Advances in Molecular Research of Tracheobronchial Tree Aging: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
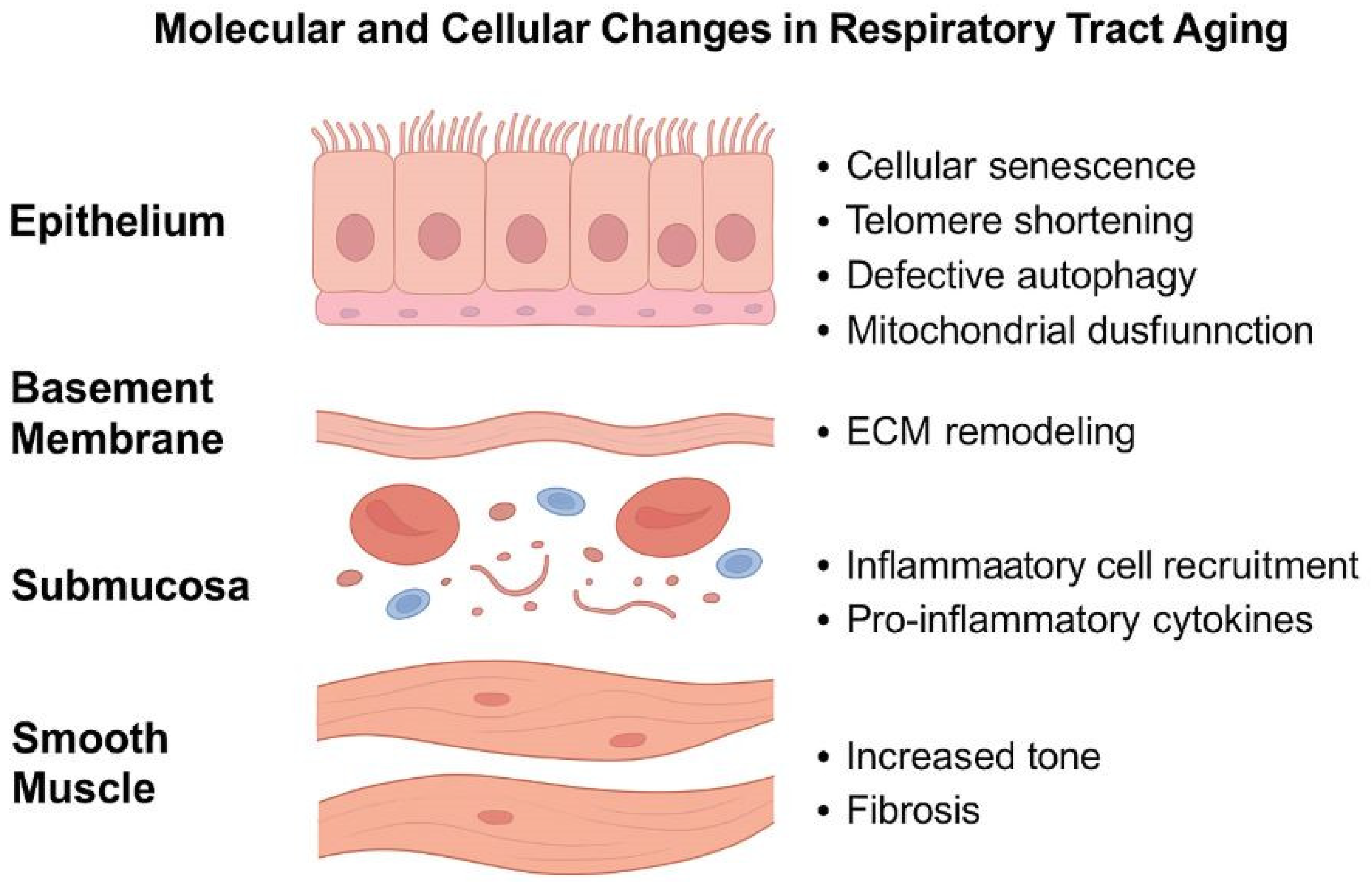
3.1. The Mucous and Submucous Layers
3.2. The Cartilaginous Layer
3.3. Airway Smooth Muscle
4. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic Regulation of Aging: Implications for Interventions of Aging and Diseases. Signal Transduct. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Salameh, Y.; Bejaoui, Y.; El Hajj, N. DNA Methylation Biomarkers in Aging and Age-Related Diseases. Front. Genet. 2020, 11, 480672. [Google Scholar] [CrossRef]
- Horvath, S. DNA Methylation Age of Human Tissues and Cell Types. Genome Biol. 2013, 14, 3156. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-A.; Mori, K.M.; McElroy, J.P.; Freudenheim, J.L.; Weng, D.Y.; Reisinger, S.A.; Brasky, T.M.; Wewers, M.D.; Shields, P.G. Accelerated Epigenetic Age, Inflammation, and Gene Expression in Lung: Comparisons of Smokers and Vapers with Non-Smokers. Clin. Epigenet. 2023, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Hernández Cordero, A.I.; Yang, C.X.; Yang, J.; Li, X.; Horvath, S.; Shaipanich, T.; MacIsaac, J.; Lin, D.; McEwen, L.; Kobor, M.S.; et al. The Relationship between the Epigenetic Aging Biomarker “Grimage” and Lung Function in Both the Airway and Blood of People Living with HIV: An Observational Cohort Study. EBioMedicine 2022, 83, 104206. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W. Is Chronic Obstructive Pulmonary Disease an Accelerated Aging Disease? Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S429–S437. [Google Scholar] [CrossRef]
- Vucic, E.A.; Chari, R.; Thu, K.L.; Wilson, I.M.; Cotton, A.M.; Kennett, J.Y.; Zhang, M.; Lonergan, K.M.; Steiling, K.; Brown, C.J.; et al. DNA Methylation Is Globally Disrupted and Associated with Expression Changes in Chronic Obstructive Pulmonary Disease Small Airways. Am. J. Respir. Cell Mol. Biol. 2014, 50, 912–922. [Google Scholar] [CrossRef]
- Hansel, C.; Jendrossek, V.; Klein, D. Cellular Senescence in the Lung: The Central Role of Senescent Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 3279. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular Senescence: From Physiology to Pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Parikh, P.; Wicher, S.; Khandalavala, K.; Pabelick, C.M.; Britt, R.D.; Prakash, Y.S. Cellular Senescence in the Lung across the Age Spectrum. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 316, L826–L842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, J.; Meng, Y.; Adcock, I.M.; Yao, X. Role of Inflammatory Cells in Airway Remodeling in COPD. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Aghasafari, P.; George, U.; Pidaparti, R. A Review of Inflammatory Mechanism in Airway Diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.; Gupta, G.; Costanzo, L.; Ahmed, H.; Wyman, A.E.; Geraghty, P. Senescence: Pathogenic Driver in Chronic Obstructive Pulmonary Disease. Medicina 2022, 58, 817. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Du, X.; Tang, S.; Wu, S.; Wang, L.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. ITGB4 Deficiency Induces Senescence of Airway Epithelial Cells through P53 Activation. FEBS J. 2019, 286, 1191–1203. [Google Scholar] [CrossRef]
