Traumatic Brain Injury and Coenzyme Q10: An Overview
Abstract
1. Introduction
2. Mitochondrial Dysfunction in Traumatic Brain Injury
3. Oxidative Stress in Traumatic Brain Injury
4. Inflammation in Traumatic Brain Injury
5. Apoptosis, Ferroptosis, and Traumatic Brain Injury
6. CoQ10 Supplementation in Animal Models of Traumatic Brain Injury
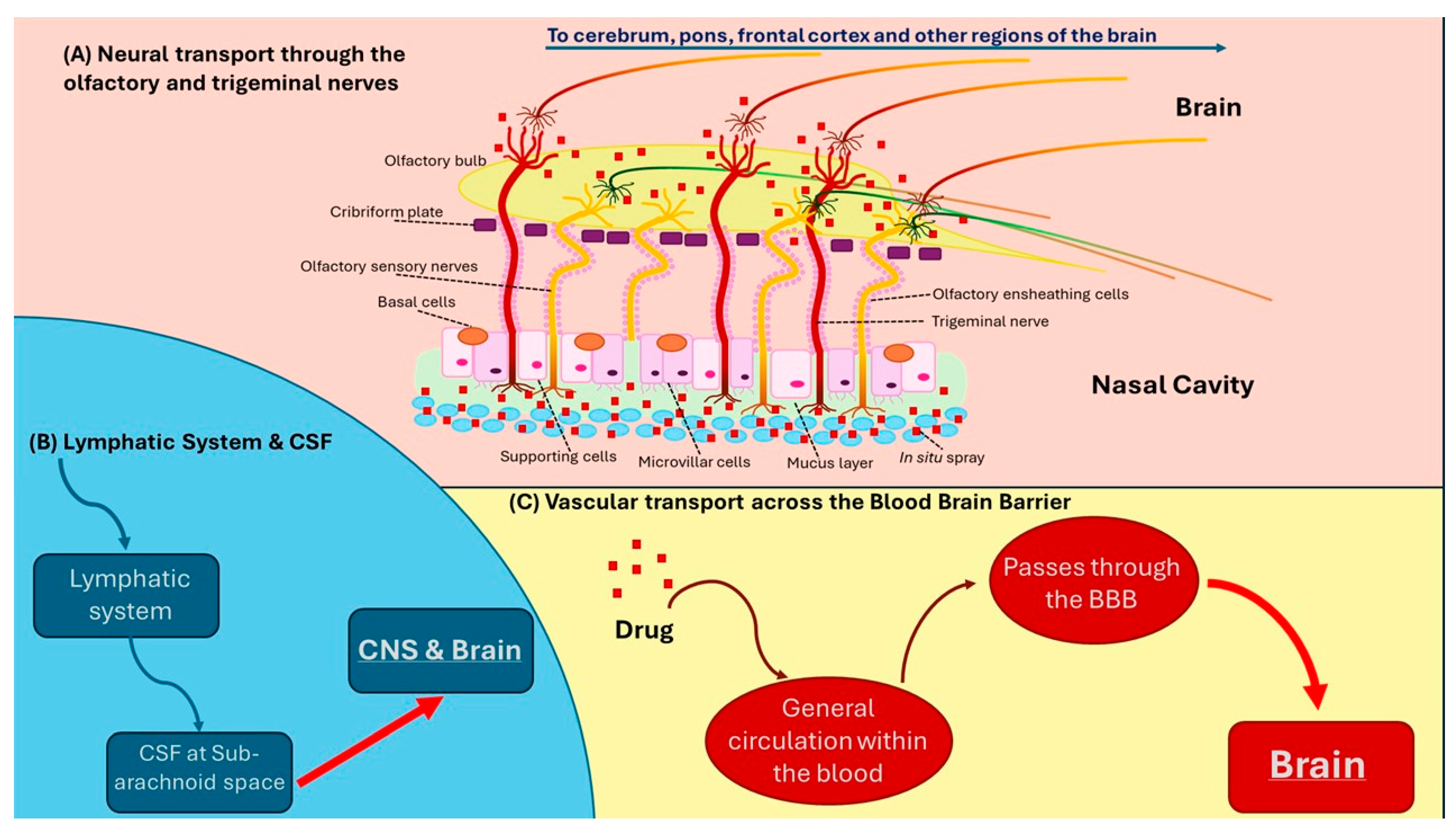
7. Transport of Coenzyme Q10 Across the Blood–Brain Barrier in Humans
8. Intranasal Delivery of CoQ10 in Traumatic Brain Injury
9. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Orr, T.J.; Lesha, E.; Kramer, A.H.; Cecia, A.; Dugan, J.E.; Schwartz, B.; Einhaus, S.L. Traumatic Brain Injury: A comprehensive review of biomechanics and molecular pathophysiology. World Neurosurg. 2024, 185, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Galgano, M.; Toshkezi, G.; Qiu, X.; Russell, T.; Chin, L.; Zhao, L.R. Traumatic brain injury: Current treatment strategies and future endeavors. Cell Transplant. 2017, 26, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, J.B.; Shen, Q.; Thimmesch, A.R.; Pierce, J.D. Traumatic brain injury and mitochondrial dysfunction. Am. J. Med. Sci. 2015, 350, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Hakiminia, B.; Alikiaii, B.; Khorvash, F.; Mousavi, S. Oxidative stress and mitochondrial dysfunction following traumatic brain injury: From mechanistic view to targeted therapeutic opportunities. Fundam. Clin. Pharmacol. 2022, 36, 612–662. [Google Scholar] [CrossRef]
- Cheng, G.; Kong, R.H.; Zhang, L.M.; Zhang, J.N. Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies. Br. J. Pharmacol. 2012, 167, 699–719. [Google Scholar] [CrossRef]
- Demers-Marcil, S.; Coles, J.P. Cerebral metabolic derangements following traumatic brain injury. Curr. Opin. Anaesthesiol. 2022, 35, 562–569. [Google Scholar] [CrossRef]
- Benaroya, H. Brain energetics, mitochondria, and traumatic brain injury. Rev. Neurosci. 2020, 31, 363–390. [Google Scholar] [CrossRef]
- Jiang, X.B.; Ohno, K.; Qian, L.; Tominaga, B.; Kuroiwa, T.; Nariai, T.; Hirakawa, K. Changes in local cerebral blood flow, glucose utilization, and mitochondrial function following traumatic brain injury in rats. Neurol. Med. Chir. 2000, 40, 16–28; discussion 28–29. [Google Scholar] [CrossRef]
- Balan, I.S.; Saladino, A.J.; Aarabi, B.; Castellani, R.J.; Wade, C.; Stein, D.M.; Eisenberg, H.M.; Chen, H.H.; Fiskum, G. Cellular alterations in human traumatic brain injury: Changes in mitochondrial morphology reflect regional levels of injury severity. J. Neurotrauma 2013, 30, 367–381. [Google Scholar] [CrossRef]
- Kumar Sahel, D.; Kaira, M.; Raj, K.; Sharma, S.; Singh, S. Mitochondrial dysfunctioning and neuroinflammation: Recent highlights on the possible mechanisms involved in Traumatic Brain Injury. Neurosci. Lett. 2019, 710, 134347. [Google Scholar] [CrossRef]
- Strogulski, N.R.; Portela, L.V.; Polster, B.M.; Loane, D.J. Fundamental neurochemistry review: Microglial immunometabolism in traumatic brain injury. J. Neurochem. 2023, 167, 129–153. [Google Scholar] [CrossRef] [PubMed]
- Signoretti, S.; Marmarou, A.; Aygok, G.A.; Fatouros, P.P.; Portella, G.; Bullock, R.M. Assessment of mitochondrial impairment in traumatic brain injury using high-resolution proton magnetic resonance spectroscopy. J Neurosurg. 2008, 108, 42–52. [Google Scholar] [CrossRef]
- Nordström, C.H.; Nielsen, T.H.; Schalén, W.; Reinstrup, P.; Ungerstedt, U. Biochemical indications of cerebral ischaemia and mitochondrial dysfunction in severe brain trauma analysed with regard to type of lesion. Acta Neurochir. 2016, 158, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Thelin, E.P.; Carpenter, K.L.; Hutchinson, P.J.; Helmy, A. Microdialysis monitoring in clinical traumatic brain injury and Its role in neuroprotective drug development. AAPS J. 2017, 19, 367–376. [Google Scholar] [CrossRef]
- Khellaf, A.; Garcia, N.M.; Tajsic, T.; Alam, A.; Stovell, M.G.; Killen, M.J.; Howe, D.J.; Guilfoyle, M.R.; Jalloh, I.; Timofeev, I.; et al. Focally administered succinate improves cerebral metabolism in traumatic brain injury patients with mitochondrial dysfunction. J. Cereb. Blood Flow. Metab. 2022, 42, 39–55. [Google Scholar] [CrossRef]
