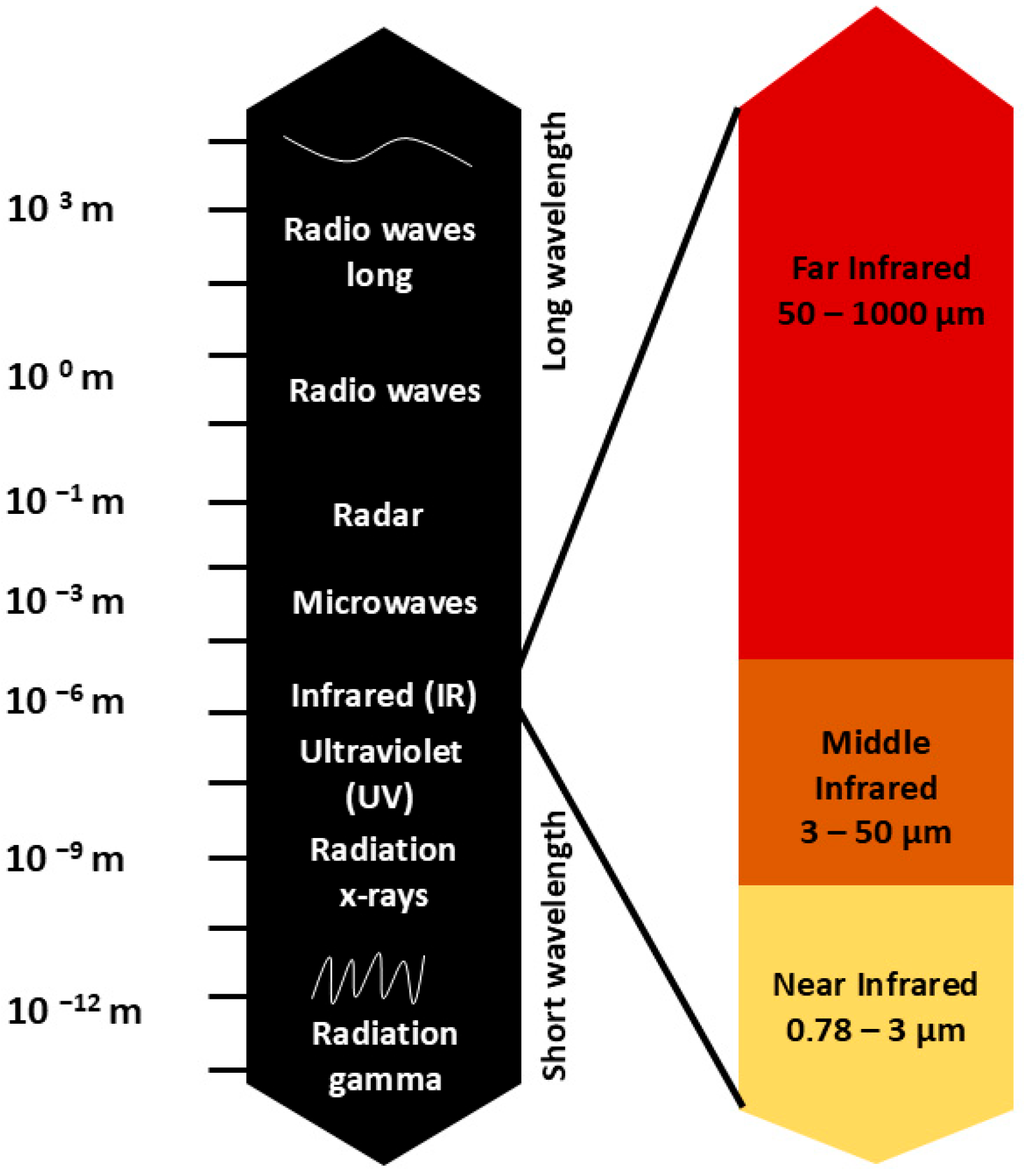
2.1. Spectral Characteristics of Infrared Radiation
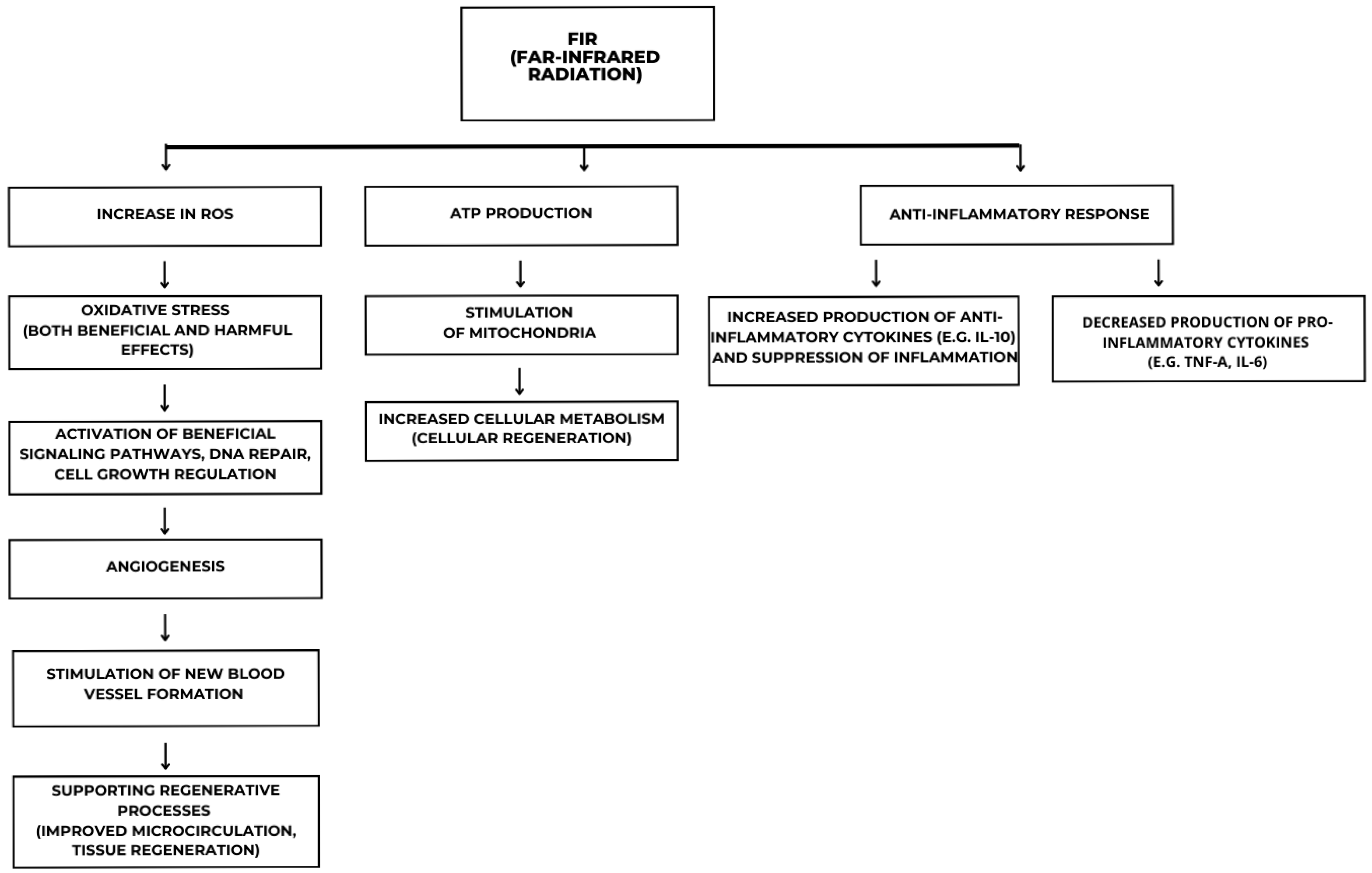
2.2. Biological Effects and Mechanisms of Action
2.3. Anti-Inflammatory and Regenerative Potential
3. The Effect of Far-Infrared Light Therapy on Insulin Resistance and Type 2 Diabetes
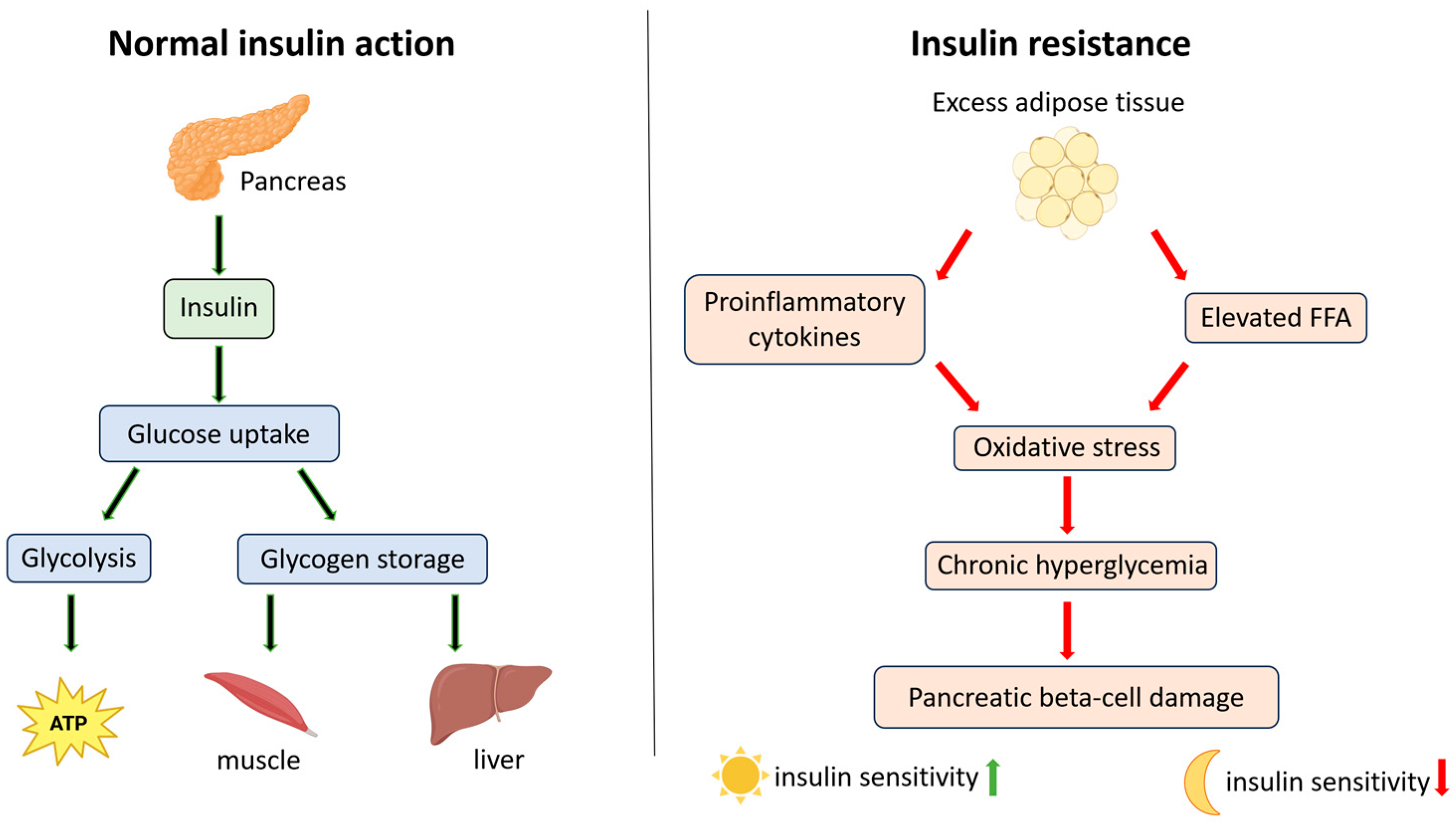
3.1. Mechanisms Underlying Insulin Resistance in Type 2 Diabetes
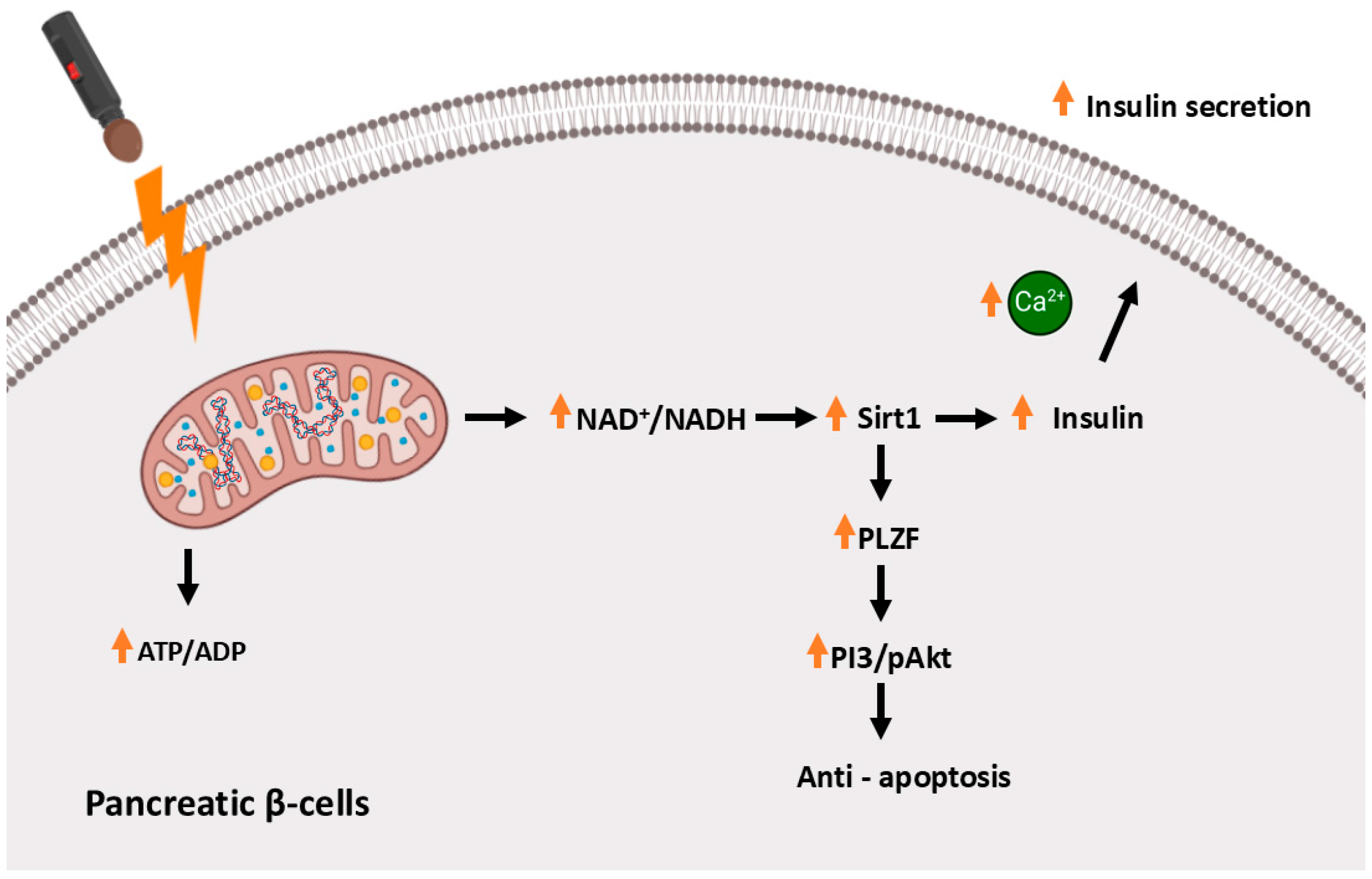
3.2. Far-Infrared Light Therapy in Animal Models of Type 2 Diabetes
3.3. Protective Effect of FIR Therapy on Endothelial Cells and AGE Accumulation
3.4. The Role of Red Light in Glucose Metabolism in Humans
4. The Effect of Far-Infrared Light Therapy on Dyslipidemia and Non-Alcoholic Fatty Liver Disease
4.1. Role of the Gut–Liver Axis and MicroRNA Regulation in Dyslipidemia and Metabolic Dysfunction
4.2. Molecular Mechanisms Linking Insulin Resistance and Dyslipidemia
4.3. Pathogenesis of Non-Alcoholic Fatty Liver Disease
4.4. Far-Infrared Light Therapy in Animal Models of Dyslipidemia
4.5. Clinical Evidence: FIR Therapy in Individuals with Dyslipidemia
5. Far-Infrared Light Therapy and Cardiovascular Diseases
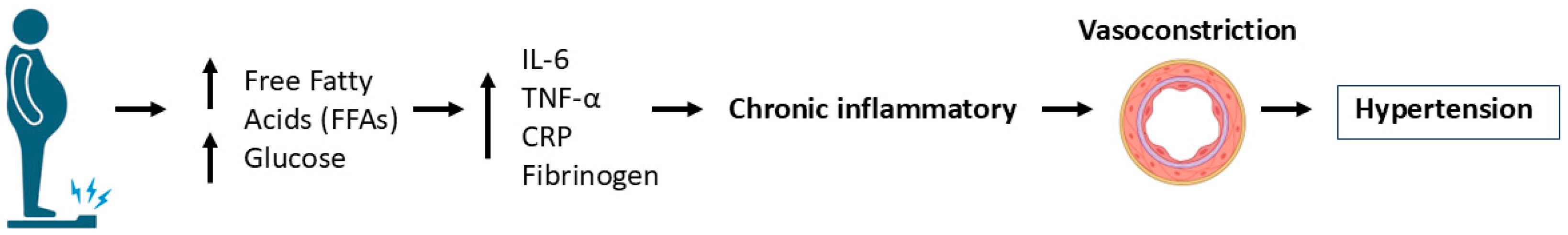
5.1. Metabolic Dysregulation as a Risk Factor for Cardiovascular Diseases
5.2. Effect of FIR Light Therapy on Blood Flow and VEGF Levels
5.3. Clinical Evidence: FIR Therapy in Patients with Cardiovascular Diseases
6. Potential Risks and Precautions with Far-Infrared Light Therapy
6.1. Cutaneous Effects and Photocarcinogenic Risk
6.2. Ocular Safety Concerns
6.3. Paradoxical Pain Sensitization
6.4. Effects on Implanted Medical Devices
6.5. Cardiovascular Considerations
6.6. Systemic Thermal Stress
6.7. Recommendations for Clinical Practice
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and Type 2 Diabetes Mellitus: Connections in Epidemiology, Pathogenesis, and Treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.X.; Chin, Y.H.; Foo, R.; Chan, M.; Muthiah, M.D. Global Burden of Metabolic Diseases: Data from Global Burden of Disease 2000–2019. A Cosortium of Metabolic Disease. Eur. Heart J. 2023, 44, ehac779.131. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; MacCallum, P.R. The Obesity, Metabolic Syndrome, and Type 2 Diabetes Mellitus Pandemic: Part I. Increased Cardiovascular Disease Risk and the Importance of Atherogenic Dyslipidemia in Persons with the Metabolic Syndrome and Type 2 Diabetes Mellitus. J. Cardiometabolic Syndr. 2009, 4, 113–119. [Google Scholar] [CrossRef]
- Miller, S.J.; Brown, S. Overweight and Obesity. APhA OTC Nutr. Nutr. Suppl. 2024. [Google Scholar] [CrossRef]
- Zang, B.; He, L.; Xue, L. Intermittent Fasting: Potential Bridge of Obesity and Diabetes to Health? Nutrients 2022, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Carracher, A.M.; Marathe, P.H.; Close, K.L. American Association of Diabetes Educators 2017. J. Diabetes 2017, 9, 1054–1057. [Google Scholar] [CrossRef]
- Seuring, T.; Archangelidi, O.; Suhrcke, M.; Suhrcke, M. The Economic Costs of Type 2 Diabetes: A Global Systematic Review. Pharm. Econ. 2015, 33, 811–831. [Google Scholar] [CrossRef]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. Exp. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Brown, J.B.; Vistisen, D.; Sicree, R.; Shaw, J.E.; Nichols, G.A. Global Healthcare Expenditure on Diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 293–301. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Bärnighausen, T.; Davies, J.; Davies, J.; et al. Global Economic Burden of Diabetes in Adults: Projections from 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Gropper, S.S. The Role of Nutrition in Chronic Disease. Nutrients 2023, 15, 664. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. Interactions between Nitric Oxide, Oxygen, Reactive Oxygen Species and Reactive Nitrogen Species. Biochem. Soc. Trans. 2006, 34, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Kim, B. Bioactive Compounds as Inhibitors of Inflammation, Oxidative Stress and Metabolic Dysfunctions via Regulation of Cellular Redox Balance and Histone Acetylation State. Foods 2023, 12, 925. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A.; Suomalainen, A. Mitochondria: In Sickness and in Health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.; de Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef]