- Schneider, J.L.; Rowe, J.H.; Garcia-de-Alba, C.; Kim, C.F.; Sharpe, A.H.; Haigis, M.C. The Aging Lung: Physiology, Disease, and Immunity. Cell 2021, 184, 1990–2019. [Google Scholar] [CrossRef]
- Cheng, P.-P.; Yu, F.; Chen, S.-J.; Feng, X.; Jia, Z.-H.; Hu, S.-H.; Cui, X.-L.; Zhou, Y.-Y.; Niu, Q.; Liang, L.-M.; et al. PM2.5 Exposure-Induced Senescence-Associated Secretory Phenotype in Airway Smooth Muscle Cells Contributes to Airway Remodeling. Environ. Pollut. 2024, 347, 123674. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Mittal, K.; Gonçalves, V.F.; Harripaul, R.; Cuperfain, A.B.; Rollins, B.; Tiwari, A.K.; Zai, C.C.; Maciukiewicz, M.; Müller, D.J.; Vawter, M.P.; et al. A Comprehensive Analysis of Mitochondrial Genes Variants and Their Association with Antipsychotic-Induced Weight Gain. Schizophr. Res. 2017, 187, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Xiao, L.; Zhang, Z.; Wang, Y.; Kouis, P.; Rasmussen, L.J.; Dai, F. Effects of Reactive Oxygen Species and Mitochondrial Dysfunction on Reproductive Aging. Front. Cell Dev. Biol. 2024, 12, 1347286. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Kim, K.; Esteves, P.; Trian, T.; Barnes, P.J. Mitochondrial Dysfunction in Lung Ageing and Disease. Eur. Respir. Rev. 2020, 29, 200165. [Google Scholar] [CrossRef]
- Delgado-Roche, L.; Mesta, F. Oxidative Stress as Key Player in Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) Infection. Arch. Med. Res. 2020, 51, 384–387. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Vareille, M.; Kieninger, E.; Edwards, M.R.; Regamey, N. The Airway Epithelium: Soldier in the Fight against Respiratory Viruses. Clin. Microbiol. Rev. 2011, 24, 210–229. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L. Aging Diminishes Mucociliary Clearance of the Lung. Adv. Geriatr. Med. Res. 2022, 4, e220005. [Google Scholar] [CrossRef]
- Koparal, M.; Kapici, Y.; Aslan, S.; Hepkarsi, S.; Karataş, M.; Yılmazer, C. Evaluation of Nasal Mucociliary Clearance as an Indicator of Nasal Function in Obsessive-Compulsive Patients: A Cross-Sectional Study. Indian J. Otolaryngol. Head Neck Surg. 2023, 75, 3263–3267. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Kharbanda, K.K.; Katafiasz, D.M.; Sisson, J.H.; Wyatt, T.A. Oxidative Stress Associated with Aging Activates Protein Kinase Cε, Leading to Cilia Slowing. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L882–L890. [Google Scholar] [CrossRef] [PubMed]
- Wansleeben, C.; Bowie, E.; Hotten, D.F.; Yu, Y.-R.A.; Hogan, B.L.M. Age-Related Changes in the Cellular Composition and Epithelial Organization of the Mouse Trachea. PLoS ONE 2014, 9, e93496. [Google Scholar] [CrossRef]
- Leopold, P.L.; O’Mahony, M.J.; Lian, X.J.; Tilley, A.E.; Harvey, B.-G.; Crystal, R.G. Smoking Is Associated with Shortened Airway Cilia. PLoS ONE 2009, 4, e8157. [Google Scholar] [CrossRef] [PubMed]
- Simet, S.M.; Sisson, J.H.; Pavlik, J.A.; DeVasure, J.M.; Boyer, C.; Liu, X.; Kawasaki, S.; Sharp, J.G.; Rennard, S.I.; Wyatt, T.A. Long-Term Cigarette Smoke Exposure in a Mouse Model of Ciliated Epithelial Cell Function. Am. J. Respir. Cell Mol. Biol. 2010, 43, 635. [Google Scholar] [CrossRef]
- Park, H.-R.; O’Sullivan, M.; Vallarino, J.; Shumyatcher, M.; Himes, B.E.; Park, J.-A.; Christiani, D.C.; Allen, J.; Lu, Q. Transcriptomic Response of Primary Human Airway Epithelial Cells to Flavoring Chemicals in Electronic Cigarettes. Sci. Rep. 2019, 9, 1400. [Google Scholar] [CrossRef]