- Svedung Wettervik, T.; Hånell, A.; Howells, T.; Enblad, P.; Lewén, A. Females exhibit better cerebral pressure autoregulation, less mitochondrial dysfunction, and reduced excitotoxicity after severe traumatic brain injury. J. Neurotrauma 2022, 39, 1507–1517. [Google Scholar] [CrossRef]
- Verweij, B.H.; Muizelaar, J.P.; Vinas, F.C.; Peterson, P.L.; Xiong, Y.; Lee, C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000, 93, 815–820. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, Q.; Cheng, H.; Yu, J.; Gao, L.; Gao, G. USP30 impairs mitochondrial quality control and aggravates oxidative damage after traumatic brain injury. Biochem. Biophys. Res. Commun. 2023, 671, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Raheja, A.; Samson, N.; Bhoi, S.; Selvi, A.; Sharma, P.; Sharma, B.S. Blood mitochondrial enzymatic assay as a predictor of long-term outcome in severe traumatic brain injury. J. Clin. Neurosci. 2016, 30, 31–38. [Google Scholar] [CrossRef]
- Vespa, P.; Bergsneider, M.; Hattori, N.; Wu, H.M.; Huang, S.C.; Martin, N.A.; Glenn, T.C.; McArthur, D.L.; Hovda, D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: A combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005, 25, 763–774. [Google Scholar] [CrossRef]
- Lai, Y.; Stange, C.; Wisniewski, S.R.; Adelson, P.D.; Janesko-Feldman, K.L.; Brown, D.S.; Kochanek, P.M.; Clark, R.S. Mitochondrial heat shock protein 60 is increased in cerebrospinal fluid following pediatric traumatic brain injury. Dev. Neurosci. 2006, 28, 336–341. [Google Scholar] [CrossRef]
- Conley, Y.P.; Okonkwo, D.O.; Deslouches, S.; Alexander, S.; Puccio, A.M.; Beers, S.R.; Ren, D. Mitochondrial polymorphisms impact outcomes after severe traumatic brain injury. J. Neurotrauma 2014, 31, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki-Zadeh, A.; Datta, D. An overview of preclinical models of traumatic brain injury (TBI): Relevance to pathophysiological mechanisms. Front. Cell Neurosci. 2024, 18, 1371213. [Google Scholar] [CrossRef]
- Wu, Q.; Xia, S.X.; Li, Q.Q.; Gao, Y.; Shen, X.; Ma, L.; Zhang, M.Y.; Wang, T.; Li, Y.S.; Wang, Z.F.; et al. Mitochondrial division inhibitor 1 (Mdivi-1) offers neuroprotection through diminishing cell death and improving functional outcome in a mouse model of traumatic brain injury. Brain Res. 2016, 1630, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wiley, C.A.; Bissel, S.J.; Lesniak, A.; Dixon, C.E.; Franks, J.; Beer Stolz, D.; Sun, M.; Wang, G.; Switzer, R.; Kochanek, P.M.; et al. Ultrastructure of Diaschisis Lesions after traumatic brain injury. J. Neurotrauma 2016, 33, 1866–1882. [Google Scholar] [CrossRef]
- Hackett, E.P.; Chen, J.; Ingle, L.; Al Nemri, S.; Barshikar, S.; da Cunha Pinho, M.; Plautz, E.J.; Bartnik-Olson, B.L.; Park, J.M. Longitudinal assessment of mitochondrial dysfunction in acute traumatic brain injury using hyperpolarized [1-13C]pyruvate. Magn. Reson Med. 2023, 90, 2432–2442. [Google Scholar] [CrossRef]
- Xiong, Y.; Gu, Q.; Peterson, P.L.; Muizelaar, J.P.; Lee, C.P. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 1997, 14, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Gilmer, L.K.; Roberts, K.N.; Joy, K.; Sullivan, P.G.; Scheff, S.W. Early mitochondrial dysfunction after cortical contusion injury. J. Neurotrauma 2009, 26, 1271–1280. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef]
- Vos, M.; Lauwers, E.; Verstreken, P. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front. Synaptic Neurosci. 2010, 2, 139. [Google Scholar] [CrossRef]