- Starkov, A.A. The Role of Mitochondria in Reactive Oxygen Species Metabolism and Signaling. Ann. N. Y. Acad. Sci. 2008, 1147, 37–52. [Google Scholar] [CrossRef]
- Malott, K.F.; Luderer, U. Toxicant Effects on Mammalian Oocyte Mitochondria. Biol. Reprod. 2021, 104, 784–793. [Google Scholar] [CrossRef]
- Pope, N.J.; Denton, M.H. Differential Effects of 808-Nm Light on Electron Transport Chain Enzymes in Isolated Mitochondria: Implications for Photobiomodulation Initiation. Mitochondrion 2022, 68, 15–24. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Tsai, S.-R.; Hamblin, M.R.; Hamblin, M.R. Biological Effects and Medical Applications of Infrared Radiation. J. Photochem. Photobiol. B-Biol. 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L. Use of Infrared as a Complementary Treatment Approach in Medicine and Aesthetic Medicine. Asp. Biomed. Clin. Case Rep. 2019, 2019, 77–81. [Google Scholar] [CrossRef]
- Liao, W.-T.; Hung, C.-H.; Liang, S.-S.; Yu, S.; Yu, S.; Lu, J.-H.; Lee, C.-H.; Chai, C.-Y.; Yu, H.-S.; Yu, H.-S.; et al. Anti-Inflammatory Effects Induced by Near-Infrared Light Irradiation through M2 Macrophage Polarization. J. Investig. Dermatol. 2021, 141, 2056–2066.e10. [Google Scholar] [CrossRef]
- Vatansever, F.; Hamblin, M.R. Far Infrared Radiation (FIR): Its Biological Effects and Medical Applications. Photonics Lasers Med. 2012, 4, 255–266. [Google Scholar] [CrossRef]
- Tasumi, M. Experimental Infrared Spectroscopy Fundamentals and Practical Methods: 1. Introduction to Infrared Spectroscopy. J. Spectrosc. Res. Jpn. 2010, 59, 27–31. [Google Scholar]
- Ou, S.M.; Hu, F.H.; Yang, W.C.; Lin, C.C. Far-Infrared Therapy as a Novel Treatment for Encapsulating Peritoneal Sclerosis. Am. J. Gastroenterol. 2014, 109, 1957–1959. [Google Scholar] [CrossRef]
- Chang, Y. The Effect of Far Infrared Radiation Therapy on Inflammation Regulation in Lipopolysaccharide-Induced Peritonitis in Mice. Sage Open Med. 2018, 6, 2050312118798941. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Applications of the Anti-Inflammatory Effects of Photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.H.; Zhuo, J.; McClellan, J.X.; Getsy, P.M.; Ryan, R.M.; Jenkins, M.J.; Lewis, S.J. Infrared Light Elicits Endothelium-Dependent Vasodilation in Isolated Occipital Arteries of the Rat via Soluble Guanylyl Cyclase-Dependent Mechanisms. Front. Physiol. 2023, 14, 1219998. [Google Scholar] [CrossRef]
- Frank, S.; Oliver, L.; Lebreton-De Coster, C.; Moreau, C.; LeCabellec, M.-T.; Michel, L.; Vallette, F.M.; Dubertret, L.; Coulomb, B. Infrared Radiation Affects the Mitochondrial Pathway of Apoptosis in Human Fibroblasts. J. Investig. Dermatol. 2004, 123, 823–831. [Google Scholar] [CrossRef]
- Quirk, B.J.; Sannagowdara, K.; Buchmann, E.; Jensen, E.S.; Gregg, D.C.; Whelan, H.T. Effect of Near-Infrared Light on in Vitro Cellular ATP Production of Osteoblasts and Fibroblasts and on Fracture Healing with Intramedullary Fixation. J. Clin. Orthop. Trauma 2016, 7, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Karu, T.I. Absorption of Monochromatic and Narrow Band Radiation in the Visible and near IR by Both Mitochondrial and Non-Mitochondrial Photoacceptors Results in Photobiomodulation. J. Photochem. Photobiol. B-Biol. 2014, 140, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Homma, K.; Villarreal, S.; Richter, C.P.; Bezanilla, F. Infrared Light Excites Cells by Changing Their Electrical Capacitance. Nat. Commun. 2012, 3, 736. [Google Scholar] [CrossRef]
- Schroeder, P.; Pohl, C.; Calles, C.; Marks, C.; Wild, S.; Krutmann, J. Cellular Response to Infrared Radiation Involves Retrograde Mitochondrial Signaling. Free. Radic. Biol. Med. 2007, 43, 128–135. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Gale, G.D.; Rothbart, P.; Li, Y. Infrared Therapy for Chronic Low Back Pain: A Randomized, Controlled Trial. Pain Res. Manag. 2006, 11, 193–196. [Google Scholar] [CrossRef]
- Barolet, A.C.; Villarreal, A.M.; Jfri, A.; Litvinov, I.V.; Barolet, D. Low-Intensity Visible and Near-Infrared Light-Induced Cell Signaling Pathways in the Skin: A Comprehensive Review. Photobiomodulation Photomed. Laser Surg. 2023, 41, 147–166. [Google Scholar] [CrossRef]
- Johnstone, D.M.; Moro, C.; Stone, J.; Benabid, A.-L.; Mitrofanis, J. Turning On Lights to Stop Neurodegeneration: The Potential of Near Infrared Light Therapy in Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2016, 9, 500. [Google Scholar] [CrossRef]