- Nettesheim, P.; Martin, D.H. Appearance of Glandlike Structures in the Tracheobronchial Tree of Aging Mice. J. Natl. Cancer Inst. 1970, 44, 687–693. [Google Scholar]
- Aros, C.J.; Vijayaraj, P.; Pantoja, C.J.; Bisht, B.; Meneses, L.K.; Sandlin, J.M.; Tse, J.A.; Chen, M.W.; Purkayastha, A.; Shia, D.W.; et al. Distinct Spatiotemporally Dynamic Wnt-Secreting Niches Regulate Proximal Airway Regeneration and Aging. Cell Stem Cell 2020, 27, 413–429.e4. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, D.H.; Besnard, V.; Lange, A.W.; Keiser, A.R.; Wert, S.E.; Bruno, M.D.; Whitsett, J.A. Sox2 Activates Cell Proliferation and Differentiation in the Respiratory Epithelium. Am. J. Respir. Cell Mol. Biol. 2011, 45, 101–110. [Google Scholar] [CrossRef]
- Li, D.; Qu, Y.; Wang, B.; Zhang, H.; Qin, L. Spatio-Temporal Expression of Sox2+ Progenitor Cells Regulates the Regeneration of Rat Submandibular Gland. Arch. Oral. Biol. 2024, 168, 106080. [Google Scholar] [CrossRef]
- Moreno-Valladares, M.; Moncho-Amor, V.; Silva, T.M.; Garcés, J.P.; Álvarez-Satta, M.; Matheu, A. KRT5+/P63+ Stem Cells Undergo Senescence in the Human Lung with Pathological Aging. Aging Dis. 2023, 14, 1013. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Zhang, T.; Wu, D.Z.; Guan, S.P.; Liew, A.-A.; Yamamoto, Y.; Wang, X.; Lim, S.J.; Vincent, M.; Lessard, M.; et al. p63+Krt5+ Distal Airway Stem Cells Are Essential for Lung Regeneration. Nature 2015, 517, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Millar-Büchner, P.; Mülleder, M.; Farztdinov, V.; Szyrwiel, L.; Addante, A.; Kuppe, A.; Rubil, T.; Drescher, M.; Seidel, K.; et al. Age-Related Differences in Structure and Function of Nasal Epithelial Cultures From Healthy Children and Elderly People. Front. Immunol. 2022, 13, 822437. [Google Scholar] [CrossRef] [PubMed]
- Ridley, C.; Thornton, D.J. Mucins: The Frontline Defence of the Lung. Biochem. Soc. Trans. 2018, 46, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, N.; O’Dowd, K.; Vahedi, S.M. Antiviral Responses against Chicken Respiratory Infections: Focus on Avian Influenza Virus and Infectious Bronchitis Virus. Cytokine 2020, 127, 154961. [Google Scholar] [CrossRef]
- Welsh, K.G.; Rousseau, K.; Fisher, G.; Bonser, L.R.; Bradding, P.; Brightling, C.E.; Thornton, D.J.; Gaillard, E.A. MUC5AC and a Glycosylated Variant of MUC5B Alter Mucin Composition in Children With Acute Asthma. Chest 2017, 152, 771–779. [Google Scholar] [CrossRef]
- Okuda, K.; Chen, G.; Subramani, D.B.; Wolf, M.; Gilmore, R.C.; Kato, T.; Radicioni, G.; Kesimer, M.; Chua, M.; Dang, H.; et al. Localization of Secretory Mucins MUC5AC and MUC5B in Normal/Healthy Human Airways. Am. J. Respir. Crit. Care Med. 2019, 199, 715–727. [Google Scholar] [CrossRef]
- Kesimer, M. Mucins MUC5AC and MUC5B in the Airways: MUCing around Together. Am. J. Respir. Crit. Care Med. 2022, 206, 1055–1057. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, S.; Kang, J.; Lin, J.; Lai, K.; Sun, Y.; Xiao, W.; Yang, L.; Yao, W.; Cai, S.; et al. Management of Airway Mucus Hypersecretion in Chronic Airway Inflammatory Disease: Chinese Expert Consensus (English Edition). Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 399–407. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Park, S.; Miller, L.; Lee, H.-C.; Langenbach, R.; Kleeberger, S.R. Role for Mucin-5AC in Upper and Lower Airway Pathogenesis in Mice. Toxicol. Pathol. 2021, 49, 1077–1099. [Google Scholar] [CrossRef]