- Hill, R.L.; Kulbe, J.R.; Singh, I.N.; Wang, J.A.; Hall, E.D. Synaptic mitochondria are more susceptible to traumatic brain injury-induced oxidative damage and respiratory dysfunction than non-synaptic mitochondria. Neuroscience 2018, 386, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Opii, W.O.; Nukala, V.N.; Sultana, R.; Pandya, J.D.; Day, K.M.; Merchant, M.L.; Klein, J.B.; Sullivan, P.G.; Butterfield, D.A. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J. Neurotrauma 2007, 24, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, Y.L.; Nguyen, L.T.; Mao, Y.; de Rosa, A.; Beh, I.T.; Chee, C.; Oliver, B.; Herok, G.; Saad, S.; et al. Moderate traumatic brain injury is linked to acute behaviour deficits and long term mitochondrial alterations. Clin. Exp. Pharmacol. Physiol. 2016, 43, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Cheng, H.L.; Huang, R.Q.; Zhuang, Z.; Shi, J.X. Quantitative detection of the expression of mitochondrial cytochrome c oxidase subunits mRNA in the cerebral cortex after experimental traumatic brain injury. Brain Res. 2009, 1251, 287–295. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, J.; Lu, D.; Zhang, Y.; Xu, J.; Wang, S.; Cheng, X.; Qin, J.; Zhang, L.; Li, H.; et al. Uqcr11 alleviates oxidative stress and apoptosis after traumatic brain injury. Exp. Neurol. 2023, 370, 114582. [Google Scholar] [CrossRef] [PubMed]
- Yonutas, H.M.; Vekaria, H.J.; Sullivan, P.G. Mitochondrial specific therapeutic targets following brain injury. Brain Res. 2016, 1640 Pt A, 77–93. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Rabchevsky, A.G.; Waldmeier, P.C.; Springer, J.E. Mitochondrial permeability transition in CNS trauma: Cause or effect of neuronal cell death? J. Neurosci. Res. 2005, 79, 231–239. [Google Scholar] [CrossRef]
- Hubbard, W.B.; Vekaria, H.J.; Velmurugan, G.V.; Kalimon, O.J.; Prajapati, P.; Brown, E.; Geisler, J.G.; Sullivan, P.G. Mitochondrial dysfunction after repeated mild blast traumatic brain injury Is attenuated by a mild mitochondrial uncoupling prodrug. J. Neurotrauma 2023, 40, 2396–2409. [Google Scholar] [CrossRef]
- Mantle, D.; Dewsbury, M.; Hargreaves, I.P. The Ubiquinone-Ubiquinol redox cycle and Its clinical consequences: An overview. Int. J. Mol. Sci. 2024, 25, 6765. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective effect of antioxidants in the brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Awasthi, D.; Church, D.F.; Torbati, D.; Carey, M.E.; Pryor, W.A. Oxidative stress following traumatic brain injury in rats. Surg. Neurol. 1997, 47, 575–581; discussion 581–582. [Google Scholar] [CrossRef] [PubMed]
- Tyurin, V.A.; Tyurina, Y.Y.; Borisenko, G.G.; Sokolova, T.V.; Ritov, V.B.; Quinn, P.J.; Rose, M.; Kochanek, P.; Graham, S.H.; Kagan, V.E. Oxidative stress following traumatic brain injury in rats: Quantitation of biomarkers and detection of free radical intermediates. J. Neurochem. 2000, 75, 2178–2189. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.C.; Chen, T.W.; Yang, T.C.; Wei, H.J.; Hsu, J.C.; Lin, C.L. Levels of F2-isoprostanes, F4-neuroprostanes, and total nitrate/nitrite in plasma and cerebrospinal fluid of patients with traumatic brain injury. Free Radic. Res. 2015, 49, 1419–1430. [Google Scholar] [CrossRef]
- Gutierrez-Mariscal, F.M.; Arenas-de Larriva, A.P.; Limia-Perez, L.; Romero-Cabrera, J.L.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 supplementation for the reduction of oxidative tress: Clinical implications in the treatment of chronic diseases. Int. J. Mol. Sci. 2020, 21, 7870. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayır, H.; Clark, R.S.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and immune function: An overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef]
- Wong, J.; Hoe, N.W.; Zhiwei, F.; Ng, I. Apoptosis and traumatic brain injury. Neurocrit. Care 2005, 3, 177–182. [Google Scholar] [CrossRef]
- Unnisa, A.; Greig, N.H.; Kamal, M.A. Inhibition of Caspase 3 and Caspase 9 mediated apoptosis: A multimodal therapeutic target in traumatic brain injury. Curr. Neuropharmacol. 2023, 21, 1001–1012. [Google Scholar] [CrossRef]
- Li, X.; Zhan, J.; Hou, Y.; Chen, S.; Hou, Y.; Xiao, Z.; Luo, D.; Lin, D. Coenzyme Q10 suppresses oxidative stress and apoptosis via activating the Nrf-2/NQO-1 and NF-κB signaling pathway after spinal cord injury in rats. Am. J. Transl. Res. 2019, 11, 6544–6552. [Google Scholar]