- Berman, M.H.; Halper, J.P.; Nichols, T.W.; Jarrett, H.; Lundy, A.; Huang, J.H. Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition. J. Neurol. Neurosci. 2017, 8, 176. [Google Scholar] [CrossRef]
- Purushothuman, S.; Johnstone, D.M.; Nandasena, C.; Mitrofanis, J.; Stone, J. Photobiomodulation with near Infrared Light Mitigates Alzheimer’s Disease-Related Pathology in Cerebral Cortex—Evidence from Two Transgenic Mouse Models. Alzheimer’s Res. Ther. 2014, 6, 2. [Google Scholar] [CrossRef]
- Kubincová, A.; Takáč, P.; Kendrová, L.; Joppa, P.; Mikuľáková, W. The Effect of Pulmonary Rehabilitation in Mountain Environment on Exercise Capacity and Quality of Life in Patients with Chronic Obstructive Pulmonary Disease (COPD) and Chronic Bronchitis. Med. Sci. Monit. 2018, 24, 6375–6386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, Y.-Y.; Wang, Y.; Lyu, P.; Hamblin, M.R.; Hamblin, M.R. Photobiomodulation of Human Adipose-Derived Stem Cells Using 810nm and 980nm Lasers Operates via Different Mechanisms of Action. Biochim. Biophys. Acta 2017, 1861, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dai, T.; Hamblin, M.R.; Hamblin, M.R. Effect of Red and Near-Infrared Wavelengths on Low-Level Laser (Light) Therapy-Induced Healing of Partial-Thickness Dermal Abrasion in Mice. Lasers Med. Sci. 2014, 29, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; De Taboada, L. Transcranial near Infrared Laser Treatment (NILT) Increases Cortical Adenosine-5′-Triphosphate (ATP) Content Following Embolic Strokes in Rabbits. Brain Res. 2010, 1306, 100–105. [Google Scholar] [CrossRef]
- Duke, A.R.; Peterson, E.; Mackanos, M.A.; Atkinson, J.B.; Tyler, D.J.; Tyler, D.J.; Jansen, E.D. Hybrid Electro-Optical Stimulation of the Rat Sciatic Nerve Induces Force Generation in the Plantarflexor Muscles. J. Neural Eng. 2012, 9, 066006. [Google Scholar] [CrossRef]
- Jenkins, M.W.; Wang, Y.T.; Doughman, Y.Q.; Watanabe, M.; Cheng, Y.; Rollins, A.M. Optical Pacing of the Adult Rabbit Heart. Biomed. Opt. Express 2013, 4, 1626–1635. [Google Scholar] [CrossRef]
- Rodríguez-Santana, E.; Santana-Blank, L.; Reyes, H.; Santana-Rodríguez, K.E.; Hunger, M.; Orellana, R.; Ortega, D. H-NMR Spin-Lattice and Correlation Times of Burned Soft-Tissue after Treatment with an Infrared Pulsed Laser Device. Lasers Surg. Med. 2003, 33, 190–198. [Google Scholar] [CrossRef]
- Naeser, M.A.; Zafonte, R.; Krengel, M.; Martin, P.I.; Frazier, J.; Hamblin, M.R.; Knight, J.; Meehan, W.P.; Baker, E. Significant Improvements in Cognitive Performance Post-Transcranial, Red/near-Infrared Light-Emitting Diode Treatments in Chronic, Mild Traumatic Brain Injury: Open-Protocol Study. J. Neurotrauma 2014, 31, 1008–1017. [Google Scholar] [CrossRef]
- Okonkwo, U.; DiPietro, L. Diabetes and Wound Angiogenesis. Int. J. Mol. Sci. 2017, 18, 1419. [Google Scholar] [CrossRef]
- Abel, E.D.; Giffin, J.; Ingelfinger, J.R.; Peek, M.; Reusch, J.E.B.; Rosen, C.J.; Sagendorf, A.; Thomas, E. Type 2 Diabetes—Controlling the Epidemic, Episode 1: Understanding and Preventing Type 2 Diabetes. N. Engl. J. Med. 2023, 389, e18. [Google Scholar] [CrossRef]
- Scherbaum, W.A. Diabetes Update 2021. Diabetologe 2021, 17, 482–493. [Google Scholar] [CrossRef]
- Roglic, G. WHO Global Report on Diabetes: A Summary. Int. J. Med. Sci. Public Health 2016, 1, 3–8. [Google Scholar] [CrossRef]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.-X.; Ren, Y.; Shan, P.F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Ferrari, P.; Weidmann, P. Insulin, Insulin Sensitivity and Hypertension. J. Hypertens. 1990, 8, 491–500. [Google Scholar] [CrossRef]
- Tokarz, V.L.; MacDonald, P.E.; Klip, A. The Cell Biology of Systemic Insulin Function. J. Cell Biol. 2018, 217, 2273–2289. [Google Scholar] [CrossRef]
- Kahn, C.R. The Molecular Mechanism of Insulin Action. Annu. Rev. Med. 1985, 36, 429–451. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr. Diabetes Rev. 2012, 9, 25–53. [Google Scholar] [CrossRef]
- Novikoff, A.; Müller, T.D. The Molecular Pharmacology of Glucagon Agonists in Diabetes and Obesity. Peptides 2023, 165, 171003. [Google Scholar] [CrossRef]
- De la Vega-Moreno, K.; Suárez-Cuenca, J.A. Understanding Glucose Transporter Type 4, Aka GLUT4: A Novel Review. Rev. Mex. Endocrinol. Metab. Nutr. 2024, 11, 53–59. [Google Scholar] [CrossRef]