- Singanayagam, A.; Footitt, J.; Kasdorf, B.T.; Marczynski, M.; Cross, M.T.; Finney, L.J.; Torralbo, M.-B.T.; Calderazzo, M.; Zhu, J.; Aniscenko, J.; et al. MUC5AC Drives COPD Exacerbation Severity through Amplification of Virus-Induced Airway Inflammation. bioRxiv 2019, 706804. [Google Scholar] [CrossRef]
- Singanayagam, A.; Footitt, J.; Marczynski, M.; Radicioni, G.; Cross, M.T.; Finney, L.J.; Trujillo-Torralbo, M.-B.; Calderazzo, M.; Zhu, J.; Aniscenko, J.; et al. Airway Mucins Promote Immunopathology in Virus-Exacerbated Chronic Obstructive Pulmonary Disease. J. Clin. Investig. 2022, 132, e120901. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Iverson, E.; Kaler, L.; Boboltz, A.; Scull, M.A.; Duncan, G.A. MUC5B Mobilizes and MUC5AC Spatially Aligns Mucociliary Transport on Human Airway Epithelium. Sci. Adv. 2022, 8, eabq5049. [Google Scholar] [CrossRef]
- Li, J.; Ye, Z. The Potential Role and Regulatory Mechanisms of MUC5AC in Chronic Obstructive Pulmonary Disease. Molecules 2020, 25, 4437. [Google Scholar] [CrossRef] [PubMed]
- Baumlin, N.; Silswal, N.; Dennis, J.S.; Niloy, A.J.; Kim, M.D.; Salathe, M. Nebulized Menthol Impairs Mucociliary Clearance via TRPM8 and MUC5AC/MUC5B in Primary Airway Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 1694. [Google Scholar] [CrossRef] [PubMed]
- Grubb, B.R.; Livraghi-Butrico, A.; Rogers, T.D.; Yin, W.; Button, B.; Ostrowski, L.E. Reduced Mucociliary Clearance in Old Mice Is Associated with a Decrease in Muc5b Mucin. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 310, L860–L867. [Google Scholar] [CrossRef]
- Hu, Y.; Riemondy, K.; Gao, B.; Hesselberth, J.; Koenigshoff, M.; Evans, C. Overproduction of MUC5B Impairs the Alveolar Repair in COPD. In D108. NEW INSIGHTS IN CELL FATE, REGENERATIVE MEDICINE, iPSCs, AND MSCs; American Thoracic Society International Conference Abstracts; American Thoracic Society: New York, NY, USA, 2022; p. A5483. [Google Scholar]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]
- Gori, A.; Brindisi, G.; Daglia, M.; Giudice, M.M.d.; Dinardo, G.; Di Minno, A.; Drago, L.; Indolfi, C.; Naso, M.; Trincianti, C.; et al. Exploring the Role of Lactoferrin in Managing Allergic Airway Diseases among Children: Unrevealing a Potential Breakthrough. Nutrients 2024, 16, 1906. [Google Scholar] [CrossRef]
- Zheng, J.; Xie, Y.; Li, F.; Zhou, Y.; Qi, L.; Liu, L.; Chen, Z. Lactoferrin Improves Cognitive Function and Attenuates Brain Senescence in Aged Mice. J. Funct. Foods 2020, 65, 103736. [Google Scholar] [CrossRef]
- Bielajew, B.J.; Hu, J.C.; Athanasiou, K.A. Collagen: Quantification, Biomechanics and Role of Minor Subtypes in Cartilage. Nat. Rev. Mater. 2020, 5, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Stephens, B.; Bergman, M.; May, A.; Chiang, T. Role of Collagen in Airway Mechanics. Bioengineering 2021, 8, 13. [Google Scholar] [CrossRef]
- Ulldemolins, A.; Narciso, M.; Sanz-Fraile, H.; Otero, J.; Farré, R.; Gavara, N.; Almendros, I. Effects of Aging on the Biomechanical Properties of the Lung Extracellular Matrix: Dependence on Tissular Stretch. Front. Cell Dev. Biol. 2024, 12, 1381470. [Google Scholar] [CrossRef] [PubMed]