- Sumi, K.; Okura, T.; Fujioka, Y.; Kato, M.; Imamura, T.; Taniguchi, S.I.; Yamamoto, K. Coenzyme Q10 suppresses apoptosis of mouse pancreatic β-cell line MIN6. Diabetol. Metab. Syndr. 2018, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Guo, Z.; Guo, R.; Ye, R.; Zhu, W.; Yan, B. Ferroptosis and traumatic brain injury. Brain Res. Bull. 2021, 172, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Fikry, H.; Saleh, L.A.; Mahmoud, F.A.; Gawad, S.A.; Abd-Alkhalek, H.A. CoQ10 targeted hippocampal ferroptosis in a status epilepticus rat model. Cell Tissue Res. 2024, 396, 371–397. [Google Scholar] [CrossRef]
- Peng, Z.; Ding, Y.N.; Yang, Z.M.; Li, X.J.; Zhuang, Z.; Lu, Y.; Tang, Q.S.; Hang, C.H.; Li, W. Neuron-targeted liposomal coenzyme Q10 attenuates neuronal ferroptosis after subarachnoid hemorrhage by activating the ferroptosis suppressor protein 1/coenzyme Q10 system. Acta Biomater. 2024, 179, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, G.; Wang, Z.; Guo, J.; Liu, Y.; Lu, Y.; Qin, Z.; Xu, Y.; Cao, C.; Wang, B.; et al. Idebenone attenuates ferroptosis by inhibiting excessive autophagy via the ROS-AMPK-mTOR pathway to preserve cardiac function after myocardial infarction. Eur. J. Pharmacol. 2023, 943, 175569. [Google Scholar] [CrossRef]
- Avcı, B.; Günaydın, C.; Güvenç, T.; Yavuz, C.K.; Kuruca, N.; Bilge, S.S. Idebenone ameliorates rotenone-induced parkinson’s disease in rats through decreasing lipid peroxidation. Neurochem. Res. 2021, 46, 513–522. [Google Scholar] [CrossRef]
- Tao, L.; Xue, Y.F.; Sun, F.F.; He, X.; Wang, H.Q.; Tong, C.C.; Zhang, C.; Xu, D.X.; Chen, X. MitoQ protects against carbon tetrachloride-induced hepatocyte ferroptosis and acute liver injury by suppressing mtROS-mediated ACSL4 upregulation. Toxicol. Appl. Pharmacol. 2024, 486, 116914. [Google Scholar] [CrossRef]
- Lazzarino, G.; Mangione, R.; Saab, M.W.; Tavazzi, B.; Pittalà, A.; Signoretti, S.; Di Pietro, V.; Lazzarino, G.; Amorini, A.M. Traumatic brain injury alters cerebral concentrations and redox states of coenzymes Q9 and Q10 in the rat. Antioxidants 2023, 12, 985. [Google Scholar] [CrossRef]
- Kalayci, M.; Unal, M.M.; Gul, S.; Acikgoz, S.; Kandemir, N.; Hanci, V.; Edebali, N.; Acikgoz, B. Effect of coenzyme Q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci. 2011, 12, 75. [Google Scholar] [CrossRef]
- Pierce, J.D.; Gupte, R.; Thimmesch, A.; Shen, Q.; Hiebert, J.B.; Brooks, W.M.; Clancy, R.L.; Diaz, F.J.; Harris, J.L. Ubiquinol treatment for TBI in male rats: Effects on mitochondrial integrity, injury severity, and neurometabolism. J. Neurosci. Res. 2018, 96, 1080–1092. [Google Scholar] [CrossRef]
- Pierce, J.D.; Shen, Q.; Peltzer, J.; Thimmesch, A.; Hiebert, J.B. A pilot study exploring the effects of ubiquinol on brain genomics after traumatic brain injury. Nurs. Outlook 2017, 65, S44–S52. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.; Elgohary, R. L-carnitine and Co Q10 ameliorate potassium dichromate -induced acute brain injury in rats targeting AMPK/AKT/NF-κβ. Int. Immunopharmacol. 2021, 101 Pt B, 107867. [Google Scholar] [CrossRef]
- El-Laithy, N.A.; Mahdy, E.M.E.; Youness, E.R.; Shafee, N.; Mowafy, M.S.S.; Mabrouk, M.M. Effect of Co Enzyme Q10 alone or in combination with Vitamin C on lipopolysaccharide-induced brain injury in rats. Biomed. Pharmacol. J. 2018, 11, 1215–1226. [Google Scholar] [CrossRef]
- Haidar, M.A.; Shakkour, Z.; Barsa, C.; Tabet, M.; Mekhjian, S.; Darwish, H.; Goli, M.; Shear, D.; Pandya, J.D.; Mechref, Y.; et al. Mitoquinone helps combat the neurological, cognitive, and molecular consequences of open head traumatic brain injury at chronic time point. Biomedicines 2022, 10, 250. [Google Scholar] [CrossRef]
- Tabet, M.; El-Kurdi, M.; Haidar, M.A.; Nasrallah, L.; Reslan, M.A.; Shear, D.; Pandya, J.D.; El-Yazbi, A.F.; Sabra, M.; Mondello, S.; et al. Mitoquinone supplementation alleviates oxidative stress and pathologic outcomes following repetitive mild traumatic brain injury at a chronic time point. Exp. Neurol. 2022, 351, 113987. [Google Scholar] [CrossRef] [PubMed]