- Chang, L.; Chiang, S.H.; Saltiel, A.R. Insulin Signaling and the Regulation of Glucose Transport. Mol. Med. 2004, 10, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-Q.; Keating, A.F. Functional Properties and Genomics of Glucose Transporters. Curr. Genom. 2007, 8, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B.; Mueckler, M. Glucose Transporters in the 21st Century. Am. J. Physiol.-Endocrinol. Metab. 2010, 298, E141–E145. [Google Scholar] [CrossRef]
- Dong, Q.; Endo, M.; Kawamura, G.; Ozawa, T. Systematic Interrogation of the Temperature Perturbation in the Insulin Signaling Pathway for Optogenetic Stimulation. Cells 2022, 11, 3136. [Google Scholar] [CrossRef]
- Longo, N.; Elsas, L.J. Human Glucose Transporters. Adv. Pediatr. 1998, 45, 293–313. [Google Scholar] [CrossRef]
- Aquilano, K.; Ceci, V.; Gismondi, A.; De Stefano, S.; Iacovelli, F.; Faraonio, R.; Di Marco, G.; Poerio, N.; Minutolo, A.; Minopoli, G.; et al. Adipocyte Metabolism Is Improved by TNF Receptor-Targeting Small RNAs Identified from Dried Nuts. Commun. Biol. 2019, 2, 317. [Google Scholar] [CrossRef] [PubMed]
- Janochova, K.; Haluzik, M.; Buzga, M. Visceral Fat and Insulin Resistance—What We Know? Biomed. Pap.-Olomouc 2019, 163, 19–27. [Google Scholar] [CrossRef]
- Fain, J.N.; Bahouth, S.W.; Madan, A.K. TNFα Release by the Nonfat Cells of Human Adipose Tissue. Int. J. Obes. 2004, 28, 616–622. [Google Scholar] [CrossRef]
- De Filippo, G.; Rendina, D.; Moccia, F.; Rocco, V.; Campanozzi, A. Interleukin-6, Soluble Interleukin-6 Receptor/Interleukin-6 Complex and Insulin Resistance in Obese Children and Adolescents. J. Endocrinol. Investig. 2015, 38, 339–343. [Google Scholar] [CrossRef]
- LaPensee, C.R.; Hugo, E.R.; Ben-Jonathan, N. Insulin Stimulates Interleukin-6 Expression and Release in LS14 Human Adipocytes through Multiple Signaling Pathways. Endocrinology 2008, 149, 5415–5422. [Google Scholar] [CrossRef]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems Associated with Glucose Toxicity: Role of Hyperglycemia-Induced Oxidative Stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.; Villar-Delfino, P.H.; Ferreira dos Anjos, P.M.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Tran, K.L.; Park, Y.I.; Pandya, S.; Muliyil, N.J.; Jensen, B.D.; Huynh, K.; Nguyen, Q.T. Overview of Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Patients with Type 2 Diabetes. Am. Health Drug Benefits 2017, 10, 178–188. [Google Scholar] [PubMed]
- Sifuentes-Franco, S.; Pacheco-Moisés, F.P.; Rodríguez-Carrizalez, A.D.; Miranda-Díaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. Exp. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef]
- Bilu, C.; Einat, H.; Zimmet, P.; Vishnevskia-Dai, V.; Vishnevskia-Dai, V.; Kronfeld-Schor, N. Beneficial Effects of Daytime High-Intensity Light Exposure on Daily Rhythms, Metabolic State and Affect. Sci. Rep. 2020, 10, 19782. [Google Scholar] [CrossRef]
- Ursino, G.; Coppari, R. Insulin under the Influence of Light. Swiss Med. Wkly. 2020, 150, w20273. [Google Scholar] [CrossRef]
- Broichhagen, J.; Schönberger, M.; Cork, S.C.; Frank, J.A.; Marchetti, P.; Bugliani, M.; Shapiro, A.M.J.; Trapp, S.; Rutter, G.A.; Hodson, D.J.; et al. Optical Control of Insulin Release Using a Photoswitchable Sulfonylurea. Nat. Commun. 2014, 5, 5116. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.C.; Chen, Y.W.; Chiu, T.H.; Kuo, Y.T.; Chen, C.H. Far-Infrared Radiation Prevents Decline in β-Cell Mass and Function in Diabetic Mice via the Mitochondria-Mediated Sirtuin1 Pathway. Metab. Clin. Exp. 2020, 104, 154143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.H.; Chen, Y.C.; Chen, T.H.; Sue, Y.M.; Cheng, T.-H.; Chen, J.R.; Chen, C.H. Far-Infrared Therapy Induces the Nuclear Translocation of PLZF Which Inhibits VEGF-Induced Proliferation in Human Umbilical Vein Endothelial Cells. PLoS ONE 2012, 7, e30674. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, T.-H.; Wu, M.Y.; Chou, T.-C.; Chen, J.-R.; Wei, M.-J.; Lee, S.-L.; Hong, L.-Y.; Zheng, C.M.; Chiu, I.-J.; et al. Far-Infrared Protects Vascular Endothelial Cells from Advanced Glycation End Products-Induced Injury via PLZF-Mediated Autophagy in Diabetic Mice. Sci. Rep. 2017, 7, 40442. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.-R.; Gao, H.; Yang, C.; Xie, C. Physiological and Pathological Characteristics of Vascular Endothelial Injury in Diabetes and the Regulatory Mechanism of Autophagy. Front. Endocrinol. 2023, 14, 1191426. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M.; Vlassara, H.; Cerami, A. Nonenzymatic Glycosylation and the Pathogenesis of Diabetic Complications. Ann. Intern. Med. 1984, 101, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol. Med. 2015, 21, S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Serban, A.I.; Stanca, L.; Geicu, O.I.; Geicu, O.I.; Dinischiotu, A. AGEs-Induced IL-6 Synthesis Precedes RAGE Up-Regulation in HEK 293 Cells: An Alternative Inflammatory Mechanism? Int. J. Mol. Sci. 2015, 16, 20100–20117. [Google Scholar] [CrossRef]