- Angelidis, I.; Simon, L.M.; Fernandez, I.E.; Strunz, M.; Mayr, C.H.; Greiffo, F.R.; Tsitsiridis, G.; Ansari, M.; Graf, E.; Strom, T.-M.; et al. An Atlas of the Aging Lung Mapped by Single Cell Transcriptomics and Deep Tissue Proteomics. Nat. Commun. 2019, 10, 963. [Google Scholar] [CrossRef]
- Devulder, J.V. Unveiling Mechanisms of Lung Aging in COPD: A Promising Target for Therapeutics Development. Chin. Med. J. Pulm. Crit. Care Med. 2024, 2, 133–141. [Google Scholar] [CrossRef]
- Córdoba-Lanús, E.; Cazorla-Rivero, S.; Espinoza-Jiménez, A.; de-Torres, J.P.; Pajares, M.J.; Aguirre-Jaime, A.; Celli, B.; Casanova, C. Telomere Shortening and Accelerated Aging in COPD: Findings from the BODE Cohort. Respir. Res. 2017, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.; Assadinia, N.; Hackett, T.-L. Airway Remodeling Heterogeneity in Asthma and Its Relationship to Disease Outcomes. Front. Physiol. 2023, 14, 1113100. [Google Scholar] [CrossRef]
- Li, F.; Zhi, J.; Zhao, R.; Sun, Y.; Wen, H.; Cai, H.; Chen, W.; Jiang, X.; Bai, R. Discovery of Matrix Metalloproteinase Inhibitors as Anti-Skin Photoaging Agents. Eur. J. Med. Chem. 2024, 267, 116152. [Google Scholar] [CrossRef]
- Christopoulou, M.-E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef]
- Koo, H.-K.; Hong, Y.; Lim, M.N.; Yim, J.-J.; Kim, W.J. Relationship between Plasma Matrix Metalloproteinase Levels, Pulmonary Function, Bronchodilator Response, and Emphysema Severity. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, Y.; Zhao, Z.; Wang, J.; Li, J.; Wang, W.; Li, S.; Song, L. Relationships of MMP-9 and TIMP-1 Proteins with Chronic Obstructive Pulmonary Disease Risk: A Systematic Review and Meta-Analysis. J. Res. Med. Sci. 2016, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Dimic-Janjic, S.; Hoda, M.A.; Milenkovic, B.; Kotur-Stevuljevic, J.; Stjepanovic, M.; Gompelmann, D.; Jankovic, J.; Miljkovic, M.; Milin-Lazovic, J.; Djurdjevic, N.; et al. The Usefulness of MMP-9, TIMP-1 and MMP-9/TIMP-1 Ratio for Diagnosis and Assessment of COPD Severity. Eur. J. Med. Res. 2023, 28, 127. [Google Scholar] [CrossRef] [PubMed]
- Linder, R.; Rönmark, E.; Pourazar, J.; Behndig, A.F.; Blomberg, A.; Lindberg, A. Proteolytic Biomarkers Are Related to Prognosis in COPD- Report from a Population-Based Cohort. Respir. Res. 2018, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Parker, M.M.; Oster, R.A.; Bowler, R.P.; Dransfield, M.T.; Bhatt, S.P.; Cho, M.H.; Kim, V.; Curtis, J.L.; Martinez, F.J.; et al. Elevated Circulating MMP-9 Is Linked to Increased COPD Exacerbation Risk in SPIROMICS and COPDGene. JCI Insight 2018, 3, e123614. [Google Scholar] [CrossRef] [PubMed]
- Prakash, Y.S. Emerging Concepts in Smooth Muscle Contributions to Airway Structure and Function: Implications for Health and Disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L1113–L1140. [Google Scholar] [CrossRef]
- Reynold, A.; Panettieri, J.; Kotlikoff, M.I.; Gerthoffer, W.T.; Hershenson, M.B.; Woodruff, P.G.; Hall, I.P.; Banks-Schlegel, S. Airway Smooth Muscle in Bronchial Tone, Inflammation, and Remodeling: Basic Knowledge to Clinical Relevance. Am. J. Respir. Crit. Care Med. 2007, 177, 248. [Google Scholar] [CrossRef]
- Wicher, S.A.; Roos, B.B.; Teske, J.J.; Fang, Y.H.; Pabelick, C.; Prakash, Y.S. Aging Increases Senescence, Calcium Signaling, and Extracellular Matrix Deposition in Human Airway Smooth Muscle. PLoS ONE 2021, 16, e0254710. [Google Scholar] [CrossRef]