- Gülşen, İ.; Ak, H.; Çölçimen, N.; Alp, H.H.; Akyol, M.E.; Demir, İ.; Atalay, T.; Balahroğlu, R.; Rağbetli, M.Ç. Neuroprotective effects of thymoquinone on the hippocampus in a rat model of traumatic brain injury. World Neurosurg. 2016, 86, 243–249. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; Álvarez-Córdoba, M.; Villalón-García, I.; Munuera-Cabeza, M.; Suárez-Carrillo, A.; Talaverón-Rey, M.; Sánchez-Alcázar, J.A. Coenzyme Q10 analogues: Benefits and challenges for therapeutics. Antioxidants 2021, 10, 236. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, L.; Hargreaves, I.P.; Georgian, A.R.; Turner, C.; Dalton, R.N.; Abbott, N.J.; Heales, S.J.R.; Preston, J.E. CoQ10 Deficient endothelial cell culture model for the investigation of CoQ10 blood-brain barrier transport. J. Clin. Med. 2020, 9, 3236. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Mantle, D.; Lopez-Lluch, G.; Hargreaves, I.P. Coenzyme Q10 metabolism: A review of unresolved issues. Int. J. Mol. Sci. 2023, 24, 2585. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P. Mitochondrial dysfunction and neurodegenerative disorders: Role of nutritional supplementation. Int. J. Mol. Sci. 2022, 23, 12603. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10, ageing and the nervous system: An overview. Antioxidants 2021, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug transport across the blood-brain barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.D.; Musyaju, S.; Modi, H.R.; Okada-Rising, S.L.; Bailey, Z.S.; Scultetus, A.H.; Shear, D.A. Intranasal delivery of mitochondria targeted neuroprotective compounds for traumatic brain injury: Screening based on pharmacological and physiological properties. J. Transl. Med. 2024, 22, 167. [Google Scholar] [CrossRef]
- Tepper, S.J.; Cady, R.K.; Silberstein, S.; Messina, J.; Mahmoud, R.A.; Djupesland, P.G.; Shin, P.; Siffert, J. AVP-825 breath-powered intranasal delivery system containing 22 mg sumatriptan powder vs. 100 mg oral sumatriptan in the acute treatment of migraines (The COMPASS study): A comparative randomized clinical trial across multiple attacks. Headache 2015, 55, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Munjal, S.; Buse, D.C.; Bennett, A.; Fanning, K.M.; Burstein, R.; Reed, M.L. Allodynia Is Associated With Initial and Sustained Response to Acute Migraine Treatment: Results from the American Migraine Prevalence and Prevention Study. Headache 2017, 57, 1026–1040. [Google Scholar] [CrossRef] [PubMed]
- Lipton, R.B.; Munjal, S.; Alam, A.; Buse, D.C.; Fanning, K.M.; Reed, M.L.; Schwedt, T.J.; Dodick, D.W. Migraine in America Symptoms and Treatment (MAST) Study: Baseline Study Methods, Treatment Patterns, and Gender Differences. Headache 2018, 58, 1408–1426. [Google Scholar] [CrossRef]
- Claxton, A.; Baker, L.D.; Hanson, A.; Trittschuh, E.H.; Cholerton, B.; Morgan, A.; Callaghan, M.; Arbuckle, M.; Behl, C.; Craft, S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J. Alzheimers Dis. 2015, 44, 897–906. [Google Scholar] [CrossRef]
- Kellar, D.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal Insulin Reduces White Matter Hyperintensity Progression in Association with Improvements in Cognition and CSF Biomarker Profiles in Mild Cognitive Impairment and Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2021, 8, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Kellar, D.; Register, T.; Lockhart, S.N.; Aisen, P.; Raman, R.; Rissman, R.A.; Brewer, J.; Craft, S. Intranasal insulin modulates cerebrospinal fluid markers of neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A randomized trial. Sci. Rep. 2022, 12, 1346. [Google Scholar] [CrossRef] [PubMed]
- Bancke, L.L.; Dworak, H.A.; Rodvold, K.A.; Halvorsen, M.B.; Gidal, B.E. Pharmacokinetics, pharmacodynamics, and safety of USL261, a midazolam formulation optimized for intranasal delivery, in a randomized study with healthy volunteers. Epilepsia 2015, 56, 1723–1731. [Google Scholar] [CrossRef] [PubMed]
- Milési, C.; Baleine, J.; Mura, T.; Benito-Castro, F.; Ferragu, F.; Thiriez, G.; Thévenot, P.; Combes, C.; Carbajal, R.; Cambonie, G. Nasal midazolam vs ketamine for neonatal intubation in the delivery room: A randomised trial. Arch. Dis. Child. Fetal Neonatal Ed. 2018, 103, F221–F226. [Google Scholar] [CrossRef]