- Goh, S.-Y.; Cooper, M.E. The Role of Advanced Glycation End Products in Progression and Complications of Diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef]
- Liu, X.-D.; Zhang, W.; Xu, Y.; Xu, X.; Jiang, Q.; Ruan, J.; Wu, Y.; Zhou, Y.; Saw, P.E.; Luo, B.-M. Targeting PI3Kγ/AKT Pathway Remodels LC3-Associated Phagocytosis Induced Immunosuppression After Radiofrequency Ablation. Adv. Sci. 2022, 9, 2102182. [Google Scholar] [CrossRef]
- Powner, M.B.; Jeffery, G. Light Stimulation of Mitochondria Reduces Blood Glucose Levels. J. Biophotonics 2024, 17, e202300521. [Google Scholar] [CrossRef]
- Martiñón-Gutiérrez, G.; Luna-Castro, M.; Hernández-Muñoz, R. Role of Insulin/Glucagon Ratio and Cell Redox State in the Hyperglycaemia Induced by Exposure to Electromagnetic Fields in Rats. Sci. Rep. 2020, 11, 11666. [Google Scholar] [CrossRef]
- Nonarath, H.J.; Hall, A.E.; SenthilKumar, G.; Abroe, B.; Eells, J.T.; Liedhegner, E.S. 670nm Photobiomodulation Modulates Bioenergetics and Oxidative Stress, in Rat Müller Cells Challenged with High Glucose. PLoS ONE 2021, 16, e0260968. [Google Scholar] [CrossRef]
- Berry, B.J.; Wojtovich, A.P. Mitochondrial Light Switches: Optogenetic Approaches to Control Metabolism. FEBS J. 2020, 287, 4544–4556. [Google Scholar] [CrossRef]
- Boicean, A.; Ichim, C.; Sasu, S.-M.; Todor, S.B. Key Insights into Gut Alterations in Metabolic Syndrome. J. Clin. Med. 2025, 14, 2678. [Google Scholar] [CrossRef] [PubMed]
- Popa, M.L.; Ichim, C.; Anderco, P.; Todor, S.B.; Pop-Lodromanean, D. MicroRNAs in the Diagnosis of Digestive Diseases: A Comprehensive Review. J. Clin. Med. 2025, 14, 2054. [Google Scholar] [CrossRef]
- Lucas Martín, A.M.; Lang, S.; Goeser, T.; Demir, M.; Steffen, H.-M.; Kasper, P. Management of Dyslipidemia in Patients with Non-Alcoholic Fatty Liver Disease. Curr. Atheroscler. Rep. 2022, 24, 533–546. [Google Scholar] [CrossRef]
- Attman, P.-O.; Samuelsson, O.; Alaupovic, P. Diagnosis and Classification of Dyslipidemia in Renal Disease. Blood Purif. 1996, 14, 49–57. [Google Scholar] [CrossRef]
- Schofield, J.; Liu, Y.; Rao-Balakrishna, P.; Malik, R.A.; Soran, H. Diabetes Dyslipidemia. Diabetes Ther. 2016, 7, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Izotov, A.I. Velká Akademická Gramatika Spisovné Češtiny. I., Morfologie. Druhy Slov, Tvoření Slov. 2 Svazky. Philol. Theory Pract. 2021, 14, 2407–2409. [Google Scholar] [CrossRef]
- Malik, V.S.; Willett, W.C.; Hu, F.B. Global Obesity: Trends, Risk Factors and Policy Implications. Nat. Rev. Endocrinol. 2013, 9, 13–27. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Abed, Y.; Rahmat, A.; Ranneh, Y.; Ali, F. Epidemiology of Obesity in Developing Countries: Challenges and Prevention. Glob. Epidemic Obes. 2014, 2, 2. [Google Scholar] [CrossRef]
- Howard, B.V. Insulin Resistance and Lipid Metabolism. Am. J. Cardiol. 1999, 84, 28–32. [Google Scholar] [CrossRef]
- Semple, R.K.; Sleigh, A.; Murgatroyd, P.R.; Adams, C.; Bluck, L.; Jackson, S.; Vottero, A.; Kanabar, D.; Charlton-Menys, V.; Durrington, P.N.; et al. Postreceptor Insulin Resistance Contributes to Human Dyslipidemia and Hepatic Steatosis. J. Clin. Investig. 2009, 119, 315–322. [Google Scholar] [CrossRef]
- Aydin, S.; Aksoy, A.; Aksoy, A.; Aydin, S.; Kalayci, M.; Yilmaz, M.; Kuloglu, T.; Citil, C.; Catak, Z. Today’s and Yesterday’s of Pathophysiology: Biochemistry of Metabolic Syndrome and Animal Models. Nutrition 2014, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cohn, G.; Valdes, G.; Capuzzi, D.M. Pathophysiology and Treatment of the Dyslipidemia of Insulin Resistance. Curr. Cardiol. Rep. 2001, 3, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Thombare, K.; Ntika, S.; Wang, X.; Krizhanovskii, C.; Krizhanovskii, C. Long Chain Saturated and Unsaturated Fatty Acids Exert Opposing Effects on Viability and Function of GLP-1-Producing Cells: Mechanisms of Lipotoxicity. PLoS ONE 2017, 12, e0177605. [Google Scholar] [CrossRef]