- Behringer, E.J.; Segal, S.S. Impact of Aging on Calcium Signaling and Membrane Potential in Endothelium of Resistance Arteries: A Role for Mitochondria. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1627. [Google Scholar] [CrossRef]
- Lopes, G.S.; Ferreira, A.T.; Oshiro, M.E.; Vladimirova, I.; Jurkiewicz, N.H.; Jurkiewicz, A.; Smaili, S.S. Aging-Related Changes of Intracellular Ca2+ Stores and Contractile Response of Intestinal Smooth Muscle. Exp. Gerontol. 2006, 41, 55–62. [Google Scholar] [CrossRef]
- Cho, S.J.; Stout-Delgado, H.W. Aging and Lung Disease. Annu. Rev. Physiol. 2020, 82, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.; Srikaram, P.; Guntupalli, V.; Hu, C.; Chen, Q.; Gao, P. Cellular Senescence in Asthma: From Pathogenesis to Therapeutic Challenges. eBioMedicine 2023, 94, 104717. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Bai, T.R.; Bates, J.H.T.; Black, J.L.; Brown, R.H.; Brusasco, V.; Chitano, P.; Deng, L.; Dowell, M.; Eidelman, D.H.; et al. Airway Smooth Muscle Dynamics: A Common Pathway of Airway Obstruction in Asthma. Eur. Respir. J. 2007, 29, 834. [Google Scholar] [CrossRef] [PubMed]
- Aghali, A.; Khalfaoui, L.; Lagnado, A.B.; Drake, L.Y.; Teske, J.J.; Pabelick, C.M.; Passos, J.F.; Prakash, Y.S. Cellular Senescence Is Increased in Airway Smooth Muscle Cells of Elderly Persons with Asthma. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2022, 323, L558. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, S.; Yi, Z.; Zhao, R.; Zhu, J.; Ding, S.; Wu, J. The Role of P21 in Cellular Senescence and Aging-Related Diseases. Mol. Cells 2024, 47, 100113. [Google Scholar] [CrossRef] [PubMed]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of P53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Ather, J.L.; Foley, K.L.; Suratt, B.T.; Boyson, J.E.; Poynter, M.E. Airway Epithelial NF-κB Activation Promotes the Ability to Overcome Inhalational Antigen Tolerance. Clin. Exp. Allergy 2015, 45, 1245–1258. [Google Scholar] [CrossRef]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of Cytokine Production in Human Alveolar Macrophages and Airway Epithelial Cells in Response to Ambient Air Pollution Particles: Further Mechanistic Studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef]
- Wu, Y.-F.; Li, Z.-Y.; Dong, L.-L.; Li, W.-J.; Wu, Y.-P.; Wang, J.; Chen, H.-P.; Liu, H.-W.; Li, M.; Jin, C.-L.; et al. Inactivation of MTOR Promotes Autophagy-Mediated Epithelial Injury in Particulate Matter-Induced Airway Inflammation. Autophagy 2020, 16, 435–450. [Google Scholar] [CrossRef]
- Tamargo-Gómez, I.; Mariño, G. AMPK: Regulation of Metabolic Dynamics in the Context of Autophagy. Int. J. Mol. Sci. 2018, 19, 3812. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; Fairlie, W.D.; Lee, E.F. BECLIN1: Protein Structure, Function and Regulation. Cells 2021, 10, 1522. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Ohbayashi, H.; Shimokata, K. Matrix Metalloproteinase-9 and Airway Remodeling in Asthma. Curr. Drug Targets Inflamm. Allergy 2005, 4, 177–181. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef] [PubMed]
- Bonser, L.R.; Erle, D.J. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J. Clin. Med. 2017, 6, 112. [Google Scholar] [CrossRef]