- Henney III, H.R.; Sperling, M.R.; Rabinowicz, A.L.; Bream, G.; Carrazana, E.J. Assessment of pharmacokinetics and tolerability of intranasal diazepam relative to rectal gel in healthy adults. Epilepsy Res. 2014, 108, 1204–1211. [Google Scholar] [CrossRef]
- Ahmad, S.; Ellis, J.C.; Kamwendo, H.; Molyneux, E. Efficacy and safety of intranasal lorazepam versus intramuscular paraldehyde for protracted convulsions in children: An open randomised trial. Lancet 2006, 367, 1591–1597. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Bitter, I.; Buyze, J.; Fagiolini, A.; Godinov, Y.; Gorwood, P.; Ito, T.; Oliveira-Maia, A.J.; Vieta, E.; Werner-Kiechle, T.; et al. Safety and tolerability of esketamine nasal spray versus quetiapine extended release in patients with treatment resistant depression. Eur. Neuropsychopharmacol. 2024, 85, 58–65. [Google Scholar] [CrossRef]
- Vitale, D.C.; Piazza, C.; Sinagra, T.; Urso, V.; Cardì, F.; Drago, F.; Salomone, S. Pharmacokinetic characterization of tizanidine nasal spray, a novel intranasal delivery method for the treatment of skeletal muscle spasm. Clin. Drug Investig. 2013, 33, 885–891. [Google Scholar] [CrossRef]
- Simmons, R.G.; Phillips, J.B.; Lojewski, R.A.; Wang, Z.; Boyd, J.L.; Putcha, L. The efficacy of low-dose intranasal scopolamine for motion sickness. Aviat Space Environ. Med. 2010, 81, 405–412. [Google Scholar] [CrossRef]
- Silachev, D.N.; Plotnikov, E.Y.; Zorova, L.D.; Pevzner, I.B.; Sumbatyan, N.V.; Korshunova, G.A.; Gulyaev, M.V.; Pirogov, Y.A.; Skulachev, V.P.; Zorov, D. Neuroprotective effects of mitochondria-targeted plastoquinone and thymoquinone in a rat model of brain ischemia/reperfusion injury. Molecules 2015, 20, 14487–14503. [Google Scholar] [CrossRef]
- Kauli, R.; Laron, Z. A vasopressin analogue in treatment of diabetes insipidus. Arch. Dis. Child. 1974, 49, 482–485. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kalra, S.; Dhingra, M. Intranasal glucagon. J. Pak. Med. Assoc. 2019, 69, 1219–1221. [Google Scholar]
- Priya, G.; Kalra, S.; Dasgupta, A.; Grewal, E. Diabetes Insipidus: A pragmatic approach to management. Cureus 2021, 13, e12498. [Google Scholar] [CrossRef] [PubMed]
- Hasanloei, M.A.V.; Zeinaly, A.; Rahimlou, M.; Houshyar, H.; Moonesirad, S.; Hashemi, R. Effect of coenzyme Q10 supplementation on oxidative stress and clinical outcomes in patients with low levels of coenzyme Q10 admitted to the intensive care unit. J. Nutr. Sci. 2021, 10, e48. [Google Scholar] [CrossRef]
- Hargreaves, I.P.; Mantle, D. Supplementation with selenium and coenzyme Q10 in critically ill patients. Br. J. Hosp. Med. 2019, 80, 589–593. [Google Scholar] [CrossRef]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q10: An overview of the absorption process and subsequent metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Kalenikova, E.I.; Gorodetskaya, E.A.; Povarova, O.V.; Medvedev, O.S. Prospects of Intravenous Coenzyme Q10 Administration in Emergency Ischemic Conditions. Life 2024, 14, 134. [Google Scholar] [CrossRef]
- Cash, A.; Theus, M.H. Mechanisms of blood-brain barrier dysfunction in traumatic brain injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef]
- Capossela, L.; Graglia, B.; Ferretti, S.; Di Sarno, L.; Gatto, A.; Calcagni, M.L.; Di Giuda, D.; Cocciolillo, F.; Romeo, D.M.; Manni, L.; et al. Intranasal human-recombinant nerve growth factor administration improves cognitive functions in a child with severe traumatic brain injury. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 4302–4312. [Google Scholar] [CrossRef]
- Huang, C.H.; Yang, C.T.; Chang, C.C. Traumatic brain injury and risk of heart failure and coronary heart disease: A nationwide population-based cohort study. PLoS ONE 2023, 18, e0295416. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, T.; Li, L.; Chopp, M.; Venkat, P.; Qian, Y.; Li, R.; Wu, R.; Li, W.; Lu, M.; et al. Immune response mediates cardiac dysfunction after traumatic brain injury. J. Neurotrauma 2019, 36, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D. Coenzyme Q10 and cardiovascular disease: An overview. Br. J. Cardiol. 2015, 22, 160. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, J.; Yu, J.; Liu, R.; Ma, H.; Zhao, Y. Nicotinamide mononucleotides alleviated neurological impairment via anti-neuroinflammation in traumatic brain injury. Int. J. Med. Sci. 2023, 20, 307–317. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Hakan, T.; Biber, N.; Solakoğlu, S.; Oğünç, A.V.; Sener, G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic. Res. 2009, 43, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Hiskens, M.I.; Li, K.M.; Schneiders, A.G.; Fenning, A.S. Repetitive mild traumatic brain injury-induced neurodegeneration and inflammation is attenuated by acetyl-L-carnitine in a preclinical model. Front. Pharmacol. 2023, 14, 1254382. [Google Scholar] [CrossRef]
| Drug | Indication | Outcome | Reference |
|---|---|---|---|
| Sumatriptan | Migraine | Greater relief in pain intensity versus oral delivery | Tepper et al. (2015) [77] |
| Sumatriptan | Migraine | Faster reduction in pain intensity versus oral delivery | Lipton et al. (2017) [78] |
| Sumatriptan | Migraine | Reduced nausea versus oral delivery | Lipton et al. (2018) [79] |
| Insulin | Alzheimer’s disease | Improved cognition | Claxton et al. (2015) [80] |
| Insulin | Alzheimer’s disease | Reduced white matter hyperintensity volume progression | Kellar et al. (2021) [81] |
| Insulin | Alzheimer’s disease | Reduced levels of CSF inflammatory markers | Kellar et al. (2022) [82] |
| Midazolam | Sedative | Improved bioavailability versus intravenous delivery | Bancke et al. (2015) [83] |
| Midazolam | Neonatal sedation | More effective than ketamine | Milesi et al. (2018) [84] |
| Diazepam | Anticonvulsant | More acceptable administration route versus rectal delivery | Henney et al. (2014) [85] |
| Lorazepam | Anticonvulsant | Less invasive alternative to intramuscular injection | Ahmad et al. (2006) [86] |
| Esketamine | Treatment resistant depression | Efficacy and safety confirmed | McIntyre et al. (2024) [87] |
| Tizanidine | Muscle spacticity | Greater bioavailability versus oral delivery | Vitale et al. (2013) [88] |
| Scopolamine | Motion sickness | More rapid absorption versus oral or transdermal delivery | Simmons et al. (2010) [89] |
| Study | Outcome | Case Model |
|---|---|---|
| Yonutas et al., 2016 [36] | Reviewed the therapeutic approaches to ameliorate mitochondrial dysfunction following brain injury. | Review |
| Sullivan et al., 2005 [37] | Reviewed the evidence relating to mitochondrial permeability transition in central nervous system trauma and evidence for therapeutically targeting the mitochondrial permeability transition in TBI. | Review |
| Hubbard et al., 2023 [38] | Reported that mild mitochondrial uncoupling can restore mitochondrial bioenergetics and oxidative balance following TBI. | Animal model using male Sprague Dawley rats at 8 weeks of age; there were six experimental groups, each with eight subjects. These groups were subjected to compressed helium-driven blasts at 11 psi to induce mTBI. The treatment groups received 8 or 80 mg/kg of MP201 (2,4-dinitrophenol prodrug, uncoupler) with administration early or delayed after mTBI. |
| Yen et al., 2015 [43] | Reported increased levels of lipid peroxidation biomarkers and the need for antioxidant protection in TBI patients. | Moderate and severe TBI patients with an age range of 15–75 years. The patients were randomly treated with 10 mg/mL propofol or 5 mg/mL midazolam for 72 h postoperation. Cerebrospinal fluid and plasma were collected from 15 patients for 6–10 days after exposure. |
| Simon et al., 2017 [46] | Found that mitochondrial dysfunction and oxidative stress contributed to the loss of control of the inflammation process in TBI patients. The loss of control of this process resulted in further tissue damage, neurological deficit, and neurodegenerative changes associated with TBI. | Review |
| Mantle et al., 2021 [47] | Reported that CoQ10 could modulate directly the action of genes involved in inflammation and may help to control the release of pro-inflammatory cytokines. | Review |
| Lin et al., 2023 [35] | Reported that the upregulation of ubiquinol–cytochrome c reductase, complex III subunit XI (Uqcr11), in a mouse model of TBI reduced neuronal apoptosis. | Review |