- Yahagi, N.; Shimano, H.; Hasty, A.H.; Amemiya-Kudo, M.; Okazaki, H.; Tamura, Y.; Iizuka, Y.; Shionoiri, F.; Ohashi, K.; Osuga, J.; et al. A Crucial Role of Sterol Regulatory Element-Binding Protein-1 in the Regulation of Lipogenic Gene Expression by Polyunsaturated Fatty Acids. J. Biol. Chem. 1999, 274, 35840–35844. [Google Scholar] [CrossRef]
- Ogawa, Y. 2. Molecular Mechanism of Metabolic Syndrome. Nihon Naika Gakkai Zasshi 2016, 105, 101b–102a. [Google Scholar] [CrossRef]
- Fukuda, T.Y.; Tanji, M.M.; da Silva, S.R.; Sato, M.N.; Plapler, H. Infrared Low-Level Diode Laser on Inflammatory Process Modulation in Mice: Pro- and Anti-Inflammatory Cytokines. Lasers Med. Sci. 2013, 28, 1305–1313. [Google Scholar] [CrossRef]
- Babiy, B.; Ramos-Molina, B.; Ocaña, L.; Sacristán, S.; Burgos-Santamaría, D.; Martínez-Botas, J.; Busto, R.; Perna, C.; Frutos, M.D.; Albillos, A.; et al. Dihydrosphingolipids Are Associated with Steatosis and Increased Fibrosis Damage in Non-Alcoholic Fatty Liver Disease. Biochim. Et Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159318. [Google Scholar] [CrossRef]
- Arnold, M.J. Nonalcoholic Fatty Liver Disease: Diagnosis and Management Guidelines from the AACE. Am. Fam. Physician 2023, 107, 554–556. [Google Scholar]
- Cotter, T.G.; Rinella, M.E. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Loomba, R.; Anstee, Q.M.; Rinella, M.E.; Bugianesi, E.; Marchesini, G.; Neuschwander-Tetri, B.A.; Serfaty, L.; Negro, F.; Caldwell, S.H.; et al. Diagnostic Modalities for Nonalcoholic Fatty Liver Disease, Nonalcoholic Steatohepatitis, and Associated Fibrosis. Hepatology 2018, 68, 349–360. [Google Scholar] [CrossRef]
- Teng, M.; Ng, C.H.; Huang, D.Q.; Chan, K.; Tan, D.J.; Lim, W.H.; Yang, J.; Tan, E.X.-X.; Muthiah, M.D. Global Incidence and Prevalence of Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2022, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Simoes, I.C.M.; Janikiewicz, J.; Bauer, J.; Karkucinska-Wieckowska, A.; Kalinowski, P.; Dobrzyn, A.; Wolski, A.; Pronicki, M.; Zieniewicz, K.; Dobrzyn, P.; et al. Fat and Sugar-A Dangerous Duet. A Comparative Review on Metabolic Remodeling in Rodent Models of Nonalcoholic Fatty Liver Disease. Nutrients 2019, 11, 2871. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Viatour, P. Hepatocellular Carcinoma: Old Friends and New Tricks. Exp. Mol. Med. 2020, 52, 1898–1907. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.-F.; She, Z.-G.; Cai, J.; Li, H. Role of Oxidative Stress in the Pathogenesis of Nonalcoholic Fatty Liver Disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Xia, J.; Chen, H.; Wang, X.; Chen, W.; Jun, L.; Xu, F.; Nie, Q.; Ye, C.; Zhong, B.; Zhao, M.; et al. Sphingosine D18:1 Promotes Nonalcoholic Steatohepatitis by Inhibiting Macrophage HIF-2α. Nat. Commun. 2024, 15, 4755. [Google Scholar] [CrossRef]
- Kulkarni, J.H. Lipid Droplet Biology in Obesity: Mechanisms of Lipid Storage and Mobilization in Metabolic Disease. INOSR Exp. Sci. 2024, 14, 6–10. [Google Scholar] [CrossRef]
- Xu, T.; Fu, H.; Zhao, W.; Shan, S. Far-Infrared Radiation Alleviates Steatohepatitis and Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease. Dent. Sci. Rep. 2024, 14, 19292. [Google Scholar] [CrossRef]
- Ishimaru, K.; Nakajima, T.; Namiki, Y.; Ryotokuji, K. Influences of Pinpoint Plantar Long-Wavelength Infrared Light Irradiation (Stress-Free Therapy) on Chorioretinal Hemodynamics, Atherosclerosis Factors, and Vascular Endothelial Growth Factor. Integr. Med. Res. 2018, 7, 103–107. [Google Scholar] [CrossRef]
- Nagy, E.N.; Ibrahim, F.M.; Jouda, A.A.; Elsayed, M. The Effect of Laser Therapy Along with Mediterranean Diet Versus Mediterranean Diet Only on Older Adults with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. J. Lasers Med. Sci. 2021, 12, e39. [Google Scholar] [CrossRef]
- Caballero, A.E. Endothelial Dysfunction in Obesity and Insulin Resistance: A Road to Diabetes and Heart Disease. Obes. Res. 2003, 11, 1278–1289. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Ricart, W. Insulin Resistance and Chronic Cardiovascular Inflammatory Syndrome. Endocr. Rev. 2003, 24, 278–301. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Fisman, E.Z.; Motro, M.; Tenenbaum, A.; Tenenbaum, A. Cardiovascular Diabetology in the Core of a Novel Interleukins Classification: The Bad, the Good and the Aloof. Cardiovasc. Diabetol. 2003, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Gili, M.; Togliatto, G.; Brizzi, M.F. Diabetes-Associated Kidney and Vascular Complications: Mechanisms of Disease Progression and Alternative Therapeutic Options. J. Mol. Genet. Med. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Hadi, H.A.R.; Carr, C.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Heistad, D.D. Arteriosclerosis, Thrombosis, and Vascular Biology. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1–2. [Google Scholar] [CrossRef]