- Fernanda de Mello Costa, M.; Weiner, A.I.; Vaughan, A.E. Basal-like Progenitor Cells: A Review of Dysplastic Alveolar Regeneration and Remodeling in Lung Repair. Stem Cell Rep. 2020, 15, 1015–1025. [Google Scholar] [CrossRef]
- Mikhed, Y.; Daiber, A.; Steven, S. Mitochondrial Oxidative Stress, Mitochondrial DNA Damage and Their Role in Age-Related Vascular Dysfunction. Int. J. Mol. Sci. 2015, 16, 15918–15953. [Google Scholar] [CrossRef]
- Campisi, J. Cellular Senescence: Putting the Paradoxes in Perspective. Curr. Opin. Genet. Dev. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Fukumoto, J.; Sidramagowda Patil, S.; Krishnamurthy, S.; Saji, S.; John, I.; Narala, V.R.; Hernández-Cuervo, H.; Alleyn, M.; Breitzig, M.T.; Galam, L.; et al. Altered Expression of P63 Isoforms and Expansion of P63- and Club Cell Secretory Protein-Positive Epithelial Cells in the Lung as Novel Features of Aging. Am. J. Physiol. Cell Physiol. 2019, 316, C492–C508. [Google Scholar] [CrossRef]
- Garcia-Vilanova, A.; Olmo-Fontánez, A.M.; Moliva, J.I.; Allué-Guardia, A.; Singh, H.; Merritt, R.E.; Maselli, D.J.; Peters, J.I.; Restrepo, B.I.; Wang, Y.; et al. The Aging Human Lung Mucosa: A Proteomics Study. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.M.d.; Gelardi, M.; Marano, P.G.; D’Ecclesia, A.; Campobasso, G.; Cariti, F.; Palumbo, A.; Loglisci, M.; Vincentiis, M.d.; Cassano, M.; et al. The Secretory Senescence of the Airway. J. Gerontol. Geriatr. 2020, 68, 61–68. [Google Scholar] [CrossRef]
- Koloko Ngassie, M.L.; De Vries, M.; Borghuis, T.; Timens, W.; Sin, D.D.; Nickle, D.; Joubert, P.; Horvatovich, P.; Marko-Varga, G.; Teske, J.J.; et al. Age-Associated Differences in the Human Lung Extracellular Matrix. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2023, 324, L799–L814. [Google Scholar] [CrossRef]
- Navarro, S.; Driscoll, B. Regeneration of the Aging Lung: A Mini-Review. Gerontology 2016, 63, 270–280. [Google Scholar] [CrossRef]
- Harvey, B.J.; McElvaney, N.G. Sex Differences in Airway Disease: Estrogen and Airway Surface Liquid Dynamics. Biol. Sex Differ. 2024, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Ekpruke, C.D.; Silveyra, P. Sex Differences in Airway Remodeling and Inflammation: Clinical and Biological Factors. Front. Allergy 2022, 3, 875295. [Google Scholar] [CrossRef]
- Reddy, K.D.; Oliver, B.G.G. Sexual Dimorphism in Chronic Respiratory Diseases. Cell Biosci. 2023, 13, 47. [Google Scholar] [CrossRef]
- Du, N.; Yang, R.; Jiang, S.; Niu, Z.; Zhou, W.; Liu, C.; Gao, L.; Sun, Q. Anti-Aging Drugs and the Related Signal Pathways. Biomedicines 2024, 12, 127. [Google Scholar] [CrossRef]
- Garatachea, N.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, C.; Morán, M.; Emanuele, E.; Joyner, M.J.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef]
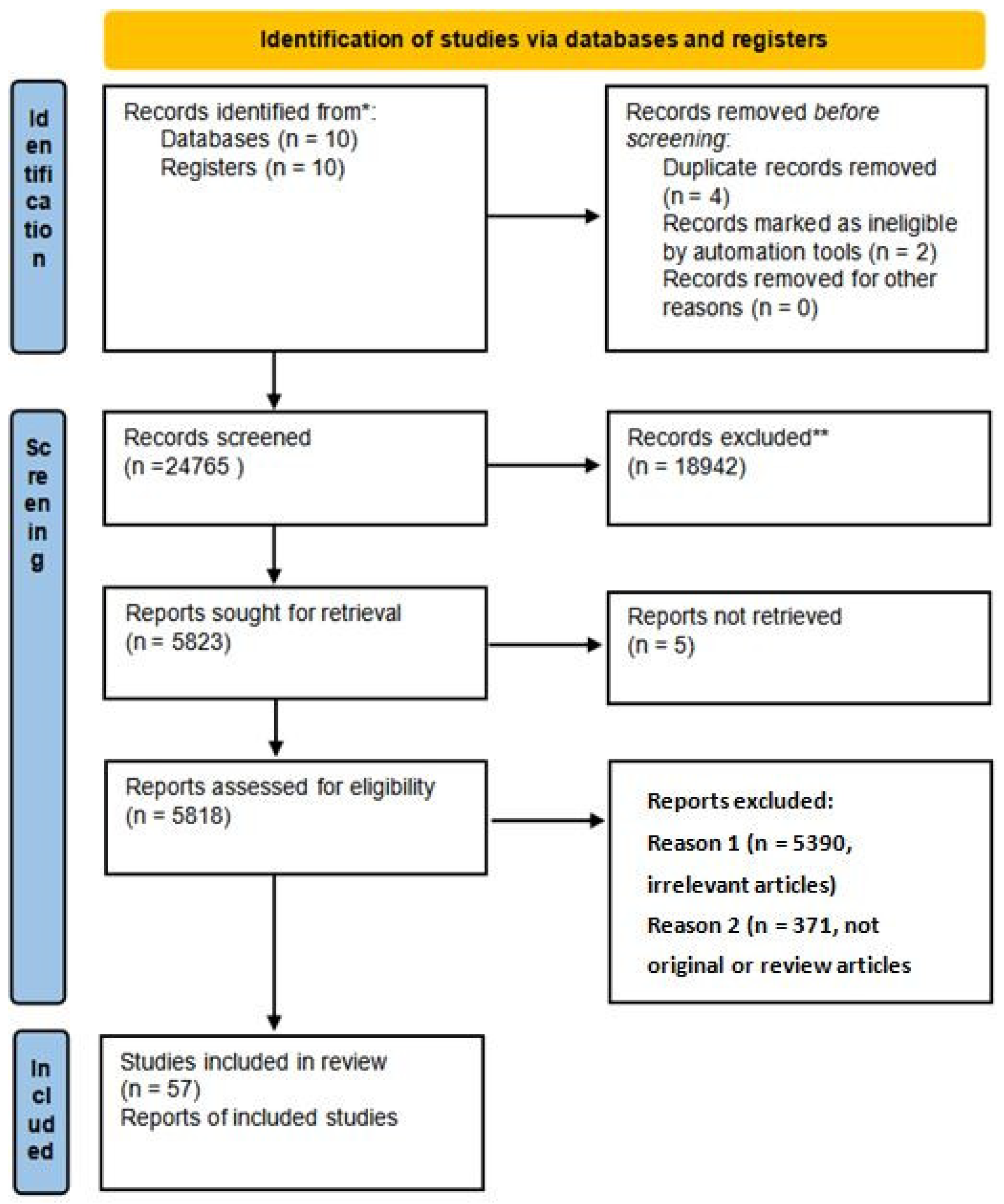
| Criteria | Stage | Count |
|---|---|---|
| 1. | Records identified | 20 |
| 2. | Records removed before screening | 6 |
| 3. | Records screened | 24,765 |
| 4. | Records excluded | 18,942 |
| 5. | Reports sought for retrieval | 5823 |
| 6. | Reports not retrieved | 5 |
| 7. | Reports assessed for eligibility | 5818 |
| 8. | Reports excluded (irrelevant articles) | 5390 |
| 9. | Reports excluded (not original/review articles) | 371 |
| 10. | Studies included in review | 57 |