| Geng et al., 2021 [52] | Reported that ferroptosis was related to the pathology of TBI and that the inhibition of ferroptosis could improve long-term outcomes of TBI. | Review |
| Fikry et al., 2023 [53] | A rat study found that CoQ10 had a beneficial targeted effect on hippocampal oxidative stress and ferroptosis. | A lithium–pilocarpine rat model was created using male Wistar rats from 6 to 8 weeks old. Seizures were induced using 0.5 mg/mL pilocarpine diluted in DMSO, injected intraperitoneally at 100 mg/kg. The CoQ10-treated group was given CoQ10 at 20 mg/kg via gavage once a day for 2 weeks before it was given a Pilo injection. |
| Lazzarino et al., 2023 [58] | A rat study found that severe TBI changed the levels and redox states of CoQ9 and CoQ10, indicating TBI-associated mitochondrial impairment affecting oxidative phosphorylation, energy generation, and antioxidant defence. | Rat study with induced graded TBIs (mild, moderate, and severe) in 26 male Wistar rats of 300–350 g body weight (b.w.). The subjects were administered 35 mg/kg b.w. ketamine and 0.25 mg/kg b.w. midazolam to indue anaesthesia prior to TBI induction. Reduced and oxidised CoQ9 and CoQ10 were determined via HPLC analysis. |
| Kalayci et al., 2011 [59] | A rat study found that CoQ10 decreased neuronal degeneration, secondary brain damage, and ischemia caused by oxidative stress in TBI rats. | The study used 28 Wistar albino male rats with a body weight between 350 and 400 g to create a brain injury model. The rats in the CoQ10 group were administered a CoQ10 dose of 10 mg/kg immediately after trauma was induced and again at the 24th hour post-trauma via gavage. |
| Pierce et al., 2018 [60] | A rat study found that ubiquinol administered before or after TBI reduced brain mitochondrial damage, apoptosis, and serum biomarkers of TBI severity. | The study used 36 adult male F344 rats with induced TBI. The rats with pre-treatment before TBI were administered 100 mg/kg b.w. ubiquinol intra-arterially 30 min before the cortical impact. The rats with post-TBI treatment were administered 100 mg/kg b.w. ubiquinol 30 min after the cortical impact. |
| Salama et al., 2021 [62] | A rat study found that CoQ10 reduced biomarkers of oxidative stress and inflammation in a rat model of brain injury. | Rat model with potassium dichromate (PD) induced brain injury via the intranasal administration of 2 mg/kg PD. Male Wister albino rats of 140–150 g were used. Starting 24 h post-PD-induced brain injury, the subjects were administered 50 mg/kg CoQ10 orally for 3 days. Prior to and after the experiment, locomotor activity was assessed, and biochemical and histopathological investigations were assessed in the brain homogenate. |
| El-Laithy et al., 2018 [63] | A rat study found that CoQ10 decreased biomarkers of brain tissue oxidative stress in a rat model of brain injury. | The study used 66 female Wistar albino rats with a body weight between 100 and 120 g at 3 months old. The groups were treated with 100 mg/kg or 200 mg/kg CoQ10. The subjects were treated with or without lipopolysaccharide (LPS) simultaneously with CoQ10 to induce brain injury. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, D.; Dewsbury, M.; Mendelow, A.D.; Hargreaves, I.P. Traumatic Brain Injury and Coenzyme Q10: An Overview. Int. J. Mol. Sci. 2025, 26, 5126. https://doi.org/10.3390/ijms26115126
Mantle D, Dewsbury M, Mendelow AD, Hargreaves IP. Traumatic Brain Injury and Coenzyme Q10: An Overview. International Journal of Molecular Sciences. 2025; 26(11):5126. https://doi.org/10.3390/ijms26115126
Chicago/Turabian StyleMantle, David, Mollie Dewsbury, Alexander David Mendelow, and Iain P. Hargreaves. 2025. "Traumatic Brain Injury and Coenzyme Q10: An Overview" International Journal of Molecular Sciences 26, no. 11: 5126. https://doi.org/10.3390/ijms26115126
APA StyleMantle, D., Dewsbury, M., Mendelow, A. D., & Hargreaves, I. P. (2025). Traumatic Brain Injury and Coenzyme Q10: An Overview. International Journal of Molecular Sciences, 26(11), 5126. https://doi.org/10.3390/ijms26115126