- Bian, K.; Doursout, M.-F.; Murad, F. Vascular System: Role of Nitric Oxide in Cardiovascular Diseases. J. Clin. Hypertens. 2008, 10, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Nava, E.; Noll, G.; Lüscher, T.F. Nitric Oxide in Cardiovascular Diseases. Ann. Med. 1995, 27, 343–351. [Google Scholar] [CrossRef]
- Tahto, E.; Jadrić, R.; Pojskić, L.; Kicic, E. Neutrophil-to-Lymphocyte Ratio and Its Relation with Markers of Inflammation and Myocardial Necrosis in Patients with Acute Coronary Syndrome. Med. Arch. 2017, 71, 312–315. [Google Scholar] [CrossRef]
- Raddino, R.; Caretta, G.; Teli, M.; Bonadei, I.; Robba, D.; Zanini, G.; Madureri, A.; Nodari, S.; Dei Cas, L. Nitric Oxide and Cardiovascular Risk Factors. Heart Int. 2007, 3, 18–26. [Google Scholar] [CrossRef]
- Leon, B.; Maddox, T.M. Diabetes and Cardiovascular Disease: Epidemiology, Biological Mechanisms, Treatment Recommendations and Future Research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.L.; de Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef]
- Scott, J. Pathophysiology and Biochemistry of Cardiovascular Disease. Curr. Opin. Genet. Dev. 2004, 14, 271–279. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, X.; Liu, Y.; Li, F.; Liu, Q.; Sun, J.; Cai, L. Dysregulation of Histone Acetyltransferases and Deacetylases in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2014, 2014, 641979. [Google Scholar] [CrossRef]
- London, G.M. Vascular Disease and Atherosclerosis in Uremia. Blood Purif. 2001, 19, 139–142. [Google Scholar] [CrossRef]
- Hayashi, K.; Naiki, T. Adaptation and Remodeling of Vascular Wall; Biomechanical Response to Hypertension. J. Mech. Behav. Biomed. Mater. 2009, 2, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Baretella, O.; Baretella, O.; Meyer, M.R.; Meyer, M.R. Obesity and Risk of Vascular Disease: Importance of Endothelium-Dependent Vasoconstriction. Br. J. Pharmacol. 2012, 165, 591–602. [Google Scholar] [CrossRef]
- Kimura, H.; Esumi, H. Reciprocal Regulation between Nitric Oxide and Vascular Endothelial Growth Factor in Angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef]
- Zachary, I. Vascular Endothelial Growth Factor. Int. J. Biochem. Cell Biol. 1998, 30, 1169–1174. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Beever, R. Do Far-Infrared Saunas Have Cardiovascular Benefits in People with Type 2 Diabetes? Can. J. Diabetes 2010, 34, 113–118. [Google Scholar] [CrossRef]
- Desmet, K.D.; Paz, D.A.; Corry, J.J.; Eells, J.T.; Wong-Riley, M.T.T.; Henry, M.M.; Buchmann, E.; Connelly, M.P.; Dovi, J.V.; Liang, H.L.; et al. Clinical and Experimental Applications of NIR-LED Photobiomodulation. Photomed. Laser Surg. 2006, 24, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous Effects of Infrared Radiation: From Clinical Observations to Molecular Response Mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Dover, J.S.; Phillips, T.J.; Arndt, K.A. Cutaneous Effects and Therapeutic Uses of Heat with Emphasis on Infrared Radiation. J. Am. Acad. Dermatol. 1989, 20, 278–286. [Google Scholar] [CrossRef]
- Zuclich, J.A.; Zwick, H.; Schuschereba, S.T.; Stuck, B.E.; Cheney, F.E. Ophthalmoscopic and Pathologic Description of Ocular Damage Induced by Infrared Laser Radiation. J. Laser Appl. 1997, 10, 114–120. [Google Scholar] [CrossRef]
- Zuclich, J.A. Ultraviolet-Induced Photochemical Damage in Ocular Tissues. Health Phys. 1989, 56, 671–682. [Google Scholar] [CrossRef]
- Loewen, E.G. Optical Surface Technology: SPIE Proceedings Vol 381. Precis. Eng.-J. Int. Soc. Precis. Eng. Nanotechnol. 1984, 6, 162–163. [Google Scholar] [CrossRef]
- Sushko, B.S.; Lymans’kyĭ, I.P.; Huliar, S.O. Action of the Red and Infrared Electromagnetic Waves of Light-Emitting Diodes on the Behavioral Manifestation of Somatic Pain. Fiziol. Zhurnal 2007, 53, 51–60. [Google Scholar]
- Wehner, H.; von Ardenne, A.; Kaltofen, S. Whole-Body Hyperthermia with Water-Filtered Infrared Radiation: Technical-Physical Aspects and Clinical Experiences. Int. J. Hyperth. 2001, 17, 19–30. [Google Scholar] [CrossRef]