| Molecule/Pathway | Affected Layer/Cell Type | Function/Alteration in Aging | Reference(s) |
|---|---|---|---|
| p53/p21 | Epithelial cells | Senescence induction, cell cycle arrest | [86,87] |
| p16INK4a/Rb | Basal epithelial and ASM cells | Permanent growth arrest, tumor suppression | [87,88] |
| NF-κB | All airway layers | Inflammatory cytokine production (SASP) | [89,90] |
| mTOR | ASM and epithelial cells | Impaired autophagy, altered cell growth | [91] |
| AMPK/Beclin-1 | Epithelial cells | Autophagy regulation, mitochondrial homeostasis | [92,93] |
| IL-6, IL-8, TNF-α | Immune and epithelial cells | Chronic inflammation, ECM remodeling | [89,94] |
| MMP-9, MMP-1 | ECM, cartilage | ECM degradation, airway remodeling | [95,96] |
| MUC5AC/MUC5B | Goblet and secretory cells | Mucus overproduction, impaired clearance | [97] |
| SOX2, KRT5, P63 | Basal progenitor cells | Reduced regeneration capacity | [41,98] |
| ROS | All layers | Oxidative stress, DNA, and mitochondrial damage | [98,99] |
| Study | Type of Article | Methodology | Model | Key Findings/Molecules |
|---|---|---|---|---|
| [66] | Original article | Single-cell transcriptomics, proteomics | Mouse | Collagen IV, XIV, XVI alterations in ECM during aging |
| [38] | Original article | Pharmacological, histological | Human/animal | KRT5+ basal stem cells; aging effects on epithelial repair niches |
| [101] | Original article | IHC, gene profiling | Animal | Increased p63, club cell markers with aging |
| [102] | Original article | Proteomics, pathway analysis | Human | MMP-8, -9, -10 upregulated with age; ECM remodeling |
| [103] | Review | Literature synthesis | - | Pro-inflammatory cytokines (IL-6, IL-1, TNF-α); secretory senescence |
| [56] | Original article | Mucin quantification, ciliary beat | Animal | Reduced MUC5B in old mice; decreased mucociliary clearance |
| [104] | Original article | Transcriptomics, proteomics | Human | COL6A1, LUM, FBLN2; structural ECM differences in aging lungs |
| [41] | Original article | IHC, IF | Human | SOX2+, KRT5+, P63+ stem cell senescence in aging lung |
| [105] | Review | Literature review | - | Aging effects on lung regeneration and epithelial stem cells |
| [16] | Review | Literature review | - | Molecular basis of lung aging and disease susceptibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahoru, C.; Hînganu, M.V.; Salahoru, P.; Hînganu, D. Advances in Molecular Research of Tracheobronchial Tree Aging: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 5128. https://doi.org/10.3390/ijms26115128
Salahoru C, Hînganu MV, Salahoru P, Hînganu D. Advances in Molecular Research of Tracheobronchial Tree Aging: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(11):5128. https://doi.org/10.3390/ijms26115128
Chicago/Turabian StyleSalahoru, Constantin, Marius Valeriu Hînganu, Paul Salahoru, and Delia Hînganu. 2025. "Advances in Molecular Research of Tracheobronchial Tree Aging: A Systematic Review" International Journal of Molecular Sciences 26, no. 11: 5128. https://doi.org/10.3390/ijms26115128
APA StyleSalahoru, C., Hînganu, M. V., Salahoru, P., & Hînganu, D. (2025). Advances in Molecular Research of Tracheobronchial Tree Aging: A Systematic Review. International Journal of Molecular Sciences, 26(11), 5128. https://doi.org/10.3390/ijms26115128