| Application | Target | Wavelength | Results | Ref. |
|---|---|---|---|---|
| Adipose Regeneration | Human adipose-derived stem cells | 0.81 μm 0.98 μm | Promote cell proliferation and differentiation | [42] |
| Neural Stimulation | HEK-293T cells | 1.889 μm | Altered the membrane electrical capacitance during optical stimulation transiently | [33] |
| In vivo models | ||||
| Wound Healing | Dermal abrasions in mice | 0.81 μm | Increased collagen deposition and improved healing effects | [43] |
| Brain Neural Regeneration | Strokes in embolized rabbits | 0.808 μm | Elevated ATP levels in the cortex | [44] |
| Neural Stimulation | Rat sciatic nerve | 1.875 μm | Hybrid electro-optical stimulation induced sustained muscle contractions while lowering laser power requirements | [45] |
| Neural Stimulation | Adult rabbit heart | 1.851 μm | Triggered optical pacing in the adult rabbit heart | [46] |
| Wound Healing | Soft tissues in rats | 0.904 μm | Enhances wound healing and affects membrane properties, as measured by 1H-NMR τc\tau_cτc | [47] |
| Clinical model | ||||
| Brain Neural Regeneration | Mild traumatic brain injury | 0.87 μm | Enhanced cognitive function, better sleep, and reduced symptoms of post-traumatic stress disorder | [48] |
| Section | Key Concepts | Pathophysiological Mechanisms | Effect of FIR Therapy | Supporting Evidence |
|---|---|---|---|---|
| 4.1 Gut–Liver Axis and miRNAs | Gut dysbiosis, leaky gut, endotoxemia, miR-122, miR-34a | Activation of TLR4 → hepatic inflammation, insulin resistance; miRNA dysregulation → lipid accumulation and fibrosis | FIR modulates miRNA expression involved in lipid metabolism and inflammation | [91,92] |
| 4.2 Insulin Resistance and Dyslipidemia | Excess FFA, SREBP-1c, ApoB-100, TNF-α, IL-6 | ↑VLDL production, ↓LDL receptor expression, impaired HDL function → atherogenesis | FIR may enhance insulin sensitivity and reduce lipid abnormalities via AMPK pathway | [93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] |
| 4.3 Pathogenesis of MASLD | Steatosis → oxidative stress → inflammation → fibrosis → NASH → HCC | Mitochondrial dysfunction, lipid peroxidation, activation of Kupffer and stellate cells | FIR activates AMPK, ↑fatty acid oxidation, ↓lipogenesis gene expression (e.g., CD36, FASN) | [107,108,110,111,112,113,114,115,116] |
| 4.4 Animal Studies | FIR-treated mice (4-week protocol) | ↓Hepatic TG and TC, ↓serum LDL-C and TG, ↑HDL-C | Improved lipid profile, reduced hepatic steatosis | [76,117,118] |
| 4.5 Human Clinical Study | 4 adults with dyslipidemia (3-week FIR intervention) | ↓LDL-C (~13%), ↓TG (~value incomplete), ↑HDL-C | Improved lipid profile | [119,120] |
| Parameter | Recommended Safe Range | Notes |
|---|---|---|
| Skin surface temperature | <42 °C | Above 42 °C, risk of burns and tissue damage increases. |
| Power density (irradiance) | 10–100 mW/cm2 | Higher values increase thermal load; individual tolerance varies. |
| Session duration | 15–30 min per session | Sessions longer than 30 min may increase dehydration and thermal risk. |
| Treatment frequency | 3–5 times per week | Adjusted based on clinical response and tolerance. |
| Distance from emitter | 20–50 cm from the skin surface | Too close proximity increases local heating and burn risk. |
| Use of protective measures | Protective eyewear, hydration before/after | Eye protection and hydration are strongly recommended. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Śniegocki, M.; Smuczyński, W.; Ziółkowska, E. Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases. Int. J. Mol. Sci. 2025, 26, 5134. https://doi.org/10.3390/ijms26115134
Nowacka A, Śniegocki M, Smuczyński W, Ziółkowska E. Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases. International Journal of Molecular Sciences. 2025; 26(11):5134. https://doi.org/10.3390/ijms26115134
Chicago/Turabian StyleNowacka, Agnieszka, Maciej Śniegocki, Wojciech Smuczyński, and Ewa Ziółkowska. 2025. "Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases" International Journal of Molecular Sciences 26, no. 11: 5134. https://doi.org/10.3390/ijms26115134
APA StyleNowacka, A., Śniegocki, M., Smuczyński, W., & Ziółkowska, E. (2025). Therapeutic Potential of Infrared and Related Light Therapies in Metabolic Diseases. International Journal of Molecular Sciences, 26(11), 5134. https://doi.org/10.3390/ijms26115134







